Introduction
Recently, the incidence of diabetic mellitus (DM)
has sharply increased; it has become one of the major diseases
affecting quality of life and represents a threat to public health
(1). The World Health Organization
has predicted that the number of patients with DM will increase to
366 million worldwide by 2030 (2).
An epidemiological study in China in 2010 reported that the
incidence of DM in adults was 11.6%, while the overall incidence of
DM in the population was 50.1% (3).
Diabetic foot ulcers (DFUs) commonly occur in
patients with DM, and represent one of the principal chronic and
severe complications associated with the disease. DFUs may arise
from peripheral neuropathy, lower limb vasculopathy-associated foot
infections and ulcerations. The most serious outcomes of DFUs are
amputation or mortality; thus, DFUs may severely affect the quality
of life of patients with DM and their families (4,5). As
the incidence of DM increases, the number of patients with DFUs is
also increasing, with ~15% of patients with DM worldwide suffering
from DFUs, and this number is even higher in China (25%) (6). The resulting series of clinical and
social problems caused by DFUs has attracted scientific interest,
and comprehensive investigation of DFU pathogenesis and refractory
mechanisms has become an important research topic.
The immunopathological effect mediated by T
lymphocytes has been reported to have an important role in the
occurrence and development of DM (7,8).
Typically, genetic and immune factors can promote damage to islet B
cells, while metabolic disorders, environmental factors and the
secretion of glucocorticoids may inhibit the function of
lymphocytes (9). Long-term
hyperglycemia and the glycosylation of cell membranes may also
damage the function of granulocytes and attenuate the response of T
cells to mitosis (10). However,
despite numerous studies having investigated immune dysfunction in
patients with type 2 DM, the underlying molecular mechanisms remain
unclear.
Human genome transcription products with no protein
coding activity have been demonstrated to exhibit biological
functions, rather than merely existing as transcriptional noise.
For example, long non-coding (lnc)RNAs are RNA molecules with
lengths of >200 nucleotides that do not exhibit protein coding
activity; however, lncRNAs do have genetic regulatory functions.
Numerous studies have demonstrated that abnormal lncRNA expression
is closely associated with DM and its complications. Sequencing of
the human β cell transcriptome identified 1,128 pancreas
islet-specific lncRNAs, a number of which were associated with
pancreatic differentiation (11).
Gao et al (12) revealed
that the expression of H19 lncRNA is significantly decreased in the
skeletal muscle of patients with type 2 DM and in animals with
insulin resistance, and suppressed expression of H19 results in
interference of the insulin signal in myocytes and reduced glucose
uptake, which suggests that H19 is involved in the glycometabolism
of DM. Furthermore, a study investigating diabetic retinopathy
demonstrated that lncRNA-p21 inhibits the proliferation of vascular
smooth muscle cells and macrophages, and induces apoptosis via
regulation of cellular tumor antigen p53 signaling (13). In addition, rs2648875, rs13447075
and rs2648862 lncRNAs are closely associated with the occurrence
and development of diabetic nephropathy (14).
Despite numerous studies having investigated
lncRNAs, DM and its complications, few studies have investigated
the immunoregulation of lncRNAs in DFUs. Therefore, in the present
study, the T lymphocyte subset and differences in the expression
levels of inflammation-associated cytokines in DFU tissues were
investigated. In addition, lncRNAs exhibiting target regulatory
associations with immune cells and relevant factors were screened
for using gene chip technology to determine the immunoregulation
and potential molecular mechanism of lncRNAs associated with the
occurrence and development of DFUs. The results of the present
study may identify molecular targets for the prevention and
treatment of DFUs.
Materials and methods
Tissue specimen collection
A total of 39 patients with DFU and 22 trauma
patients receiving debridement at Yijishan Hospital, Wannan Medical
College (Wuhu, China) between January and February 2017 were
enrolled. A total of 3 patients with DFU and 3 trauma patients were
randomly divided into an ulcer group and a control group. The ulcer
group contained one man and two women aged 50–69 years, and the
control group contained one man and two women aged 48–65 years.
There were no significant differences between the two groups.
Patients with DM were excluded from the control group, which
included patients whose wounds were caused by trauma, and for whom
debridement indications were clear. Patients with a confirmed
diagnosis of type 2 DM were included in the ulcer group. Their
lower limb ulcers had persisted for longer than 1 month with no
healing following medical treatment. Debridement indications were
again clear for this group.
Complete skin tissues (2×2 cm) were collected from
the center of the wound surfaces from patients in the two groups.
Following washing, a number of tissue samples were fixed in 4%
paraformaldehyde for 48 h at 25°C and embedded in paraffin. The
remaining tissues were stored at −80°C. The present study was
approved by the Ethics Committee of Yijishan Hospital, and all
patients provided written informed consent.
Pathological staining
Wound surface tissues were fixed with 4%
paraformaldehyde, washed in purified water, dehydrated in ethanol,
cleared by purified water, immersed in paraffin, embedded and
sectioned at 5 µm. The sections were further dewaxed at 25°C,
hydrated at 25°C and stained using hematoxylin and eosin (HE) for
30 sec at 25°C and Masson's trichome for 40 sec at 25°C (Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) to observe
histological changes, including alterations associated with blood
capillaries, collagen and fibroblasts.
Immunofluorescent staining of tissue
specimens
Skin tissues were frozen for 30 min at −20°C and cut
into sections (8 µm). The sections were fixed in paraformaldehyde
for 1 day at 25°C, washed a number of times with distilled water
and subsequently dried at room temperature. Following this, the
sections were treated with pure acetone at 4°C for 10 min and dried
prior to further immunostaining. The sections were washed with PBS
and 0.3% Triton X-100 for 30 min at 37°C, washed again with PBS and
blocked using 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.) for 30 min at 37°C. Primary antibodies against
forkhead box P3 (1:100; Abcam, Cambridge, UK; cat. no. ab4728),
cluster of differentiation (CD)3 (1:200; Abcam; cat. no. ab5690),
CD4 (1:200; Abcam; cat. no. ab203034) or CD8 (1:150; Abcam; cat.
no. ab4055) were added and incubated with the sections at 4°C
overnight. Following washing with PBS, membranes were incubated at
37°C for 30 min with an appropriate fluorescent secondary antibody
(1:500; Invitrogen; Thermo Fisher Scientific, Inc.; cat. no.
A-11034). Finally, sections were stained with DAPI for 5 min at
37°C, washed with PBS and observed via laser confocal scanning
microscopy at ×100 magnification (Leica Microsystems GmbH, Wetzlar,
Germany).
Western blot analysis
The total protein was extracted from the skin tissue
using radioimmunoprecipitation assay buffer (Invitrogen; Thermo
Fisher Scientific, Inc.), and its concentration was measured using
a bicinchoninic acid assay kit (Invitrogen; Thermo Fisher
Scientific, Inc.). The remaining protein was degenerated by boiling
at 99°C for 10 min, and was subsequently stored at −20°C. Protein
samples (20 µg) were run on 10% SDS-PAGE gels and transferred onto
polyvinylidene fluoride membranes for 80 min. Membranes were
blocked with 5% bovine serum albumin (Invitrogen; Thermo Fisher
Scientific, Inc.) at 25°C for 1 h and incubated at 4°C overnight
with primary antibodies (all purchased from Abcam) against the
following proteins: Transforming growth factor-β (TGF-β; 1:5,000;
cat. no. ab92486), interleukin (IL)-1β (1:1,000; cat. no. ab2105),
IL-2 (1:1,000; cat. no. ab180780), IL-6 (1:500; cat. no. ab6672),
IL-10 (1:1,000; cat. no. ab34843), IL-8 (1:1,000; cat. no. ab7747),
interferon (IFN)-γ (1:400; cat. no. ab25101), tumor necrosis factor
(TNF)-α (1:2,000; cat. no. ab6671) and GAPDH (1:5,000; cat. no.
ab8245), β-actin (1:5,000; cat. no. ab8226). Following this, the
membranes were washed three times with TBS containing 0.1% Tween-20
(TBST; 10 min/wash) and incubated with a secondary antibody
(1:5,000; Cell Signaling Technology, Inc., Danvers, MA, USA) at
room temperature for 1 h. Membranes were subsequently washed three
times with TBST (10 min/wash) and developed using a gel imaging
system (EMD Millipore, Billerica, MA, USA).
Microarray analysis of the lncRNA
expression profile
There were at least three replicates in the control
test. Therefore, three tissue specimens were randomly selected from
each group to conduct the lncRNA gene chip assay. The other tissue
specimens from the patients were used for further test and verify.
RNA was extracted from three randomly selected skin tissue samples
obtained from the control and ulcer groups using TRIzol®
reagent (Invitrogen; Thermo Fisher Scientific, Inc.). Quantified
RNA was transcribed into fluorescent-labeled complementary (c)RNA
using the Quick Amp Labeling kit (Agilent Technologies, Inc., Santa
Clara, CA, USA) at 65°C. Following this, cRNAs were hybridized onto
the Arraystar Human LncRNA Microarray v4.0 (Arraystar Inc.,
Rockville, MD, USA) and the fluorescence intensity was determined
using an Agilent G2565BA microarray scanner (Agilent Technologies,
Inc.). Images were input into Agilent Feature Extraction software
version 10.5.1, and the data underwent quantile normalization and
further analysis using Agilent Gene Spring software GX 12.0 (both
Agilent Technologies, Inc.). A total of 20 lncRNAs and
co-expressing mRNAs exhibiting maximum levels of upregulation and
downregulation were screened for and verified via reverse
transcription quantitative polymerase chain reaction (RT-qPCR),
using GAPDH as an internal reference.
RT-qPCR
Total RNA was extracted from control and ulcer
tissues using TRIzol®, according to the manufacturer's
instructions. lncRNA and mRNA expression levels were determined
using the Ribo™ SYBR Green mRNA/lncRNA RT-qPCR Starter
kit (Guangzhou RiboBio Co., Ltd., Guangzhou, China), using GAPDH as
an internal reference. Realtime PCR system (2XMaster Mix: 5 µl, 10
µM PCR-specific primer F: 0.5 µl, 10 µM PCR-specific primer R: 0.5
µl water added for total volume: 8 µl) was used to prepare all the
DNA samples for PCR reaction. The conditions for RT-qPCR were: 95°C
for 10 min; 40 cycles, then 95°C for 10 sec and 60°C for 60 sec.
Dissociation curves revealed no nonspecific amplification. The
primers used for the RT-qPCR assay are listed in Tables I and II. The results were analyzed using the
2−∆∆Cq method (15),
and all experiments were repeated in triplicate.
 | Table I.Primer sequences for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primer sequences for reverse
transcription-quantitative polymerase chain reaction.
| Gene | Primer sequence
(5′-3′) |
|---|
| TGF-β | F:
CGCGTGCTAATGGTGGAAA |
|
| R:
CGCTTCTCGGAGCTCTGATG |
| TNF-α | F:
GCCAGAGGGCTGATTAGAGA |
|
| R:
TCAGCCTCTTCTCCTTCCTG |
| IFN-γ | F:
GCTCTAGAGATTTCAACTTCTTTGGCTTA |
|
| R:
TTGTCGACGCAGGCAGGACAACCATTACT |
| IL-1β | F:
CCGACCACCACTACAGCAAG |
|
| R:
TGGACCAGACATCACCAAGC |
| IL-2 | F:
ATGTACAGGATGCAACTCCTG |
|
| R:
TCAAGTCAGTGTTGAGATGATGCTTTGACAAAA |
| IL-6 | F:
ATGAACTCCTTCTCCACAAGC |
|
| R:
CTACATTTGCCGAAGAGCCCTCAGGCTGGACTG |
| IL-8 | F:
ATGACTTCCAAGCTGGCCGTG |
|
| R:
TTATGAATTCTCAGCCCTCTTCAAAAACTTCTC |
| IL-10 | F:
AACCTGCCTAACATGCTTCG |
|
| R:
GCAAGGACTCCTTTAACAACAA |
| GAPDH | F:
GCACCGTCAAGGCTGAGAAC |
|
| R:
TGGTGAAGACGCCAGTGGA |
 | Table II.Primer sequences for reverse
transcription-quantitative polymerase chain reaction of long
non-coding (lnc)RNAs. |
Table II.
Primer sequences for reverse
transcription-quantitative polymerase chain reaction of long
non-coding (lnc)RNAs.
| lncRNAs | Primer sequence
(5′-3′) |
|---|
|
| A, Upregulated |
|
| T199454 | F:
GAGATTGAGAAACTGAGGTCGTG |
|
| R:
CCCACCACTGATAAGGGATGT |
| T118424 | F:
GCCTTCTAAATCACAGACCCC |
|
| R:
AGACTCCCATTTGGAGAGCCTA |
| ENST00000447028 | F: AACACTGGAAACACCCA
GCTCTC |
|
| R:
TCACCACTGCTCAGCCCAGACCTCCCT |
| ENST00000585911 | F:
ATCAACTTGTAAGAGAGCTGGGGTTCT |
|
| R:
AGTTAAACTAGGCCATGATGACAC |
|
ENST00000556606 | F:
AGAGGTCCCTCAGTGCCAGGGCCT |
|
| R:
CATGAGACTTATTTTGACT |
|
TCONS_l2_00009699 | F:
CCACCCAACTCTAAGCATCA |
|
| R:
GGTCAAGTAGGCAGTCAGGTAT |
| T299457 | F:
GCTCCTGTGGGGAATAGACT |
|
| R:
CCTCACCTCCTCTACTACCAAGA |
| NR_029393 | F:
AACACACTGATTCCCATGGCTGAATA |
|
| R:
TCCTTGAAGATCACAGGGGTGGT |
| T381695 | F:
CATGAGACTTATTTTGACTTCTAC |
|
| R:
GAAGTGGGTGCAGAGGCTGGG |
|
ENST00000566954 | F:
GCCCTCTTCTTCAAGGATGC |
|
| R:
GCGGGCACATTTCACAGAT |
| β-actin | F:
GTGGCCGAGGACTTTGATTG |
|
| R:
CCTGTAACAACGCATCTCATATT |
|
| B,
Downregulated |
|
| TCONS_00019680 | F:
ACCACTATGCCTGTGGTGGC |
|
| R:
ATATCTAGATCTGTGTGC |
| T173832 | F:
CAATGACGCACAGGAGAAAG |
|
| R:
AGATAAGCTTCTTGCCTGT |
|
ENST00000416861 | F:
AGCAGGCACCTCTTATGCT |
|
| R:
TTCTCCACATTATTCTCCT |
|
ENST00000527239 | F:
GGTTGCTGCTCTCCATGAG |
|
| R:
ATCAGGCAGAGAGAGA |
|
ENST00000561322 | F:
TACCGGTACAGCCGGGCTTCAAT |
|
| R:
CTGGCAGCCAACCACGCAGA |
| T182081 | F:
GCCGACCCCCTGAGGCTCGC |
|
| R:
CACTCCATCCGGACCAGGG |
|
ENST00000411554 | F:
ATAAAGTTTTACTTTATACG |
|
| R:
TACCAGGAGACATGAGA |
| T071762 | F:
CTCACAGCAGATCTCTCTGGCTTAAC |
|
| R:
GTGCTCCAGTTCTTATGGTGTT |
|
ENST00000430816 | F:
TGCCCAGAAGGCTCTGGAAGA |
|
| R:
TTCTGGAAGTAAGCACGG |
|
ENST00000606648 | F:
GTGTTGTAGAATAGGAGGGTCCTGG |
|
| R:
ATATGAAAACCCAAATGGAGTGAAT |
| β-actin | F:
GTGGCCGAGGACTTTGATTG |
|
| R:
CCTGTAACAACGCATCTCATATT |
lncRNA fluorescence in situ
hybridization (FISH)
An lncRNA antisense probe was synthesized by
Novatech Enterprise Co., Ltd. (Wuxi, China). Skin tissues were
embedded in paraffin, cut into sections (4 µm), dewaxed and sealed
with sealing solution for 15 min. Pre-hybridization solution
(100–150 µl, Novatech Enterprise Co., Ltd. Wuxi, China) was added
to the sections, which were incubated at 37°C for 1 h. The lncRNA
antisense probe was diluted in pre-hybridization solution,
degenerated at 65°C for 5 min and rapidly cooled in iced water for
10 min. Following this, the pre-hybridization solution was
discarded from the sections, which were uniformly covered with
100–150 µl degenerated probe and incubated at 4°C overnight. The
sections were subsequently washed, sealed for 30 min with sealing
solution at 37°C and then stained with DAPI for 1–5 min at room
temperature. Following this, the sections were washed with running
water, air-dried, sealed with neutral gum and observed under a
fluorescence microscope (Leica Microsystems GmbH) at ×100
magnification. Peptidyl-prolyl cis-trans isomerase B (Aviva Systems
Biology, San Diego, CA, USA) was used as the internal control.
Candidate lncRNA length sequence and
verification of protein coding function
Full-length candidate lncRNAs were established via
5′ and 3′ rapid amplification of cDNA ends (RACE) using a
FirstChoice® RLM-RACE kit (Invitrogen; Thermo Fisher
Scientific, Inc.). Candidate lncRNA non-coding verification was
performed by cloning the full-length ENST00000411554 sequence into
pcDNA3.1 vectors (Guangzhou RiboBio Co., Ltd.). A total of three
Myc-tagged proteins encoding three different sequences were
inserted into the C-terminus of the lnc-testicular cell adhesion
molecule 1, pseudogene. In addition, the full-length
ENST00000411554 sequence was also cloned into pFlag-CMV-2 vectors
(Guangzhou RiboBio Co., Ltd.) containing Flag-tagged protein at the
N-terminus using three different cloning techniques (16). The plasmids were mixed with
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) and transferred into 293T cells
(1×106/vector, Invitrogen; Thermo Fisher Scientific,
Inc.). pcDNA3.1 vectors containing a Myc-tagged green fluorescent
protein (GFP) or pFlag-CMV-2 containing a Flag-linked GFP were used
as positive controls. An empty plasmid was used as the negative
control. The total RNA was isolated and subjected to RT-qPCR to
determine the vector-mediated gene transduction 48 h
post-transfection. After a 72-h time interval, the protein was
extracted and Myc-tagged or Flag-tagged proteins were detected via
western blotting as described above.
Statistical analysis
All experiments were repeated at least three times.
All statistical data are expressed as the mean ± standard
deviation. Statistical analysis was performed using the SPSS 18.0
software package (SPSS, Inc., Chicago, IL, USA). Comparisons of the
measurement data between two groups were performed using a
Student's t-test. Pearson's test was used for correlation analysis.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Histopathological changes at the wound
surface of the control and ulcer groups
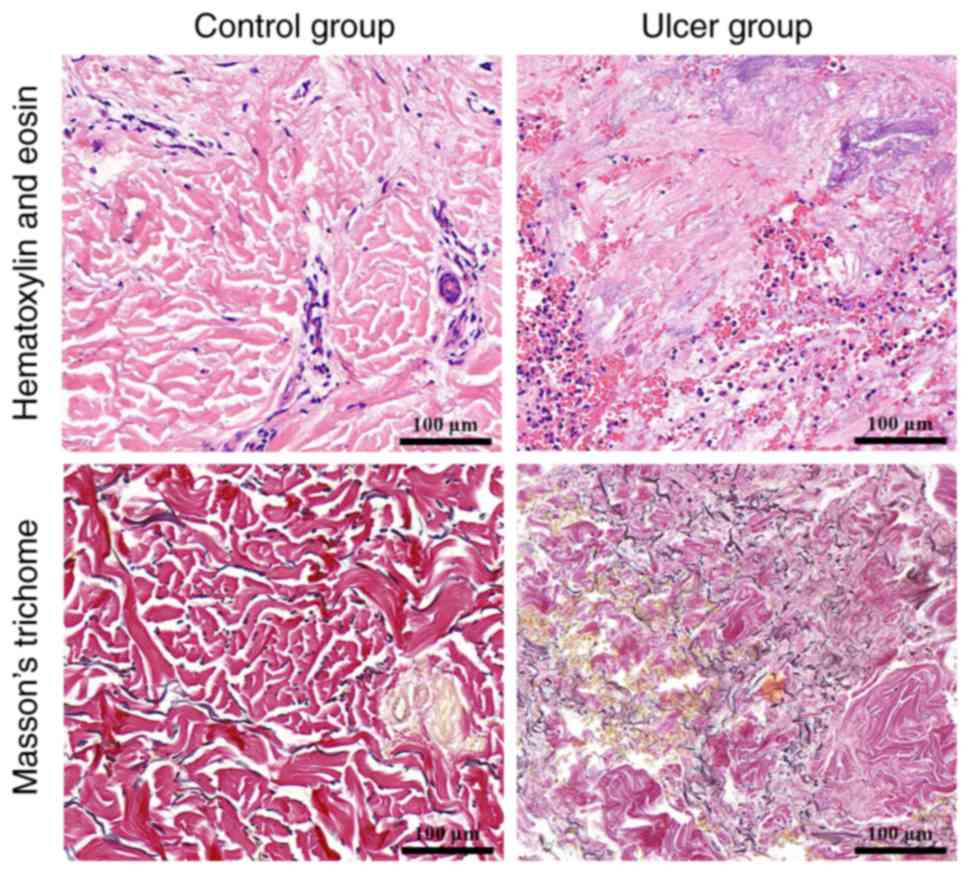
HE staining of the control wound surface revealed a
clear tissue structure with numerous neutrophil granulocytes and
fibroblast infiltration, while Masson staining revealed thin
collagenous fibers in a loose arrangement. HE staining of the ulcer
group indicated a disordered tissue structure, the formation of
granulated tissue, increased numbers of neutrophil granulocytes and
fibroblast infiltration. Masson staining of the ulcer group
revealed atrophic and decreased numbers of collagenous fibers in a
disordered arrangement (Fig.
1).
Differential expression of a T
lymphocyte subset in the wound surface of the control and ulcer
groups
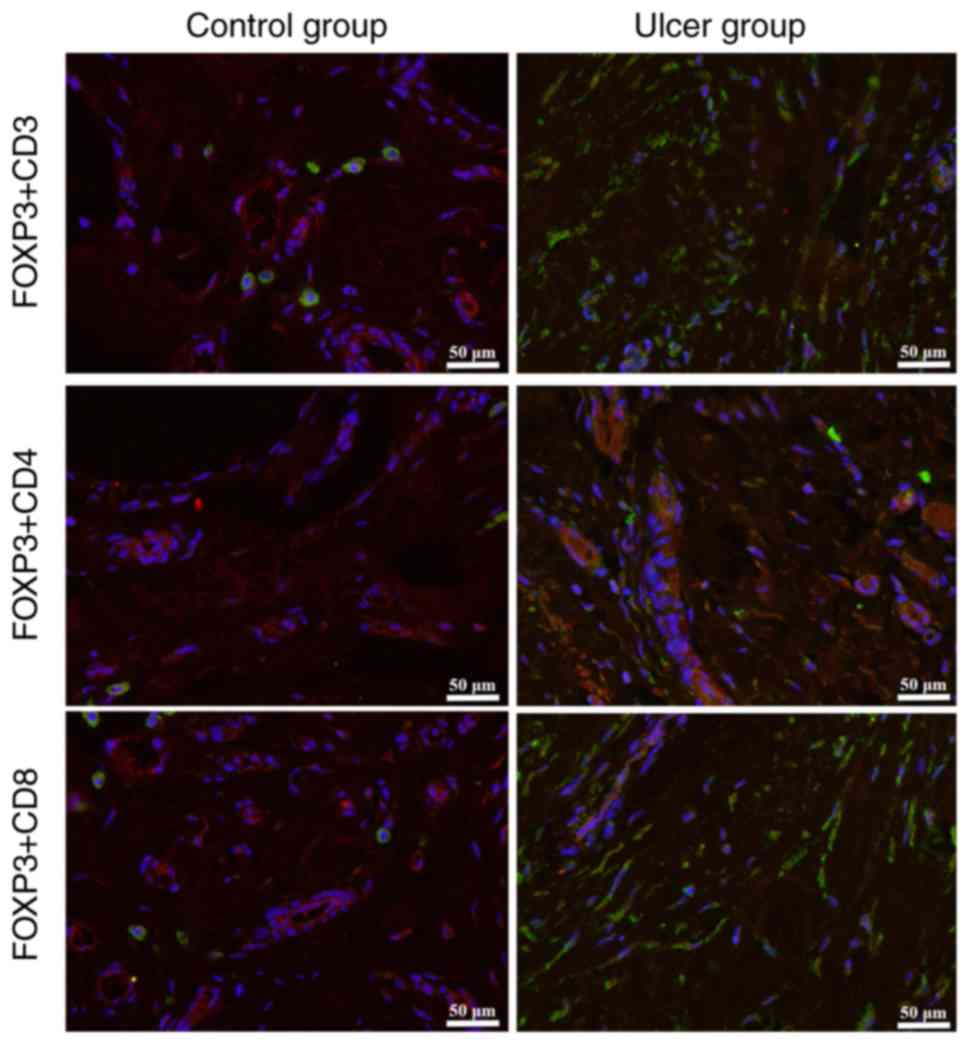
Immunofluorescence staining revealed increased
expression of CD3 and CD8 in the wound surface of tissues obtained
from the ulcer group compared with the control group; however,
there was no marked difference in the levels of CD4 expression
between the two groups (Fig. 2).
These results indicated a decreased CD4:CD8 ratio in DFU tissues,
which suggested that cellular immune dysfunction was present in
patients with type 2 DM and DFUs.
Differential expression of
inflammatory factors in the wound surface of the control and ulcer
groups
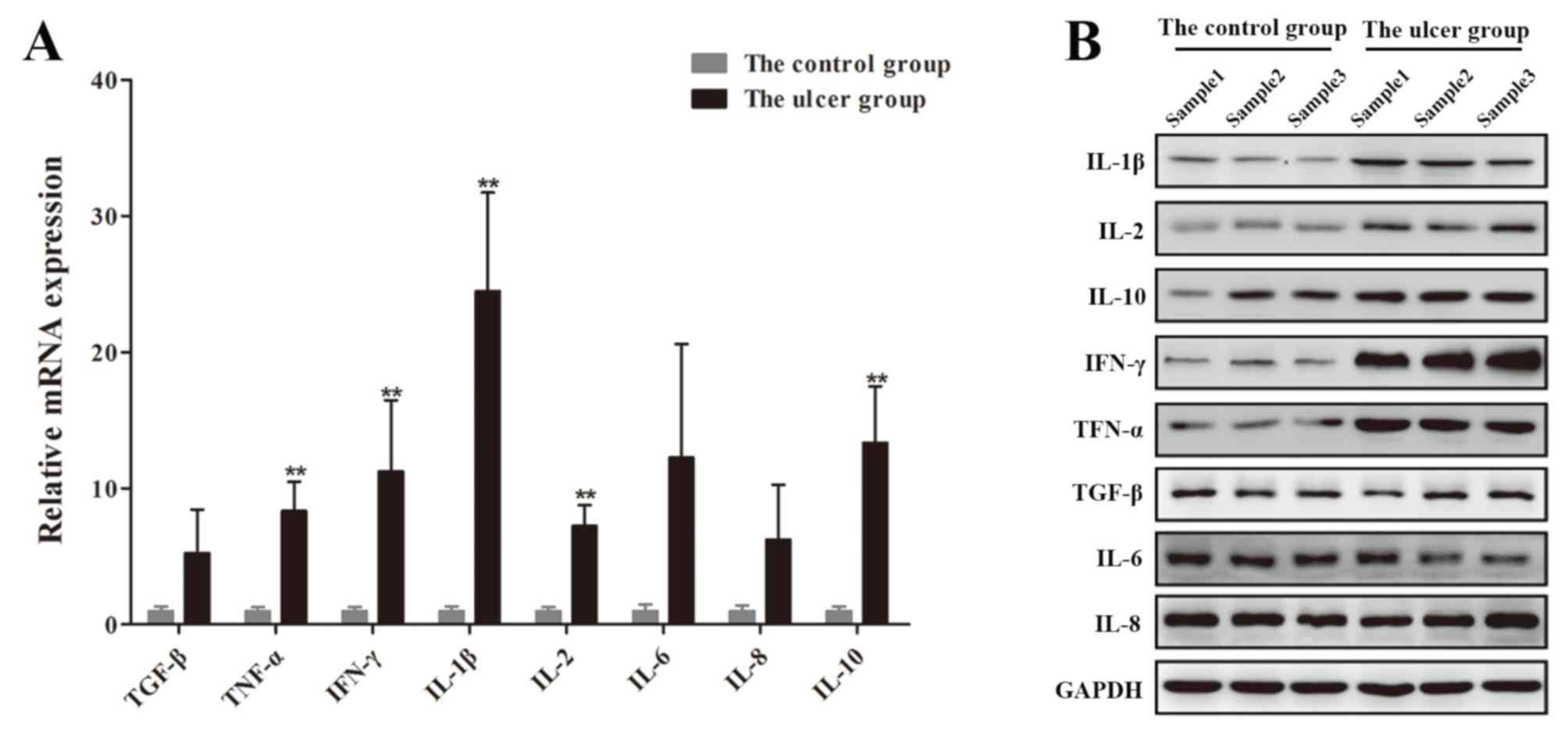
RT-qPCR and western blotting demonstrated that
IL-1β, IL-2, IL-10, IFN-γ and TNF-α expression levels were
significantly upregulated in skin tissues obtained from the ulcer
group compared with the control group; however, no significant
differences in the expression levels of TGF-β, IL-6 and IL-8 were
revealed between the two groups (Fig.
3). These results suggested that IL-1β, IL-2, IL-10, IFN-γ and
TNF-α may be associated with the onset of DFUs.
Differential expression of lncRNAs in
the wound surface of the control and ulcer groups
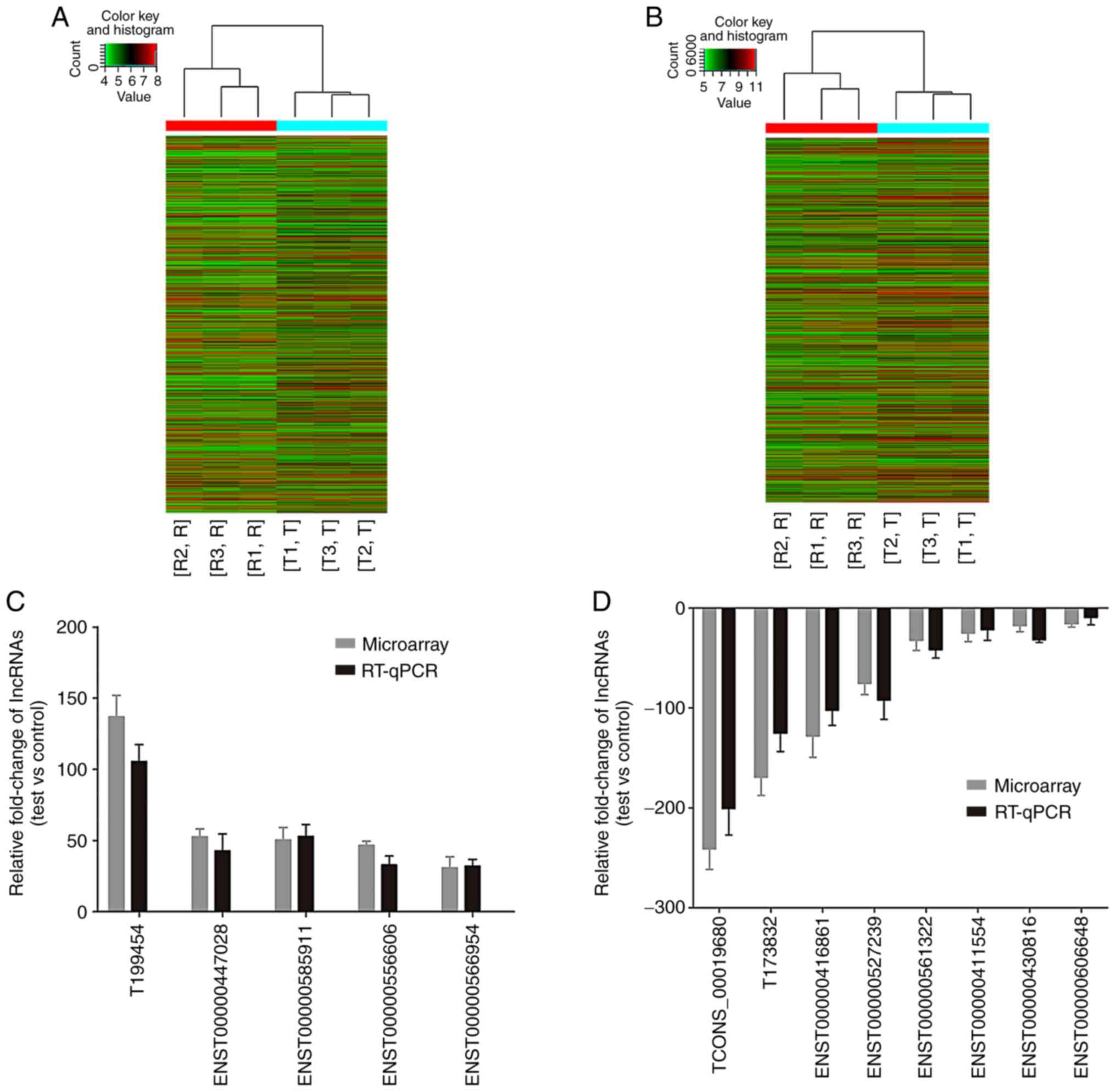
To investigate the involvement of lncRNA in DFUs,
lncRNA/mRNA gene chip technology was used to detect lncRNA and mRNA
expression in the wound surface tissues obtained from the ulcer and
control groups. The expression levels of 2,142 lncRNAs were
revealed to be upregulated, while 1,332 lncRNAs were downregulated
with a differential multiple of >2. A total of 20 lncRNAs
exhibiting significant differential expression between the two
groups were selected for subsequent RT-qPCR analysis. The results
demonstrated that five lncRNAs were upregulated, while eight
lncRNAs were downregulated, which was consistent with the results
of the chip screening analyses (Fig.
4).
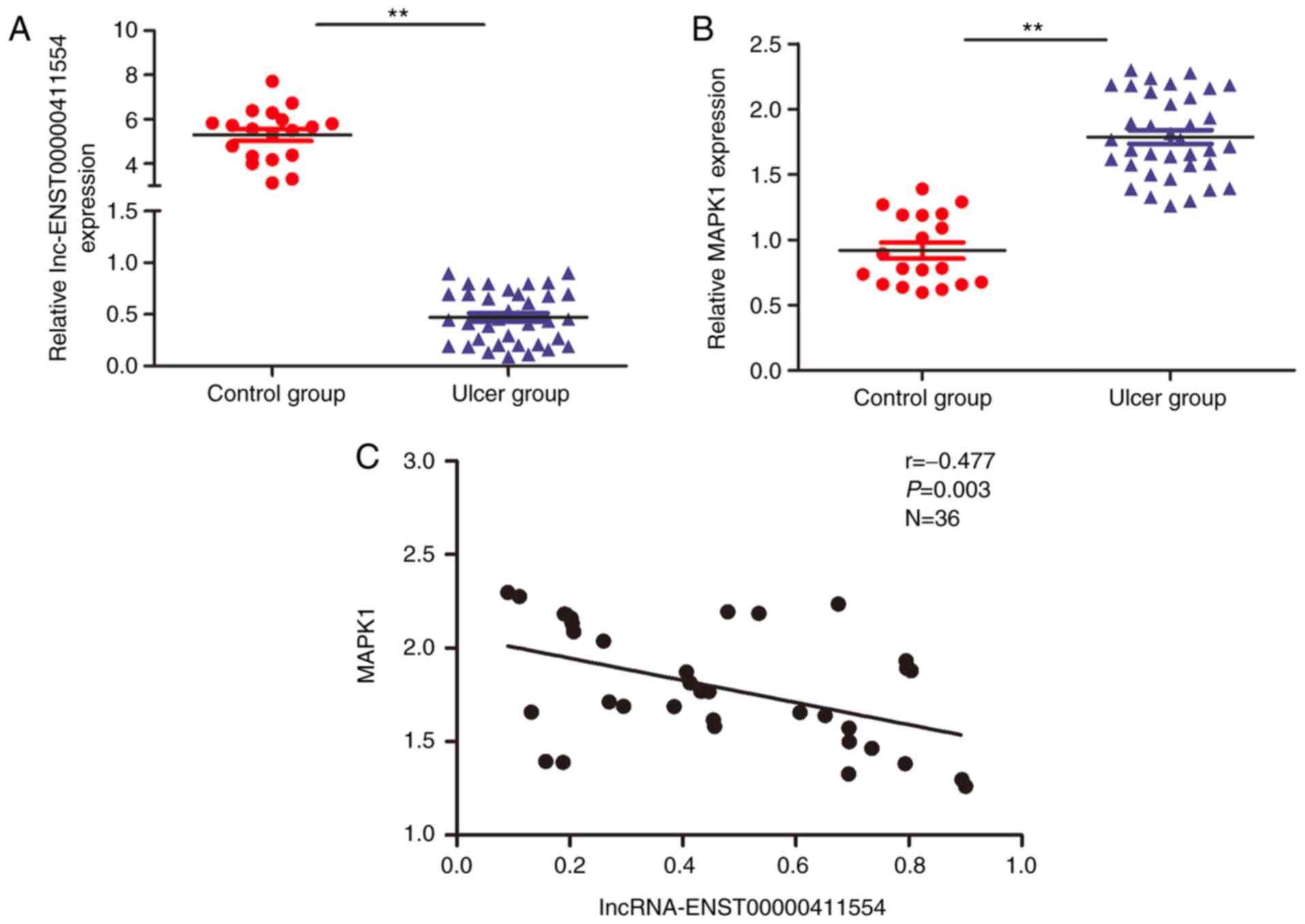
Differential expression of
lncRNA-ENST00000411554/mitogen activated protein kinase 1 (MAPK1)
in control and DFU tissues
Following the analysis of the aforementioned 13
lncRNAs and target genes, it was revealed that
lncRNA-ENST00000411554 (location, chromosome 22:
22,390,743–22,394,463; transcript length, 779 bp) was located in a
key upstream regulatory region of target gene, MAPK1. Of the
numerous signaling pathways involved in the regulation of DFU, the
MAPK signal transduction pathway is one of the most important
(17). Therefore, to investigate
whether there was a negative correlation between
lncRNA-ENST00000411554 and MAPK1 expression in control and
DFU tissues, an additional 19 control and 36 ulcer wound surface
tissue samples were investigated via lncRNA FISH and RT-qPCR. The
results revealed that lncRNA-ENST00000411554 expression was
significantly downregulated and MAPK1 expression was
significantly upregulated in DFU tissues compared with the control
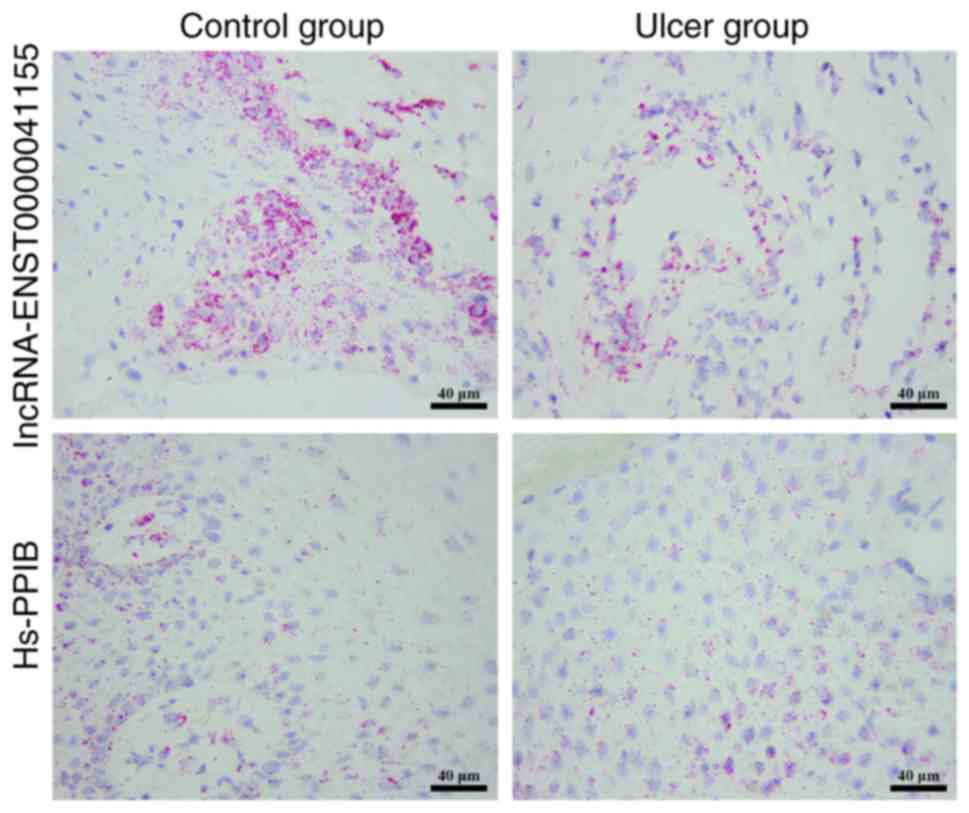
tissues, and thus exhibited a negative correlation (Fig. 5). lncRNA FISH also revealed
differential expression of lncRNA-ENST00000411554 in control and
DFU tissues (Fig. 6).
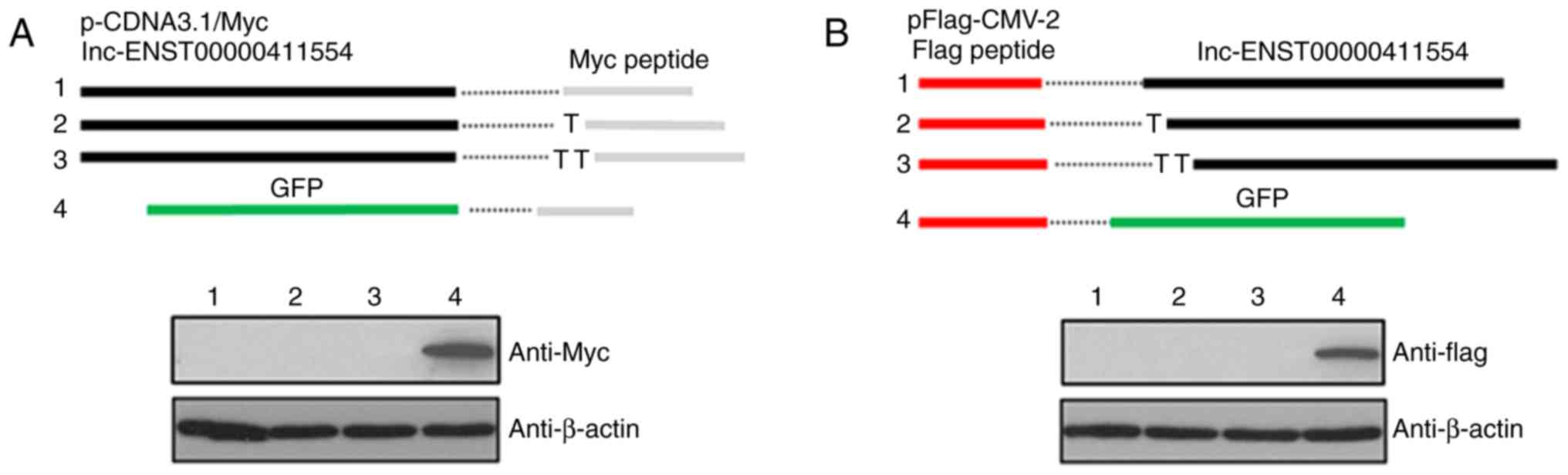
lncRNA-ENST00000411554 exhibits
non-coding activity
To verify the non-coding characteristics of
lncRNA-ENST00000411554, lncRNA-ENST00000411554 was cloned into
pcDNA3.1 and pFlag-CMV-2 vectors and subsequently tagged with Myc
or Flag, respectively. Following this, these vectors were
transfected into 293T cells and investigated via western blotting.
The results did not reveal any detection of Myc- or Flag-tagged
proteins via western blotting, which suggested that
lncRNA-ENST00000411554 does not have any protein-coding abilities
(Fig. 7).
Discussion
The sharp increase in DM incidence worldwide has
resulted in DFUs becoming an increasingly common complication, with
an estimated development risk of 15–25% in patients with DM
(6). Although efforts have been
made to control the deterioration of DF, patients with existing
DFUs face possible amputation, and the number of associated
mortalities is increasing annually. DM involves the immune system
and abnormal inflammatory responses, in addition to aberrant
adhesion, uptake, chemotaxis and the germicidal function of
neutrophil granulocytes, which have been observed in the peripheral
blood of patients with DM (18,19).
In addition, reduced immunocompetence is one of the risk factors
associated with type 2 DM and diabetic macrovascular and
microvascular complications (20).
Dysregulated levels of pro-inflammatory cytokines are one of the
principal factors associated with poor healing of DFU wound
surfaces, and thus represent a key focus of treatment.
Previous studies have indicated that T lymphocytes,
vasculopathy and DFU infection are closely associated in patients
with DM (21). The T lymphocyte
subset is the most important immune cell population (22). CD3, CD4 and CD8 expression levels
are determined to investigate whole T cells, helper T lymphocytes
and suppressor/cytotoxic T lymphocytes, respectively. The
regulatory association between CD4 and CD8 expression has both
adjuvant and inhibitory effects, and their ratio is important for
immunoregulation (23). Following
the stimulation of CD8 cells by extrinsic antigens, cell-mediated
killing is induced, which directly destroys target cells. However,
following the sensitization of CD4 cells by extrinsic antigens,
numerous cytokines are generated to induce the proliferation of T
cells, B cells and macrophages.
In the present study, the results demonstrated that
the expression levels of CD3 and CD8 in the ulcer group were
increased; whereas levels of CD4 did not exhibit a significant
difference between the ulcer group and the control group.
Furthermore, the CD4/CD8 ratio was decreased in patients with DFU,
which suggested that patients in the ulcer group suffered from
severe immune dysfunction. Despite inflammatory factors having an
important role in the immune response, it is unclear which T
lymphocyte subset infiltration has the major effect and which
cytokines are involved in inflammation. To investigate this,
expression levels of T lymphocyte-associated inflammatory factors
were detected, and the results revealed that IL-2, IFN-γ and TNF-α
expression levels were significantly upregulated in the ulcer group
compared with the control group. Therefore, it may be suggested
that following the infection of keratinocytes surrounding the ulcer
wound surface, TNF-α is released from inflammatory infiltrating
cells and subsequently activates CD8+ T cells and IL-2,
which further enhances the secretion of TNF-α and TNF-γ.
Among the various signaling pathways involved in the
regulation of DFUs, the MAPK signal transduction pathway is
particularly important (24). The
extracellular signal-related kinase (ERK) pathway is also
considered to be an important MAPK signaling pathway, and has been
revealed to have a crucial role in the inflammatory response
induced by T cells by regulating the expression of inflammatory
factors. Kremer et al (25)
demonstrated that the ERK pathway activates activator protein-1
(AP-1) in T cells via a phosphorylation cascade, and that p-AP-1
upregulates IL-10 expression by directly binding to its promoter
region. IFN-γ and IL-1β pro-inflammatory factors are also regulated
by the ERK pathway (26,27). In the present study, the results
revealed that MAPK1, IFN-γ and IL-1β were upregulated in DFU
tissues, which suggested that MAPK1 promoted the occurrence and
development of DFUs. The analysis of lncRNA/mRNA expression profile
changes via gene chip technology demonstrated that
lncRNA-ENST00000411554 binds to MAPK1. Therefore, it may be
suggested that lncRNA-ENST00000411554 induces the upregulation of
MAPK1 at the DFU wound surface. Furthermore, the results revealed
that lncRNA-ENST0000041155 exhibited non-coding characteristics,
and was negatively correlated with MAPK1 expression in DFU
tissues.
The present study had certain limitations. Only 6
patients were selected for inclusion in the lncRNA gene chip
analysis. Future studies may expand the number of study
participants. In addition, there was no corresponding lncRNA
knockout and functional verification test to further clarify the
function of lncRNA-ENST0000041155. This may be included in
subsequent studies.
In conclusion, cellular immune dysfunction is an
important factor associated with the occurrence and development of
DFUs. Furthermore, the results of the present study suggested that
activation of the MAPK signal transduction pathway mediated by
lncRNA-ENST00000411554/MAPK1 is associated with an imbalance in DFU
immune regulation; however, this requires further
investigation.
Acknowledgements
Not applicable.
Funding
The present study was supported by a grant from The
National Natural Science Foundation of China (grant no. 81572185),
The Natural Science Foundation of Anhui Province of China (grant
no. 1708085MH185), The Chinese Anhui province Education Department
key Fund Project (grant no. KJ2018A0252) and The Chinese Wuhu City
Scientific and Technological Achievements Transformation Project
(grant no. 2017CG27).
Availability of data and materials
The datasets used and analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SX conceived the idea of the study. XW, YW, DL, MZ
and FZ performed the research. YS analysed data and interpreted the
results. SX wrote the paper. All authors discussed the results and
revised the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Yijishan Hospital (Wuhu, China), and all patients
provided written informed consent.
Patient consent for publication
This study was approved by the Ethics Committee of
Yijishan Hospital (Wuhu, China). Written informed consent was
obtained from all enrolled patients. All the subsequent research
analyses were carried out in accordance with the approved
guidelines.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Leung PC: Diabetic foot ulcers-a
comprehensive review. Surgeon. 5:219–231. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Unwin N: The diabetic foot in the
developing world. Diabetes Metab Res Rev. 24 Suppl 1:S31–S33. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xu Y, Wang L, He J, Bi Y, Li M, Wang T,
Wang L, Jiang Y, Dai M, Lu J, et al: Prevalence and control of
diabetes in Chinese adults. JAMA. 310:948–959. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Izumi Y, Satterfield K, Lee S and Harkless
LB: Risk of reamputation in diabetic patients stratified by limb
and level of amputation: A 10-year observation. Diabetes Care.
29:566–570. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lepore G, Maglio ML, Cuni C, Dodesini AR,
Nosari I, Minetti B and Trevisan R: Poor glucose control in the
year before admission as a powerful predictor of amputation in
hospitalized patients with diabetic foot ulceration. Diabetes Care.
29:19852006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Boulton AJ: The diabetic foot: A global
view. Diabetes Metab Res Rev. 16 Suppl 1:S2–S5. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zykova SN, Svartberg J, Seljelid R,
Iversen H, Lund A, Svistounov DN and Jenssen TG: Release of
TNF-alpha from in vitro-stimulated monocytes is negatively
associated with serum levels of apolipoprotein B in patients with
type 2 diabetes. Scand J Immunol. 60:535–542. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shau H, Gupta RK and Golub SH:
Identification of a natural killer enhancing factor (NKEF) from
human erythroid cells. Cell Immunol. 147:1–11. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Thorsby P, Undlien DE, Berg JP, Thorsby E
and Birkeland KI: Diabetes mellitus-a complex interaction between
heredity and environment. Tidsskr Nor Laegeforen. 118:2519–2524.
1998.(In Norwegian). PubMed/NCBI
|
|
10
|
Bouter KP, Meyling FH, Hoekstra JB,
Masurel N, Erkelens DW and Diepersloot RJ: Influence of blood
glucose levels on peripheral lymphocytes in patients with diabetes
mellitus. Diabetes Res. 19:77–80. 1992.PubMed/NCBI
|
|
11
|
Morán I, Akerman I, van de Bunt M, Xie R,
Benazra M, Nammo T, Arnes L, Nakić N, García-Hurtado J,
Rodríguez-Seguí S, et al: Human β cell transcriptome analysis
uncovers lncRNAs that are tissue-specific, dynamically regulated,
and abnormally expressed in type 2 diabetes. Cell Metab.
16:435–448. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gao Y, Wu F, Zhou J, Yan L, Jurczak MJ,
Lee HY, Yang L, Mueller M, Zhou XB, Dandolo L, et al: The H19/let-7
double-negative feedback loop contributes to glucose metabolism in
muscle cells. Nucleic Acids Res. 42:13799–13811. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu G, Cai J, Han Y, Chen J, Huang ZP, Chen
C, Cai Y, Huang H, Yang Y, Liu Y, et al: LincRNA-p21 regulates
neointima formation, vascular smooth muscle cell proliferation,
apoptosis, and atherosclerosis by enhancing p53 activity.
Circulation. 130:1452–1465. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Alvarez ML and DiStefano JK: Functional
characterization of the plasmacytoma variant translocation 1 gene
(PVT1) in diabetic nephropathy. PLoS One. 6:e186712011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huang JZ, Chen M, Chen, Gao XC, Zhu S,
Huang H, Hu M, Zhu H and Yan GR: A peptide encoded by a putative
lncRNA HOXB-AS3 suppresses colon cancer growth. Mol Cell.
68:171–184 e6. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang CT, Chen L, Chen WL, Li N, Chen MJ,
Li X, Zheng X, Zhao YZ, Wu YX, Xian MA and Liu J: Hydrogen sulfide
primes diabetic wound to close through inhibition of NETosis. Mol
Cell Endocrinol. 480:74–82. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lipsky BA, Peters EJ, Berendt AR,
Senneville E, Bakker K, Embil JM, Lavery LA, Urbančič-Rovan V and
Jeffcoate WJ; International Working Group on Diabetic Foot, :
Specific guidelines for the treatment of diabetic foot infections
2011. Diabetes Metab Res Rev. 28 Suppl 1:S234–S235. 2012.
View Article : Google Scholar
|
|
19
|
Boulton AJ: The pathogenesis of diabetic
foot problems: An overview. Diabet Med. 46 Suppl 2:S12–S16. 1996.
View Article : Google Scholar
|
|
20
|
Navarro-González JF and Mora-Fernández C:
The role of inflammatory cytokines in diabetic nephropathy. J Am
Soc Nephrol. 19:433–442. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Moura J, Rodrigues J, Goncalves M, Amaral
C, Lima M and Carvalho E: Impaired T-cell differentiation in
diabetic foot ulceration. Cell Mol Immunol. 14:758–769. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tanaka S, Isoda F, Ishihara Y, Kimura M
and Yamakawa T: T lymphopaenia in relation to body mass index and
TNF-alpha in human obesity: Adequate weight reduction can be
corrective. Clin Endocrinol (Oxf). 54:347–354. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Keane WF and Lyle PA: Reduction of
Endpoints in NIDDM with the Angiotensin II Receptor Antagonist
Losartan study: Recent advances in management of type 2 diabetes
and nephropathy: Lessons from the RENAAL study. Am J Kidney Dis. 41
(3 Suppl 1):S22–S25. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yuan X, Han L, Fu P, Zeng H, Lv C, Chang
W, Runyon RS, Ishii M, Han L, Liu K, et al: Cinnamaldehyde
accelerates wound healing by promoting angiogenesis via
up-regulation of PI3K and MAPK signaling pathways. Lab Invest.
98:783–798. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kremer KN, Kumar A and Hedin KE:
Haplotype-independent costimulation of IL-10 secretion by
SDF-1/CXCL12 proceeds via AP-1 binding to the human IL-10 promoter.
J Immunol. 178:1581–1588. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jin E, Ren M, Liu W, Liang S, Hu Q, Gu Y
and Li S: Effect of boron on thymic cytokine expression, hormone
secretion, antioxidant functions, cell proliferation, and apoptosis
potential via the extracellular Signal-regulated kinases 1 and 2
signaling pathway. J Agric Food Chem. 65:11280–11291. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang XL and Sun Q: Photodynamic therapy
with 5-aminolevulinic acid suppresses IFN-γ-induced K17 expression
in HaCaT cells via MAPK pathway. Eur Rev Med Pharmacol Sci.
21:4694–4702. 2017.PubMed/NCBI
|