Introduction
Infantile hemangiomas (IHs), the most common
neoplasms in infants, are characterized by their early appearance
following birth and their eventual regression over time. These
benign tumors are estimated to occur in approximately 4–5% of the
general population (1,2) and are usually harmless. However, a
small percentage of these tumors are destructive, disfiguring and
even life-threatening, depending on their size and location
(3). At present, the primary
candidate for cellular sources of IHs are hemangioma stem cells
(HemSCs), and cluster of differentiation (CD)133+ HemSCs
also differentiate into a number of cell lineages, including
adipocytes (4,5). For this reason, investigating the
mechanism of adipogenesis in HemSCs is of considerable clinical
interest.
One potential regulator§ of HemSC adipogenesis is
insulin-like growth factor 1 (IGF-1), since expression of this
polypeptide in the majority of tissues is known to regulate
adipogenesis (6). IGF-1 activity
is mediated by the IGF-1 receptor (IGF-1R), a member of the
tyrosine kinase family of growth factor receptors that contains two
extracellular α-subunits and two membrane-spanning β-subunits that
constitute an intracellular tyrosine kinase (7). IGF-1 controls the mass of adipose
tissue by regulating adipogenesis (8), and it is also known to stimulate fat
formation (9,10). Binding of IGF-1 to its receptor
upregulates the phosphorylation of cyclic AMP-responsive
element-binding protein (CREB) through the phosphoinositide
3-kinase (PI3K)/RAC-α serine/threonine-protein kinase (AKT) pathway
(9). Activated CREB increases the
expression of peroxisome proliferator-activated receptor-γ2
(PPARγ2), a key factor that directs the differentiation of
adipocytes by controlling the expression of specific
differentiation-associated adipocyte genes (6,11).
Other studies have demonstrated that IGF-1 promotes adipocyte
proliferation and differentiation from adipose stromal cells and
human mesenchymal stem cells (12,13).
The effect of IGF-1 on HemSCs in IH is currently unclear.
The present study investigated the function of IGF-1
in HemSC proliferation and adipogenesis in IH. The PI3K signaling
cascades in HemSCs were also studied to obtain a better
understanding of the possible signaling mechanisms that may be
involved and that may be used to develop new therapeutic approaches
for the treatment of patients with IH.
Materials and methods
Preparation of hemangioma
specimens
Ethics committee approval was obtained for the
collection of abscised human hemangiomas from The Second Affiliated
Hospital of Anhui Medical University (Hefei, China; no.
PJ-bb2017-026). The tissues were immediately used for cell
isolation and culture for in vitro experiments.
Isolation and identification of
HemSCs
The proliferating IH tissues removed from the
patients were immediately immersed in growth medium consisting of
high glucose Dulbecco's modified Eagle's medium (GE Healthcare Life
Sciences, Shanghai, China), 10% fetal bovine serum (FBS; Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA), and 1%
penicillin-streptomycin (PS; Beyotime Institute of Biotechnology,
Haimen, China) at 4°C and quickly taken to the laboratory. The fat
or skin tissues were removed and the samples were rinsed three
times in PBS. The tissues were minced and digested with 0.2%
collagenase (SERVA Electrophoresis GmbH, Heidelberg, Germany) at
37°C for 2 h until the samples were chylous, and were passed
through a 100-µm cell strainer. The cells expressing CD133 were
separated from the resulting single-cell suspension using a
magnetic bead technique (Miltenyi Biotec, Inc., Auburn, CA, USA)
and cultured on fibronectin-coated plates in endothelial growth
medium-2 (EGM-2; Lonza Group, Ltd., Basel, Switzerland)
supplemented with 20% FBS and 1% PS in a 37°C incubator with 5%
CO2.
Proliferation assay
HemSCs in the logarithmic growth phase were seeded
into 96-well tissue culture plates (Corning, Inc., Corning, NY,
USA) at an initial density of 2×103 cells. After 24 h of
serum starvation, IGF-1 (Peprotech, Inc., Rocky Hill, NJ, USA),
dissolved in EBM-2 containing 5% FBS, was added at 0, 10, 20, 100
and 200 ng/ml. After 72 h of culture, Cell Counting kit-8 (CCK-8)
reagent (Dojindo Molecular Technologies, Inc., Kumamoto, Japan) was
added to each well and the absorbance at 490 nm was read using a
microplate reader (BioTek ELx 800; BioTek Instruments, Inc.,
Winooski, VT, USA).
The growth kinetics of IGF-1-treated HemSCs were
examined by seeding HemSCs into 96-well plates (Corning, Inc.) at a
density of 1.5×103 cells/well. Following serum
starvation, the cells were treated with 100 ng/ml IGF-1 containing
5% FBS. CCK-8 was added after 0, 1, 3, 5 and 7 days of culture and
the cells were incubated at 37°C for a further 4 h. The optical
density at 490 nm was measured with a microplate reader (BioTek ELx
800).
Immunohistochemistry
Cells (1×104) cultured on coverslips were
fixed in 4% poly-formaldehyde for 15 min at room temperature.
Following air-drying for 5 min, the cells were permeabilized in
0.5% Triton X-100 for 10 min. Endogenous peroxidase activity was
inactivated by treatment with 3% hydrogen peroxide at room
temperature for 5–10 min. The slides were blocked with 10% FBS for
15–20 min at room temperature, and the blocking solution was
aspirated without further washing. The cells were washed with PBS,
and the slides were incubated overnight at 4°C with the following
primary antibodies: Anti-IGF-1 (1:500, cat. no. bs-0014R),
anti-IGF-1R (1:500, cat. no. bs-0227R) and anti-β-actin (1:500,
cat. no. bs-0016R; all BIOSS, Beijing, China). Antibodies were
replaced with PBS in the negative control group. Horseradish
peroxidase-conjugated secondary antibody working solution (cat. no.
PV-6000; OriGene Technologies, Inc., Beijing, China) were incubated
for 10–15 min at 37°C. The cells were observed by bright-field
microscopy (×400) for 3–10 min immediately following addition of
diaminobenzidine (DAB) solution (50 µl DAB:1 ml DAB substrate;
stored in the dark). The cells were hematoxylin-stained for 0.5–1
min at room temperature, and gum-sealed following drying. The cells
were photographed under bright field illumination (×400, Olympus
IX71; Olympus Corporation, Tokyo, Japan).
Oil Red O staining
The adipogenic differentiation of IGF-1-treated
HemSCs was evaluated by seeding 5.0×104 cells in 6-well
plates in EGM-2/10% FBS. When cells were oversaturated, the
original medium was switched to an adipogenic differentiation media
(Cyagen Biosciences, Inc., Santa Clara, CA, USA) containing either
IGF-1 (100 ng/ml), IGF-1 (100 ng/ml) plus OSI-906 (1 µM; Selleck
Chemicals, Houston, TX, USA; an IGF-1R inhibitor), IGF-1 (100
ng/ml) plus LY294002 (10 µM; MedChemExpress, Monmouth Junction, NJ,
USA), LY294002 (10 µM; MedChemExpress; PI3K inhibitor), or no
treatment, and the cells were incubated for 10 days. At room
temperature, the cells were washed gently with PBS twice, fixed
with 4% fresh formaldehyde in PBS for 15 min and stained with Oil
Red O for 30 min. Cells were examined with an inverted microscope
(×200), and the numbers determined using Image J software (1.51s;
National Institutes of Health, Bethesda, MD, USA). The cells were
photographed using an Eclipse E800 microscope (Nikon Corporation,
Tokyo, Japan).
Western blotting
HemSCs were grown on 10-cm tissue culture plates
(Corning, Inc.) in EGM-2/10% FBS containing IGF-1 (100 ng/ml),
IGF-1 (100 ng/ml) plus OSI-906 (1 µM), IGF-1 (100 ng/ml) plus
LY294002 (10 µM), or no treatment. Total protein was extracted
using radioimmunoprecipitation assay lysis buffer (1 mM; BIOSS),
and protein concentration was determined with a Bradford Protein
Assay kit (Beyotime Institute of Biotechnology). Cell protein
extracts (5 µl/lane) were subjected to SDS-PAGE on 10%
polyacrylamide gels. The samples were transferred to polyvinylidene
fluoride membranes, which were blocked with 5% skim milk for 2 h,
incubated with primary antibodies overnight at 4°C, and
subsequently incubated with IRDye 800CW-conjugated goat anti-rabbit
IgG secondary antibody (cat. no. bs-40295G-IRDye8; 1:20,000; BIOSS)
for 1 h at room temperature. The primary antibodies were:
Anti-PPARγ (cat. no. bs-0530P; 1:500; BIOSS),
anti-CCAAT/enhancer-binding protein (C/EBPα; cat. no. bs-0016R;
1:1,000), anti-C/EBPβ (cat. no. bs-1396R; 1:1,000),
anti-adiponectin (cat. no. bs-0471R; 1:1,000; BIOSS), anti-IGF-1
(cat. no. bs-0014R; 1:1,000; all BIOSS), anti-phosphorylated (p)AKT
(cat. no. 9271; 1:500), anti-AKT (cat. no. 9272; 1:500) and
anti-β-actin (cat. no. 3700; 1:500; all Cell Signaling Technology,
Inc., Danvers, MA, USA). Finally, the signal was detected using
enhanced chemiluminescence reagents (Beyotime Institute of
Biotechnology), and band density was analyzed with ImageJ software
(1.51s; National Institutes of Health, Bethesda, MD, USA).
Statistical analyses
The data are expressed as the mean ± standard
deviation (n=3 for each experiment). SPSS version 23 (IBM Corp.,
Armonk, NY, USA) was used to perform statistical analysis. Where
appropriate, an unpaired Student's t-test was used to assess the
differences between two groups, and one-way analysis of variance
followed by Fisher's Least Significant Difference test was applied
for the analysis of the mean values among multiple groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
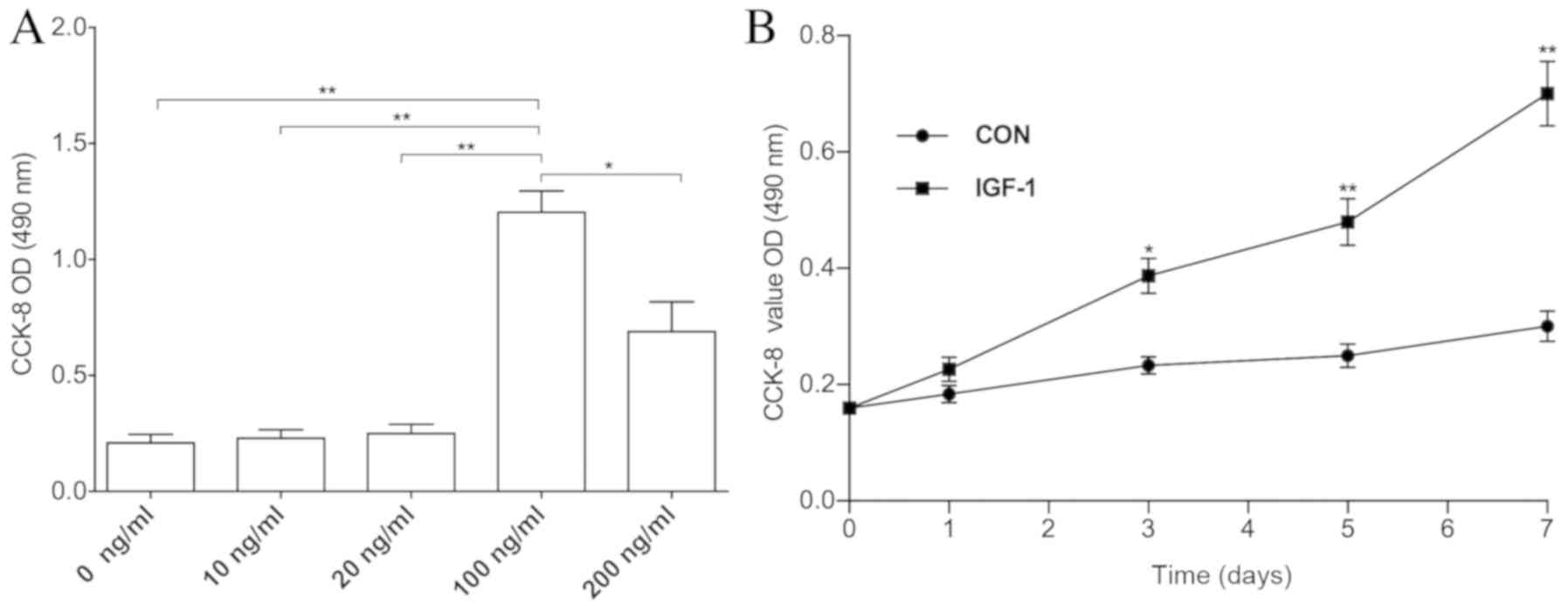
IGF-1 promotes cell proliferation in
IH HemSCs
The CCK-8 assay was performed to determine whether
IGF-1 affected in vitro HemSC proliferation. The addition of
IGF-1 at 100–200 ng/ml stimulated the proliferation of HemSCs, with
the 100 ng/ml concentration exhibiting the best response among all
the groups (Fig. 1A). Cell growth
curves confirmed that treatment with 100 ng/ml IGF-1 promoted cell
proliferation between days 3 and 7 when compared with the
non-treatment group (Fig. 1B).
Expression of IGF-1 and IGF-1R in
HemSCs
Fig. 2A presents
the positive expression of IGF-1 and IGF-1R in HemSCs. IGF-1 was
localized primarily to the cytoplasm, while IGF-1R was localized to
the cytoplasm and the cell membrane.
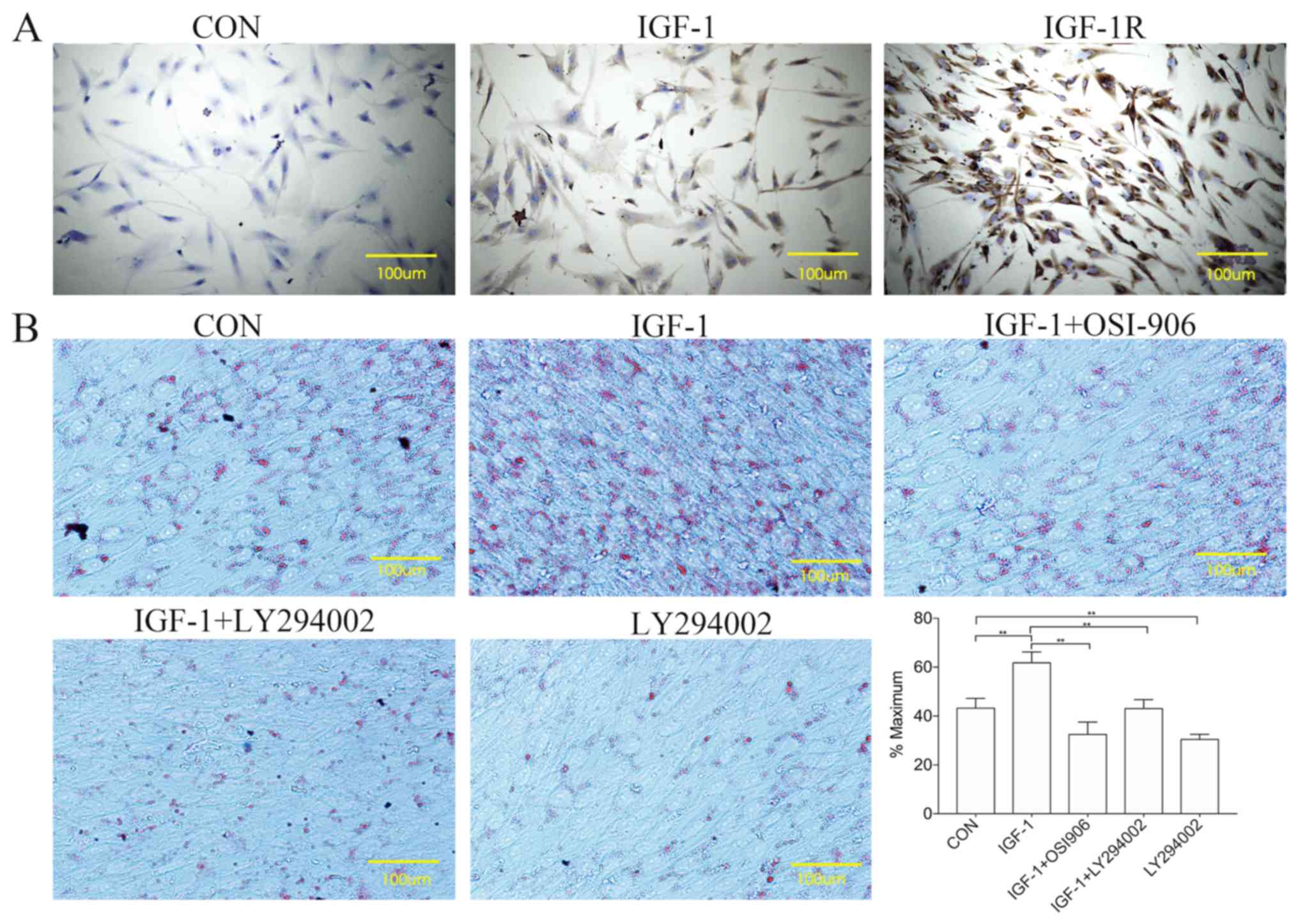 | Figure 2.Expression of IGF-1 and its receptors
in HemSCs, and the effect of IGF-1 on the adipogenesis of HemSCs.
(A) Immunohistochemistry of HemSCs using antibodies specific for
IGF-1 and IGF-1R. (B) IGF-1 was able to induce the differentiation
of HemSCs into adipocytes. CON, HemSCs that were cultivated for 10
days in adipogenic differentiation medium; IGF-1, adipogenic
differentiation medium with IGF-1 (100 ng/ml); IGF-1+OSI-906,
adipogenic differentiation medium with IGF-1 (100 ng/ml) plus
OSI-906 (1 µM); IGF-1+LY294002, adipogenic differentiation medium
with IGF-1 (100 ng/ml) plus LY294002 (1 µM); LY294002, adipogenic
differentiation medium with LY294002 (1 µM). Following Oil Red O
staining, phase-contrast microscopy was used to observe the lipid
droplets. The Oil Red O-stained cells were quantified using Image J
software. Data are expressed as the average percentage of the
maximum signal %. Values are presented as the mean ± standard
deviation. n=3. **P<0.01. CON, control; IGF-1, insulin-like
growth factor-1; IGF-1R, IGF-1 receptor; HemSCs, hemangioma stem
cells. |
IGF-1 promotes the differentiation of
HemSCs into adipocytes and enhances lipogenesis
The HemSCs exhibited increased cell numbers and
greater lipid droplet density when treated with adipogenic
differentiation medium containing IGF-1, compared with adipogenic
differentiation medium alone or with media containing IGF-1 plus
OSI-906, IGF-1 plus LY294002, or LY294002 alone (Fig. 2B). The IGF-1 treatment thus
appeared to stimulate adipogenesis and lipid accumulation in the
HemSCs, whereas treatment with the OSI-906 IGF-1R inhibitor or
LY294002, a specific inhibitor of PI3K, suppressed this
IGF-1-induced lipid accumulation.
OSI-906 and LY294002 suppress
IGF-1-enhanced adipogenic differentiation and AKT protein
phosphorylation
Western blotting confirmed an increase in C/EBPα,
C/EBPβ, PPARγ and adiponectin expression in response to IGF-1 when
compared with the control group. This increase in transcription
factors and adipocyte-specific proteins indicated that IGF-1
enhanced adipogenesis in HemSCs and this enhancement was suppressed
in the presence of OSI-906 or LY294002. In addition, LY294002 alone
was able to suppress HemSC adipogenesis and inhibit the expression
of C/EBPα, C/EBPβ, PPARγ and adiponectin proteins (Fig. 3A). The IGF-1 treatment increased
the phosphorylation of AKT, but this increase was suppressed by
co-treatment with LY294002 or OSI-906, whereas the amounts of total
AKT protein were unaltered (Fig.
3B). Therefore, IGF-1 appeared to induce adipocyte
differentiation in HemSCs by upregulating the phosphorylation of
AKT via an IGF-1R-PI3K signaling pathway.
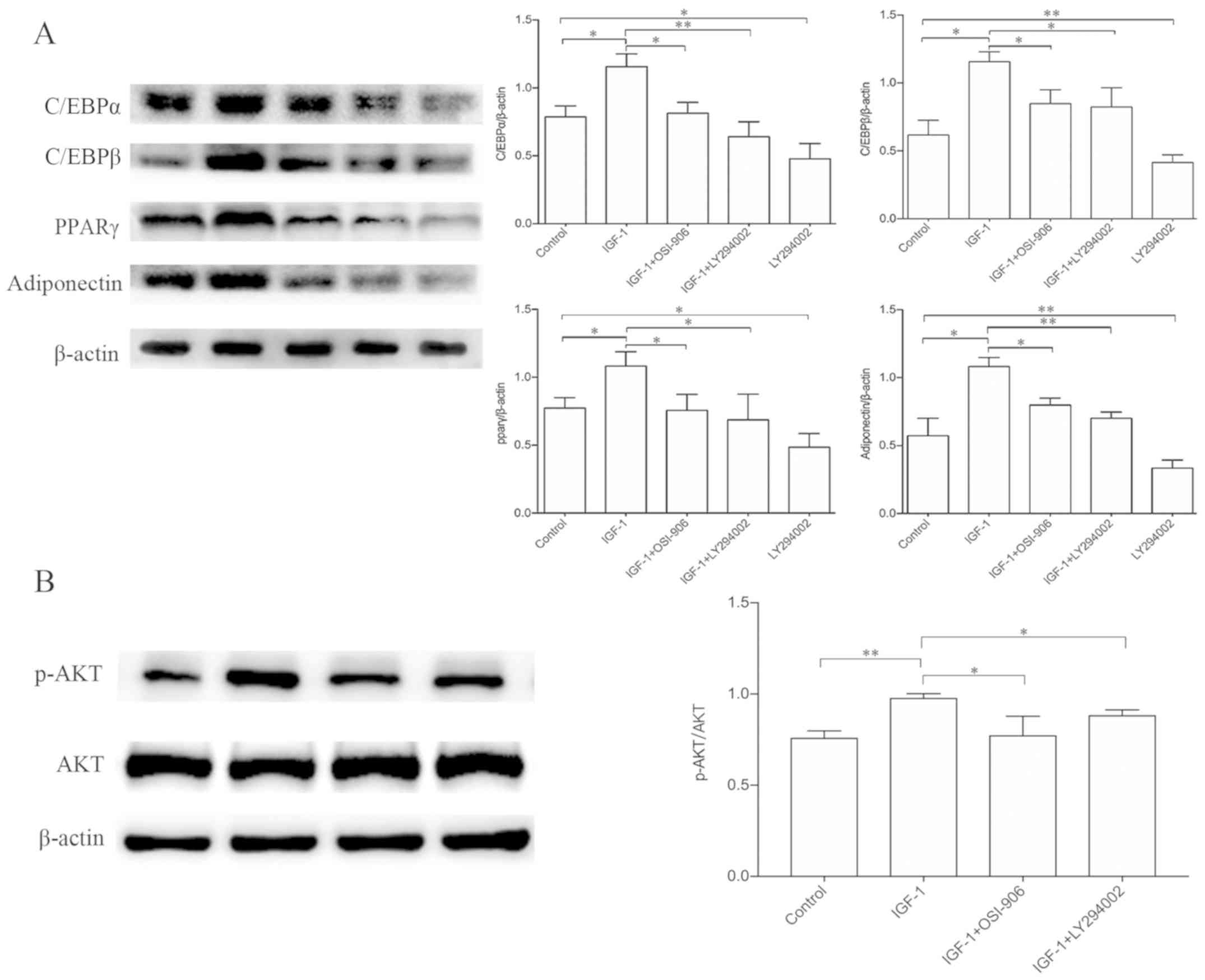 | Figure 3.Effects of IGF-1 on the expression of
adipogenic markers in HemSCs. The C/EBPα, C/EBPβ, PPARγ and
adiponectin protein expression levels were analyzed by western
blotting. (A) Western blot analysis demonstrating the protein
expression levels of C/EBPα, C/EBPβ, PPARg and adiponectin in
HemSCs treated with IGF-1 (100 ng/ml), IGF-1 (100 ng/ml) plus
OSI-906 (1 µM), IGF-1 (100 ng/ml) plus LY294002 (10 µM), and
LY294002 alone (10 µM). β-actin was used as a loading control. (B)
Western blot analysis demonstrating the expression levels of
phosphorylated AKT (p-AKT) and total AKT in confluent HemSC
cultures exposed to the indicated treatments for 1 h.
Quantification of the p-AKT protein expression levels indicated a
significant increase in the IGF-1 group (P<0.05). No differences
were detected in the total AKT protein levels among the groups.
Values are presented as the mean ± standard deviation. n=3.
*P<0.05, **P<0.01. IGF-1, insulin-like growth factor-1;
HemSCs, hemangioma stem cells; C/EBP, CCAAT/enhancer-binding
protein; PPARγ, peroxisome proliferator-activated receptor-γ; AKT,
RAC-a serine/threonine-protein kinase; p, phosphorylated. |
Discussion
The cellular components of hemangiomas alter as
their growth increases and subsides during their life cycle
(1). Endothelial cells predominate
in the proliferative phase but their numbers gradually decrease
throughout the degeneration process (2). Adipocyte numbers increase and
eventually dominate in the involuted phase. This accumulation of
adipocytes is also reflected in the gene expression patterns in the
involuting hemangioma (14). A
number of genes associated with lipid metabolism are also
overexpressed in the involuting hemangioma (14). This increased accumulation of
adipocytes provides a novel perspective for the study of
hemangiomas.
A previous study indicated the involvement of IGF in
the differentiation of stem cells into adipocytes (12), but no studies have yet been
performed on hemangioblast stem cells. The data presented here
indicate that IGF-1 significantly and dose-dependently promotes
in vitro proliferation and lipid accumulation in HemSCs, and
that adipogenesis in these cells proceeds in response to known
signaling pathways.
The process of adipogenesis is regulated by
transcription factors, including the PPARs and CCAAT/enhancer
binding protein (C/EBP) family members, which form a complex
network of signaling pathways (15). PPARγ is a nuclear hormone receptor
that serves a key role in adipogenesis. For example, its ectopic
expression stimulates the differentiation of cultured fibroblasts
(16) and a moderate reduction in
PPARγ in mice prevents the obesity caused by high-fat diets
(17). Numerous molecules serve a
role in the regulation of adipogenesis through PPARγ (17–19).
In the present study, it was identified that IGF-1 upregulated the
expression levels of PPARγ, C/EBPα and C/EBPβ, and increased lipid
accumulation, as demonstrated by Oil Red O staining.
IGF-1 serves an important role in the proliferation
and differentiation of various cells, including adipocytes
(20). IGF-1 binds primarily to
IGF-1R on the cell surface, and subsequent activation results in
phosphorylation of the major substrate (21). The two major signal transduction
pathways initiated by IGF-1/IGF-1R are mitogen-activated protein
kinase (MAPK)/extracellular signal-regulated kinase (ERK) and
PI3K/AKT. Previous studies have reported that the MAPK/ERK pathway
stimulates 3T3-L1 preadipocyte proliferation, while its inhibition
blocks IGF-1-stimulated mitosis, but not the differentiation of
these cells (18,22). Other studies have reported an
influence of the PI3K/AKT component of the IGF-1/IGF-1R signaling
pathway on adipose tissue differentiation, as treatment of 3T3-L1
preadipocytes with PI3K inhibitors results in a complete blockade
of adipocyte differentiation (23,24).
The present findings indicated that IGF-1 potently stimulated HemSC
proliferation and adipocyte differentiation, while treatment of
HemSCs with the PI3K inhibitor LY294002 or the IGF-1R inhibitor
OSI-906 suppressed the expression of C/EBPα, C/EBPβ, PPARγ and
adiponectin induced by IGF-1. Treatment with LY294002 alone also
suppressed adipocyte differentiation and the expression of these
proteins. The IGF-1 treatment also promoted the phosphorylation of
AKT, while treatment with OSI-906 or LY294002 suppressed this
phosphorylation. These findings indicated that IGF-1 regulates the
adipocyte differentiation of HemSCs from IH through the
IGF-1R/PI3K/AKT pathway.
The present study had certain limitations. One is
the small number of patients included in the study. In addition,
only the in vitro effects of exogenous IGF-1 on adipogenesis
in HemSCs were analyzed, and the study did not address endogenous
or in vivo effects. Nevertheless, our findings indicated
that IGF-1 was able to modulate the proliferation and
differentiation of HemSCs, and that adipogenesis was enhanced by
the IGF-1R and PI3K pathways. Therefore, IGF-1 serves an important
role in fat formation in HemSCs arising from IH and may be of
interest for the treatment of IH.
Acknowledgements
The authors would like to thank the Central
Laboratory of the Second Affiliated Hospital of Anhui Medical
University.
Funding
The present study was funded by the Natural Science
and Technology Fund Project of Anhui Province (grant no.
1808085MH282).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DC, XH, FW and HL conceived and designed the
experiments. FW and HL performed the experiments. FW produced the
manuscript. FW and HL conducted data analysis. YL, HL and JX
provided tissue specimens. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
the second Hospital of Anhui Medical University (no.
PJ-bb2017-026). Written informed consent was obtained from the
patients and/or guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kilcline C and Frieden IJ: Infantile
hemangiomas: How common are they? A systematic review of the
medical literature. Pediatr Dermatol. 25:168–173. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Munden A, Butschek R, Tom WL, Marshall JS,
Poeltler DM, Krohne SE, Alió AB, Ritter M, Friedlander DF,
Catanzarite V, et al: Prospective study of infantile haemangiomas:
Incidence, clinical characteristics and association with placental
anomalies. Br J Dermatol. 170:907–913. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pandey A, Gangopadhyay AN and Upadhyay VD:
Evaluation and management of infantile hemangioma: An overview.
Ostomy Wound Manage. 54:16–18, 20, 22–26, 28–29. 2008.PubMed/NCBI
|
|
4
|
Khan ZA, Boscolo E, Picard A, Psutka S,
Melero-Martin JM, Bartch TC, Mulliken JB and Bischoff J:
Multipotential stem cells recapitulate human infantile hemangioma
in immunodeficient mice. J Clin Invest. 118:2592–2599.
2008.PubMed/NCBI
|
|
5
|
Roach EE, Chakrabarti R, Park NI, Keats
EC, Yip J, Chan NG and Khan ZA: Intrinsic regulation of hemangioma
involution by platelet-derived growth factor. Cell Death Dis.
3:e3282012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhao P, Deng Y, Gu P, Wang Y, Zhou H, Hu
Y, Chen P and Fan X: Insulin-like growth factor 1 promotes the
proliferation and adipogenesis of orbital adipose-derived stromal
cells in thyroid-associated ophthalmopathy. Exp Eye Res. 107:65–73.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Laviola L, Natalicchio A and Giorgino F:
The IGF-I signaling pathway. Curr Pharm Des. 13:663–669. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Garten A, Schuster S and Kiess W: The
insulin-like growth factors in adipogenesis and obesity. Endocrinol
Metab Clin North Am. 41:283–295, v-vi. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen Q, Shou P, Zheng C, Jiang M, Cao G,
Yang Q, Cao J, Xie N, Velletri T, Zhang X, et al: Fate decision of
mesenchymal stem cells: Adipocytes or osteoblasts? Cell Death
Differ. 23:1128–1139. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
James AW: Review of signaling pathways
governing MSC osteogenic and adipogenic differentiation.
Scientifica (Cairo). 2013:6847362013.PubMed/NCBI
|
|
11
|
Tontonoz P, Hu E and Spiegelman BM:
Regulation of adipocyte gene expression and differentiation by
peroxisome proliferator activated receptor gamma. Curr Opin Genet
Dev. 5:571–576. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Scavo LM, Karas M, Murray M and Leroith D:
Insulin-like growth factor-I stimulates both cell growth and
lipogenesis during differentiation of human mesenchymal stem cells
into adipocytes. J Clin Endocrinol Metab. 89:3543–3553. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yu Y, Wylie-Sears J, Boscolo E, Mulliken
JB and Bischoff J: Genomic imprinting of IGF2 is maintained in
infantile hemangioma despite its high level of expression. Mol Med.
10:117–123. 2004.PubMed/NCBI
|
|
14
|
Yu Y, Fuhr J, Boye E, Gyorffy S, Soker S,
Atala A, Mulliken JB and Bischoff J: Mesenchymal stem cells and
adipogenesis in hemangioma involution. Stem Cells. 24:1605–1612.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kubota N, Terauchi Y, Miki H, Tamemoto H,
Yamauchi T, Komeda K, Satoh S, Nakano R, Ishii C, Sugiyama T, et
al: PPAR gamma mediates high-fat diet-induced adipocyte hypertrophy
and insulin resistance. Mol Cell. 4:597–609. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Siersbaek R, Nielsen R and Mandrup S:
PPARgamma in adipocyte differentiation and metabolism-novel
insights from genome-wide studies. FEBS Lett. 584:3242–3249. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang C, Zhang X, Ma L, Peng F, Huang J
and Han H: Thalidomide inhibits adipogenesis of orbital fibroblasts
in Graves' ophthalmopathy. Endocrine. 41:248–255. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Boney CM, Gruppuso PA, Faris RA and
Frackelton AR Jr: The critical role of Shc in insulin-like growth
factor-I-mediated mitogenesis and differentiation in 3T3-L1
preadipocytes. Mol Endocrinol. 14:805–813. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hers I: Insulin-like growth factor-1
potentiates platelet activation via the IRS/PI3Kalpha pathway.
Blood. 110:4243–4252. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Boney CM, Smith RM and Gruppuso PA:
Modulation of insulin-like growth factor I mitogenic signaling in
3T3-L1 preadipocyte differentiation. Endocrinology. 139:1638–1644.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Boney CM, Sekimoto H, Gruppuso PA and
Frackelton AR Jr: Src family tyrosine kinases participate in
insulin-like growth factor I mitogenic signaling in 3T3-L1 cells.
Cell Growth Differ. 12:379–386. 2001.PubMed/NCBI
|
|
22
|
Smith TJ: Insulin-like growth factor-I
regulation of immune function: A potential therapeutic target in
autoimmune diseases? Pharmacol Rev. 62:199–236. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Peng XD, Xu PZ, Chen ML, Hahn-Windgassen
A, Skeen J, Jacobs J, Sundararajan D, Chen WS, Crawford SE, Coleman
KG and Hay N: Dwarfism, impaired skin development, skeletal muscle
atrophy, delayed bone development, and impeded adipogenesis in mice
lacking Akt1 and Akt2. Genes Dev. 17:1352–1365. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xu J and Liao K: Protein kinase B/AKT 1
plays a pivotal role in insulin-like growth factor-1 receptor
signaling induced 3T3-L1 adipocyte differentiation. J Biol Chem.
279:35914–35922. 2004. View Article : Google Scholar : PubMed/NCBI
|