Introduction
At present, colorectal cancer (CRC, also known as
bowel cancer) is one of the leading cause of cancer-associated
mortality, which is newly diagnosed in 1.4 million people and
resulted in 694,000 deaths worldwide in 2012 (1). The development of CRC is
characterized by the uncontrolled growth of transformed cells
associated with complex interactions, including genetic
alterations, environmental carcinogens and dysregulation in host
immunity (2). Despite several
recent developments, the therapeutic options for treatment during
the metastatic stages of this disease remain limited (3). Local recurrence following the
resection of CRC is difficult to treat and has been associated with
severe complications. A previous study demonstrated poor prognosis
following local recurrence, with a survival duration of <18
months (4). Therefore, therapeutic
approaches that target the control of growth (metastasis, invasion
and apoptosis), angiogenesis, as well as cell cycle-regulating
signals, are necessary for the treatment of patients with CRC
(5). Preoperative
chemoradiotherapy is commonly used as a major treatment modality
for advanced CRC (6). Previous
studies have demonstrated that preoperative chemoradiotherapy was
associated with decreased local recurrence, improved survival rate
and increased anal preservation rate (7,8).
Matrix metalloproteinases (MMPs) are a group of
matrix-degrading proteins, which include >22 human
zinc-dependent proteolytic enzymes (9,10).
MMPs produced by tumor cells or by adjacent stromal cells are
involved in the metastatic process (11). Among the MMPs, MMP1 is the most
ubiquitously expressed interstitial collagenase (12). Additionally, MMP1 expression in CRC
cells has been reported to be associated with poor prognosis
(13). Numerous studies have
demonstrated the association between MMP1 and CRC (14–16);
however, the effects of radiotherapy on MMP1 expression levels,
cell viability and migration require further investigation.
The small bowel only tolerates limited dosages in
pelvic radiotherapy (17). Bowel
displacement devices have been adopted in the clinic to reduce
bowel volume under high-dose pelvic radiation fields (18). Therefore, it is of great importance
to understand the effects of various radiation dosages on the
viability and migration of CRC cells. The present study was
performed with two aims: i) To detect the effects of MMP1 on the
viability and migration of CRC cells; and ii) to investigate the
effects of MMP1 on cell viability and migration under various doses
of X-ray radiation. The results of the present study may provide a
theoretical foundation for the clinical application of
MMP1-targeted therapy in the treatment of CRC.
Materials and methods
Cell culture
The CRC cell line SW620 was obtained from the Cell
Bank of Type Culture Collection of Chinese Academy of Sciences
(Shanghai, China); cells were cultured in Dulbecco's modified
Eagles medium (DMEM) supplemented with 10% fetal bovine serum
(FBS), 1% penicillin and 1% streptomycin (all Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA). Cells were incubated under 5%
CO2 at a constant temperature of 37°C in an incubator.
Following culturing for a period of time, the cells in logarithmic
phase were obtained for the following experiments.
Detection of cell viability
The effects of various doses of X-ray radiation on
cell viability were detected via an MTT assay. Briefly, CRC cells
were plated at a density of 104 cells/well into a
96-well plate. Cells were incubated in DMEM at 37°C for 48 h
following exposure to radiation of different doses of X-ray (0,
0.1, 0.5, 1, 3 and 6 Gy). Then, cells in each well were incubated
with 10 µl MTT for 4 h at 37°C. Following the removal of media, 100
µl dimethyl sulfoxide was added to dissolve the formazan crystals.
In addition, the optical density was measured at a wavelength of
570 nm using a NanoDrop spectrophotometer (Thermo Fisher
Scientific, Inc., Wilmington, DE, USA).
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was isolated from CRC cells with an RNA
rapid extraction kit (Takara Biotechnology Co., Ltd., Dalian,
China) according to the manufacturer's protocols. The concentration
and purity of RNA products were measured via spectrophotometry.
Then, 0.5 µg total RNA was reverse transcribed into cDNA using the
Prime Script® RT reagent kit (Takara Biotechnology Co.,
Ltd.); the reaction was incubated at 37°C for 15 min, and 85°C for
5 sec. Using SYBR Green Master Mix (Takara Biotechnology Co., Ltd.,
Dalian, China), qPCR was then performed to detect the expression
levels of MMP1. The 20 µl reaction system for qPCR was: 10 µl SYBR
Premix Ex Taq (X2), 8 µl cDNA template (diluted to a uniform
level), 1 µl forward primer (10 µM), and 1 µl reverse primer (10
µM). Each sample had three repeats. The thermocycling conditions
were as follows: 50°C for 3 min, 95°C for 3 min, and 40 cycles of
95°C for 10 sec and 60°C for 30 sec. A melting curve was
subsequently generated. The sequences of primers used for
amplification are listed in Table
I. To evaluate the mRNA expression levels of MMP1, the
2−ΔΔCq method (19) was
employed. β-actin was taken as the reference gene for normalizing
MMP1 expression.
 | Table I.Primer sequences employed in the
present study. |
Table I.
Primer sequences employed in the
present study.
| Primer | Sequence (5′-3′) |
|---|
| MMP1 forward |
AAGAATGATGGGAGGCAAGT |
| MMP1 reverse |
GGTTTCAGCATCTGGTTTCC |
| β-actin forward |
GGAGATTACTGCCCTGGCTCCTA |
| β-actin reverse |
GACTCATCGTACTCCTGCTTGCTG |
Small interfering (si)-RNA
transfection
After the SW620 cells were digested by pancreatin
(Gibco; Thermo Fisher Scientific, Inc.), centrifuged at 15,000 × g
at 4°C and counted, they were plated into a 6-well plate
(2×105 cells/well) and DMEM supplemented with 10% FBS
(both Gibco; Thermo Fisher Scientific, Inc.) added. Then, 4 siRNAs
were designed for silencing MMP1, and the cells were randomly
divided into siRNA1-transfected cell group, siRNA2-transfected cell
group, siRNA3-transfected cell group, control siRNA (Con siRNA)
transfection cell group, and negative control (NC) cell group.
SiRNA-mediated gene silencing of MMP1 was conducted according to
the protocols of the manufacturer of Lipofectamine 2000™. Firstly,
siRNA (final concentration 66 nM; GenePharma, Shanghai, China) and
Lipofectamine 2000™ (Invitrogen; Thermo Fisher Scientific, Inc.)
were diluted with 250 µl Opti-Minimal Essential Medium (Gibco;
Thermo Fisher Scientific, Inc.), respectively. Then, the
transfection reagent was mixed with the diluted siRNA and incubated
for 20 min at room temperature for complex formation. The complexes
were added into each well for transfection with 5% CO2
under a constant temperature of 37°C in an incubator. Media were
changed into DMEM supplemented with 10% FBS (both Gibco; Thermo
Fisher Scientific, Inc.) after 48 h. Untreated control cells
received the same concentration of buffer, but no siRNA (NC). The
silencing effects of siRNAs were detected using RT-qPCR and western
blotting, and siRNA2 was employed for subsequent analysis.
Cell migration assay
Cell migration was evaluated using a 6-well
Transwell filter with a polyvinylidene fluoride membrane (Merck
KGaA, Darmstadt, Germany). SW620 cells were diluted by DMEM
containing 0.1% bovine serum albumin (BSA; both Gibco; Thermo
Fisher Scientific, Inc.) and then were seeded on 6-well Transwell
filter (3×105 cell/well). The Transwell filter was
placed in a 24-well plate with 500 µl DMEM containing 10% FBS (both
Gibco; Thermo Fisher Scientific, Inc.), and then was cultured under
5% CO2 at 37°C in an incubator. Following 24 h
incubation, the filter was submerged in 500 µl of 4%
paraformaldehyde, washed twice with 500 µl PBS, stained with 0.1%
crystal violet at room temperature for 10 min, and washed with
water. Prior to imaging using a fluorescence microscope
(magnification, ×100; 5 fields; Olympus Corporation, Tokyo, Japan),
cells that had not migrated were removed with a cotton swab from
the upper layer of the filter.
Western blotting
Cultured cells were washed 3 times with PBS and
lysed in phenylmethanesulfonyl fluoride (Sangon Biotech Co., Ltd.,
Shanghai, China) on ice for 20 min. The lysates were centrifuged at
15,000 × g at 4°C for 10 min and the supernatants were collected.
The protein concentration was measured using a Bicinchoninic Acid
protein assay kit (Sangon Biotech Co., Ltd.), with 2 mg/ml BSA
(Gibco; Thermo Fisher Scientific, Inc.) as the standard.
Subsequently, 50 µg proteins were separated by 10% SDS-PAGE and
transferred onto a polyvinylidene fluoride membrane (Merck KGaA).
The membranes were blocked in 5% nonfat dried milk at room
temperature for 1 h, and then incubated at 4°C with primary
antibodies (anti-MMP1 antibody; 1:10,000; cat. no. sc-58377; Santa
Cruz Biotechnology, Inc., Dallas, TX, USA and anti-β-actin
antibody, 1:10,000; cat. no. 115035003; Jackson ImmunoResearch
Laboratories, Inc., West Grove, PA, USA) overnight. Following
washing with Tris buffered saline with Tween-20 (1:1,000) 4 times,
the blots were incubated with horseradish peroxidase-conjugated
goat-anti-rabbit antibody (1:5,000; cat. no. 111035047; Jackson
ImmunoResearch Laboratories, Inc.) at 37°C for 1 h, and then
developed with enhanced chemiluminescence detection reagents (Merck
KGaA). Visualization was performed with a gel imaging analysis
system (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Statistical analysis
There were three repeats for each experiment. The
data were presented as the mean ± standard error of mean. One-way
analysis of variance followed by a Bonferroni post-hoc test was
applied for statistical analysis using SPSS 13.0 software (SPSS,
Inc., Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
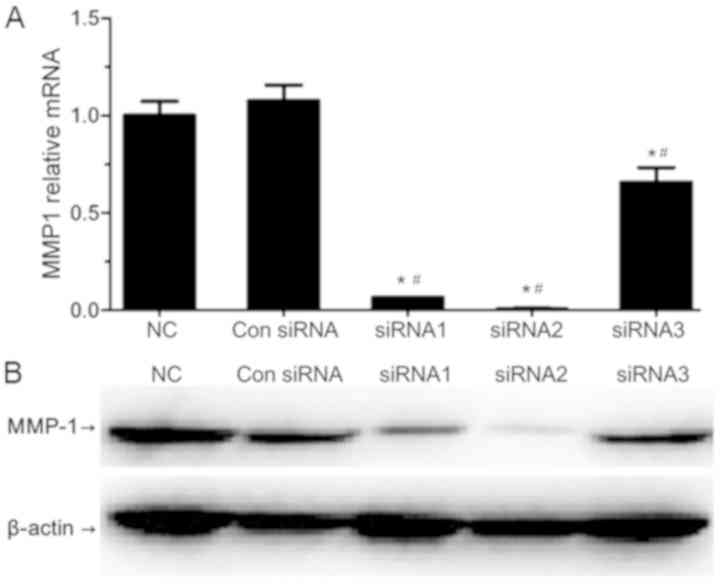
Suppression of MMP1 expression levels
by siRNA
The mRNA and protein expression levels of MMP1 were
evaluated by RT-qPCR and western blotting, respectively (Fig. 1). All of the three designed siRNAs
(siRNA1, siRNA2 and siRNA3) significantly reduced the expression
levels of MMP1 when compared with the NC and Con siRNA transfection
cell groups, which confirmed the success of transfection. SiRNA1
and siRNA2 resulted in a significant decrease in MMP1 expression in
SW620 cells when compared with the siRNA3-transfected cell group.
SiRNA2 was most efficient in silencing MMP1, and thus was selected
for subsequent use.
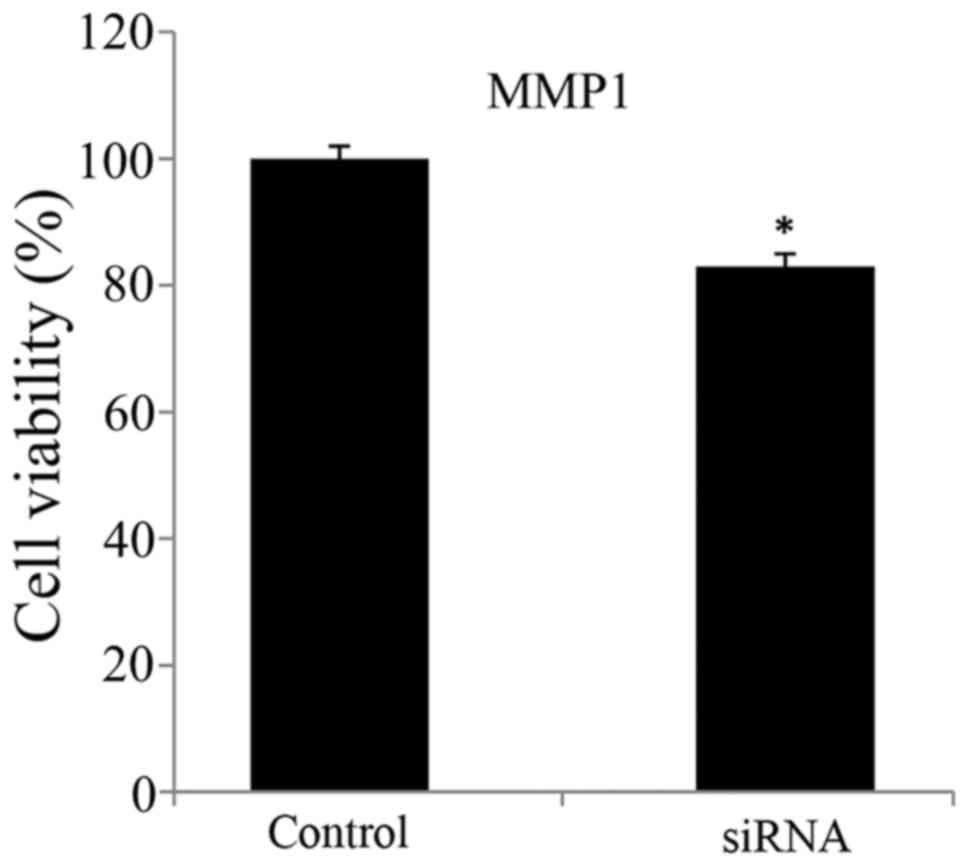
Viability and migration of
MMP1-silenced CRC cells
The viability and migration of MMP1-silenced CRC
cells were presented in Figs. 2
and 3, respectively. The
application of siRNA to silence MMP1 in SW620 cells resulted in
significantly reduced cell viability and migration ability compared
with in the control untransfected group. That is, the viability and
migration ability of SW620 cells were suppressed by siRNA-induced
MMP1 silencing.
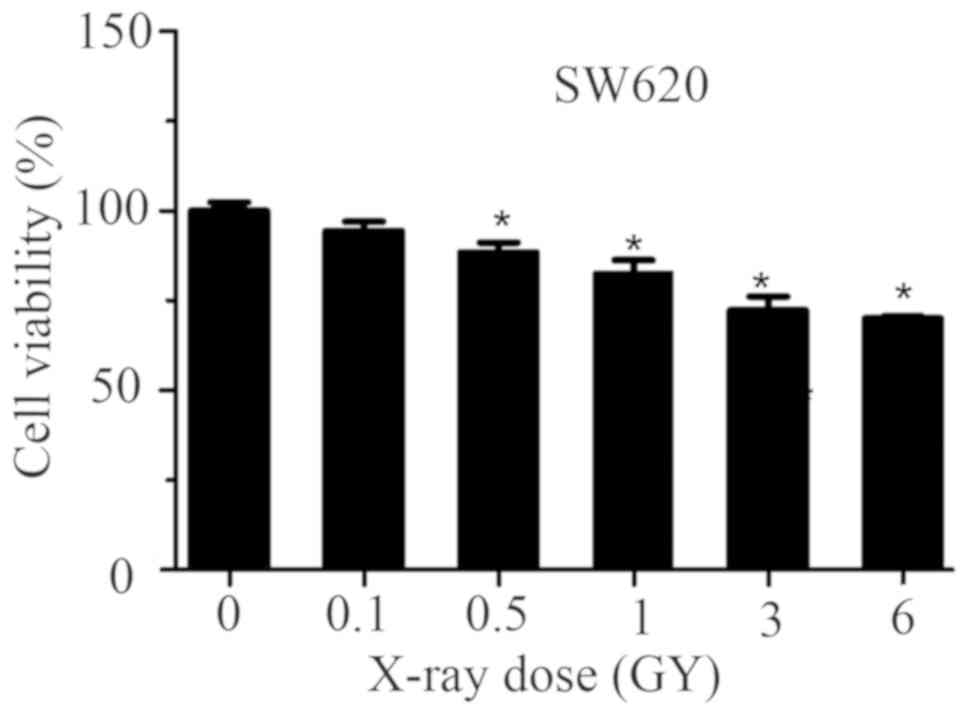
Effects of radiation on cell viability
and migration
The effects of various doses of X-ray exposure on
SW620 cells is presented in Fig.
4. The results of the present study revealed that cell
viability decreased in a dose-dependent manner following exposure
to X-ray radiation. Compared with the control group, cell viability
was significantly reduced following exposure to 0.5, 1, 3 and 6 Gy
X-ray radiation in a dose-dependent manner. The results also
demonstrated that cell viability was markedly decreased under
treatment with 3 and 6 Gy X-ray radiation when compared with 0.5
and 1 Gy treatment. These results suggested that different doses of
X-ray radiation could reduce the cell viability of SW620 cells to
different extents, and a higher dose of X-ray radiation had a
greater inhibiting effect.
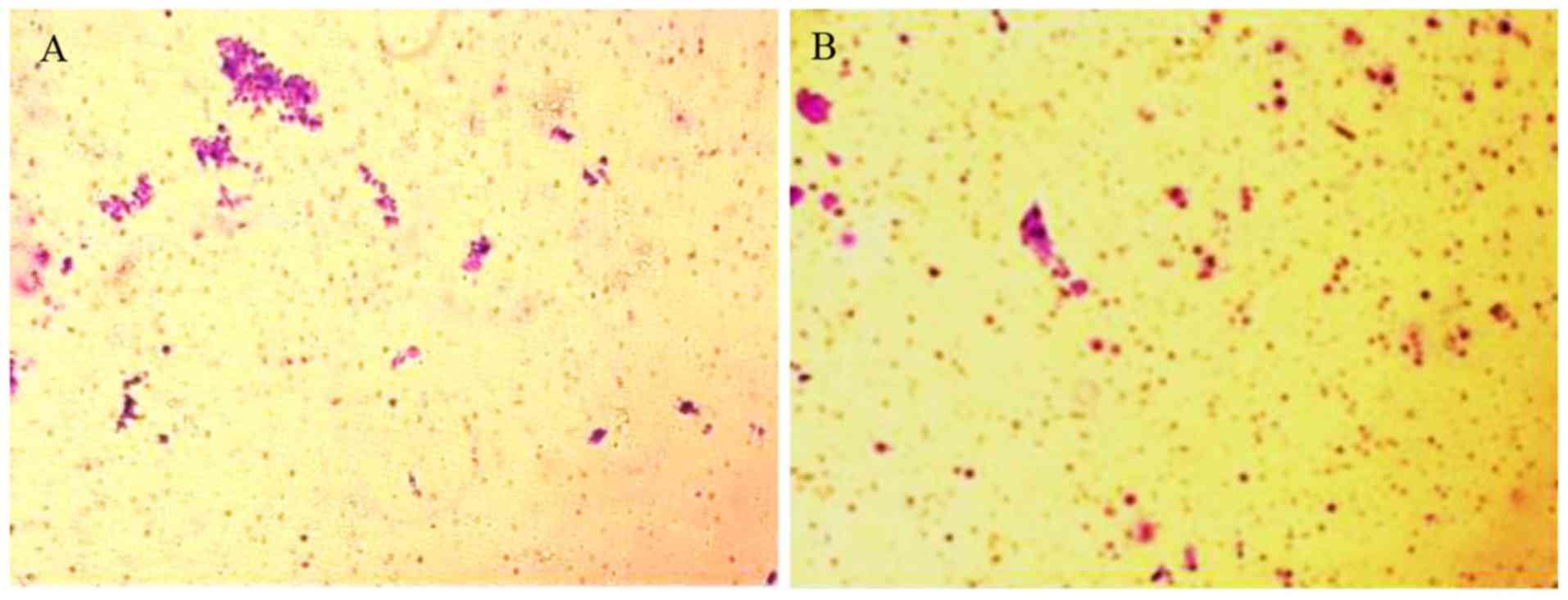
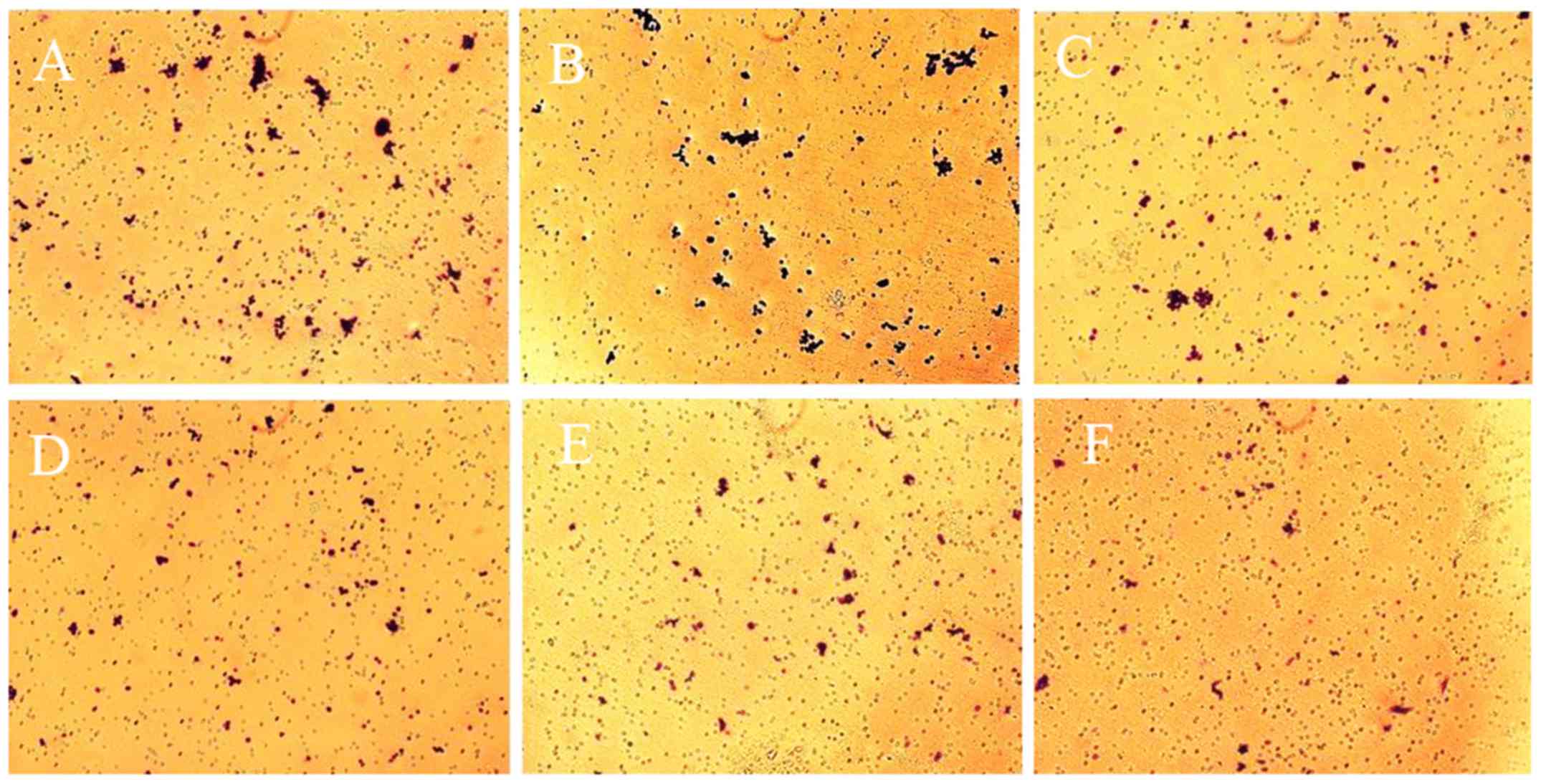
In addition, cell migration was evaluated via a
Transwell assay. As presented in Fig.
5, compared with control and 0.1 Gy X-ray treated cells
(Fig. 5A and B), the number of
migrated cells was markedly lower in the experimental groups
(Fig. 5C-E) the average number of
migrated cells within 5 fields in the experimental groups (0.5, 1,
3 and 6 Gy X-ray treated cells) was significantly lower than those
of the blank control and the 0.1 Gy X-ray treated cells. Therefore,
different doses of X-ray radiation could markedly repress the
migration of SW620 cells.
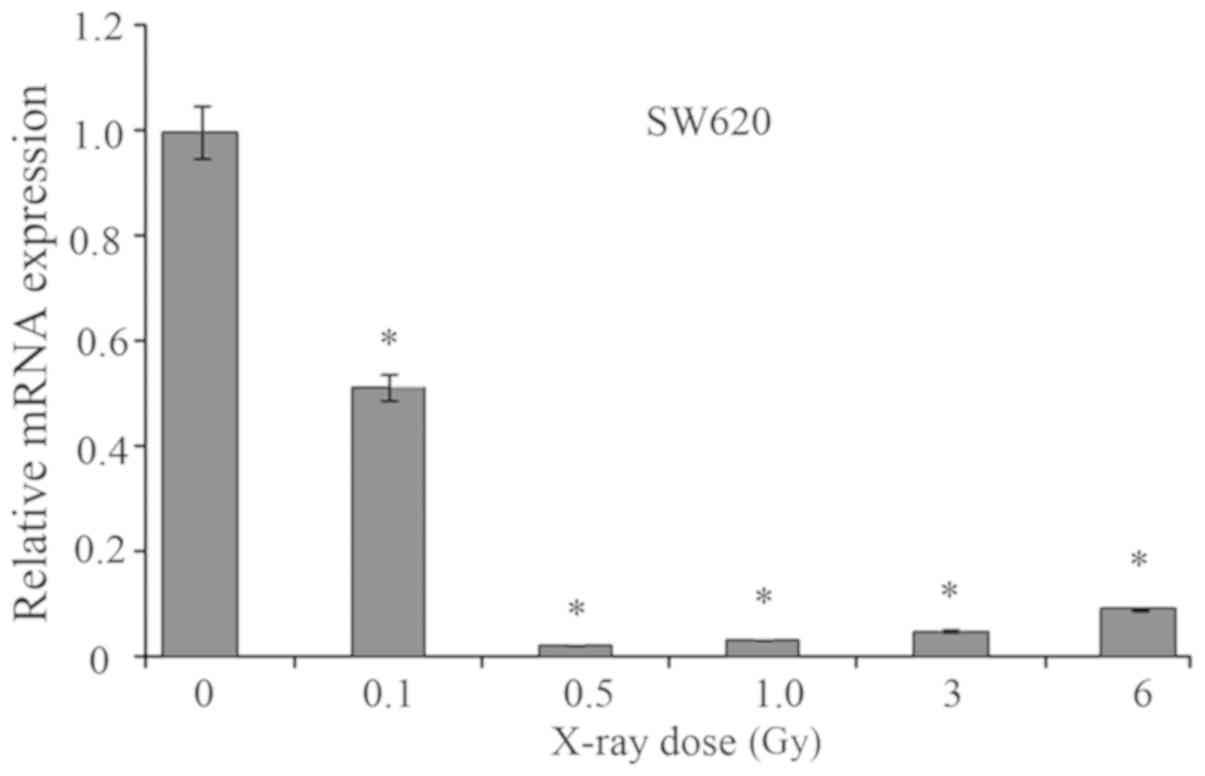
Effects of radiation on MMP1
expression
As presented in Fig.
6, the effects of radiation on the mRNA expression levels of
MMP1 were determined. As a result, when exposed to X-ray radiation,
the mRNA expression levels of MMP1 in SW620 cells were
significantly suppressed when compared with the untreated group.
The cells exposed to 0.5 Gy X-ray exhibited the lowest mRNA
expression levels of MMP1 compared with the remaining groups. With
increasing doses of X-ray radiation (from 0.5 to 6 Gy), the mRNA
expression levels of MMP1 were markedly elevated. Thus, X-ray
radiation could significantly decrease the mRNA expression levels
of MMP1.
Discussion
The development of CRC is a complex process that
involves multistage carcinogenesis (20). Preoperative radiotherapy is
commonly used in the clinic and has been reported to be associated
with decreases in recurrence rate; however, the effects of
radiotherapy on CRC cells at the molecular level requires further
investigation (21). MMP1 serves
an important role in degrading tumor cells (22). In the present study, the SW620 cell
line was selected to investigate the association between MMP1, cell
viability and migration. Classified as Duke's type B level cancer,
SW620 cells originate from mesenteric lymph node metastasis, with
high invasive and malignancy potentials (23). Therefore, the SW620 cell line may
be suitable for the detection of cell viability and migration.
MMP1 serves an important role in the degradation of
collagenous extracellular matrix in a variety of physiological and
pathological situations (24). The
association of MMP1 and CRC has also been reported previously
(13,25). For example, Vogelstein et al
(20) investigated the genotype of
patients with CRC and revealed that MMP1 promoter polymorphisms
affected the susceptibility of developing CRC due to abnormal
alterations in the expression of MMP1. Additionally, MMP1 has been
proposed as a prognostic factor of CRC; high MMP1 expression levels
may indicate poor prognosis (13,25).
To detect the effects of MMP1 on cell viability and migration, MTT
and Transwell assays were performed in the present study. The
viability and migration of SW620 cells were significantly
suppressed following MMP1 downregulation in the present study,
which indicated that MMP1 may serve a key role in cell
migration.
The association between preoperative radiotherapy
and MMP1 in CRC cells has not been well established. MMP1 has been
reported to be differentially expressed following preoperative
chemoradiotherapy (26). This has
also been observed in patients with breast cancer, in which MMP1
protein expression levels were downregulated following preoperative
radiotherapy (27). The results of
present study were consistent with the aforementioned findings,
which revealed that MMP1 was significantly decreased following
X-ray radiation. However, from gene expression profile studies,
alterations in the expression of MMP1 have not been observed in
patients with CRC following radiotherapy (28,29).
This may be due to varying sensitivities of radiation in different
growth stages of tumors. Therefore, it appears necessary to
consider tumor stages when combining MMP1-targeted therapy with
current radiotherapy regimens.
Radiation dosage serves an important role in
radiotherapy for patients with cancer (30). In the present study, it was
reported that the mRNA expression levels of MMP1 varied in response
to different doses of X-ray radiation. The results indicated that
radiation dosage was associated with gene expression levels, which
may affect the therapeutic efficiency. There is limited information
regarding the radiation dosage of preoperative chemoradiotherapy on
CRC. A high radiation dose (45 Gy) has been reported to affect the
pathological complete response level of patients with rectal cancer
(31). A previous study
investigated the effects of X-ray doses (0, 2, 4, 6 and 8 Gy) on
the expression of microRNA (miR) −221 and p57kip2 in CRC
cells, which revealed that radiation dose can affect the
miR-221/p57kip2 pathway (27). This may enhance the
radiosensitivity of CRC cells (32). In the present study, the mRNA
expression levels of MMP1 were significantly decreased in SW620
cells when exposed to X-ray radiation. Additionally, the viability
and migration of SW620 cells prior to MMP1-silencing were
significantly reduced in a dose-dependent manner. The results of
the present study may provide novel insight into the radiotherapy
of CRC in the clinic.
In conclusion, expression of MMP1 was associated
with the promotion of the viability and migration of SW620 cells.
X-ray radiation of 6 Gy significantly reduced cell viability. It
also appears necessary to consider tumor stages when applying
combined MMP1-targeted therapy with current radiotherapy regimens.
In the future, investigation may be conducted with in vivo
models or samples obtained from patients with CRC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
FJ conceived and designed the study; NL acquired the
data; WW analyzed and interpreted the data; HY performed
statistical analysis; FJ and NL drafted the manuscript, and WW and
HY revised the manuscript for important intellectual content. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
McGuire S: World cancer report 2014.
Geneva, Switzerland: World Health Organization, International
Agency for Research on Cancer, WHO press, 2015. Adv Nutr.
7:418–419. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fearon ER and Vogelstein B: A genetic
model for colorectal tumorigenesis. Cell. 61:759–767. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lange F, Franz B, Maletzki C, Linnebacher
M, Hühns M and Jaster R: Biological and molecular effects of small
molecule kinase inhibitors on low-passage human colorectal cancer
cell lines. Biomed Res Int. 2014:5686932014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Holm T, Cedermark B and Rutqvist LE: Local
recurrence of rectal adenocarcinoma after ‘curative’ surgery with
and without preoperative radiotherapy. Br J Surg. 81:452–455. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hanahan D and Weinberg RA: The hallmarks
of cancer. Cell. 100:57–70. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Park IJ and Yu CS: Current issues in
locally advanced colorectal cancer treated by preoperative
chemoradiotherapy. World J Gastroenterol. 20:2023–2029. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Braendengen M, Tveit KM, Berglund A,
Birkemeyer E, Frykholm G, Påhlman L, Wiig JN, Byström P, Bujko K
and Glimelius B: Randomized phase III study comparing preoperative
radiotherapy with chemoradiotherapy in nonresectable rectal cancer.
J Clin Oncol. 26:3687–3694. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kao PS, Chang SC, Wang LW, Lee RC, Liang
WY, Lin TC, Chen WS, Jiang JK, Yang SH, Wang HS and Lin JK: The
impact of preoperative chemoradiotherapy on advanced low rectal
cancer. J Surg Oncol. 102:771–777. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Parsons SL, Watson SA, Brown PD, Collins
HM and Steele RJ: Matrix metalloproteinases. Br J Surg. 84:160–166.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Visse R and Nagase H: Matrix
metalloproteinases and tissue inhibitors of metalloproteinases:
Structure, function, and biochemistry. Circ Res. 92:827–839. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zucker S and Vacirca J: Role of matrix
metalloproteinases (MMPs) in colorectal cancer. Cancer Metastasis
Rev. 23:101–117. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Arakaki PA, Marques MR and Santos MC:
MMP-1 polymorphism and its relationship to pathological processes.
J Biosci. 34:313–320. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Murray GI, Duncan ME, O'Neil P, Melvin WT
and Fothergill JE: Matrix metalloproteinase-1 is associated with
poor prognosis in colorectal cancer. Nat Med. 2:461–462. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zinzindohoué F, Lecomte T, Ferraz JM,
Houllier AM, Cugnenc PH, Berger A, Blons H and Laurent-Puig P:
Prognostic significance of MMP-1 and MMP-3 functional promoter
polymorphisms in colorectal cancer. Clin Cancer Res. 11:594–599.
2005.PubMed/NCBI
|
|
15
|
Bendardaf R, Buhmeida A, Ristamäki R,
Syrjänen K and Pyrhönen S: MMP-1 (collagenase-1) expression in
primary colorectal cancer and its metastases. Scand J
Gastroenterol. 42:1473–1478. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Han G, Wei Z, Lu Z, Cui H, Bai X, Ge H and
Zhang W: Association between matrix metalloproteinase 1 −1607
1G>2G polymorphism and cancer risk: A meta-analysis including
19706 subjects. Int J Clin Exp Med. 7:2992–2999. 2014.PubMed/NCBI
|
|
17
|
Drzymala M, Hawkins M, Henrys A, Bedford
J, Norman A and Tait D: The effect of treatment position, prone or
supine, on dose-volume histograms for pelvic radiotherapy in
patients with rectal cancer. Br J Radiol. 82:321–327. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim JY, Kim DY, Kim TH, Park SY, Lee SB,
Shin KH, Pyo H, Kim JY and Cho KH: Intensity-modulated radiotherapy
with a belly board for rectal cancer. Int J Colorectal Dis.
22:373–379. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vogelstein B, Fearon ER, Hamilton SR, Kern
SE, Preisinger AC, Leppert M, Nakamura Y, White R, Smits AM and Bos
JL: Genetic alterations during colorectal-tumor development. N Engl
J Med. 319:525–532. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Häfner MF and Debus J: Radiotherapy for
colorectal cancer: Current standards and future perspectives. Visc
Med. 32:172–177. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tahara K, Mimori K, Iinuma H, Iwatsuki M,
Yokobori T, Ishii H, Anai H, Kitano S and Mori M: Serum
matrix-metalloproteinase-1 is a bona fide prognostic marker for
colorectal cancer. Ann Surg Oncol. 17:3362–3369. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Teran BL and Thomas J: Antimycin induces
apoptosis in rapamycin resistant SW620 colorectal cancer cells.
FASEB J. 27:793–798. 2013.PubMed/NCBI
|
|
24
|
Westermarck J and Kähäri VM: Regulation of
matrix metalloproteinase expression in tumor invasion. FASEB J.
13:781–792. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Langenskiöld M, Ivarsson ML, Holmdahl L,
Falk P, Kåbjörn-Gustafsson C and Angenete E: Intestinal mucosal
MMP-1-a prognostic factor in colon cancer. Scand J Gastroenterol.
48:563–569. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nishioka M, Shimada M, Kurita N, Iwata T,
Morimoto S, Yoshikawa K, Higashijima J and Miyatani T: Gene
expression profile can predict pathological response to
preoperative chemoradiotherapy in rectal cancer. Cancer Genomics
Proteomics. 8:87–92. 2011.PubMed/NCBI
|
|
27
|
Artacho-Cordón F, Ríos-Arrabal S, Lara PC,
Artacho-Cordón A, Calvente I and Núñez MI: Matrix
metalloproteinases: Potential therapy to prevent the development of
second malignancies after breast radiotherapy. Surg Oncol.
21:143–151. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Angenete E, Oresland T, Falk P, Breimer M,
Hultborn R and Ivarsson ML: Preoperative radiotherapy and
extracellular matrix remodeling in rectal mucosa and tumour matrix
metalloproteinases and plasminogen components. Acta Oncol.
48:1144–1151. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Supiot S, Gouraud W, Campion LC, Jezéquel
P, Buecher B, Charrier J, Heymann MF, Mahé MA, Rio E and Chérel M:
Early dynamic transcriptomic changes during preoperative
radiotherapy in patients with rectal cancer: A feasibility study.
World J Gastroenterol. 19:3249–3254. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jo S, Choi Y, Park SK, Kim JY, Kim HJ, Lee
YH, Oh WY, Cho H and Ahn KJ: Efficacy of dose-escalated
radiotherapy for recurrent colorectal cancer. Ann Coloproctol.
32:66–72. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sanghera P, Wong DW, McConkey CC, Geh JI
and Hartley A: Chemoradiotherapy for rectal cancer: An updated
analysis of factors affecting pathological response. Clin Oncol (R
Coll Radiol). 20:176–183. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sun K, Zhang X, Deng H, et al: Effects of
X-ray dose on expression of microRNA-221 and p57~ (kip2) in human
colorectal carcinoma cells. Cancer Res Prev Treat. 40:921–924.
2013.
|