Introduction
Gut microbiota play a pivotal role in various
aspects of host physiology including the immune system, metabolism,
hormonal secretion and gastrointestinal (GI) motility (1). The mechanism by which gut microbiota
affects such physiological functions remains largely unclear.
However, immune cells appear to be key players in mediating the
interaction between gut microbiota and host tissues. Among various
immune cells, macrophages have been highlighted due to their
polarization into different phenotypes, M1 and M2, whose profiles
are closely associated with specific cytokines (2,3).
Indeed, the T helper cell type 1 (Th1) signature cytokine,
interferon-γ (IFN-γ), is associated with M1 polarization towards a
proinflammatory phenotype (4),
whereas macrophages exposed to Th2 signature cytokines, such as
IL-4, assume an anti-inflammatory phenotype referred to as M2
polarization (5). M1 and M2
macrophages produce mainly inflammatory and anti-inflammatory
cytokines, respectively (6).
Furthermore, M1 and M2 macrophages have been reported to share
dominancy of their roles, including antigen presentation,
phagocytosis, and growth factor/cytokine secretion to enteric
neurons (7).
Antibiotic treatment is a critical factor causing
imbalance of the gut microbiota profile (dysbiosis). Antibiotics
are dispensable for the treatment of infection, but the dysbiosis
they cause leads to not only Clostridium difficile colitis
(8) but also adiposity, insulin
resistance or functional gastrointestinal disorders (9). In particular, it is noteworthy that
dysbiosis occurring in the early stage of life may be a significant
factor in the development of disorders of metabolism and
gastrointestinal motility (10).
In this context, we prepared an animal model subjected to treatment
with the antibiotic vancomycin that has been widely used and its
related data is well accumulated. Thereafter, we investigated the
resulting alterations of body metabolism and gastrointestinal
motility in relation to the macrophage profile in the colon.
Materials and methods
Antibiotic treatment
Specific pathogen-free (SPF) mice (ICR, 6 weeks old,
female) were obtained from Clea Japan (Tokyo, Japan). To create
dysbiotic conditions for gut microbiota, the SPF mice were orally
administered vancomycin (0.2 mg/ml; Sigma, St. Louis, MO, USA) in
drinking water for five weeks, whereas controls were supplied with
untreated water (11). Body weight
and 24-h food intake were monitored weekly. At the end point of the
experiments, the mice were fasted for 4 h before sacrifice. The
length of the small intestine and colon, and the weight of the
cecal content, were measured. The GI tissues were removed from the
mice, cut open along the longitudinal axis, rinsed with saline, and
fixed in neutral aqueous phosphate-buffered 10% formalin for
histological examination or stored in nitrogen liquid for RT-qPCR.
The experimental protocol was approved by the Animal Use and Care
Committee at Hyogo College of Medicine.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from the colonic tissues with
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). Total RNA (4 µg) was reverse-transcribed using
oligo(dT) primer (Applied Biosystems, Branchburg, NJ, USA), and
RT-qPCR was performed using a 7900H Fast Real-Time PCR System
(Applied Biosystems) as described previously (12). The set of primers for mouse
IL-4, IL-6, IL-10, IL-12, IFN-γ and
glyceraldehydes-3-phosphate dehydrogenase (GAPDH) were
prepared as shown in Table I.
RT-qPCR assays were carried out with 200 ng of RNA equivalent cDNA,
SYBR-Green Master Mix (Applied Biosystems), and 500 nmol/l
gene-specific primers. The PCR cycling conditions were 50°C for 15
sec and 60°C for 60 sec. The intensity of the fluorescent dye was
determined, and the expression levels of target gene mRNA were
normalized to the expression level of GAPDH mRNA.
 | Table I.Primers for reverse
transcription-polymerase chain reaction analysis. |
Table I.
Primers for reverse
transcription-polymerase chain reaction analysis.
| Genes | Direction | Sequences |
|---|
| IL-4 | Forward |
5′-GAATGTACCAGGAGCGATATC-3′ |
|
| Reverse |
5′-CTCAGTACTACGAGTAATCCA-3′ |
| IL-6 | Forward |
5′-CCAGTTGCCTTCTTGGGACT-3′ |
|
| Reverse |
5′-GGTCTGTTGGGAGTGGTATCC-3′ |
| IL-10 | Forward |
5′-TGGACAACATACTGCTAACCG-3′ |
|
| Reverse |
5′-GGATCATTTCCGATAAGGCT-3′ |
| IL-12 | Forward |
5′-CAACATCAAGAGCAGTAGCAG-3′ |
|
| Reverse |
5′-TACTCCCAGCTGACCTCCAC-3′ |
| IFN-γ | Forward |
5′-GCATCTTGGCTTTGCAGCT-3′ |
|
| Reverse |
5′-CCTTTTTCGCCTTGCTGTTG-3′ |
| GAPDH | Forward |
5′-GGAGAAACCTGCCAAGTATG-3′ |
|
| Reverse |
5′-TGGGAGTTGCTGTTGAAGTC-3′ |
Immunohistochemistry
Immunohistochemical staining for CD80 (a marker of
M1 polarized macrophages) and CD163 (a marker of M2 polarized
macrophages) was performed with an Envision Kit (Dako, Kyoto,
Japan) in accordance with the manufacturer's protocol (13). Polyclonal anti-CD80 antibody
(1:10,000) and polyclonal anti-CD163 antibody (1:1,000) (both from
Abcam, Cambridge, UK) were used as the primary antibodies. In
brief, the sections were treated by microwave heating for 20 min in
1 Dako REAL Target Retrieval Solution (Dako Denmark, Glostrup,
Denmark), preincubated with 0.3% H2O2 in
methanol for 20 min at room temperature, and incubated with the
primary antibodies for 60 min at room temperature. The slides were
then washed in PBS, incubated with a secondary antibody for 30 min,
visualized by 3,3′-diaminobenzidine tetrahydrochloride with 0.05%
H2O2 for 3 min, and then counterstained with
Mayer's hematoxylin. The numbers of CD80-positive and
CD163-positive cells were evaluated as follows: Four sections in
each mouse were prepared for the colon. The positive cells were
counted in the lamina propria and muscular layer in at least five
different visual fields in a 1,000-µm stretch of the entire length
using well-oriented tissue sections, and the average was calculated
for each mouse.
GI transit time (GITT)
GITT was measured as described previously (14). In brief, the mice were orally
treated with 0.3 ml of 0.5% methylcellulose solution including 6%
carmine red (Wako Pure Chemical Industries, Ltd., Osaka, Japan).
They were then allowed access to food and water ad libitum until
the first red fecal pellet appeared. GITT was determined as the
time period between oral gavage and the appearance of the first red
fecal pellet.
Statistical analysis
All values were expressed as the mean ± standard
error of the mean. Significance of differences between the two
animal groups was analyzed by Mann-Whitney U test. Correlations
among GITT, CD80 expression and CD163 expression were assessed by
linear regression analysis. P<0.05 was considered to indicate a
statistically significant difference.
Results
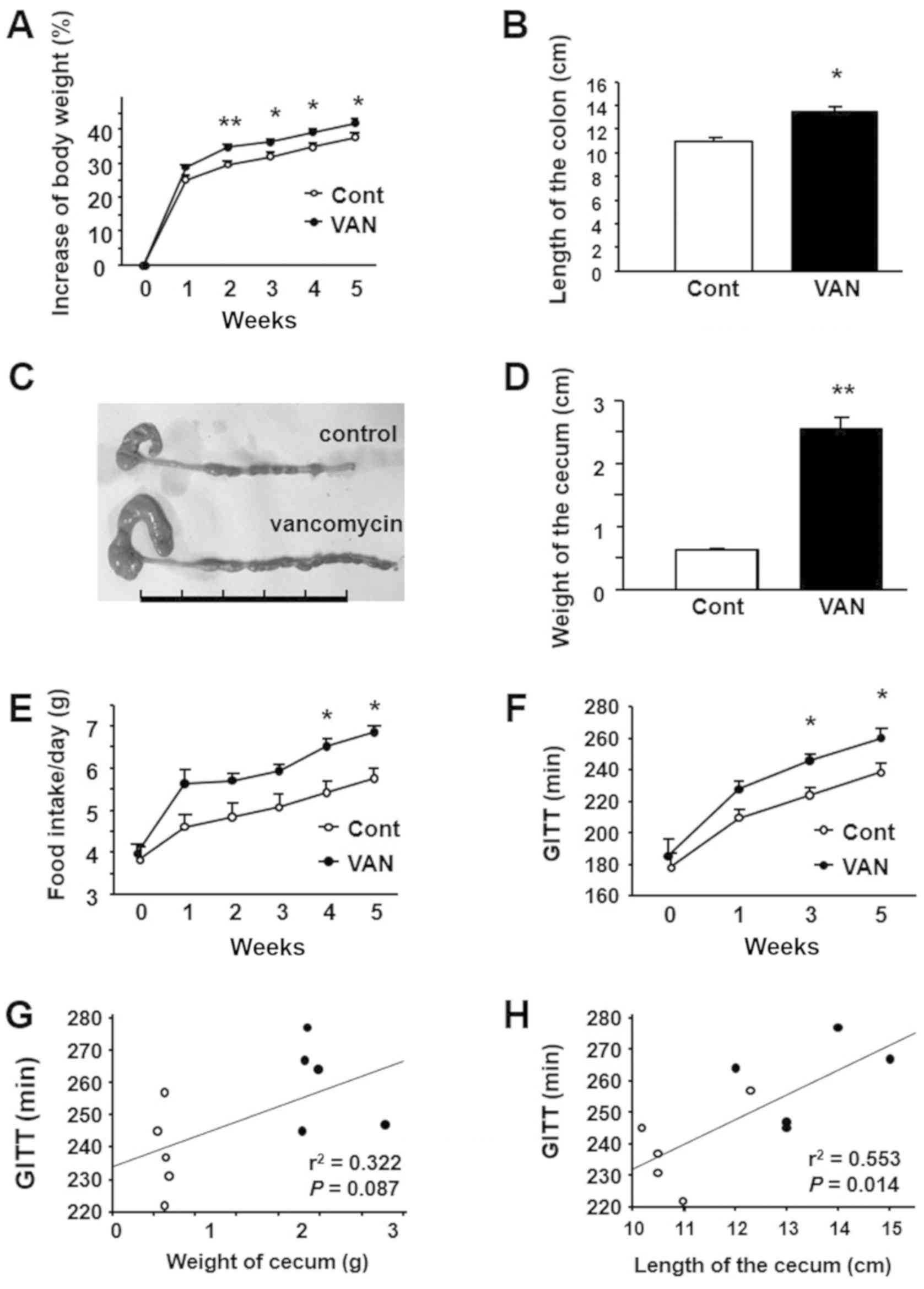
Effect of vancomycin treatment on body
weight, food intake, gastrointestinal motility and colonic
morphology in mice
Body weight increased according to body growth in
both the control and the vancomycin-treated groups. The percentage
increase in body weight was significantly greater in
vancomycin-treated mice from 2 weeks after the start of the
experiment (Fig. 1A). Regarding
the morphology of the colon, its length was significantly longer in
vancomycin-treated mice than in controls (Fig. 1B). Additionally, we detected an
apparent enlargement of the cecum in vancomycin-treated mice
relative to the controls (Fig.
1C). Compatible with this finding, the weight of the cecum was
significantly greater in the vancomycin-treated mice (Fig. 1D). We also investigated food intake
and gastrointestinal motility in the two groups. As shown in
Fig. 1E, vancomycin-treated mice
showed a significant increase of food intake relative to the
controls at 2 weeks or later from the start of the experiment.
Furthermore, we observed that GITT was significantly prolonged in
vancomycin-treated mice than in the controls beyond 4 weeks after
the start of the treatment (Fig.
1F). Regarding the relationship between GITT and intestinal
morphology, GITT tended to correlate to the weight of cecum
(Fig. 1G) and significantly
correlated with the length of colon (Fig. 1H).
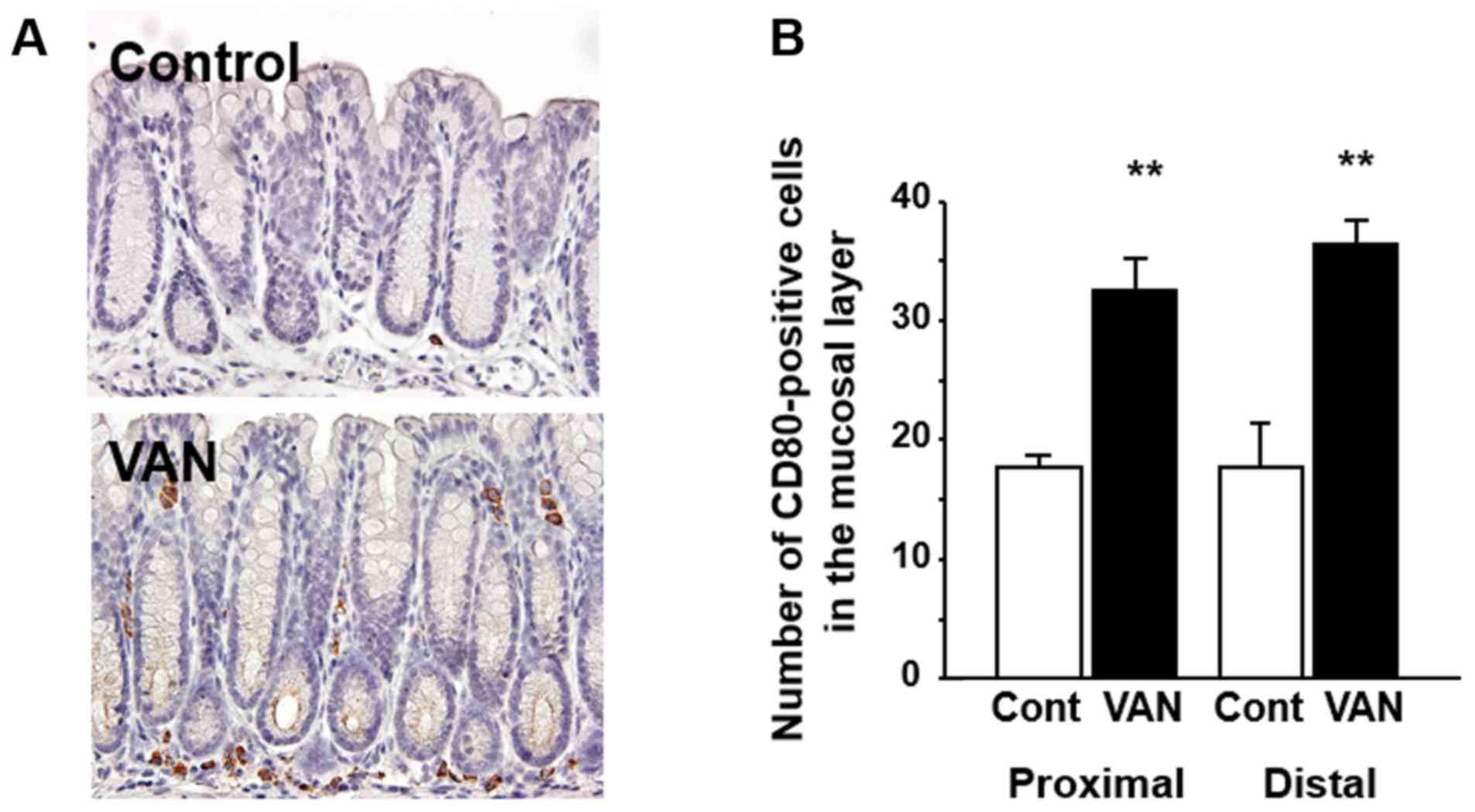
Expression of CD80 in the colon of
mice treated with vancomycin
CD80 expression, as a marker of M1 macrophages, was
investigated in the colonic tissues by immunohistochemistry.
Immunoreactivity for CD80 was detected in immune cells in the
lamina propria of the colonic mucosa (Fig. 2A) but not in the muscular layer in
either control or vancomycin treated mice (data not shown). In
control mice, the CD80-positive macrophages were localized mainly
at the bottom of the mucosal layer. On the other hand, in
vancomycin-treated mice, CD80-positive macrophages were distributed
not only at bottom but also the upper part of the colonic mucosal
layer (Fig. 2A). The number of
CD80-positive macrophages was significantly greater in both the
proximal and distal colon in vancomycin-treated mice relative to
the controls (Fig. 2B).
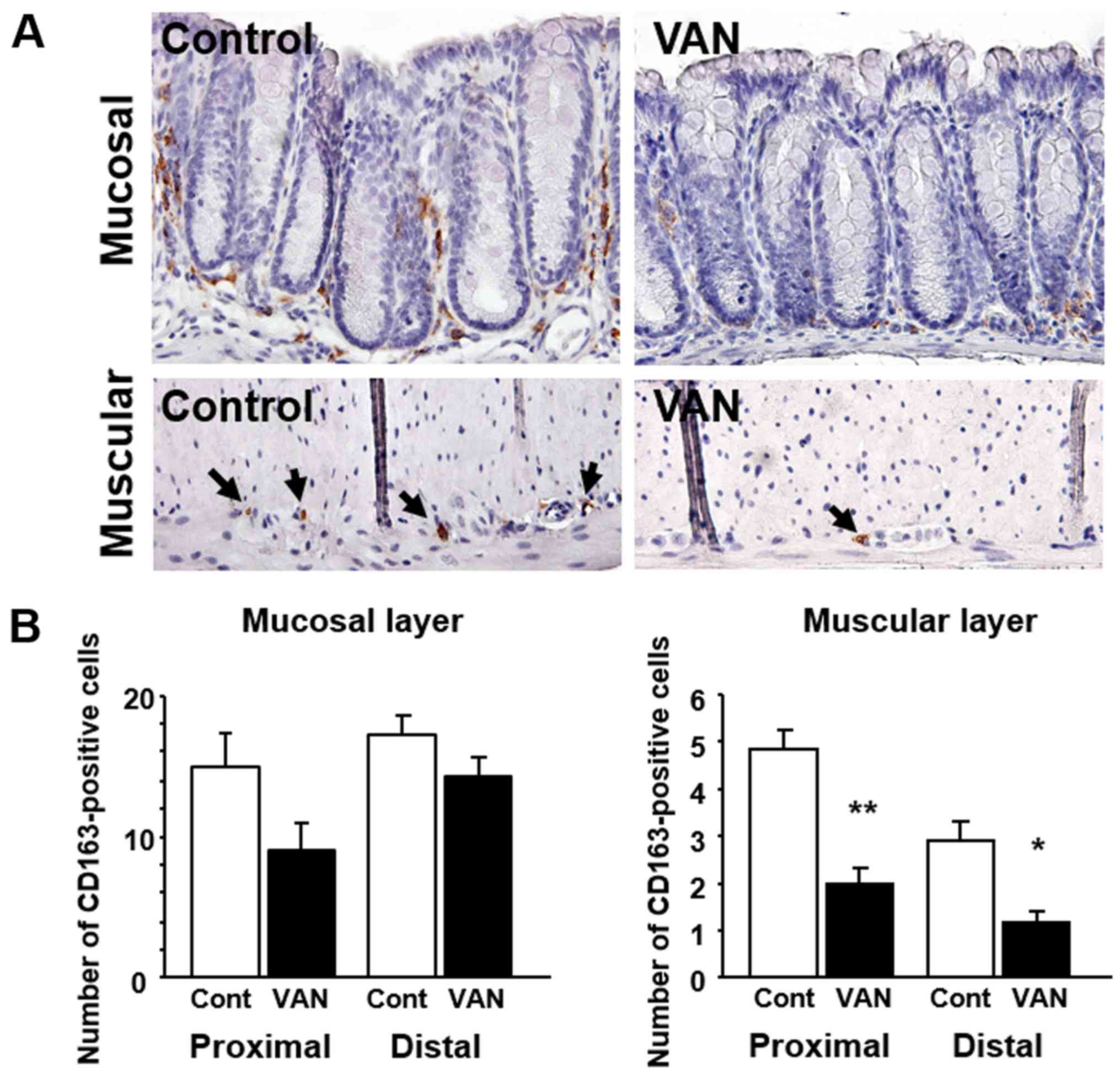
Expression of CD163 in the colon of
mice treated with vancomycin
CD163 expression, as a marker of M2 macrophages, was
also investigated in the colonic tissues by immunohistochemistry.
Immunoreactivity for CD163 was detected in immune cells in the
lamina propria of the colonic mucosa (Fig. 3A). Although their number was small,
CD163-positive macrophages were detected in the muscular layer of
the colon (Fig. 3A). The number of
CD163-positive cells tended to be greater in both the proximal and
distal colonic mucosa in vancomycin-treated mice (P=0.075 and
0.251, respectively) (Fig. 3B). In
the muscular layer, the number of CD163-positive cells was
significantly increased in both the proximal and distal colonic
mucosa in vancomycin-treated mice (Fig. 3B).
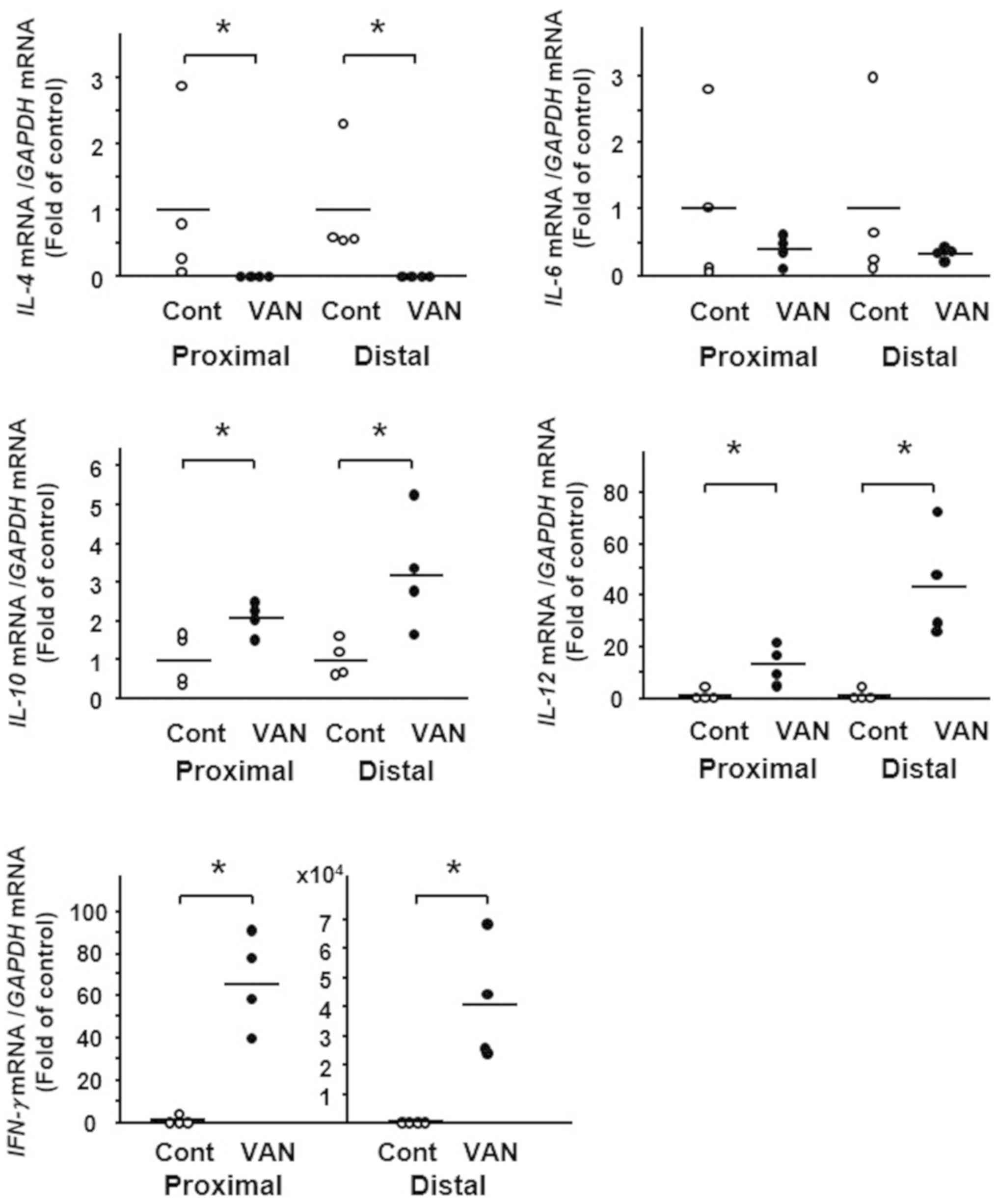
Expression of cytokines in the colon
of mice treated with vancomycin
We next examined the expression of cytokines in the
colonic tissue of vancomycin-treated mice. Although the expression
of several cytokines differed between the controls and
vancomycin-treated mice, marked differences were detected in the
expression of IL-4, IL-12 and IFN-γ. In the colon of
vancomycin-treated mice, IL-4 expression was significantly
decreased whereas expression of IL-12 and IFN-γ was strongly
increased (Fig. 4).
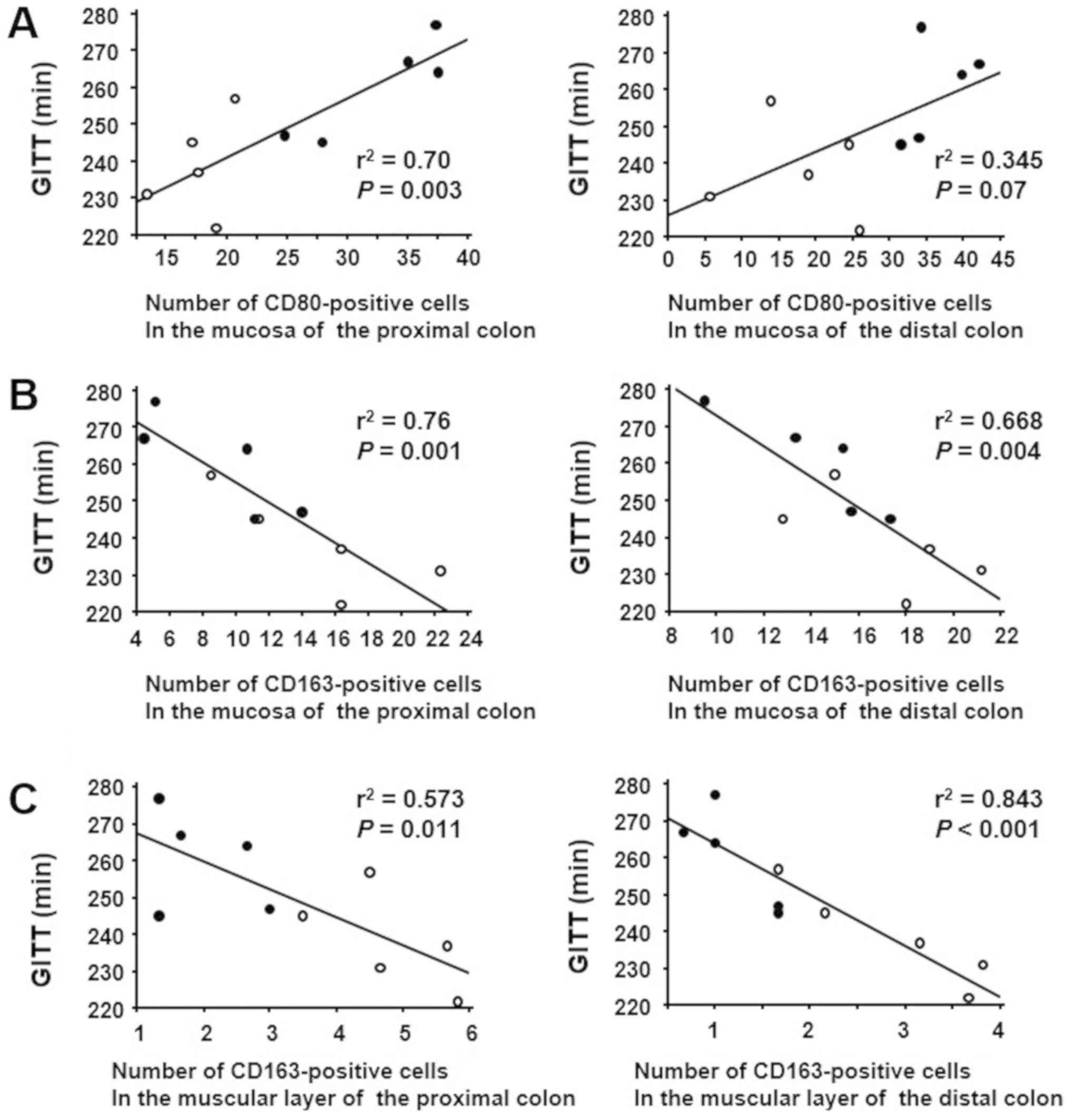
Correlation between GITT and CD80 or
CD163 expression in the colon of mice
To investigate the effect of macrophage phenotype
alteration on GI motility, we analyzed the correlation between GITT
and CD80 or CD163 expression in the experimental mice by linear
regression analysis. GITT was positively correlated with the number
of CD80-positive cells in the colonic mucosa (Fig. 5A). On the other hand, GITT was
negatively correlated with the number of CD163-positive cells in
the colonic mucosal layer (Fig.
5B). Similarly, GITT was negatively correlated with the number
of CD163-positive cells in the colonic muscular layer (Fig 5C).
Discussion
It has been accepted that antibiotic-related
dysbiosis is associated with the development of metabolic disorder
and functional gastrointestinal disorders (15). In the present study, we
demonstrated that vancomycin treatment clearly promoted a gain of
body weight in mice. In addition, we observed that food intake was
significantly greater in vancomycin-treated mice, suggesting that
vancomycin may promote appetite, thus leading to an increase of
body weight gain. Although the types of microbiota that are
responsible for induction of obesity remains unclear, our
preliminary analyses had shown that the ratio of
Lactobacillus was markedly high in vancomycin-treated mice
(data not shown), being compatible with previous reports (11,16).
Interestingly, it has been reported that Lactobacillus is
increased in obese (17,18) and moreover, Lactobacillus
species are widely used as growth promoters in the farm industry
(19). Together, we are tempting
to speculate that the increase of Lactobacillus species
associated with vancomycin treatment may be involved at least in
part in the obesity phenotype in mice with vancomycin treatment. On
the other hand, we clarified that GI motility in mice was
suppressed by vancomycin treatment. In this context, since we have
recently clarified that intestinal macrophages play a pivotal role
in GI motility (20), we
investigated the significance of macrophage phenotype alterations
in this experimental model.
We first observed the distribution and population of
M1 and M2 macrophages in the colonic tissues of mice treated with
vancomycin. Although it is impossible to distinguish M1 and M2
macrophage using available markers in vivo tissues, we used
the CD80 and CD163 that are widely used as a marker for M1 and M2
marker, respectively. Interestingly, we found that M1 macrophages
were increased in the colonic mucosa of these mice, and conversely
M2 macrophages were decreased in both the colonic mucosa and
muscular layer of those mice. These findings suggest that
vancomycin-induced dysbiosis greatly affects the macrophage
phenotypic profile in colonic tissues. On the whole, macrophage
polarization was dominantly shifted toward an M1 phenotype in the
colon of this animal model. M1 macrophages are key players in the
proinflammatory reaction downstream of IFN-γ stimulation (21), and indeed interact with not only
other immune cells but also neural, muscle or epithelial cells
using proinflmmatory cytokines as mediators (22). On the other hand, M2 macrophages
are known to interact with those cells in an anti-inflammatory
context (23). Thus, the profile
of cytokines expression in vancomycin-treated mice is interesting.
The enhancement of proinflammatory IFN-γ and IL-12 expression is
well compatible with the dominant shift to M1 macrophages in
vancomycin-treated mice, and moreover, the decrease of
anti-inflammatory IL-4 expression is also consistent with reduction
of M2 macrophages in these mice.
Then, what is the influence of the M1-dominant shift
of macrophage polarization on the metabolism and GI function?
Lumeng et al (24) have
reported that obesity induces a phenotypic switch in adipose tissue
macrophage polarization, and that thereafter, involvement of M1
macrophages and its associated proinflammatory cytokines play a
dominant role in the progression of obesity (25). In particular, the insulin
resistance induced by M1 macrophages-associated proinflammatory
cytokines are considered as a key mechanism (24,26,27).
In this regard, it is interesting that IFN-γ expression to activate
M1 macrophage is increased and furthermore, M1
macrophage-associated pro-inflammatory cytokine IL-12 was increased
in our experimental animals. On the other hand, vancomycin-induced
imbalance of macrophage phenotypes may be involved in the
alteration of GI motility. Indeed, the present study revealed that
an increase of M1 macrophages was significantly correlated with
prolongation of the GITT. In fact, previous studies have suggested
that M1 macrophages suppress GI motility through the effects of
associated proinflammatory cytokines on smooth muscle and the
enteric nervous system in an ileus model (28), although this mechanism may not be
applicable to our present dysbiosis model. Furthermore, it is
noteworthy that M2 macrophages were suppressed not only in the
mucosa but also in the muscular layer in the colon of mice treated
with vancomycin. Muller et al (7) and others have reported that
muscularis macrophages play a pivotal role in GI motility through
cross-talk with enteric nerve cells via cytokines (29,30).
Accordingly, the muscularis M2 macrophages in our experimental mice
must have played some roles in GI motility. In this context, we
have recently demonstrated that M2 macrophages migrating into the
GI muscular layer may be involved in acceleration of GI motility in
germ-free mice subjected to commensal bacterial transplantation
(20). In contrast, we have shown
that reduction of M2 macrophages is correlated with suppression of
GI motility in vancomycin-treated mice, which seems reasonable in
view of previous work. However, the mechanism by which M2
macrophages regulate GI motility remains to be resolved.
In summary, we have shown that treatment with the
antibiotic vancomycin promotes body weight gain and food intake and
prolongs the GITT in mice. Moreover, polarization of macrophages
shows a shift to M1 phenotype dominance and upregulation of M1
macrophage-associated proinflammatory cytokines in the colon of
vancomycin-treated mice. Of note, enhancement of M1 polarization
was positively correlated with inhibition of GI motility, whereas
suppression of M2 polarization was negatively correlated. These
findings suggest that antibiotic treatment may affect body
metabolism and GI motility accompanied by alteration of macrophage
polarization and the cytokine profile in the colon.
Acknowledgements
The authors would like to thank Miss Chiyomi Ito and
Miss Mayumi Yamada (Hyogo College of Medicine, Nishinomiya, Japan)
for their technical assistance.
Funding
This study was supported in part by Grants-in-aid
for Scientific Research (grant no. 17K09363) from the Ministry of
Education, Culture, Sports, Science and Technology, Japan.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YI, HF, XX, YR and HM conceived and designed the
experiments. YI, HF, XX and YR performed the experiments. YI, HF,
XX, YR, TT, TO and JW analyzed the data. YI, HF, XX, YR, TT and JW
contributed reagents/materials/analysis tools. YI, HF, XX and HM
were involved in the drafting of the manuscript and revising it
critically for important intellectual content. All the authors
critically reviewed and approved the manuscript for
publication.
Ethics approval and consent to
participate
All experimental protocols were approved by the
Animal Use and Care Committee at Hyogo College of Medicine.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
GI
|
gastrointestinal
|
|
GITT
|
gastrointestinal transit time
|
References
|
1
|
Tremaroli V and Bäckhed F: Functional
interactions between the gut microbiota and host metabolism.
Nature. 489:242–249. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Canton J, Neculai D and Grinstein S:
Scavenger receptors in homeostasis and immunity. Nat Rev Immunol.
13:621–634. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jämsen E, Kouri VP, Olkkonen J, Cör A,
Goodman SB, Konttinen YT and Pajarinen J: Characterization of
macrophage polarizing cytokines in the aseptic loosening of total
hip replacements. J Orthop Res. 32:1241–1246. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mosser DM: The many faces of macrophage
activation. J Leukoc Biol. 73:209–212. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Stein M, Keshav S, Harris N and Gordon S:
Interleukin 4 potently enhances murine macrophage mannose receptor
activity: A marker of alternative immunologic macrophage
activation. J Exp Med. 176:287–292. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Novoselov VV, Sazonova MA, Ivanova EA and
Orekhov AN: Study of the activated macrophage transcriptome. Exp
Mol Pathol. 99:575–580. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Muller PA, Koscsó B, Rajani GM, Stevanovic
K, Berres ML, Hashimoto D, Mortha A, Leboeuf M, Li XM, Mucida D, et
al: Crosstalk between muscularis macrophages and enteric neurons
regulates gastrointestinal motility. Cell. 158:300–313. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Theriot CM, Koenigsknecht MJ, Carlson PE
Jr, Hatton GE, Nelson AM, Li B, Huffnagle GB, Z Li J and Young VB:
Antibiotic-induced shifts in the mouse gut microbiome and
metabolome increase susceptibility to Clostridium difficile
infection. Nature Commun. 5:31142014. View Article : Google Scholar
|
|
9
|
Mahana D, Trent CM, Kurtz ZD, Bokulich NA,
Battaglia T, Chung J, Müller CL, Li H, Bonneau RA and Blaser MJ:
Antibiotic perturbation of the murine gut microbiome enhances the
adiposity, insulin resistance, and liver disease associated with
high-fat diet. Genome Med. 8:482016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cho I, Yamanishi S, Cox L, Methé BA,
Zavadil J, Li K, Gao Z, Mahana D, Raju K, Teitler I, et al:
Antibiotics in early life alter the murine colonic microbiome and
adiposity. Nature. 488:621–626. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sekirov I, Tam NM, Jogova M, Robertson ML,
Li Y, Lupp C and Finlay BB: Antibiotic-induced perturbations of the
intestinal microbiota alter host susceptibility to enteric
infection. Infect Immun. 76:4726–4736. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sun C, Fukui H, Hara K, Kitayama Y, Eda H,
Yang M, Yamagishi H, Tomita T, Oshima T, Watari J, et al:
Expression of Reg family genes in the gastrointestinal tract of
mice treated with indomethacin. Am J Physiol Gastrointest Liver
Physiol. 308:G736–G744. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kitayama Y, Fukui H, Hara K, Eda H, Kodani
M, Yang M, Sun C, Yamagishi H, Tomita T, Oshima T, et al: Role of
regenerating gene I in claudin expression and barrier function in
the small intestine. Transl Res. 173:92–100. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang M, Fukui H, Eda H, Xu X, Kitayama Y,
Hara K, Kodani M, Tomita T, Oshima T, Watari J and Miwa H:
Involvement of gut microbiota in association between GLP-1/GLP-1
receptor expression and gastrointestinal motility. Am J Physiol
Gastrointest Liver Physiol. 312:G367–G373. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mendall MA and Kumar D: Antibiotic use,
childhood affluence and irritable bowel syndrome (IBS). Eur J
Gastroenterol Hepatol. 10:59–62. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cheng RY, Li M, Li SS, He M, Yu XH, Shi L
and He F: Vancomycin and ceftriaxone can damage intestinal
microbiota and affect the development of the intestinal tract and
immune system to different degrees in neonatal mice. Pathog Dis.
75:2017. View Article : Google Scholar
|
|
17
|
Armougom F, Henry M, Vialettes B, Raccah D
and Raoult D: Monitoring bacterial community of human gut
microbiota reveals an increase in Lactobacillus in obese
patients and Methanogens in anorexic patients. PLoS One.
4:e71252009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Million M, Angelakis E, Paul M, Armougom
F, Leibovici L and Raoult D: Comparative meta-analysis of the
effect of Lactobacillus species on weight gain in humans and
animals. Microb Pathog. 53:100–108. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Khan M, Raoult D, Richet H, Lepidi H and
La Scola B: Growth-promoting effects of single-dose
intragastrically administered probiotics in chickens. Br Poult Sci.
48:732–735. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang M, Fukui H, Eda H, Kitayama Y, Hara
K, Kodani M, Tomita T, Oshima T, Watari J and Miwa H: Involvement
of gut microbiota in the association between gastrointestinal
motility and 5-HT expression/M2 macrophage abundance in the
gastrointestinal tract. Mol Med Rep. 16:3482–3488. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hu X, Herrero C, Li WP, Antoniv TT,
Falck-Pedersen E, Koch AE, Woods JM, Haines GK and Ivashkiv LB:
Sensitization of IFN-gamma Jak-STAT signaling during macrophage
activation. Nat Immunol. 3:859–866. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gordon S: Alternative activation of
macrophages. Nat Rev Immunol. 3:23–25. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhao A, Urban JF Jr, Anthony RM, Sun R,
Stiltz J, van Rooijen N, Wynn TA, Gause WC and Shea-Donohue T: Th2
cytokine-induced alterations in intestinal smooth muscle function
depend on alternatively activated macrophages. Gastroenterology.
135:217–225. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lumeng CN, Bodzin JL and Saltiel AR:
Obesity induces a phenotypic switch in adipose tissue macrophage
polarization. J Clin Invest. 117:175–184. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lumeng CN, Deyoung SM, Bodzin JL and
Saltiel AR: Increased inflammatory properties of adipose tissue
macrophages recruited during diet-induced obesity. Diabetes.
56:16–23. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fujisaka S, Usui I, Bukhari A, Ikutani M,
Oya T, Kanatani Y, Tsuneyama K, Nagai Y, Takatsu K, Urakaze M, et
al: Regulatory mechanisms for adipose tissue M1 and M2 macrophages
in diet-induced obese mice. Diabetes. 58:2574–2582. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Olefsky JM and Glass CK: Macrophages,
inflammation, and insulin resistance. Annu Rev Physiol. 72:219–246.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wehner S, Behrendt FF, Lyutenski BN,
Lysson M, Bauer AJ, Hirner A and Kalff JC: Inhibition of macrophage
function prevents intestinal inflammation and postoperative ileus
in rodents. Gut. 56:176–185. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Avetisyan M, Rood JE, Huerta Lopez S,
Sengupta R, Wright-Jin E, Dougherty JD, Behrens EM and Heuckeroth
RO: Muscularis macrophage development in the absence of an enteric
nervous system. Proc Natl Acad Sci USA. 115:4696–4701. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cipriani G, Gibbons SJ, Kashyap PC and
Farrugia G: Intrinsic gastrointestinal macrophages: Their phenotype
and role in gastrointestinal motility. Cell Mol Gastroenterol
Hepatol. 2:120–130.e1. 2016. View Article : Google Scholar : PubMed/NCBI
|