Introduction
Chronic kidney disease (CKD) is a progressive loss
in kidney function over a period of months or years. There is a
high global prevalence of CKD, which imposes an enormous
socioeconomic burden on individuals with the disease and society
(1–3). Renal fibrosis is thought to be the
common final outcome of almost all CKD (4). In the present study, a murine
unilateral ureteral obstruction (UUO) model was used to generate
renal fibrosis in vivo (5).
Macrophages are important immune cells, which can be
divided into two main subtypes, namely M1 and M2. They serve a
crucial role under both pathological and physiological conditions.
In the context of kidney disease, M1 macrophages have been
demonstrated to exert a pathogenic function in renal inflammation
and may represent a possible therapeutic target (6). M2 macrophages appear to suppress
inflammation and promote injury repair; therefore, representing a
potential treatment for renal disease. However, M2 macrophages may
also function as a fibrosis promoter (7). Studies have indicated that targeting
macrophages, including macrophage depletion, disruption of
macrophage recruitment and genetic alteration of macrophage
activity, may be used as novel therapeutic approaches in a range of
diseases (8,9), including inflammation and cancer.
Peroxisome proliferator-activated receptor γ
(PPARγ), which forms a heterodimer with the retinoid X receptor on
peroxisome response elements, is a ligand-activated transcription
factor that regulates glucose and lipid metabolism, immune
responses, and inflammation (10).
PPARγ is expressed in several cell types, including immune cells
and various epithelial and muscle-like cells (10). The PPARγ agonist pioglitazone is
used worldwide to treat patients with diabetes (11). Pioglitazone takes part in several
physiopathologic processes, including glucose metabolism,
lipogenesis, inflammation, proliferation, apoptosis and fibrosis,
vascular reactivity (12). A
previous study demonstrated that macrophage PPARγ was necessary for
accelerating pioglitazone-mediated recovery from dextran sodium
sulfate colitis (13). However,
the specific mechanisms underlying the effect of pioglitazone on
macrophages requires further study.
In the present study, the aim was to determine
whether pioglitazone may influence macrophages through PPARγ and to
investigate its role on renal fibrosis in vivo.
Materials and methods
Cells and reagents
Bone marrow cells were obtained by flushing the
femurs and tibias of normal male C57/BL6 mice in Dulbecco's
modified Eagle's medium (DMEM; GE Healthcare Life Sciences, Logan,
UT, USA) supplemented with 10% fetal bovine serum (FBS; Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) and 30% L929
conditioned medium at 37°C. The medium was changed at day 3 and day
5. L929 conditioned medium was the supernatant from growing L929
cells in DMED-containing 10% FBS for 5 days. After 7 days
incubation, bone marrow-derived macrophages (BMDMs, M0) were
harvested. 100 ng/ml lipopolysaccharide (LPS) and 6 ng/ml
interferon (IFN)-γ were used to stimulate M0 and M1 macrophages
which were harvested after 24 h. In addition, M2 macrophages were
induced by stimulating M0 macrophages with 10 ng/ml interleukin
(IL)-4 and 10 ng/ml IL-13 for 24 h. IL-4, IL-13, LPS and IFN-γ were
purchased from PeproTech, Inc. (Rocky Hill, NJ, USA).
Pioglitazone and PPARγ antagonist GW9662 were
purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). M0
macrophages were stimulated with pioglitazone (5 µm) for 24 h in
the presence or absence of GW9662. PPARγ inhibitor GW9662 (5 µm)
was added 6 h before treatment with pioglitazone.
Western blot analysis
Cells and mouse kidney tissues were lysed using
radio immunoprecipitation assay buffer containing a protease
inhibitor cocktail (Wuhan Saiweier Biotechnology Co., Ltd., Wuhan,
China) on ice for 30 min, then centrifuged at 16,500 × g for 30 min
at 4°C to collect the supernatant. Protein concentration was
quantified by a BCA protein assay kit (Beyotime Institute of
Biotechnology, Shanghai, China). A total of 20 µg protein was
separated by 10% SDS-PAGE and transferred to polyvinylidene
fluoride membranes (EMD Millipore, Billerica, MA, USA). The
membranes containing cellular protein were blocked with 5% bovine
serum albumin (BSA; Sigma-Aldrich; Merck KGaA) for 1 h at room
temperature. Then they were incubated overnight at 4°C with rabbit
anti-arginase1 (Arg1; cat. no. sc-20150; 1:400; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), rabbit anti-vascular
endothelial growth factor receptor 3 (VEGFR3; cat. no. sc-321;
1:400; Santa Cruz Biotechnology, Inc.), mouse anti-inducible nitric
oxide synthase (iNOS; cat. no. sc-7271; 1:200; Santa Cruz
Biotechnology, Inc.) and rabbit anti-α-tubulin (1:5,000; cat. no.
ab18251; Abcam, Cambridge, MA, USA). The membranes containing
tissue protein were incubated overnight at 4°C with rabbit
anti-α-smooth muscle actin (SMA; cat. no. ab5694; 1:1,000; Abcam),
rabbit anti-platelet-derived growth factor receptor (PDGFR)-β (cat.
no. ab32570; 1:1,000; Abcam), rabbit anti-PPARγ (cat. no. AP20705a;
1:1,000; Abgent, Inc., San Diego, CA, USA), rabbit
anti-phosphorylated (p)-PPARγ (cat. no. ab195925; 1:1,000; Abcam)
and mouse anti-GAPDH (1:3,000; Abcam; cat. no. ab8245). Following
cultivated with goat anti-rabbit or goat anti-mouse secondary
antibodies (1:10,000; cat. no. A27036SAMPLE; cat. no. A28177SAMPLE;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) at 37°C for 1 h,
membranes were incubated with horseradish peroxidase
(HRP)-conjugated immunoglobulin G (1:5,000; cat. no. 323065021;
Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA) for
1 min at room temperature. The protein bands were detected using a
ChemiDOC™ XRS+ system (Bio-Rad Laboratories, Inc., Hercules, CA,
USA). Densitometric analysis was performed using Image Lab™
software version 6.0.1(Bio-Rad Laboratories, Inc).
UUO mouse model
MaleC57/BL6 mice (n=18, weight 20–22 g; age, 6–8
weeks) were obtained from the Hua Fukang Experimental Animal Center
(Beijing, China) and housed at the animal facilities at Tongji
Medical Collage in an air-controlled room (temperature 23±1%,
humidity 55±5%) under a 12-h light/dark cycle with free access to
standard food and water. All the animal raising and handling
protocols approved by the Institutional Animal Care and Use
Committee of Tongji Medical College, Huazhong University of Science
and Technology. The study was approved by the Ethics Committee of
Tongji Hospital, Tongji Medical College, Huazhong University of
Science and Technology (Wuhan, China). Animals were divided into
three groups: Sham group (n=4), UUO group (n=7) and UUO +
pioglitazone group (n=7). The UUO renal fibrosis model was induced
as previously described (14).
Briefly, an incision was made in the midline of the abdomen, and
the left proximal ureter was exposed and was ligated at two
separate locations using 4-0 silk suture. Mice in the sham group
underwent the same surgical procedures except for ligation. The
sham and UUO groups were administered a daily oral gavage of
saline, whereas the UUO + pioglitazone group received 20 mg/kg
pioglitazone. The dose of pioglitazone was determined according to
previous studies (14).
Subsequently, mice were sacrificed 14 days after UUO operation.
Kidneys were excised, fixed with 4% paraformaldehyde 24 h in room
temperature, then dehydrated in a graded series of alcohol (Wuhan
Goodbio Technology Co., Ltd., Wuhan, China) and finally embedded in
paraffin (Wuhan Goodbio Technology Co., Ltd.) as previously
described (15).
Immunofluorescence staining
The 3 µm paraffin sections were treated with xylene
and hydrated with graded ethanol. Following incubation with 10 mM
sodium citrate buffer (pH 6) at 100°C for 20 min for antigen
revival, the sections were blocked with normal goat serum (cat. no.
ab7481; Abcam) for 20 min at room temperature as previously
described (15). Subsequently,
sections were incubated with mouse anti-F4/80 primary antibodies
(cat. no. sc-377009; 1:50; Santa Cruz Biotechnology, Inc.) and
rabbit anti-VEGFR3 primary antibodies (cat. no. sc-321; 1:50; Santa
Cruz Biotechnology, Inc.) at 37°C for 120 min. Alexa Fluor 488- or
Cy3-conjugated secondary antibodies goat anti-rabbit, or Donkey
anti-mouse immunoglobulins (cat. no. GB25303/GB21401; 1:200; Wuhan
Saiweier Biotechnology Co., Ltd.) were used to visualize
antigen-antibody complexes at 37°C for 30 min. Nuclei were stained
with DAPI at room temperature for 5 min (2 µg/ml; cat. no. D9542;
Sigma-Aldrich; Merck KGaA). Digital images were captured with a
fluorescent microscope (Olympus Corporation, Tokyo, Japan).
Immunohistochemistry
Following antigen retrieval with citrate buffer
(pH=6.0) as above, the 3-µm paraffin sections were incubated with
3% H2O2 for 10 min at room temperature, and
then blocked with normal goat serum (cat. no. ab7481; Abcam) for 30
min at room temperature. Sections were incubated with primary
antibodies against α-SMA (cat. no. ab5694; 1:300; Abcam), F4/80
(cat. no. sc-377009; 1:50; Santa Cruz Biotechnology, Inc.) and
Collagen-4 (cat. no. ab6586; 1:300; Abcam) overnight at 4°C. Then,
biotinylated goat anti-rabbit secondary antibody (cat. no. GB23204;
1:100; Wuhan Saiweier Biotechnology Co., Ltd.) was used added for
30 min at room temperature followed by horseradish
peroxidase-conjugated streptavidin (OriGene Technologies, Inc.,
Beijing, China) and 3,3′-diaminobenzidine staining (OriGene
Technologies, Inc.) for several seconds at room temperature, until
the sections changed to brown, when the reaction was halted with
water. Digital images were captured with an optical microscope
(Olympus Corporation, Tokyo, Japan).
Statistical analysis
All statistical analyses were conducted using SPSS
version 12.0 (SPSS, Inc., Chicago, IL, USA). The data are expressed
as the mean ± standard error of the mean from 3 independent
experiments. Multiple group comparisons were performed using
one-way analysis of variance followed by Dunnett's multiple
comparison test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Identification of macrophages induced
by LPS/IFN-γ and IL-4/IL-13
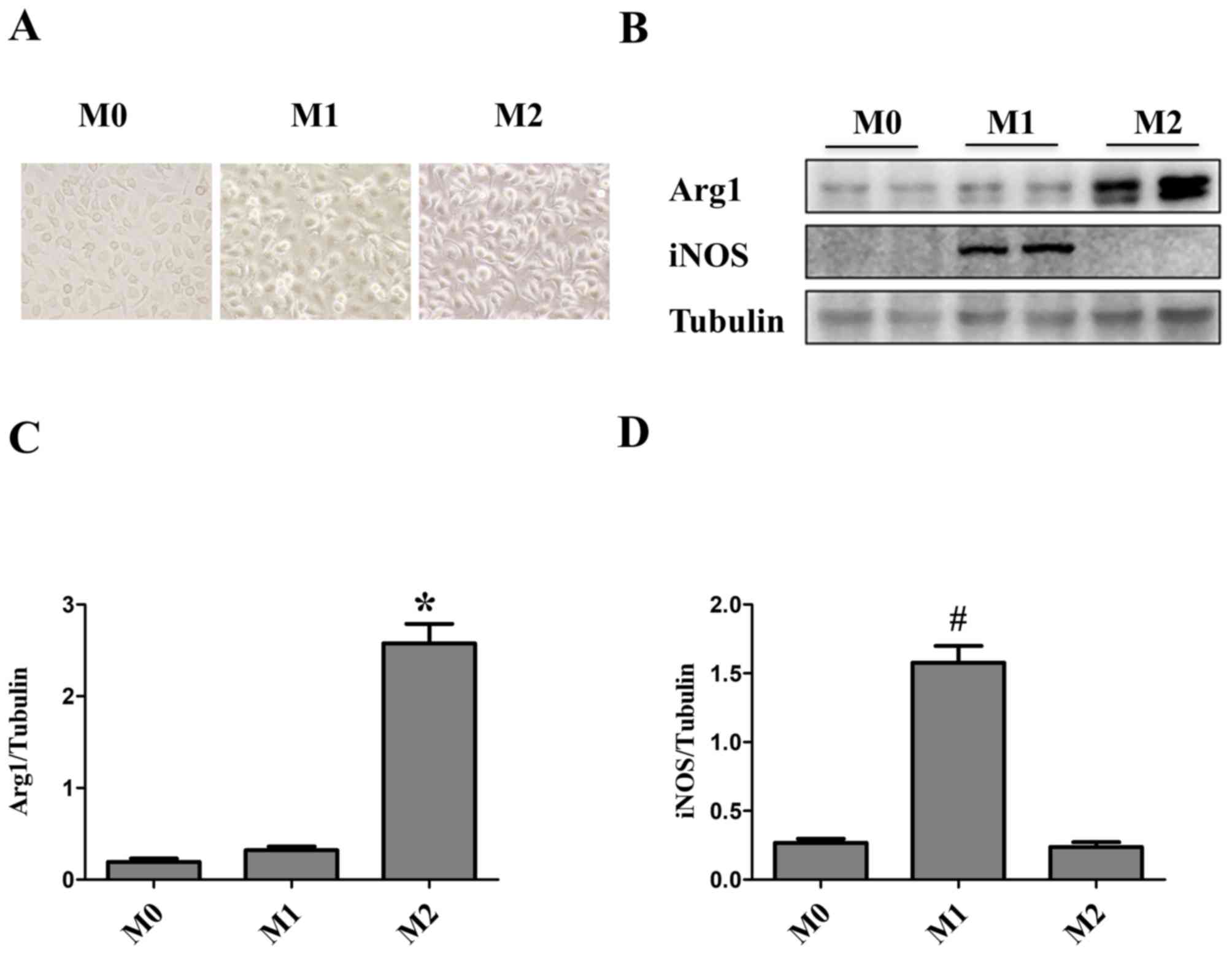
Macrophage maturity and subsequent polarization were
confirmed by phase-contrast microscopy. Bone marrow cells were
differentiated into macrophages over the course of 7 days.
Initially, Macrophages were small, round and formed colonies, which
were firmly adherent to the cell culture plate. Macrophage
differentiation and subsequent polarization had a marked impact on
cell morphology. The majority of M0 macrophages were elongated and
spindle-shaped, whereas M1 macrophages were round and M2
macrophages were cone-shaped (16)
(Fig. 1A). M1 and M2 macrophages
induced by LPS/IFN-γ and IL-4/IL-13, respectively, were
characterized by western blot analysis. Both cell cultures were
assessed for Arg1 and iNOS. iNOS protein expression levels were
significantly upregulated in M1 macrophages, whereas Arg1 was
significantly upregulated in M2-polarized macrophages (Fig. 1B-D).
Pioglitazone promotes M2 macrophage
polarization
To identify the effect of pioglitazone on M0 to
M1/M2 macrophage polarization, M0 macrophages were treated with the
drug. Pioglitazone had no effect on M0 to M1 macrophage
polarization according to M1 Marker iNOS (Fig. 2A and D). However, pioglitazone
could polarize M0 macrophages towards an M2 phenotype. Notably,
this process was not inhibited by GW9662 (a specific antagonist of
PPARγ), which indicated that an alternative pathway that does not
require PPARγ was involved in regulating this process (Fig. 2A-C). It was found that the
expression of VEGFR3 in M0 macrophages was similar to Arg1 with
pioglitazone or GW9662 (Fig. 2A and
B).
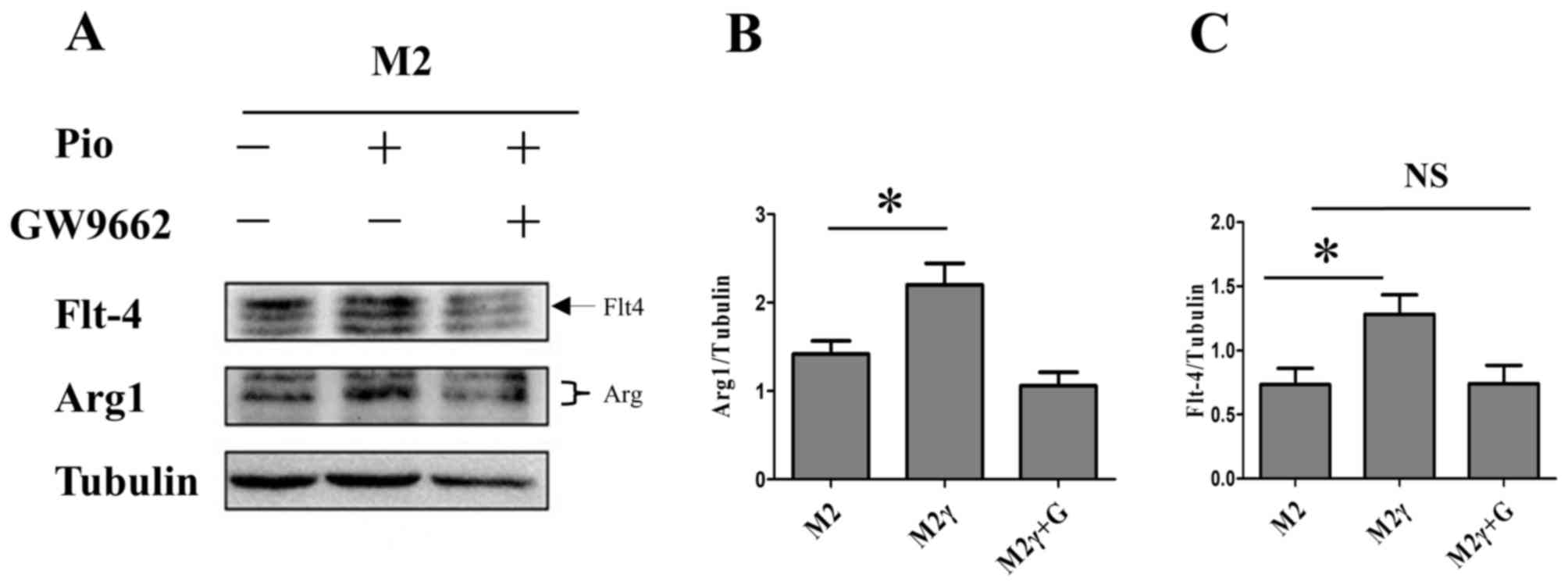
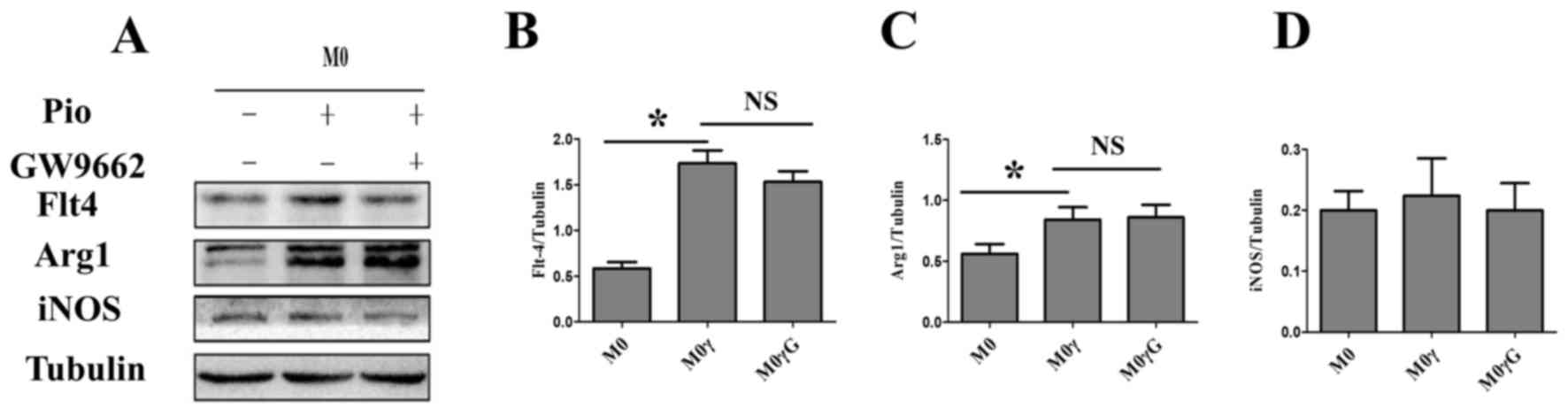 | Figure 2.Pioglitazone regulates the
polarization of M0 macrophages. M0 macrophages stimulated with
pioglitazone (5 µm) for 24 h in the presence or absence of GW9662.
PPARγ inhibitor GW9662 (5 µm) was added 6 h before treatment with
pioglitazone. (A) Western blot analysis was performed to determine
the protein expression levels of iNOS (M1 macrophage marker), Arg1
(M2 macrophage marker) and VEGFR3, in M0 macrophages treated with
pioglitazone in the presence or absence of GW9662. Tubulin was used
as the loading control. (B-D) Quantification of the western blot
results. The data are presented as the mean ± standard error of the
mean. *P<0.05. Arg1, arginase1; iNOS, inducible nitric oxide
synthase; M, macrophage; M2γ, M2 treated with pioglitazone; G,
GW9662; NS, not significant; Pio, pioglitazone; VEGFR3, vascular
endothelial growth factor receptor 3. |
Pioglitazone promotes M2 macrophage
activation and upregulates VEGFR3
To explore whether pioglitazone is associated with
M2 macrophage activation in vitro. M0 macrophages were
cultured with different concentrations of pioglitazone. The western
blot results demonstrated that M2 macrophage maker was increased,
particularly in the 20 µM group (Fig.
3A and B). M1 macrophages activation were not be affected by
pioglitazone (Fig. 3D and E). In
addition, the protein expression levels of VEGFR3 were increased in
M2 macrophages (Fig. 3C). However,
this was not observed in M1 macrophages (Fig. 3F).
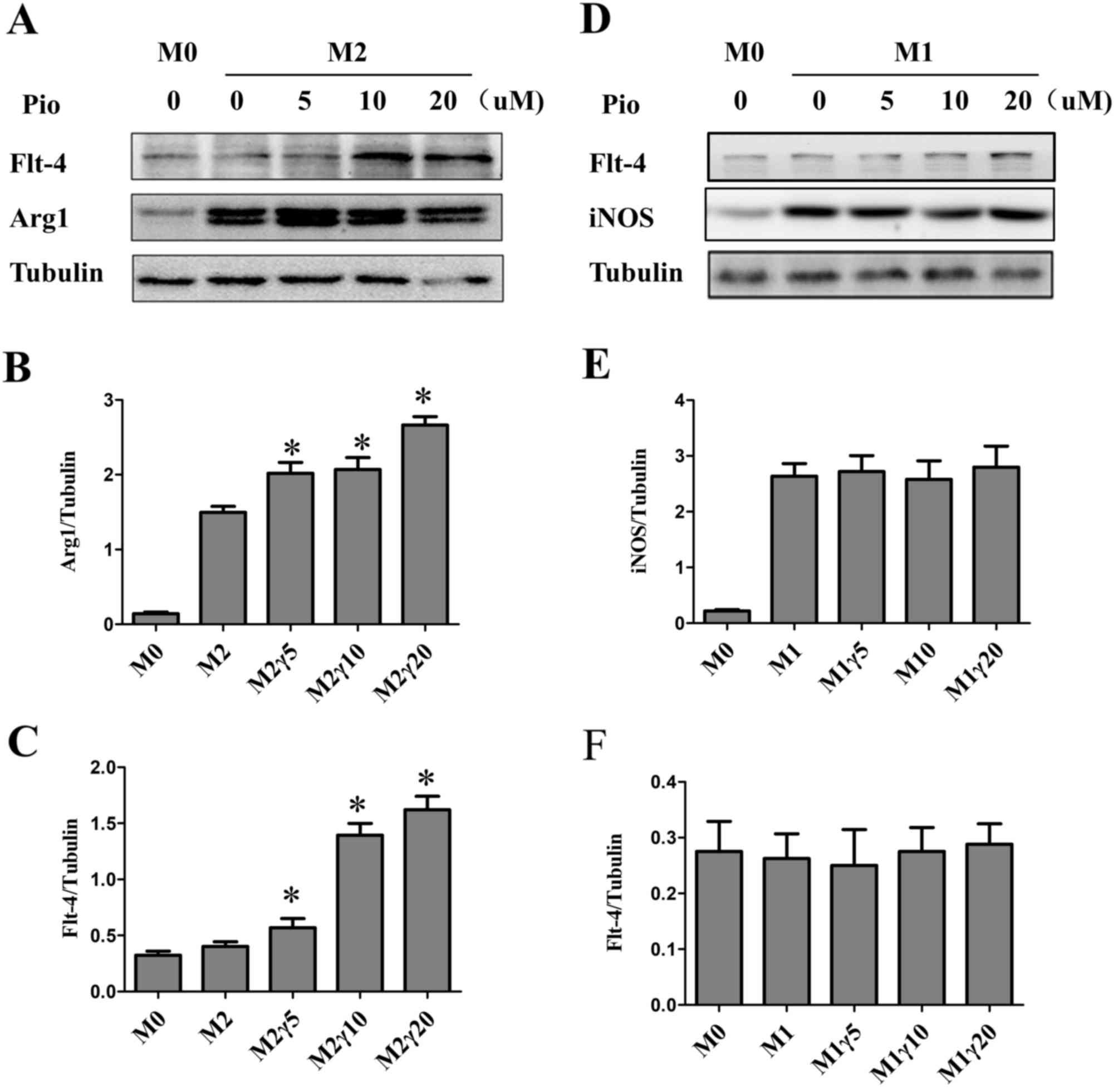 | Figure 3.Effect of pioglitazone on M1 and M2
macrophages. (A) M2 and (D) M1 macrophages were treated with
different concentrations of pioglitazone (0, 5, 10 and 20 µM) for
24 h and western blot analysis was performed to determine protein
expression levels of VEGFR3, Arg1 and iNOS. Tubulin was used as the
loading control. (B, C, E and F) Quantification of the western blot
results. The data are presented as the mean ± standard error of the
mean. *P<0.05 vs. M0. Arg1, arginase1; iNOS, inducible nitric
oxide synthase; M, macrophage; Pio, pioglitazone; VEGFR3, vascular
endothelial growth factor receptor 3. |
Pioglitazone-induced upregulation of
VEGFR3 is PPARγ- dependent
Subsequently, it was investigated whether
pioglitazone-induced upregulation of VEGFR3 in M2 macrophages is
related to PPARγ. The results demonstrated that pioglitazone could
promote M2 activation, which was inhibited by GW9662, a specific
antagonist of PPARγ (Fig. 4A and
B). VEGFR3 protein expression levels were increased in M2
macrophages following pioglitazone treatment, which was reversed by
GW9662 (Fig. 4A and C). This
confirmed that pioglitazone may enhance the expression of VEGFR3
through PPARγ.
Pioglitazone upregulates the
expression of p-PPARγ and promotes macrophage proliferation of in
vivo
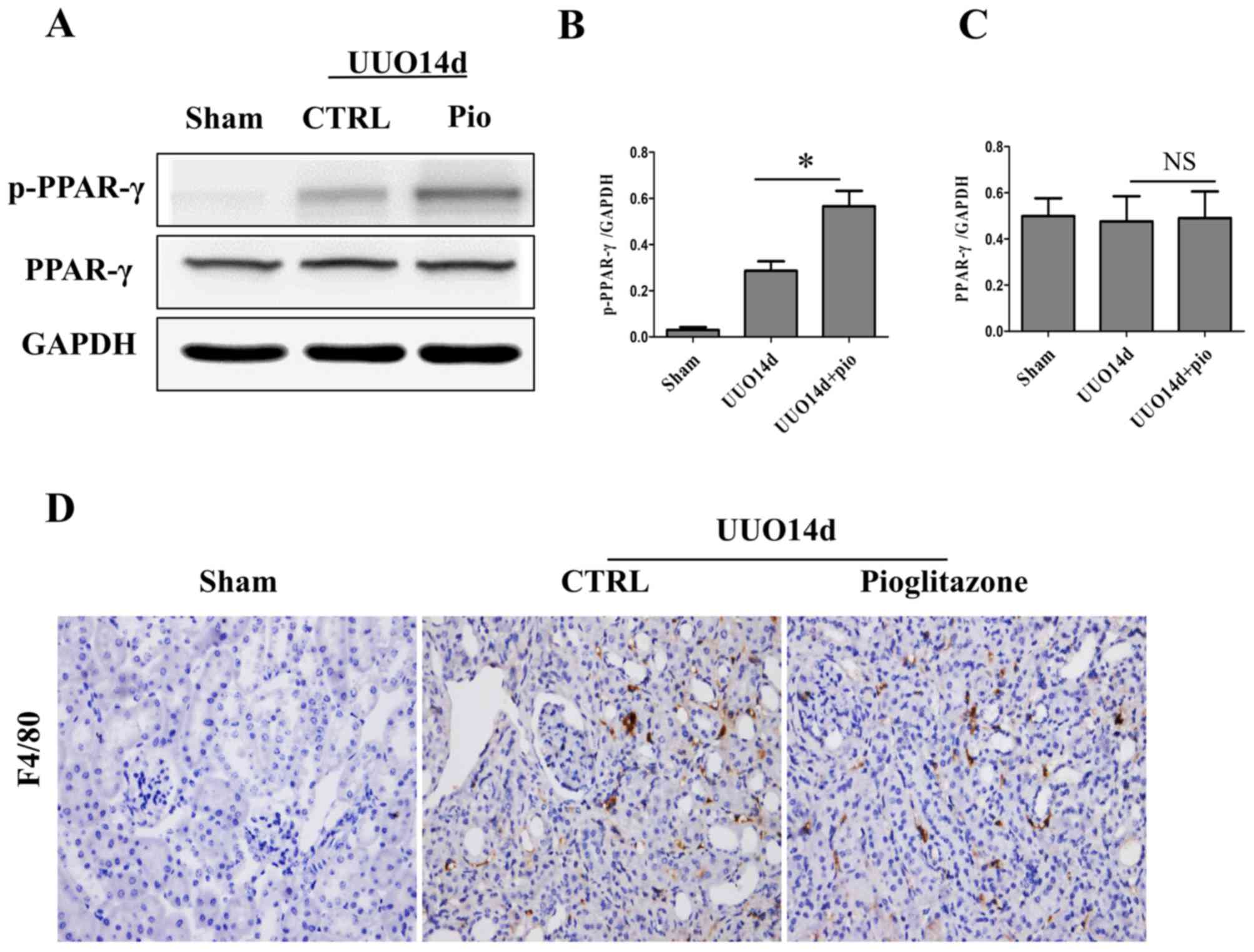
To determine the effect of pioglitazone in
vivo, a UUO mouse model was established, and the p-PPARγ and
total PPARγ expression levels in the kidney tissue were measured by
western blot analysis. As shown in Fig. 5A-C the total PPARγ expression
levels did not change in the presence or absence of pioglitazone;
however, the p-PPARγ expression levels were significantly
upregulated in the UUO + pioglitazone group compared with the UUO
group (Fig. 5A-B). Furthermore,
pioglitazone promoted the proliferation and infiltration of
macrophages (Fig. 5D).
Pioglitazone increases the expression
of VEGFR3 in macrophages in vivo
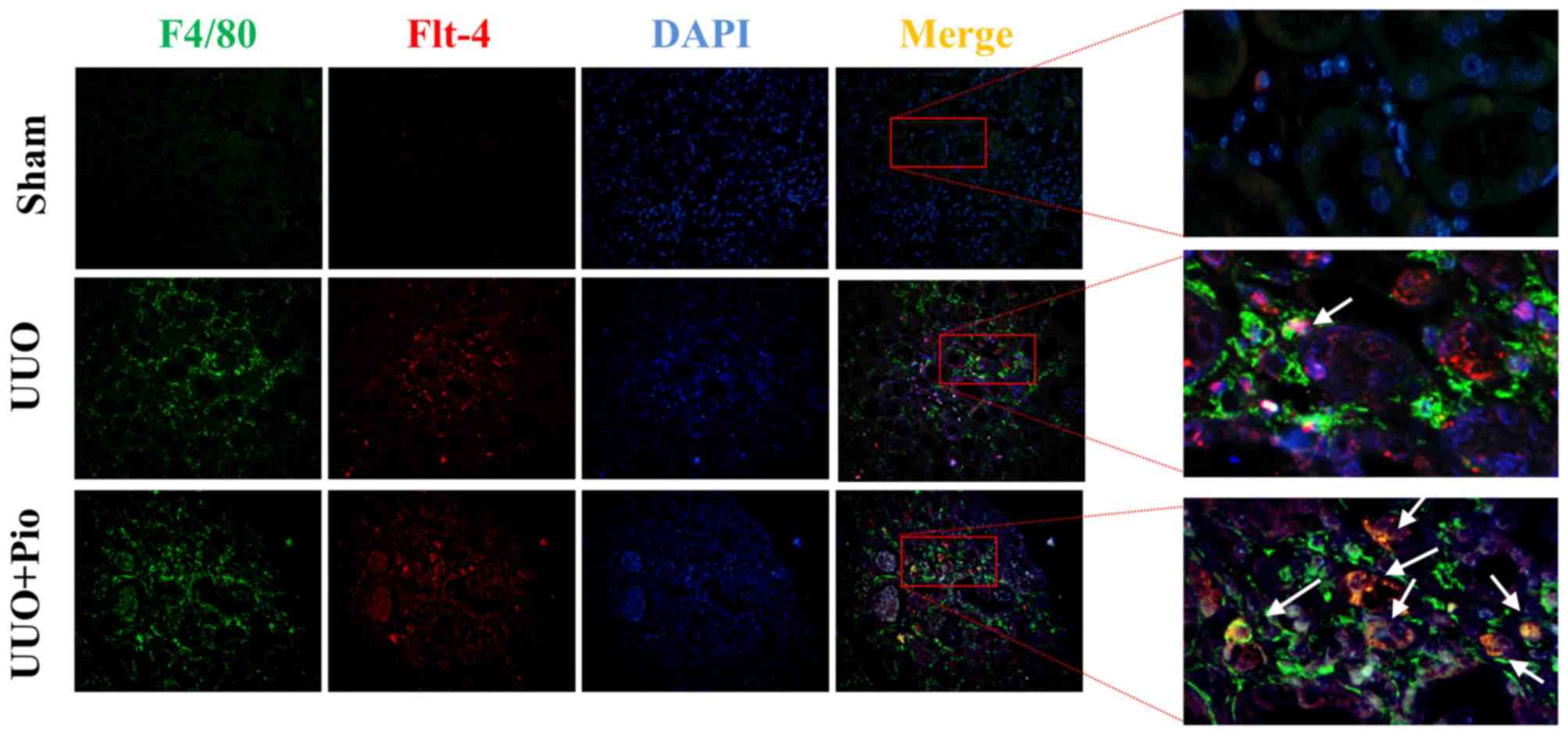
To further determine whether pioglitazone could
increase the expression of VEGFR3 in macrophages in vivo,
double immunofluorescence staining of F4/80 and VEGFR3 was
performed on kidney tissues from the sham, UUO and UUO +
pioglitazone groups. In the sham group, the expression of F4/80 and
VEGFR3 was low, whereas F4/80 and VEGFR3 expression levels were
increased in the UUO model. Following pioglitazone treatment, the
expression of F4/80 was increased, which was in line with the
immunohistochemistry results. In addition, the level of VEGFR3 was
greater in the UUO + pioglitazone group compared with in the UUO
group, and expression was localized in the macrophages (Fig. 6).
Pioglitazone has no therapeutic effect
on renal fibrosis in CKD
To further explore the therapeutic effect of
pioglitazone on renal fibrosis in vivo, collagen-4, α-SMA
and PDGFR-β were detected by immunohistochemistry and western blot
analysis. In the sham group, α-SMA was only expressed in the small
arteries (Fig. 7A). Following UUO,
α-SMA was upregulated in the renal interstitium, which was not
reduced by pioglitazone treatment. Collagen-4 was visible in the
renal interstitium of the sham group; however, its expression was
higher in the UOO group. In addition, pioglitazone treatment did
not decrease collagen-4 expression in the UOO model. Western
blotting also demonstrated similar results. No significant changes
in protein expression levels of PDGFR-β and α-SMA were observed
following pioglitazone treatment compared with UUO group, and these
two proteins were lower in sham compared with the UUO group
(Fig. 7B-D).
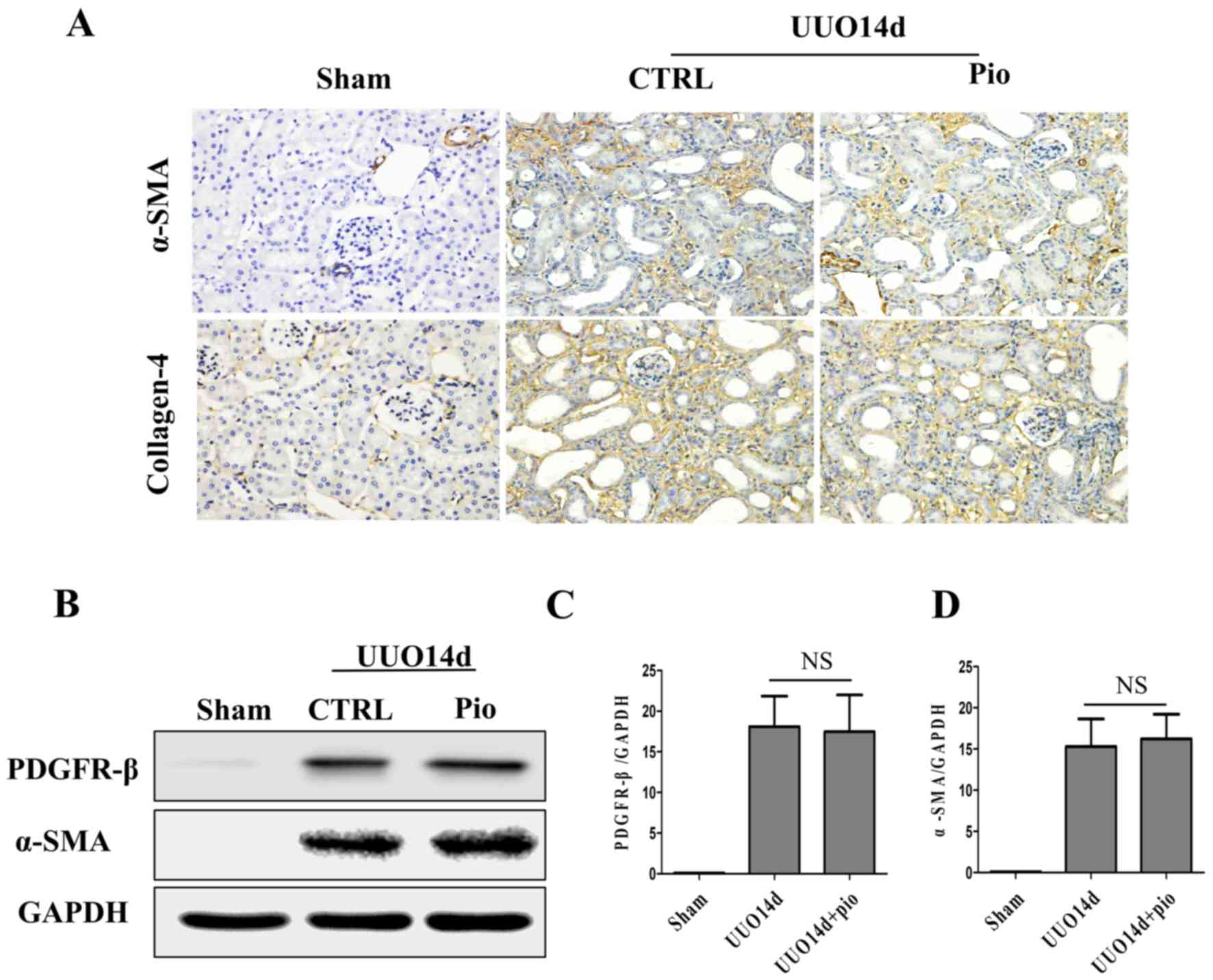 | Figure 7.Pioglitazone has no therapeutic
effect on renal fibrosis in chronic kidney disease. (A)
Immunohistochemical staining of fibrosis markers, α-SMA and
collagen-4, in kidney tissues of mice in the sham, UUO and UUO +
pioglitazone groups. Scale bar, 100 µm. (B) Western blot analysis
was performed to determine the protein expression levels of
fibrosis markers, PDGFR-β and α-SMA, in kidney tissues of mice in
the sham, UUO and UUO + pioglitazone groups. GAPDH was used as the
loading control. (C and D) Quantification of the western blot
results. The data are presented as the mean ± standard error of the
mean (n=7). α-SMA, α-smooth muscle actin; CTRL, control; NS, not
significant; PDGFR-β, platelet-derived growth factor receptor-β;
Pio, pioglitazone; UUO, unilateral ureteral obstruction. |
Discussion
In the present study, it was demonstrated that
pioglitazone promoted the polarization of M2 macrophages.
Stimulation of M2 macrophages with pioglitazone increased
expression of VEGFR3, which was dependent on PPARγ. Furthermore,
pioglitazone increased the expression levels of p-PPARγ in an in
vivo UUO model, which was accompanied by increased infiltration
of VEGFR3-expressing macrophages. However, pioglitazone did not
appear to have a therapeutic effect on renal fibrosis.
Macrophages are heterogeneous populations that serve
an important role in kidney homeostasis, but can also be activated
to cause renal injury, or promote chronic fibrosis, when there is
an ongoing renal insult (17).
There are a number of mechanisms by which macrophages can promote
renal fibrosis, including macrophage-to-myofibroblast transition
(18,19). The present study revealed that
pioglitazone promoted M0-M2 macrophage polarization, which was not
mediated by PPARγ. However, pioglitazone has been identified as a
high affinity ligand for PPARγ, and may also activate other PPAR
subtypes, including PPARα, albeit with weak affinity (20). These discrepancies in results may
be attributed to a difference in pathologic state, and further
studies are required to confirm the underlying mechanisms.
Furthermore, pioglitazone enhanced the activation in M2 macrophages
by activating PPARγ, which was consistent with a previous study
where BMDM were co-cultured with cancer cells (21). Pioglitazone has a strong ability to
promote proliferation and infiltration of macrophages in
vivo, which may promote fibrogenic activities (22).
Vascular endothelial growth factor C (VEGF-C) and
its receptor, VEGFR3, also known as Fms related tyrosine kinase 4,
are the central pathway for proliferation, migration and survival
of lymphatic endothelial cells (LECs) (23). A previous study indicated that the
VEGF-C/VEGFR-3 axis serves a vital role in not only LECs, but also
a variety of other cells, including tumor cells, DCs and
macrophages (24). Previous
studies have also reported the beneficial effects of the
VEGF-C/VEGFR3 pathway in mediating M0 polarization to M1/M2 and
ameliorating experimental inflammatory bowel disease (25,26).
However, VEGFR3 in macrophages in the context of renal fibrosis
requires further investigation. To the best of our knowledge, the
present study revealed for the first time that treatment of M2
cells with pioglitazone increased the expression levels of VEGFR3
via a PPARγ-dependent pathway. However, the effect of pioglitazone
on renal fibrosis remains unclear.
PPARγ has an important role in regulating metabolic
homeostasis, and pioglitazone has been reported to induce
activation of PPARγ and exert anti-inflammatory effects (27,28).
As a type of antidiabetic drug, PPARγ agonists not only serves a
reno-protective role in diabetic nephropathy but also has potential
therapeutic effects in non-metabolic kidney disease (29). A previous study reported that
pioglitazone treatment serves a potential protective role in
non-metabolic nephropathy, such as aging-related progressive renal
injure (30). In experimental
mammal kidney disease studies, pioglitazone prevented the NF-κB
activation and reduced the kidney damage in cisplatin-treated mice
(12), attenuated renal
ischemia-reperfusion injury through its anti-inflammation effect
(31) and decrease the renal cyst
growth in a rat model of polycystic kidney disease (32). However, the effect of pioglitazone
on renal fibrosis is limited and controversial (33–35).
A recent study reported that pioglitazone protects against renal
fibrosis in 5/6 nephrectomized rats (36). Conversely, in our previous study
pioglitazone failed to attenuate non-diabetic UUO-induced renal
fibrosis, although it partially regulated CD4+ T
lymphocyte-associated cytokines (14). In addition, the present study
further demonstrated that pioglitazone had no therapeutic effect on
renal fibrosis in a UUO model. Recent studies indicated that M2
macrophages may have a pro-fibrotic role and inhibition of M2
macrophages may decrease fibrosis in obstructive nephropathy
(37). Therefore, activation of M2
macrophages may explain why pioglitazone failed to attenuate
UUO-induced renal fibrosis in the present study.
In conclusion, the present study demonstrated that
pioglitazone induced polarization of M0 macrophages to M2
macrophages, which may represent a promising therapeutic target for
macrophage related inflammatory disease. In addition, pioglitazone
upregulated VEGFR3 expression in M2 macrophages via PPAR-γ.
Although pioglitazone had no therapeutic effect on renal fibrosis,
further investigations on the role of VEGFR3+ M2
macrophages in renal fibrosis, may provide novel insights and
treatment strategies for renal fibrosis.
Acknowledgements
The authors would like to thank the Tongji Medical
College, Huazhong University of Science and Technology and Animal
House of the College for research infrastructure.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81372244 and
81470948), Hubei Provincial Health and Family Planning Youth
Project of China (grant no. WJ2015Q007), Tongji Hospital New
Technology and New Business (grant no. SJS201102).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ChZ, YZ, YyL and GX designed the study. CoZ and YZ
performed the animal surgeries, and YgL performed image analysis
and histology. CoZ wrote the first draft of the manuscript and all
authors contributed in revising the manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Tongji Hospital, Tongji Medical College, Huazhong University of
Science and Technology (Wuhan, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ma YC, Zuo L, Chen JH, Luo Q, Yu XQ, Li Y,
Xu JS, Huang SM, Wang LN, Huang W, et al: Modified glomerular
filtration rate estimating equation for chinese patients with
chronic kidney disease. J Am Soc Nephrol. 17:2937–2944. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Levey AS, de Jong PE, Coresh J, El Nahas
M, Astor BC, Matsushita K, Gansevoort RT, Kasiske BL and Eckardt
KU: The definition, classification, and prognosis of chronic kidney
disease: A KDIGO controversies conference report. Kidney Int.
80:17–28. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Coresh J, Selvin E, Stevens LA, Manzi J,
Kusek JW, Eggers P, van Lente F and Levey AS: Prevalence of chronic
kidney disease in the United States. JAMA. 298:2038–2047. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fogo AB: Mechanisms of progression of
chronic kidney disease. Pediatr Nephrol. 22:2011–2022. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chevalier RL, Forbes MS and Thornhill BA:
Ureteral obstruction as a model of renal interstitial fibrosis and
obstructive nephropathy. Kidney Int. 75:1145–1152. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang Y and Harris DC: Macrophages in renal
disease. J Am Soc Nephrol. 22:21–27. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Murray PJ and Wynn TA: Protective and
pathogenic functions of macrophage subsets. Nat Rev Immunol.
11:723–737. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Owen MR, Stamper IJ, Muthana M, Richardson
GW, Dobson J, Lewis CE and Byrne HM: Mathematical modeling predicts
synergistic antitumor effects of combining a macrophage-based,
hypoxia-targeted gene therapy with chemotherapy. Cancer Res.
71:2826–2837. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Spitler LE, Grossbard ML, Ernstoff MS,
Silver G, Jacobs M, Hayes FA and Soong SJ: Adjuvant therapy of
stage III and IV malignant melanoma using granulocyte-macrophage
colony-stimulating factor. J Clin Oncol. 18:1614–1621. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ahmadian M, Suh JM, Hah N, Liddle C,
Atkins AR, Downes M and Evans RM: PPARγ signaling and metabolism:
The good, the bad and the future. Nat Med. 19:557–566. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Murphy GJ and Holder JC: PPAR-γ agonists:
Therapeutic role in diabetes, inflammation and cancer. Trends
Pharmacol Sci. 21:469–474. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang J, Zhang Y, Xiao F, Liu Y, Wang J,
Gao H, Rong S, Yao Y, Li J and Xu G: The peroxisome
proliferator-activated receptor γ agonist pioglitazone prevents
NF-κB activation in cisplatin nephrotoxicity through the reduction
of p65 acetylation via the AMPK-SIRT1/p300 pathway. Biochem
Pharmacol. 101:100–111. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hontecillas R, Horne WT, Climent M, Guri
AJ, Evans C, Zhang Y, Sobral BW and Bassaganya-Riera J:
Immunoregulatory mechanisms of macrophage PPAR-γ in mice with
experimental inflammatory bowel disease. Mucosal Immunol.
4:304–313. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang Y, Wang J, Zhou QD, Zhang CH, Li Q,
Huang S, Zhan J, Wang K, Liu YY and Xu G: Peroxisome
proliferator-activated receptor-γ agonist pioglitazone fails to
attenuate renal fibrosis caused by unilateral ureteral obstruction
in mice. J Huazhong Univ Sci Technol Med Sci. 36:41–47. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu Y, Wang K, Liang X, Li Y, Zhang Y,
Zhang C, Wei H, Luo R, Ge S and Xu G: Complement C3 produced by
macrophages promotes renal fibrosis via IL-17A secretion. Front
Immunol. 9:23852018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ying W, Cheruku PS, Bazer FW, Safe SH and
Zhou B: Investigation of macrophage polarization using bone marrow
derived macrophages. J Vis Exp. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Duffield JS: Macrophages and immunologic
inflammation of the kidney. Semin Nephrol. 30:234–254. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nikolic-Paterson DJ, Wang S and Lan HY:
Macrophages promote renal fibrosis through direct and indirect
mechanisms. Kidney Int Suppl (2011). 4:34–38. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang YY, Jiang H, Pan J, Huang XR, Wang
YC, Huang HF, To KF, Nikolic-Paterson DJ, Lan HY and Chen JH:
Macrophage-to-myofibroblast transition contributes to interstitial
fibrosis in chronic renal allograft injury. J Am Soc Nephrol.
28:2053–2067. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tuccori M, Filion KB, Yin H, Yu OH, Platt
RW and Azoulay L: Pioglitazone use and risk of bladder cancer:
Population based cohort study. BMJ. 352:i15412016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li H, Sorenson AL, Poczobutt J, Amin J,
Joyal T, Sullivan T, Crossno JT Jr, Weiser-Evans MC and Nemenoff
RA: Activation of PPARγ in myeloid cells promotes lung cancer
progression and metastasis. PLoS One. 6:e281332011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhu Z, Ding J, Ma Z, Iwashina T and
Tredget EE: Alternatively activated macrophages derived from THP-1
cells promote the fibrogenic activities of human dermal
fibroblasts. Wound Repair Regen. 25:377–388. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tammela T and Alitalo K:
Lymphangiogenesis: Molecular mechanisms and future promise. Cell.
140:460–476. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Su JL, Yen CJ, Chen PS, Chuang SE, Hong
CC, Kuo IH, Chen HY, Hung MC and Kuo ML: The role of the
VEGF-C/VEGFR-3 axis in cancer progression. Br J Cancer. 96:541–545.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
D'Alessio S, Correale C, Tacconi C,
Gandelli A, Pietrogrande G, Vetrano S, Genua M, Arena V, Spinelli
A, Peyrin-Biroulet L, et al: VEGF-C-dependent stimulation of
lymphatic function ameliorates experimental inflammatory bowel
disease. J Clin Invest. 124:3863–3878. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Beghdadi W, Madjene LC, Claver J, Pejler
G, Beaudoin L, Lehuen A, Daugas E and Blank U: Mast cell chymase
protects against renal fibrosis in murine unilateral ureteral
obstruction. Kidney Int. 84:317–326. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sugawara A, Uruno A, Kudo M, Matsuda K,
Yang CW and Ito S: Effects of PPARγ on hypertension,
atherosclerosis, and chronic kidney disease. Endocr J. 57:847–852.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rosen ED and Spiegelman BM: PPARgamma: A
nuclear regulator of metabolism, differentiation, and cell growth.
J Biol Chem. 276:37731–37734. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fogo AB: PPARγ and chronic kidney disease.
Pediatr Nephrol. 26:347–351. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang HC, Deleuze S, Zuo Y, Potthoff SA, Ma
LJ and Fogo AB: The PPARgamma agonist pioglitazone ameliorates
aging-related progressive renal injury. J Am Soc Nephrol.
20:2380–2388. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Singh AP, Singh N and Bedi PM:
Pioglitazone ameliorates renal ischemia reperfusion injury through
NMDA receptor antagonism in rats. Mol Cell Biochem. 417:111–118.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Blazer-Yost BL, Haydon J, Eggleston-Gulyas
T, Chen JH, Wang X, Gattone V and Torres VE: Pioglitazone
attenuates cystic burden in the PCK rodent model of polycystic
kidney disease. PPAR Res. 2010:2743762010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ochodnicky P, Mesarosova L, Cernecka H,
Klimas J, Krenek P, Goris M, van Dokkum RP, Henning RH and
Kyselovic J: Pioglitazone, a PPARγ agonist, provides comparable
protection to angiotensin converting enzyme inhibitor ramipril
against adriamycin nephropathy in rat. Eur J Pharmacol. 730:51–60.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Higashi K, Oda T, Kushiyama T, Hyodo T,
Yamada M, Suzuki S, Sakurai Y, Miura S and Kumagai H: Additive
antifibrotic effects of pioglitazone and candesartan on
experimental renal fibrosis in mice. Nephrology (Carlton).
15:327–335. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Han JY, Kim YJ, Kim L, Choi SJ, Park IS,
Kim JM, Chu YC and Cha DR: PPARgamma agonist and angiotensin II
receptor antagonist ameliorate renal tubulointerstitial fibrosis. J
Korean Med Sci. 25:35–41. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sun L, Yuan Q, Xu T, Yao L, Feng J, Ma J,
Wang L, Lu C and Wang D: Pioglitazone improves mitochondrial
function in the remnant kidney and protects against renal fibrosis
in 5/6 nephrectomized rats. Front Pharmacol. 8:5452017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Nishida M and Hamaoka K: Macrophage
phenotype and renal fibrosis in obstructive nephropathy. Nephron
Exp Nephrol. 110:e31–36. 2008. View Article : Google Scholar : PubMed/NCBI
|