Introduction
Breast cancer is the most frequently diagnosed
cancer type and the fifth most common cause of mortality among
women worldwide (1). It was
estimated that there were 268,600 newly diagnosed cases and 69,500
patients that succumbed to this disease in China in 2015, which
accounts for 15.1% of all cancer incidences and 6.9% of
cancer-related mortalities in women (2). Although the mortality rates due to
breast cancer have declined, the incidence rates of this disease
have increased in China over the past decades (3). Breast cancer is a heterogeneous
disease that can be classified into several forms based on
histological, clinical and genetic features. Numerous attempts of
categorizing this heterogeneous disease have led to a molecular
classification into five subgroups by gene-expression profiling
(4,5).
Circular RNAs (circRNAs) are large non-coding RNAs
that are present ubiquitously in the cytoplasm of eukaryotic cells
and function as competing endogenous RNAs (6). Numerous circRNAs serve important
‘sponge’ roles through their effect on microRNA (miR/miRNA)
activity. For example, circRNA ciRS-7 is a newly identified human
circRNA that is involved in resistance to miRNA-mediated target
destabilization, by strongly binding and suppressing the activity
of miR-7 (7). A previous report
also revealed the crucial role of circRNAs in regulating gene
transcription (8). Accumulating
evidences have demonstrated that circRNAs have remarkable
regulatory roles in the generation and development of various
diseases, including atherosclerosis, nervous system disorders and
cancers, including leukemia and solid tumors (9–11). A
recent study demonstrated that chondrocyte extracellular
matrix-related ciRNA (circRNA-CER) expression increases with
increased levels of interleukin-1 and tumor necrosis factor in
chondrocytes, and silencing of circRNA-CER using small interfering
RNA (siRNA) suppresses matrix metalloproteinase 13 (MMP13)
expression and increases extracellular matrix formation (12). Yao et al suggested that the
expression of circRNA-CER is significantly associated with lymph
node metastasis, overall survival and tumor stage in non-small cell
lung cancer (13). However, the
function of circRNA-CER in breast cancer remains largely unknown
and requires further investigation.
The present study aimed to evaluate the expression
profiles of circRNA-CER in breast cancer and investigate their
potential role in the disease. The data demonstrated that the
expression levels of circRNA-CER in breast cancer tissue were
significantly higher compared with in adjacent non-tumor tissue,
and that circRNA-CER may function as a competing endogenous RNA to
regulate the expression of MMP13 by competing with miR-136 in
breast cancer cells. To the best of our knowledge the present study
demonstrated for the first time a positive circRNA-CER/MMP13
association, and crosstalk among miR-136, circRNA-CER and MMP13,
which provides novel insight into the treatment of breast
cancer.
Materials and methods
Patients and tissue samples
A total of 24 surgically resected tissue specimens
from female patients with breast cancer were collected from the
First Affiliated Hospital of Jiamusi University (Jiamusi, China)
from April 2016 to March 2018. Patients who had received local or
systemic therapy prior to surgery were excluded from the study, and
the ages of the patients ranged from 38–73 years old. Pathological
examination of the tissue specimens was conducted independently by
three pathologists, without prior knowledge of the patients'
medical records. The present study was approved by the Ethics
Committee of Jiamusi University (approval no. JMSU-215) and all
patients provided written informed consent prior to enrolment.
Immunohistochemistry
The tissue samples were fixed in 10% buffered
formalin at room temperature for 24 h, embedded in paraffin, and
cut into 5 µm-thick sections. Following deparaffinization in xylene
at 37°C for 30 min, the sections were dehydrated in a graded series
of ethanol, subjected to antigen retrieval with 0.01 M citrate
buffer (cat. no. AR0024; Wuhan Boster Biological Technology, Ltd.,
Wuhan, China) at 100°C for 18 min in a high-pressure cooker and
washed in PBS (cat. no. AR0030; Wuhan Boster Biological Technology,
Ltd.) at room temperature for 5 min. Endogenous peroxidase activity
was subsequently quenched by incubating the sections with 3%
hydrogen peroxide in PBS for 15 min at room temperature, followed
by blocking with 5% bovine serum albumin (BSA, cat. no. AR0004;
Wuhan Boster Biological Technology, Ltd.) at 37°C for 60 min. Then,
the slides were incubated with a rabbit polyclonal anti-MMP13
primary antibody (1:150; cat. no. ab39012; Abcam, Cambridge, MA,
USA) overnight at 4°C. As a negative control, the primary antibody
was replaced with the same amount of normal rabbit serum (cat. no.
AR0010; Wuhan Boster Biological Technology, Ltd.). Following
several washes in PBS (3×3 min) to remove the excess of antibody, a
biotin-conjugated goat anti-rabbit secondary antibody (cat. no.
SA1022; Wuhan Boster Biological Technology, Ltd.) was applied at
37°C for 20 min, followed by washing of slides in PBS (3×3 min).
Next, the slides were incubated in avidin-biotin peroxidase complex
(cat. no. SA1022; Wuhan Boster Biological Technology, Ltd.) for 20
min at 37°C. Then, 3,3′-diaminobenzidine (Beijing Biosynthesis
Biotechnology Co., Ltd, Beijing, China) was used for chromogenic
detection and slides were counterstained with hematoxylin at room
temperature for 30 min for cell nuclear detection.
MMP13 protein expression was quantified based on the
staining intensity and proportion of stained cells, according to Li
et al (14) with slight
modifications. Briefly, MMP13 staining intensity was scored from 0
to 3 as follows: 0, absent immunopositivity; 1, low
immunopositivity; 2, moderate immunopositivity; 3, intense
immunopositivity. The extent of total staining was scored from 0 to
4 as follows: 0, negative; 1, 1–25% of cells; 2, 26–50% of cells;
3, 51–75% of cells; and 4, 76–100% of cells. On average, three
random fields were observed for each tissue at ×400 magnification
under an Olympus BX-60 fluorescence microscope (Olympus
Corporation, Tokyo, Japan). The final staining score was the sum of
the intensity and extent scores.
Cell lines, cell culture and
transfection
The MCF-7 and ZR-75-30 breast cancer cell lines were
purchased from the Shanghai Institute of Biochemistry and Cell
Biology (Shanghai, China). Cells were cultured in RPMI-1640 medium
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc.), 100 U/ml penicillin and 100 mg/ml streptomycin
(Invitrogen; Thermo Fisher Scientific, Inc.) at 37°C with 5%
CO2 in a humidified atmosphere. miR-136 mimics, miR-136
inhibitors and the corresponding negative controls were designed
and constructed by Shanghai Gene Pharma Co., Ltd. (Shanghai,
China). siRNA targeting circRNA-CER (si-circRNA-CER) and its
negative controls were purchased from Guangzhou RiboBio Co., Ltd.
(Guangzhou, China). The sequences for miR-136 mimics, miR-136
inhibitors and si-circRNA-CER were: 5′-ACUCCAUUUGUUUUGAUGAUGGA-3′,
5′-CAUCAUCGUCUCAAAUGAGUCU-3′ and 5′-CCCACGCUCCUACAAUGUU-3′.
Transfection of MCF-7 cells was performed using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's instructions.
Briefly, Lipofectamine® 2000 was incubated with miRNAs
at a concentration of 100 nmol/l or siRNAs at a concentration of
200 nmol/l for 20 min, and the complex was then added to each well.
Following transfection for 24 h, the subsequent experiments were
performed.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Specific primers for circRNA-CER, β-actin, miRNA-136
and U6 were purchased from GeneCopoeia, Inc. (Rockville, MD,
USA). The primers used in this investigation were: circRNA-CER
forward: 5′-CTGGTGCAGTGGAAGCAGAG-3′, reverse:
5′-CGACCCTCCATTGCTCTTCT-3′; β-actin forward:
5′-CTCCATCCTGGCCTCGCTGT-3′, reverse: 5′-GCTGTCACCTTCACCGTTCC-3′;
miRNA-136 forward: 5′-ACUCCAUUUGUUUUGAUGAUGGA-3′, reverse:
5′-UCCAUCAUCAAAACAAAUGGAGU-3′, U6 forward:
5′-GCTTCGGCAGCACATATACTAAAAT-3′, reverse:
5′-CGCTTCACGAATTTGCGTGTCAT-3′. For detection of circRNA-CER, total
RNA from cells and tissues was isolated using TRIzol®
reagent (Invitrogen; Thermo Fisher Scientific, Inc.), and reverse
transcribed using the iScript™ cDNA Synthesis kit (Bio-Rad
Laboratories, Inc., Hercules, CA, USA), according to the
manufacturer's protocols. circRNA-CER PCR amplifications were
performed using a Super Real Pre Mix Color (SYBR Green) Real Time
PCR kit(cat. no. FP215; Tiangen Biotech Co., Ltd., Beijing, China)
with the following conditions: Denaturation at 95°C for 10 min,
followed by 40 cycles of 95°C for 10 sec, 60°C for 20 sec and 72°C
for 10 sec. Relative expression of miR-136 was determined with the
All-in-One™ miRNA qRT-PCR Detection kit based on SYBR Green dye
method (cat. no. AOMD-Q020; GeneCopoeia, Inc.), according to the
manufacturer's protocol. miR-136 PCR amplifications were performed
using the following conditions: Denaturation at 95°C for 10 min,
followed by 40 cycles of 95°C for 15 sec, 60°C for 20 sec and 72°C
for 12 sec. The expression levels of circRNA-CER and miR-136 were
evaluated using the comparative quantification method (15), and normalized to β-actin and U6
expression levels, respectively.
Cell proliferation assay
Cell proliferation was determined using the Cell
Counting Kit-8 (CCK-8; cat. no. AR1160; Wuhan Boster Biological
Technology, Ltd.), according to the manufacturer's protocols.
Briefly, cells were plated in a 96-well plate (1×102
cells/well) and cultured in 100 µl RPMI-1640 medium supplemented
with 10% FBS, 100 U/ml penicillin and 100 µg/ml streptomycin.
Following incubation at 37°C for 24, 48, 72 or 96 h, 10 µl CCK-8
reagent was added to each well and cells were further incubated for
1 h. Cell proliferation analysis was subsequently performed by
measuring absorbance at wavelength 450 nm using a spectrophotometer
(BioTek Instruments, Inc., Winooski, VT, USA).
Cell migration assay
Migration of MCF-7 cells was evaluated using
Transwell inserts (pore size, 8 µm; Corning, Inc., Corning, NY,
USA) in a 24-well plate. Briefly, 2×104 cells (for
miR-136 mimics and si-circRNA-CER assays) or 5×103 cells
(for miR-136 inhibitors assays) were resuspended in 100 µl
serum-free RPMI-1640 medium and seeded in the top chamber of each
Transwell insert in triplicate. A total of 600 µl RPMI-1640 medium
containing 10% FBS was added to the lower chamber. After 48 h of
incubation at 37°C, cells in the top chamber were removed by
scraping with a cotton swab, and the inserts were fixed with 4%
paraformaldehyde at room temperature for 30 min and stained with
0.5% crystal violet at room temperature for 15 min. Cells were
visualized under a light microscope at ×100 magnification (Olympus
Corporation) and migrating cells in five random microscopic fields
were counted.
Western blot analysis
Western blotting was performed to detect MMP13
protein expression. Total protein was extracted from cells using
RIPA lysate (cat. no. AR0105; Wuhan Boster Biological Technology,
Ltd.). Following quantification of protein concentration using a
BCA assay kit (cat. no. AR0146A; Wuhan Boster Biological
Technology, Ltd.), a total of 50 µg total protein was subjected to
12% SDS-PAGE. Following transfer to polyvinylidene fluoride
membranes (EMD Millipore, Billerica, MA, USA), the membranes were
blocked with 5% non-fat milk at 37°C for 2 h and probed with rabbit
polyclonal anti-MMP13 (1:5,000; cat. no. ab39012; Abcam) and mouse
monoclonal anti-GAPDH (1:1,000; cat. no. ab8245; Abcam) primary
antibodies at 37°C for 2 h. The membranes were washed twice for 5
min in tris-buffered saline containing 0.05% Tween-20, and
subsequently incubated with horseradish peroxidase-conjugated goat
anti-rabbit or goat anti-mouse secondary antibodies (1:10,000; cat.
nos. BA1054 and BA1050; Wuhan Boster Biological Technology, Ltd.)
with gentle shaking at 37°C for 25 min. The protein bands were
visualized using the Beyo ECL Star kit (cat. no. P0018A; Beyotime
Institute of Biotechnology, Jiangsu, China) and quantified by the
Image Quant TL software (GE Healthcare Life Sciences, Little
Chalfont, UK).
Statistical analysis
All experiments were repeated three times. The data
are presented as the mean ± standard deviation. Comparison of two
means was performed using Student's t-test, whereas comparison of
multiple means was conducted with analysis of variance followed by
Newman-Keuls post hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
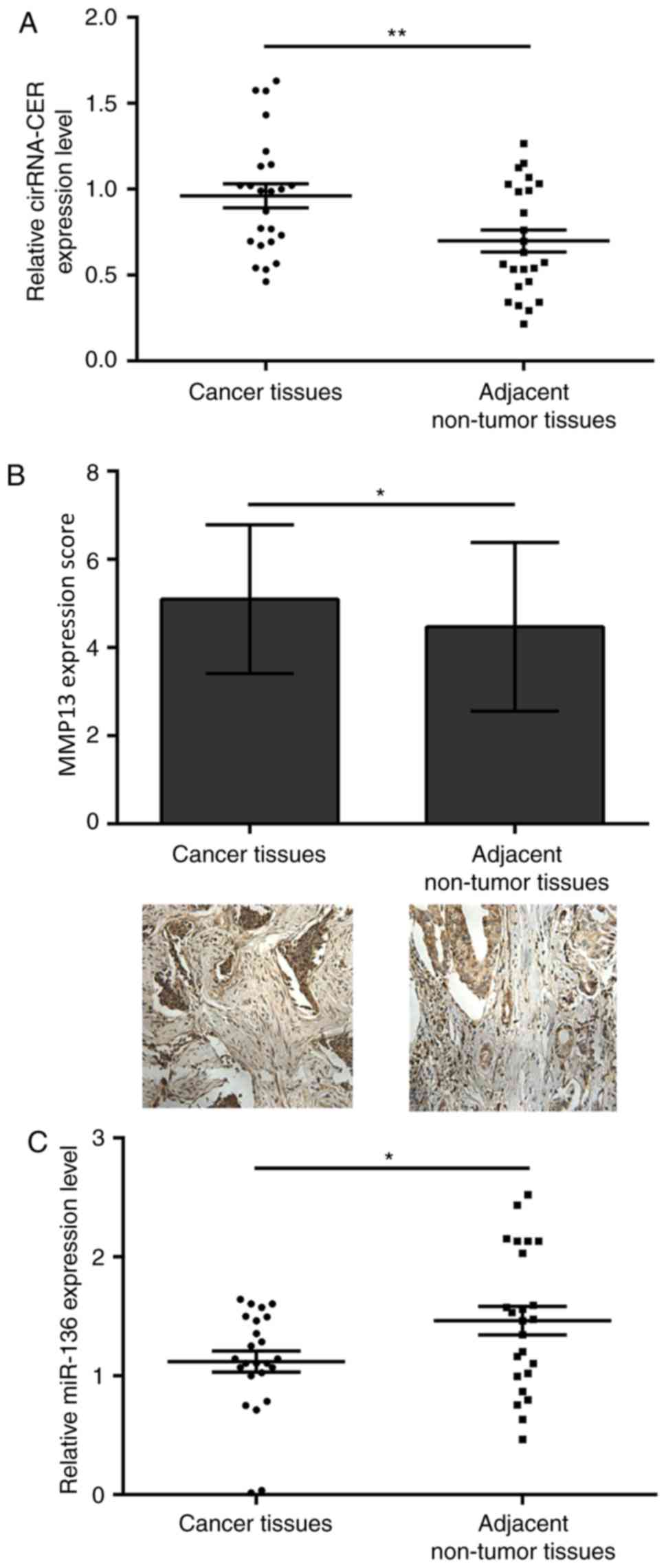
circRNA-CER expression is increased in
breast cancer tissues
To determine the expression levels of circRNA-CER,
MMP13 and miR-136 in breast cancer tissues, circRNA-CER and miR-136
were quantified by RT-qPCR, whereas MMP13 was detected by IHC. The
results revealed that the expression levels of circRNA-CER
(Fig. 1A) and MMP13 (Fig. 1B) were significantly elevated in
breast cancer tissues compared with in adjacent non-tumor tissues.
Conversely, miR-136 expression was decreased in breast cancer
tissues compared with in adjacent non-tumor tissues (Fig. 1C).
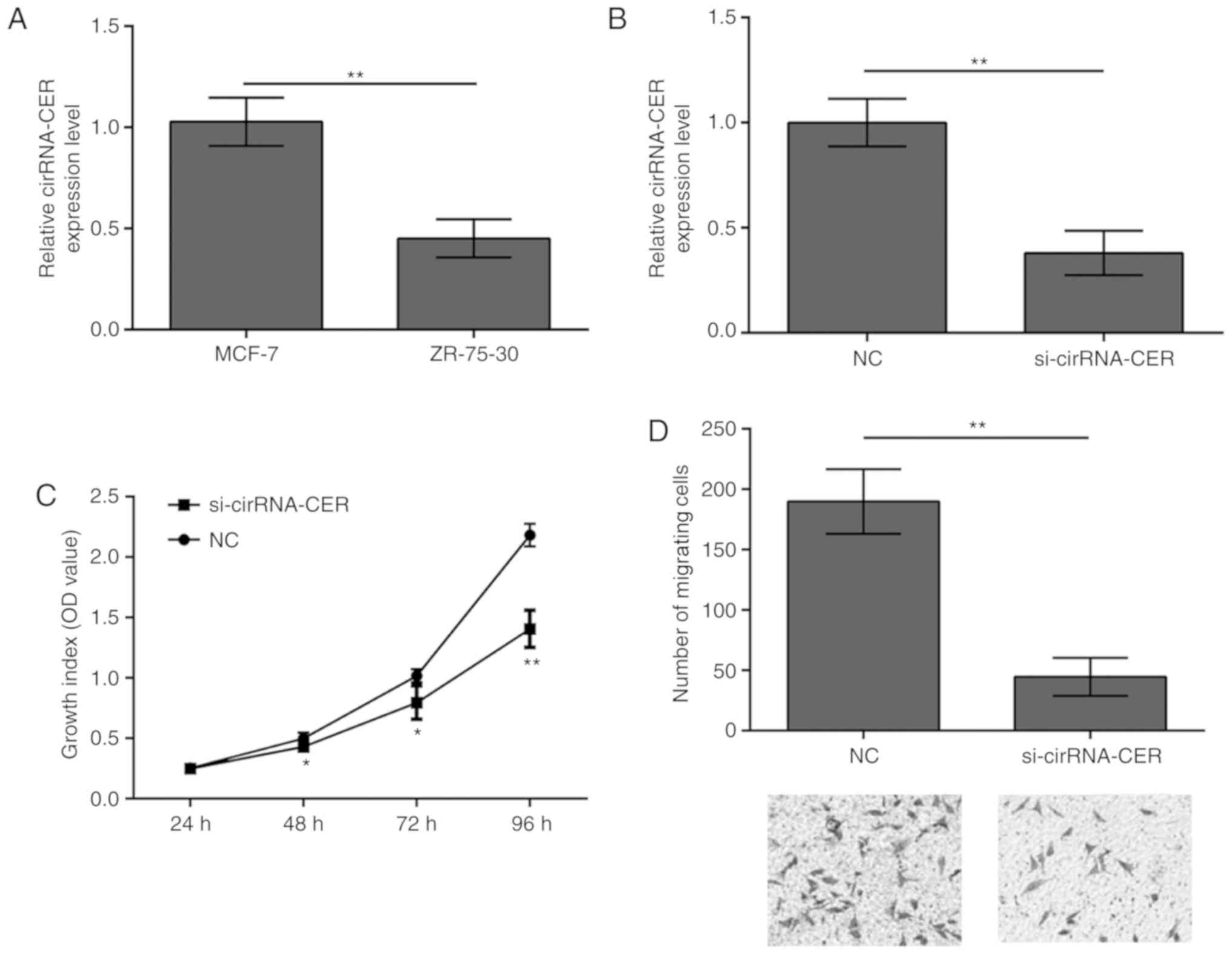
circRNA-CER knockdown inhibits MCF-7
cell proliferation and migration
To examine whether circRNA-CER mediates the
malignant phenotype of breast cancer, its role in breast cancer
cell proliferation and migration was investigated. RT-qPCR revealed
that circRNA-CER expression was significantly higher in the MCF-7
cell line compared with in the ZR-75-30 cell line (Fig. 2A). Based on this observation, the
MCF-7 cell line was selected for conducting silencing experiments,
and the results demonstrated that silencing of circRNA-CER
efficiently suppressed the expression of circRNA-CER (Fig. 2B). In addition, the results from
the CCK-8 and Transwell assays indicated that knockdown of
circRNA-CER significantly suppressed proliferation (Fig. 2C) and migration (Fig. 2D) of MCF-7 cells.
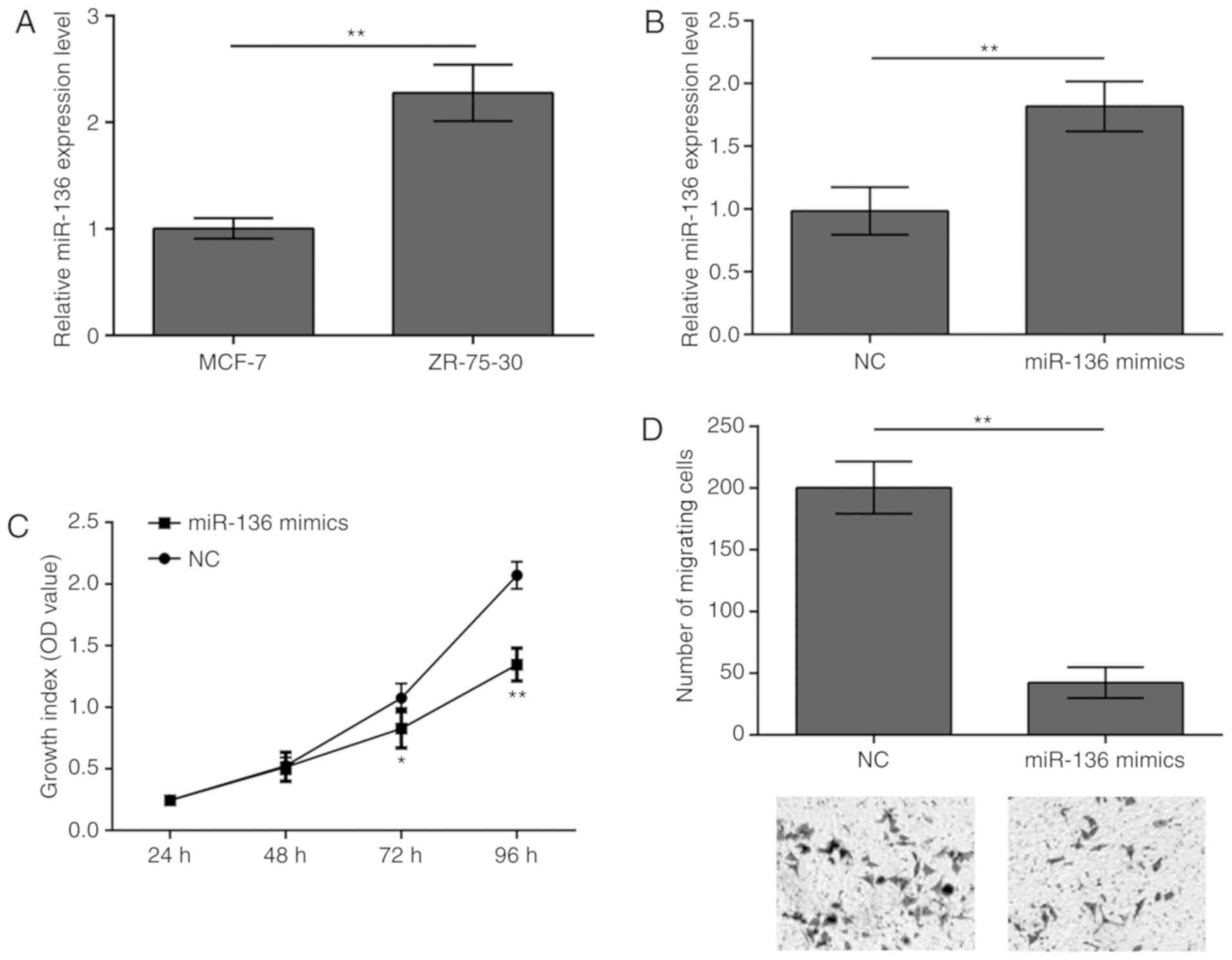
Overexpression of miR-136 inhibits
MCF-7 cell proliferation and migration
The present study revealed that miR-136 expression
levels were significantly lower in MCF-7 cells compared with in
ZR-75-30 cells (Fig. 3A). Thus,
miR-136 was overexpressed in MCF-7cells to evaluate the potential
effects of miR-136 on breast cancer (Fig. 3B). The results demonstrated that
overexpression of miR-136 in MCF-7 cells inhibited both cell
proliferation (Fig. 3C) and
migration (Fig. 3D).
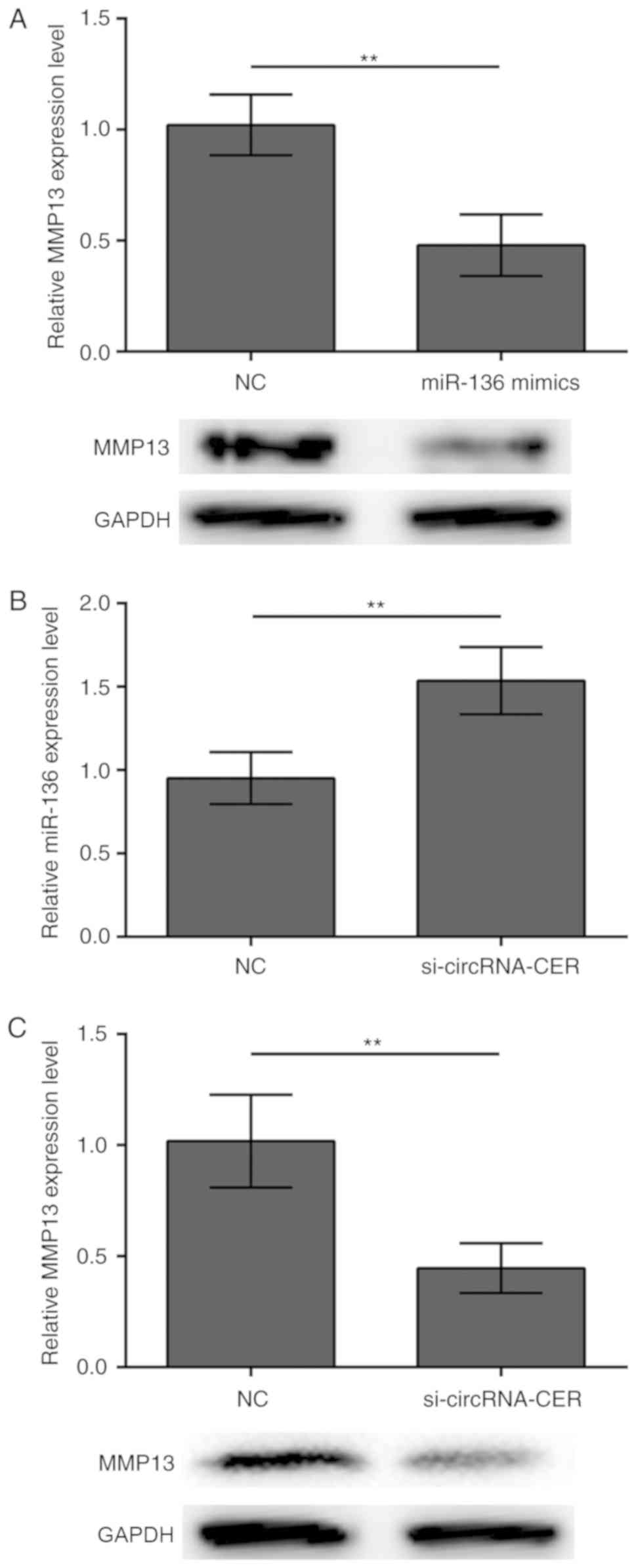
circRNA-CER knockdown regulates the
expression of miR-136 and its target gene MMP13
It is accepted that MMP13 is a target of miR-136 in
human cartilage degradation (12).
In accordance with this notion, the present study demonstrated that
transfection with miR-136 mimics suppressed MMP13 protein
expression in MCF-7 breast cancer cells (Fig. 4A). In addition, the results
demonstrated that silencing of circRNA-CER increased the expression
levels of miR-136 (Fig. 4B) and
inhibited the protein expression of MMP13 (Fig. 4C), suggesting that miR-136/MMP13
crosstalk is regulated by circRNA-CER.
Knockdown of circRNA-CER suppresses
MCF-7 cell proliferation and migration by targeting miR-136
To further demonstrate the association between
circRNA-CER and miR-136, the present study conducted transfection
experiments using miR-136 inhibitors to downregulate miR-136
expression in MCF-7 cells. The results revealed that miR-136
expression levels were successfully decreased; however, the
presence of circRNA-CER knockdown reversed this effect (Fig. 5A). In addition, downregulation of
miR-136 expression promoted MCF-7 cell proliferation and migration,
and these biological effects were abolished upon silencing of
circRNA-CER (Fig. 5B and C).
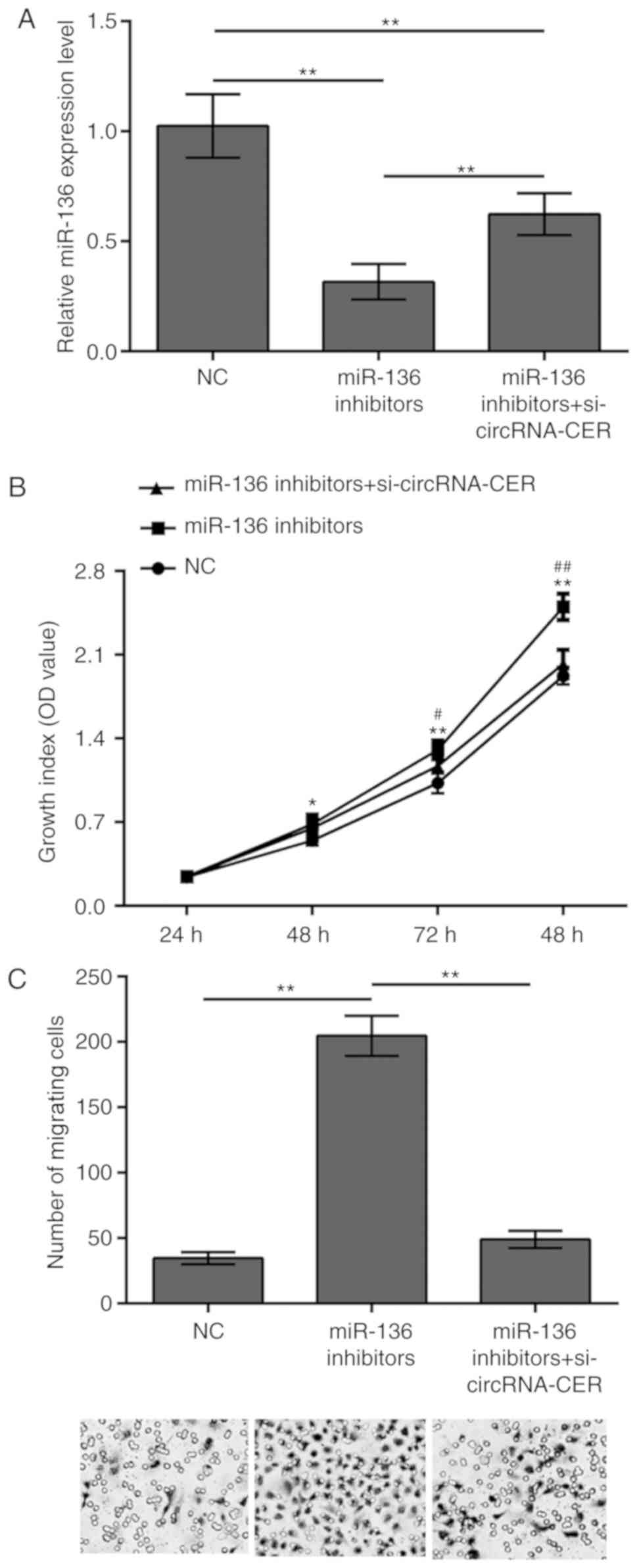 | Figure 5.circRNA-CER knockdown suppresses MCF-7
cell proliferation and migration by targeting miR-136. (A) MCF-7
cells were transfected, with miR-136 inhibitors or miR-136
inhibitors + si-circRNA-CER, and miR-136 expression levels were
determined by RT-qPCR. (B) CCK-8 and (C) Transwell migration assays
(magnification, ×200) were performed using MCF-7 cells transfected
miR-136 inhibitors and miR-136 inhibitors + si-circRNA-CER.
*P<0.05, **P<0.01 vs. NC; #P<0.05,
##P<0.01 vs. miR-136 inhibitors + si-circRNA-CER.
**P<0.01. circRNA-CER, chondrocyte extracellular matrix-related
circular RNA; miR, microRNA; MMP13, matrix metalloproteinase 13;
NC, negative control; OD, optical density; si, small
interfering. |
Discussion
Non-coding RNAs (ncRNAs), which include miRNAs, long
ncRNAs and circRNAs, are involved in the development of several
diseases including pathological cardiac remodeling, nervous system
diseases and several cancers (16–19).
circRNAs are a novel type of ncRNA characterized by a stable
structure and highly specific tissue expression. circRNAs are more
stable than linear RNAs due to their covalently closed continuous
loop without a free 3′ or 5′ end and their polyadenylated tail
(11). Several studies have
proposed that circRNAs mediate diverse biological processes,
including miRNA ‘sponging’, splicing and transcription regulation,
protein binding and RNA transport (13).
The present study revealed that the levels of
circRNA-CER were higher in breast cancer tissues compared with
adjacent non-tumor tissues. Consistent with our findings, a
previous study identified that among 101 patients with non-small
cell lung cancer, the expression levels of circRNA-CER were
significantly elevated in cancer tissues, which was markedly
associated with lymph node metastasis, overall survival and tumor
stage (13). To understand whether
circRNA-CER is involved in the malignant progression of breast
cancer, the present study conducted CCK-8 and Transwell assays. The
results demonstrated that silencing of circRNA-CER inhibited the
proliferation and migration of MCF-7 cells. In addition, silencing
of circRNA-CER enhanced and decreased the expression levels of
miR-136 and MMP13, respectively. Previously, Liu et al
(12) demonstrated that
circRNA-CER harbors binding sites form iRNAs, including miR-136,
which can bind to the 3′-UTR of MMP13. Furthermore, downregulation
of circRNA-CER by transfection with siRNA reduced MMP13 expression
and increased extracellular matrix formation (12). These findings suggested that there
exists crosstalk between circRNA-CER and the miR-136/MMP13 axis. To
further confirm this, the present study performed proliferation and
migration assays to assess the relationship between circRNA-CER and
miR-136. The results revealed that silencing of miR-136 using
miR-136 inhibitors resulted in an increase in MCF-7 cell
proliferation and migration. The biological effects caused by
miR-136 inhibitors were reversed by co-transfection with
circRNA-CER siRNA, suggesting that circRNA-CER serves as a ‘sponge’
and competitively prevents miR-136 activity.
miRNAs have been demonstrated to serve a crucial
role in regulation of human tumor initiation, development and
metastasis (20). Several miRNAs
are recognized to serve as potential tumor suppressors and their
low expression levels are associated with upregulation of oncogenic
genes in several types of cancer (21). An miRNA microarray expression assay
identified that miR-136 is upregulated in human and murine lung
cancers (22), and miR-136 was
able to target the tumor suppressor phosphatase and tensin homolog
(23), suggesting a potential role
of miR-136 in the development of cancer. However, it is now well
accepted that miR-136 may also function as a tumor suppressor,
since it promotes the apoptosis of glioma cells by targeting
astrocyte elevated gene-1 and B-cell lymphoma 2 (24). In breast cancer, miR-136 is
considered as an anti-invasive miRNA due to its suppressive role in
the mesenchymal migration and metastasis of breast cancer cells.
Furthermore, its expression is downregulated in breast cancer
tissues and is negatively correlated with World Health Organization
grades (25). In accordance with
the above findings on miR-136 expression in breast cancer, the
present study observed that miR-136 was downregulated, whereas its
downstream molecule MMP13 was upregulated, in breast cancer
tissues. MMP13 is a member of the human matrix metalloproteinase
family, which comprises of 26 zinc-dependent transmembrane and
secreted neutral endopeptidases that contribute to the homeostasis
of the extracellular matrix (26).
MMP13 is considered a potential new tumor marker for breast cancer
diagnosis (27), and its elevated
expression is closely associated with decreased overall survival
and lymph node metastasis in breast cancer (28). By contrast, a selective inhibitor
targeting MMP13 delays tumor growth and reduces the severity of
tumor-associated osteolytic lesions in experimental models of
breast cancer, indicating a potential therapeutic role for
MMP13-selective inhibitors in primary breast cancer and
cancer-induced bone osteolysis (29). In conclusion, the present study
demonstrated that circRNA-CER expression was upregulated in breast
cancer tissues compared with adjacent non-tumor tissues, and
provided insight into the potential mechanisms of circRNA-CER in
regulating the activity of the miR-136/MMP13 axis. The results
supported the value of circRNA-CER as a possible molecular target
for the diagnosis and treatment of breast cancer.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Research
Project of the Education Department of Heilongjiang Province (grant
no. 2016-KYYWF-0594).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HS designed the study and contributed to revision of
the manuscript. YQ and PD performed cellular and molecular biology
experiments and wrote the manuscript. MH participated in sample
collection and immunohistochemistry experiments. JX performed
cellular experiments. WX participated in the molecular biology
experiments and data analysis. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Jiamusi University (approval no. JMSU-215) and all
patients provided written informed consent prior to enrolment.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interest.
References
|
1
|
Zuo TT, Zheng RS, Zeng HM, Zhang SW and
Chen WQ: Female breast cancer incidence and mortality in China,
2013. Thorac Cancer. 8:214–218. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zheng R, Zeng H, Zhang S, Chen T and Chen
W: National estimates of cancer prevalence in China, 2011. Cancer
Lett. 370:33–38. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cancer Genome Atlas Network: Comprehensive
molecular portraits of human breast tumours. Nature. 490:61–70.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Perou CM, Sørlie T, Eisen MB, van de Rijn
M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA,
et al: Molecular portraits of human breast tumours. Nature.
406:747–752. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Conn SJ, Pillman KA, Toubia J, Conn VM,
Salmanidis M, Phillips CA, Roslan S, Schreiber AW, Gregory PA and
Goodall GJ: The RNA binding protein quaking regulates formation of
circRNAs. Cell. 160:1125–1134. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Memczak S, Jens M, Elefsinioti A, Torti F,
Krueger J, Rybak A, Maier L, Mackowiak SD, Gregersen LH, Munschauer
M, et al: Circular RNAs are a large class of animal RNAs with
regulatory potency. Nature. 495:333–338. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang Y, Zhang XO, Chen T, Xiang JF, Yin
QF, Xing YH, Zhu S, Yang L and Chen LL: Circular intronic long
noncoding RNAs. Mol Cell. 51:792–806. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jeck WR, Sorrentino JA, Wang K, Slevin MK,
Burd CE, Liu J, Marzluff WF and Sharpless NE: Circular RNAs are
abundant, conserved, and associated with ALU repeats. RNA.
19:141–157. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
You X, Vlatkovic I, Babic A, Will T,
Epstein I, Tushev G, Akbalik G, Wang M, Glock C, Quedenau C, et al:
Neural circular RNAs are derived from synaptic genes and regulated
by development and plasticity. Nat Neurosci. 18:603–610. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Guarnerio J, Bezzi M, Jeong JC, Paffenholz
SV, Berry K, Naldini MM, Lo-Coco F, Tay Y, Beck AH and Pandolfi PP:
Oncogenic Role of Fusion-circRNAs derived from cancer-associated
chromosomal translocations. Cell. 165:289–302. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu Q, Zhang X, Hu X, Dai L, Fu X, Zhang J
and Ao Y: Circular RNA related to the chondrocyte ECM regulates
MMP13 expression by functioning as a miR-136 ‘Sponge’ in human
cartilage degradation. Sci Rep. 6:225722016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yao JT, Zhao SH, Liu QP, Lv MQ, Zhou DX,
Liao ZJ and Nan KJ: Over-expression of circRNA 100876 in non-small
cell lung cancer and its prognostic value. Pathol Res Pract.
213:453–456. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li RK, Zhao WY, Fang F, Zhuang C, Zhang
XX, Yang XM, Jiang SH, Kong FZ, Tu L, Zhang WM, et al: Lysyl
oxidase-like 4 (LOXL4) promotes proliferation and metastasis of
gastric cancer via FAK/Src pathway. J Cancer Res Clin Oncol.
141:269–281. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gao J, Xu W, Wang J, Wang K and Li P: The
role and molecular mechanism of Non-Coding RNAs in pathological
cardiac remodeling. Int J Mol Sci. 18:E6082017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Soreq H: Novel roles of non-coding brain
RNAs in health and disease. Front Mol Neurosci. 7:552014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang Y, Du Y, Liu X and Cho WC:
Involvement of Non-coding RNAs in the signaling pathways of
colorectal cancer. Adv Exp Med Biol. 937:19–51. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hou LD and Zhang J: Circular RNAs: An
emerging type of RNA in cancer. Int J Immunopathol Pharmacol.
30:1–6. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Farazi TA, Spitzer JI, Morozov P and
Tuschl T: miRNAs in human cancer. J Pathol. 223:102–115. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Valastyan S, Reinhardt F, Benaich N,
Calogrias D, Szász AM, Wang ZC, Brock JE, Richardson AL and
Weinberg RA: A pleiotropically acting microRNA, miR-31, inhibits
breast cancer metastasis. Cell. 137:1032–1046. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu X, Sempere LF, Ouyang H, Memoli VA,
Andrew AS, Luo Y, Demidenko E, Korc M, Shi W, Preis M, et al:
MicroRNA-31 functions as an oncogenic microRNA in mouse and human
lung cancer cells by repressing specific tumor suppressors. J Clin
Invest. 120:1298–1309. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee DY, Jeyapalan Z, Fang L, Yang J, Zhang
Y, Yee AY, Li M, Du WW, Shatseva T and Yang BB: Expression of
versican 3′-untranslated region modulates endogenous microRNA
functions. PLoS One. 5:e135992010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang Y, Wu J, Guan H, Cai J, Fang L, Li J
and Li M: miR-136 promotes apoptosis of glioma cells by targeting
AEG-1 and Bcl-2. FEBS Lett. 586:3608–3612. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yan M, Li X, Tong D, Han C, Zhao R, He Y
and Jin X: miR-136 suppresses tumor invasion and metastasis by
targeting RASAL2 in triple-negative breast cancer. Oncol Rep.
36:65–71. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Martin MD and Matrisian LM: The other side
of MMPs: Protective roles in tumor progression. Cancer Metastasis
Rev. 26:717–724. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chang HJ, Yang MJ, Yang YH, Hou MF, Hsueh
EJ and Lin SR: MMP13 is potentially a new tumor marker for breast
cancer diagnosis. Oncol Rep. 22:1119–1127. 2009.PubMed/NCBI
|
|
28
|
Zhang B, Cao X, Liu Y, Cao W, Zhang F,
Zhang S, Li H, Ning L, Fu L, Niu Y, et al: Tumor-derived matrix
metalloproteinase-13 (MMP-13) correlates with poor prognoses of
invasive breast cancer. BMC Cancer. 8:832008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shah M, Huang D, Blick T, Connor A, Reiter
LA, Hardink JR, Lynch CC, Waltham M and Thompson EW: An
MMP13-selective inhibitor delays primary tumor growth and the onset
of tumor-associated osteolytic lesions in experimental models of
breast cancer. PLoS One. 7:e296152012. View Article : Google Scholar : PubMed/NCBI
|