Introduction
Psoriasis is a lifelong disease that severely
reduces the quality of life of patients (1,2).
Recently, psoriasis has been suggested to result from the abnormal
communication between keratinocytes and immunocytes (3–5). It
is also widely accepted that psoriasis has a strong genetic
background and numerous psoriasis susceptibility long noncoding
(lnc) RNAs has been identified (3,6).
Several susceptible genes of psoriasis as detected via genome-wide
association analysis, RNA-sequencing (RNA-seq) or microarray
comprise noncoding DNA and RNA (7–9);
however, little is known regarding the association of specific
lncRNAs in the prevalence of this disease.
LncRNAs are >200 nucleotides and can be expressed
as alternatively spliced variants; however, they cannot encode
proteins (10). LncRNAs include
antisense, intronic, intergenic, pseudogenes and retrotransposon
transcripts (11). In addition,
lncRNAs serve important functional roles in epigenetic,
transcriptional or post-transcriptional regulation by acting on
cis and trans-regulated target genes (12–14),
and form networks of ribonucleoprotein complexes (12). A total of ~1% of the mammalian
genome is expressed as mRNA; however, 70–90% is transcribed into
lncRNA during development (11,15,16).
LncRNAs serve important roles in regulating immune-mediated
inflammatory disorders and autoimmunity (17–19).
Dysregulation of lncRNAs has been studied in numerous immune
diseases, including systemic lupus erythematosus (20), rheumatoid arthritis (21), Crohn's disease, ulcerative colitis
(22), multiple sclerosis
(23) and psoriasis (7,24,25).
This suggests that lncRNAs have crucial roles in the regulation of
immune-mediated disease and contribute to the pathogenesis of
psoriasis by regulating the expression of protein-coding genes, or
by altering chromatin structure (15,26).
LncRNAs are emerging as key molecules in the genesis
and progression of psoriasis. Several studies have been conducted
on the expression and function of lncRNAs in psoriasis; however,
these studies have used RNA sequencing (7,12,27).
The understanding of the exact molecular mechanism is limited; no
studies have performed an lncRNA microarray to investigate their
expression and potential functions.
In the present study, an lncRNA microarray was
performed to determine the expression profiles between psoriasis
tissue and paired control tissue. In addition, reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) was
conducted to detect 10 dysregulated lncRNAs based on the microarray
results. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and
Genomes (KEGG) pathway analyses were conducted to study the
potential function of the differentially expressed genes. A
co-expression network analysis was also performed to study
molecular interactions; cis- and trans-regulated
targets of lncRNAs were predicted. The results of the present study
revealed that dysregulation of lncRNAs and mRNAs may contribute to
the pathogenesis of psoriasis. Furthermore, lncRNAs may be used as
potential biomarkers and therapeutic targets for the treatment of
psoriasis.
Materials and methods
Tissues
A total of 15 psoriasis specimens were collected
from patients with psoriasis (7 males and 8 females; aged
23–39-years-old) from Qilu Hospital of Shandong University
(September 2016 to August 2017). Patients did not receive systemic
drugs, phototherapy or externally applied drugs during the last 3
months prior to sample collection. All tissues were obtained from
the trunks; half of each tissue sample was frozen immediately at
−80°C. The remaining tissue was embedded in paraffin for
pathological examination. In addition, 15 normal tissues were
collected from healthy volunteers (8 males and 7 females; aged
25–40-years-old) and tissue specimens were stored at −80°C; three
samples of the psoriasis and normal tissues were used for
microarray analysis, and the remaining 12 pairs of tissue were used
for RT-qPCR. The present study was approved by the Ethics Committee
of Shandong University, Qilu Hospital (Jinan, China). All patients
enrolled in the present study provided written informed
consent.
LncRNA and mRNA microarray
The microarray [SBC Human (4×180K) competing
endogenous (ce)RNA microarray; Shanghai Biotechnology Corporation,
Shanghai, China] used in the present study could detect 68,432
lncRNAs and 18,853 mRNAs sourced from the most authoritative
databases, including University of California Santa Cruz
(https://genome.ucsc.edu/), GENCODE v21/Ensembl
(https://www.gencodegenes.org/human/release_21.html),
LNCipedia v3.1 (28), Noncode v4
(29) and Lncrnadb v2.0 (30,31).
The lncRNA and mRNA probes were designed according to the latest
genome version [human/GRCh38 (hg38)].
RNA extraction
Total RNAs from skin samples were extracted using an
RNeasy mini kit (cat. no. 74106; Qiagen GmBH, Hilden, Germany)
according to the manufacturer's protocols. Following this, RNA
integration was investigated using an Agilent 2100 Bioanalyzer
(Agilent Technologies, Inc., Santa Clara, CA, USA).
RNA labelling
Total RNAs from skin samples were amplified and
labeled using a Low Input Quick Amp WT Labeling kit (cat. no.
5190-2943; Agilent Technologies, Inc.) according to the
manufacturer's protocols. Furthermore, a RNeasy mini kit (cat. no.
74106; Qiagen GmBH) was used to purify the labelled cRNAs according
to the manufacturer's protocols.
Cy3-labelled hybridization
The RNA of each skin sample in the present study was
hybridized with 1.65 µg Cy3-labelled cRNA using a commercialized
Gene Expression Hybridization kit (cat. no. 5188-5242; Agilent
Technologies, Inc.) according to the manufacturer's protocols.
Following this, the slides were washed with a Gene Expression Wash
Buffer kit (cat. no. 5188-5327; Agilent Technologies, Inc.) 17 h
later.
Data analysis
Each slide was scanned using an Agilent Microarray
Scanner (Dye channel, Green; Scan resolution=3 µm; photoelectric
multi-plication tube 100%, 20 bit; Agilent Technologies, Inc). The
experimental data were collected using Feature Extraction software
10.7 (Agilent Technologies, Inc.), which were then normalized using
the limma packages in R (version 3.8) (32).
Function analysis of dysregulated
genes
GO analysis was performed to detect the functions of
the dysregulated genes. The KEGG database (https://www.genome.jp/kegg/) was used to investigate
the functions of these dysregulated genes in the pathways.
Target prediction of dysregulated
lncRNAs
As previously described (33), two methods were used to identify
target mRNAs of dysregulated lncRNAs. Firstly, cis-acting target
mRNAs were searched. Cis-target genes were considered as
transcribed within a 10 kbp window upstream or downstream of
lncRNAs. Furthermore, target genes were identified according to the
sequence of mRNA and RNA duplex energy prediction. BLAST
(https://blast.ncbi.nlm.nih.gov/Blast.cgi) was used in
the present study for screening and parameters were set (e-value,
1e-20; identity, ≥95%). RNAplex software (https://omictools.com/rnaplex-tool) and starBase v3.0
(http://starbase.sysu.edu.cn/) were also
used in the present study to screen trans-regulated predicted
target mRNAs and microRNAs, respectively (e≤-30) (34).
Weighted gene co-expression network
analysis
Weighted gene co-expression network analysis (WGCNA)
was performed as described in previous studies (35,36).
RT-qPCR
Total RNA extracted from skin samples was reverse
transcribed using a ReverTra Ace qPCR kit (cat. no. FSQ-101; Toyobo
Life Science, Osaka, Japan) according to the manufacturer's
protocols. The expression levels of 10 dysregulated lncRNAs in 12
psoriasis and normal control tissue samples were detected by
RT-qPCR using Power SYBR-Green PCR Master Mix (cat. no. 4368708) on
the 7900 HT Sequence Detection System (both Applied Biosystems;
Thermo Fisher Scientific, Inc., Waltham, MA, USA), according to the
manufacturer's protocols. The thermocycling conditions were as
follows: Stage 1, 50°C for 2 min; stage 2, 95°C for 10 min; stage 3
(40 cycle) 95°C for 15 sec, 60°C for 1 min, and stage 4, 95°C for
15 sec, 60°C for 15 sec and 95°C for 15 sec. GAPDH was used as an
internal control. The primers employed in the present study are
listed in Table I. All the
experiments were repeated three times (37).
 | Table I.Primers used for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primers used for reverse
transcription-quantitative polymerase chain reaction.
| LncRNA, mRNA or
gene | Forward primer
(5′-3′) | Reverse primer
(5′-3′) |
|---|
|
ENST00000547006 |
CATTGTTGCTTTTCGGGTCT |
GGCCACGTAGCAATGACTGT |
| lnc-HSFY2-10:1 |
ACCCACGCAGATTTATTCCA |
CCTTGGTGGTGGCATTAGTT |
| lnc-PERP-2:7 |
CCATTTTTCTGTGGTCAGGTC |
GCTGTTGAAGAGCACTGTGAG |
|
ENST00000557691 |
CATGCTGAGTCTGCAAGGAC |
CAAAAACCCCGATGATAGGA |
| lnc-THRSP-6:1 |
GTTCCATTGATCCAGCCACT |
ACAAGAGGCCACTGACTGCT |
| NONHSAT066260 |
CACTGCACTTGGCTGTGATT |
GGCCGAGAAGCCTAGAAGAA |
| lnc-MGMT-2:1 |
GGAAAAACACAGCAGCCAGT |
ACGCCAACACCCTGTAGACT |
|
lnc-AP000769.1-1:2 |
CACCAGCTTCTCCAATCTTCCT |
GGAGTAAGCAAGTCACAATGTTGAG |
| lnc-POLR3E-3:3 |
TACTGAGAATGGGGAAGGAAAC |
CTCTTGCTTACTGCCTGCTGA |
| lnc-PXDNL-4:1 |
CAGGTCAGGCTGTTGGATTCT |
AGGATGTGCTTCTTGGGAGTC |
| GAPDH |
TGACTTCAACAGCGACACCCA |
CACCCTGTTGCTGTAGCCAAA |
Statistical analyses
Data in the present study were presented as the mean
± standard deviation from three independent experiments.
Differences between groups were analyzed using Student's t-test and
Benjamini-Hochberg correction with SPSS 17.0 analysis software
(SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Dysregulated lncRNAs
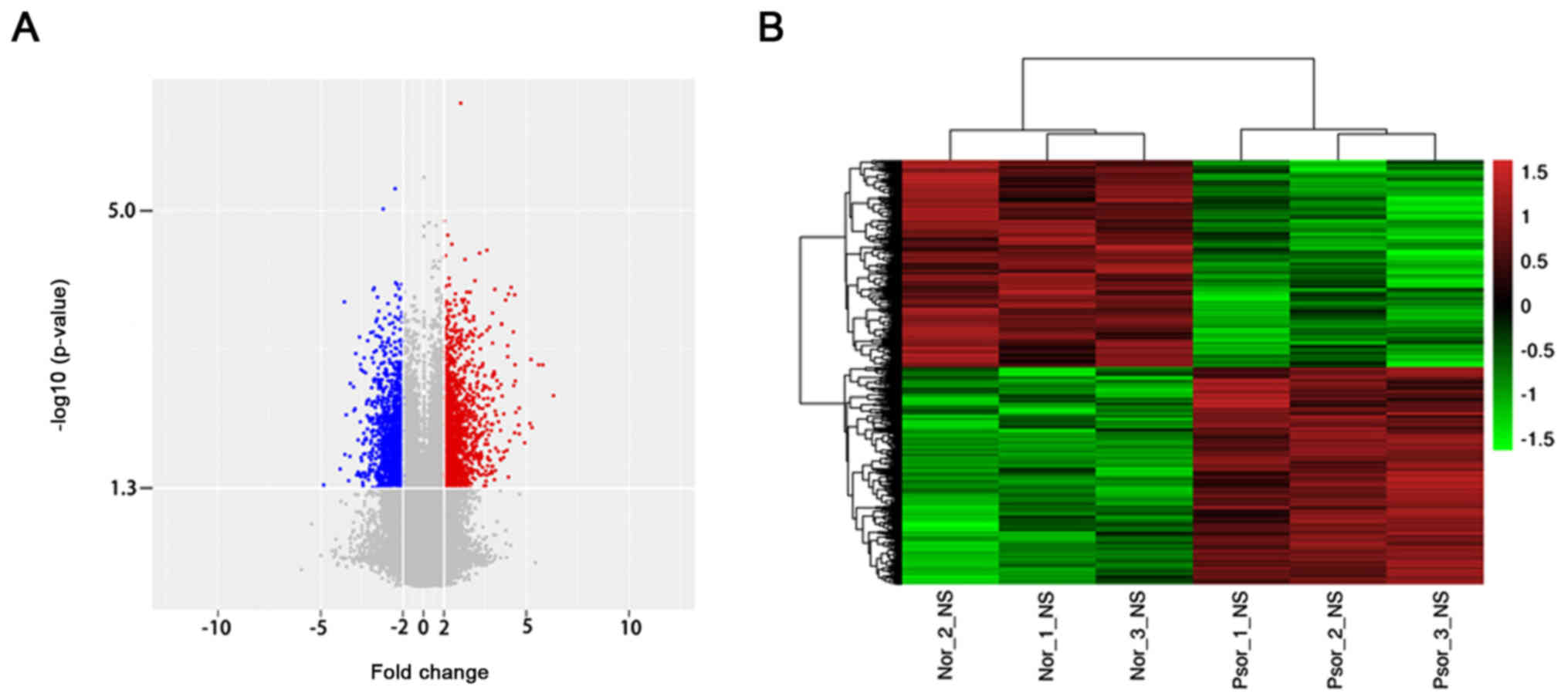
To investigate the dysregulated lncRNAs in
psoriasis, an lncRNA microarray analysis was performed using
psoriasis lesions and normal tissue. According to the lncRNA
microarray results, 2,194 lncRNAs were dysregulated (≥2.0 fold,
P<0.05). Among these lncRNAs, 1,123 lncRNAs were upregulated and
1,071 lncRNAs were downregulated (Fig.
1A). Hierarchical clustering analysis was used to arrange
specimens in the present study according to the level of expression
(Fig. 1B). The top 10 upregulated
and downregulated lncRNAs of the microarray results were presented
in Table II. Among these,
lnc-SLC6A14-1:1 (79.87306773-fold) was the most overexpressed
lncRNA and NONHSAT044111 (29.0815707-fold) was the most
downregulated lncRNA. The results of microarray analysis revealed
that the number of upregulated lncRNAs was greater than that of
downregulated lncRNAs.
 | Table II.Top 10 differentially expressed
lncRNAs in psoriasis tissues compared with normal control. |
Table II.
Top 10 differentially expressed
lncRNAs in psoriasis tissues compared with normal control.
| A, Upregulated
lncRNAs |
|---|
|
|---|
| LncRNA | P-value | Fold change | Source |
|---|
|
lnc-SLC6A14-1:1 | 0.00292 | 79.87 | LNCipedia |
| NR_003062 | 0.00114 | 55.64 | RefSeq |
|
lnc-SERPINB3-4:1 | 0.00113 | 48.26 | LNCipedia |
| NONHSAT006518 | 0.00775 | 38.64 | NONCODE |
| lnc-IGFL3-6:1 | 0.00096 | 37.3 | LNCipedia |
|
ENST00000472053 | 0.00687 | 36.05 | ENSEMBL |
| NONHSAT006509 | 0.01240 | 30.2 | NONCODE |
| NR_030617 | 0.00942 | 25.21 | RefSeq |
|
lnc-RAPGEF2-3:1 | 0.00427 | 25.13 | LNCipedia |
| lnc-RPP40-3:3 | 0.00719 | 24.61 | LNCipedia |
|
| B, Downregulated
lncRNAs |
|
| LncRNA | P-value | Fold
change | Source |
|
| NONHSAT044111 | 0.0344 | 29.08 | NONCODE |
| lnc-PPM1N-1:1 | 0.0600 | 16.66 | LNCipedia |
| lnc-GRHL2-11:1 | 0.0692 | 14.45 | LNCipedia |
|
lnc-JAKMIP2-1:1 | 0.0704 | 14.21 | LNCipedia |
| lnc-GGTLC1-2:1 | 0.0734 | 13.62 | LNCipedia |
| NONHSAT025181 | 0.0799 | 12.52 | NONCODE |
|
ENST00000447257 | 0.0832 | 12.01 | ENSEMBL |
|
ENST00000623414 | 0.0843 | 11.863 | ENSEMBL |
|
ENST00000415656 | 0.0943 | 10.61 | ENSEMBL |
|
lnc-LINC00273-22:1 | 0.0959 | 10.43 | LNCipedia |
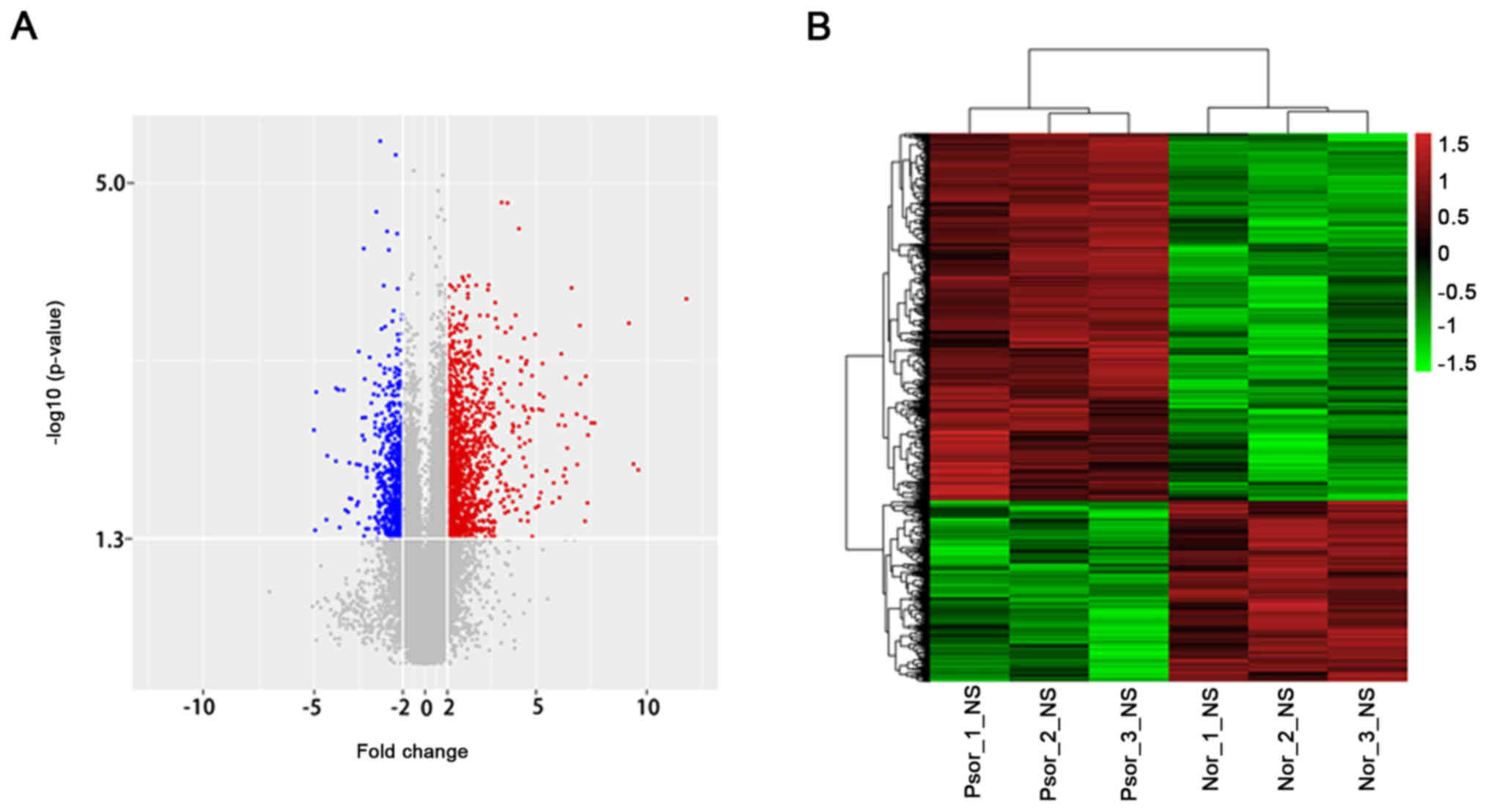
Dysregulated mRNAs
Based on the microarray results, 1,725 dysregulated
mRNAs were identified. Among these, 1,157 mRNAs were overexpressed
and 568 mRNAs were downregulated (Fig.
2). The top 10 upregulated and downregulated mRNAs were
indicated (Table III). DEFB4A
(3,505.251346 fold) was the most overexpressed lncRNA and WIF1
(32.27749933 fold) was the most downregulated lncRNA. The
microarray results suggested that that there was a greater number
of upregulated mRNAs.
 | Table III.Top 10 differentially expressed mRNAs
in psoriasis tissues compared with normal control. |
Table III.
Top 10 differentially expressed mRNAs
in psoriasis tissues compared with normal control.
| A, Upregulated
mRNAs |
|---|
|
|---|
| mRNA | P-value | Fold change | Source |
|---|
| DEFB4A | 0.00016 | 3505.25 | RefSeq |
| SERPINB4 | 0.00967 | 780.42 | RefSeq |
| S100A7A | 0.00840 | 672.76 | RefSeq |
| S100A12 | 0.00029 | 582.68 | RefSeq |
| PI3 | 0.00315 | 199.59 | RefSeq |
| SERPINB3 | 0.00314 | 180.61 | RefSeq |
| SPRR2G | 0.00420 | 162.24 | RefSeq |
| S100A9 | 0.02130 | 159.63 | RefSeq |
| LCE3A | 0.00102 | 152.00 | RefSeq |
| IL36A | 0.00276 | 149.66 | RefSeq |
|
| B, Downregulated
mRNAs |
|
| mRNA | P-value | Fold
change | Source |
|
| WIF1 | 0.0310 | 32.28 | RefSeq |
| MT4 | 0.0322 | 31.05 | RefSeq |
| BTC | 0.0333 | 30.05 | RefSeq |
| CACNA1H | 0.0457 | 21.87 | RefSeq |
| CYP2W1 | 0.0470 | 21.29 | RefSeq |
| KRT77 | 0.0615 | 16.27 | RefSeq |
| FAM166B | 0.0617 | 16.20 | RefSeq |
| NEUROD2 | 0.0671 | 14.90 | RefSeq |
| SPINK1 | 0.0693 | 32.28 | RefSeq |
| ZBTB16 | 0.0780 | 31.05 | RefSeq |
Function analysis of dysregulated
mRNAs
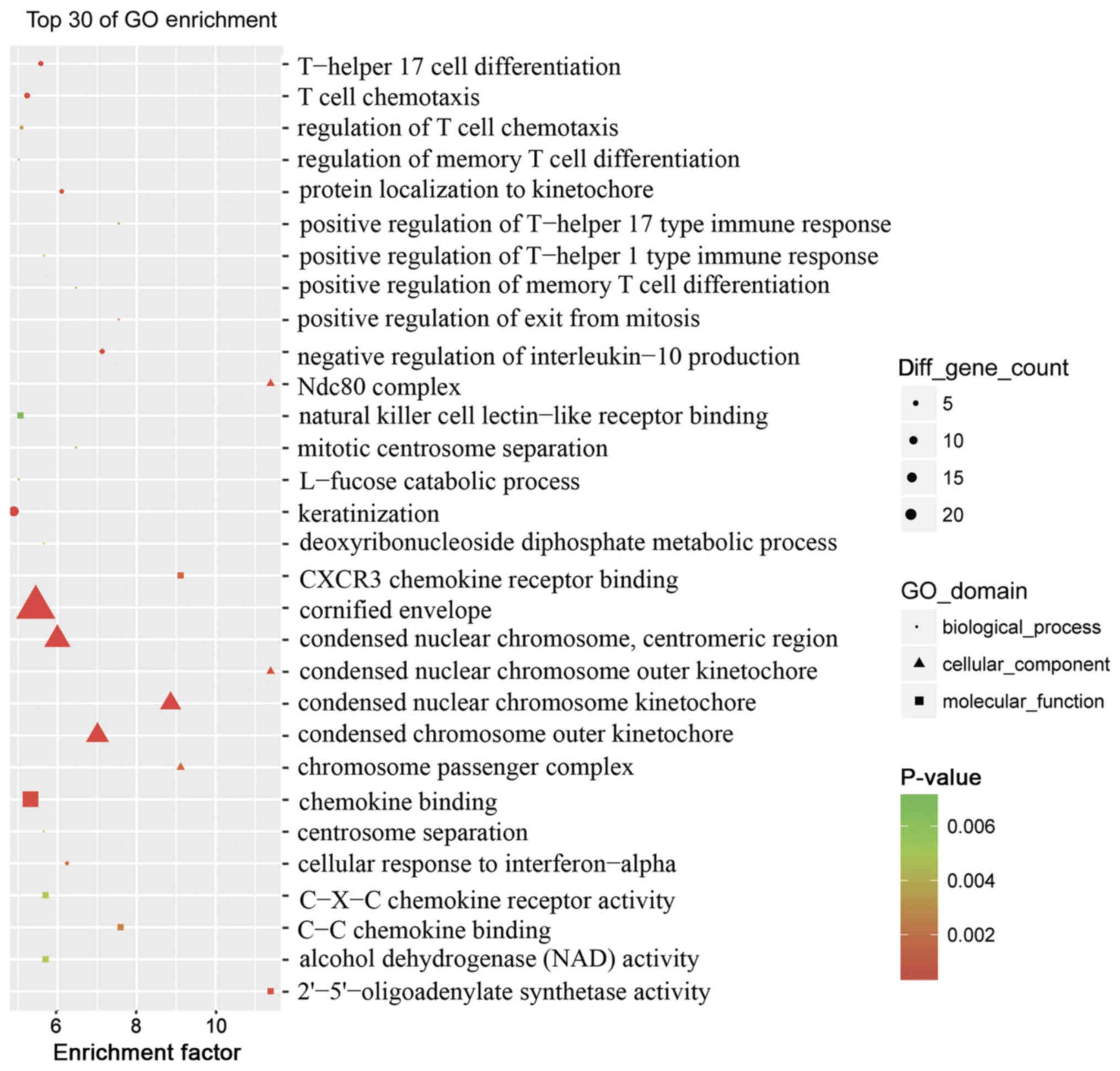
To assess the pathogenesis of psoriasis, the top 30
GO terms between the psoriasis and control groups were obtained via
enrichment analysis (Fig. 3). The
association of dysregulated mRNAs with ‘molecular function’,
‘biological processes’ and ‘cellular component’ in the GO database
was assessed. Numerous dysregulated epigenetic and genetic genes
associated with ‘T-helper 17 cell differentiation’, ‘T-cell
chemotaxis’, ‘regulation of T-cell chemotaxis’, ‘keratinization’,
‘positive regulation of T-helper 17’ and ‘T-helper 1 type immune
response’ were identified. This suggested that a variety of
specific genes were involved in the pathogenesis of psoriasis,
particularly genes associated with pro-inflammatory cells and
molecules.
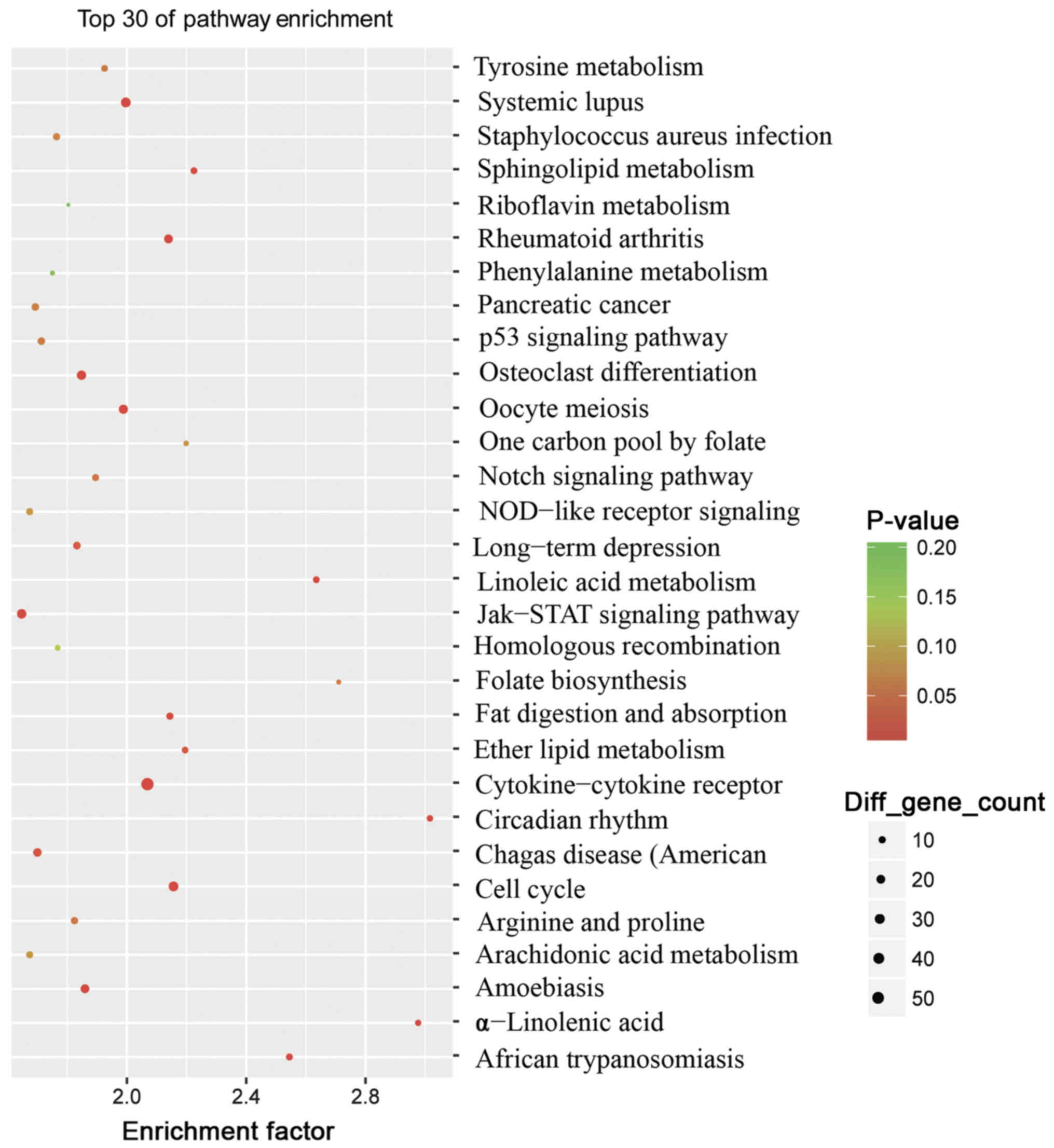
To further investigate the pathogenesis of
psoriasis, analysis of the top 30 KEGG pathways between the
psoriasis and control groups was performed (Fig. 4). The results revealed that 23
pathways were identified to be significantly enriched (P<0.05).
The majority of these pathways were associated with inflammation
and lipid metabolism, including the ‘Janus kinase (Jak)-signal
transducers and activators of transcription (STAT)’ signaling
pathway (associated with 26 genes), the ‘cytokine-cytokine
receptor’ interaction signaling pathway (associated with 56 genes),
the ‘cell cycle’ signaling pathway (associated with 28 genes), the
‘Toll-like receptor’ signaling pathway (associated with 17 genes),
the ‘Wnt’ signaling pathway (associated with 24 genes) and the
‘chemokine’ signaling pathway (associated with 30 genes) (data not
shown). These findings also reinforced the fact that psoriasis is a
chronic, immune-mediated disorder.
Potential targets and function of the
dysregulated lncRNAs
To investigate whether the differentially expressed
lncRNAs regulate genes and determine the signaling pathways
associated with psoriasis, target prediction programs were used to
predict the potential targets of the dysregulated lncRNAs (data not
shown). It was revealed that 1,549 dysregulated lncRNAs had
predicted target genes in the present study. Among these, 1,447
dysregulated lncRNAs had cis-regulated target genes, 397
dysregulated lncRNAs had trans-regulated target genes and 295
dysregulated lncRNAs had trans-and cis-regulated target genes.
Notably, 3,740 mRNAs may be regulated by the
dysregulated lncRNAs. Among these, 1,460 mRNAs may be
cis-regulated, 2,588 mRNAs may be trans-regulated,
and 308 mRNAs may be cis-and trans-regulated. Upon
further analysis and integration with the profile of differentially
expressed lncRNAs, a total of 489 mRNAs were dysregulated.
Furthermore, the results suggested that these mRNAs may be
regulated by dysregulated cis- or trans-acting
lncRNAs. Among these, 289 dysregulated mRNAs could be regulated by
dysregulated cis-acting lncRNAs and 262 dysregulated mRNAs
could be regulated by dysregulated trans-acting lncRNAs.
These results indicated that the dysregulated
lncRNAs were involved in the onset of psoriasis by regulating their
potential target mRNAs and its associated signaling pathways.
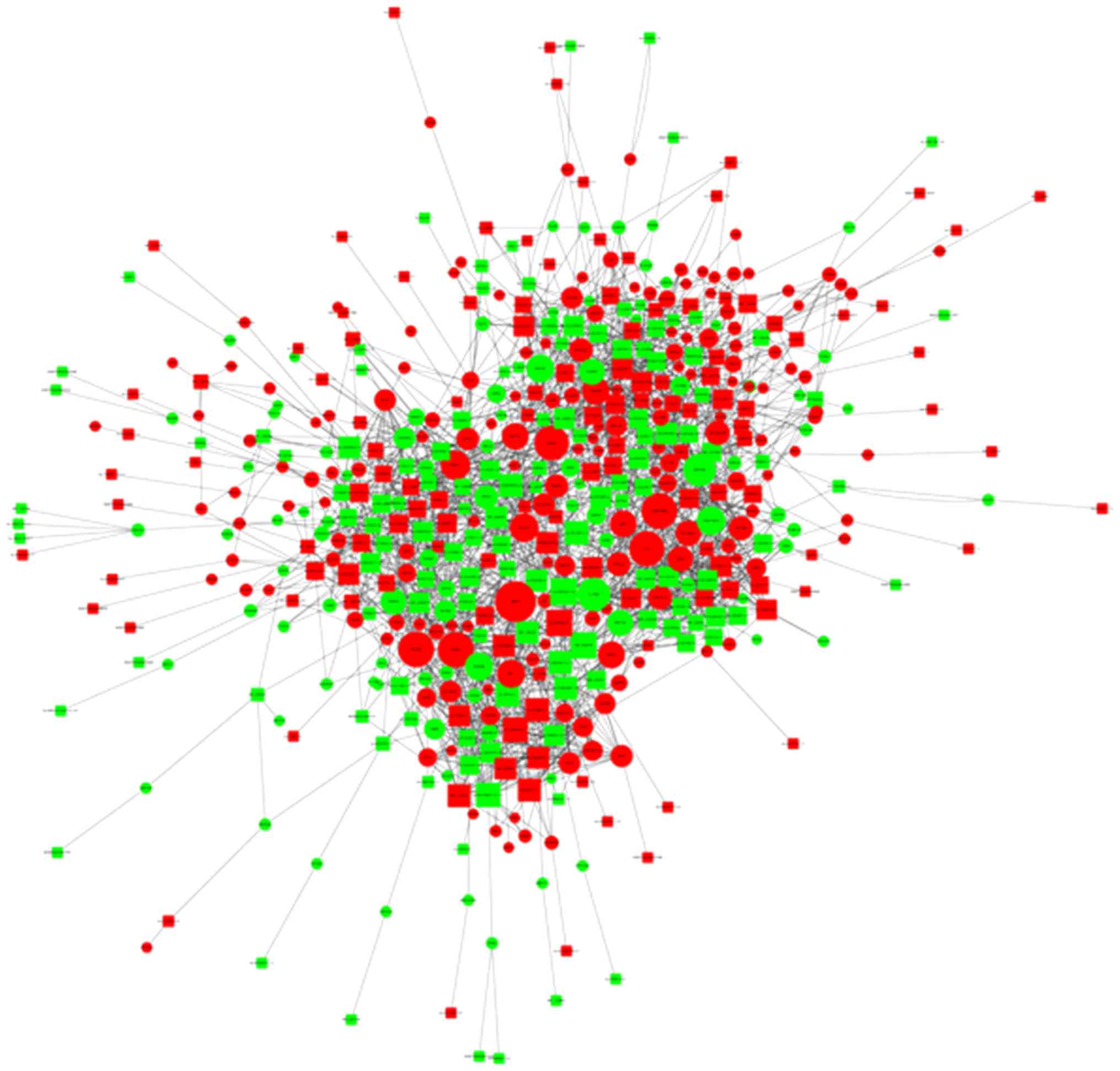
Co-expression network of lncRNAs and
mRNAs
Aided by the microarray data and a weighted gene
co-expression network analysis approach, numerous networks of
coordinately expressed genes were identified in psoriasis tissues
compared with the normal control tissues (Fig. 5). The co-expression results in the
present study strongly support a network model in which lncRNAs and
mRNAs function together in psoriasis. Furthermore, the findings
suggested that lncRNAs do not act alone but rather work in concert
with other lncRNAs and mRNAs.
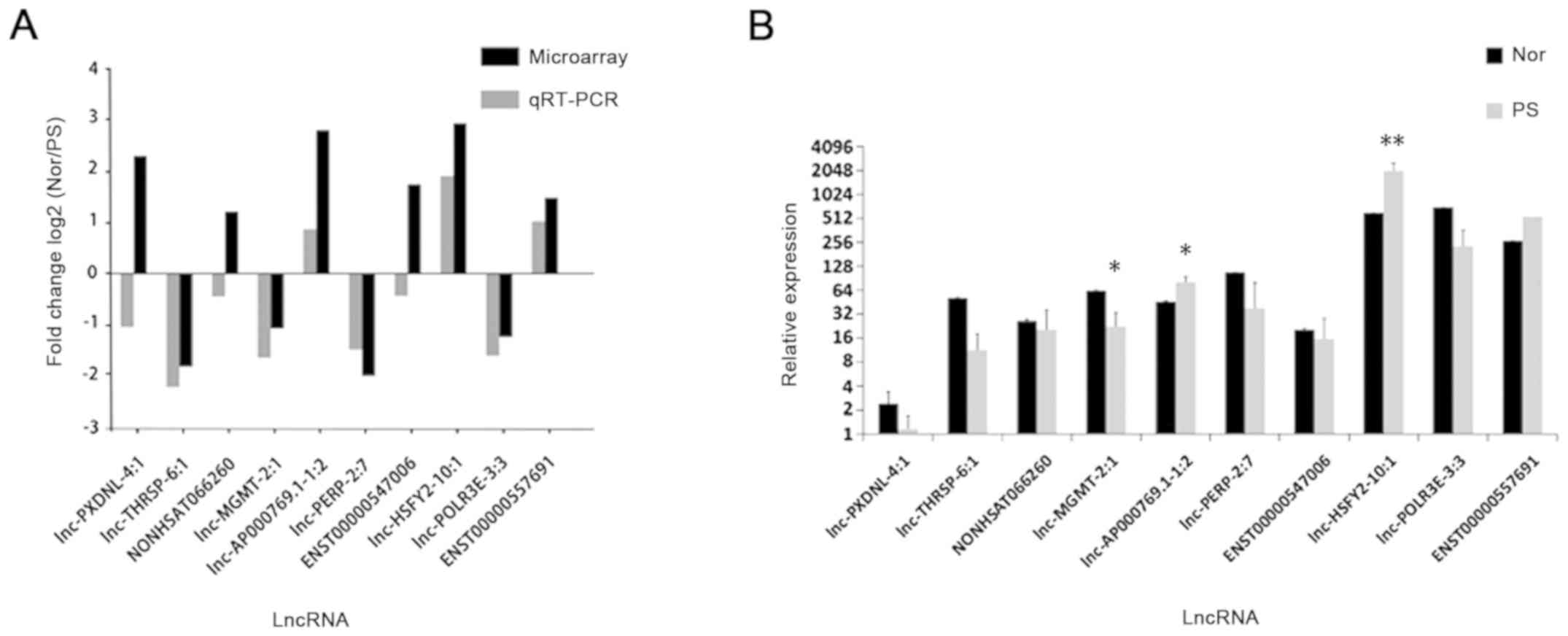
RT-qPCR analysis
In order to detect the lncRNA and mRNA microarray
results, 10 dysregulated lncRNAs were randomly selected for RT-qPCR
analysis. Based on the results of the microarray data, 6
upregulated lncRNAs (lnc-AP000769.1–1:2, NONHSAT066260,
lnc-PXDNL-4:1, ENST00000557691, lnc-HSFY2-10:1 and ENST00000547006)
and 4 downregulated lncRNAs (lnc-MGMT-2:1, lnc-POLR3E-3:3,
lnc-THRSP-6:1 and lnc-PERP-2:7) were selected.
The expression levels of dysregulated lncRNAs were
validated by RT-qPCR (n=12). The expression levels of 7 lncRNAs
were consistent with the microarray results, which exhibited the
same dysregulation profiles (up-/down-regulated, ≥2.0 fold)
(Fig. 6A). Of note, 3 lncRNAs
(lnc-AP000769.1-1:2, ENST00000557691 and lnc-HSFY2-10:1) were
upregulated and 4 lncRNAs (lnc-MGMT-2:1, lnc-POLR3E-3:3,
lnc-THRSP-6:1 and lnc-PERP-2:7) were downregulated (≥2.0 fold)
(Fig. 6A). The expression levels
of lnc-MGMT-2:1 (P<0.05), lnc-AP000769.1-1:2 (P<0.05) and
lnc-HSFY2-10:1 (P<0.01) were significantly different between the
psoriasis and normal tissues (Fig.
6B); however, lnc-PXDNL-4:1 was observed to be downregulated,
and the expression levels of NONHSAT066260 and ENST00000547006 in
psoriasis and normal tissues exhibited 0.5<fold change<2
(Fig. 6B).
Discussion
It is well known that psoriasis has a strong genetic
predisposition (38–40); however, the underlying genetic
mechanisms remain unknown. Previous transcriptomic studies in
psoriasis have analyzed the expression levels of mRNA to
investigate the functions of their translated proteins, including
protein-coding genes associated with the interleukin-17 signaling
pathway (41), the nuclear factor
(NF)-κB signaling pathway (42,43),
the Jak-STAT signaling pathway (44) and the mitogen-activated protein
kinase (MAPK) signaling pathway (45,46).
LncRNAs were once considered as transcriptional
noise or junk but have recently begun to attract attention in
research (15,47). LncRNAs serve important roles in
regulating various functions of the immune system (18,48,49).
Increasing evidence suggests lncRNA dysregulation may have a vital
role in the pathogenesis of psoriasis (8,25,50).
At present, few lncRNAs associated with psoriasis have been
investigated. Psoriasis associated non-protein coding RNA induced
by stress (PRINS), an lncRNA gene, exhibited the highest expression
levels in non-psoriatic skin lesions, indicating that it may serve
an important role in the susceptibility of psoriasis (51). Széll et al (25) reported that overexpression of PRINS
in non-lesional psoriatic skin alters the stress response and
contributes to disease pathogenesis. Furthermore, Szegedi et
al (52) reported that the
dysregulation of PRINS contributed to the onset of psoriasis,
resulting in decreased keratinocyte apoptosis. Another lncRNA gene
associated with psoriasis, psoriasis susceptibility 1 candidate 3,
has been considered to be strongly associated with psoriasis
(53). Using RNA-seq, Kretz et
al (54) reported terminal
differentiation-induced lncRNA, which controls human epidermal
differentiation via posttranscriptional regulation. Therefore,
similar to mRNAs, lncRNAs may function as proinflammatory or
inducers of proliferation in psoriasis by regulating
transcriptional regulation or altering the expression levels of
their target mRNAs.
To the best of our knowledge, the present study is
the first to determine lncRNA and mRNA expression profiles in
psoriasis compared with normal healthy volunteers using a
microarray. GO and KEGG pathway analyses were conducted to
determine the potential functions and co-expression networks of
lncRNAs to study the molecular interactions.
To validate the microarray results and predict the
possible role of lncRNAs in psoriasis, 10 dysregulated lncRNAs were
randomly selected for RT-qPCR analysis. The results of RT-qPCR were
consistent with the data of microarray results: 3 lncRNAs
(lnc-AP000769.1-1:2, ENST00000557691 and lnc-HSFY2-10:1) were
upregulated and 4 lncRNAs (lnc-MGMT-2:1, lnc-POLR3E-3:3,
lnc-THRSP-6:1 and lnc-PERP-2:7) were downregulated. By predicting
cis- and trans-acting lncRNAs, it was revealed that
lnc-AP000769.1-1:2 (upregulated) and lnc-MGMT-2:1 (downregulated)
have a common predicted target gene, jak3, which suggested
they may be involved in the pathogenesis of psoriasis by regulating
jak3. ENST00000557691 (upregulated) and lnc-PERP-2:7
(downregulated) may regulate the MAPK signaling pathway by
regulating their predicted target gene, mapk kinase kinase
9. Lnc-HSFY2-10:1 (upregulated) was predicted to regulate
microRNA-145 (starBase v3.0). Of note, it has been reported that
miR-145 negatively regulates cell proliferation and proinflammatory
cytokine release, and inhibits the downstream genes of NF-κB and
mechanistic target of rapamycin (mTOR) (55,56).
Thus, lnc-HSFY2-10:1 may be involved in the pathogenesis of
psoriasis via ceRNA regulation. Lnc-POLR3E-3:3 (downregulated),
which possibly regulates interferon regulatory factor 1
(irf1) in trans, may be involved in regulating T
regulatory (Treg) cell differentiation, as irf1 has been
considered as a regulator of Treg cells by inhibiting Forkhead box
P3 in mice (57). Lnc-THRSP-6:1,
which has been predicted to potentially regulate C-C motif
chemokine ligand 5 (ccl5) (data not shown) via RNAplex
software, was downregulated in the present study. This suggested
that the predicted target gene proinflammation chemokine,
ccl5, may be overexpressed and that lnc-THRSP-6:1
contributes to the pathogenesis of psoriasis. However, the
expression levels of lnc-PXDNL-4:1, as determined by RT-qPCR, were
not in agreement with the results of microarray analysis in the
present study. The lncRNA and mRNA probes were designed according
to the latest genome version [human/GRCh38 (hg38)] and the primers
were carefully selected; thus, this discrepancy may be due to the
small sample size (n=12). This suggests that further investigation
using a larger sample is required. In addition, the expression
levels of NONHSAT066260 and ENST00000547006 were not significantly
different, which indicated that they may not be involved in the
onset of psoriasis.
The results microarray and RT-qPCR analyses
suggested that lncRNAs were involved in the pathogenesis of
psoriasis, possibly by regulating the differentiation and function
of Treg cells, the NF-κΒ signaling pathway, the mTOR signaling
pathway and the MAPK signaling pathway, the release of cytokines
and chemokines, and the JAK-STAT signaling pathway.
GO and KEGG analyses were performed to investigate
the biological functions that were enriched among the dysregulated
mRNAs. GO analysis identified ‘T-helper 17 cell differentiation’,
‘T-cell chemotaxis’ and the ‘T-helper 17 type immune response’ to
be involved in the development of psoriasis. In addition, pathway
analysis identified several pathways associated with psoriasis,
including the ‘JAK-STAT’ signaling pathway, the ‘cytokine-cytokine’
receptor interaction signaling pathway, the ‘cell cycle’ signaling
pathway, the ‘Wnt’ signaling pathway and the ‘MAPK’ signaling
pathway. These results further supported the data obtained from the
microarray analysis.
Co-expression analyses emphasized the importance of
integrating the expression profiles of lncRNA and mRNA to obtain
more generalized information. Genes with strong associations are
more likely to be co-regulated with their co-expressed genes
(58). The results of the
co-expression analyses indicated that the dysregulated lncRNA may
collaboratively function the pathogenesis of psoriasis.
In addition, it is well reported that the detection
of lncRNAs on the array platform is more stable than RNA-seq
(59,60). Therefore, the study of lncRNA and
mRNA microarray expression in psoriasis is valuable and may improve
the understanding of the pathogenesis of psoriasis.
In conclusion, to the best of our knowledge, the
present study is the first to conduct a microarray to investigate
lncRNA expression in psoriasis. The findings also revealed that
numerous lncRNAs were dysregulated in psoriasis; several networks
of mRNA and lncRNA were associated with the pathogenesis of
psoriasis, including pathways that have not been previously
identified. RT-qPCR, target prediction and co-expression analyses
revealed that the lncRNAs did not act alone, but rather functioned
in concert with other lncRNAs, which may facilitate the development
of psoriasis. Despite the limitations of the present study,
including the small sample size, and that no functional or
mechanical investigations using the biopsies were conducted, the
results may provide insight for further into the development and
treatment of psoriasis.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81573046), and the
Science and Technology Research Project of Shandong (grant no.
2014GSF118125).
Availability of data and materials
The datasets generated and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
JY and JS made substantial contributions to the
design and experiments of the present study and should be regarded
as co-first authors. MQ, RL and XZ made substantial contributions
in analysing and interpreting patient data. JJ and QS made
substantial contributions to the clinical diagnosis and
histological examination during the experiment. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Shandong University, Qilu Hospital. All patients
enrolled in the present study provided written informed
consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Yan S, Xu Z, Lou F, Zhang L, Ke F, Bai J,
Liu Z, Liu J, Wang H, Zhu H, et al: NF-κB-induced microRNA-31
promotes epidermal hyperplasia by repressing protein phosphatase 6
in psoriasis. Nat Commun. 6:76522015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nestle FO, Kaplan DH and Barker J:
Psoriasis. N Engl J Med. 361:496–509. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Srivastava A, Nikamo P, Lohcharoenkal W,
Li D, Meisgen F, Xu Landén N, Ståhle M, Pivarcsi A and Sonkoly E:
MicroRNA-146a suppresses IL-17-mediated skin inflammation and is
genetically associated with psoriasis. J Allergy Clin Immun.
139:550–561. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lowes MA, Suárez-Fariñas M and Krueger JG:
Immunology of psoriasis. Annu Rev Immunol. 32:227–255. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nestle FO, Di Meglio P, Qin JZ and
Nickoloff BJ: Skin immune sentinels in health and disease. Nat Rev
Immunol. 9:679–691. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Harden JL, Krueger JG and Bowcock AM: The
immunogenetics of Psoriasis: A comprehensive review. J Autoimmun.
64:66–73. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gupta R, Ahn R, Lai K, Mullins E, Debbaneh
M, Dimon M, Arron S and Liao W: Landscape of long noncoding RNAs in
psoriatic and healthy skin. J Invest Dermatol. 136:603–609. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tsoi LC, Iyer MK, Stuart PE, Swindell WR,
Gudjonsson JE, Tejasvi T, Sarkar MK, Li B, Ding J, Voorhees JJ, et
al: Analysis of long non-coding RNAs highlights tissue-specific
expression patterns and epigenetic profiles in normal and psoriatic
skin. Genome Biol. 16:242015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yan JJ, Qiao M, Li RH, Zhao XT, Wang XY
and Sun Q: Downregulation of miR-145-5p contributes to
hyperproliferation of keratinocytes and skin inflammation in
psoriasis. Br J Dermatol. 180:365–372. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hrdlickova B, Kumar V, Kanduri K,
Zhernakova DV, Tripathi S, Karjalainen J, Lund RJ, Li Y, Ullah U,
Modderman R, et al: Expression profiles of long non-coding RNAs
located in autoimmune disease-associated regions reveal immune
cell-type specificity. Genome Med. 6:882014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee JT: Epigenetic regulation by long
noncoding RNAs. Science. 338:1435–1439. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tsoi LC, Iyer MK, Stuart PE, Swindell WR,
Gudjonsson JE, Tejasvi T, Sarkar MK, Li BS, Ding J, Voorhees JJ, et
al: Analysis of long non-coding RNAs highlights tissue-specific
expression patterns and epigenetic profiles in normal and psoriatic
skin. Genome Biol. 16:242015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pacifici M, Kadri F, Jeansonne D and
Peruzzi F: Tat-mediated changes of malat1 long non-coding Rna
affects the structure and function of Sc35 nuclear speckles domains
in neurons. J Neuroimmune Pharmacol. 8:427–428. 2013.
|
|
14
|
Ponting CP, Oliver PL and Reik W:
Evolution and functions of long noncoding RNAs. Cell. 136:629–641.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lan X, Zhang H, Wang Z, Dong W, Sun W,
Shao L, Zhang T and Zhang D: Genome-wide analysis of long noncoding
RNA expression profile in papillary thyroid carcinoma. Gene.
569:109–117. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Eddy SR: Non-coding RNA genes and the
modern RNA world. Nat Rev Genet. 2:919–929. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu GC, Pan HF, Leng RX, Wang DG, Li XP, Li
XM and Ye DQ: Emerging role of long noncoding RNAs in autoimmune
diseases. Autoimmun Rev. 14:798–805. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Heward JA and Lindsay MA: Long non-coding
RNAs in the regulation of the immune response. Trends Immunol.
35:408–419. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sigdel KR, Cheng A, Wang Y, Duan L and
Zhang Y: The emerging functions of long noncoding RNA in immune
cells: Autoimmune diseases. J Immunol Res. 2015:8487902015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wu Y, Zhang F, Ma J, Zhang X, Wu L, Qu B,
Xia S, Chen S, Tang Y and Shen N: Association of large intergenic
noncoding RNA expression with disease activity and organ damage in
systemic lupus erythematosus. Arthritis Res Ther. 17:1312015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Messemaker TC, Frank-Bertoncelj M, Marques
RB, Adriaans A, Bakker AM, Daha N, Gay S, Huizinga TW, Toes RE,
Mikkers HM and Kurreeman F: A novel long non-coding RNA in the
rheumatoid arthritis risk locus TRAF1-C5 influences C5 mRNA levels.
Genes Immun. 17:85–92. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mirza AH, Berthelsen CH, Seemann SE, Pan
X, Frederiksen KS, Vilien M, Gorodkin J and Pociot F:
Transcriptomic landscape of lncRNAs in inflammatory bowel disease.
Genome Med. 7:392015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Santoro M, Nociti V, Lucchini M, De Fino
C, Losavio FA and Mirabella M: Expression profile of long
non-coding RNAs in serum of patients with multiple sclerosis. J Mol
Neurosci. 59:18–23. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ahn R, Gupta R, Lai K, Chopra N, Arron ST
and Liao W: Network analysis of psoriasis reveals biological
pathways and roles for coding and long non-coding RNAs. BMC
Genomics. 17:8412016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Széll M, Danis J, Bata-Csörgő Z and Kemény
L: PRINS, a primate-specific long non-coding RNA, plays a role in
the keratinocyte stress response and psoriasis pathogenesis.
Pflugers Arch. 468:935–943. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Braconi C, Kogure T, Valeri N, Huang N,
Nuovo G, Costinean S, Negrini M, Miotto E, Croce CM and Patel T:
microRNA-29 can regulate expression of the long non-coding RNA gene
MEG3 in hepatocellular cancer. Oncogene. 30:4750–4756. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li B, Tsoi LC, Swindell WR, Gudjonsson JE,
Tejasvi T, Johnston A, Ding J, Stuart PE, Xing X, Kochkodan JJ, et
al: Transcriptome analysis of psoriasis in a large case-control
sample: RNA-Seq provides insights into disease mechanisms. J Invest
Dermatol. 134:1828–1838. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Volders PJ, Verheggen K, Menschaert G,
Vandepoele K, Martens L, Vandesompele J and Mestdagh P: An update
on LNCipedia: A database for annotated human lncRNA sequences.
Nucleic Acids Res. 43:Database Issue. D174–D180. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xie C, Yuan J, Li H, Li M, Zhao G, Bu D,
Zhu W, Wu W, Chen R and Zhao Y: NONCODEv4: Exploring the world of
long non-coding RNA genes. Nucleic Acids Res. 42:Database Issue.
D98–D103. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Quek XC, Thomson DW, Maag JL, Bartonicek
N, Signal B, Clark MB, Gloss BS and Dinger ME: lncRNAdb v2.0:
Expanding the reference database for functional long noncoding
RNAs. Nucleic Acids Res. 43:Database Issue. D168–D173. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Amaral PP, Clark MB, Gascoigne DK, Dinger
ME and Mattick JS: lncRNAdb: A reference database for long
noncoding RNAs. Nucleic Acids Res. 39:Database Issue. D146–D151.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ritchie ME, Phipson B, Wu D, Hu Y, Law CW,
Shi W and Smyth GK: limma powers differential expression analyses
for RNA-sequencing and microarray studies. Nucleic Acids Res.
43:e472015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Han L, Zhang K, Shi Z, Zhang J, Zhu J, Zhu
S, Zhang A, Jia Z, Wang G, Yu S, et al: LncRNA profile of
glioblastoma reveals the potential role of lncRNAs in contributing
to glioblastoma pathogenesis. Int J Oncol. 40:2004–2012.
2012.PubMed/NCBI
|
|
34
|
Tafer H and Hofacker IL: RNAplex: A fast
tool for RNA-RNA interaction search. Bioinformatics. 24:2657–2663.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sundarrajan S and Arumugam M: Weighted
gene co-expression based biomarker discovery for psoriasis
detection. Gene. 593:225–234. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang H, Gu L, Zhang X, Liu M, Jiang H, Cai
R, Zhao Y and Cheng B: Global transcriptome and weighted gene
co-expression network analyses reveal hybrid-specific modules and
candidate genes related to plant height development in maize. Plant
Mol Biol. 98:187–203. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Stawczyk-Macieja M, Szczerkowska-Dobosz A,
Rębała K and Purzycka-Bohdan D: Genetic background of skin barrier
dysfunction in the pathogenesis of psoriasis vulgaris. Postepy
Dermatol Alergol. 32:123–126. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ekman AK, Verma D, Fredrikson M, Bivik C
and Enerbäck C: Genetic variations of NLRP1: Susceptibility in
psoriasis. Br J Dermatol. 171:1517–1520. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Nikamo P, Lysell J and Ståhle M:
Association with genetic variants in the IL-23 and NF-κB pathways
discriminates between mild and severe psoriasis skin disease. J
Invest Dermatol. 135:1969–1976. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lynde CW, Poulin Y, Vender R, Bourcier M
and Khalil S: Interleukin 17A: Toward a new understanding of
psoriasis pathogenesis. J Am Acad Dermatol. 71:141–150. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Nair RP, Duffin KC, Helms C, Ding J,
Stuart PE, Goldgar D, Gudjonsson JE, Li Y, Tejasvi T, Feng BJ, et
al: Genome-wide scan reveals association of psoriasis with IL-23
and NF-kappaB pathways. Nat Genet. 41:199–204. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Goldminz AM, Au SC, Kim N, Gottlieb AB and
Lizzul PF: NF-κB: An essential transcription factor in psoriasis. J
Dermatol Sci. 69:89–94. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Johansen C, Rittig AH, Mose M, Bertelsen
T, Weimar I, Nielsen J, Andersen T, Rasmussen TK, Deleuran B and
Iversen L: STAT2 is involved in the pathogenesis of psoriasis by
promoting CXCL11 and CCL5 production by keratinocytes. PLoS One.
12:e01769942017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Haase I, Hobbs RM, Romero MR, Broad S and
Watt FM: A role for mitogen-activated protein kinase activation by
integrins in the pathogenesis of psoriasis. J Clin Invest.
108:527–536. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Mavropoulos A, Rigopoulou EI, Liaskos C,
Bogdanos DP and Sakkas LI: The role of p38 MAPK in the
aetiopathogenesis of psoriasis and psoriatic arthritis. Clin Dev
Immunol. 2013:5697512013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Eades G, Zhang YS, Li QL, Xia JX, Yao Y
and Zhou Q: Long non-coding RNAs in stem cells and cancer. World J
Clin Oncol. 5:134–141. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Carpenter S, Aiello D, Atianand MK, Ricci
EP, Gandhi P, Hall LL, Byron M, Monks B, Henry-Bezy M, Lawrence JB,
et al: A long noncoding RNA mediates both activation and repression
of immune response genes. Science. 341:789–792. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Fitzgerald KA and Caffrey DR: Long
noncoding RNAs in innate and adaptive immunity. Curr Opin Immunol.
26:140–146. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wu GC, Pan HF, Leng RX, Wang DG, Li XP, Li
XM and Ye DQ: Emerging role of long noncoding RNAs in autoimmune
diseases. Autoimmun Rev. 14:798–805. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Sonkoly E, Bata-Csorgo Z, Pivarcsi A,
Polyanka H, Kenderessy-Szabo A, Molnar G, Szentpali K, Bari L,
Megyeri K, Mandi Y, et al: Identification and characterization of a
novel, psoriasis susceptibility-related noncoding RNA gene, PRINS.
J Biol Chem. 280:24159–24167. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Szegedi K, Sonkoly E, Nagy N, Németh IB,
Bata-Csörgo Z, Kemény L, Dobozy A and Széll M: The anti-apoptotic
protein G1P3 is overexpressed in psoriasis and regulated by the
non-coding RNA, PRINS. Exp Dermatol. 19:269–278. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Holm SJ, Sánchez F, Carlén LM, Mallbris L,
Ståhle M and O'Brien KP: HLA-Cw*0602 associates more strongly to
psoriasis in the Swedish population than variants of the novel
6p21.3 gene PSORS1C3. Acta Derm Venereol. 85:2–8. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Kretz M, Siprashvili Z, Chu C, Webster DE,
Zehnder A, Qu K, Lee CS, Flockhart RJ, Groff AF, Chow J, et al:
Control of somatic tissue differentiation by the long non-coding
RNA TINCR. Nature. 493:231–235. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Shi J, Jiang K and Li Z: MiR-145
ameliorates neuropathic pain via inhibiting inflammatory responses
and mTOR signaling pathway by targeting Akt3 in a rat model.
Neurosci Res. 134:10–17. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
O'Leary L, Sevinç K, Papazoglou IM, Tildy
B, Detillieux K, Halayko AJ, Chung KF and Perry MM: Airway smooth
muscle inflammation is regulated by microRNA-145 in COPD. FEBS
Lett. 590:1324–1334. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Perazzio AS, Oliveira JS, Figueiredo VL
and Chauffaille ML: Increase of IRF-1 gene expression and
impairment of T regulatory cells suppression activity on patients
with myelodysplastic syndrome: A longitudinal one-year study. Leuk
Res. 55:6–17. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Azuaje FJ: Selecting biologically
informative genes in co-expression networks with a centrality
score. Biol Direct. 9:122014. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Nazarov PV, Muller A, Kaoma T, Nicot N,
Maximo C, Birembaut P, Tran NL, Dittmar G and Vallar L: RNA
sequencing and transcriptome arrays analyses show opposing results
for alternative splicing in patient derived samples. BMC Genomics.
18:4432017. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Antonini D, Mollo MR and Missero C:
Research techniques made simple: Identification and
characterization of long noncoding RNA in dermatological research.
J Invest Dermatol. 137:e21–e26. 2017. View Article : Google Scholar : PubMed/NCBI
|