Introduction
Prostate cancer is the most frequently diagnosed
non-cutaneous cancer in males in western countries (1). The majority of prostate cancer cases
are diagnosed at a local stage and have a 5-year survival rate of
almost 100% (2). However, prostate
cancer remains a major cause of cancer-associated mortality due to
its heterogeneous nature, ranging from asymptomatic to a rapidly
fatal systemic malignancy. One of the most challenging aspects of
prostate cancer is that the androgen receptor-dependent tumors
inevitably progress to highly aggressive castration-resistant
tumors following initial androgen-ablation therapy.
Only a limited number of effective therapeutic
options are available for advanced prostate cancer. Docetaxel
(Doc), a semi-synthetically taxane analogue, has displayed
promising therapeutic potential in treating advanced-stage prostate
cancer. In clinical practice, it is recommended that combination of
Doc and prednisone should be used in the treatment of prostate
cancer patients to improve overall survival and disease control
(3). However, progression is
ultimately observed after 6–8 months in patients treated with Doc
due to inherent or acquired drug resistance (4,5).
Doc is considered to function as a microtubule
inhibitor by binding to β-tubulin and preventing microtubule
disassembly. Hence, Doc is able to arrest cells in the
G2/M-phase of the cell cycle and induce cell death. A
number of studies have suggested that Doc induces phosphorylation
of B-cell lymphoma 2 (Bcl-2) and Bcl-xL members, thus inactivating
their anti-apoptotic capacities (6). In addition, the mitogen-activated
protein kinase pathway is considered to be involved in the
development of drug resistance in prostate cancer cells (7,8).
Nevertheless, the association between the expression of Bcl-2
family members and Doc resistance remains controversial (9). Overexpression of pro-apoptotic
proteins caspase-9 and Bcl-2 interacting protein 3 in resistant
prostate cancer cells was observed in previous studies (10,11).
However, few studies have carefully examined the regulation of
these mechanisms in response to initial treatment with Doc. Thus,
identification of the mechanistic basis of Doc-induced cell death
in prostate cancer will improve our understanding on the resistance
mechanism.
In the present study, the antiproliferative effect
of Doc on androgen-dependent and androgen-independent human
prostate cancer cells was investigated. In addition, we also
investigated signaling pathways involved in Doc-induced cell
apoptosis.
Materials and methods
Reagents
MTT was purchased from Sigma-Aldrich (Merck KGaA,
Darmstadt, Germany). Dulbecco's modified Eagle's medium (DMEM) and
Annexin V-FITC/propidium iodide (PI) were purchased from Beyotime
Institute of Biotechnology (Hangzhou, China). Fetal bovine serum
(FBS) was obtained from Sijingqing Biotechnology Co., Ltd.
(Hangzhou, China). Monoclonal antibodies against total protein
kinase B (Akt; 1:1,000; cat. no. ab179463; Abcam, Cambridge, UK),
phospho-Akt (1:1,000; cat. no. ab133458; Abcam), Bcl-2 (1:1,000;
cat. no. ab59348; Abcam), Bcl-2-associated death promoter (Bad;
1:1,000; cat. no. ab32445; Abcam), caspase-3 (1:1,000; cat. no.
ab4051; Abcam), caspase-9 (1:1,000; cat. no. ab52298; Abcam) and
β-actin (1:1,000; cat. no. ab16039; Abcam) were used as primary
antibodies. Horseradish peroxidase-conjugated goat anti-rabbit
immunoglobulin G (1:1,000; cat. no. 31460; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) was used as secondary
antibody.
Cell culture
Three human prostate cancer cell lines, namely PC-3
[androgen receptor (AR)-negative], DU-145 (AR-negative) and LNCaP
(AR-sensitive) were purchased from The American Type Culture
Collection (Manassas, VA, USA) and were maintained in DMEM
supplemented with 10% (v/v) FBS and antibiotics at 37°C with 5%
CO2. After 3–4 days of culture, the cells were detached
from the surface by trypsinization and reseeded into the
appropriate plates for use in further experiments.
MTT assay
Cell proliferation was evaluated by an MTT assay.
Briefly, cells were seeded into 96-well plates at a density of
4×103 cells per well in 100 µl medium and cultured for
~24 h. Next, PC-3, DU-145 and LNCaP cells were treated with Doc at
the doses of 0–64, 0–40 and 0–8 nM, respectively. Six replicated
samples were assayed for each concentration. Cells were cultured
for 48 h, followed by the addition of 20 µl MTT in each well. After
4 h, the supernatant were discarded, 200 µl dimethyl sulfoxide
(DMSO) was added to each well, and the plate was placed on a shaker
for 10 min to completely dissolve DMSO. Absorbance was then
measured at wavelengths of 490 and 630 nM. The half maximal
inhibitory concentration (IC50) was calculated using
Origin software, version 8.5 (OriginLab Corporation, Northampton,
MA, USA).
Cell apoptosis assay
Cells in the logarithmic growth phase were seeded in
6-well plates at a density of 2×105 cells per well. The
three cell lines were divided into three groups each and then
incubated with two different concentrations of Doc (4 and 10 nM) as
the positive controls and in the absence of Doc as the negative
control. After 48 h, cells were harvested, washed in cold
phosphate-buffered saline (PBS), and then treated with Annexin
V-FITC and PI using an Apoptosis Detection kit with PI, according
to the manufacturer's protocol. Annexin V-FITC and PI binding was
detected with an Attune flow cytometer (Thermo Fisher Scientific,
Inc.), and flow cytometric analysis was performed using BD
CellQuest software (BD Biosciences, Franklin Lakes, NJ, USA).
Cell cycle analysis
The DNA content during the cell cycle phases was
evaluated by flow cytometry. Briefly, 4×105 PC-3, DU-145
and LNCaP cells were seeded and were treated with Doc at
concentrations of 4, 10 and 2 nM, respectively. Cells were then
incubated for 48 h, and cell pellets were washed and fixed in cold
70% ethanol at 4°C overnight. On the following day, the cell
pellets were washed three times and resuspended in PBS, followed by
treatment with RNase (50 µg/ml) for 1 h. Next, 5 µl PI (1 mg/ml)
was added to a final concentration of 50 µg/ml and incubated for 1
h at room temperature in the dark. Subsequent to staining of DNA by
PI, samples were evaluated using the Attune flow cytometer, and
analysis was performed using the BD CellQuest software.
Protein extraction and western
blotting
Following treatment for 24 or 48 h, total protein
was extracted using a cell lysis buffer (Thermo Fisher Scientific,
Inc.). Protein concentration in the supernatant was determined
using a Bio-Rad protein assay kit (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). Next, protein (20 µg) was subjected to 12%
SDS-PAGE and transferred to a polyvinylidene difluoride membrane.
Subsequent to blocking in non-fat dry milk for 2 h at room
temperature, the membrane was incubated overnight at 4°C with total
Akt, phospho-Akt, Bcl-2, Bad, caspase-3 and caspase-9 primary
antibodies. The membrane was then washed three times with
Tris-buffered saline (10 min each time), followed by incubation
with secondary antibody for 2 h at room temperature. Signal
development was performed using an enhanced chemiluminescence kit.
Signals were captured and band densities were quantified using
Bandscan software, version 5.0 (ProZyme; Agilent Technologies,
Inc., Santa Clara, CA, USA).
Statistical analysis
Data are presented as the mean ± standard deviation.
Analysis of variance with Tukey-Kramer test adjustment was used for
comparisons among multiple groups, while Student' test was used to
examine comparisons between two groups. All analyses were performed
using SPSS software, version 11.0 (SPSS, Inc., Chicago, IL, USA).
Probability values of P<0.05 were considered to denote
statistically significant differences.
Results
Effects of Doc on the growth of human
prostate cancer cells
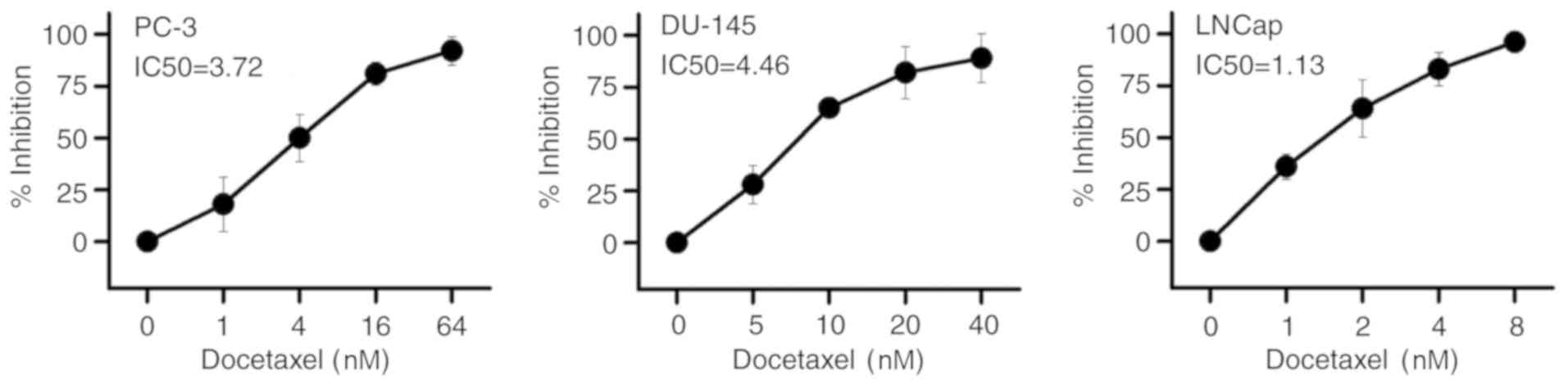
Following treatment for 48 h, the inhibitory effects
of Doc on the three prostate cancer cell lines PC-3, DU-145 and
LNCaP were explored by an MTT assay. The results presented in
Fig. 1 demonstrate that, upon
treatment with Doc, cell viability in the three cell lines
decreased in a concentration-dependent manner. The IC50
values of the effect of Doc on PC-3, DU-145 and LNCaP cells were
3.72, 4.46 and 1.13 nM, respectively. Among the three cell lines,
LNCaP is an androgen-dependent prostate cancer cell line, while the
other two cell lines are androgen-independent (12). The study results indicated that
PC-3 and DU-145 cells were more resistant to Doc treatment, as
their IC50 values were approximately threefold and
fourfold higher in comparison with that of LNCaP cells,
respectively.
Effects of Doc on the apoptosis of
human prostate cancer cells
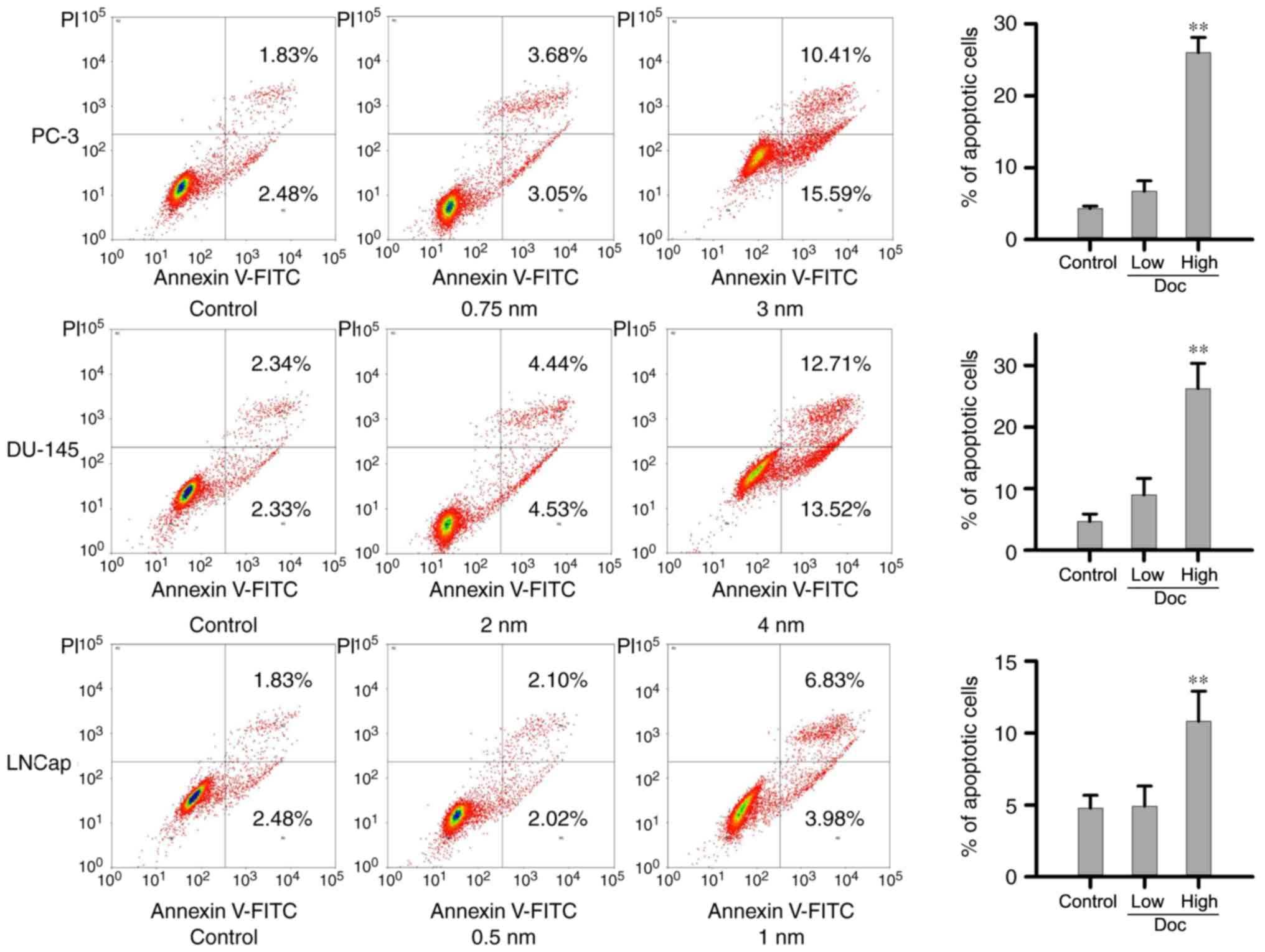
To examine the mechanism underlying the antitumor
effect of Doc, flow cytometric analysis with Annexin V/PI staining
was performed. Cells were exposed to low and high concentrations of
Doc, which were determined according to the IC50 value
of each cell line. The low Doc dose for PC-3, DU-145 and LNCaP
cells was 0.75, 2 and 0.5 nM, respectively, while the high dose was
3, 4 and 1 nM, respectively. As presented in Fig. 2, low concentrations of Doc had no
effect on cell death in all cells lines. Compared with the negative
control and with the cells treated with a low concentration of Doc,
treatment with high dose of Doc significantly increased the
proportion of Annexin V+ apoptotic cells.
Effect of Doc on cell cycle phase
distribution
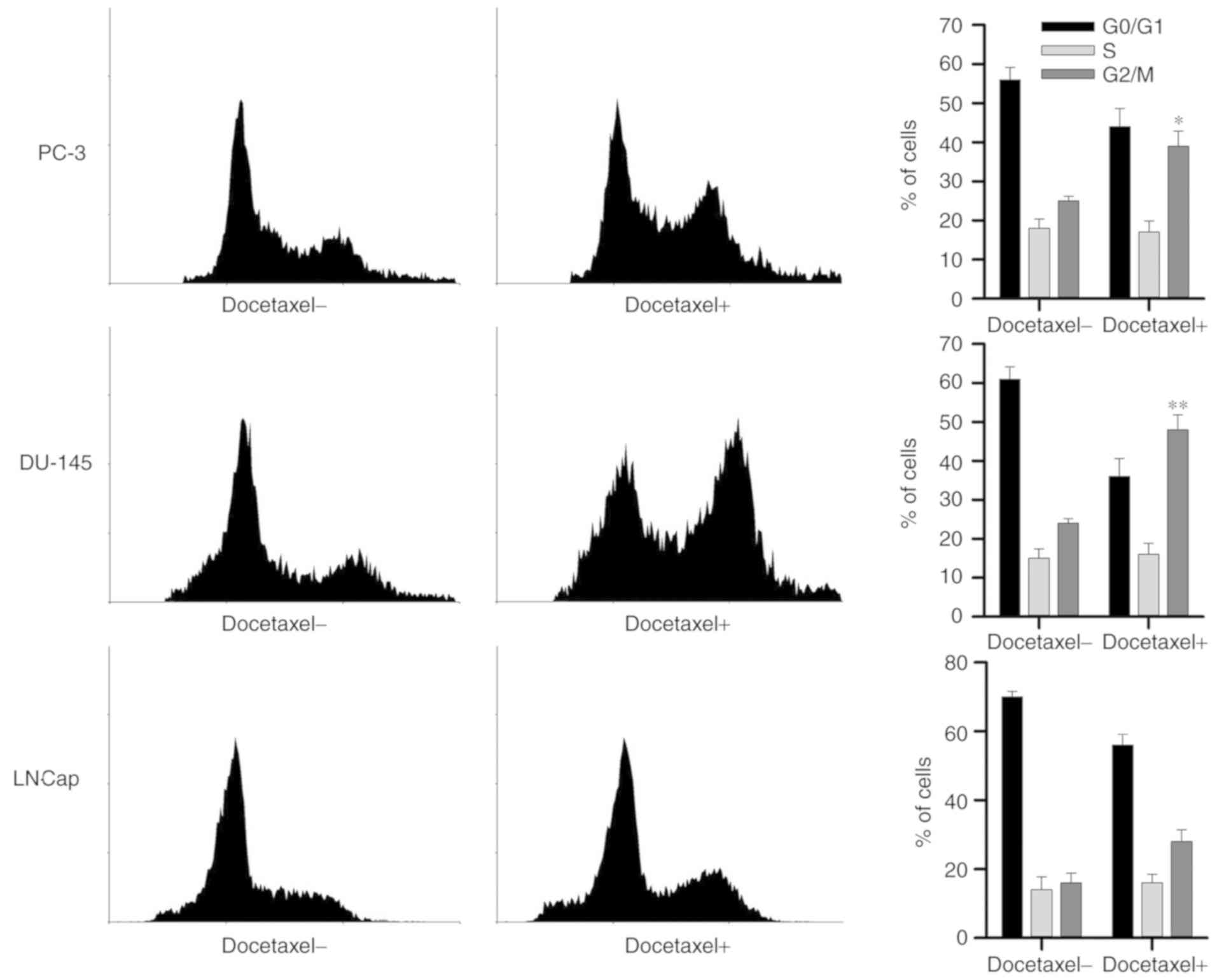
Cultured human prostate cancer PC-3, DU-145 and
LNCaP cells were exposed to Doc at the concentrations of 4, 10 and
2 nM, respectively. As presented in Fig. 3, it was observed that treatment
with Doc led to marked cell cycle arrest at G2/M phase,
as observed by the significant increase in the percentage of cells
at this phase in PC-3 and DU145 cells, as compared with the
untreated cells.
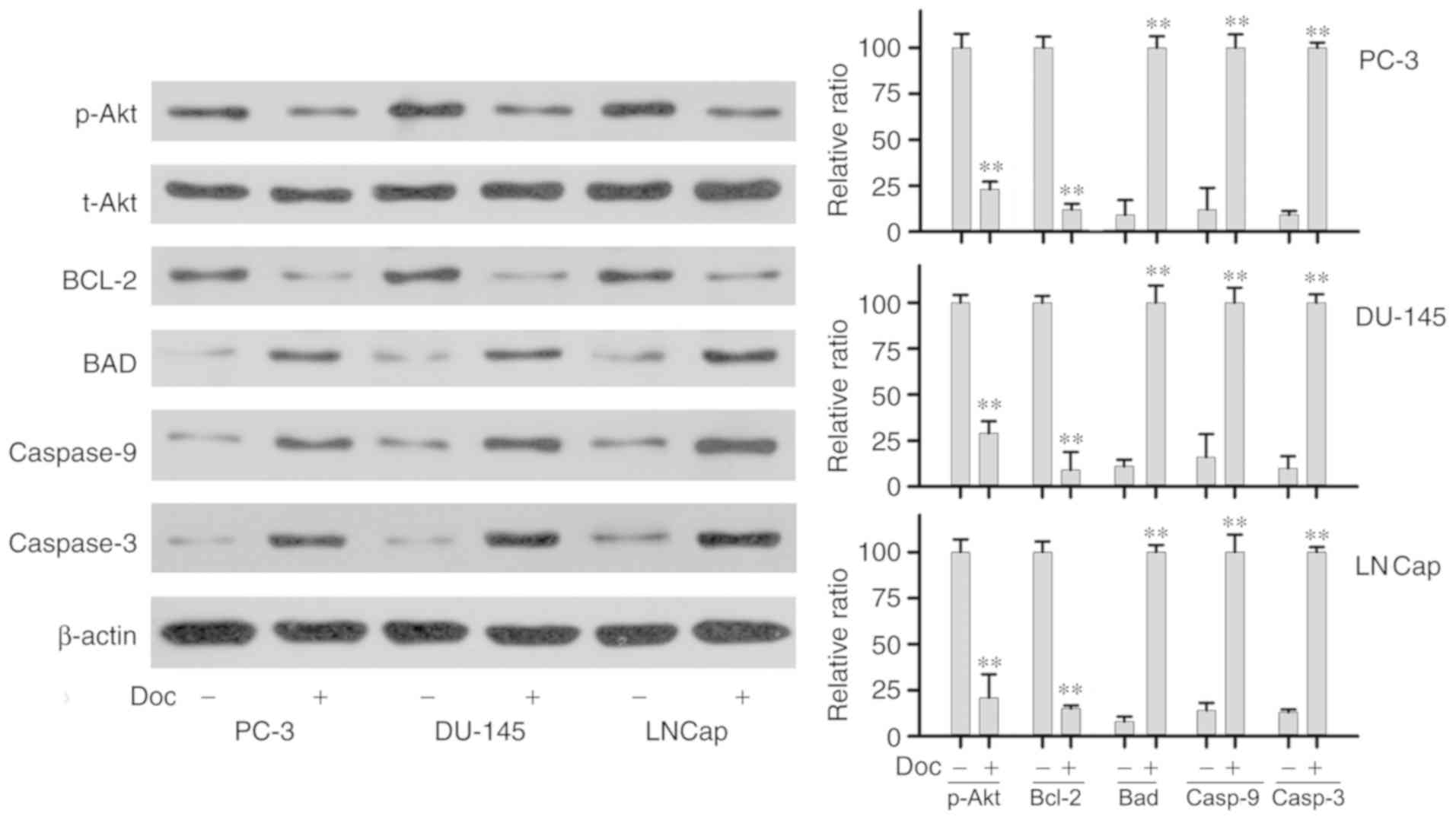
Effects of Doc on the protein levels
of Bcl-2, Bad, phospho-Akt and caspase-3/9
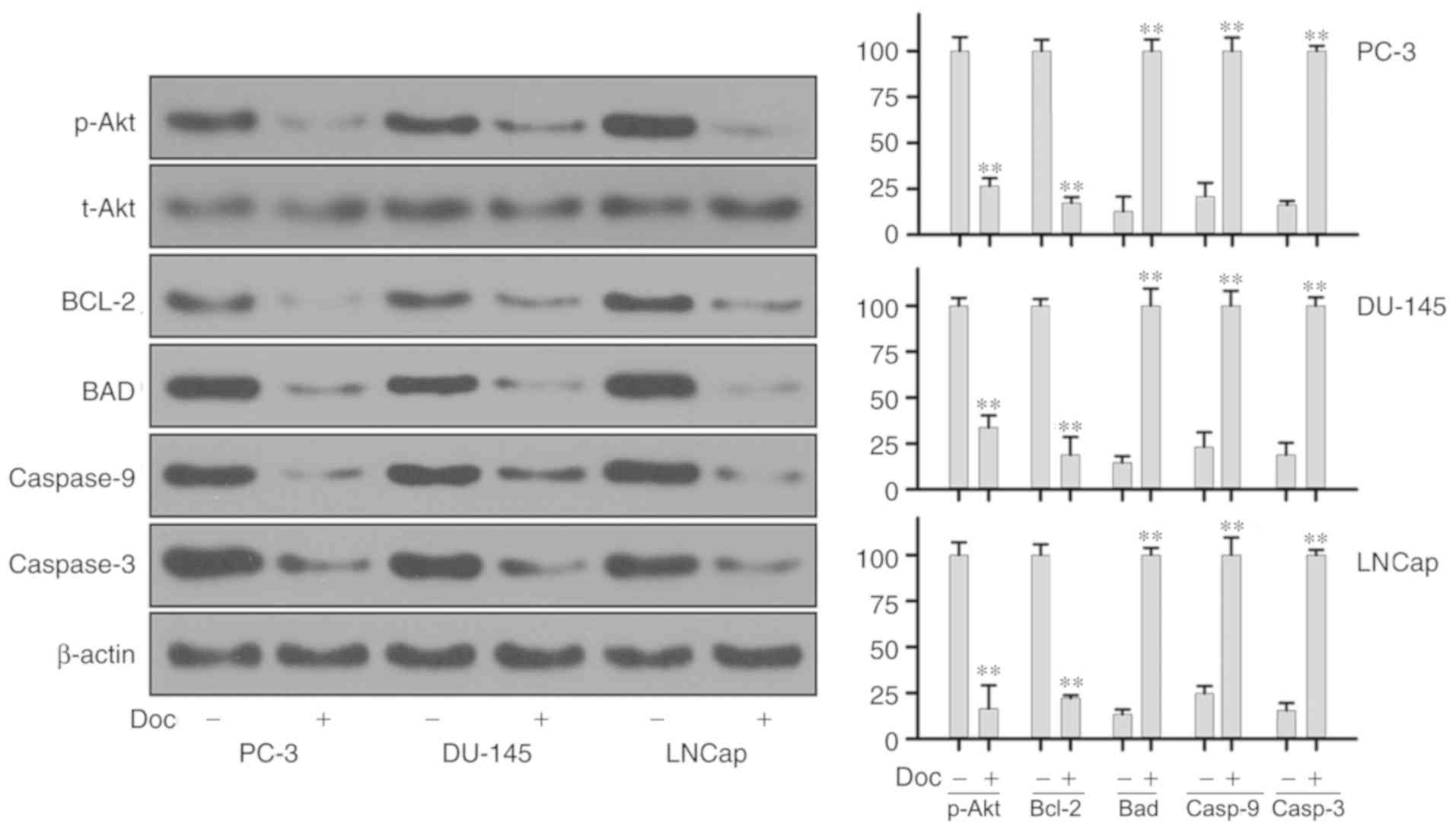
The study next explored the expression of proteins
associated with the development of Doc resistance in prostate
cancer cells. Western blot assay was used to assess the expression
of Bcl-2, total Akt, phospho-Akt, Bcl-2, Bad, caspase-3 and
caspase-9 at the protein levels after 24 and 48 h of Doc treatment.
As shown in Fig. 4, compared with
the control group, treatment with Doc for 24 h led to significantly
decreased expression of anti-apoptotic Bcl-2 protein. The activity
of p-Akt was decreased significantly, while total Akt level
remained stable. Treatment of PC-3, DU-145 and LNCaP cell lines
with Doc also resulted in significant increase in the levels of
caspase-3, caspase-9 and pro-apoptotic protein Bad. Protein
expression was further examined at 48 h, and similar results were
obtained (Fig. 5).
Discussion
In spite of the significantly improved survival,
prostate cancer remains a clinical challenge due to the fact that,
in a certain portion of patients, prostate cancer may progress to
metastatic castration-resistant prostate cancer with no curative
treatment options (2,3). Doc is an effective cytotoxic agent
that provides survival benefits for patients with
castration-resistant tumors (13).
However, clinical resistance to Doc remains a challenge in clinical
practice. Growing evidence has demonstrated the complexity of the
mechanism underlying Doc resistance, which includes cellular
anti-apoptotic, AR-mediated redox signaling pathways (14–16).
In the current study, the inhibitory and apoptotic effects of Doc
on androgen-dependent or androgen-independent prostate cancer cells
were initially examined. The study then attempted to explore the
regulation of Bcl-2 and phosphoinositide 3-kinase (PI3K)/Akt
signaling pathway in Doc-induced apoptosis.
The results reported in the present study confirmed
that Doc exerted an inhibitory effect on the growth of cultured
prostate cancer cells in a dose-independent manner. The
IC50 values for the effect of Doc in PC-3, DU-145 and
LNCaP cells were 3.72, 4.46 and 1.13 nM, respectively. These
results indicated that Doc had a stronger inhibitory effect on the
AR-dependent LNCaP cells, as compared with that on AR-independent
prostate cancer cells PC-3 and DU-145. In respect to Doc-induced
apoptosis, the current data are in agreement with previous findings
indicating that a low dose of Doc causes no apoptotic cell death,
which is accompanied by senescence necrosis and mitotic catastrophe
(17–19). AR signaling is known to serve a
crucial role in the progression of prostate cancer. Dysregulation
of AR signaling and transcriptional activity stimulates resistance
to Doc (20). Aberrant AR pathway
activation and various splice variant isoforms are frequently
observed in advanced prostate cancer (21). At present, the molecular mechanism
of the differences in Doc sensitivity is unknown. In a small scale
clinical trial, Doc was proven to exert a therapeutic effect on
early hormone-sensitive prostate cancer, without affecting
testosterone levels (22). In
future studies, it would be of interest to explore the impact of
low-dose Doc treatment on hormone-sensitive prostate cancer
compared with the effect of traditional anti-androgen therapy. It
would also be interesting to explore the mechanical differences
between inherent and therapy-induced AR activation upon initial
treatment with Doc.
To examine whether the strong effects of Doc
treatment on the three prostate cancer cell lines were mediated
through apoptosis-associated signaling pathways, the expression
levels of associated genes were assessed at the protein levels in
the present study. Previous studies have demonstrated that taxanes
inhibit the anti-apoptotic protein Bcl-2 by stabilization of
microtubules, leading to apoptosis (20,23).
Bcl-2, a proto-oncogene, is one of the most widely studied negative
regulators of apoptotic cascades. It is considered that Bcl-2
prevents the release of cytochrome c and consequently blocks
the activation of the caspase cascade (24). In the present study, it was
observed that treatment of prostate cancer cells lines PC-3, DU-145
and LNCaP with Doc significantly inhibited Bcl-2 activity, and
further upregulated the pro-apoptotic Bad, caspase-3 and caspase-9
proteins. Recent studies suggested that activation of Akt directly
affects the apoptosis pathway by targeting and downregulating the
levels of Bcl-2 family members, including Bad and Bcl-2-associated
X protein, resulting in cell survival (25,26).
Emerging evidence revealed a negative feedback link between
PI3K/Akt and AR (25,26). Furthermore, activation of Akt
induced the phosphorylation of AR, resulting in inhibition of the
AR-induced apoptotic pathway, whereas AR inhibition was reported to
activate the PI3K/Akt signaling pathway (25,26).
The results of the present study revealed that treatment of the
three prostate cancer cell lines with Doc markedly decreased the
level of phospho-Akt. A certain decrease, although not significant,
was also observed in AR-dependent prostate cancer cells (namely
LNCaP), as compared with that detected in AR-independent prostate
cancer cells, implying a potential interplay between AR and
PI3K/Akt when prostate cancer cells are treated with Doc. Given the
central role of PI3K/Akt signaling pathway on the growth,
proliferation, motility, survival and angiogenesis of tumor cells
(27,28), there is great need for
understanding the associations between PI3K/Akt and AR pathway in
Doc-induced apoptosis. In addition, other pathways may participate
in Doc-induced apoptosis through AR-dependent or AR-independent
pathways; however, further studies are required to investigate the
involvement of other pathways.
It has been reported that the development of Doc
resistance in prostate cancer is associated with AR activation
(29), which is consistent with
the findings of the present study, further confirming the
reliability of our data. Nevertheless, compared with a 2D cell
culture system, a 3D cell culture system that exhibits a more
similar behavior to in vivo conditions (30) should be performed to further
confirm the conclusions of the present study. Doc has been widely
used in the clinical treatment of prostate cancer (31), and multiple pathways have been
proven to be involved in the Doc-mediated therapeutic response in
this disease, such as p53/p21WAF1/CIP1, p27KIP1 and Notch pathways
(32,33). However, the involvement of Akt
signaling in this process is rarely studied. Thus, the present
study systemically investigated the role of Akt signaling in
Doc-mediated therapeutic responses in prostate cancer. A number of
pathways, such as type I insulin-like growth factor (34), have been proven to be involved in
androgen-dependent and androgen-independent prostate cancer, and
our future studies will focus on these pathways.
In conclusion, the present study demonstrated that
Doc strongly inhibited the growth and induced the apoptosis of
human prostate cancer cells. AR-dependent prostate cancer cells
were more sensitive to Doc in comparison with androgen-independent
cells. The effects of Doc on growth inhibition and apoptosis in
prostate cancer cells were associated with inhibition of PI3K/Akt
activation, decreased levels of Bcl-2 and increased caspase-3/9
activation.
Acknowledgements
Not applicable.
Funding
The present study was funded by The Agricultural and
Social Class Science and Technology Plan Project of Ninghai County
(grant no. 2013B11).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JJ and CY designed and conceived the study. CY and
WZ performed experiments and collected the data. JW and PC analyzed
and interpreted the data. JJ wrote the manuscript. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Eyre H, Kahn R and Robertson RM;
ACS/ADA/AHA Collaborative Writing Committee, : Preventing cancer,
cardiovascular disease, and diabetes: A common agenda for the
American cancer society, the American diabetes association, and the
American heart association. CA Cancer J Clin. 54:190–207. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Brunelli A, Kim AW, Berger KI and
Addrizzo-Harris DJ: Physiologic evaluation of the patient with lung
cancer being considered for resectional surgery: Diagnosis and
management of lung cancer, 3rd ed: American College of Chest
Physicians evidence-based clinical practice guidelines. Chest. 143
(Suppl 5):e166S–e190S. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee JL, Kim JE, Ahn JH, Lee DH, Lee J, Kim
CS, Hong JH, Hong B, Song C and Ahn H: Efficacy and safety of
docetaxel plus prednisolone chemotherapy for metastatic
hormone-refractory prostate adenocarcinoma: Single institutional
study in Korea. Cancer Res Treat. 42:12–17. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Petrylak DP, Tangen CM, Hussain MH, Lara
PN Jr, Jones JA, Taplin ME, Burch PA, Berry D, Moinpour C, Kohli M,
et al: Docetaxel and estramustine compared with mitoxantrone and
prednisone for advanced refractory prostate cancer. N Engl J Med.
351:1513–1520. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Montero A, Fossella F, Hortobagyi G and
Valero V: Docetaxel for treatment of solid tumours: A systematic
review of clinical data. Lancet Oncol. 6:229–239. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
West KA, Castillo SS and Dennis PA:
Activation of the PI3K/Akt pathway and chemotherapeutic resistance.
Drug Resist Updat. 5:234–248. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pritchard AL and Hayward NK: Molecular
pathways: Mitogen-activated protein kinase pathway mutations and
drug resistance. Clin Cancer Res. 19:2301–2309. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mohammadian J, Sabzichi M, Molavi O,
Shanehbandi D and Samadi N: Combined treatment with stattic and
docetaxel alters the Bax/Bcl-2 gene expression ratio in human
prostate cancer cells. Asian Pac J Cancer Prev. 17:5031–5035.
2016.PubMed/NCBI
|
|
10
|
Yoo NJ, Kim MS and Park SW: Expression
analysis of caspase-6, caspase-9 and BNIP3 in prostate cancer.
Tumori. 96:138–142. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Winter RN, Kramer A, Borkowski A and
Kyprianou N: Loss of caspase-1 and caspase-3 protein expression in
human prostate cancer. Cancer Res. 61:1227–1232. 2001.PubMed/NCBI
|
|
12
|
Webber MM, Bello D and Quader S:
Immortalized and tumorigenic adult human prostatic epithelial cell
lines: Characteristics and applications Part 2. Tumorigenic cell
lines. Prostate. 30:58–64. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Basch EM, Somerfield MR, Beer TM, Carducci
MA, Higano CS, Hussain MH and Scher HI; American Society of
Clinical Oncology, : American Society of Clinical Oncology
endorsement of the cancer care ontario practice guideline on
nonhormonal therapy for men with metastatic hormone-refractory
(castration-resistant) prostate cancer. J Clin Oncol. 25:5313–5318.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hughes C, Murphy A, Martin C, Sheils O and
O'Leary J: Molecular pathology of prostate cancer. J Clin Pathol.
58:673–684. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Muenchen HJ, Poncza PJ and Pienta KJ:
Different docetaxel-induced apoptotic pathways are present in
prostate cancer cell lines LNCaP and PC-3. Urology. 57:366–370.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hwang C: Overcoming docetaxel resistance
in prostate cancer: A perspective review. Ther Adv Med Oncol.
4:329–340. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Morse DL, Gray H, Payne CM and Gillies RJ:
Docetaxel induces cell death through mitotic catastrophe in human
breast cancer cells. Mol Cancer Ther. 4:1495–1504. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fabbri F, Amadori D, Carloni S,
Brigliadori G, Tesei A, Ulivi P, Rosetti M, Vannini I, Arienti C,
Zoli W and Silvestrini R: Mitotic catastrophe and apoptosis induced
by docetaxel in hormone-refractory prostate cancer cells. J Cell
Physiol. 217:494–501. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fabbri F, Carloni S, Brigliadori G, Zoli
W, Lapalombella R and Marini M: Sequential events of apoptosis
involving docetaxel, a microtubule-interfering agent: A cytometric
study. BMC Cell Biol. 7:62006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kahn B, Collazo J and Kyprianou N:
Androgen receptor as a driver of therapeutic resistance in advanced
prostate cancer. Int J Biol Sci. 10:588–595. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Attar RM, Takimoto CH and Gottardis MM:
Castration-resistant prostate cancer: Locking up the molecular
escape routes. Clin Cancer Res. 15:3251–3255. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gravis G, Fizazi K, Joly F, Oudard S,
Priou F, Esterni B, Latorzeff I, Delva R, Krakowski I, Laguerre B,
et al: Androgen-deprivation therapy alone or with docetaxel in
non-castrate metastatic prostate cancer (GETUG-AFU 15): A
randomised, open-label, phase 3 trial. Lancet Oncol. 14:149–158.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Loriot Y and Fizazi K: Taxanes: Still a
major weapon in the armamentarium against prostate cancer. Eur
Urol. 63:983–985. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Akinleye A, Avvaru P, Furqan M, Song Y and
Liu D: Phosphatidylinositol 3-kinase (PI3K) inhibitors as cancer
therapeutics. J Hematol Oncol. 6:882013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Morgan TM, Koreckij TD and Corey E:
Targeted therapy for advanced prostate cancer: Inhibition of the
PI3K/Akt/mTOR pathway. Curr Cancer Drug Targets. 9:237–249. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lin HK, Yeh S, Kang HY and Chang C: Akt
suppresses androgen-induced apoptosis by phosphorylating and
inhibiting androgen receptor. Proc Natl Acad Sci USA. 98:7200–7205.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Thomas C, Lamoureux F, Crafter C, Davies
BR, Beraldi E, Fazli L, Kim S, Thaper D, Gleave ME and Zoubeidi A:
Synergistic targeting of PI3K/AKT pathway and androgen receptor
axis significantly delays castration-resistant prostate cancer
progression in vivo. Mol Cancer Ther. 12:2342–2355. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Osaki M, Oshimura M and Ito H: PI3K-Akt
pathway: Its functions and alterations in human cancer. Apoptosis.
9:667–676. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Komura K, Jeong SH, Hinohara K, Qu F, Wang
X, Hiraki M, Azuma H, Lee GS, Kantoff PW and Sweeney CJ: Resistance
to docetaxel in prostate cancer is associated with androgen
receptor activation and loss of KDM5D expression. Proc Natl Acad
Sci USA. 113:6259–6264. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Souza AG, Silva IBB, Campos-Fernandez E,
Barcelos LS, Souza JB, Marangoni K, Goulart LR and Alonso-Goulart
V: Comparative assay of 2D and 3D cell culture models:
Proliferation, gene expression and anticancer drug response. Curr
Pharm Des. 24:1689–1694. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
James ND and Mason M: Docetaxel and/or
zoledronic acid for hormone-naïve prostate cancer: First survival
results from STAMPEDE. J Clin Oncol. 33:50012015. View Article : Google Scholar
|
|
32
|
Singh SK, Banerjee S, Acosta EP, Lillard
JW and Singh R: Resveratrol induces cell cycle arrest and apoptosis
with docetaxel in prostate cancer cells via a p53/p21WAF1/CIP1 and
p27KIP1 pathway. Oncotarget. 8:17216–17228. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cui D, Dai J, Keller JM, Mizokami A, Xia S
and Keller ET: Notch pathway inhibition using PF-03084014, a
γ-secretase inhibitor (GSI), enhances the antitumor effect of
docetaxel in prostate cancer. Clin Cancer Res. 21:4619–4629. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wu JD, Odman A, Higgins LM, Haugk K,
Vessella R, Ludwig DL and Plymate SR: In vivo effects of the human
type I insulin-like growth factor receptor antibody A12 on
androgen-dependent and androgen-independent xenograft human
prostate tumors. Clin Cancer Res. 11:3065–3074. 2005. View Article : Google Scholar : PubMed/NCBI
|