Introduction
A lack of blood flow into the heart muscle results
in a disruption to the supply and demand of oxygen, termed
ischemia, which leads to damage or dysfunction of the heart tissue.
The subsequent reperfusion that occurs following ischemia can also
cause injury. This event is named myocardial ischemia reperfusion
injury (IRI). Myocardial IRI, as a common physiological and
pathological phenomenon, was first described by Jennings et
al (1) in 1960 and often
occurs in myocardial infarction and numerous types of cardiac
surgeries. In addition, it also causes inflammation, which leads to
further harm to the normal tissues around the infarct site.
Therefore, myocardial IRI is a major challenge in organ
transplantation and surgery (2).
Although significant progress has been made in treating
ischemia/reperfusion (I/R) mechanisms based on the acute myocardial
infarction model, the results of clinical studies have been largely
unsatisfactory, which may be due to an inadequate understanding of
the mechanisms involved. Hypoxia/reoxygenation (H/R) injury, a
mimic in vitro model of myocardial I/R injury, has been
widely used to explore the underlying molecular mechanism of
myocardial I/R injury (3–5). At present, a number of studies have
explored myocardial H/R injury from the perspectives of the
inflammatory response (6), cell
apoptosis (7) and cell signal
transduction (8), but the exact
molecular mechanism remains unknown. Therefore, it is important to
explore the potential molecular mechanisms of myocardial IRI.
Emerging evidence has suggested that microRNAs
(miRNAs/miRs) function as regulators in cells development,
differentiation, immunity and cell cycle (9). In addition, miRNAs have been
demonstrated to serve vital roles in improving the therapeutic
outcomes of myocardial infarction (10), arrhythmia (11) and inhibition of atrial fibrillation
(12). miR-155, as a typical
multifunctional RNA, has been identified to be associated with
homeostasis, atherogenesis, immune system and inflammation function
(13). In addition, previous
studies have observed that miR-155 was also involved in processes
other than hematopoiesis and immune system, including
cardiovascular disease (14),
tumor and other pathological processes (15). It has previously been demonstrated
that the inhibition of miR-155 ameliorated cardiac fibrosis in the
process of angiotensin II-induced cardiac remodeling. In addition,
previous data has identified that miR-155 functions as a vital
moderator of cardiac damage and inflammation in atherosclerosis by
repressing Bcl-6 in macrophages (16), and that miR-155 may aggravate
ischemia-reperfusion injury via regulation of inflammatory cell
recruitment and the respiratory oxidative burst (17). Downregulation of miR-155 may
stimulate sevoflurane-mediated cardio protection against myocardial
ischemia/reperfusion injury via binding to SIRT1 in mice (18). Furthermore, miR-155 may aggravate
liver ischemia/reperfusion injury through limiting suppressor of
cytokine signaling 1 in mice (19). However, data concerning how miR-155
functions in myocardial I/R injury, its potential molecular
mechanism and the signaling pathway involved, are limited.
The present study identified that miR-155 was
notably upregulated in a myocardial H/R in vitro model.
Following overexpression of miR-155, cell viability was markedly
decreased, the number of apoptotic cells was significant increased,
and the expression of apoptosis-associated proteins caspase 3 and
caspase 9 were markedly upregulated; inhibition of miR-155 resulted
in a reversal of all of these events. In addition, high expression
levels of key proteins involved in the mitogen-activated protein
kinase (MAPK)/JNK pathway caused by H/R were attenuated by miR-155
inhibitor. BAG family molecular chaperone regulator 5 (BAG5), which
was expressed at a decreased level in the H/R model, was confirmed
to be a target of miR-155 and to be negatively regulated by
miR-155. A co-transfection assay demonstrated that the
overexpression of BAG5 may promote the mitigative effect of miR-155
inhibition on the cell damage induced by H/R. In addition, the high
expression level of hypoxia-inducible factor 1-α (HIF-1α) and low
expression level of von Hippel-Lindau protein (VHL) induced by H/R
were suppressed by miR-155 inhibition. These data suggested that
knockdown of miR-155 may alleviate the cell damage caused by H/R by
mediating BAG5 and the MAPK/JNK pathway. The function of
miR-155/BAG5 on myocardial H/R injury represents a novel avenue of
research for understanding the mechanism of I/R and provides a
theoretical reference for the identification of clinical
therapeutic targets in the future.
Materials and methods
Construction of a myocardial ischemia
model in vitro
Rat H9c2 myocardial cells were obtained from the
American Type Culture Collection and were used to construct the H/R
model in vitro. H9c2 cells were washed with PBS and rendered
quiescent in serum-free Dulbecco's modified Eagle's medium (DMEM,
Sigma-Aldrich; Merck KGaA) for 24 h prior to experimentation. The
cells were cultured in DMEM with 10% fetal bovine serum (FBS,
Gibco; Thermo Fisher Scientific, Inc.) and gentamicin. Then, the
medium was replaced with DMEM without FBS and the cells were
cultured in a hypoxic incubator with 95% N2 and 5%
CO2 at 37°C. Following culture in the hypoxic incubator
for 6 h, the cells were transferred to a normal incubator with 95%
O2 and 5% CO2 at 37°C for 24 h, in order to
reoxygenate the cells.
Cell transfection
miR-155 mimic negative control (NC), miR-155 mimics,
miR-155 inhibitor NC, and miR-155 inhibitors were synthesized by
Shanghai GenePharma Co., Ltd. When cell coverage reached 80%,
Lipofectamine 3000 (Invitrogen; Thermo Fisher Scientific, Inc.) was
used to transfect miR-155 mimic (200 nM,
5′-UUAAUGCUAAUCGUGAUAGGGGU-3′), inhibitor (100 nM,
3′-CCCUAUCACGAUUAGCAUUAAUU-3′) and NC (100 nM,
5′-UUGUCCUACACCUCACAGUCCUG-3′) into H9c2 cells following
manufacturer's protocol to generate the knockout and overexpression
cell models. Transfected cells were cultured at 37°C for 6 h,
followed by incubation with complete medium. After 24 h, subsequent
experiments were performed.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total cellular RNA was extracted from the treated
cells using TRIzol® (Thermo Fisher Scientific, Inc.)
according to manufacturer's protocol, and then reverse transcribed
into cDNA using an iScript™ cDNA Synthesis kit (Bio-Rad
Laboratories, Inc.) following the manufacturer's protocol. The
mRNA/miRNA relative expression was assessed by qPCR using the
SsoFast™ EvaGreen® Supermix (Bio-Rad Laboratories,
Inc.). The thermocycler conditions were as follows: 95°C for 2 min,
followed by 40 cycles at 95°C for 5 min, 95°C for 30 sec and 60°C
for 45 sec, and then 72°C for 30 min. The 2−ΔΔCq method
(20) was used to detect the
relative fold-changes, and GAPDH and U6 were used as internal
controls to detect mRNA and miRNA expression. The primer sequences
were as follows: BAG5 forward, 5′-GCAAGTGGTTGGCTTCAGTG-3′; BAG5
reverse, 5′-CACGCATGATAAGTGCCTGC-3′; GAPDH forward,
5′-GCCAGCCTCGTCTCATAGAC-3′; GAPDH reverse,
5′-AGTGATGGCATGGACTGTGG-3′; miR-155 forward
5′-AATGCTAATTGTGATAGGGG-3′; miR-155 reverse,
5′-GAACATGTCTGCGTATCTC-3′; U6 forward, 5′-CCTGCTTCGGCAGCACAT-3′;
and U6 reverse, 5′-GCGTGAAGCGTTCCATG-3′.
Western blot analysis
Transfected cells were lysed using RIPA buffer
(Invitrogen; Thermo Fisher Scientific, Inc.) with 1% protease
inhibitor. A bicinchoninic acid kit (Beyotime Institute of
Biotechnology) was used to measure the concentration of total
proteins following the manufacturer's instruction. Equal amounts of
proteins (20 µg) were electrophoresed using 12% SDS-PAGE gels, and
then transferred onto PVDF membranes (Invitrogen; Thermo Fisher
Scientific, Inc.). The membranes were blocked with 5% non-fat milk
for 1 h at room temperature, and then incubated with primary
antibodies purchased from Abcam: BAG5 (1:1,000; cat. no. ab97660),
cleaved-caspase 3 (1:1,000; cat. no. ab2303), cleaved-caspase 9
(1:1,000; cat. no. ab2324), mitogen-activated protein kinase 11
(P38; 1:1,000; cat. no. ab170099), phosphorylated (p)-P38 (1:1,000;
cat. no. ab31828), JNK (1:1,000; cat. no. ab126424), p-JNK
(1:1,000; cat. no. ab176662), HIF-1α (1:1,000; cat. no. ab221610),
VHL (1:4,000, ab140989) and GAPDH (1:1,000; cat. no. ab181602)
overnight at 4°C. The membranes were then washed with TBS + 0.1%
Tween-20 3 times, and then cultured with the goat anti-rabbit
IgG-HRP secondary antibody conjugated with horseradish peroxidase
(1:10,000, cat. no. ab6721, Abcam) for 1 h at room temperature.
Finally, the protein bands were visualized using
electrochemiluminescence according to manufacturer's protocol and
analyzed using Quantity One software version 4.6.6 (Bio-Rad
Laboratories, Inc.).
Cell viability assay
Cell proliferation was determined using a Cell
Counting Kit-8 (CCK-8) assay. According to the manufacturer's
protocol, cells were seeded onto 96-well plates at the density of
1,000 cells/well. A total of 10 µl CCK-8 solution was added to each
well and incubated at 37°C for 1.5 h; the cell activity was
detected at 24 h intervals and the absorbance values at 450 nm
wavelength were measured with a microplate spectrophotometer
(Bio-Rad Laboratories, Inc.). All experiments were performed in
triplicate.
Detection of apoptosis by Annexin
V-fluorescein isothiocyanate (FITC)/propidium iodide (PI)
double-staining
Cell apoptosis was examined using the Annexin-V-FITC
Apoptosis Detection kit. The myocardial cells (1–5×106)
were collected and washed in PBS following H/R treatment. Then, the
myocardial cells were resuspended in binding buffer with 5 µl
Annexin V-FITC and 10 µl PI double-stain, following the
manufacturer's protocol. Finally, the samples were analyzed by a
flow cytometer (BD Biosciences) and FlowJo version 7.6.3 software
(Flow Jo LLC).
miR-155 target gene prediction and
luciferase activity assay
Bioinformatics analysis tools including miRanda
(21), miRWalk (22) and TargetScan (23) were used to predict the possible
target mRNA of miR-155. A luciferase reporter gene assay was
conducted using the Dual-Luciferase Reporter Assay System (Promega
Corporation) according to the manufacturer's protocol. The 3′UTR
segments of the BAG5 including the wild type (WT) or mutant (Mut)
miR-155 binding sites were inserted into pGL3 luciferase vector
(Promega Corporation). The transfected cells were seeded onto
24-well plates at a density of 1×104/well. 293 cells
were then co-transfected with WT-BAG5/Mut-BAG5 plasmids and miR-155
mimic/NC by Lipofectamine® 3000 (Invitrogen; Thermo
Fisher Scientific, Inc.). Cultured for 48 h, transfected cells were
harvested and the luciferase activity was detected using a Dual
Luciferase™ reporting system (Promega Corporation). The luciferase
activity was normalized to that of Renilla luciferase
activity.
Statistical analysis
SPSS v.22.0 (IBM Corp.) software and GraphPad Prism
v.5.0 (GraphPad Software, Inc.) were used to analyze the
experimental data. Student's t-test was used to evaluate the
difference between two groups. A one-way analysis of variance
followed by Dunnett's post-hoc test, to compare all groups with the
control group, or Tukey's post-hoc test, to compare all pairs of
groups, was performed to assess the differences between multiple
groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
Increased expression of miR-155 is
exhibited in myocardial cells exposed to H/R
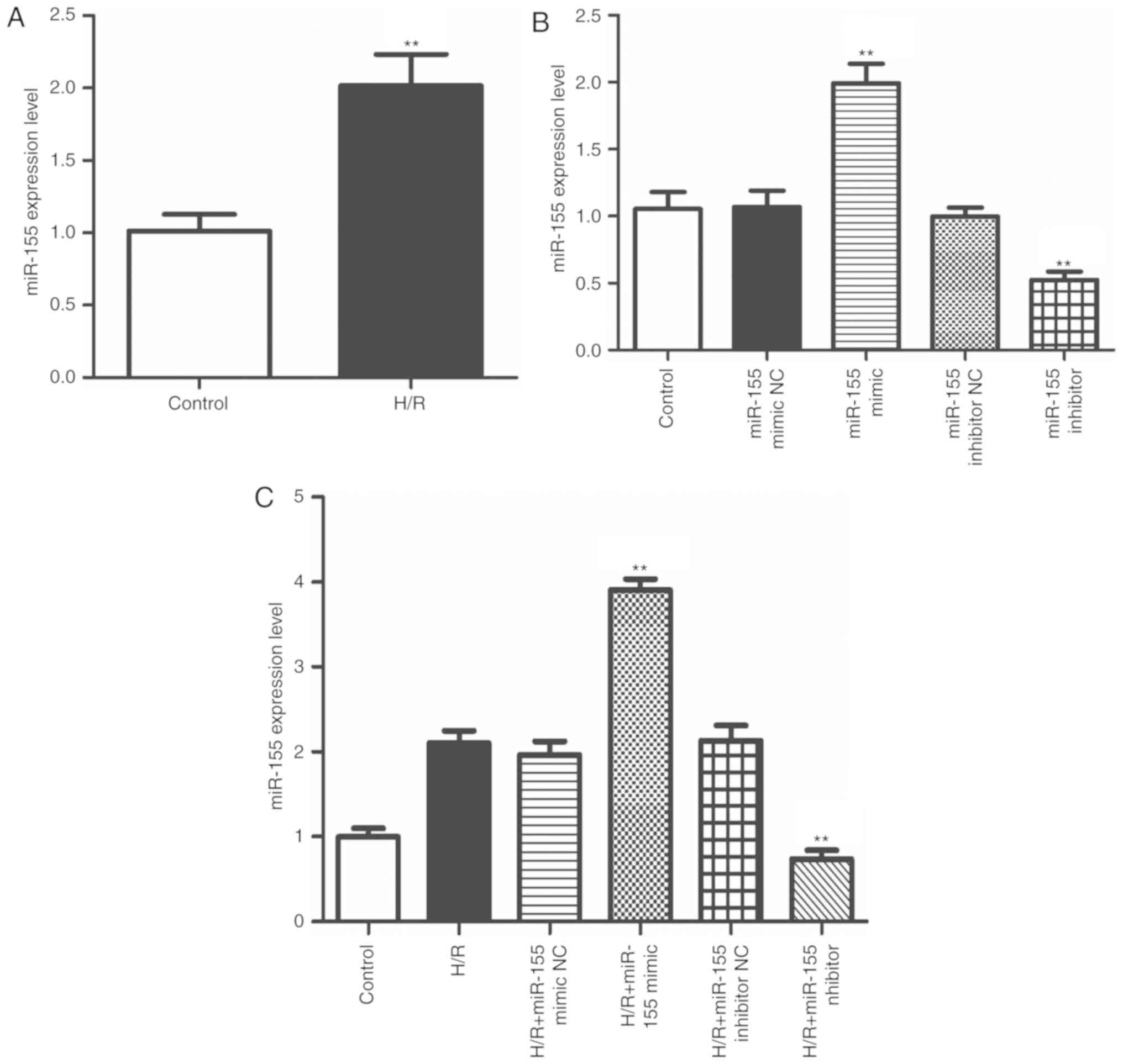
To explore the function of miR-155 in myocardial
IRI, a model of H/R in myocardial cells was first established to
simulate the cells in myocardial IRI in vivo. The RT-qPCR
data indicated that miR-155 was notably upregulated in the H/R
model cells compared with the untreated cells (Fig. 1A; P<0.01). Then, miR-155 mimics
and inhibitors were applied to regulate the expression of miR-155.
As indicated in Fig. 1B, the
expression of miR-155 was successfully up- or downregulated by
miR-155 mimics or inhibitors, respectively. Similarly, in the H/R
model, the results demonstrated that the relative expression of
miR-155 was up- or downregulated following transfection with the
miR-155 mimics or inhibitors, respectively (Fig. 1C; P<0.01). These results
suggested that miR-155 was highly expressed in myocardial cells in
the H/R model and was successfully regulated by the miR-155 mimics
and inhibitors.
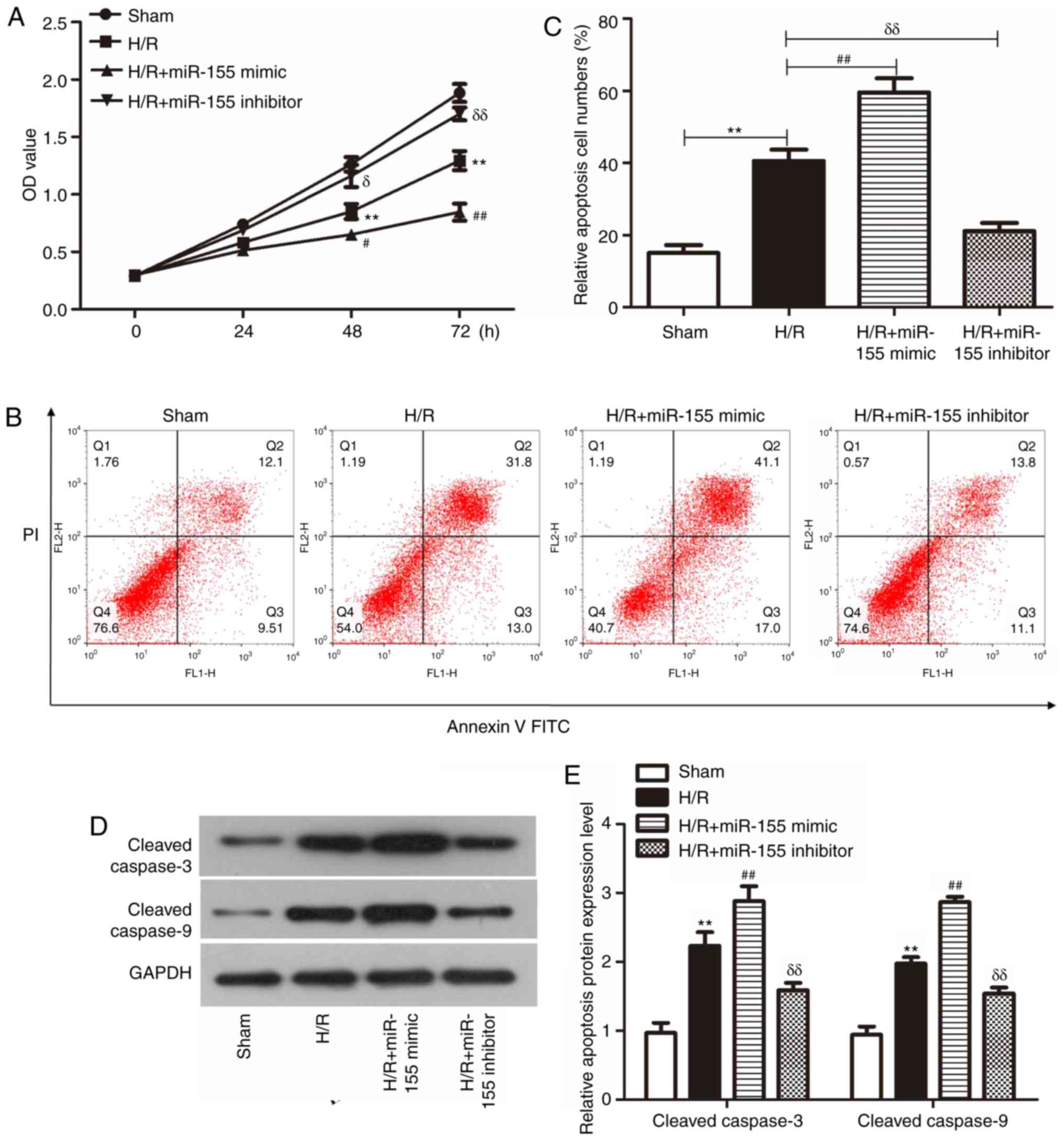
Effect of miR-155 on the activity and
apoptosis of injured H/R-stimulated myocardial cells
To additionally identify the type of cell injury
induced by H/R stimulation, a CCK-8 assay was performed to examine
cell viability. The results indicated that cell viability was
significantly decreased in the H/R model compared with the sham
group. Following transfection with the miR-155 mimic, the cell
viability was additionally decreased compared with the
non-transfected H/R cells. However, following transfection with the
miR-155 inhibitor, cell viability was markedly increased compared
with the non-transfected H/R cells (Fig. 2A; P<0.05).
Flow cytometry analysis was conducted to detect the
levels of cell apoptosis. As demonstrated in Fig. 2B and C, the number of apoptotic
cells was increased >2-fold in the H/R cells compared with the
sham group. Following transfection with the miR-155 mimic, the
number of apoptotic cells was markedly increased compared with the
non-transfected H/R model cells. However, transfection with the
miR-155 inhibitor resulted in a marked decrease in the number of
apoptotic cells compared with the non-transfected H/R model
cells.
Western blot analysis was used to identify the
expression levels of the apoptotic proteins cleaved caspase 3 and 9
following different treatments. The data from Fig. 2D and E indicated that the
expression of cleaved caspase-3 and 9 were markedly increased in
the H/R-treated cells compared with the sham group. Following
transfection with the miR-155 mimic, the expression levels of
cleaved caspase-3 and 9 were markedly increased compared with the
non-transfected H/R group. However, following transfection with
miR-155 mimic, the expression of cleaved caspase-3 and 9 were
markedly decreased compared with the non-transfected H/R group.
Based on these results, it was identified that the overexpression
of miR-155 aggravated myocardial cell injury in a H/R model.
Conversely, miR-155 downregulation reversed these effects.
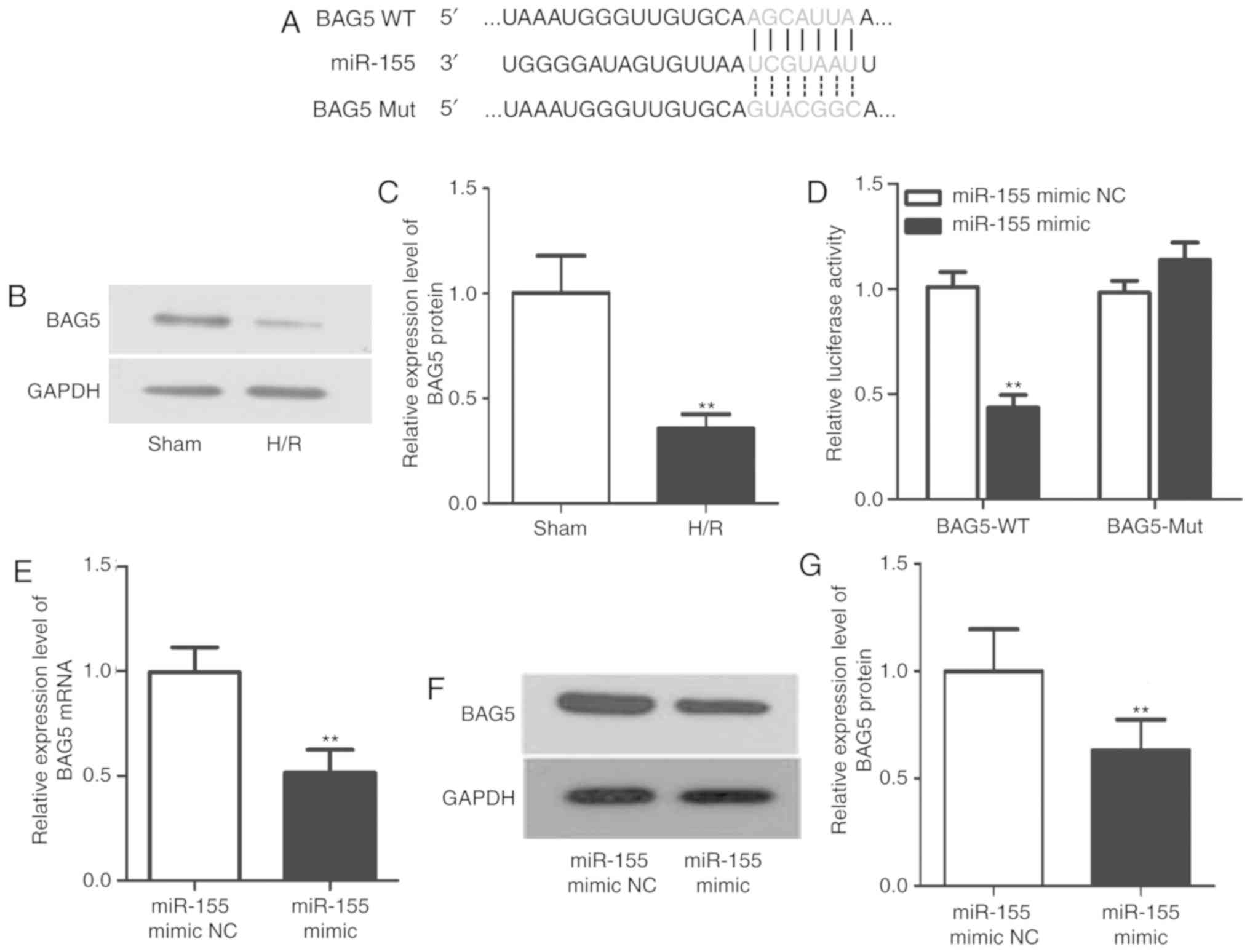
miR-155 directly targets BAG5 to
function in H/R
It is well-known that miRNAs serve various roles by
regulating their downstream target genes. Bioinformatics analysis
tools including miRanda, miRWalk and TargetScan were applied to
predict the possible target mRNA of miR-155. BAG5 was predicted as
a target of miR-155. Each miRNA was predicted to have multiple
target mRNAs. BAG5 was selected due to its involvement in a number
of important physiological and pathological processes, including
the development of tumors and the treatment of Parkinson's disease
(24), but few studies have been
performed on ischemia-reperfusion injury. Therefore, the present
study focused on BAG5. The sequences of WT-BAG5, Mut-BAG5 and
miR-155 are presented in Fig. 3A.
The data from luciferase reporter assay (Fig. 3D) indicated that the luciferase
activity was decreased in WT-BAG5 group following transfection with
the miR-155 mimic, but that the luciferase activity was nearly
unchanged in the Mut-BAG5 group, which confirmed the association
between BAG5 and miR-155. Then, the protein expression level of
BAG5 in the H/R-treated cells was examined. The data demonstrated
that BAG5 was downregulated in the H/R group compared with the sham
group (Fig. 3B and C; P<0.01).
Finally, the mRNA and protein expression levels of BAG5 were
detected in the miR-155 NC and mimic groups. In the miR-155 mimic
group, it was identified that the expression of BAG5 was
significant decreased at both mRNA and protein levels compared with
the NC group (Fig. 3E-G;
P<0.01). These results indicated that BAG5, which was expressed
at a decreased level in the H/R-treated group, was a confirmed
target of miR-155 and was negatively regulated by miR-155.
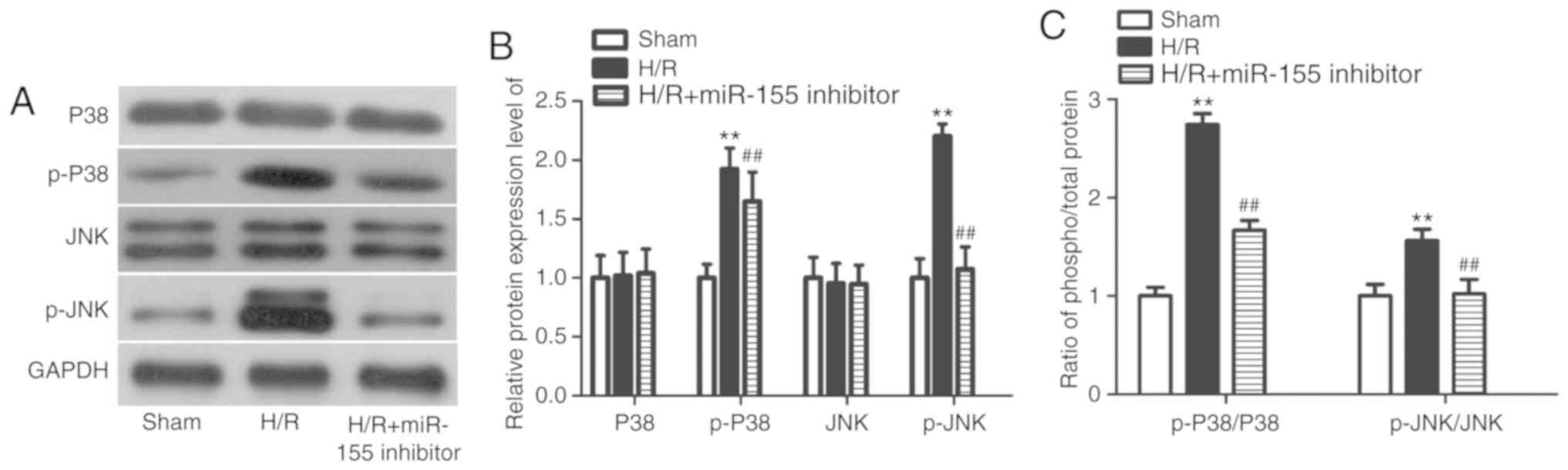
Cell injury caused by H/R is
attenuated by miR-155 inhibition partly through MAPK pathway
The MAPK signaling pathway is one of the most
important signal transduction systems in vivo, and is
involved in mediating various physiological and pathological
processes such as cell growth, development, division and
differentiation. MAPK subfamilies in mammals primarily include ERK,
JNK and P38. However, JNK and P38 have been demonstrated to
regulate cell apoptosis (25),
proliferation (26) or pressor
response (27). In addition, JNK
and P38 have been revealed to participate the process of
ischemia/reperfusion injury in rats (28). Therefore, p-JNK and p-P38 were
selected for examination in the present study. In the H/R model, it
was identified that the protein expression levels of p-P38 and
p-JNK were significantly increased. Following transfection with the
miR-155 inhibitor, the protein expression of p-P38 and p-JNK were
markedly decreased (Fig. 4A and B;
P<0.01). In addition, the increased ratios of phosphorylated to
total P38 and JNK proteins in the H/R model was suppressed
following miR-155 inhibition. This result suggested that the
overexpression of p-P38 and p-JNK in the H/R model cells was
inhibited following transfection with the miR-155 inhibitor,
suggesting that miR-155 functions as a regulator in myocardial H/R
injury, partly through the MAPK signaling pathway.
Overexpression of BAG5 enhances the
effect of miR-155 inhibitor on H/R-induced myocardial cells
impairment
Following on from the aforementioned results, it was
demonstrated that miR-155 silencing promoted the protective effects
on myocardial injury caused by H/R. However, the effects of
miR-155/BAG5 on myocardial cell injury caused by H/R were unclear.
The miR-155 inhibitor and pcDNA3.1-BAG5 were co-transfected into
the H9c2 cells to evaluate the effect of miR-155/BAG5 on cells
viability and apoptosis. As presented in Fig. 5A, the expression of BAG5 was
significantly upregulated after transfection with pcDNA3.1-BAG5
compared with the sham or empty vector. The CCK-8 assay data
indicated that BAG5 may promote the mitigative effect of miR-155
inhibition on the H9c2 cells damage induced by H/R (Fig. 5B; P<0.05). The flow cytometry
data demonstrated that overexpression of BAG5 promoted the
inhibitory effect of miR-155 inhibitor on the apoptosis of H9c2
cells (Fig. 5C and D;
P<0.01).
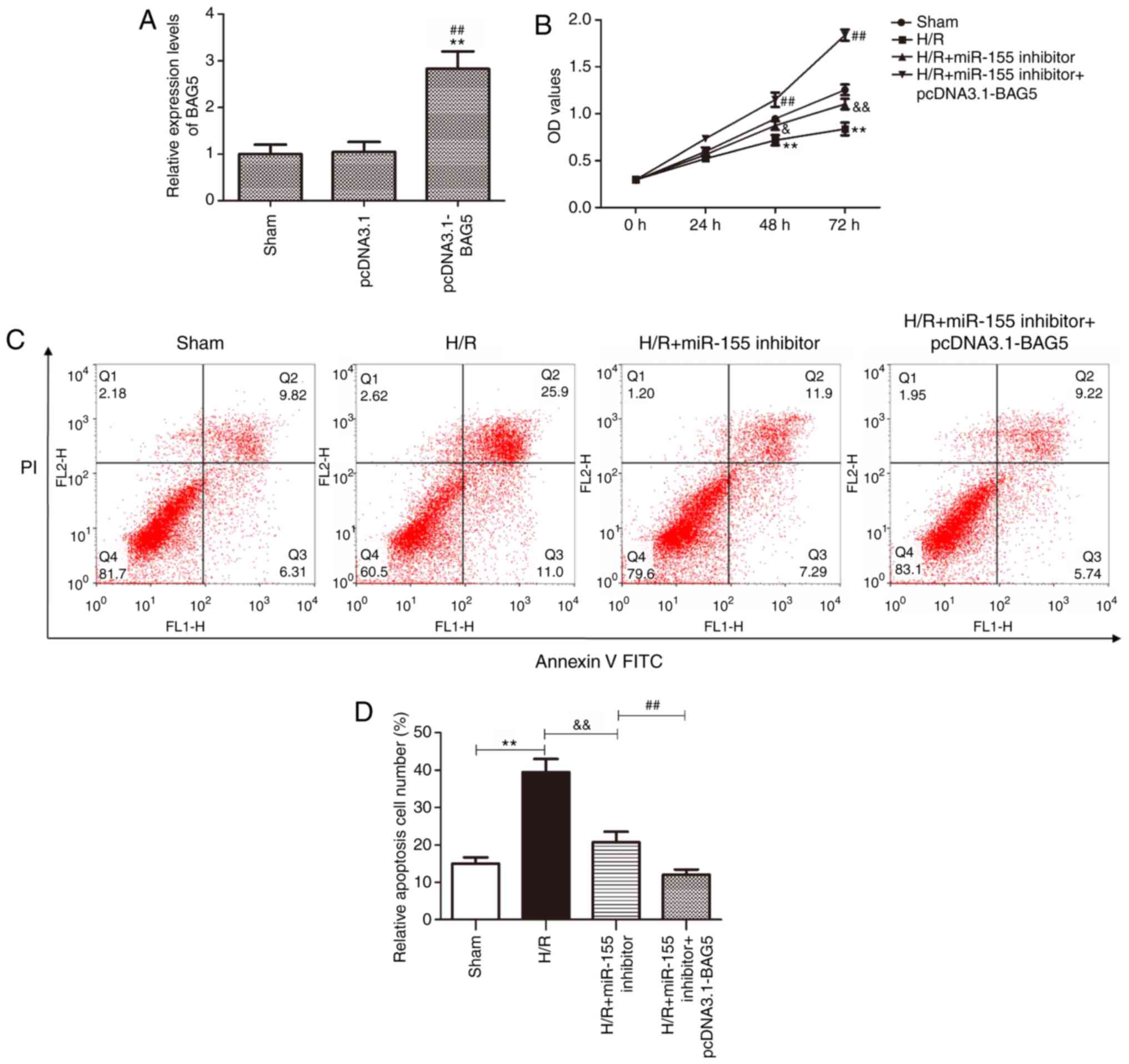 | Figure 5.Overexpression of BAG5 enhances the
protective effect of the miR-155 inhibitor on myocardial cells
damage in the H/R model. (A) The transfection efficiency of
pcDNA3.1-BAG5 was measured by reverse transcription-quantitative
polymerase chain reaction. **P<0.01 vs. sham,
##P<0.01 vs. pcDNA3.1. (B) Cell viability was
detected using CCK-8 assay following transfection with miR-155
inhibitor and pcDNA3.1-BAG5 in the H/R model. δP<0.05
and δδP<0.01 vs. H/R, **P<0.01 vs. sham,
##P<0.01 vs. H/R + miR-155 inhibitor. (C) The level
of cell apoptosis was measured by flow cytometry following
transfection with miR-155 inhibitor and pcDNA3.1-BAG5 in the H/R
model. (D) Quantified flow cytometry data. **P<0.01 vs. sham,
##P<0.01 vs. H/R + miR-155 inhibitor,
δδP<0.01 vs. H/R. BAG5, BAG family molecular
chaperone regulator 5; miR, microRNA; H/R, hypoxia/reoxygenation;
FITC, fluorescein isothiocyanate; PI, propidium iodide. |
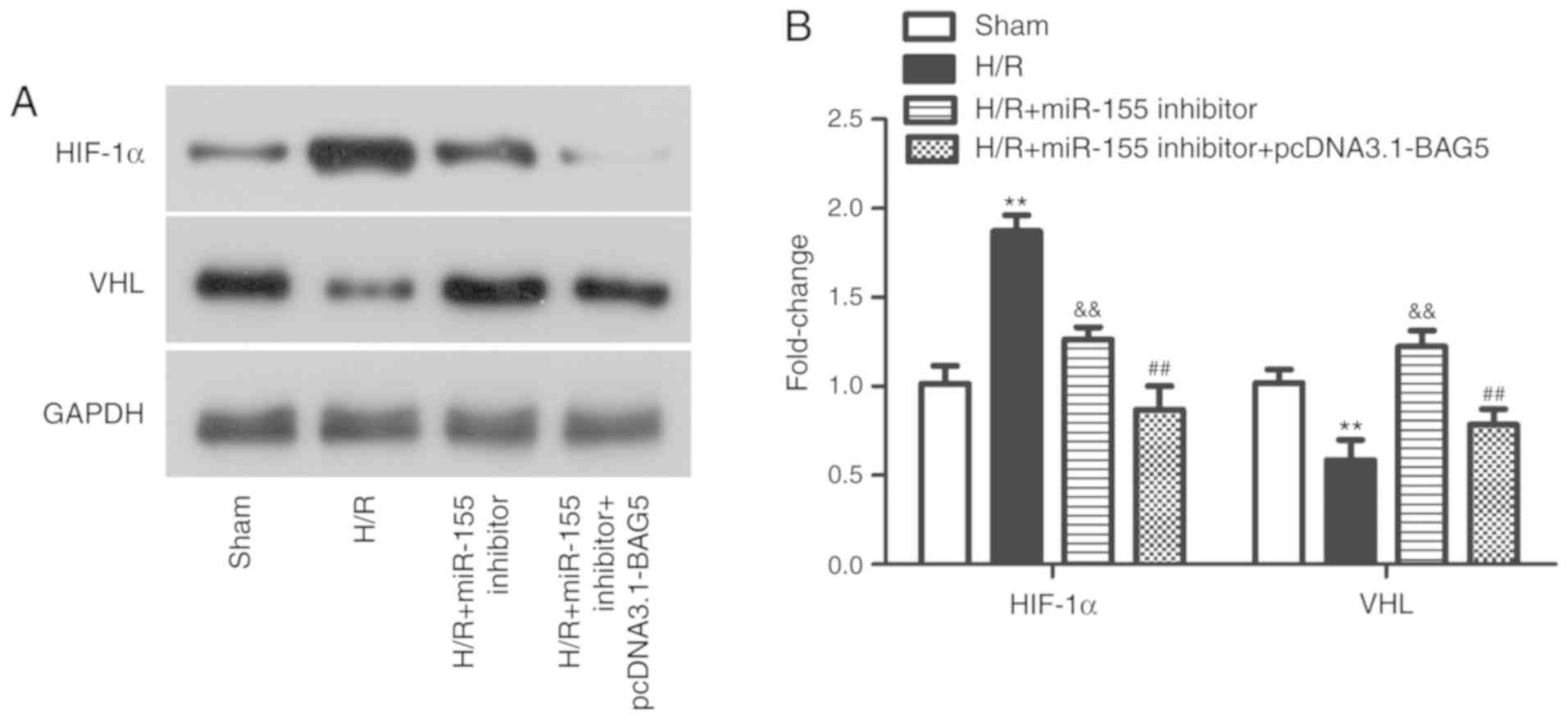
It is well-known that HIF regulates cellular
protection against decreased oxygen delivery via modulating
cellular pathways and functions (29,30).
The oxygen-regulated HIF-1α subunit is continuously synthesized in
cells but promptly degraded in the presence of oxygen following
hydroxylation. Hydroxylated HIF is targeted for proteasomal
degradation after binding to the VHL E3 ubiquitin ligase (31). Therefore, the expression levels of
HIF-1α and VHL in the H/R model of the present study were detected
following different treatment. In the H/R model, the expression of
HIF-1α was increased, while the expression of VHL was decreased
compared with the sham group. However, this phenomenon was reversed
after transfection with miR-155 inhibitor or miR-155 inhibitor +
pcDNA3.1-BAG5 (Fig. 6A and B;
P<0.01). Altogether, the results demonstrated that
co-transfection with pcDNA3.1-BAG5 and miR-155 inhibitor decreased
the level of myocardial cells apoptosis and increased the level of
proliferation compared with the cells only transfected with miR-155
inhibitor, through altering the expression patterns of HIF-1α and
VHL, suggesting that depletion of miR-155 may attenuate cell damage
in the myocardial H/R model by targeting BAG5 and regulating the
expression of HIF-1α and VHL.
Discussion
Reperfusion injury is one of the most significant
complications in the recovery of ischemic myocardial blood flow.
The toxic and side effects of reperfusion injury are caused by
numerous complex pathological mechanism (32). Various forms of myocardial IRI have
been identified, including reperfusion induced arrhythmias,
myocardial coma, microvascular obstruction and fatal myocardial
reperfusion injury; only the first two of these are reversible
(33). The underlying mechanisms
of IRI are not entirely clear (34), but the modification of molecular,
cellular and tissues through processes such as oxidative stress,
cell death, neurohumoral activation and inflammation are regarded
critical in the development of IRI (35,36).
Although a complete understanding of myocardial IRI completely
remains a challenge, active reperfusion remains the most important
method and is being vigorously initiated. The present study
identified that depletion of miR-155 improved myocardial cells
injury caused by H/R. The data from the CCK-8 assay demonstrated
that the downregulation of miR-155 enhanced the myocardial cells
activity, the flow cytometry analysis data revealed that miR-155
inhibition attenuated myocardial cells apoptosis, and the western
blot analysis data indicated that depletion of miR-155 decreased
the protein expression levels of key apoptotic proteins caspase 3
and caspase 9. Using Bioinformatics prediction software programs
and a dual-luciferase reporter assay, BAG5 was confirmed as a
direct functional target of miR-155. In addition, BAG5 was
downregulated in the H/R model cells and was negatively regulated
by miR-155. The data from the co-transfection assay suggested that
overexpression of BAG5 could promote the protective effect of
miR-155 inhibitor on cell damage caused by H/R through regulating
the expression of HIF-1α and VHL. Taken together, the results of
the present study indicated that the inhibition of miR-155
expression improved the outcomes of myocardial IRI through
mediating BAG5 and the MAPK signaling pathway.
miR-155, as a multifunctional RNA, has been
identified to function in numerous pathological and physiological
processes including viral infection (37), hematopoietic lineage
differentiation (38),
cardiovascular disease (39),
immunity (40), cancer (41), inflammation (42) and Down syndrome (43). In the present study, it was
identified that miR-155 was highly expressed in the myocardial H/R
model, and that overexpression of miR-155 decreased myocardial
cells activity, increased the number of apoptotic cells and
increased the expression of pro-apoptotic proteins. However,
silencing miR-155 improved myocardial cell viability, decreased the
number of apoptotic cells and attenuated the expression levels of
pro-apoptotic proteins. These results indicated that miR-155
functioned as a crucial modulator in myocardial IRI.
It is well-known that mature miRNA regulate gene
expression through binding to complementary sites in the
3′-untranslated region of their target genes. According to the
analysis results of bioinformatics prediction software, BAG5 was
selected as a specific target gene of miR-155. BAG5, as an
important member of the BAG family, has been reported to
demonstrate anti-apoptotic effects in prostate cancer (44). A previous study identified that
BAG5 protected neuronal cells from amyloid β-induced cell death in
Alzheimer's disease (45), and it
has been demonstrated that BAG5 decreased the degradation of PTEN
and maintained its stability through an ubiquitylation-dependent
pathway (46). It has previously
been observed that BAG5 may promote the accumulation of mutant p53
in tumors, and increase the gain-of-functions of mutant p53
(47). The study conducted by
Kalia et al (48)
established that BAG5 may inhibit parkin and enhance dopaminergic
neuron degeneration. In addition, it has been suggested that BAG5
may function together with miR-127-3p to inhibit epithelial ovarian
cancer cell growth (49). The
present study demonstrated that BAG5 was a direct target gene of
miR-155, and was downregulated in H/R. Concomitantly,
overexpression of miR-155 was demonstrated to attenuate the levels
of BAG5 expression, and overexpression of BAG5 in the H/R model
promoted the protective effects of the miR-155 inhibitor on
H/R-induced cell damage. A limitation of the present study was that
only the basic phenomena were identified, and that cell models
in vitro cannot fully simulate in vivo scenarios;
further in vivo experiments are required to explore the
specific mechanism.
In the process of myocardial H/R, the
pathophysiological significance of myocardial cell apoptosis is
important. It has been suggested that the JNK/MAPK signaling
pathway is associated with various pathophysiological processes
during apoptosis and oxidative stress. Bourke et al
(50) demonstrated that, in an
in vitro H/R injury model, anti-phospholipid antibodies
enhanced the levels of apoptosis of newborn rat cardiac myocytes
through p38 MAPK. It has previously been observed that myocardial
cells were protected from H/R injury through inhibiting JNK
(51). In concordance with
previous data, the present study identified that the expression
levels of MAPK/JNK-associated proteins p-P38 and p-JNK were
markedly decreased following transfection with the miR-155
inhibitor, indicating that miR-155 regulated myocardial H/R injury
through the MAPK/JNK signaling pathway.
The results from the present study demonstrated that
miR-155/BAG5 function in myocardial H/R injury partly through the
MAPK/JNK signaling pathway, suggesting that miR-155/BAG5 may be
promising targets for the development of novel therapies for
myocardial IRI.
In conclusion; the present study demonstrated that
miR-155 was highly expressed in a myocardial H/R model, leading to
decreased levels of myocardial cell proliferation and increased
levels of apoptosis. Bioinformatics prediction software and a
luciferase reporter assay confirmed that BAG5 was a specific target
gene of miR-155. In addition, co-transfection of the miR-155
inhibitor and pcDNA3.1-BAG5 further indicated that miR-155
inhibition alleviated the myocardial H/R injury through targeting
BAG5, via the MAPK/JNK signaling pathway.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and /or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
JX conceived, designed, and performed the
experiments and edited the manuscript. QL performed the experiments
and provided technical support. BL analyzed the data and edited the
manuscript. NL conceived, supervised and supported the study and
edited the manuscript. All authors have read, revised and approved
the final version of the manuscript. All authors agree to be
accountable for all aspects of the work in ensuring that questions
related to the accuracy or integrity of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jennings RB, Sommers HM, Smyth GA, Flack
HA and Linn H: Myocardial necrosis induced by temporary occlusion
of a coronary artery in the dog. Arch Pathol. 70:68–78.
1960.PubMed/NCBI
|
|
2
|
Eltzschig HK and Eckle T: Ischemia and
reperfusion-from mechanism to translation. Nat Med. 17:1391–401.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sun G, Lu Y, Li Y, Mao J, Zhang J, Jin Y,
Li Y, Sun Y, Liu L and Li L: miR-19a protects cardiomyocytes from
hypoxia/reoxygenation-induced apoptosis via PTEN/PI3K/p-Akt
pathway. Biosci Rep. 37:BSR201708992017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang X, Ha T, Hu Y, Lu C, Liu L, Zhang X,
Kao R, Kalbfleisch J, Williams D and Li C: MicroRNA-214 protects
against hypoxia/reoxygenation induced cell damage and myocardial
ischemia/reperfusion injury via suppression of PTEN and Bim1
expression. Oncotarget. 7:86926–86936. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Qiu R, Li W and Liu Y: MicroRNA-204
protects H9C2 cells against hypoxia/reoxygenation-induced injury
through regulating SIRT1-mediated autophagy. Biomed Pharmacother.
100:15–19. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kawaguchi M, Takahashi M, Hata T, Kashima
Y, Usui F, Morimoto H, Izawa A, Takahashi Y, Masumoto J, Koyama J,
et al: Inflammasome activation of cardiac fibroblasts is essential
for myocardial ischemia/reperfusion injury. Circulation.
123:594–604. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Feng R, Liu J, Wang Z, Zhang J, Cates C,
Rousselle T, Meng Q and Li J: The structure-activity relationship
of ginsenosides on hypoxia-reoxygenation induced apoptosis of
cardiomyocytes. Biochem Biophys Res Commun. 494:556–568. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li Y, Shi X, Li J, Zhang M and Yu B:
Knockdown of KLF11 attenuates hypoxia/reoxygenation injury via
JAK2/STAT3 signaling in H9c2. Apoptosis. 22:510–518. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tutar Y: miRNA and cancer; computational
and experimental approaches. Curr Pharm Biotechnol. 15:4292014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lesizza P, Prosdocimo G, Martinelli V,
Sinagra G, Zacchigna S and Giacca M: Single-dose intracardiac
injection of Pro-regenerative MicroRNAs improves cardiac function
after myocardial infarction. Circ Res. 120:1298–1304. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li YG, Zhang PP, Jiao KL and Zou YZ:
Knockdown of microRNA-181 by lentivirus mediated siRNA expression
vector decreases the arrhythmogenic effect of skeletal myoblast
transplantation in rat with myocardial infarction. Microvasc Res.
78:393–404. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang J, Bai Y, Li N, Ye W, Zhang M, Greene
SB, Tao Y, Chen Y, Wehrens XH and Martin JF: Pitx2-microRNA pathway
that delimits sinoatrial node development and inhibits
predisposition to atrial fibrillation. Proc Natl Acad Sci USA.
111:9181–6. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rodriguez A, Vigorito E, Clare S, Warren
MV, Couttet P, Soond DR, van Dongen S, Grocock RJ, Das PP, Miska
EA, et al: Requirement of bic/microRNA-155 for normal immune
function. Science. 316:608–611. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Elton TS, Selemon H, Elton SM and
Parinandi NL: Regulation of the MIR155 host gene in physiological
and pathological processes. Gene. 532:1–12. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Faraoni I, Antonetti FR, Cardone J and
Bonmassar E: miR-155 gene: A typical multifunctional microRNA.
Biochim Biophys Acta. 1792:497–505. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nazari-Jahantigh M, Wei Y, Noels H, Akhtar
S, Zhou Z, Koenen RR, Heyll K, Gremse F, Kiessling F, Grommes J, et
al: MicroRNA-155 promotes atherosclerosis by repressing Bcl6 in
macrophages. J Clin Invest. 122:4190–202. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Eisenhardt SU, Weiss JB, Smolka C,
Maxeiner J, Pankratz F, Bemtgen X, Kustermann M, Thiele JR, Schmidt
Y, Bjoern Stark G, et al: MicroRNA-155 aggravates
ischemia-reperfusion injury by modulation of inflammatory cell
recruitment and the respiratory oxidative burst. Basic Res Cardiol.
110:322015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nederlof R, Eerbeek O, Hollmann MW,
Southworth R and Zuurbier CJ: Targeting hexokinase II to
mitochondria to modulate energy metabolism and reduce
ischaemia-reperfusion injury in heart. Br J Pharmacol.
171:2067–2079. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tan L, Jiang W, Lu A, Cai H and Kong L:
miR-155 aggravates liver Ischemia/reperfusion injury by suppressing
SOCS1 in mice. Transplant Proc. 50:3831–3839. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Enright AJ, John B, Gaul U, Tuschl T,
Sander C and Marks DS: MicroRNA targets in drosophila. Genome Biol.
5:R12003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dweep H and Gretz N: miRWalk2.0: A
comprehensive atlas of microRNA-target interactions. Nat Methods.
12:6972015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lewis BP, Burge CB and Bartel DP:
Conserved seed pairing, often flanked by adenosines, indicates that
thousands of human genes are microRNA targets. Cell. 120:15–20.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kalia SK, Kalia LV and McLean PJ:
Molecular chaperones as rational drug targets for Parkinson's
disease therapeutics. CNS Neurol Disord Drug Targets. 9:741–753.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Harper SJ and LoGrasso P: Signalling for
survival and death in neurones: The role of stress-activated
kinases, JNK and p38. Cell Signal. 13:299–310. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lamb JA, Ventura JJ, Hess P, Flavell RA
and Davis RJ: JunD mediates survival signaling by the JNK signal
transduction pathway. Mol Cell. 11:1479–1489. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chan SH, Hsu KS, Huang CC, Wang LL, Ou CC
and Chan JY: NADPH oxidase-derived superoxide anion mediates
angiotensin II-induced pressor effect via activation of p38
mitogen-activated protein kinase in the rostral ventrolateral
medulla. Circ Res. 97:772–780. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shao Z, Bhattacharya K, Hsich E, Park L,
Walters B, Germann U, Wang YM, Kyriakis J, Mohanlal R, Kuida K, et
al: c-Jun N-terminal kinases mediate reactivation of Akt and
cardiomyocyte survival after hypoxic injury in vitro and in vivo.
Circ Res. 98:111–118. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kaelin WG Jr and Ratcliffe PJ: Oxygen
sensing by metazoans: The central role of the HIF hydroxylase
pathway. Mol Cell. 30:393–402. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Semenza GL: HIF-1 inhibitors for cancer
therapy: From gene expression to drug discovery. Curr Pharm Des.
15:3839–3843. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Epstein AC, Gleadle JM, McNeill LA,
Hewitson KS, O'Rourke J, Mole DR, Mukherji M, Metzen E, Wilson MI,
Dhanda A, et al: C. elegans EGL-9 and mammalian homologs define a
family of dioxygenases that regulate HIF by prolyl hydroxylation.
Cell. 107:43–54. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Brennan J: Reperfusion injury of cardiac
myocytes: Mechanisms, treatment, and implications for advanced
practice nursing. AACN Clin Issues. 11:252–260. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hausenloy DJ and Yellon DM: Myocardial
ischemia-reperfusion injury: A neglected therapeutic target. J Clin
Invest. 123:92–100. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Baines CP: How and when do myocytes die
during ischemia and reperfusion: The late phase. J Cardiovasc
Pharmacol Ther. 16:239–243. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Braunwald E: The war against heart
failure: The lancet lecture. Lancet. 385:812–824. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhao ZQ: Oxidative stress-elicited
myocardial apoptosis during reperfusion. Curr Opin Pharmacol.
4:159–165. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Podsiad A, Standiford TJ, Ballinger MN,
Eakin R, Park P, Kunkel SL, Moore BB and Bhan U: MicroRNA-155
regulates host immune response to postviral bacterial pneumonia via
IL-23/IL-17 pathway. Am J Physiol Lung Cell Mol Physiol.
310:L465–L475. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bayraktar R and Van Roosbroeck K: miR-155
in cancer drug resistance and as target for miRNA-based
therapeutics. Cancer Metastasis Rev. 37:33–44. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gangwar RS, Rajagopalan S, Natarajan R and
Deiuliis JA: Noncoding RNAs in cardiovascular disease: Pathological
relevance and emerging role as biomarkers and therapeutics. Am J
Hypertens. 31:150–165. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhai A, Qian J, Kao W, Li A, Li Y, He J,
Zhang Q, Song W, Fu Y, Wu J, et al: Borna disease virus encoded
phosphoprotein inhibits host innate immunity by regulating miR-155.
Antiviral Res. 98:66–75. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Cao S, Wang Y, Li J, Lv M, Niu H and Tian
Y: Tumor-suppressive function of long noncoding RNA MALAT1 in
glioma cells by suppressing miR-155 expression and activating FBXW7
function. Am J Cancer Res. 6:2561–2574. 2016.PubMed/NCBI
|
|
42
|
Teramura T and Onodera Y: Stem cell
depletion by inflammation-associated miR-155. Aging (Albany NY).
10:17–18. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Tili E, Mezache L, Michaille JJ, Amann V,
Williams J, Vandiver P, Quinonez M, Fadda P, Mikhail A and Nuovo G:
microRNA 155 up regulation in the CNS is strongly correlated to
Down's syndrome dementia. Ann Diagn Pathol. 34:103–109. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Bruchmann A, Roller C, Walther TV, Schafer
G, Lehmusvaara S, Visakorpi T, Klocker H, Cato AC and Maddalo D:
Bcl-2 associated athanogene 5 (Bag5) is overexpressed in prostate
cancer and inhibits ER-stress induced apoptosis. BMC Cancer.
13:962013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Guo K, Li L, Yin G, Zi X and Liu L: Bag5
protects neuronal cells from amyloid β-induced cell death. J Mol
Neurosci. 55:815–820. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ying Z, Haiyan G and Haidong G: BAG5
regulates PTEN stability in MCF-7 cell line. BMB Rep. 46:490–494.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yue X, Zhao Y, Huang G, Li J, Zhu J, Feng
Z and Hu W: A novel mutant p53 binding partner BAG5 stabilizes
mutant p53 and promotes mutant p53 GOFs in tumorigenesis. Cell
Discov. 2:160392016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kalia SK, Lee S, Smith PD, Liu L, Crocker
SJ, Thorarinsdottir TE, Glover JR, Fon EA, Park DS and Lozano AM:
BAG5 inhibits parkin and enhances dopaminergic neuron degeneration.
Neuron. 44:931–945. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Bi L, Yang Q, Yuan J, Miao Q, Duan L, Li F
and Wang S: MicroRNA-127-3p acts as a tumor suppressor in
epithelial ovarian cancer by regulating the BAG5 gene. Oncol Rep.
36:2563–2570. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Bourke LT, McDonnell T, McCormick J,
Pericleous C, Ripoll VM, Giles I, Rahman A, Stephanou A and Ioannou
Y: Antiphospholipid antibodies enhance rat neonatal cardiomyocyte
apoptosis in an in vitro hypoxia/reoxygenation injury model via p38
MAPK. Cell Death Dis. 8:e25492017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Li Q, Xiang Y, Chen Y, Tang Y and Zhang Y:
Ginsenoside Rg1 protects cardiomyocytes against
hypoxia/reoxygenation injury via activation of Nrf2/HO-1 signaling
and inhibition of JNK. Cell Physiol Biochem. 44:21–37. 2017.
View Article : Google Scholar : PubMed/NCBI
|