Introduction
Colorectal cancer (CRC) is one of the main causes of
cancer deaths and the fourth most frequent cancer worldwide, with
about 1,096,601 newly diagnosed cases and 551,269 deaths in 2018
(1). Despite the increase in the
incidence of CRC over the last 20 years, CRC mortality has
decreased in many countries, probably due to prevention strategies,
early detection and more effective treatment (1). Surgery is the gold standard treatment
for early colorectal tumors: Indeed it is associated with 5-year
cancer-specific survival rates of 91.4 and 70.2% in stage II and
III forms, respectively (2).
Treatment of CRC is defined based on tumor stage. Very early tumors
can be treated using local excision, while neoadjuvant
chemora-diotherapy is indicated in locally-advanced rectal cancer
(3,4). Metastatic disease (stage IV) is
usually treated with chemotherapy with a combination of
5-fluorouracil and leucovorin (e.g. oxaliplatin- FOLFOX,
irinotecan-FOLFIRI). In addition, two monoclonal antibodies against
the epidermal growth factor receptor are now used in combination
with well-established CRC-treatment schedules (3,5–7).
According to the American Cancer Society Guidelines, radiation
therapy can be considered for colon cancer therapy to promote
cancer reduction before surgery. It can also be used after surgery
in cases in which the tumor adheres to other organs and cannot be
totally removed surgically. Moreover, the so-called intraoperative
radiation therapy can be used during surgery to kill cancer cells
in their location. Lastly, radiotherapy combined with chemotherapy
can be used for unresectable cancer or to attenuate symptoms in
advanced cancers and in case of metastases (8,9).
Although anticancer therapies have yielded a good success rate, in
terms of overall survival, they fail to kill cancer cells in over
90% of patients with advanced CRC due to the development of therapy
resistance. Metastatic cancer cells are characterized by
mesenchymal and stemness features conferring them aberrant survival
capacity and evasion of apoptosis that represent the major
mechanisms responsible for cancer resistance to therapy (10).
LiCl is the most well studied GSK-3β inhibitor. It
exerts its effect through a direct and indirect mechanism. In the
first case, it competes with the GSK-3β cofactor Mg2+, thereby
inhibiting the enzyme's activity, whereas in the second case, it
increases the inhibition of phosphorylation of GSK-3β Ser9
(11). In addition to its role in
the regulation of GSK-3β, LiCl emerged as a promising drug for
various diseases, such as neurological diseases, cancer, and
inflammation (12–14). We previously demonstrated that LiCl
induces the differentiation and the mesenchymal-to-epithelial
transition (MET) of primary colon cancer cells, thereby reducing
migration and stemness features (15,16).
Since mesenchymal and stemness features are the main causes of
aberrant survival capacity and evasion of apoptosis during cancer
progression, we suggested that LiCl could sensitize colon cancer
cells to chemo-radiotherapy. To address this issue, we investigated
the effects of LiCl treatment on the viability of primary
mesenchymal colon cancer cells in combination with radiation
therapy. We observed that LiCl and high-energy photon irradiation
had an additive effect both on the viability of mesenchymal colon
cancer cells, and on the induction of apoptosis. Finally, at
molecular level, we found that LiCl induced strong Survivin
down-regulation and p53 and Bax up-regulation. We believe that
these molecular changes could contribute to LiCl-mediated
sensitization to high-energy photon irradiation in CRC.
Materials and methods
Sample collection
T88 primary CRC cells were previously isolated and
characterized (15,16). The patient study was approved by
the ethics committe of the University of Naples Federico II,
‘Comitato etico per le attività Biomediche-Carlo Romano’, with
protocol no. 35/17. The patient provided written informed consent
to the study. All methods were performed in accordance with the
relevant guidelines and regulations.
Cell culture and treatments
Primary T88 cells were cultured as reported
elsewhere (15,16). Subsequently, cell suspensions (500
µl containing 240×103 cells) were plated on 100 mm
tissue culture treated plates and cells were alternatively
incubated with LiCl (30 mmol/l) for 48 h, irradiated with 2 or 7 Gy
of high-energy photon beams or irradiated and pretreated with LiCl.
Untreated cells were compared with treated cells for subsequent
cell analysis. RKO cells were from ATCC and grown in Eagle's
Minimum Essential Medium (M22791L, Sigma) supplemented with 10%
FBS, 100 U/ml penicillin and 100 µg/ml streptomycin.
MTT assay
Untreated cells, LiCl-treated cells, irradiated
cells and cells irradiated after LiCl pretreatment were analyzed
immediately after (T0) and 48 hours after (T48 h) treatments. Cells
were washed and incubated for three hours in 100 µl DMEM (ECB7501L;
Euroclone) supplemented with 0.45 mg/ml MTT reagent; the medium was
then replaced by 100 µl of 0.1 M HCl in isopropanol and cells were
incubated 30 min for lysis. Insoluble formazan was resuspended, and
optical densities were measured at a wavelength of 570 nm with a
Microplate Reader (Biotek Synergy Microplate Reader), according to
the MTT manufacturer's protocol. Data are expressed as mean ± SEM
of three experiments.
Western blot analysis
Total protein extracts were isolated from untreated
T88 cells, LiCl treated cells, irradiated cells and cells
irradiated after LiCl pretreatment; all cells were lysed at time
T48h. Cell lysates were prepared as reported previously (17), proteins were separated by
SDS-polyacrylamide gel electrophoresis, and blots were prepared as
reported previously (18). Primary
antibodies against Survivin (rabbit polyclonal anti-human; cat. no.
2803; dilution 1:1,000), β-catenin (rabbit polyclonal anti-human;
cat. no. 9562; dilution 1:1,000) p53 (rabbit monoclonal anti-human;
cat. no. 2527) and Bax (rabbit monoclonal antihuman; cat. no.
BK2772T) were from Cell Signaling Technology; the anti-GAPDH
(monoclonal anti-mouse; sc-393358) antibody was from Santa Cruz
Biotechnology. Membranes were probed with peroxidase-conjugated
secondary antibodies against rabbit or goat IgG, and immunoreactive
bands were detected as described before (19). The experiment was repeated three
times with similar results. Densitometric analyses were performed
using Image J software.
Flow cytometry analysis
Untreated cells, LiCl-treated cells, irradiated
cells and irradiated cells pretreated with LiCl, were harvested at
time T48h, fixed and stored in ice-cold ethanol at 20°C. Propidium
iodide (PI) (Applichem) was used for cell cycle analysis, as
described previously (20).
Briefly, cells were washed twice in ice-cold PBS and then
resuspended at a concentration of about 1 million/ml of cells in
0.1% Na-citrate, 50 mg/ml RNase, 50 mg/ml propidium iodide and
incubated for 30 min in the dark at room temperature. PI
fluorescence intensity was measured using the BD C6 Accuri
cytometer and data were analyzed using the BD C6 Accuri Software
(Becton Dickinson).
Statistical analysis
All data were obtained from at least three
independent experiments and are reported as the mean ± SEM.
Statistical differences between groups was determined by the t test
and/or Kruskal-Wallis test followed by a Dunn's post hoc test at a
significance level of P<0.05.
Results
LiCl decreases the viability of
irradiated cancer cells
Using the MTT assay, we first evaluated the effect
of high-energy photon irradiation on the viability of T88 primary
colon cancer cells and of commercially available RKO colon cancer
cells. We initially irradiated cells with 2 Gy, which is the dose
generally used in clinical practice. However, neither the T88
primary colon cancer cells or the commercially available colon
cancer RKO cells responded to this treatment (data not shown).
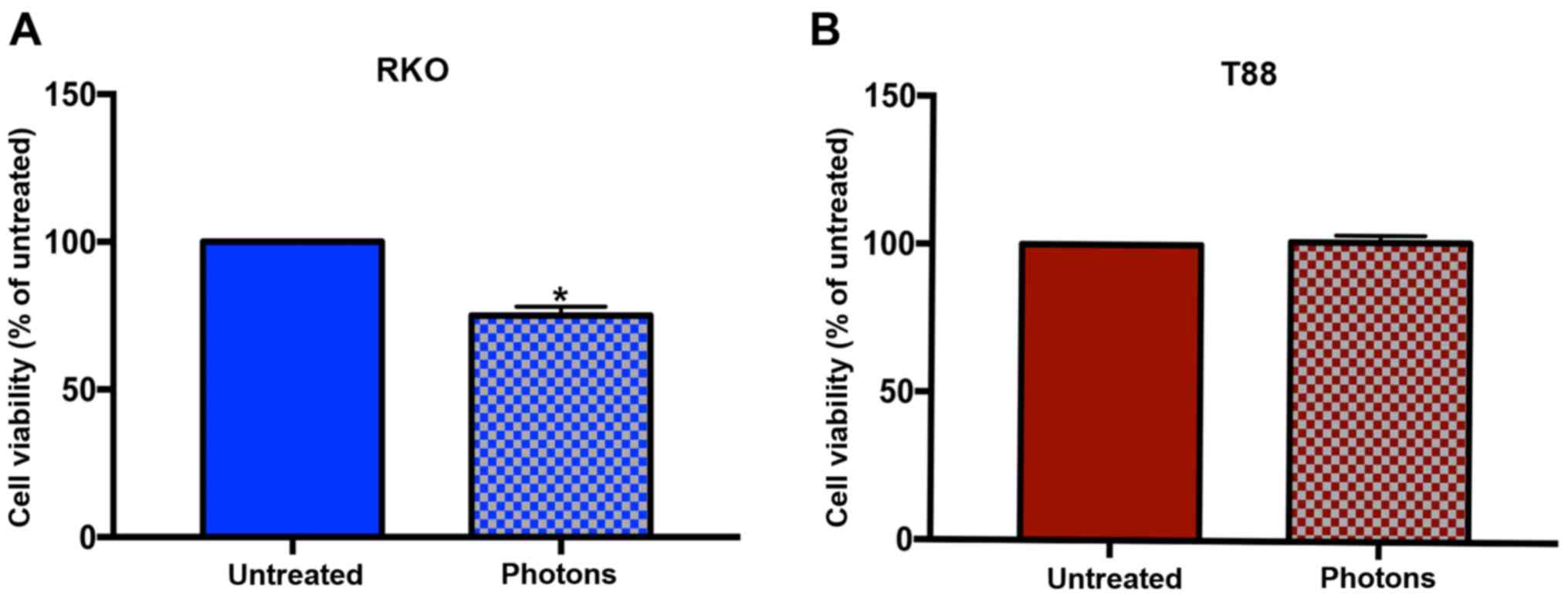
Thus, we irradiated cells with 7 Gy of high-energy photon beams,
and obtained a response, in terms of cell viability, only in RKO
cells. As shown in Fig. 1, the
photon irradiation affected the viability of RKO cells, but not
that of T88 cells, which appear completely unresponsive. To
determine whether LiCl would sensitize T88 cells to high-energy
photon irradiation, we evaluated the viability of T88 cells after
i) incubation with LiCl, ii) high energy photon irradiation and
iii) combined treatment immediately after completion of treatment
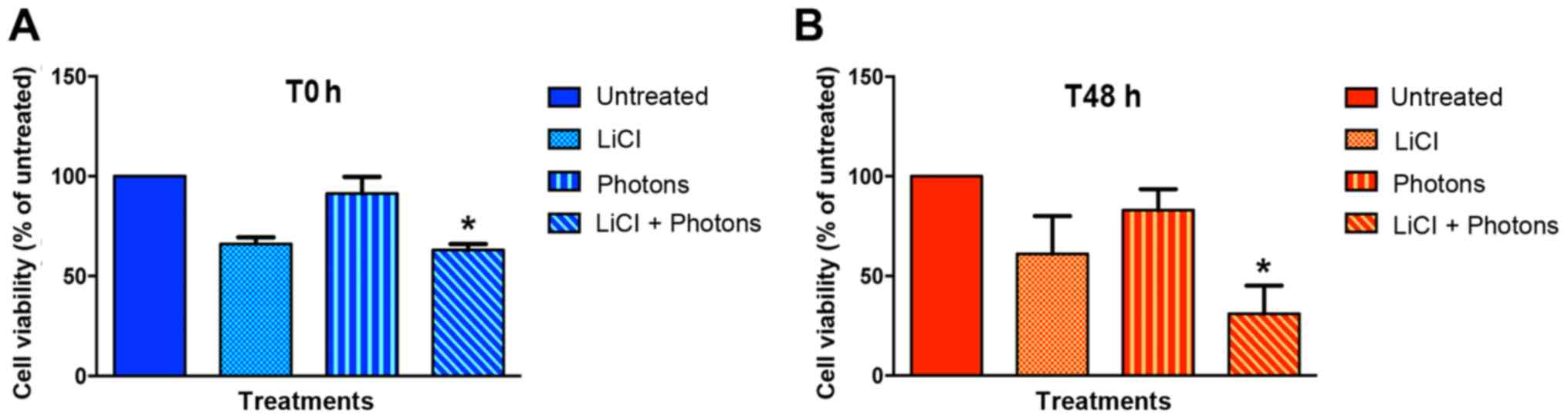
(T0) and 48 h after completion of treatment (T48). As shown in
Fig. 2 and Table I, LiCl decreased the viability of
T88 cells versus untreated cells, at both T0 (Fig. 2A) and T48 h (Fig. 2B). As expected, high-energy photon
irradiation affected cell viability only at T48. Notably, the
maximum effect on cell viability was observed when cells were
irradiated after LiCl pretreatment, which suggests that LiCl and
high-energy photon irradiation may have an additive effect.
 | Table I.Statistical analysis of MTT
assay. |
Table I.
Statistical analysis of MTT
assay.
| Statistic | T0 | T48h |
|---|
| Un. (mean ±
SEM) | 100.00 | 100.00 |
| LiCl (mean ±
SEM) | 66.00±3.46 | 61.00±19.08 |
| High energy photons
(mean ± SEM) | 91.33±8.41 | 83.00±10.60 |
| LiCl + high energy
photons (mean ± SEM) | 63.00±3.00 | 31.00±14.18 |
| P-value: Un. vs.
LiCl | 0.1161 | 0.3312 |
| P-value: Un. vs.
high energy photons | >0.9999 | >0.9999 |
| P-value: Un. vs.
LiCl+high energy photons | 0.0476a | 0.0262a |
LiCl sensitizes colon cancer cells to
high energy photon irradiation altering the balance between
pro-apoptotic and survival signaling
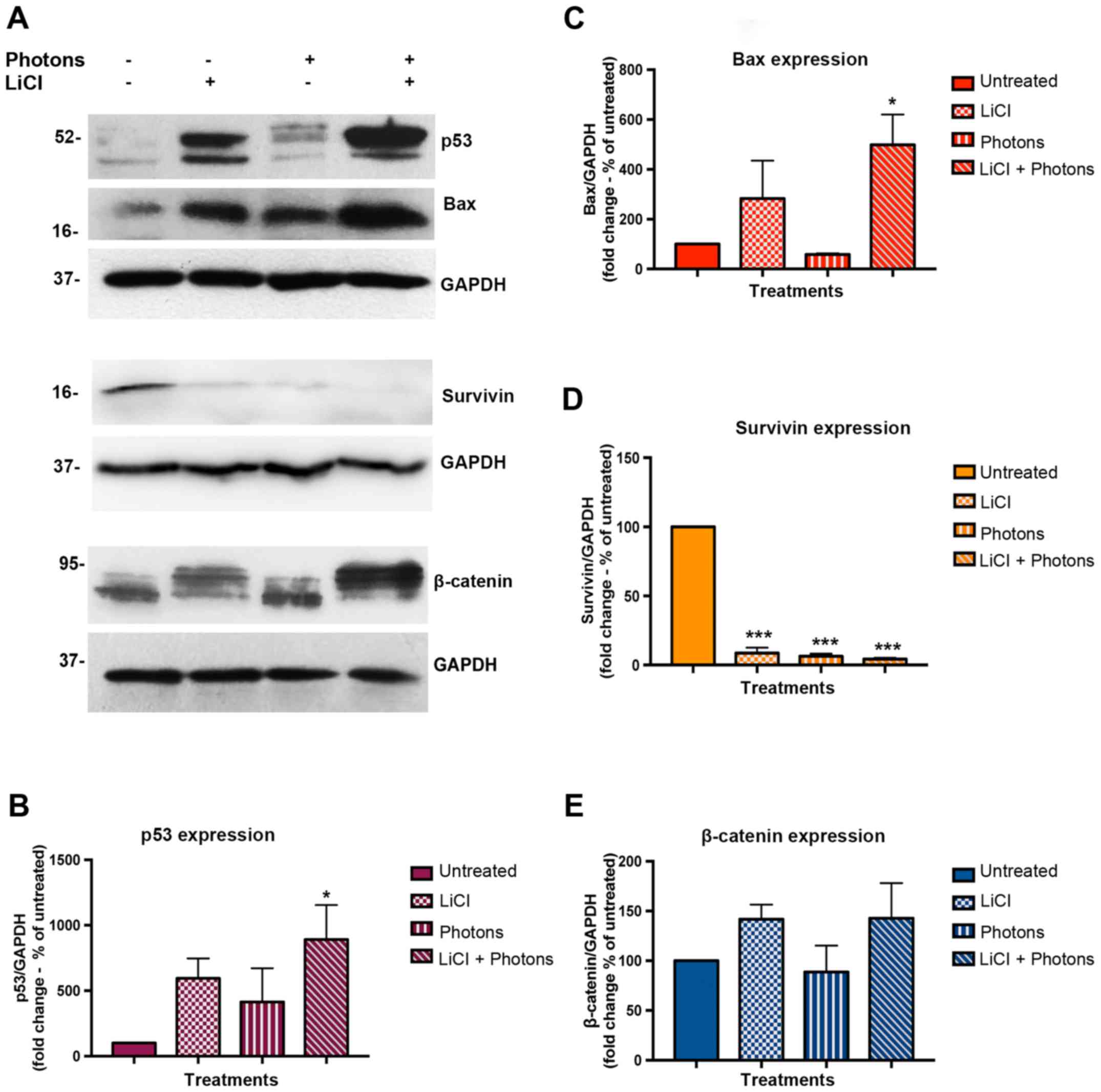
Since both LiCl and high-energy photonare known to
activate pro-apoptotic signals in colon cancer cells, we
investigated the effects of each of these treatments alone and in
combination on the expression of proteins involved in apoptosis and
death escape, such as p53, Bax, and Survivin. To this aim, we
performed Western blot assay on total protein extracts from
untreated cells, cells treated with LiCl or high energy photons,
and cells irradiated with high-energy photons after pretreatment
with LiCl. Samples were collected 48 h after completion of
treatments. As shown in Fig. 3A-C,
p53 and Bax protein expression was upregulated in both LiCl and
LiCl plus high-energy photon treated cells, and the highest
increase was observed after combined treatment. On the contrary,
Survivin expression was greatly reduced after each treatment
(Fig. 3A and D). As LiCl induces
GSK-3β inhibition, we investigated β-catenin expression level in
treated and untreated cells. As shown in Fig. 3A and in Fig. 3E, β-catenin expression was
stabilized in both LiCl-treated cells and in cells treated with
LiCl plus high-energy irradiation. Interestingly, in LiCl-treated
cells, western blot immunostaining showed upregulation of an
isoform of β-catenin a little higher in molecular weight than that
observed in cells irradiated without LiCl pre-treatment. Additional
experiments are necessary to shed light on this result. To verify
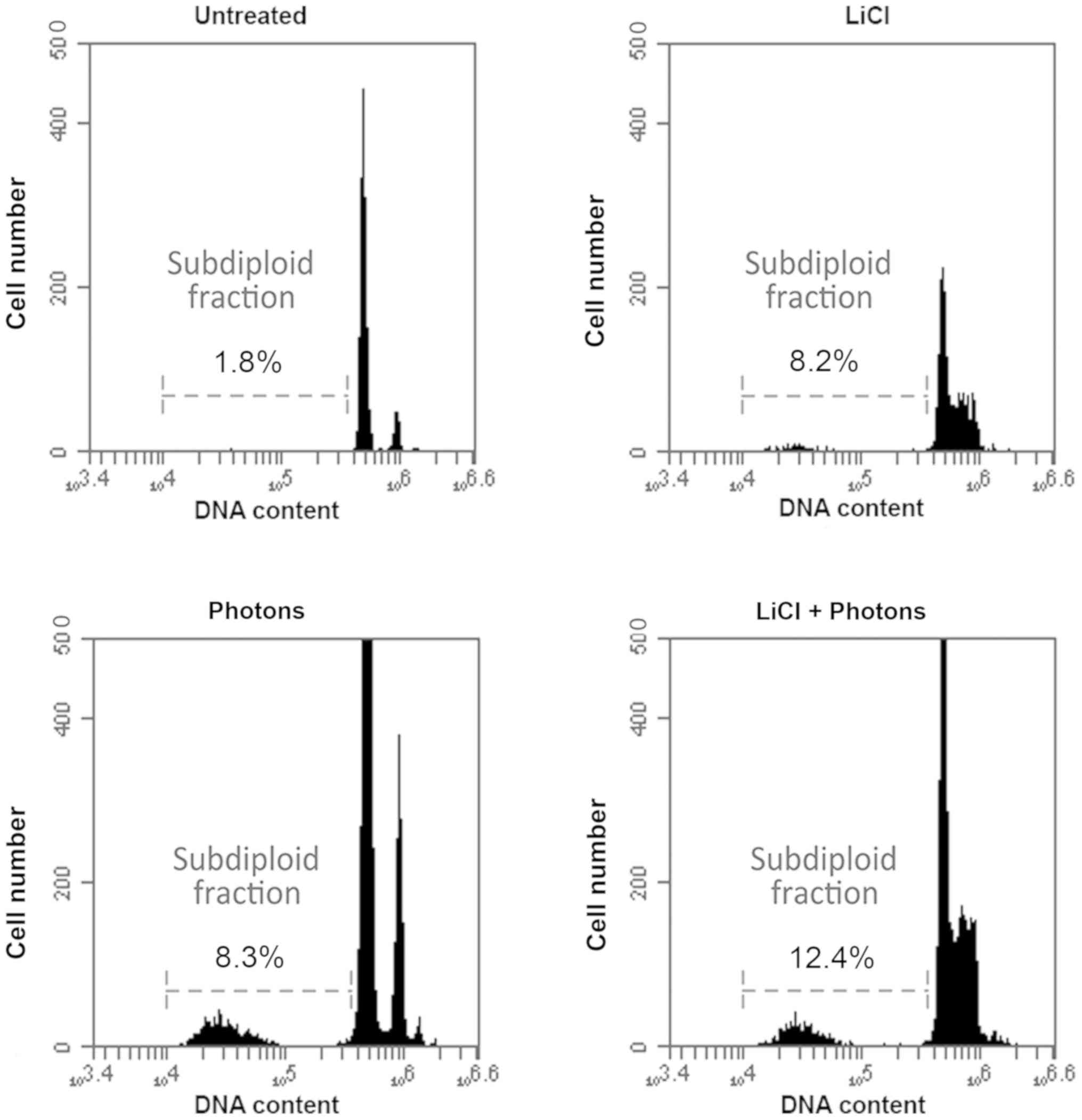
whether these molecular changes result in differences in apoptosis
levels, we also performed a FACS analysis to evaluate the
percentage of subdiploid cells. As expected, the percentage of
subdiploid cells increased significantly after the combined LiCl
and high-energy photon treatment versus control cells (Fig. 4). Taken together, these data
indicate that LiCl sensitizes T88 primary CRC cells to the effects
of high-energy photon treatment by altering the expression pattern
of pro-apoptotic and pro-survival proteins.
Discussion
The epithelial-to-mesenchymal transition, a
biological process by which epithelial cancer cells lose their
epithelial phenotype and acquire a mesenchymal phenotype, is a
physiological mechanism developed by cancer cells during cancer
progression and metastases. Indeed, cells that have undergone the
EMT-transcription factors (EMT-TFs) acquire all the features needed
to complete the metastatic process, such as, motility, stem cell
features, cell plasticity, resistance to apoptosis and to therapy.
This process is orchestrated by the EMT-TFs TWIST1 and Snail. It
has been suggested that high levels of these EMT-TFs can also cause
resistance to apoptosis by altering the TGF-β- and p53-mediated
programmed cell death pathways (21–24).
Apoptosis is an energy-dependent mechanism of programmed cell death
by which organisms maintain tissue homeostasis (25). Several pathways regulate apoptosis
and several mechanisms are used by tumor cells to survive,
suppressing apoptotic program. The most frequent apoptotic pathways
are the extrinsic and intrinsic pathways. Both are usually
characterized by early activation of the caspases proteolytic
cascade that causes cleavage of cellular proteins and of other
components essential for cell survival, thereby triggering
programmed cell death. In the extrinsic pathway, death
domain-containing proteins, such as the tumor necrosis factor
family of receptors, which are direct targets of caspase cleavage,
are activated (26). In the
intrinsic pathway, mitochondria play an essential role in
triggering apoptotic signals by releasing cytochrome-c into the
cytosol, which induces ‘apoptosome’ assembly. Subsequently, the
‘apoptosome’ complex is able to activate the caspases proteolytic
cascade. This pathway is known as the ‘BCL-2-regulated apoptotic
pathway’, because the cell death program is triggered by the
upregulation of the BCL-2 protein family, that, in turn, activates
the cell death effectors Bax and Bak (27). Cytochrome c can also induce the
release of other proteins, i.e., endonuclease G and
apoptosis-inducing factor, that may promote caspase-independent
cell death (28,29).
The intrinsic pathway is altered in most cancer
cells and is closely regulated by cellular metabolism. Indeed
metabolic changes occurring in cancer cells, consequent to
oncogenic activation or stress-induced therapy, promote resistance
to apoptosis and therapy via alterations of BCL-2 family expression
(25). P53, a protein often
altered in tumor progression, has been implicated in the activation
of the intrinsic and extrinsic pathways, and its ability to induce
apoptosis depends on NF-κB activation (30). Furthermore, alteration in the
extrinsic and intrinsic pathways could cause resistance to anoikis,
which is programmed cell death induced by detachment of cells from
the extracellular matrix, that is often altered in cancer cells
with metastatic potential (31–34).
Upon activation of proapoptotic cellular pathways, the survivin
protein, a member of a family of apoptosis inhibitors, is released
from mitochondria and inhibits caspase-9 thereby enhancing the
effects of inhibitor of apoptosis proteins (35).
In this scenario, we previously isolated and
characterized epithelial colon cancer cells endowed with
mesenchymal features, and together with high EMT-TFs expression, in
the attempt of identifying a therapeutic target able to sensitize
colon cancer cells to specific therapies. These cells were able to
grow for long periods in suspension as tumorspheres, which suggests
they are anoikis-resistant (16).
We have also demonstrated that LiCl induces differentiation, MET,
and downregulation of the EMT-TFs TWIST-1 and Snail, in primary
colon cancer cells (15). In the
present study, we demonstrate that T88 primary colon cancer cells
are completely unresponsive to high-energy photon irradiation in
terms of cell viability, and that LiCl sensitizes them to such
treatment.
Development of radiation resistance has been
correlated with two main mechanisms, one consisting in
disequilibrium between pro-apoptotic signaling transduction
pathways (mediated by p53 and Bax) and pathways mediating cell
survival, in which the protein Survivin plays an essential role
(36–38). On the other hand, DNA double strand
break repair pathways, which maintain genomic integrity and prevent
mis-repair and chromosomal rearrangements, could be responsible for
radio-resistance and failure of radiation treatment. Rouhani et
al (39) reported that LiCl
increases radio-sensitivity in breast cancer cells in vitro by
abrogating DNA repair. Indeed, in contrast to the expected effect
of LiCl, i.e., GSK-3β inactivation and β-catenin stabilization, the
authors observed GSK-3β upregulation and β-catenin down-regulation
together with mRNA downregulation of its transcriptional target
MR11, which is a crucial protein of DSB repair system (39). As expected, we observed that LiCl
induces apoptosis by activating cell death signaling and
down-regulating survival signaling in T88 cells. Indeed, LiCl
induced upregulation of the p53 and Bax proteins and strong
downregulation of the survivin protein. In addition, combined cell
treatment with LiCl and high energy photons was more effective than
high energy photons or LiCl used alone in reducing the viability of
colon cancer cells. In fact, under combined treatment, cells show
the highest percentage of apoptotic subdiploid cells between all
treatments analyzed, associated with the highest expression of p53
and Bax protein, and absence of survivin protein expression. A
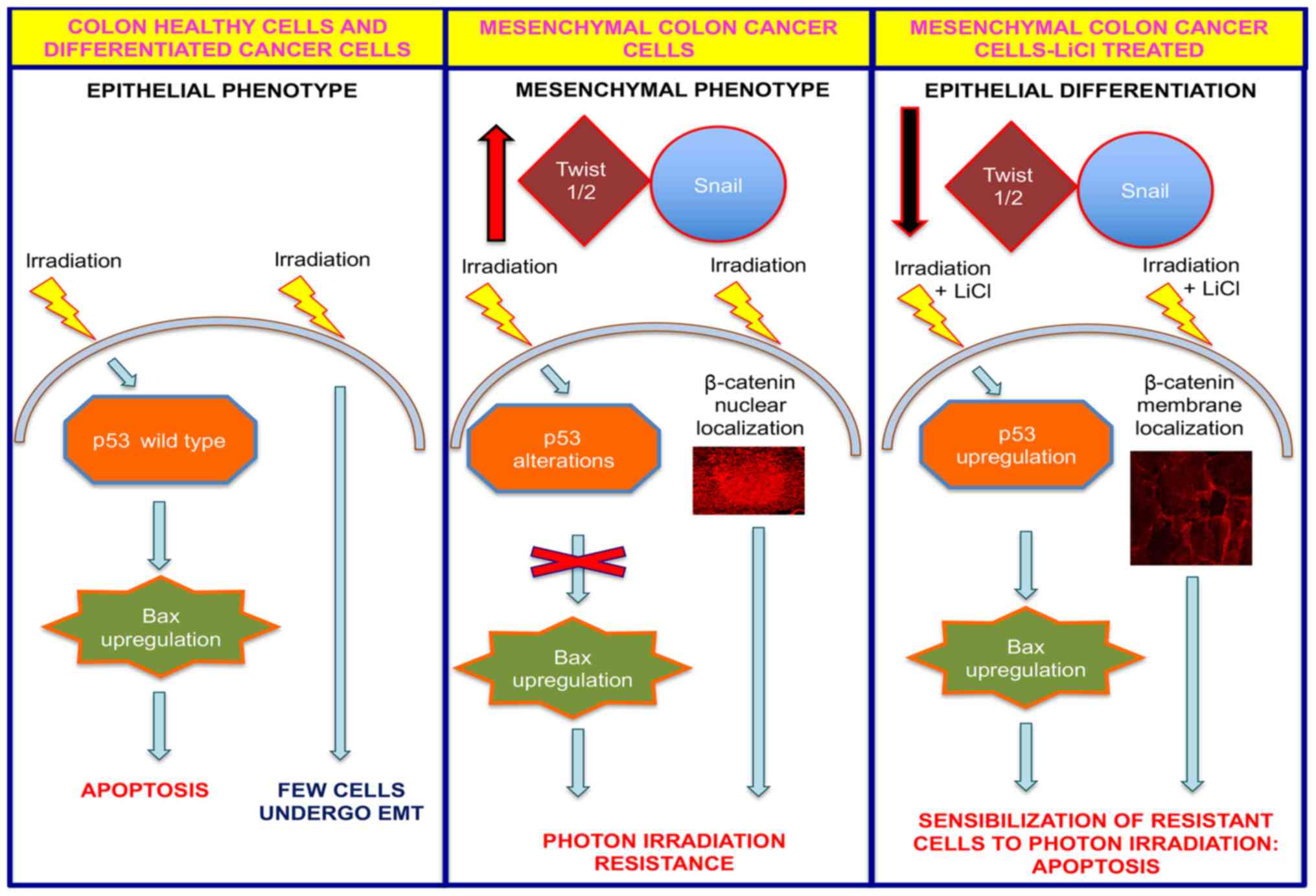
diagrammatic summary representing a model for the action of LiCl by
which it sensitizes resistant colon cancer cells to radiotherapy is
shown in Fig. 5. In contrast to
breast cancer (39), but in
accordance with the LiCl mechanism of action, we observed
stabilization of high molecular weight β-catenin isoforms in cells
treated with LiCl alone and in cells treated with LiCl plus high
energy photons. We speculated that these isoforms could be those
phosphorylated by pyruvate dehydrogenase kinase 1 in Thr112 and
Thr120, which are selectively directed to the plasma membrane,
where they interact with the E-cadherin protein (40). We previously observed that the
effect exerted by LiCl on the level of β-catenin expression was
time-dependent and LiCl promoted β-catenin membrane localization
(15,16). Furthermore, β-catenin is
heterogeneously distributed in CRC cells; indeed,
well-differentiated parenchymal cells, located in the tumor center,
retain β-catenin membranous expression comparable to that of normal
colon epithelium, while nuclear β-catenin expression predominates
in tumor cells localized at the invasion front (41). It would be interesting to
investigate further the role of β-catenin in LiCl-induced
sensitization to photon irradiation therapy and, more generally, in
the antineoplastic effect of LiCl in colon cancer. Could the
LiCl-sensitizing effect to photon irradiation on colon cancer cells
be also mediated by a decrease in the activity of the DSB repair
system, as observed in breast cancer cells? Additional experiments
are required to shed light on these intriguing and controversial
points.
As we discussed in a previous paper (15), cell culture models obviously do not
mimic the complex interactions that occur in the intestinal mucosa,
and a functional change in cell cultures may not equate with the
effect observed in vitro. On the other hand, complex
functions can be reproduced in cell cultures and thus become
amenable to investigation. However, the data reported herein could
have important clinical significance and clinical applications and
need, in a next future, to be improved and reinforced with animal
models experiments.
In conclusion, we demonstrate that T88 mesenchymal
colon cancer cells are resistant to radiotherapy, and that LiCl
sensitizes these cells to apoptosis in response to high-energy
photons, probably by acting on the balance between pro-apoptotic
and survival signaling transduction pathways. In light of our
finding, we suggest that LiCl could be used to increase sensitivity
of resistant colon cancer cells to radiotherapy.
Acknowledgements
The authors would like to thank Dr Jaen Ann Gilder
for the text editing.
Funding
The present study was supported by a grant from
Fondo Straordinario di Ateneo-2018, Università di Napoli Federico
II; POR Campania FESR 2014–2020 ‘SATIN’.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
GMP, MDR and PR designed the study. FC, AC, AA, VV
and MT performed the cellular and molecular experiments. PM, GA and
PR performed the high energy photon irradiation. MDR and PR
performed the statistical analysis of the data. PD provided the
surgical sample for primary cell isolation. GMP and MDR coordinated
the work. GMP, MDR, PI and PD contributed to data interpretation.
PI provided funding. MDR, PI and PD wrote the manuscript. GMP and
MT critically revised the manuscript. All authors edited and
approved the final version of the manuscript.
Ethics approval and consent to
participate
The patient study was approved by the Ethics
Committe of the University of Naples Federico II, ‘Comitato etico
per le attività Biomediche-Carlo Romano’ (no. 35/17). The patient
provided written informed consent for the study. All methods were
performed in accordance with the relevant guidelines and
regulations.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siani LM and Pulica C: Stage I–IIIC right
colonic cancer treated with complete mesocolic excision and central
vascular ligation: Quality of surgical specimen and long term
oncologic outcome according to the plane of surgery. Minerva Chir.
69:199–208. 2014.PubMed/NCBI
|
|
3
|
De Rosa M, Rega D, Costabile V, Duraturo
F, Niglio A, Izzo P, Pace U and Delrio P: The biological complexity
of colorectal cancer: Insights into biomarkers for early detection
and personalized care. Therap Adv Gastroenterol. 6:861–886. 2016.
View Article : Google Scholar
|
|
4
|
Schmoll HJ, Van Cutsem E, Stein A,
Valentini V, Glimelius B, Haustermans K, Nordlinger B, Van de Velde
CJ, Balmana J, Regula J, et al: ESMO consensus guidelines for
management of patients with colon and rectal cancer. A personalized
approach to clinical decision making. Ann Oncol. 23:2479–2516.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tveit K, Guren T, Glimelius B, Pfeiffer P,
Sorbye H, Pyrhonen S, Kure E, Ikdahl T, Skovlund E, Fokstuen T, et
al: Phase III trial of cetuximab with continuous or intermittent
fluorouracil, leucovorin, and oxaliplatin (Nordic FLOX) versus FLOX
alone in first-line treatment of metastatic colorectal cancer: The
NORDIC-VII study. J Clin Oncol. 30:1755–1762. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Peeters M, Price T, Cervantes A, Sobrero
A, Ducreux M, Hotko Y, André T, Chan E, Lordick F, Punt CJ, et al:
Randomized phase III study of panitumumab with fluorouracil,
leucovorin, and irinotecan (FOLFIRI) compared with FOLFIRI alone as
second-line treatment in patients with metastatic colorectal
cancer. J Clin Oncol. 28:4706–4713. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Van Cutsem E, Köhne C, Hitre E, Zaluski J,
Chang Chien C, Makhson A, D'Haens G, Pintér T, Lim R, Bodoky G, et
al: Cetuximab and chemotherapy as initial treatment for metastatic
colorectal cancer. N Engl J Med. 360:1408–1417. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Martenson JA Jr, Willett CG, Sargent DJ,
Mailliard JA, Donohue JH, Gunderson LL, Thomas CR Jr, Fisher B,
Benson AB III, Myerson R and Goldberg RM: Phase III study of
adjuvant chemotherapy and radiation therapy compared with
chemotherapy alone in the surgical adjuvant treatment of colon
cancer: Results of intergroup protocol 0130. J Clin Oncol.
22:3277–3283. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ma B, Gao P, Wang H, Xu Q, Song Y, Huanget
X, Sun J, Zhao J, Luo J, Sun Y and Wang Z: What has preoperative
radio(chemo)therapy brought to localized rectal cancer patients in
terms of perioperative and long-term outcomes over the past
decades? A systematic review and meta-analysis based on 41,121
patients. Int J Cancer. 141:1052–1065. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang Y and Weinberg RA:
Epithelial-to-mesenchymal transition in cancer: Complexity and
opportunities. Front Med. 12:361–373. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jope RS: Lithium and GSK-3: One inhibitor,
two inhibitory actions, multiple outcomes. Trends Pharmacol Sci.
24:441–443. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Post RM: The new news about lithium: An
underutilized treatment in the United States.
Neuropsychopharmacology. 43:1174–1179. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Forlenza OV, De-Paula VJ and Diniz BS:
Neuroprotective effects of lithium: Implications for the treatment
of Alzheimer's disease and related neurodegenerative disorders. ACS
Chem Neurosci. 5:443–450. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Medić B, Stojanović M, Štimec B, Divac N,
Vujović KS, Stojanović R, Čolović M, Krstić DZ and Prostran M:
Lithium-pharmacological and toxicological aspects: The current
state of the art. Curr Med Chem. Sep 4–2018.(Epub ahead of print).
View Article : Google Scholar
|
|
15
|
Costabile V, Duraturo F, Delrio P, Rega D,
Pace U, Liccardo R, Rossi GB, Genesio R, Nitsch L, Izzo P and De
Rosa M: Lithium chloride induces mesenchymal-to-epithelial
reverting transition in primary colon cancer cell cultures. Int J
Oncol. 46:1913–1923. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Turano M, Costabile V, Cerasuolo A,
Duraturo F, Liccardo R, Delrio P, Pace U, Rega D, Dodaro CA, Milone
M, et al: Characterisation of mesenchymal colon tumour-derived
cells in tumourspheres as a model for colorectal cancer
progression. Int J Oncol. 53:2379–2396. 2018.PubMed/NCBI
|
|
17
|
Rinaldo C, Moncada A, Gradi A, Ciuffini L,
D'Eliseo D, Siepi F, Prodosmo A, Giorgi A, Pierantoni GM, Trapasso
F, et al: HIPK2 controls cytokinesis and prevents tetraploidization
by phosphorylating histone H2B at the midbody. Mol Cell. 47:87–98.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Galatola M, Paparo L, Duraturo F, Turano
M, Rossi GB, Izzo P and De Rosa M: Beta catenin and cytokine
pathway dysregulation in patients with manifestations of the ‘PTEN
hamartoma tumor syndrome’. BMC Med Genet. 13:282012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Valentino T, Palmieri D, Vitiello M,
Pierantoni GM, Fusco A and Fedele M: PATZ1 interacts with p53 and
regulates expression of p53-target genes enhancing apoptosis or
cell survival based on the cellular context. Cell Death Dis.
4:e9632013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Di Maio N, Vicidomini R, Angrisani A,
Belli V, Furia M and Turano M: A new role for human dyskerin in
vesicular trafficking. FEBS Open Bio. 7:1453–1468. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ong BA, Vega KJ and Houchen CW: Intestinal
stem cells and the colorectal cancer microenvironment. World J
Gastroenterol. 20:1898–1909. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Vichalkovski A, Gresko E, Hess D,
Restuccia DF and Hemmings BA: PKB/AKT phosphorylation of the
transcription factor Twist-1 at Ser42 inhibits p53 activity in
response to DNA damage. Oncogene. 29:3554–3565. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang Y, Pan X, Lei W, Wang J and Song J:
Transforming growth factor-beta1 induces epithelial-to-mesenchymal
transition and apoptosis via a cell cycle-dependent mechanism.
Oncogene. 25:7235–7244. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Vega S, Morales AV, Ocaña OH, Valdés F,
Fabregat I and Nieto MA: Snail blocks the cell cycle and confers
resistance to cell death. Genes Dev. 18:1131–1143. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sharma A, Boise LH and Shanmugam M: Cancer
metabolism and the evasion of apoptotic cell death. Cancers
(Basel). 11(pii): E11442019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hassan M, Watari H, AbuAlmaaty A, Ohba Y
and Sakuragi N: Apoptosis and molecular targeting therapy in
cancer. Biomed Res Int. 2014:1508452014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Aubrey BJ, Kelly GL, Janic A, Herold MJ
and Strasser A: How does p53 induce apoptosis and how does this
relate to p53-mediated tumour suppression? Cell Death Differ.
25:104–113. 2018. View Article : Google Scholar
|
|
28
|
Chauhan D, Hideshima T, Rosen S, Reed JC,
Kharbanda S and Anderson KC: Apaf-1/cytochrome c-independent and
Smac-dependent induction of apoptosis in multiple myeloma (MM)
cells. J Biol Chem. 276:24453–24456. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kroemer G and Reed JC: Mitochondrial
control of cell death. Nat Med. 6:513–519. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ryan KM, Ernst MK, Rice NR and Vousden KH:
Role of NF-kappaB in p53-mediated programmed cell death. Nature.
404:892–897. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ionov Y, Yamamoto H, Krajewski S, Reed JC
and Perucho M: Mutational inactivation of the proapoptotic gene BAX
confers selective advantage during tumor clonal evolution. Proc
Natl Acad Sci USA. 97:10872–10877. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Miyashita T, Krajewski S, Krajewska M,
Wang HG, Lin HK, Liebermann DA, Hoffman B and Reed JC: Tumor
suppressor p53 is a regulator of bcl-2 and bax gene expression in
vitro and in vivo. Oncogene. 9:1799–1805. 1994.PubMed/NCBI
|
|
33
|
Vaux DL: Immunopathology of
apoptosis-introduction and overview. Springer Semin Immunopathol.
19:271–278. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Frischand SM and Screaton RA: Anoikis
mechanisms. Curr Opinin Cell Biol. 13:555–562. 2001. View Article : Google Scholar
|
|
35
|
Mita AC, Mita MM, Nawrocki ST and Giles
FJ: Survivin: Key regulator of mitosis and apoptosis and novel
target for cancer therapeutics. Clin Cancer Res. 14:5000–5005.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Pawlik TM and Keyomarsi K: Role of cell
cycle in mediating sensitivity to radiotherapy. Int J Radiat Oncol
Biol Phys. 59:928–942. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pfeifer D, Wallin A, Holmlund B and Sun
XF: Protein expression following gamma-irradiation relevant to
growth arrest and apoptosis in colon cancer cells. J Cancer Res
Clin Oncol. 135:1583–1592. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang J, Yang L, Yang J, Kuropatwinski K,
Wang W, Liu XQ, Hauser J and Brattain MG: Transforming growth
factor beta induces apoptosis through repressing the
phosphoinositide 3-kinase/AKT/survivin pathway in colon cancer
cells. Cancer Res. 68:3152–3160. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Rouhani M, Goliaei B, Khodagholi F and
Nikoofar A: Lithium increases radiosensitivity by abrogating DNA
repair in breast cancer spheroid culture. Arch Iran Med.
17:352–360. 2014.PubMed/NCBI
|
|
40
|
Du C, Jaggi M, Zhang C and Balaji KC:
Protein kinase D1-mediated phosphorylation and subcellular
localization of beta-catenin. Cancer Res. 69:1117–1124. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Fodde R and Brabletz T: Wnt/beta-catenin
signaling in cancer stemness and malignant behavior. Curr Opin Cell
Biol. 19:150–158. 2007. View Article : Google Scholar : PubMed/NCBI
|