Introduction
Intestinal ischemia-reperfusion (IIR) may occur in
healthy individuals and clinical patients (1,2).
Under physiological conditions, IIR may help induce blood
redistribution to important organs and tissues, resulting in
intestinal hypoperfusion and decreased mesenteric blood flow
(3). However, under pathological
conditions, including major surgery, trauma, inflammatory bowel
disease or hypovolemia, IIR may lead to severe mesenteric artery
embolism or phlebothrombosis (4).
A previous study identified that the injury of distant organs and
tissues caused by intestinal ischemia does not mainly come from
ischemic injury itself, but bacterial translocation, endotoxin
translocation and massive release of inflammatory cytokines during
the recovery of intestinal blood supply and blood flow. This can
result in multiple organ injury to the liver, kidney and lung,
which leads to systemic inflammatory reaction, systemic multiple
organ dysfunction syndrome and even mortality (5). The clinical classification of IIR
comprises acute mesenteric ischemia, chronic mesenteric ischemia
and ischemic colitis (6). Patients
with a certain specific medical history, including congestive heart
failure, arrhythmia, atherosis, sepsis and collagen deposition
disease, have an elevated morbidity rate when faced with IIR
(6,7). Prompt mesenteric blood flow recovery
and ischemic tissue reoxygenation are the dominant treatment
principles for IIR; however, they may increase vessel and tissue
damage (7,8). Previous studies have indicated that
GYY4137, as a novel type of hydrogen sulfide (H2S)
donor, is able to stably induce sustained release of H2S
under physiological pH and temperature, which may effectively
simulate the release process of H2S (9,10).
Furthermore, GYY4137 has been demonstrated to protect myocardial
cells from damage caused by high glycemia-induced cytotoxicity, and
reduce vascular inflammation and oxidative stress (10–12).
The effects of GYY4137 on IIR have so far remained elusive;
therefore, the present study assessed the effects of GYY4137 on a
Sprague Dawley (SD) rat model of IIR and investigated the
underlying mechanisms.
Materials and methods
Animals and experimental
procedure
The protocols of the present study were approved by
the Institutional Animal Care and Use Committee of Wuhan University
(approval ID. WHU20110312). A total of 40 male specific
pathogen-free SD rats (aged 30–35 days; weight, 120–150 g) were
obtained from the Experimental Animal Center of Wuhan University.
All rats were selected and kept under conventional conditions in an
environmentally controlled room (temperature, 22–25°C; humidity,
50–70%; 12-h light/dark cycle) for 7 days prior to the procedure.
GYY4137 was obtained from Abcam (cat. no. ab142145). All rats were
randomly divided into the following four groups (n=10/group): Group
A, a sham-surgery group; group B, an IIR group; group C, an IIR
group that was administered an abdominal injection of low-dose
GYY4137 (40 mg/kg); and group D, an IIR group that was administered
high-dose GYY4137 (80 mg/kg). For groups C and D, abdominal
injection of 40 or 80 mg/kg GYY4137, respectively, was administered
daily for 3 consecutive days prior to surgery. After 7 days
acclimation, sodium pentobarbital (50 mg/kg) was used for
anesthesia prior to surgery. For all groups, regular disinfection
was conducted and a 2-cm incision was made on the central
hypogastrium; layer-by-layer cutting and isolation of the
perimesenteric adipose tissue were performed in sequence.
Subsequently, for groups B, C and D, a microvascular clip was used
to obstruct the superior mesenteric artery (intestinal appearance
changed from red to maroon) for 45 min, in order to generate the
ischemia model. Conversely, no clipping was performed in group A.
Following the removal of the clip (reperfusion) in groups B, C and
D, all four groups were euthanized by an overdose of1 anesthesia.
For each rat, 5 mm of the jejunum was obtained for biochemical and
pathological evaluation. In addition, blood was collected from the
aorta abdominalis for further analysis.
Hematoxylin and eosin (H&E)
staining
According to standard protocols, tissues were
embedded in paraffin (paraffin I, 60°C, 1 h; paraffin II, 60°C, 1
h; paraffin III, 60°C, 1 h). After cutting into 4-µm sections,
tissues were dewaxed, hydrated and stained with hematoxylin at 25°C
for 5–7 min. Sections were observed at a magnification of ×200
using an Olympus BX53 light microscope (Olympus Corporation).
Biochemical analysis
Tissues were homogenized and centrifuged (1,000 × g
at 25°C for 15 min) prior to analysis. Malondialdehyde (MDA)
content (nmol/g protein) and superoxide dismutase (SOD) activity
(U/mg protein) were measured spectrophotometrically through
thiobarbituric acid and xanthine oxidase methods, respectively,
according to the manufacturer's protocols (MDA Assay kit, cat. no.
A003-1; SOD Assay kit, cat. no. A001-3-2; Nanjing Jiancheng
Bioengineering Institute).
Terminal
deoxynucleotidyl-transferase-mediated dUTP nick end labeling
(TUNEL) assay
TUNEL staining using the in situ Cell Death
Detection kit, Fluorescein (Roche Diagnostics) was performed to
detect apoptosis of intestinal mucous epithelial cells according to
manufacturer's protocol. TUNEL-positive cells (nuclei stained
brown) were observed under a Zeiss LSM 510 confocal laser scanning
microscope (Zeiss AG). Apoptotic index (AI) was expressed as
AI=(TUNEL-positive cells/total cells) ×100%.
Immunohistochemistry
Rabbit polyclonal anti-caspase-3 (cat. no. sc-7148;
Santa Cruz Biotechnology, Inc.) and rabbit polyclonal anti-Bax
(cat. no. sc-493; Santa Cruz Biotechnology, Inc.) antibodies were
used for immunohistochemical staining. Tissue treatment prior to
staining consisted of several steps, including dehydration (25°C,
75% ethanol 4 h, 85% ethanol 2 h, 90% ethanol 1.5 h, 100% ethanol I
0.5 h, 100% ethanol II 0.5 h), permeabilization (25°C,100%
ethanol:xylol 1:1, 10 min; xylol I 10 min, xylol II 7 min),
paraffin embedding, slicing, and baking and dewaxing. Subsequently,
sections (4 µm) underwent antigen retrieval, endogenous peroxidase
blocking and serum blocking. For antigen retrieval, sections were
boiled with the retrieval solution (0.01 M diluted citric acid
buffer solution, pH 6.0) at 100°C for 10–15 min. The solution was
then left to cool at room temperature for 40 min and washed with
PBS (pH 7.4) three times (3 min/wash). For peroxidase blocking,
sections were incubated with 3% hydrogen peroxide (usually diluted
with methanol or distilled water for 3% hydrogen peroxide) at room
temperature for 15 min, then washed with PBS three times (3
min/wash). For serum blocking, sections were incubated with 10%
normal goat serum (Dako; Agilent Technologies, Inc.) at room
temperature for 30 min.
Subsequently, sections were incubated with primary
antibodies at 4°C for 15 h, and with goat anti-rabbit/mouse
secondary antibodies (cat. no. k500711-2; Dako; Agilent
Technologies, Inc.) at 37°C for 20 min. Counterstaining was
conducted using Harris hematoxylin for 30 sec to 1 min, after
which, sections were washed with 1% hydrochloric acid and then with
tap water or PBS. Staining evaluation was performed under an
Olympus BX50 light microscope (Olympus Corporation). The results
were evaluated by comparing staining intensity upon microscopic
examination.
Western blot analysis
Proteins were extracted from tissues using RIPA
buffer (cat. no. 9806S; Cell Signaling Technology, Inc.) and PMSF
(cat. no. 8553; Cell Signaling Technology, Inc.), and quantified
using the bicinchoninic acid assay. Briefly, equivalent protein
samples (40 µg/lane) were separated by SDS-PAGE on 10% gels and
were transferred to nitrocellulose membranes. Subsequently, the
membranes were blocked with 5% non-fat milk in Tris-buffered saline
with (10%) Tween 20 at 25°C for 30 min and were then incubated with
primary antibodies at 4°C overnight. After extensive washing with
TBST, the membranes were incubated with horseradish
peroxidase-conjugated secondary goat anti-rabbit or anti-mouse
antibodies (cat. nos. LK2001 and LK2003; 1:100; Tianjin Sungene
Biotech, Co., Ltd.) for 1 h at 25°C. All specific bands were
visualized using an ECL system kit (Pierce; Thermo Fisher
Scientific, Inc.). Optical densities were detected using ImageJ
software (version 1.48; National Institutes of Health). The
following primary antibodies were used: Bax (cat. no. sc-493;
1:500; Santa Cruz Biotechnology, Inc.), cleaved-caspase-3 (cat. no.
sc-7148; 1:1,000; Santa Cruz Biotechnology, Inc.) Bcl-2 (cat. no.
sc-509; 1:100; Santa Cruz Biotechnology, Inc.) and (GAPDH, 37 kDa,
anti-rabbit, AB-P-R 001; 1:1,000 Hangzhou Goodhere Biotechnology
Co., Ltd.).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from 100 mg intestinal
tissue sample using TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) and RNA purity was detected by
spectrophotometry. Subsequently, first-strand cDNA was synthesized
using random primers and M-MLV Reverse Transcriptase (Promega
Corporation). RT temperature conditions were as follows: 25°C for 5
min, 50°C for 15 min, 85°C for 5 min and 4°C for 10 min. cDNA was
amplified by qPCR using an Applied Biosystems SYBR Green mix kit
(Applied Biosystems, Inc.) and the ABI 7900 Real-Time PCR system
(Applied Biosystems, Inc.). Thermocycling conditions were as
follows: 50°C for 2 min, 95°C for 10 min; 95°C for 30 sec, 60°C for
30 sec all for 40 cycles. mRNA expression was normalized to GAPDH
(13). Primer sequences were as
follows: Caspase-3, forward 5′-TGGACTGCGGTATTGAGACA-3′, reverse
5′-GCGCAAAGTGACTGGATGAA-3′; Bax, forward 5′
TGAACTGGACAACAACATGGAG-3, reverse 5′ AGCAAAGTAGAAAAGGGCAACC-3′;
Bcl-2, forward 5′-ATGCTTCAGACCTCCCTT-3′, reverse
5′-CTCCACCAACTATCTCCACT-3′; and GAPDH, forward
5′-ACAGCAACAGGGTGGTGGAC-3′ and reverse
5′-TTTGAGGGTGCAGCGAACTT-3′.
The conditions for RT and qPCR were as follows: i)
RT system: RNA, 3.192 µg; Oligo (dT) 15 (10 µM), 2 µl; dNTP (2.5
mM), 4 µl; 5X Hiscript Buffer, 4 µl; Hiscript Reverse
Transcriptase, 1 µl; Ribonuclease Inhibitor, 0.5 µl;
ddH2O (RNase-free), ≤20 µl. ii) Semi-quantitative
RT-PCR; DNA products were run on a 10% agarose gel containing
ethidium bromide at 120V and 100 mA for 30 min. DNA bands were
visualized using an ultraviolet analytical instrument (Beijing
Junyi-Dongfang Electrophoresis Equipment Co., Ltd., JY02S) and
quantified using Image Lab 3.0 (Bio-Rad Laboratories, Inc.) with
GAPDH as the internal reference gene: Forward primer (10 µM), 0.5
µl; reverse primer (10 µM), 0.5 µl; dNTP (2.5 mM), 2 µl; Ex Taq,
0.25 µl; 10X Ex Taq E buffer, 2.5 µl; cDNA, 1 µl; ddH2O,
≥25 µl. Temperature conditions: 94°C for 4 min; 30 cycles at 94°C
for 30 sec, 56°C for 30 sec, 72°C for 25 sec; 72°C for 4 min and
4°C for 4 min. iii) RT-qPCR: cDNA, 4 µl; forward primer (100 µM),
0.4 µl; reverse primer (100 µM), 0.4 µl; SYBR Green/Fluorescein
qPCR Master Mix (2X), 10 µl; H2O, 5.2 µl.
Statistical analysis
Experiments were repeated three times. Data are
expressed as the mean ± standard deviation. SPSS 19.0 (IBM Corp.)
was used for statistical analysis. Comparisons between different
groups were performed by one-way analysis followed by the
Student-Newman-Keuls test. P<0.05 was considered to indicate a
statistically significant difference.
Result
Pathological alterations in intestinal
tissues
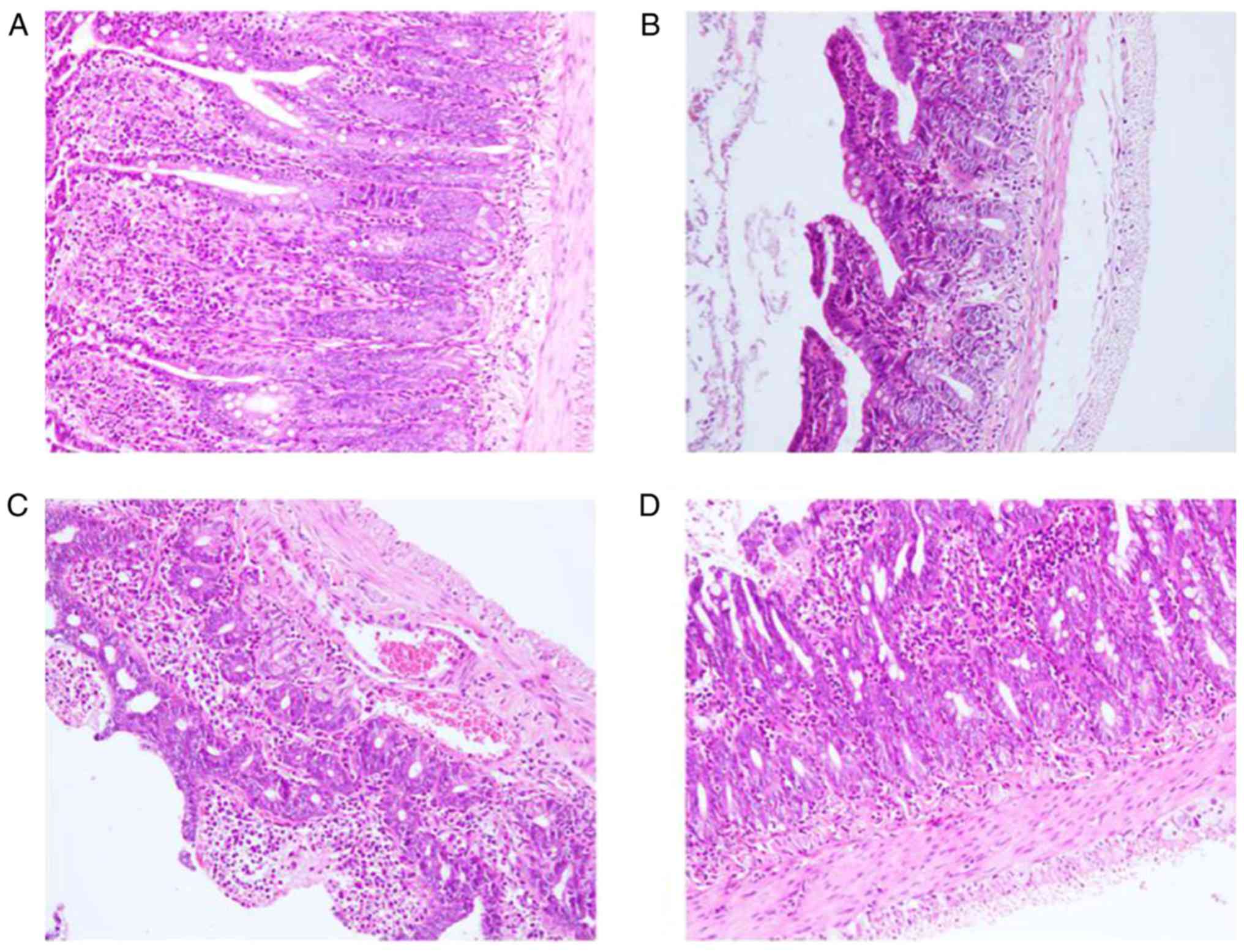
In group A, aligned mucosal epithelium and
morphological structural integrity were observed without any
histopathological abnormalities. In group B, disordered alignment
of the epithelium was detected and villi were obviously swollen; in
addition, necrosis of the intestinal mucosa was observed.
Conversely, group C exhibited reduced histopathological damage
compared with group B (partially swollen villi and partial mucosal
necrosis). Compared with group C, group D exhibited reduced
histopathological damage, which presented as mild mucosal necrosis
without any swollen villi (Fig.
1).
MDA content and SOD activity
In group B, MDA content was markedly increased
compared with in group A, whereas it was markedly decreased in
groups C and D compared with in group B (P<0.05). Furthermore,
in group B, SOD activity was significantly decreased compared with
in group A, whereas it was markedly increased in groups C and D
compared with in group B (P<0.05; Table I).
 | Table I.MDA and SOD levels following
intestinal ischemia-reperfusion and GYY4137 intervention. |
Table I.
MDA and SOD levels following
intestinal ischemia-reperfusion and GYY4137 intervention.
| Group | No. | MDA (nmol/mg) | SOD (U/mg) |
|---|
| A | 10 | 2.83±0.36 | 135.37±3.34 |
| B | 10 |
9.23±0.78a |
76.45±1.39a |
| C | 10 |
4.97±0.45b |
95.13±1.64a,b |
| D | 10 |
3.51±1.05c |
115.13±2.54a–c |
Apoptosis of intestinal mucous
epithelial cells
Epithelial cells in group B had significantly
increased AI compared with in group A (P<0.05). However, in
groups C and D, AI of epithelial cells was significantly decreased
compared with in group B (P<0.05; Table II and Fig. 2).
 | Table II.AI of intestinal mucous epithelial
cells. |
Table II.
AI of intestinal mucous epithelial
cells.
| Group | No. | AI (%) |
|---|
| A | 10 | 4.53±0.28 |
| B | 10 |
21.73±1.17a |
| C | 10 |
9.53±0.96a,b |
| D | 10 |
6.53±0.76a–c |
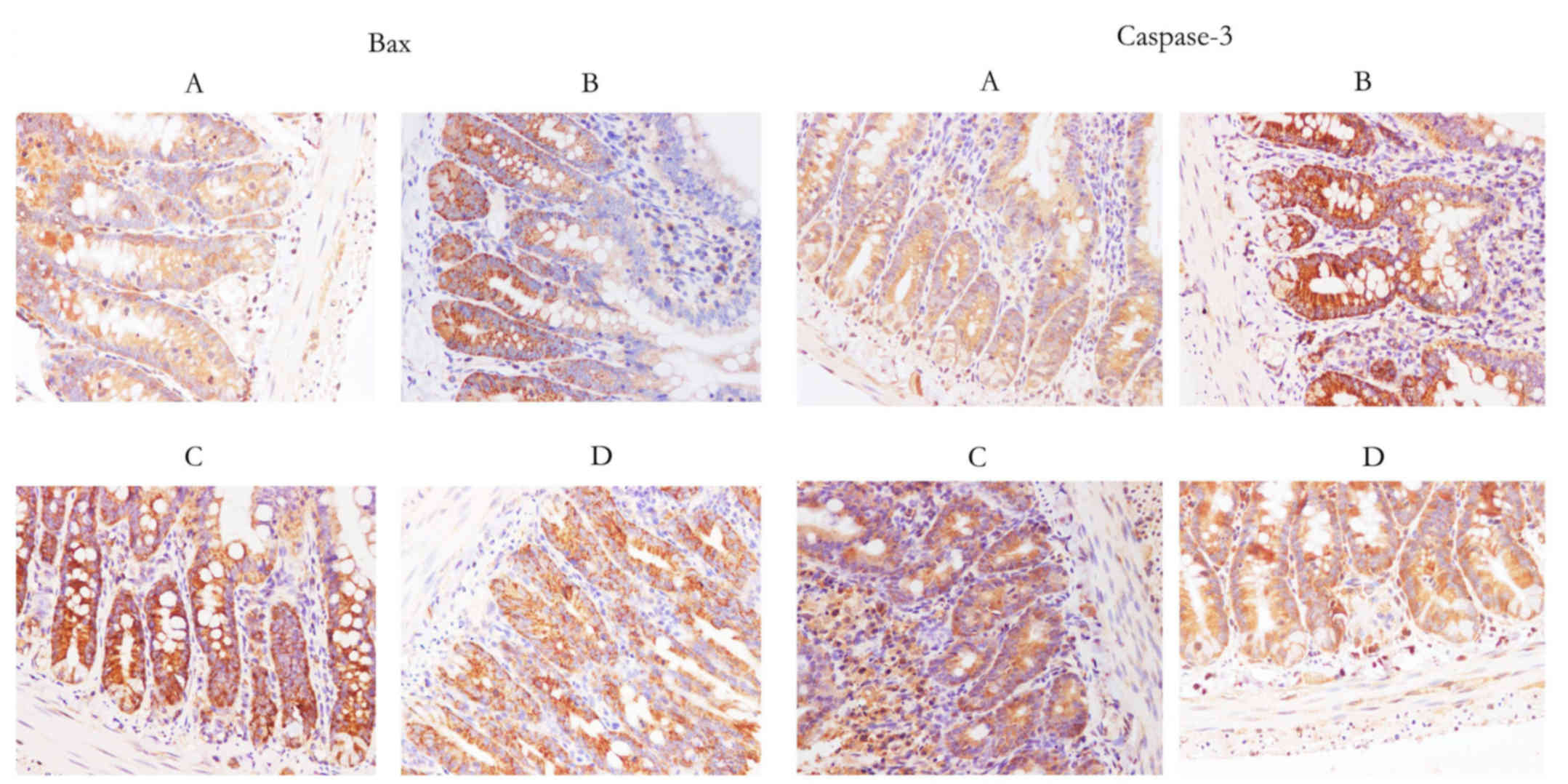
Expression of caspase-3 and Bax, as
determined by immunohistochemistry
A marked reduction in caspase-3 and Bax expression
was identified in groups C and D compared with in group B (Fig. 3).
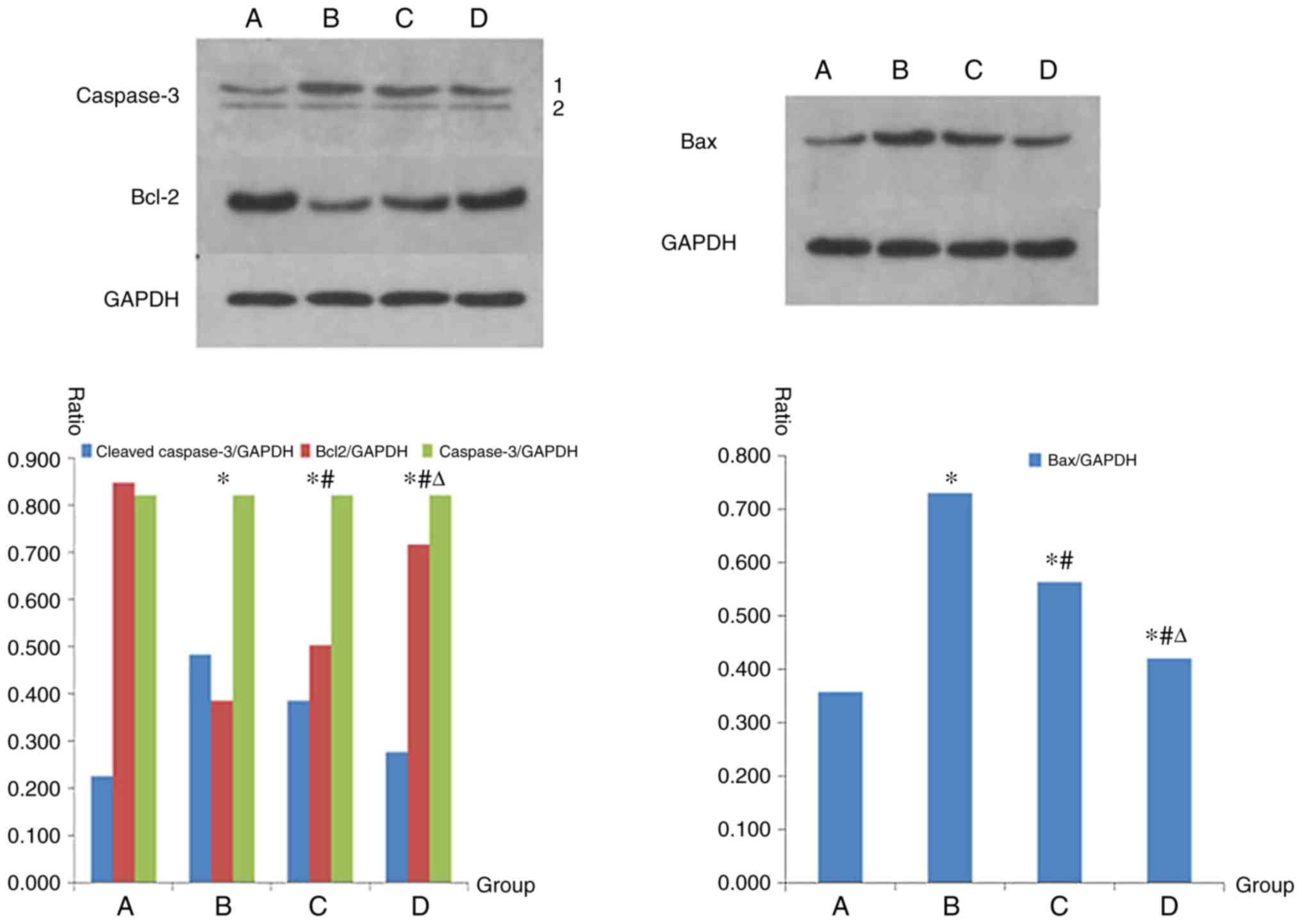
Protein expression levels of
caspase-3, Bax and Bcl-2, as determined by western blot
analysis
Group B exhibited a marked increase in the protein
expression levels of Bax and cleaved caspase-3 compared with in
group A. Compared with in group B, in groups C and D, protein
expression levels of Bax and cleaved caspase-3 were decreased.
Statistically significant differences were detected between the
groups (P<0.05). Conversely, in group B, the protein expression
levels of Bcl-2 were markedly decreased compared with in group A,
whereas the protein expression levels of Bcl-2 were increased in
groups C and D compared with in group B. Statistically significant
differences between the groups were detected (P<0.05; Fig. 4).
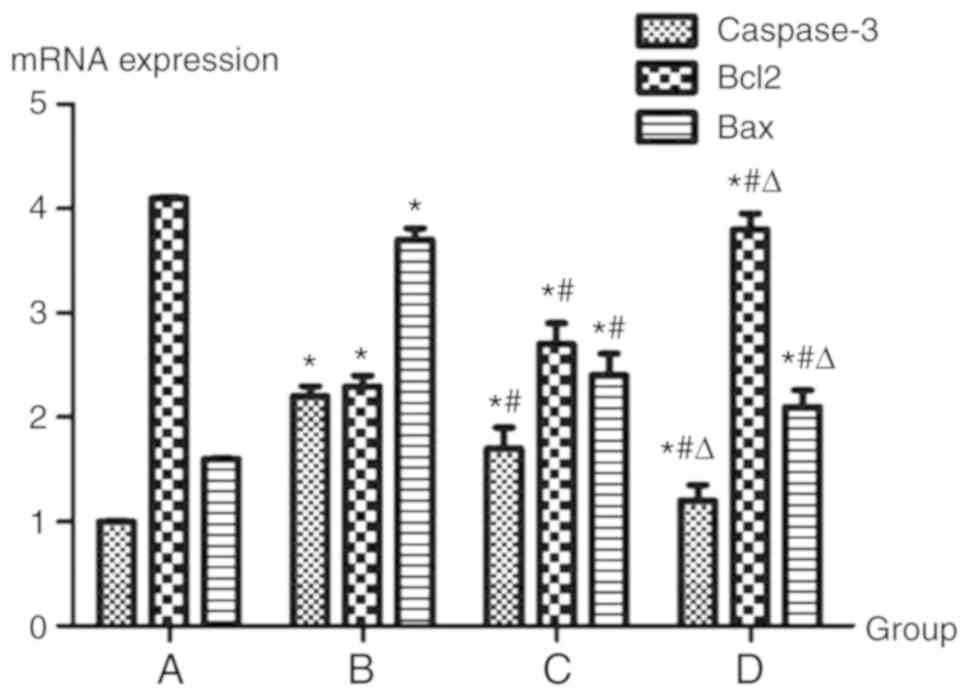
mRNA expression levels of caspase-3,
Bax and Bcl-2
Group B exhibited a marked increase in the mRNA
expression levels of Bax and caspase-3 compared with in group A.
Compared with in group B, groups C and D exhibited decreased mRNA
expression of Bax and caspase-3. Statistically significant
differences between the groups were detected (P<0.05).
Conversely, in group B, the mRNA expression levels of Bcl-2 were
markedly decreased compared with in group A, whereas in groups C
and D, the mRNA expression levels of Bcl-2 were increased compared
with in group B. Statistically significant differences were
detected between the groups (P<0.05; Fig. 5).
Discussion
GYY4137 is a water-soluble, slow-releasing
H2S donor, which may activate endothelial cells to
secrete growth factors, promote the proliferation of smooth muscle
cells and reduce oxidative stress. Previous studies have indicated
that GYY4137 exerts anti-apoptotic and anti-inflammatory effects
that reduce hepatocellular and brain cell injury (12–15).
In accordance with previous studies, the present study demonstrated
that pretreatment with GYY4137 had a marked protective effect on
intestinal tissues subjected to IR.
MDA, as the final product of lipid peroxide
decomposition, is frequently used to evaluate the extent of
cellular damage under oxidative stress. In addition, SOD has an
important role in cell growth and differentiation, and its
antioxidant capacity may attenuate the damage associated with IIR
(14–17). The results of the present study
indicated a decreased MDA content and enhanced SOD activity in the
groups with GYY4137 intervention, which may lead to attenuated
oxidative stress damage following IIR.
As a normal physiological phenomenon, apoptosis is a
cell death program regulated by genetic mechanisms (16,18).
Due to consumption of energy, generation of a large number of
active metabolites and activation of the mitochondrial signal
transduction pathway, IIR may induce massive cell apoptosis and
tissue damage (16,17,19).
In the process of IIR injury, the increased level of oxidative
stress may further induce abnormal apoptosis of mucosal epithelial
cells (18,20). Caspase-3 is a key mediator enzyme
of apoptosis and a marker of the irreversible phase of apoptosis
(18,19,21).
The pro-apoptotic factor Bax and anti-apoptotic factor Bcl-2 are a
pair of closely linked apoptotic genes (20–23).
In the present study, compared with in group A, AI was
significantly increased in group B (IIR group), whereas cell
apoptosis in groups C and D was significantly reduced compared with
in group B. The protein and mRNA expression levels of Bax and
caspase-3 were significantly higher in group B than in group A. In
the groups with GYY4137 intervention (groups C and D), the mRNA
expression levels of Bax and caspase-3 were reduced compared with
in group B. The decrease was more obvious in group D than in group
C, and a statistically significant difference was identified among
the groups. Conversely, the protein and mRNA expression levels of
Bcl-2 exhibited the opposite trend to those of caspase-3 and
Bax.
Regarding the strengths and limitations of the
present study, the experiment focused on the effects of GYY4137 on
IIR. It was performed in vitro without any in vivo
studies, and the regulation of upstream and downstream signaling
pathways was not studied in depth, which represents a
limitation.
In conclusion, the present study indicated that,
compared with in the IIR group, GYY4137 intervention significantly
decreased the expression of Bax and caspase-3, and AI, whereas the
expression of Bcl-2 was significantly increased. It may therefore
be suggested that GYY4137 has a protective effect against IIR
injury.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
NC wrote the manuscript and analyzed data. HL
performed data analysis. YZ designed the study. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Nadatani Y, Watanabe T, Shimada S, Otani
K, Tanigawa T and Fujiwara Y: Microbiome and intestinal
ischemia/reperfusion injury. J Clin Biochem Nutr. 63:26–32. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bradbury AW, Brittenden J, McBride K and
Ruckley CV: Mesenteric ischaemia: A multidisciplinary approach. Br
J Surg. 82:1446–1459. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
van Wijck K, Lenaerts K, van Loon LJ,
Peters WH, Buurman WA and Dejong CH: Exercise-induced splanchnic
hypoperfusion results in gut dysfunction in healthy men. PLoS One.
6:e223662011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hatoum OA, Binion DG, Otterson MF and
Gutterman DD: Acquired microvascular dysfunction in inflammatory
bowel disease: Loss of nitric oxide-mediated vasodilation.
Gastroenterology. 125:58–69. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lin ZL, Yu WK, Tan SJ, Duan KP, Dong Y,
Bai XW, Xu L and Li N: Protective effects of terminal ileostomy
against bacterial translocation in a rat model of intestinal
ischemia/reperfusion injury. World J Gastroenterol. 20:17905–17913.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yasuhara H: Acute mesenteric ischemia: The
challenge of gastroenterology. Surg Today. 35:185–195. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Granger DN, Richardson PD, Kvietys PR and
Mortillaro NA: Intestinal blood flow. Gastroenterology. 78:837–863.
1980. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Parks DA and Granger DN: Contributions of
ischemia and reperfusion to mucosal lesion formation. Am J Physiol.
250:G749–G753. 1986.PubMed/NCBI
|
|
9
|
Li L, Whiteman M, Guan YY, Neo KL, Cheng
Y, Lee SW, Zhao Y, Baskar R, Tan CH and Moore PK: Characterization
of a novel, water-soluble hydrogen sulfide-releasing
molecule(gyy4137): New insights into the biology of hydrogen
sulfide. Circulation. 117:2351–2360. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hayley Robinson and Susan Wray: A new slow
releasing, H2S generating compound, GYY4137 relaxes spontaneous and
oxytocin-stimulated contractions of human and rat pregnant
myometrium. PLoS One. 7:e46272012.
|
|
11
|
Wei WB, Hu X, Zhuang XD, Liao LZ and Li
WD: GYY4137, a novel hydrogen sulfide-releasing molecule, likely
protects against high glucose-induced cytotoxicity by activation of
the AMPK/mTOR signal pathway in H9c2 cells. Mol Cell Biochem.
389:249–256. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu Z, Han Y, Li L, Lu H, Meng G, Li X,
Shirhan M, Peh MT, Xie L, Zhou S, et al: The hydrogen sulfide
donor, GYY4137, exhibits anti-atherosclerotic activity in high fat
fed apolipoprotein E(−/-) mice. Br J Pharmacol. 169:1795–1809.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bernth Jensen JM, Petersen MS, Stegger M,
Østergaard LJ and Møller BK: Real-time relative qPCR without
reference to control samples and estimation of run-specific PCR
parameters from run-internal mini-standard curves. PLoS One.
5:e117232010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Galaly SR, Ahmed OM and Mahmoud AM:
Thymoquinone and curcumin prevent gentamicin-induced liver injury
by attenuating oxidative stress, inflammation and apoptosis. J
Physiol Pharmacol. 65:823–832. 2014.PubMed/NCBI
|
|
15
|
Shao YY, Li B, Huang YM, Luo Q, Xie YM and
Chen YH: Thymoquinone attenuates brain injury via an anti-oxidative
pathway in a status epilepticus rat model. Transl Neurosci. 8:9–14.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jiang D, Wu D, Zhang Y, Xu B, Sun X and Li
Z: Protective effects of hydrogen rich saline solution on
experimental testicular ischemia-reperfusion injury in rats. J
Urol. 187:2249–2253. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cobourne-Duval MK, Taka E, Mendonca P,
Bauer D and Soliman KF: The antioxidant effects of thymoquinone in
activated BV-2 murine microglial cells. Neurochem Res.
41:3227–3238. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Karimian A, Ahmadi Y and Yousefi B:
Multiple functions of p21 in cell cycle, apoptosis and
transcriptional regulation after DNA damage. DNA Repair (Amst).
42:63–71. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang ZX, Shek K, Wang S, Huang X, Lau A,
Yin Z, Sun H, Liu W, Garcia B, Rittling S and Jevnikar AM:
Osteopontin expressed in tubular epithelial cells regulates NK
cell-mediated kidney ischemia reperfusion injury. J Immunol.
185:967–973. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kojima M, Iwakiri R, Wu B, Fujise T,
Watanabe K, Lin T, Amemori S, Sakata H, Shimoda R, Oguzu T, et al:
Effects of antioxidative agents on apoptosis induced by
ischaemia-reperfusion in rat intestinal mucosa. Aliment Pharmacol
Ther. 18 (Suppl 1):S139–S145. 2003. View Article : Google Scholar
|
|
21
|
Arnoult D, Parone P, Martinou JC,
Antonsson B, Estaquier J and Ameisen JC: Mitochondrial release of
apoptosis-inducing factor occurs downstream of cytochrome c release
in response to several proapoptotic stimuli. J Cell Biol.
159:923–929. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Guo J, Wang SB, Yuan TY, Wu YJ, Yan Y, Li
L, Xu XN, Gong LL, Qin HL, Fang LH and Du GH: Coptisine protects
rat heart against myocardial ischemia/reperfusion injury by
suppressing myocardial apoptosis and inflammation. Atherosclerosis.
231:384–391. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Moya A, Sakamaki K, Mason BM, Huisman L,
Forêt S, Weiss Y, Bull TE, Tomii K, Imai K, Hayward DC, et al:
Functional conservation of the apoptotic machinery from coral to
man: The diverse and complex Bcl-2 and caspase repertoires of
Acropora millepora. BMC Genomics. 17:622016. View Article : Google Scholar : PubMed/NCBI
|