Introduction
The main function of ovarian granulosa cells (GCs)
under physiological conditions is maintenance of the proper courses
of folliculogenesis and oogenesis (1). In recent years, it has been
demonstrated that human (h)GCs exhibit stem-like properties under
conditions of long-term in vitro culture; therefore, they
can differentiate into other cell types, such as osteoblasts,
chondrocytes and muscle cells, under the influence of appropriate
factors (2–4). A number of previous studies seem to
suggest that GCs may also differentiate towards neural cells,
despite the lack of any obvious link between these two cell types
(5).
For a number of years, it was thought that the
process of neurogenesis occurs only during embryonic and perinatal
stages in mammals (6).
Neurogenesis is defined as the process of functional neuron
generation from precursor cells (7). During embryogenesis, the neural plate
is formed, with its invaginations built from neuroepithelium. In
subsequent stages of development, the neuroepithelium lines the
inner layer of the neural tube, which gives rise to specific parts
of the central nervous system. A characteristic feature of
neuroepithelial cells is the ability to proliferate rapidly,
resulting in separation of the sub-ventricular zone, which is the
center of the brain ventriculi. The sub-ventricular zone contains
numerous multipotent stem cells that are capable of transforming
into neuron, astrocyte or oligodendrocyte progenitors (8). In the subsequent stages of brain
development, neuroblasts are transformed into proneurons that move
along radial astrocytes to the developing brain and spinal cord
(9). Neurons of the peripheral
nervous system arise from multipotent neural crest cells that
migrate to target sites in an intercellular manner. At the
destination, one neuronal tip becomes the next axon with the growth
cone at the end. This cone has the ability to move in a
quadriplegic motion towards the innervated organ. The complex
process of neurogenesis depends on a number of factors, including
the key role played by proteins belonging to the transforming
growth factor β (TGFβ) family (10,11).
One of the main goals of numerous research groups is
the production of stable, functional neural cell lines that may be
used to restore damage to the nervous system. In recent years, a
number of attempts have been made to differentiate cells of
stem-like potential towards neural lineage (5). These studies primarily used induced
pluripotent stem cells (iPSCs) (12–14).
Despite the difficulties, an increasing number of scientists are
trying to find more sources of functional nerve cells (15–18).
The search has moved away from the model based on cells taken from
the embryo, as well as iPSCs, as this is considered an unstable
model that exhibits a tendency towards tumorigenesis (19). New research focuses primarily on
the reprogramming of stem-like somatic cells towards neurons.
There are a number of reports on the possibility of
differentiation of mesenchymal stem cells towards neural stem cells
(5,20,21).
Herman et al (20) was the
first to present six protocols describing the reprogramming of bone
marrow-derived human mesenchymal stem cells into neural stem cells.
The process of this differentiation was possible by adding
appropriate growth factors including brain-derived neurotrophic
factor, platelet-derived growth factor, epidermal growth factor,
fibroblast growth factor 2 and retinoic acid to the culture medium
(14,20). Another important model for
obtaining neuronal cells is the transdifferentiation of somatic
cells. Transdifferentiation is the reprogramming of somatic cells
into another cell type, omitting the pluripotent stem cell stage.
Transdifferentiation of somatic cells towards neurons seems to be
particularly important. This method could become a novel strategy
in acquiring neural cells, creating new treatment options for
neurodegenerative diseases, and injuries to the central or
peripheral nervous system (22).
Recent studies have indicated that stem cells could become a tool
for the effective treatment of neurological conditions (23–25).
The use of stem cells can have a number of positive effects not
necessarily associated with the artificial growth of new nerves.
Mesenchymal stem cells in particular could be used to treat a
number of neurodegenerative diseases, including Parkinson's
disease, Alzheimer's disease and age-related macular degeneration,
as well as traumatic brain injury and glioblastoma (24,26).
Parkinson's disease manifests as a movement disorder due to loss of
the substantia nigra dopaminergic neurons. In recent years, one of
the most promising tools for effective treatment of this disease is
based on the application of stem cells within the striatum
(27). Stem cell therapy has also
been used to treat stroke. Administration of stem cells within the
first 24 h significantly increases the chances of patient recovery
(28). In addition, cell therapy
has an immunosuppressive and angiogenic effect; therefore, patients
have improved health after the application of stem cells.
Another important issue, the mechanisms of which are
not yet fully elucidated, is neurogenesis in adult mammals. For a
long time, studies have indicated that new neurons are formed in
the subgranular zone of the dentate gyrus of the mammalian
hippocampus (29,30) during learning, remembering or
stress (31). Neurogenesis in
adults is often discussed and it is believed that ~700 new neurons
are created every day in the cusp of the human hippocampus
(32,33), while other studies have suggested
that the neurogenesis process decreases with age (34,35).
It is suggested that the number of developing progenitor cells in
the dentate gyrus drops sharply in the first year of life, as in
healthy adults and patients with neurological pathology (epilepsy)
no ‘young’ neurons have been detected in the hippocampal dentate
gyrus (30). A previous rodent
study indicated that mice maintained in a sensory enriched
environment exhibited significantly more new neurons in the
hippocampus than mice bred in cages (36).
The main objective of the present study was to
identify the potential molecular markers characteristic of GC
differentiation, and to develop a cell line possessing neuronal
characteristics after 30 days of cultivation. The new properties of
hGCs may also be used in the context of reconstruction of tissues
following injury. In the present study, the most important finding
was the identification of genes that perform a large role in the
process of nerve cell formation during long-term in vitro
culture. This knowledge might serve as a basic molecular entry into
further in vitro and clinical studies.
Materials and methods
Previous work
Part of the materials and methods is based on other
publications from the same research team, presenting results from
the cycle of studies related to human ovarian GCs (37–41).
Patient clinical evaluations and GC
collection
A total of 20 female patients (18–40 years; mean
age, 27 years) assigned to the procedure of in vitro
fertilization (IVF) at the Division of Infertility and Reproductive
Endocrinology, Poznań University of Medical Sciences (Poznań,
Poland) qualified for the present study. Patients were recruited
between May 2017 and August 2019. Patients underwent controlled
ovarian hyperstimulation with human recombinant
follicle-stimulating hormone (rFSH; Gonal-F®; Merck
KGaA) and highly purified human menopausal gonadotropin (hMG-HP;
Menopur®; Ferring B.V.) and gonadotropin-releasing
hormone (GnRH) antagonist (Cetrotide®; Merck KGaA).
Ovulation was induced by subcutaneous injection of 6,500 IU human
chorionic gonadotropin (hCG; Ovitrelle®; Merck KGaA).
The doses of gonadotropins and GnRH antagonist were controlled and
recorded for every patient. The follicular fluid (FF) containing
oocytes and GCs was collected and transferred to a qualified
embryologist who extracted all oocytes from the fluid and passed it
on to the further stages of IVF. At this point, it was re-verified
that FF did not contain oocytes. Subsequently, the GC-containing FF
(this sample is usually discarded at this stage) was transferred
for further laboratory testing. The FF containing GCs was collected
during transvaginal ultrasound-guided oocyte pickup, 36 h after
administration of hCG. GCs suspended in FF were obtained from
follicles with a diameter >16 mm.
The exclusion criteria for the study were: i) A
potential risk of inadequate ovarian stimulation according to the
Bologna criteria of poor ovarian responders, published by the
European Society of Human Reproduction and Embryology in 2011
(42); ii) serum antimullerian
hormone 0.7 ng/ml as a cut-off value; iii) patients with a serum
level of FSH >15 mU/ml on the 2nd-3rd day of the cycle; iv)
patients with polycystic ovary syndrome; or v) patients with
endometriosis. The present study was approved by resolution 558/17
of the Poznań University of Medical Sciences Bioethical Committee.
All participating patients were informed about the course of the
study and expressed their written consent to use the material
collected from them during the IVF procedure.
Long-term primary in vitro cell
culture
The GC-containing FF was washed twice using
Dulbecco's phosphate-buffered saline (Sigma-Aldrich; Merck KGaA)
and centrifuged at 200 × g for 10 min at room temperature. The
culture medium consisted of Dulbecco's modified Eagle's medium
(Sigma-Aldrich; Merck GaA), 2% fetal bovine serum (Sigma-Aldrich;
Merck KGaA), 4 mM L-glutamine (stock 200 mM; Gibco; Thermo Fisher
Scientific, Inc.), 10 mg/ml gentamicin (Gibco; Thermo Fisher
Scientific, Inc.), 10,000 U/ml penicillin and 10,000 µg/ml
streptomycin (Gibco; Thermo Fisher Scientific, Inc.). GCs were
cultivated at 37°C under aerobic conditions (5% CO2).
The cultures used samples in which necrotic and apoptotic cells
accounted for <5%. Once adherent cells were >90% confluent,
they were detached with 0.05% trypsin-EDTA (Gibco; Thermo Fisher
Scientific, Inc.) for 1–3 min and counted using a fluorescence
automatic cell counter (ADAM-MC; NanoEnTek America, Inc.). GCs were
then cultivated for 30 days; total RNA was isolated after 1, 7, 15
and 30 days (38,39,43).
The medium was replaced every 72 h. Cell morphology was observed
after 1, 7, 15 and 30 days of culture under an inverted light
microscope (Olympus IXC73; Olympus Corporation).
Total RNA isolation
Total RNA was isolated after 1, 7, 15 and 30 days of
culture. The Chomczyński-Sacchi method was used for total RNA
extraction (44). The GCs, after
trypsin treatment, were suspended in a 1-ml mixture of guanidine
thiocyanate and phenol in monophase solution (TRI
Reagent®; Sigma-Aldrich; Merck KGaA); 1 ml TRI
Reagent® was used to lyse 5–10×106 cells,
with subsequent storage of the samples at −80°C. After thawing, 0.2
ml chloroform per ml TRI Reagent® was added to the
samples, which were then gently mixed (15 sec), and left to stand
for 15 min at room temperature. After centrifugation (12,000 × g,
15 min, room temperature), three phases were visible: Red organic
phase (containing protein), interphase (containing DNA) and
colorless upper phase (containing RNA). The aqueous phase that
contained the RNA was precipitated with 0.5 ml 2-propanol (cat. no.
I9516; Sigma-Aldrich; Merck KGaA) per ml TRI Reagent®
and incubated for 10 min at room temperature. Finally, the RNA
pellet was washed with 75% ethanol. The resulting RNA was used for
further analysis. The total mRNA was determined from the optical
density at 260 nm, and the RNA purity was estimated using the
260/280 nm absorption ratio (NanoDrop spectrophotometer; NanoDrop;
Thermo Fisher Scientific, Inc.). Only samples with a 260/280
absorbance ratio >1.8 were used in the present study.
Microarray expression analysis
Total RNA (100 ng) from each pooled sample was
subjected to two rounds of sense cDNA amplification (Ambion WT
Expression kit; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. The obtained cDNA was used for biotin
labeling and fragmentation using the Affymetrix GeneChip WT
Terminal Labeling and Hybridization kit (Affymetrix; Thermo Fisher
Scientific, Inc.). Biotin-labeled fragments of cDNA (5.5 µg) were
hybridized to the Affymetrix Human Genome U219 Array (HgU 219;
48°C/20 h; Affymetrix; Thermo Fisher Scientific, Inc.). Microarrays
were then washed and stained according to the technical protocol
using the Affymetrix GeneAtlas Fluidics Station (Affymetrix; Thermo
Fisher Scientific, Inc.). The array strips were scanned using the
Imaging Station of the GeneAtlas system (Affymetrix; Thermo Fisher
Scientific, Inc.). Preliminary analysis of the scanned chips was
performed using Affymetrix GeneAtlas Operating Software v.
2.0.0.460 (Affymetrix; Thermo Fisher Scientific, Inc.). The quality
of gene expression data was confirmed according to the quality
control criteria provided by the software. The obtained CEL files
were imported into downstream data analysis software (38,45).
Reverse transcription-quantitative
(RT-q)PCR
RT-qPCR was performed to validate microarray
results, using the same cDNA samples. A total of 20 genes were
selected from the 131 selected genes: 10 represent the highest
change in expression, and 10 represent the lowest change in
expression, but all 20 had been upregulated in relation to day 1 of
primary culture. Each reaction was repeated three times, with three
replicates per group. For RT, 1 µg each RNA sample was used. RT was
conducted based on the protocols and reagents of the RT2
First Stand kit (cat. no. 330401; Qiagen, Inc.), using a Veriti
96-well Thermal Cycler (Applied Biosystems; Thermo Fisher
Scientific, Inc.). PCR was performed using the Light
Cycler® 96 (Roche Diagnostics GmbH), RT2 SYBR
Green ROX qPCR Master Mix (Qiagen, Inc.) and sequence-specific
primers (Table I). The reaction
cocktail used for the test contained 3 µl nuclease-free water
(Invitrogen; Thermo Fisher Scientific, Inc.), 5 µl RT2
SYBR Green ROX qPCR Master Mix (Qiagen, Inc.), 0.5 µl forward
primers, 0.5 µl reverse primers and 1 µl cDNA. GAPDH,
β-actin (ACTB) and hypoxanthine phosphoribosyltransferase 1
(HPRT1) were used as reference genes. Thermocycling
conditions were as follows: Preincubation at 37°C for 30 sec;
3-step amplification (95°C for 15 sec, 58°C for 15 sec, 72°C for 15
sec) for 45 cycles; melting (95°C for 60 sec, 40°C for 60 sec, 70°C
for 1 sec, 95°C for 1 sec); cooling at 37°C for 30 sec. Gene
expression was analyzed using the 2−ΔΔCq method. The
qPCR primers were designed using Primer3Plus software (http://primer3plus.com/cgi-bin/dev/primer3plus.cgi).
 | Table I.Oligonucleotide sequences of primers
used for reverse transcription-quantitative polymerase chain
reaction analysis. |
Table I.
Oligonucleotide sequences of primers
used for reverse transcription-quantitative polymerase chain
reaction analysis.
| Gene | Primer sequences
(5′-3′) | Product size
(bp) |
|---|
| NTN4 | F:
GGCCTGGAAGATGATGTTGT | 234 |
|
| R:
TTGAGGCTCTTCGTTCAGGT |
|
| CRIM1 | F:
GGAAGGAGAAACGTGGAACA | 247 |
|
| R:
GTCAGGCTTCCAGGACTCAG |
|
| NANOS1 | F:
GCTCCTGGAACGACTACCTG | 209 |
|
| R:
GTCGTCGTCCTCGTCGTAGT |
|
| FRY | F:
CCAGCACAGTGACCTCTCAA | 232 |
|
| R:
AACAAGGACGTTGGAGTTGG |
|
| CD9 | F:
TTGGTGATATTCGCCATTGA | 160 |
|
| R:
ACGCATAGTGGATGGCTTTC |
|
| ITGA3 | F:
GCCTGCCAAGCTAATGAGAC | 247 |
|
| R:
AGAAGCTTTGTAGCCGGTGA |
|
| ATP8B1 | F:
TGCATACGAGGATTGGTTCA | 189 |
|
| R:
ACCCCATGCAACAAGCTTAC |
|
| DFNA5 | F:
AGGTGGCTTCGAGAACAAGA | 234 |
|
| R:
AATAGGACCGCCTGGAAGAT |
|
| OXTR | F:
TTCTTCGTGCAGATGTGGAG | 234 |
|
| R:
GGACGAGTTGCTCTTTTTGC |
|
| CLDN11 | F:
CTGGTGGACATCCTCATCCT | 190 |
|
| R:
CCAGCAGAATGAGCAAAACA |
|
| RPM1 | F:
GGAGGAATTGGTGTTGCTGT | 235 |
|
| R:
GCTGCTCTTCCTTTCCTGTG |
|
| SRGAP2 | F:
ACTAAAGGAGGCGGAGAAGC | 220 |
|
| R:
GTACTCATTCCGGGCTTTGA |
|
| LRFN4 | F:
GGACTGGTGGACCTGACACT | 194 |
|
| R:
GATGAGGTGCTGCAGATTGA |
|
| INPP5J | F:
TTCAACTTCGTGCTGGTGAG | 248 |
|
| R:
TTCAGGAAGCAGAGCATGTG |
|
| RITA1 | F:
CCCTCACACCAAGGAAGAAG | 204 |
|
| R:
CTCTGTCTTGGAGGGACCAG |
|
| PPP3CA | F:
TGCATCAATTCTTCGACAGG | 162 |
|
| R:
AAGGCCCACAAATACAGCAC |
|
| CTNND1 | F:
TCTGCCATAGCTGACCTCCT | 208 |
|
| R:
GGAGTTCTGCTGTCCTCCTG |
|
| CASP2 | F:
GACGCAGGATATTGGGAGTG | 170 |
|
| R:
GGCAGCAAGTTGAGGAGTTC |
|
| VIM | F:
GAGAACTTTGCCGTTGAAGC | 199 |
|
| R:
TCCAGCAGCTTCCTGTAGGT |
|
|
KIDINS220 | F:
CTGATGATAGCTGCCGAACA | 191 |
|
| R:
GAGCTGTCCATCCTCCCATA |
|
| RRM1 | F:
GGAGGAATTGGTGTTGCTGT | 235 |
|
| R:
GCTGCTCTTCCTTTCCTGTG |
|
| GAPDH |
F:TCAGCCGCATCTTCTTTTGC | 90 |
|
|
R:ACGACCAAATCCGTTGACTC |
|
| β-actin |
F:AAAGACCTGTACGCCAACAC | 132 |
|
|
R:CTCAGGAGGAGCAATGATCTTG |
|
| HPRT1 |
F:TGGCGTCGTGATTAGTGATG | 141 |
|
|
R:ACATCTCGAGCAAGACGTTC |
|
Statistical analysis
All of the presented analyses and graphs of
microarray expression were performed and generated using
Bioconductor (v. 3.10; http://www.bioconductor.org/) and R programming
language (v 3.5.1; www.r-project.org). Each CEL file was merged with a
description file. In order to correct background, normalize and
summarize results, the Robust Multiarray Averaging algorithm was
used. To determine the statistical significance of the analyzed
genes, moderated t-statistics from the empirical Bayes method were
performed. The obtained P-value was corrected for multiple
comparisons using Benjamini and Hochberg's false discovery rate.
The selection of significantly altered genes was based on P<0.05
and expression >2-fold. The differentially expressed gene list
(separated for up- and downregulated genes) was uploaded to the
Database for Annotation, Visualization and Integrated Discovery
(DAVID, v 6.8) software to investigate their mutual relations
(46–48). DAVID was used for extraction of the
genes belonging to ‘neurogenesis’, ‘neuronal precursor cell
proliferation’ and ‘nervous system development’ GO BP terms. Up-
and downregulated gene sets were subjected to the DAVID search
separately and only gene sets with adjusted P<0.05 were
selected.
Subsequently, mutual interactions between the genes
belonging to the selected Gene Ontology (GO) biological process
(BP) terms were investigated using the GOplot package (49). Finally, the functional interactions
(FIs) between genes that belong to the chosen GO BP terms were
investigated by REACTOME FIViz application in the Cytoscape 3.6.0
software (https://cytoscape.org/). The
ReactomeFIViz application is designed to find pathways and network
patterns related to cancer and other types of diseases. This
application accesses the pathways stored in the Reactome database,
allowing pathway enrichment analysis for a set of genes,
visualizing hit pathways using manually laid-out pathway diagrams
directly in Cytoscape, and investigating functional relationships
among genes in hit pathways. The application can also access the
Reactome FI network, a highly reliable, manually curated
pathway-based protein FI network covering >60% of human
proteins. The results of the experiments refer to three separate
biological replicates, and represent the average measurements (mean
± standard error of mean) from each time period of the cell
cultures and mRNA measurements, as determined by RT-qPCR. In the
case of RT-qPCR, biological replicates were divided into three
technical repetitions. As an internal control, HPRT1, GAPDH
and ACTB were used, for which the levels of transcripts
analyzed were standardized in each sample. Relative quantification
was performed using the 2−ΔΔCq method to determine
target cDNA quantification (50).
Statistical analysis of the RT-qPCR results was performed for all
samples considered separately (Student's t-test was corrected using
Benjamini and Hochberg coefficient). P<0.05 was considered to
indicate a statistically significant difference. The analysis was
performed using the Real Statistics Resource Pack for MS Excel 2016
(Microsoft Corporation).
Results
Overview
Microarray analysis allows the identification of
groups of genes related to the development of the nervous system
and neurogenesis. Three ontological groups: ‘neurogenesis’,
‘neuronal precursor cell proliferation’ and ‘nervous system
development’ were chosen. The microarray results provided 131 genes
from three heat maps. The highest change in expression was
demonstrated by claudin 11 (CLDN11), oxytocin receptor
(OXTR), gasdermin E (DFNA5), ATPase phospholipid
transporting 8B1 (ATP8B1), integrin subunit α3
(ITGA3), CD9, FRY microtubule binding protein
(FRY), nanos C2HC-type zinc finger 1 (NANOS1),
cysteine rich transmembrane BMP regulator 1 (CRIM1) and
netrin 4 (NTN4). The first part of the results focuses on
the 131 genes belonging to all three selected ontological groups.
The second part of the results focuses on the genes with the
highest change in expression.
Microarray analysis
Whole transcriptome profiling by Affymetrix
microarray allowed the analysis of transcriptomic changes of the
GCs during long-term in vitro culture after 24 h (1 day), 7,
15 and 30 days of culture. Using Affymetrix® Human HgU
219 Array, the expression levels of 22,480 transcripts were
examined. Genes with fold change >2 and corrected P<0.05 were
considered as differentially expressed. This set of genes consisted
of 2,278 different transcripts and is available as from the GEO
database (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE129919).
The DAVID software analysis demonstrated that differentially
expressed genes belong to 582 GO groups.
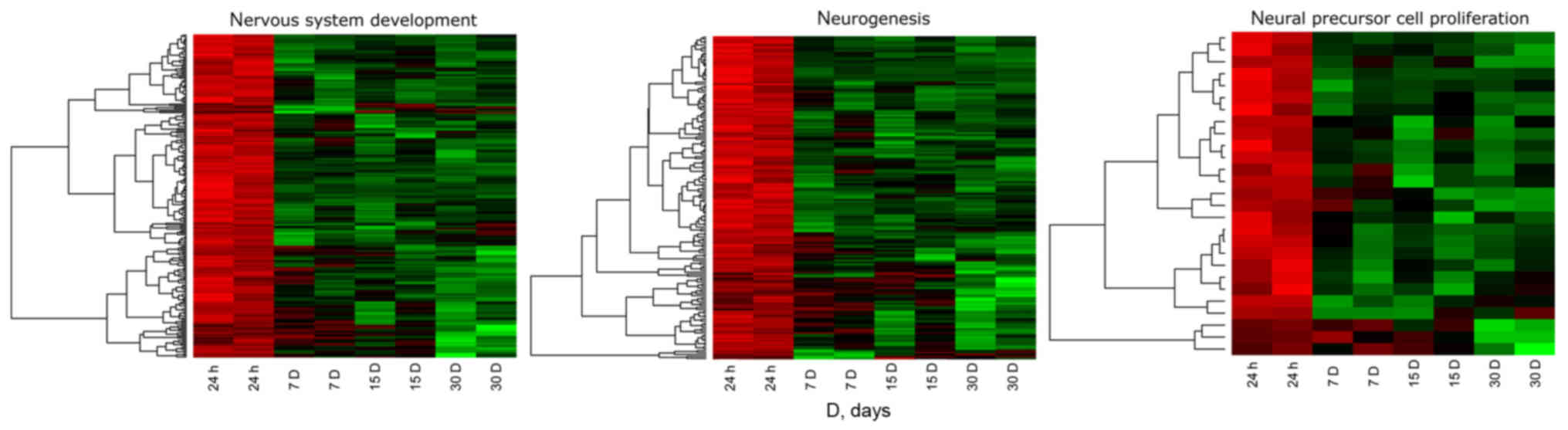
Fig. 1 shows the
hierarchical clustering of all genes belonging to the selected
ontological groups, presented as heat maps. The heat maps indicate
a large number of genes involved in neurogenesis-related processes,
and show the levels of expression of a given gene at particular
time intervals of long-term primary in vitro culture. The
gene symbols, fold changes in expression, Entrez gene IDs and
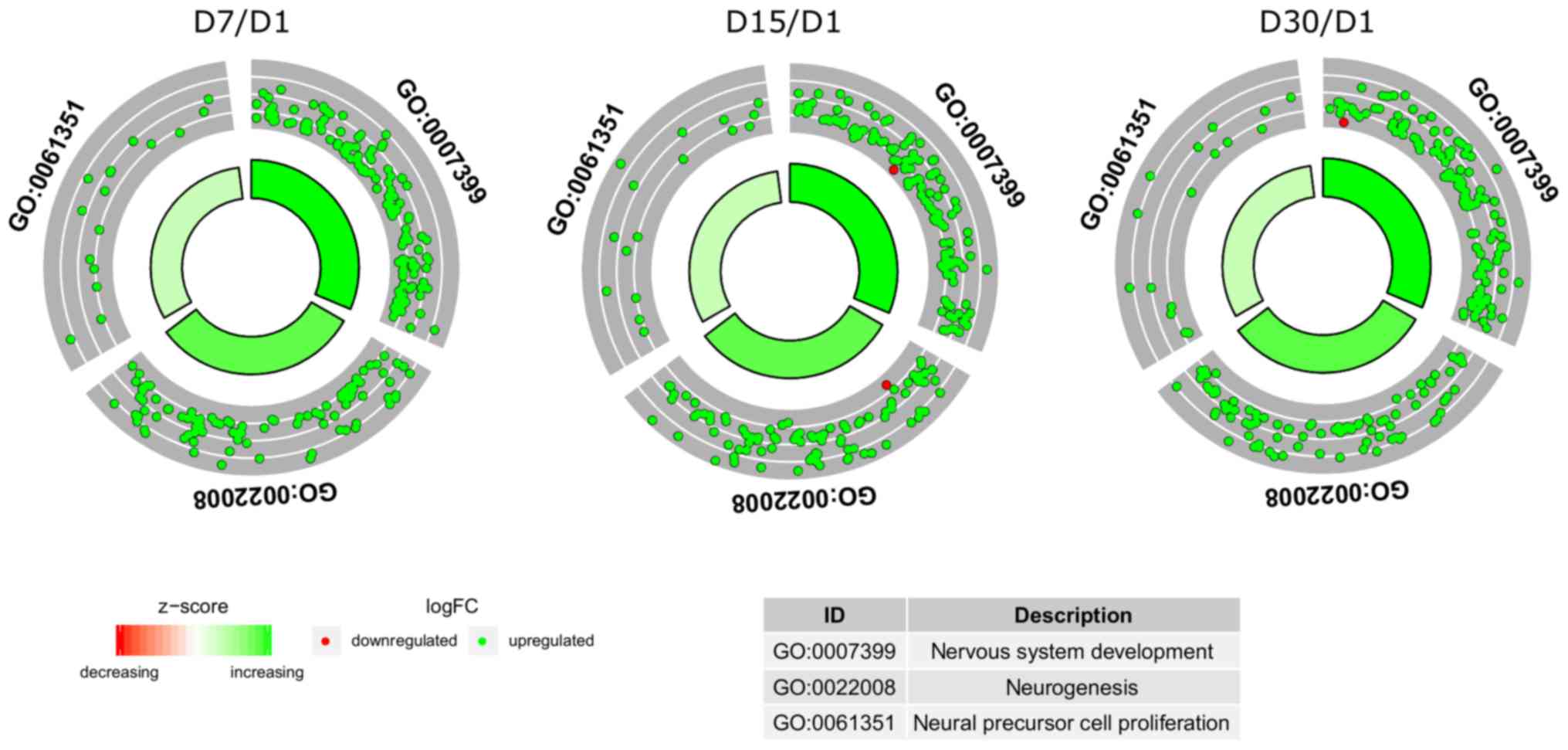
corrected P-values of the genes are shown in Table SI. The enrichment of each GO BP
term was calculated as a z-score and shown on a circle diagram
(Fig. 2). The aforementioned
circle graph combines data on the expression of all genes belonging
to the listed ontological groups and the enrichment of gene
annotations. It also demonstrates the spread of gene expression
data.
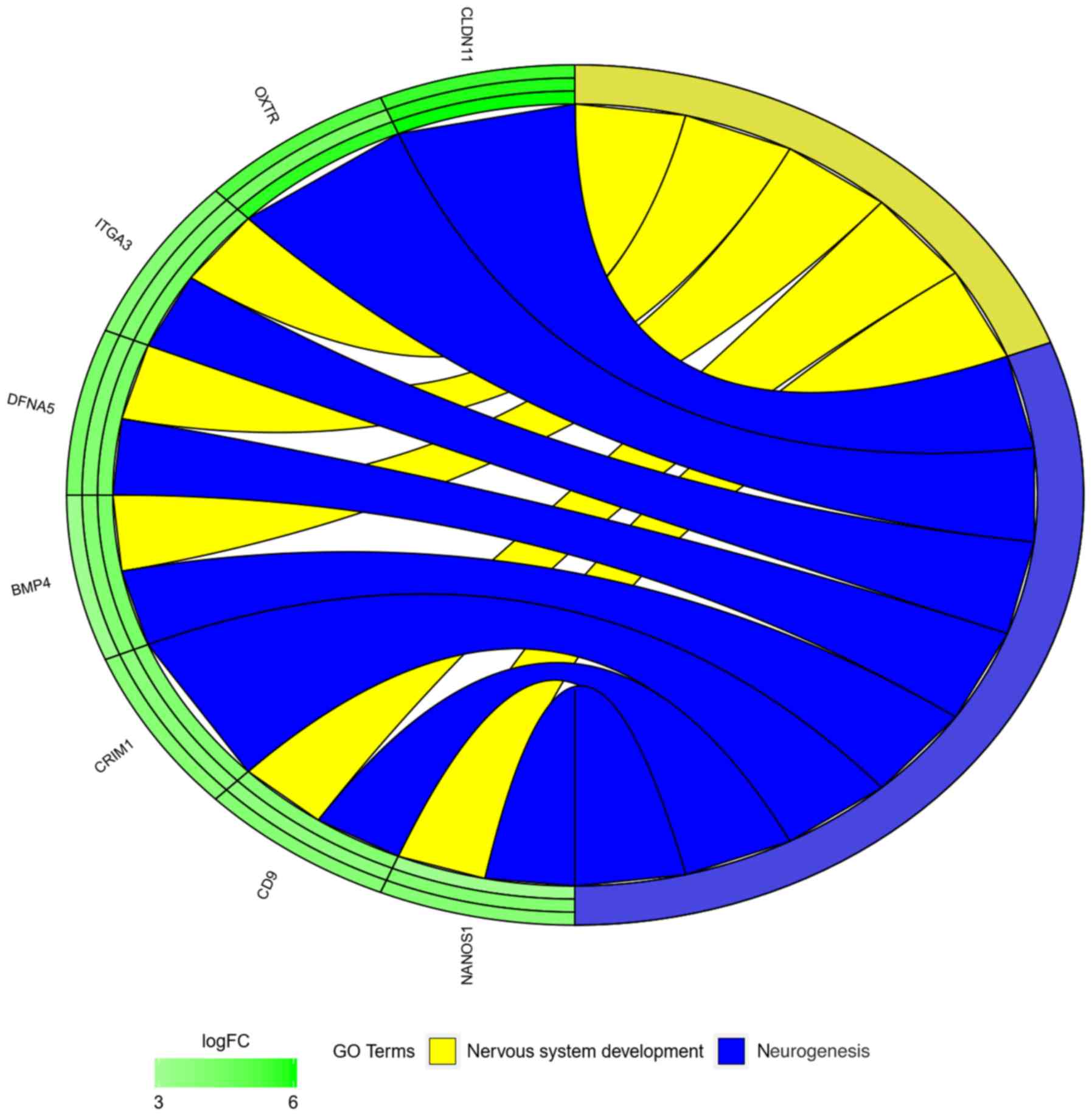
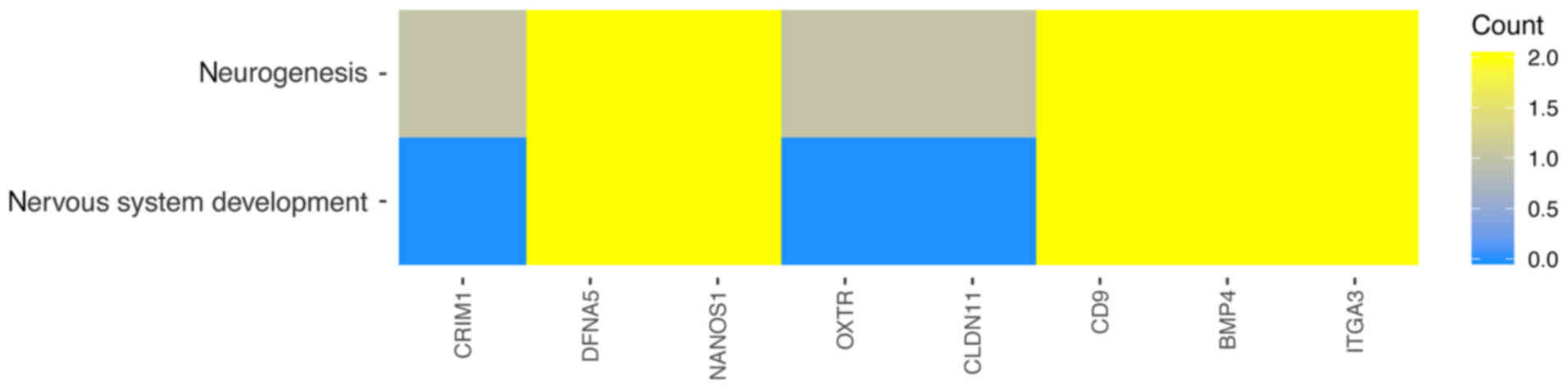
In the GO database, genes that are associated with
one particular GO term can also belong to other GO term categories.
For this reason, the gene intersections between the selected GO BP
terms were explored. The next stage of statistical analysis was
focused on analyzing the relationships between genes with the
highest change in expression, closely related to the process of
neurogenesis. From this group, eight genes were selected, based on
their assumed importance in the process of interest. The relation
between those GO BP terms was presented as a circle plot (Fig. 3) as well as heat maps (Fig. 4).
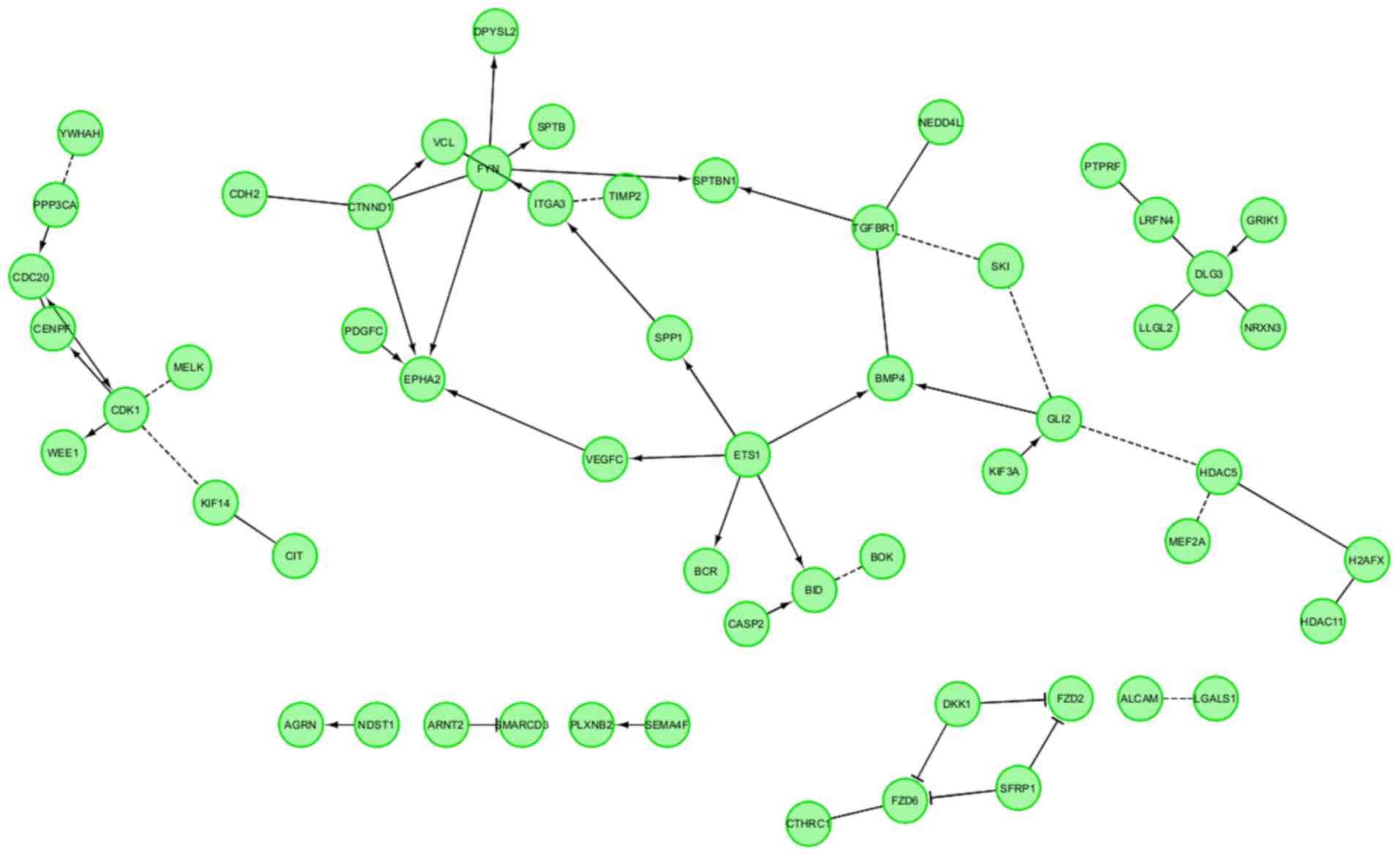
Finally, the FIs between chosen genes were
investigated with REACTOME FIViz application in Cytoscape 3.6.0
software. This statistical analysis concerned the interaction
between genes involved in neurogenesis-related processes. All genes
belonging to selected ontological groups were considered in this
study. The results are shown in Fig.
5. Notably, all of the genes were involved in interaction with
other representatives of the analyzed group. There were four larger
groups of interacting genes, as well as several genes that only
exhibited singular interactions.
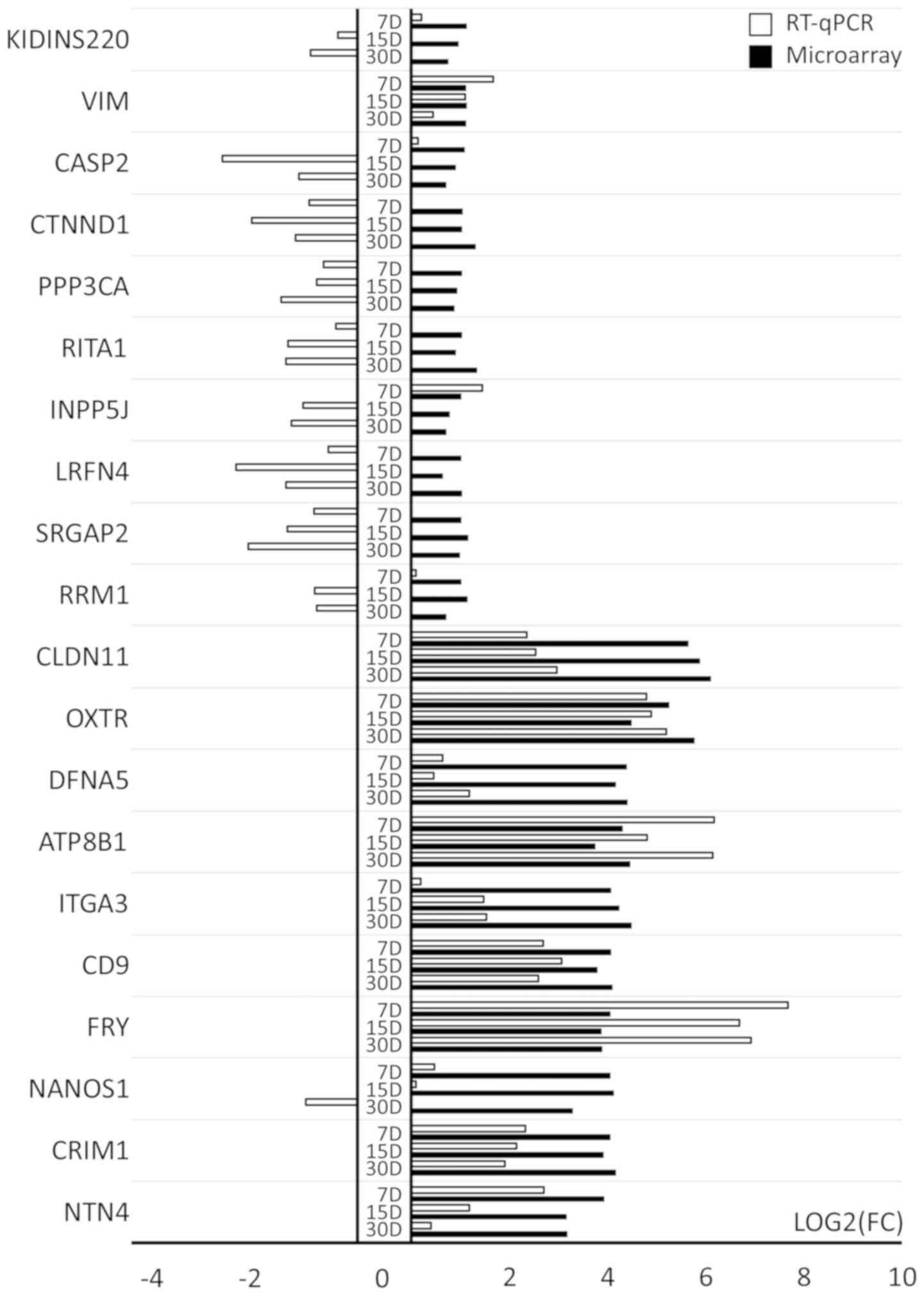
RT-qPCR results
The 20 genes with increased expression were
subjected to validation and 10 of these genes exhibited the highest
changes in expression compared to day 1 of primary culture
(CLDN11, OXTR, DFNA5, ATP8B1, ITGA3, CD9, FRY, NANOS1, CRIM1
and NTN4). The remaining 10 genes (RRM1, SRGAP2, LRFN4,
INPP5J, RITA1, PPP3CA, CTNND1, CASP2, VIM and KIDINS220)
exhibited the lowest level of upregulation, being on the border of
selection for this study with a fold change only slightly >2. As
shown in Fig. 6, the RT-qPCR
method confirmed the direction of change in the expression of the
top 10 genes with the highest transcript expression in microarray
analysis in most of the cases, with the exception of CR1M1,
which exhibited discrepancies at day 30. Differences in the scale
of change are due to the sensitivity of the methods used. For some
genes, the direction of change in expression was not confirmed by
RT-qPCR (Fig. 6). This discrepancy
can be explained in two ways: On one hand, this may be due to the
fact that the RT-qPCR method is much more sensitive to quantitative
changes, on the other hand it may indicate that selected genes
exhibited very low upregulation of transcript levels during the
microarray analysis. Such discrepancies confirm that whole
transcriptomic screening requires extensive quantitative
validation.
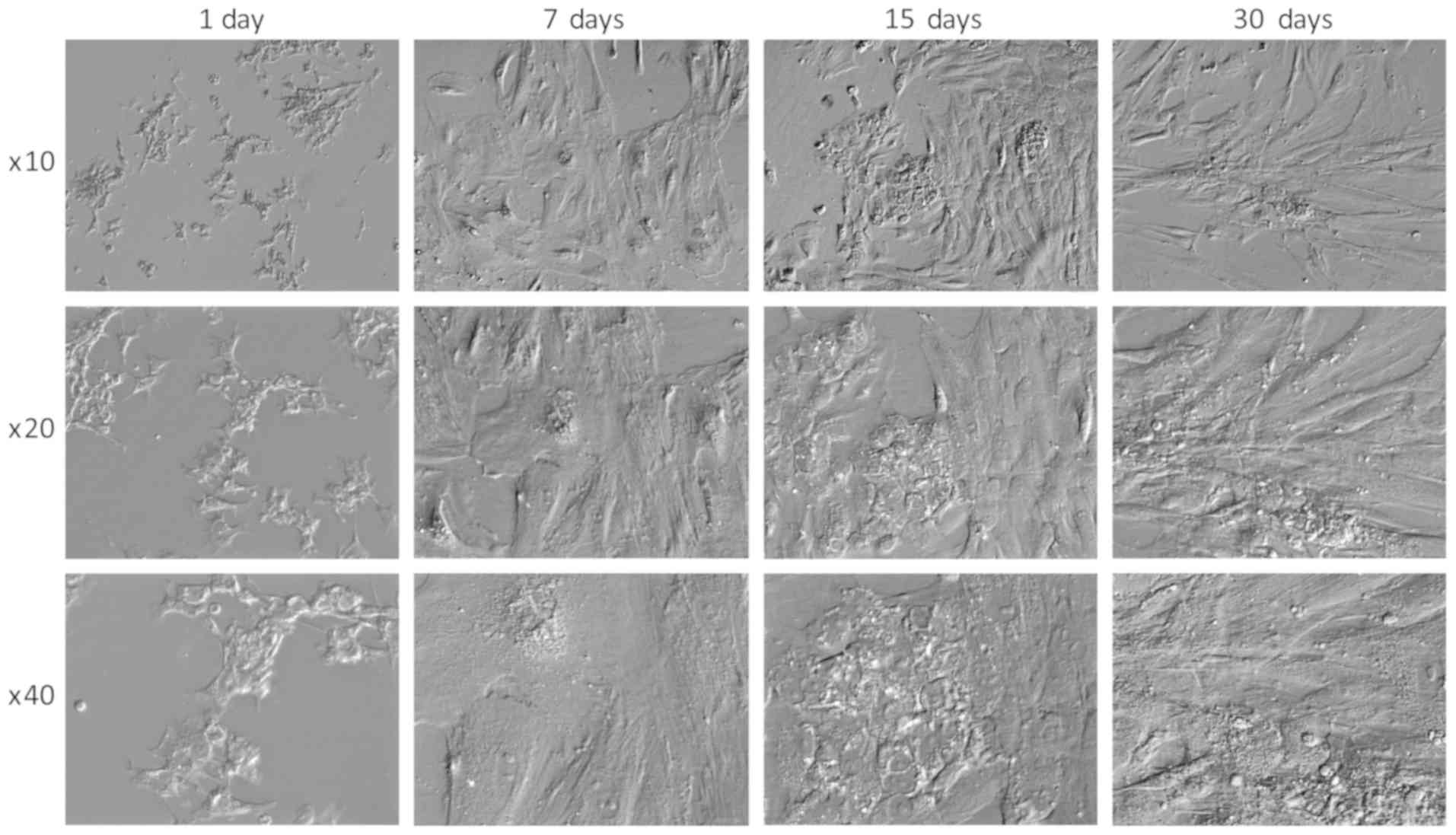
Cell morphology
Notably, it has been suggested that GCs exhibit
morphology similar to that of nerve cells in long-term in
vitro culture conditions and exhibit molecular markers
characteristic of neuronal cells (51). The morphology of GCs in the present
study also changed. Initially, the cells had a stellate shape,
after which, they transformed (regardless of confluence) into
spindle-shaped cells (Fig. 7).
Such changes have also been confirmed in other studies (37,39).
Discussion
In the present study, a group of genes responsible
for processes associated with neurogenesis, nervous system
development and neural precursor cell proliferation were selected
for analysis during long-term in vitro culture of GCs. The
results indicated that these cells have the potential to
differentiate towards neurons, as they express
neural-differentiation specific genes, providing further proof for
their stem-like potential (14,20).
A number of studies have reported that it is possible to obtain
neuronal-like cells during the differentiation/transdifferentiation
of other cell types (5,39,43,52).
Notably, neural-like cells can be obtained through adipocyte
differentiation. These adipocyte-derived cells of neural lineage
exhibited high levels of CLDN11 (oligodendrocyte-specific protein),
which is a marker of glial cells (53). The CLDN11 gene exhibited the
highest expression at the individual time intervals in the present
study. CLDNs are membrane proteins that belong to the peripheral
myelin protein 22 superfamily. One of the most important functions
of these membrane proteins is to co-create tight junction
connections (54). The presence of
CDLN proteins in epithelial cells and endothelium is regulated
hormonally, and is also subject to changes depending on ovarian
dysfunction (54). In addition,
the administration of hormonal stimulation affects the expression
of CLDN in the ovaries, with hCG administration causing an increase
in the expression of CLDN11 at the transcript level, which
indicates the expression of this gene during ovulation (55). It is also believed that this gene
may be involved in the growth of ovarian follicles in cattle
(56). In addition, this gene is
highly expressed in neurons (53).
Another gene that was highly expressed during the 30
days of in vitro culture was OXTR. In vivo, it
is primarily associated with fertility and reproductive behavior
(57). It has also been suggested
that this gene is involved in the formation of the heart and
cardiac muscle cells (58).
Therefore, its expression has also been discussed in a previous
study regarding the differentiation of GCs to myocardial cells
(39). Many scientists believe
that neurogenesis also occurs in adult mammals (59–64).
Research by Lin et al (65)
indicated that oxytocin may stimulate neurogenesis occurring in the
hippocampal gyrus in adult humans. Oxytocin neurons located in the
paraventricular nucleus connect directly to the hippocampus
(65). Oxytocin is assumed to
perform a key role in the neurogenesis of adult mammals, including
humans (65). However, another
study indicated that OXTR is not expressed in neuronal
progenitor cells derived from the subgranular dentate gyrus
(66).
Another gene with high expression in this study was
DFNA5. This gene encodes proteins located in a number of
organs, including the cortex of the brain and the ovaries (67–70).
In the cortex, this gene is expressed at RNA and protein levels. A
previous study by Croes et al (71) suggested that DFNA5 may be
considered a breast cancer biomarker, as there are significant
differences between the expression of DFNA5 in women with
breast cancer and healthy controls. It has also been shown that the
expression of this gene is influenced by the presence of the
estrogen receptor (71). Other
studies revealed that an increase in the expression DFNA5
may be affected by the hormonal stimulation of patients (72–74).
Assou et al (72) indicated
that DFNA5 expression is increased in cumulus cells obtained
from patients following rFSH stimulation, compared to cells
obtained following hMG-HP stimulation. These findings indicated
that the high expression of DFNA5 detected in GCs in this
study may be the result of stimulation with rFSH. In the present
study, patients were also stimulated with hMG-HP, so both
substances may influence the aforementioned results. Previous
research has indicated the possibility of the differentiation of
GCs towards neurons. In addition, DFNA5 expression is also
increased in another type of MSC, in bone marrow cells during their
differentiation into neuronal cells (73,74).
The ITGA3 gene, which encodes a protein from
the integrin family, was also highly expressed in GCs in this
study. Integrins, as the main extracellular matrix receptors, are
poorly understood in the nervous system. Non-neural tissue is the
subject of the majority of research into these proteins (75). It is well known that during
embryonic development, integrins serve an important role in
neurogenesis (76). The
extracellular matrix and its receptors, which influence the process
of nerve cell formation as well as synaptic connections, seem
particularly important (75).
Under physiological conditions, integrins have a role in the
differentiation of individual GC populations during
folliculogenesis and are also present in endometrial cells
(77). The results of the present
study suggested that the ITGA3 gene may be involved in the
process of differentiation and formation of neuronal precursor
cells. To the best of our knowledge, this study is the first to
indicate this and this finding is not confirmed elsewhere in the
literature.
In the present study, the CRIM1 gene
exhibited increased expression at particular time intervals of
human GC culture, relative to the control (1st day of culture).
Overexpression of CRIM1 in mice increased the activity of
integrins (including ITGA1), and induced phosphorylation of focal
adhesion kinase and ERK. It is suggested that this gene may
participate in the formation of motor neurons and regulate their
viability through interaction with various growth factors. Kolle
et al (78) reported that
development of the central nervous system is dependent on the
CRIM1 gene; CRIM1 is expressed in early motor neurons
as well as in the developing spinal cord. It is believed that
CRIM1 encodes a transmembrane protein that contains the
sequence of the IGF binding protein. In addition, this
transmembrane protein contains numerous cysteine-rich repeats
(78,79). It has also been suggested that this
gene interacts with bone morphogenetic proteins (BMPs). The
interaction between CRIM1 and BMP/TGFβ may be functionally
important for the proper development of the central nervous system
(78). In addition, it has been
suggested that this gene is involved in the survival ability of
motor neurons in the embryo and following injury in adult animals
(80).
While not presented in detail in this study, as it
was not one of the 10 genes that exhibited the biggest change in
any direction, BMP4 was also differentially expressed in GC
long-term in vitro culture. The protein encoded by this gene
serves a number of functions during embryonic and postnatal
development, including bone development, mineralization,
neurogenesis, adipogenesis and ovarian primordial follicle
development (81–83). Co-expression of this gene with
other factors (CRIM1) in this study suggests that long-term
in vitro cultivation may result in the process of GC
differentiation to neuronal cells. The presented results from our
study (expression of CRIM1 and BMP4) suggested that
GCs may have the potential to differentiate into neural-like
cells.
Takao et al (84) suggested that the expression of
genes responsible for integrin formation is correlated with the
presence of CD9 on surface of the GCs. In the present study
regarding GCs, CD9 and ITGA3 were highly upregulated,
but correlation studies do not show any link between them (84). CD9 is a marker of mesenchymal stem
cells; a previous report demonstrated that cells expressing this
marker can differentiate towards neuronal cells and express neural
cell-specific proteins (vimentin, glial fibrillary acidic protein,
nuclear factor, neuron specific enolase and nestin) (85). CD9 is primarily found in stem cell
exosomes, and a previous study revealed that exosomes can also
serve an important role in neurogenesis and can function as
information relays between stem and neural cells (86). These findings suggested that the
presence of this marker in GCs may indicate their potential to
differentiate into neural-like cells.
The studied cells also expressed the NANOS1
gene (87). NANOS1 is
primarily responsible for the coding of proteins involved in the
development of embryonic stem cells in one or both sexes of model
organisms, such as Drosophila melanogaster (88,89)
or Caenorhabditis elegans (90). The human equivalent of this gene
exhibits a high expression in germinal stem cells (91), as well as in oocytes at various
levels of maturity (92). In
addition, high expression levels of this gene have been detected in
the fetal brain (93), which is
important with regards to the findings of the present study.
Through analysis of the obtained results and
available literature, it was concluded that GCs may possess
stem-like functions and might be capable of differentiating into
neuronal cells under long-term in vitro cultures. This
suggestion was supported by the high expression of genes associated
with processes, such as neurogenesis, nervous system development
and neural precursor cell proliferation. The present study was
carried out at the transcriptome level. Obtained results, despite
validation using quantitative methods, require confirmation at the
protein level, as well as an analysis of factors released during
the potential differentiation process. In addition, it should be
noted that analysis of the GC transcriptome using microarrays is a
largely qualitative method, which can be seen in the validation
carried out by RT-qPCR. Variable results might be caused by
differences in microarray probe and RT-qPCR primer design, and
their specificity and responsiveness to particular transcript
variants. They can also result from the potential interactions
between cDNAs present in the analyzed samples, leading to false
positive/negative results. This further emphasizes the need of
protein validation of the results, which could also account for the
processes that underlie the discrepancies between transcriptomic
and proteomic results, including alternative splicing, selective
translation and post transcriptional regulation processes.
In conclusion, the present study analyzed the
expression profile of genes belonging to three ontological groups:
‘neurogenesis’ (GO:0022008), ‘neuronal precursor cell
proliferation’ (GO:0061351) and ‘nervous system development’
(GO:0007399). The results appear to support the suggestion that GCs
may be able to develop into a cell line showing neuronal
characteristics after 30 days of cultivation, and may serve as a
basic transcriptomic entry for further research that could fully
confirm this ability. Given the proven plasticity of FF GCs in the
context of the current trend to consider treatment of nervous
system disorders with stem cells, GCs may be considered a promising
tool in the treatment of these diseases, as they appear to exhibit
significant stem-like properties. Hence, it is necessary to take
into account the number of mechanisms triggered by the
administration of stem cells, which can be beneficial when treating
diseases, due to their anti-inflammatory or immunomodulatory
effects. The results of the present study are a promising
introduction to further research on the mechanisms and factors that
result in the ability of the analyzed cells to express neuronal
features. In the future, GCs could not only become a tool of direct
neuronal regeneration but also a producer of factors that may find
application in the treatment of neurodegenerative diseases. Our
long-term project aims to examine the further molecular mechanisms
associated with genes of interest, with next stages of research
planned, in which the cells in the present study will be cultured
with neural differentiation medium. Furthermore, the authors will
aim to compare the transcriptome of GCs before and after exposure
to neural differentiation medium.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Science Centre (grant no. 2018/31/B/NZ5/02475) and the Poznań
University of Medical Sciences (grant no.
502-14-02227367-10694).
Availability of data and materials
The datasets used and/or analyzed during the
current study are available from the corresponding author on
reasonable request. In addition, the datasets generated and/or
analyzed during the current study are available in the Gene
Expression Omnibus repository, [https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE129919].
Authors' contributions
MBrą designed and performed the experiments, chose
the models, prepared part of the medical methodology, and drafted
and wrote parts of the manuscript. WK wrote parts of the
manuscript, and prepared and validated the RNA isolation protocol.
PC performed data analysis, prepared figures and wrote parts of the
manuscript. MJ performed data analysis and language corrections.
HPK was responsible for software, experimental design and model
analysis. LP made substantial contributions to conception of
experiments, revised it critically for important intellectual
content. MBru supervised the project, provided technical advice and
conducted analyses of the raw data. MZ revised the methodology,
optimizing the methods of culture and analysis, and assisted in
writing the manuscript. MN designed the methodology, and assisted
in writing and revision of the manuscript. BK contributed to
project supervision and design, revision of methodology, editorial
supervision and major assistance. All authors approved the final
article.
Ethics approval and consent to
participate
This study has been approved with resolution 558/17
by Poznań University of Medical Sciences Bioethical Committee. All
participating patients were informed about the course of the study
and expressed their written consent to use the material collected
from them during the IVF procedure.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rybska M, Knap S, Jankowski M, Jeseta M,
Bukowska D, Antosik P, Nowicki M, Zabel M, Kempisty B and Jaśkowski
JM: Characteristic of factors influencing the proper course of
folliculogenesis in mammals. Med J Cell Biol. 6:33–38. 2018.
View Article : Google Scholar
|
|
2
|
Dzafic E, Stimpfel M and Virant-Klun I:
Plasticity of granulosa cells: On the crossroad of stemness and
transdifferentiation potential. J Assist Reprod Genet.
30:1255–1261. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Brevini TAL, Pennarossa G, Rahman MM,
Paffoni A, Antonini S, Ragni G, deEguileor M, Tettamanti G and
Gandolfi F: Morphological and molecular changes of human granulosa
cells exposed to 5-azacytidine and addressed toward muscular
differentiation. Stem Cell Rev Reports. 10:633–642. 2014.
View Article : Google Scholar
|
|
4
|
Kossowska-Tomaszczuk K, Pelczar P, Güven
S, Kowalski J, Volpi E, De Geyter C and Scherberich A: A novel
three-dimensional culture system allows prolonged culture of
functional human granulosa cells and mimics the ovarian
environment. Tissue Eng Part A. 16:2063–2073. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kossowska-Tomaszczuk K, De Geyter C, De
Geyter M, Martin I, Holzgreve W, Scherberich A and Zhang H: The
multipotency of luteinizing granulosa cells collected from mature
ovarian follicles. Stem Cells. 27:210–219. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ming G and Song H: Adult neurogenesis in
the mammalian central nervous system. Annu Rev Neurosci.
28:223–250. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ming GL and Song H: Adult neurogenesis in
the mammalian brain: Significant answers and significant questions.
Neuron. 70:687–702. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kriegstein A and Alvarez-Buylla A: The
Glial nature of embryonic and adult neural stem cells. Annu Rev
Neurosci. 32:149–184. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pistorius LR: Imaging of the embryonic and
fetal central nervous system. Facts Views Vis Obgyn. 1:66–71.
2009.PubMed/NCBI
|
|
10
|
Liu A and Niswander LA: Bone morphogenetic
protein signalling and vertebrate nervous system development. Nat
Rev Neurosci. 6:945–954. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rybska M, Knap S, Stefańska K, Jankowski
M, Chamier-Gliszczyńska A, Popis M, Jeseta M, Bukowska D, Antosik
P, Kempisty B, et al: Transforming growth factor (TGF)-is it a key
protein in mammalian reproductive biology? Med J Cell Biol.
6:125–130. 2018. View Article : Google Scholar
|
|
12
|
D'Aiuto L, Zhi Y, Kumar Das D, Wilcox MR,
Johnson JW, McClain L, MacDonald ML, Di Maio R, Schurdak ME, Piazza
P, et al: Large-scale generation of human iPSC-derived neural stem
cells/early neural progenitor cells and their neuronal
differentiation. Organogenesis. 10:365–377. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Denham M and Dottori M: Neural
differentiation of induced pluripotent stem cells. Methods Mol
Biol. 793:99–110. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Samoilova EM, Kalsin VA, Kushnir NM,
Chistyakov DA, Troitskiy AV and Baklaushev VP: Adult neural stem
cells: Basic research and production strategies for
neurorestorative therapy. Stem Cells Int. 2018:48354912018.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Anchan R, Gerami-Naini B, Lindsey JS, Ho
JW, Kiezun A, Lipskind S, Ng N, LiCausi JA, Kim CS, Brezina P, et
al: Efficient differentiation of steroidogenic and germ-like cells
from epigenetically-related iPSCs derived from ovarian granulosa
cells. PLoS One. 10:e01192752015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Son EY, Ichida JK, Wainger BJ, Toma JS,
Rafuse VF, Woolf CJ and Eggan K: Conversion of mouse and human
fibroblasts into functional spinal motor neurons. Cell Stem Cell.
9:205–218. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Miyoshi N, Ishii H, Nagano H, Haraguchi N,
Dewi DL, Kano Y, Nishikawa S, Tanemura M, Mimori K, Tanaka F, et
al: Reprogramming of mouse and human cells to pluripotency using
mature microRNAs. Cell Stem Cell. 8:633–638. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Brouwer M, Zhou H and Nadif Kasri N:
Choices for induction of pluripotency: Recent developments in human
induced pluripotent stem cell reprogramming strategies. Stem Cell
Rev Rep. 12:54–72. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Attwood SW and Edel MJ: iPS-cell
technology and the problem of genetic instability-can it ever be
safe for clinical use? J Clin Med. 8(pii): E2882019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hermann A, Liebau S, Gastl R, Fickert S,
Habisch HJ, Fiedler J, Schwarz J, Brenner R and Storch A:
Comparative analysis of neuroectodermal differentiation capacity of
human bone marrow stromal cells using various conversion protocols.
J Neurosci Res. 83:1502–1514. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zheng B, Wang C, He L, Xu X, Qu J, Hu J
and Zhang H: Neural differentiation of mesenchymal stem cells
influences chemotactic responses to HGF. J Cell Physiol.
228:149–162. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mollinari C, Zhao J, Lupacchini L, Garaci
E, Merlo D and Pei G: Transdifferentiation: A new promise for
neurodegenerative diseases. Cell Death Dis. 9:8302018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fujimoto Y, Abematsu M, Falk A, Tsujimura
K, Sanosaka T, Juliandi B, Semi K, Namihira M, Komiya S, Smith A
and Nakashima K: Treatment of a mouse model of spinal cord injury
by transplantation of human induced pluripotent stem cell-derived
long-term self-renewing neuroepithelial-like stem cells. Stem
Cells. 30:1163–1173. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Song CG, Zhang YZ, Wu HN, Cao XL, Guo CJ,
Li YQ, Zheng MH and Han H: Stem cells: A promising candidate to
treat neurological disorders. Neural Regen Res. 13:1294–1304. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gancheva MR, Kremer KL, Gronthos S and
Koblar SA: Using dental pulp stem cells for stroke therapy. Front
Neurol. 10:4222019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang B, Gaiteri C, Bodea LG, Wang Z,
McElwee J, Podtelezhnikov AA, Zhang C, Xie T, Tran L, Dobrin R, et
al: Integrated systems approach identifies genetic nodes and
networks in late-onset Alzheimer's disease. Cell. 153:707–720.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Soldner F, Hockemeyer D, Beard C, Gao Q,
Bell GW, Cook EG, Hargus G, Blak A, Cooper O, Mitalipova M, et al:
Parkinson's disease patient-derived induced pluripotent stem cells
free of viral reprogramming factors. Cell. 136:964–977. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hess DC, Wechsler LR, Clark WM, Savitz SI,
Ford GA, Chiu D, Yavagal DR, Uchino K, Liebeskind DS, Auchus AP, et
al: Safety and efficacy of multipotent adult progenitor cells in
acute ischaemic stroke (MASTERS): A randomised, double-blind,
placebo-controlled, phase 2 trial. Lancet Neurol. 16:360–368. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Altman J and Das GD: Autoradiographic and
histological evidence of postnatal hippocampal neurogenesis in
rats. J Comp Neurol. 124:319–335. 1965. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sorrells SF, Paredes MF, Cebrian-Silla A,
Sandoval K, Qi D, Kelley KW, James D, Mayer S, Chang J, Auguste KI,
et al: Human hippocampal neurogenesis drops sharply in children to
undetectable levels in adults. Nature. 555:377–381. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
van Praag H, Kempermann G and Gage FH:
Running increases cell proliferation and neurogenesis in the adult
mouse dentate gyrus. Nat Neurosci. 2:266–270. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Spalding KL, Bergmann O, Alkass K, Bernard
S, Salehpour M, Huttner HB, Boström E, Westerlund I, Vial C,
Buchholz BA, et al: Dynamics of hippocampal neurogenesis in adult
humans. Cell. 153:1219–1227. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Eriksson PS, Perfilieva E, Björk-Eriksson
T, Alborn AM, Nordborg C, Peterson DA and Gage FH: Neurogenesis in
the adult human hippocampus. Nat Med. 4:1313–1317. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dennis CV, Suh LS, Rodriguez ML, Kril JJ
and Sutherland GT: Human adult neurogenesis across the ages: An
immunohistochemical study. Neuropathol Appl Neurobiol. 42:621–638.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Knoth R, Singec I, Ditter M, Pantazis G,
Capetian P, Meyer RP, Horvat V, Volk B and Kempermann G: Murine
features of neurogenesis in the human hippocampus across the
lifespan from 0 to 100 years. PLoS One. 5:e88092010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kempermann G, Kuhn HG and Gage FH: More
hippocampal neurons in adult mice living in an enriched
environment. Nature. 386:493–495. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kranc W, Brązert M, Budna J, Celichowski
P, Bryja A, Nawrocki MJ, Ożegowska K, Jankowski M, Chermuła B,
Dyszkiewicz-Konwińska M, et al: Genes responsible for
proliferation, differentiation, and junction adhesion are
significantly up-regulated in human ovarian granulosa cells during
a long-term primary in vitro culture. Histochem Cell Biol.
151:125–143. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kranc W, Brązert M, Ożegowska K, Nawrocki
MJ, Budna J, Celichowski P, Dyszkiewicz-Konwińska M, Jankowski M,
Jeseta M, Pawelczyk L, et al: Expression profile of genes
regulating steroid biosynthesis and metabolism in human ovarian
granulosa cells-A primary culture approach. Int J Mol Sci. 18(pii):
E26732017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kranc W, Brązert M, Celichowski P, Bryja
A, Nawrocki MJ, Ożegowska K, Jankowski M, Jeseta M, Pawelczyk L,
Bręborowicz A, et al: ‘Heart development and morphogenesis’ is a
novel pathway for human ovarian granulosa cell differentiation
during long-term in vitro cultivation-a microarray approach. Mol
Med Rep. 19:1705–1715. 2019.PubMed/NCBI
|
|
40
|
Bryja A, Dyszkiewicz-Konwińska M,
Jankowski M, Celichowski P, Stefańska K, Chamier-Gliszczyńska A,
Borowiec B, Mehr K, Bukowska D, Antosik P, et al: Cation
homeostasis and transport related gene markers are differentially
expressed in porcine buccal pouch mucosal cells during long-term
cells primary culture in vitro. Med J Cell Biol. 6:83–90. 2018.
View Article : Google Scholar
|
|
41
|
Borys-Wójcik S, Kocherova I, Celichowski
P, Popis M, Jeseta M, Bukowska D, Antosik P, Nowicki M and Kempisty
B: Protein oligomerization is the biochemical process highly
up-regulated in porcine oocytes before in vitro maturation (IVM).
Med J Cell Biol. 6:155–162. 2018. View Article : Google Scholar
|
|
42
|
Ferraretti AP, La Marca A, Fauser BCJM,
Tarlatzis B, Nargund G and Gianaroli L; ESHRE working group on Poor
Ovarian Response Definition, : ESHRE consensus on the definition of
‘poor response’ to ovarian stimulation for in vitro fertilization:
The Bologna criteria. Hum Reprod. 26:1616–1624. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kranc W, Budna J, Dudek M, Bryja A,
Chachuła A, Ciesiółka S, Borys S, Dyszkiewicz-Konwińska M, Jeseta
M, Porowski L, et al: The origin, in vitro differentiation, and
stemness specificity of progenitor cells. J Biol Regul Homeost
Agents. 31:365–369. 2017.PubMed/NCBI
|
|
44
|
Chomczynski P and Sacchi N: Single-step
method of RNA isolation by acid guanidinium
thiocyanate-phenol-chloroform extraction. Anal Biochem.
162:156–159. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Brązert M, Iżycki D, Kranc W, Borowiec B,
Popis M, Ożegowska K, Bręborowicz A, Rachoń D, Nowicki M and
Kempisty B: Genes involved in hormone metabolism and cellular
response in human ovarian granulosa cells. J Biol Regul Homeost
Agents. 33:461–468. 2019.PubMed/NCBI
|
|
46
|
Huang DW, Sherman BT, Tan Q, Kir J, Liu D,
Bryant D, Guo Y, Stephens R, Baseler MW, Lane HC and Lempicki RA:
DAVID bioinformatics resources: Expanded annotation database and
novel algorithms to better extract biology from large gene lists.
Nucleic Acids Res. 35((Web Server Issue)): W169–W175. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Huang da W, Sherman BT and Lempicki RA:
Systematic and integrative analysis of large gene lists using DAVID
bioinformatics resources. Nat Protoc. 4:44–57. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Huang da W, Sherman BT and Lempicki RA:
Bioinformatics enrichment tools: Paths toward the comprehensive
functional analysis of large gene lists. Nucleic Acids Res.
37:1–13. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Walter W, Sánchez-Cabo F and Ricote M:
GOplot: An R package for visually combining expression data with
functional analysis. Bioinformatics. 31:2912–2914. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kossowska-Tomaszczuk K and De Geyter C:
Cells with stem cell characteristics in somatic compartments of the
ovary. Biomed Res Int. 2013:3108592013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Dzafic E, Stimpfel M, Novakovic S,
Cerkovnik P and Virant-Klun I: Expression of mesenchymal stem
cells-related genes and plasticity of aspirated follicular cells
obtained from infertile women. Biomed Res Int. 2014:5082162014.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Poloni A, Maurizi G, Foia F, Mondini E,
Mattiucci D, Ambrogini P, Lattanzi D, Mancini S, Falconi M, Cinti
S, et al: Glial-like differentiation potential of human mature
adipocytes. J Mol Neurosci. 55:91–98. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Zhang L, Feng T and Spicer LJ: The role of
tight junction proteins in ovarian follicular development and
ovarian cancer. Reproduction. 155:183–198. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Wissing ML, Kristensen SG, Andersen CY,
Mikkelsen AL, Høst T, Borup R and Grøndahl ML: Identification of
new ovulation-related genes in humans by comparing the
transcriptome of granulosa cells before and after ovulation
triggering in the same controlled ovarian stimulation cycle. Hum
Reprod. 29:997–1010. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Hatzirodos N, Hummitzsch K, Irving-Rodgers
HF and Rodgers RJ: Transcriptome profiling of the theca interna in
transition from small to large antral ovarian follicles. PLoS One.
9:e974892014. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Takayanagi Y, Yoshida M, Bielsky IF, Ross
HE, Kawamata M, Onaka T, Yanagisawa T, Kimura T, Matzuk MM, Young
LJ and Nishimori K: Pervasive social deficits, but normal
parturition, in oxytocin receptor-deficient mice. Proc Natl Acad
Sci USA. 102:16096–16101. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Gutkowska J and Jankowski M: Oxytocin
revisited: Its role in cardiovascular regulation. J
Neuroendocrinol. 24:599–608. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Briones BA and Gould E: Adult neurogenesis
and stress. In Stress: Physiology, biochemistry, and pathology.
Elsevier; 3. pp. pp79–92. 2019
|
|
60
|
Peng L and Bonaguidi MA: Function and
dysfunction of adult hippocampal neurogenesis in regeneration and
disease. Am J Pathol. 188:23–28. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Yoo S and Blackshaw S: Regulation and
function of neurogenesis in the adult mammalian hypothalamus. Prog
Neurobiol. 170:53–66. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Soares R, Ribeiro FF, Xapelli S, Genebra
T, Ribeiro MF, Sebastião AM, Rodrigues CMP and Solá S:
Tauroursodeoxycholic acid enhances mitochondrial biogenesis, neural
stem cell pool, and early neurogenesis in adult rats. Mol
Neurobiol. 55:3725–3738. 2018.PubMed/NCBI
|
|
63
|
Boldrini M, Fulmore CA, Tartt AN, Simeon
LR, Pavlova I, Poposka V, Rosoklija GB, Stankov A, Arango V, Dwork
AJ, et al: Human hippocampal neurogenesis persists throughout
aging. Cell Stem Cell. 22:589–599.e5. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Danzer SC: Adult neurogenesis in the human
brain: Paradise lost? Epilepsy Curr. 18:329–331. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Lin YT, Chen CC, Huang CC, Nishimori K and
Hsu KS: Oxytocin stimulates hippocampal neurogenesis via oxytocin
receptor expressed in CA3 pyramidal neurons. Nat Commun. 8:5372017.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Zimmerman EA, Nilaver G, Hou-Yu A and
Silverman AL: Vasopressinergic and oxytocinergic pathways in the
central nervous system. Fed Proc. 43:91–96. 1984.PubMed/NCBI
|
|
67
|
Busch-Nentwich E, Söllner C, Roehl H and
Nicolson T: The deafness gene dfna5 is crucial for ugdh expression
and HA production in the developing ear in zebrafish. Development.
131:943–951. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Stoll G, Ma Y, Yang H, Kepp O, Zitvogel L
and Kroemer G: Pro-necrotic molecules impact local
immunosurveillance in human breast cancer. Oncoimmunology.
6:e12993022017. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Webb MS, Miller AL and Thompson EB: In CEM
cells the autosomal deafness gene dfna5 is regulated by
glucocorticoids and forskolin. J Steroid Biochem Mol Biol.
107:15–21. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Yao X, Buhi WC, Alvarez IM, Curtis LM and
Rarey KE: De novo synthesis of glucocorticoid hormone regulated
inner ear proteins in rats. Hear Res. 86:183–188. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Croes L, Beyens M, Fransen E, Ibrahim J,
Vanden Berghe W, Suls A, Peeters M, Pauwels P, Van Camp G and Op de
Beeck K: Large-scale analysis of DFNA5 methylation reveals its
potential as biomarker for breast cancer. Clin Epigenetics.
10:512018. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Assou S, Haouzi D, Dechaud H, Gala A,
Ferrières A and Hamamah S: Comparative gene expression profiling in
human cumulus cells according to ovarian gonadotropin treatments.
Biomed Res Int. 2013:3545822013. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Chen Y, Teng FY and Tang BL: Coaxing bone
marrow stromal mesenchymal stem cells towards neuronal
differentiation: Progress and uncertainties. Cell Mol Life Sci.
63:1649–1657. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Qin X, Han W and Yu Z: Neuronal-like
differentiation of bone marrow-derived mesenchymal stem cells
induced by striatal extracts from a rat model of Parkinson's
disease. Neural Regen Res. 7:2673–2680. 2012.PubMed/NCBI
|
|
75
|
Lilja J and Ivaska J: Integrin activity in
neuronal connectivity. J Cell Sci. 131(pii): jcs2128032018.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Loulier K, Lathia JD, Marthiens V, Relucio
J, Mughal MR, Tang SC, Coksaygan T, Hall PE, Chigurupati S, Patton
B, et al: beta1 integrin maintains integrity of the embryonic
neocortical stem cell niche. PLoS Biol. 7:e10001762009. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Honda T, Fujiwara H, Ueda M, Maeda M and
Mori T: Integrin alpha 6 is a differentiation antigen of human
granulosa cells. J Clin Endocrinol Metab. 80:2899–2905. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Kolle G, Georgas K, Holmes GP, Little MH
and Yamada T: CRIM1, a novel gene encoding a cysteine-rich repeat
protein, is developmentally regulated and implicated in vertebrate
CNS development and organogenesis. Mech Dev. 90:181–193. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Prenkert M, Uggla B, Tidefelt U and Strid
H: CRIM1 is expressed at higher levels in drug-resistant than in
drug-sensitive myeloid leukemia HL60 cells. Anticancer Res.
30:4157–4161. 2010.PubMed/NCBI
|
|
80
|
Iwasaki Y, Shiojima T, Tagaya N, Kobayashi
T and Kinoshita M: Effect of transforming growth factor β1 on
spinal motor neurons after axotomy. J Neurol Sci. 147:9–12. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Mira H, Andreu Z, Suh H, Lie DC,
Jessberger S, Consiglio A, San Emeterio J, Hortigüela R,
Marqués-Torrejón MA, Nakashima K, et al: Signaling through BMPR-IA
regulates quiescence and long-term activity of neural stem cells in
the adult hippocampus. Cell Stem Cell. 7:78–89. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Blázquez-Medela AM, Jumabay M and Boström
KI: Beyond the bone: Bone morphogenetic protein signaling in
adipose tissue. Obes Rev. 20:648–658. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Nilsson EE and Skinner MK: Bone
morphogenetic protein-4 acts as an ovarian follicle survival factor
and promotes primordial follicle development. Biol Reprod.
69:1265–1272. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Takao Y, Fujiwara H, Yamada S, Hirano T,
Maeda M, Fujii S and Ueda M: CD9 is expressed on the cell surface
of human granulosa cells and associated with integrin alpha61. Mol
Hum Reprod. 5:303–310. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Hayati AR, Nur Fariha MM, Tan GC, Tan AE
and Chua K: Potential of human decidua stem cells for angiogenesis
and neurogenesis. Arch Med Res. 42:291–300. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Yang Y, Ye Y, Su X, He J, Bai W and He X:
MSCs-derived exosomes and neuroinflammation, neurogenesis and
therapy of traumatic brain injury. Front Cell Neurosci. 11:552017.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Virant-Klun I, Rožman P, Cvjeticanin B,
Vrtacnik-Bokal E, Novakovic S, Rülicke T, Dovc P and Meden-Vrtovec
H: Parthenogenetic embryo-like structures in the human ovarian
surface epithelium cell culture in postmenopausal women with no
naturally present follicles and oocytes. Stem Cells Dev.
18:137–150. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Asaoka-Taguchi M, Yamada M, Nakamura A,
Hanyu K and Kobayashi S: Maternal Pumilio acts together with Nanos
in germline development in Drosophila embryos. Nat Cell Biol.
1:431–437. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
89
|
Wang Z and Lin H: Nanos maintains germline
stem cell self-renewal by preventing differentiation. Science.
303:2016–2019. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Subramaniam K and Seydoux G: nos-1 and
nos-2, two genes related to Drosophila nanos, regulate primordial
germ cell development and survival in Caenorhabditis
elegans. Development. 126:4861–4871. 1999.PubMed/NCBI
|
|
91
|
Jaruzelska J, Kotecki M, Kusz K, Spik A,
Firpo M and Reijo Pera RA: Conservation of a Pumilio-Nanos complex
from Drosophila germ plasm to human germ cells. Dev Genes Evol.
213:120–126. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Virant-Klun I, Knez K, Tomazevic T and
Skutella T: Gene expression profiling of human oocytes developed
and matured in vivo or in vitro. Biomed Res Int. 2013:8794892013.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Julaton VT and Reijo Pera RA: NANOS3
function in human germ cell development. Hum Mol Genet.
20:2238–2250. 2011. View Article : Google Scholar : PubMed/NCBI
|