Introduction
Irritable bowel syndrome (IBS) is a common chronic
gastrointestinal disorder with a worldwide prevalence of 5–20%
(1,2); it is characterized by chronic
abdominal pain and discomfort, as well as changes in bowel habits.
The pathogenic mechanisms of IBS remain unknown; however,
accumulating evidence has indicated that abnormal gastrointestinal
motility and visceral hypersensitivity are two important
pathophysiologic features of IBS (3,4), and
that the latter contributes to abdominal pain in patients.
Currently, there is no satisfactory management method available for
patients with IBS. Hellström et al (5) demonstrated that the glucagon-like
peptide-1 (GLP-1) analogue, ROSE-010, could effectively relieve IBS
pain exhibited by patients. However, the underlying mechanisms
governing this remain poorly understood. Recent research has
indicated that the GLP-1 analogue liraglutide alters the visceral
sensation in patients with IBS (6), indicating that GLP-1 may be useful as
a treatment for this disease.
GLP-1 is a common incretin hormone that is released
from intestinal L-cells in response to nutrient ingestion (7,8).
GLP-1 can enhance insulin secretion, delay gastric emptying,
inhibit motility and exert antispasmodic effects (9). A previous study indicated that
exendin-4, a GLP-1 analogue, reduced visceral hypersensitivity by
increasing serotonin-selective reuptake transporter (SERT)
expression, a consequence of decreasing serotonin
(5-hydroxytryptamine; 5-HT) levels (10). The short half-life of GLP-1
presents a considerable barrier to its therapeutic use, whereas
exendin-4, which has 53% homology with GLP-1, exhibits a longer
half time and can be used to mimic the effects of GLP-1 (11). GLP-1 exerts biological functions by
binding to its specific receptor, GLP-1R, in the stomach, intestine
and brain (11,12). GLP-1 also stimulates cyclic
adenosine monophosphate (cAMP) formation and subsequently induces
protein kinase A (PKA) activity by binding to GLP-1R (13). SERT expression can be regulated by
a variety of stimuli, including cAMP (14,15).
However, the GLP-1/GLP-1R/cAMP signaling pathway in intestinal
epithelial cells has, to the best of our knowledge, not yet been
investigated.
SERT is expressed in intestinal epithelial cells,
and this expression is significantly decreased in the colon and
rectum of patients with IBS (16,17);
the removal of 5-HT by SERT is important in inhibiting 5-HT
activity (18). 5-HT is a
neurotransmitter that has been examined in rodents and humans, and
is expressed at high levels in the intestinal mucosa of patients
with IBS with constipation (IBS-C) (19). 5-HT is released from
enterochromaffin cells in response to mucosal stimuli, and it can
initiate motor reflexes (20) and
visceral sensation (21); 5-HT can
be inactivated by SERT-mediated uptake into enterocytes or neurons
(18). Abnormal serotonergic
signaling can contribute to visceral hypersensitivity in IBS, and a
number of serotonergic drugs can relieve IBS symptoms (22).
In a previous study, immunochemistry results
demonstrated that GLP-1R expression was located in the colonic
mucosa layer in rodent models of IBS, especially in IBS-C models
(23). Furthermore, it has been
shown that in colonic sensitized rats, SERT expression was
decreased, and exendin-4 treatment reduced visceral
hypersensitivity by increasing SERT expression and decreasing 5-HT
content (10). These results
demonstrated that GLP-1 binding to GLP-1R may be associated with
SERT expression and 5-HT content, a consequence of the formation of
visceral hypersensitivity in IBS models. However, the underlying
intracellular mechanisms governing this are yet to be determined.
The current study aimed to investigate whether the GLP-1 analogue
exendin-4 was able to modulate SERT expression via the adenosine
cyclophosphate (AC)/PKA/SERT signaling pathway in IEC-6 rat
intestinal epithelial cells.
Materials and methods
Cell culture
Normal rat intestinal epithelial cell line IEC-6
were cultured in completed DMEM (Thermo Fisher Scientific, Inc.)
supplemented with 10% FBS (Thermo Fisher Scientific, Inc.) (v/v), 2
mM L-glutamine, 1% antibiotic (v/v) solution containing penicillin
G (10,000 U/ml) and streptomycin (10,000 U/ml), 1.5 g/l
NaHCO3, 2 g/l HEPES and 0.01 mg/ml insulin, and
maintained in a 37°C homothermal incubator with a 5% CO2
atmosphere. IEC-6 cells were seeded into six-well plates and
cultured for 48 h (to 80% confluence) in complete DMEM. Cells were
then cultured in serum-free DMEM for 12 h, and then exposed to
exendin-4 at different concentrations (0, 0.1, 1, 10 and 100 nM)
for 12 h at 37°C to select the optimal concentration, as described
previously (24). Subsequent
experiments were conducted at 37°C for a number of time periods (0,
3, 6, 12 and 24 h). In certain experiments, the GLP-1R antagonist
exendin-9 (10 µM; Sigma-Aldrich; Merck KGaA) was used 1 h prior to
exendin-4 treatment at 37°C. The IEC-6 cells were pre-stimulated
with H89 (10 µM; Beyotime Institute of Biotechnology) and SQ22536
(10 µM; Cayman Chemical Company) for 30 min at 37°C with or without
exendin-4 treatment. Forskolin (5 µM; Cayman Chemical Company) was
administrated for 12 h at 37°C with or without SQ22536
pre-treatment in IEC-6 cells. All experiments were performed in
triplicate.
PKA activity assay
IEC-6 cells were lysed in cold PKA extraction buffer
containing 25 mM Tris-HCl (pH 7.4), 0.5 mM EDTA, 0.5 mM EGTA, 10 mM
β-mercaptoethanol and 50-fold diluted proteinase inhibitor cocktail
(25). The homogenates were
centrifuged at 20,000 × g for 5 min at 4°C, and PKA activity in the
supernatants was subsequently assessed using a non-radioactive PKA
kinase assay kit, Type I (ImmuneChem Pharmaceuticals, Inc.),
according to the manufacturer's protocols.
[3H]−5-HT re-uptake
The serum-free medium was removed by aspiration, and
the cells were washed with 1 ml of Krebs-Ringer's (KRH) buffer (130
mM NaCl; 1.3 mM KCl; 2.2 mM CaCl2; 1.2 mM
MgSO4; 1.2 mM KH2PO4; 1.8 g/l
glucose and 10 mM HEPES, pH 7.4). A total of 1 ml KRH buffer
containing 100 µM pargyline and 100 µM ascorbic acid with or
without exendin-4 was subsequently added to IEC-6 cells for 10 min
in a 37°C chamber with a 5% CO2 atmosphere. The
[3H]−5-HT reuptake assays were initiated by adding 100
nM [3H]−5-HT (27.9 Ci/mmol; NET498; PerkinElmer, Inc.)
and the cells were then incubated in a 37°C chamber for a
subsequent 10 min. The cells were washed rapidly with cold KRH
buffer to terminate the reuptake assays and solubilized in 600 µl
1% Triton. The cell lysate (50 µl) was mixed in OptiPhase Supermix
scintillation mixture for radioactivity counting (Wallac Liquid
Scintillation Counter; PerkinElmer, Inc.). Non-specific uptake
[3H]−5-HT was defined using 100 µM paroxetine (a SERT
inhibitor; Enzo Life Sciences, Inc.). The specific SERT-mediated
[3H]−5-HT was determined by subtracting the non-specific
uptake. The remaining cell lysate was used to determine protein
concentration by the Bradford method (Beyotime Institute of
Biotechnology), with BSA as a standard.
Western blot analysis for GLP-1R and
SERT
IEC-6 cells were washed with ice-cold PBS three
times and lysed in 100 µl RIPA lysis buffer (Beyotime Institute of
Biotechnology) with a protease inhibitor cocktail. Following
centrifugation at 12,000 × g for 10 min at 4°C, supernatants were
collected and transferred to 1.5 ml centrifuge tubes. The protein
concentration was determined using a BCA assay (26) according to the manufacturer's
protocols (Thermo Fisher Scientific, Inc.). Total proteins were
dissolved in lithium dodecyl sulfate sample buffer (0.5% lithium
dodecyl sulfate; 62.5 mM Tris·HCl; 2.5% glycerol; 0.125 mM EDTA; pH
8.5). Samples (30 µg/lane) were separated by 10% (w/v) SDS-PAGE.
After being transferred to PVDF membrane, washed and blocked with
5% non-fat dry milk in 0.1% Tween/Tris-Buffered Saline (Beyotime
Institute of Biotechnology) for 1 h at room temperature, the
membranes were incubated with goat polyclonal anti-GLP-1R primary
antibody (1:500; cat. no. sc-34637; Santa Cruz Biotechnology,
Inc.), rabbit polyclonal anti-SERT primary antibody (1:500; cat.
no. AB9726; EMD Millipore) and mouse monoclonal anti-GAPDH primary
antibody (1:5,000; cat. no. M0002; CMC Scientific) overnight at
4°C. Membranes were then washed three times and incubated with
horseradish peroxidase-conjugated goat anti-rabbit (1:3,000; cat.
no. BS13278; Bioworld Technology, Inc.), goat anti-mouse (1:3,000;
cat. no. BS12478; Bioworld Technology, Inc.) or rabbit anti-goat
(1:3,000; cat. no. BS10008; Bioworld Technology, Inc.) secondary
antibodies for 60 min at 37°C. Protein bands were visualized using
the ECL Western Blotting Substrate (Thermo Fisher Scientific, Inc.)
and images captured using a Bio-Rad Gel Imaging system (Bio-Rad
Laboratories, Inc.). Protein expressions were normalized to GAPDH
and were analyzed using Image Lab 3.0 (Bio-Rad Laboratories,
Inc.).
Reverse transcription-quantitative
(RT-q)PCR for the detection of SERT mRNA
Total RNA was extracted from the cells using
TRIzol® reagent (Shanghai Pufei Biotechnology Co.,
Ltd.), according to the manufacturer's protocol, under RNase-free
conditions. RNA concentrations were determined using NanoDrop™ 2000
Spectrophotometer (Thermo Fisher Scientific, Inc.). cDNA was
synthesized from total RNA (500 ng/each sample) using the
PrimeScript RT Master mix (Takara Biotechnology Co., Ltd.). The
reverse transcription conditions are as follows: 37°C for 15 min
and 85°C for 5 sec. cDNA (2 µl) was amplified by PCR using the
following primers: SERT, forward 3′-GACTCCTCCCCTCTAAGCCA-5′,
reverse 3′-CACGGAAAGAAGTGGTCGGA-5′; β-actin, forward
3′-CTAAGGCCAACCGTGAAAAG-5′, reverse 3′-TCTCAGCTGTGGTGGTGAAG-5′.
qPCR was performed using a 20 µl reaction volume and
SYBR® Premix Ex Taq™ (Takara Biotechnology Co., Ltd.)
with an Applied Biosystems 7500 Real-Time PCR system (Applied
Biosystems; Thermo Fisher Scientific, Inc.) and the following
thermocycling conditions: Initial denaturation at 95°C for 30 sec,
followed by 40 cycles of 95°C for 5 sec and 60°C for 34 sec. The
quantitation cycle (Cq) values of all genes were obtained; the
expression values of investigated genes were normalized to β-actin
mRNA levels, and the relative expression was determined using the
2−∆∆Cq method (27).
All reactions were performed in triplicate.
Statistical analysis
Statistical analysis was performed using SPSS
Statistics 18.0.0 software (SPSS, Inc.). Data are expressed as the
mean ± SD. For multiple comparisons, a parametric one-way ANOVA,
two-way ANOVA and Bonferroni's post-hoc test were used. GraphPad
Prism software 5.0 (Prism; GraphPad Software, Inc.) was used for
plotting figures. P<0.05 was considered to indicate a
statistically significant result.
Results
Effects of exendin-4 on SERT
expression and 5-HT reuptake in IEC-6 cells
In a previous in vivo study, the GLP-1
analogue exendin-4 was revealed to upregulate SERT expression and
decrease 5-HT levels in the intestinal mucosa of colonic sensitized
rats (10). However, the
mechanisms governing this are yet to be determined. The present
study aimed to investigate whether exendin-4 modulated SERT
expression and to identify the intracellular signaling mechanisms
behind this in vitro. IEC-6 cells were treated for 12 h with
exendin-4 at doses of 0, 0.1, 1, 10 and 100 nM; the results
demonstrated that SERT protein and mRNA expression levels were
significantly increased compared with the untreated cells (Fig. 1A and B, respectively), and
expression was highest when doses were 10 and 100 nM. To determine
whether exendin-4 regulated SERT expression in a time-dependent
manner, IEC-6 cells were treated with 10 nM exendin-4 for 0, 3, 6,
12 and 24 h. The results demonstrated that the expression levels of
SERT protein and mRNA were significantly increased following
exendin-4 treatment for 6, 12 and 24 h (Fig. 1C and D, respectively).
Additionally, to observe the effect of exendin-4 on SERT activity,
a kinetic study was performed to identify 5-HT reuptake rates; the
results revealed that 5-HT reuptake as significantly enhanced at 3
and 6 h in IEC-6 cells treated with 10 nM exendin-4 (Fig. 1E).
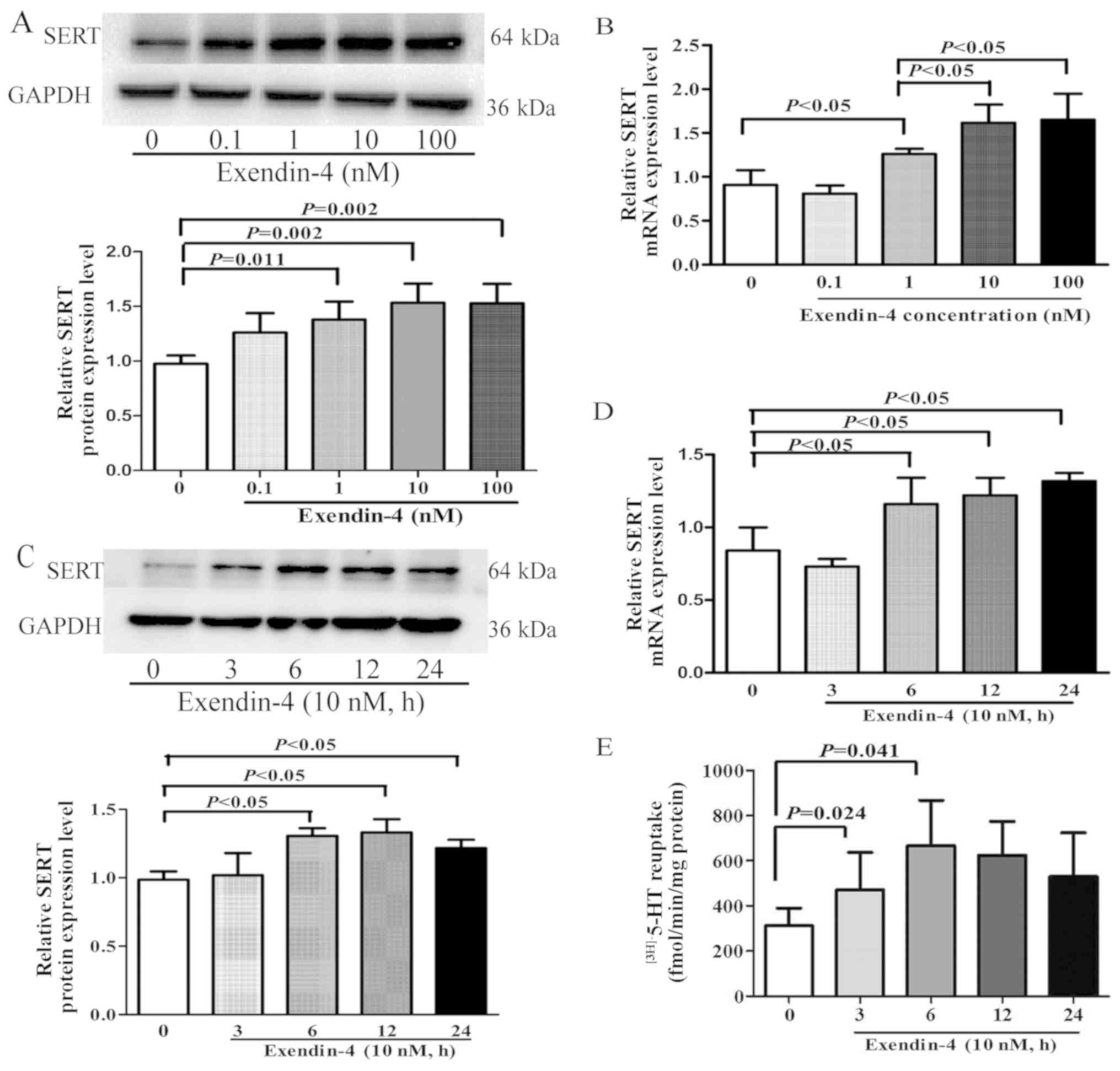 | Figure 1.Effects of exendin-4 on SERT function
and expression in IEC-6 cells. (A and B) IEC-6 rat intestinal
epithelial cells were treated with various concentrations of
exendin-4 (0, 0.1, 1, 10 and 100 nM) for 12 h and the (A) protein
and (B) mRNA expression levels of SERT were determined by western
blotting and RT-qPCR analysis, respectively. (C-E) IEC-6 cells were
treated with exendin-4 (10 nM) for 0, 3, 6, 12 and 24 h and the (C)
protein and (D) mRNA expression levels of SERT were determined by
western blotting and RT-qPCR analysis, respectively. (E) 5-HT
reuptake in IEC-6 cells measured. GAPDH was used as a loading
control for western blotting; β-actin was used as an internal
control for RT-qPCR. Values are presented as the mean ± SD of 3
independent experiments. 5-HT, 5-hydroxytryptamine (serotonin);
RT-qPCR, reverse transcription-quantitative PCR; SERT,
serotonin-selective reuptake transporter. |
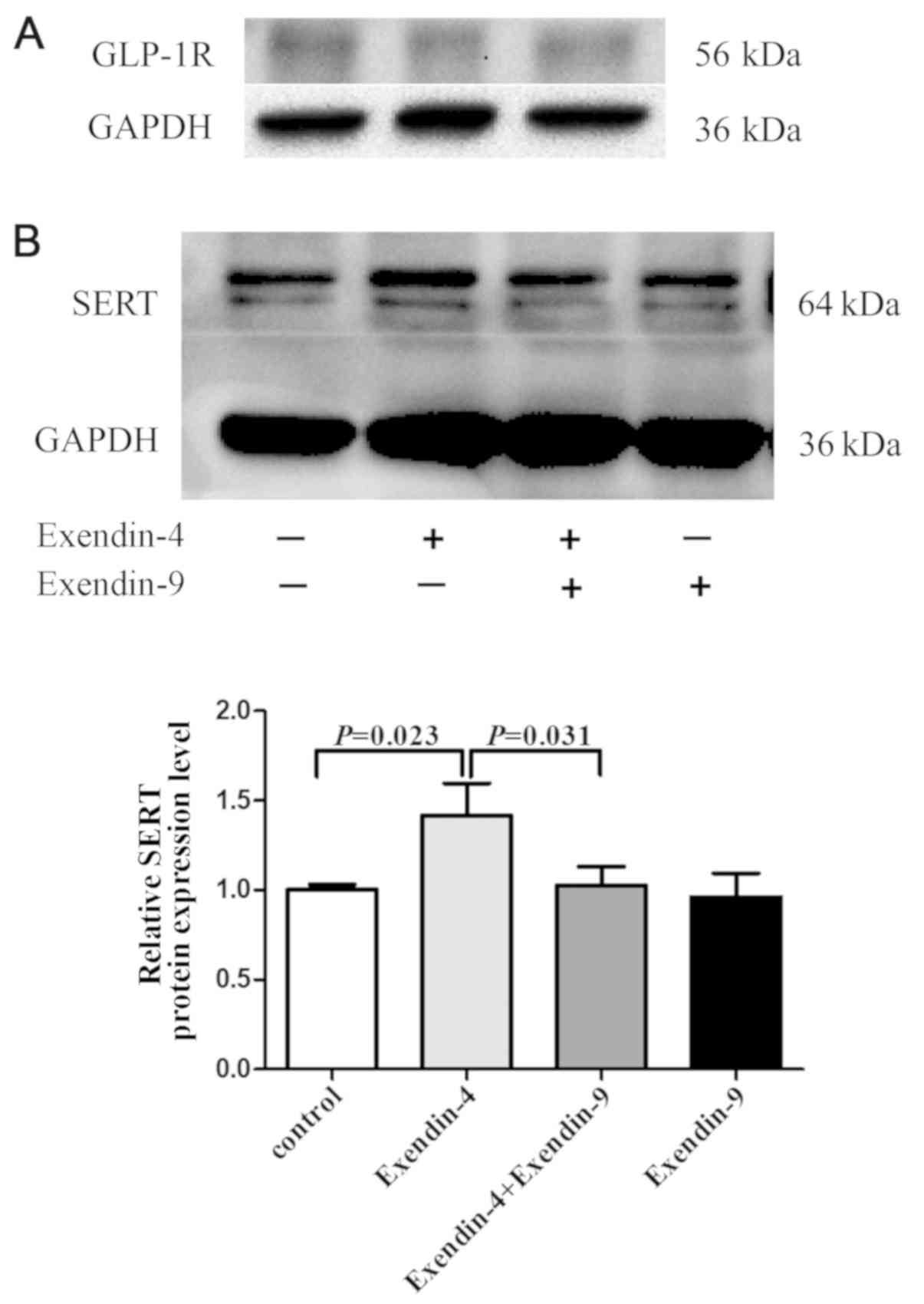
Exendin-4 effects SERT protein
expression through GLP-1R
To examine whether exendin-4 upregulated SERT
expression by binding to GLP-1R, the GLP-1R antagonist exendin-9
was used to pre-treat IEC-6 cells. GLP-1R was expressed in IEC-6
cells (Fig. 2A). SERT protein
expression in IEC-6 cells significantly increased after exendin-4
treatment, and this effect was blocked by pretreatment with the
GLP-1R antagonist exendin-9 (Fig.
2B). These results suggested that SERT stimulation was
partially affected by GLP-1R-mediated signaling, indicating that
the upregulatory effect of exendin-4 on SERT expression was
mediated through GLP-1R.
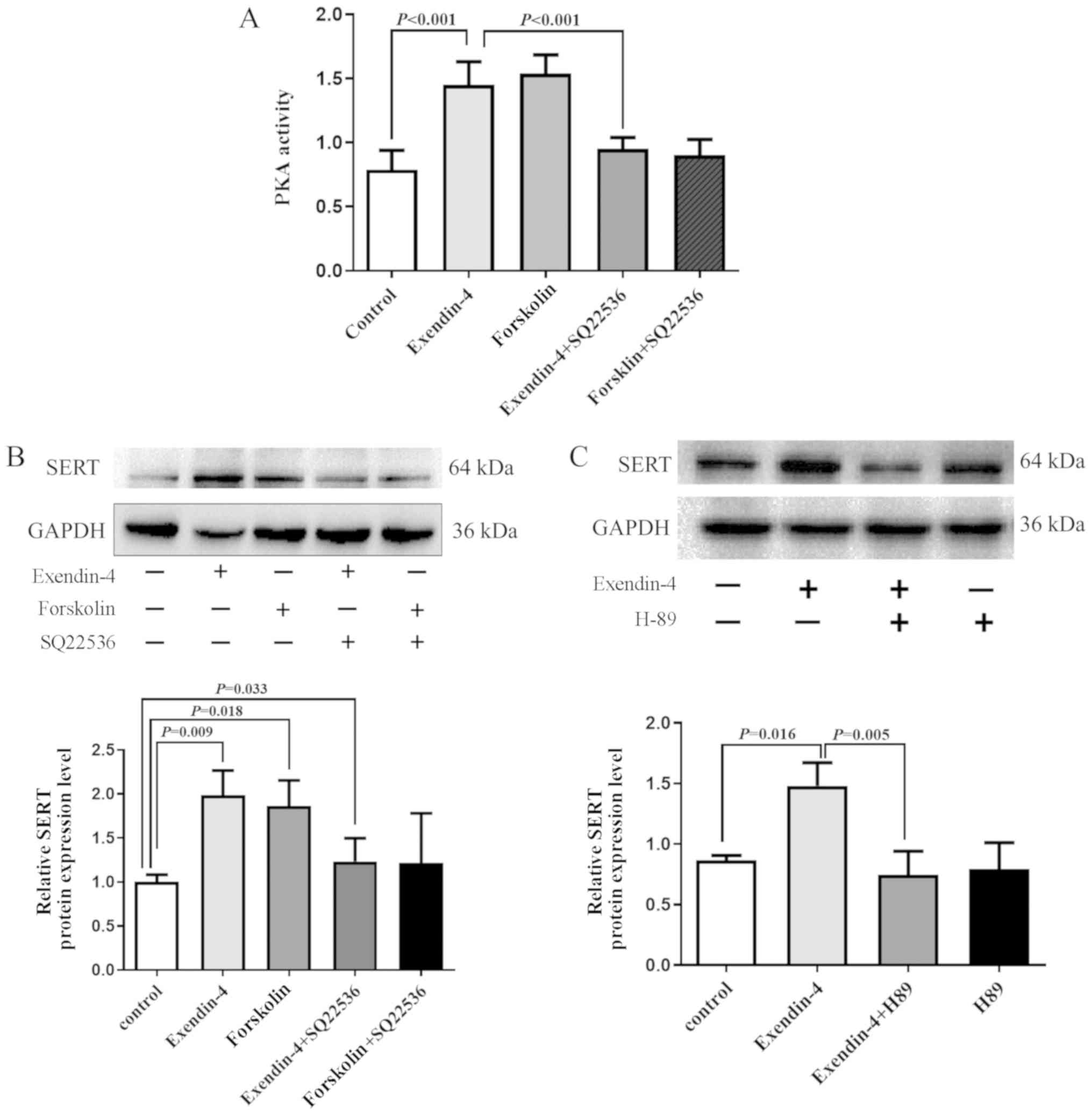
AC/PKA signaling pathway serves a role
in the upregulation of SERT expression by exendin-4
To determine whether exendin-4 influenced SERT
expression via the AC/PKA signaling pathway, IEC-6 cells were
treated with exendin-4 (10 nM) in the presence or absence of the AC
activator forskolin or the AC inhibitor SQ22536. PKA activity was
significantly upregulated in cells treated with exendin-4. and the
effect was reversed by pretreatment with AC inhibitor SQ22536 for
30 min (Fig. 3A). Treatment with
forskolin or exendin-4 significantly increased SERT protein
expression in IEC-6 cells (Fig.
3B). The results indicated that the exendin-4induced SERT
expression was also inhibited by pretreatment with SQ22536. These
results demonstrated that SQ22356 inhibited exendin-4-induced SERT
expression (Fig. 3B). Treatment
with PKA inhibitor H89 (10 µM) also exhibited a similar blocking
effect in IEC-6 cells (Fig.
3C).
Discussion
In our previous study, the GLP-1 analogue exendin-4
was found to reduce visceral hypersensitivity and increase SERT
protein expression in colonic sensitized rats (10). As previously reported, 5-HT is
associated with the visceral sensitivity of the gastrointestinal
tract (28,29). Therefore, decreased SERT expression
may led to a decreased capacity to remove 5-HT from the
interstitial space.
IBS animal models and patient studies have
previously revealed decreased SERT expression in the colon,
suggesting that abnormal serotonergic signaling may contribute to
visceral hypersensitivity during IBS (17,30).
In a previous study, decreased SERT protein expression was observed
in colonic sensitized rats, and after exendin-4 treatment, SERT
protein expression increased and 5-HT levels decreased in colonic
sensitized rats (10). The present
study demonstrated that in IEC-6 cells, exendin-4 upregulated SERT
protein expression and mRNA level in vitro. The 5-HT
reuptake rate was significantly enhanced, but the expression of
SERT mRNA and protein was not significantly increased in IEC-6
cells 3 h after treatment with 10 nM exendin-4. This observed
effect may be correlated to elevated SERT activity. Considering
SERT activity in intestinal epithelial cells, this regulation may
be an effect on the allosteric site of the membrane protein
(29). Additionally, the results
of the current study revealed that SERT mRNA and protein expression
increased following exendin-4 treatment at 6, 12 and 24 h, and SERT
expression reached a peak at 6 h following treatment. The 5-HT
re-uptake rate was also significantly increased at 6 h following
treatment with 10 nM exendin-4. These results, which were in
accordance with those from the studies of Gill et al
(31) and Kerckhoffs et al
(16), suggested that the
augmented expression of SERT led to an elevated capacity to remove
5-HT from the interstitial space.
To investigate whether exendin-4 regulated SERT
protein expression through GLP-1R, the GLP-1R specific antagonist
exendin-9 was used. The results indicated that exendin-9 reversed
the upregulation of SERT protein expression induced by exendin-4,
and this suggested that exendin-4 upregulated SERT protein
expression through GLP-1R. It has previously been shown that GLP-1R
activation can stimulate cAMP formation and subsequently induce PKA
activity (13). SERT expression
can be regulated by a variety of stimuli, including cAMP, hormones
and epidermal growth factor (14,15,32,33).
Therefore, the AC/PKA signaling pathway was investigated to
determine whether exendin-4 could trigger intracellular signaling.
The present results indicated that the AC/PKA signaling pathway
mediated SERT expression induced by exendin-4.
There are a number of limitations of the present
study. As this was an in vitro study involving a single cell
line, further investigations using different cell lines is
required. Additionally further studies into the effect of
extendin-4 on 5-HT in animal models is required. Another limitation
of this study is that this study did not focus on specific subtypes
of IBS. As the majority of patients with IBS suffer from abdominal
pain that is thought is to be caused by visceral sensitivity, this
study was based on the assumption that extendin-4 and its influence
on SERT expression and function is a common characteristic of all
types of IBS. However, further investigation is needed to verify
this.
In conclusion, exendin-4 may modulate SERT
expression and 5-HT reuptake in intestinal epithelial cells.
Combined with the results of previous studies, the
GLP-1R/AC/PKA/SERT signaling pathway may be the signaling pathway
of exendin-4 that is used to reduce visceral hypersensitivity.
However, the mechanism by which exendin-4 alleviates visceral
sensitivity in rat IBS models requires further study.
Acknowledgements
Not applicable.
Funding
This work was supported by The National Natural
Science Foundation of China (grant no. 81770553) and The Key
Medical Personnel of Jiangsu Province (grant no. ZDRCB2016001).
Availability of data and materials
The data used and analyzed in this study are
available from the corresponding author on reasonable request.
Authors' contributions
XC participated in the study design, data
acquisition and analysis, and drafting, interpretation and writing
of the manuscript. XZhao, YW and YY participated in the statistical
analysis. HZhang participated in the study concept and design,
study supervision, interpretation of data, and critical revision of
the manuscript for important intellectual content. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gwee KA, Gonlachanvit S, Ghoshal UC, Chua
ASB, Miwa H, Wu J, Bak YT, Lee OY, Lu CL, Park H, et al: Second
Asian consensus on irritable bowel syndrome. J Neurogastroenterol
Motil. 25:343–362. 2019. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Quigley EM, Abdel-Hamid H, Barbara G,
Bhatia SJ, Boeckxstaens G, De Giorgio R, Delvaux M, Drossman DA,
Foxx-Orenstein AE, Guarner F, et al: A global perspective on
irritable bowel syndrome: A consensus statement of the world
gastroenterology organisation summit task force on irritable bowel
syndrome. J Clin Gastroenterol. 46:356–366. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kanazawa M, Hongo M and Fukudo S: Visceral
hypersensitivity in irritable bowel syndrome. J Gastroenterol
Hepatol. 26 (Suppl 3):S119–S121. 2011. View Article : Google Scholar
|
|
4
|
Carrasco-Labra A, Lytvyn L, Falck-Ytter Y,
Surawicz CM and Chey WD: AGA technical review on the evaluation of
functional diarrhea and diarrhea-predominant irritable bowel
syndrome in adults (IBS-D). Gastroenterology. 157:859–880. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hellström PM, Hein J, Bytzer P, Björnssön
E, Kristensen J and Schambye H: Clinical trial: The glucagon-like
peptide-1 analogue ROSE-010 for management of acute pain in
patients with irritable bowel syndrome: A randomized,
placebo-controlled, double-blind study. Aliment Pharmacol Ther.
29:198–206. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nozu T, Miyagishi S, Kumei S, Nozu R,
Takakusaki K and Okumura T: Glucagon-like peptide-1 analog,
liraglutide, improves visceral sensation and gut permeability in
rats. J Gastroenterol Hepatol. 33:232–239. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Smith NK, Hackett TA, Galli A and Flynn
CR: GLP-1: Molecular mechanisms and outcomes of a complex signaling
system. Neurochem Int. 128:94–105. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hellström PM: GLP-1 playing the role of a
gut regulatory compound. Acta Physiol (Oxf). 201:151–156. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Smits MM, Tonneijck L, Muskiet MH, Kramer
MH, Cahen DL and van Raalte DH: Gastrointestinal actions of
glucagon-like peptide-1-based therapies: Glycaemic control beyond
the pancreas. Diabetes Obes Metab. 18:224–235. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang Y, Cui X, Chen Y, Wang Y, Li X, Lin L
and Zhang H: Exendin-4, an analogue of glucagon-like peptide-1,
attenuates hyperalgesia through serotonergic pathways in rats with
neonatal colonic sensitivity. J Physiol Pharmacol. 65:349–357.
2014.PubMed/NCBI
|
|
11
|
Hellström PM, Smithson A, Stowell G,
Greene S, Kenny E, Damico C, Leone-Bay A, Baughman R, Grant M and
Richardson P: Receptor-mediated inhibition of small bowel migrating
complex by GLP-1 analog ROSE-010 delivered via pulmonary and
systemic routes in the conscious rat. Regul Pept. 179:71–76. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wei Y and Mojsov S: Distribution of GLP-1
and PACAP receptors in human tissues. Acta Physiol Scand.
157:355–357. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xiao YF, Nikolskaya A, Jaye DA and Sigg
DC: Glucagon-like peptide-1 enhances cardiac L-type Ca2+ currents
via activation of the cAMP-dependent protein kinase A pathway.
Cardiovasc Diabetol. 10:62011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Morikawa O, Sakai N, Obara H and Saito N:
Effects of interferon-alpha, interferon-gamma and cAMP on the
transcriptional regulation of the serotonin transporter. Eur J
Pharmacol. 349:317–324. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rumajogee P, Madeira A, Vergé D, Hamon M
and Miquel MC: Up-regulation of the neuronal serotoninergic
phenotype in vitro: BDNF and cAMP share Trk B-dependent mechanisms.
J Neurochem. 83:1525–1528. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kerckhoffs AP, ter Linde JJ, Akkermans LM
and Samsom M: SERT and TPH-1 mRNA expression are reduced in
irritable bowel syndrome patients regardless of visceral
sensitivity state in large intestine. Am J Physiol Gastrointest
Liver Physiol. 302:G1053–G1060. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
El-Salhy M, Wendelbo I and Gundersen D:
Serotonin and serotonin transporter in the rectum of patients with
irritable bowel disease. Mol Med Rep. 8:451–455. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wade PR, Chen J, Jaffe B, Kassem IS,
Blakely RD and Gershon MD: Localization and function of a 5-HT
transporter in crypt epithelia of the gastrointestinal tract. J
Neurosci. 16:2352–2364. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Miwa J, Echizen H, Matsueda K and Umeda N:
Patients with constipation-predominant irritable bowel syndrome
(IBS) may have elevated serotonin concentrations in colonic mucosa
as compared with diarrhea-predominant patients and subjects with
normal bowel habits. Digestion. 63:188–194. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bertrand PP and Bertrand RL: Serotonin
release and uptake in the gastrointestinal tract. Auton Neurosci.
153:47–57. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Grundy D: 5-HT system in the gut: Roles in
the regulation of visceral sensitivity and motor functions. Eur Rev
Med Pharmacol Sci. 12 (Suppl 1):S63–S67. 2008.
|
|
22
|
Salaga M, Binienda A, Tichkule RB, Thakur
GA, Makriyannis A, Storr M and Fichna J: The novel peripherally
active cannabinoid type 1 and serotonin type 3 receptor agonist
AM9405 inhibits gastrointestinal motility and reduces abdominal
pain in mouse models mimicking irritable bowel syndrome. Eur J
Pharmacol. 836:34–43. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen Y, Li Z, Yang Y, Lin L and Zhang H:
Role of glucagon-like peptide-1 in the pathogenesis of experimental
irritable bowel syndrome rat models. Int J Mol Med. 31:607–613.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu Z, Zhang M, Zhou T, Shen Q and Qin X:
Exendin-4 promotes the vascular smooth muscle cell
re-differentiation through AMPK/SIRT1/FOXO3a signaling pathways.
Atherosclerosis. 276:58–66. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Franzellitti S and Fabbri E: Cyclic-AMP
mediated regulation of ABCB mRNA expression in mussel haemocytes.
PLoS One. 8:e616342013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Smith PK, Krohn RI, Hermanson GT, Mallia
AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ and
Klenk DC: Measurement of protein using bicinchoninic acid. Anal
Biochem. 150:76–85. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Camilleri M: Serotonergic modulation of
visceral sensation: Lower gut. Gut. 51 (Suppl 1):i81–i86. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Matheus N, Mendoza C, Iceta R, Mesonero JE
and Alcalde AI: Melatonin inhibits serotonin transporter activity
in intestinal epithelial cells. J Pineal Res. 48:332–339. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Coates MD, Mahoney CR, Linden DR, Sampson
JE, Chen J, Blaszyk H, Crowell MD, Sharkey KA, Gershon MD, Mawe GM
and Moses PL: Molecular defects in mucosal serotonin content and
decreased serotonin reuptake transporter in ulcerative colitis and
irritable bowel syndrome. Gastroenterology. 126:1657–1664. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gill RK, Anbazhagan AN, Esmaili A, Kumar
A, Nazir S, Malakooti J, Alrefai WA and Saksena S: Epidermal growth
factor upregulates serotonin transporter in human intestinal
epithelial cells via transcriptional mechanisms. Am J Physiol
Gastrointest Liver Physiol. 300:G627–G636. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ramamoorthy S, Cool DR, Mahesh VB, Leibach
FH, Melikian HE, Blakely RD and Ganapathy V: Regulation of the
human serotonin transporter. Cholera toxin-induced stimulation of
serotonin uptake in human placental choriocarcinoma cells is
accompanied by increased serotonin transporter mRNA levels and
serotonin transporter-specific ligand binding. J Biol Chem.
268:21626–21631. 1993.PubMed/NCBI
|
|
33
|
Cui XF, Zhou WM, Yang Y, Zhou J, Li XL,
Lin L and Zhang HJ: Epidermal growth factor upregulates serotonin
transporter and its association with visceral hypersensitivity in
irritable bowel syndrome. World J Gastroenterol. 20:13521–13529.
2014. View Article : Google Scholar : PubMed/NCBI
|