Introduction
Mesenchymal stem cells (MSCs) are a group of
multipotent cells that can mature into cells with various
immunomodulatory functions. Additionally, MSCs exert regulatory
effects on the maturation and differentiation of immune cells, such
as naive T cells and dendritic cells. They also exert effects on
the secretion of different cytokines and the expression of surface
receptors in these immune cells (1). During the differentiation process of
naive T cells, there are two types of cells that have gained
increasing attention in recent years: Regulatory T cells (Tregs)
that inhibit the inflammatory immune response, and T helper 17
(Th17) cells that exert a proinflammatory effect and secrete
various inflammatory cytokines, including interleukin (IL)-17, IL-6
(2). Özdemir et al
(3) reported that MSCs can promote
the proliferation and transformation of Treg cells and inhibit the
proliferation of Th17 cells. A shift in the immune balance between
Treg/Th17 cells towards Treg cells can result in an escape from the
immune response from the host, and it can help to maintain
homeostasis and induce immune tolerance (4).
In an animal study on liver transplantation, the
postoperative survival time and liver function of rats that were
treated with tacrolimus + MSCs were improved compared with the rats
treated with a standard dose of tacrolimus alone (5). MSCs can inhibit Th1 and Th17 cells,
promote the expression of anti-inflammatory cytokines in Th2 cells
(6) and induce the differentiation
of immature T cells into Treg cells (7). A shift in the Treg/Th17 balance
towards Th17 cells and increased IL-17 production may underlie
graft rejection (8). Therefore,
the effects of MSCs on the Treg/Th17 balance is of notable interest
to potentially increase tissue acceptance in transplant surgeries.
However, the mechanism by which MSCs regulate Treg/Th17 balance and
its function on immunosuppression are still unclear.
In the present study, co-cultures of different
quantities of bone marrow derived (BM)-MSCs and CD4+ T
lymphocytes were used to investigate the effect of BM-MSCs on the
balance of Treg/Th17 under various conditions via the addition of
different immunosuppressive agents and cytokine blockers. The aim
of the present study was to provide an experimental basis for the
use of MSCs in certain clinical conditions.
Materials and methods
Animals
Male Wistar rats (n=18; age, 3 weeks; weight, 50–55
g) were used for isolation of MSCs for culture. Male Wistar rats
(n=12; age, 6 weeks; weight, 180–210 g) were used for isolation of
CD4+ T lymphocytes. Rats were obtained from the
experimental animal center of the Chinese Academy of Military
Medical Sciences (license no. SCXK). Animals were housed in a
pathogen-free environment at 20-25°C with 50–70% humidity, ad
libitum access to food and water, and 12-h light/dark cycles.
The present study was approved by the Ethics Committee of Tianjin
First Center Hospital (Tianjin, China) and was performed in
accordance with the principles of 3Rs and those described in the
Experimental Animal Welfare Ethics Review Guide of China (GB/T
35892-2018).
Materials
Foxp3 transcription factor staining buffer kit,
IL-17 intracellular staining buffer kit, monoclonal antibodies
against CD4, CD25, Foxp3 and IL-17, rat anti- transforming growth
factor-β (TGF-β) antibody, and ProcartaPlex™ cytokine
detection kits for IL-6, IL-10, IL-17 and TGF-β were all purchased
from eBioscience; Thermo Fisher Scientific, Inc. Mouse anti-IL-2
antibody and monoclonal antibodies against CD29, CD45 and CD90 were
purchased from Becton, Dickinson and Company. Rat CD4+ T
lymphocyte magnetic beads, MS sorting column and magnetic cell
sorter were purchased from (Miltenyi Biotec GmbH). All samples were
tested on a FACSCanto™ II flow cytometer (Becton,
Dickinson and Company).
Extraction, culture and identification
of BM-MSCs
Bone marrow cell suspension was obtained from the
femur of a 50 g male Wistar rat. Male Wistar rats were sacrificed
by cervical dislocation and sterilized in 75% ethanol for 10 min at
room temperature. Subsequently, the femur and tibia were obtained
by aseptic operation. After the bone marrow cavity was exposed, the
bone marrow cell suspension was obtained by rinsing the marrow
cavity four times with DMEM/F12 complete culture medium (Gibco;
Thermo Fisher Scientific, Inc.). The cells were cultured in
DMEM/F12 medium with 5% CO2 at 37°C with 100% humidity.
Anti-CD29 (cat. no. 562801), anti-CD45 (cat. no. 561588) and
anti-CD90 (cat. no. 561409) antibodies were used to label the
cells, and the purity of BM-MSCs cells was assessed by flow
cytometry using Flow Jo software (version 7.6.1; Flow Jo LLC). The
average ratio of CD90+, CD29+ and
CD45+ BM-MSCs were 99.83±0.01, 97.50±0.10 and
4.06±0.47%, respectively. The number of extracted CD4+ T
lymphocytes was ~5.7±0.3×106/ml and the purity was
>95%, which was considered satisfactory for all subsequent
experiments.
Extraction of CD4+ T
lymphocytes
A suspension of mononuclear cells was obtained from
the grinded spleen of Wistar rats by Ficoll-Hypaque density
gradient centrifugation at 838 × g for 25 min at room temperature
and then at 503 × g for 15 min at room temperature. Subsequently,
the supernatant was discarded and CD4+ T lymphocyte
magnetic beads were added. After thoroughly mixing and incubating
at 4°C for 15 min, the cell suspension was extracted using an MS
sorting column and a magnetic cell sorter. Subsequently,
CD4+ T lymphocytes were obtained and the purity was
measured by flow cytometry.
Co-culture of BM-MSCs with
CD4+ T lymphocytes
The BM-MSCs were plated into a 6-well plate at a
density of 1×105 cells/well (0.5×Gr), 2×105
cells/well (1×Gr), 4×105 cells/well (2×Gr) or
8×105 cells/well (4×Gr). The extracted CD4+ T
lymphocytes were added to the culture plates with the BM-MSCs and 5
mg/ml of PHA and 50 µg/ml of IL-2 was added. After 72 h, the
culture medium was collected, centrifuged at 503 × g for 10 min at
room temperature, and the supernatant was collected and stored at
−80°C. The residual cells were incubated with anti-CD4 (cat. no.
11-0040-82) and anti-CD25 (cat. no. 46-0390-82) for 60 min at room
temperature, and anti-Foxp3 (cat. no. 12-5773-82) and anti-IL-17A
(cat. no. 12-7177-81) for 30 min at room temperature. Subsequently,
cells were analyzed by flow cytometry (Figs. S1 and S2).
Immunosuppressant treatment
Tacrolimus (8 or 12 ng/ml) or rapamycin (100 or 150
nmol/l) were used as immunosuppressants and added to the
co-cultures of BM-MSCs and CD4+ T lymphocytes and
incubated for 72 h. The supernatant was stored at −80°C for
assessment of the presence of cytokines and the cells were analyzed
using flow cytometry.
Cytokine inhibitors
BM-MSCs were co-cultured with CD4+ T
lymphocytes as described above and treated with varying
concentrations of TGF-β inhibitors (10 or 20 µg/ml) or different
concentrations of IL-2 inhibitors (2 or 4 µg/ml). Treated
co-cultured cells were incubated for 72 h. The supernatant was
collected after centrifugation at 503 × g for 10 min at 4°C and
stored at −80°C refrigerator to measure levels of cytokines. The
proportions of Treg and Th17 cells were analyzed using flow
cytometry. The TGF-β blocker used was anti-TGF-β functional grade
purified (cat. no. 16-9243-85; eBioscience; Thermo Fisher
Scientific, Inc.) and IL-2 blocker used was purified mouse anti-rat
CD25 (cat. no. 559980; Becton, Dickinson and Company; Thermo Fisher
Scientific, Inc.). The dosage of TGF-β used was 20 µg/ml in the
higher concentration group and 10 µg/ml in the lower concentration
group, respectively. The dosage of IL-2 was 4 µg/ml in the higher
concentration group and 2 µg/ml in the lower concentration
group.
Detection of
CD4+CD25+Foxp3+Treg cells and
CD4+IL-17+Th17 cells
The collected cells were tested by immunophenotyping
for surface or intracellular markers with monoclonal antibodies
specific for each protein.
Measurement of expression of
cytokines
The concentration of IL-6, IL-10, IL-17 and TGF-β
were measured. After diluting the antibody magnetic beads 1:50, 50
µl of the sample was added to each well in a 96-well plate and the
plate was placed on a magnetic rack. After 2 min, the supernatant
was discarded and washed with wash buffer once. Additional care was
taken not to detach the 96-well plate from the magnetic plate rack
during the process to prevent the magnetic beads from falling off.
The provided reagents in each kit were dissolved according to the
manufacturer's protocol and different dilutions were added to the
96-well plates. DMEM/F12 complete medium was added to the final
well as the blank control group. Experiments where performed with 6
repeats per each condition and 2 groups were used to plot the
standard curve. A total of 50 µl of sample was added to the
remaining wells and 25 µl of wash buffer was added to these wells.
The plate was incubated for 2 h at room temperature to allow the
magnetic beads to bind to the corresponding cytokines. After
incubation, the wells were washed with the special solution three
times and 25 µl of a diluted solution of the corresponding cytokine
antibody was added, incubated at room temperature in the dark for
30 min, rinsed with special solution, 50 µl of phycoerythrin dye
was added and incubated on an oscillator for 30 min at room
temperature. After the incubation, the buffer was cleaned once with
the special solution and 120 µl reading buffer was added to each
well. The buffer was placed on the oscillator for 5 min to ensure
the solution was adequately mixed prior to taking measurements.
Statistical analysis
For normally distributed data, data are expressed as
the mean ± standard deviation. For non-normally distributed data,
data are expressed as the median and interquartile range (Q25 and
Q75). One-way ANOVA followed by Tukey's post hoc test was used for
comparison of multiple groups if the data were normally
distributed. Kruskal-Wallis test followed by Dunn's post hoc test
was performed to assess comparisons among multiple groups if the
data were not normally distributed. P<0.05 was considered to
indicate a statistically significant difference. Statistical
analyses were performed using SPSS version 19.0 (IBM, Corp.).
Results
Effects of BM-MSCs on the Treg/Th17
balance
There was a significant difference in the proportion
of Treg cells amongst the co-cultures with different numbers of
BM-MSCs added (P=0.001; Fig. 1).
Amongst these, 2×Gr had the highest proportion of Treg cells
(9.24±2.68%), which was significantly increased compared with
0.5×Gr (3.87±0.38%, P=0.002), 1×Gr (5.16±1.69%, P=0.017) and 4×Gr
(3.86±0.36%, P=0.002). The differences in the proportion of Treg
cells in the other three groups were not statistically significant
(P>0.05). The proportion of Th17 cells was significantly
different amongst the co-cultures with different numbers of BM-MSCs
added (P<0.001). The proportion of Th17 cells in the 1×Gr
(0.89±0.08%) was the highest and was significantly increased
compared with 0.5×Gr (0.64±0.15%, P=0.020) and 4×Gr (0.37±0.10%,
P<0.001), but there was no significant difference compared with
2×Gr (0.83±0.06%, P=0.796). The proportion of Th17 cells in the
0.5×Gr was not reduced compared with 2×Gr (P=0.095), but was
increased compared with 4×Gr (P=0.013).
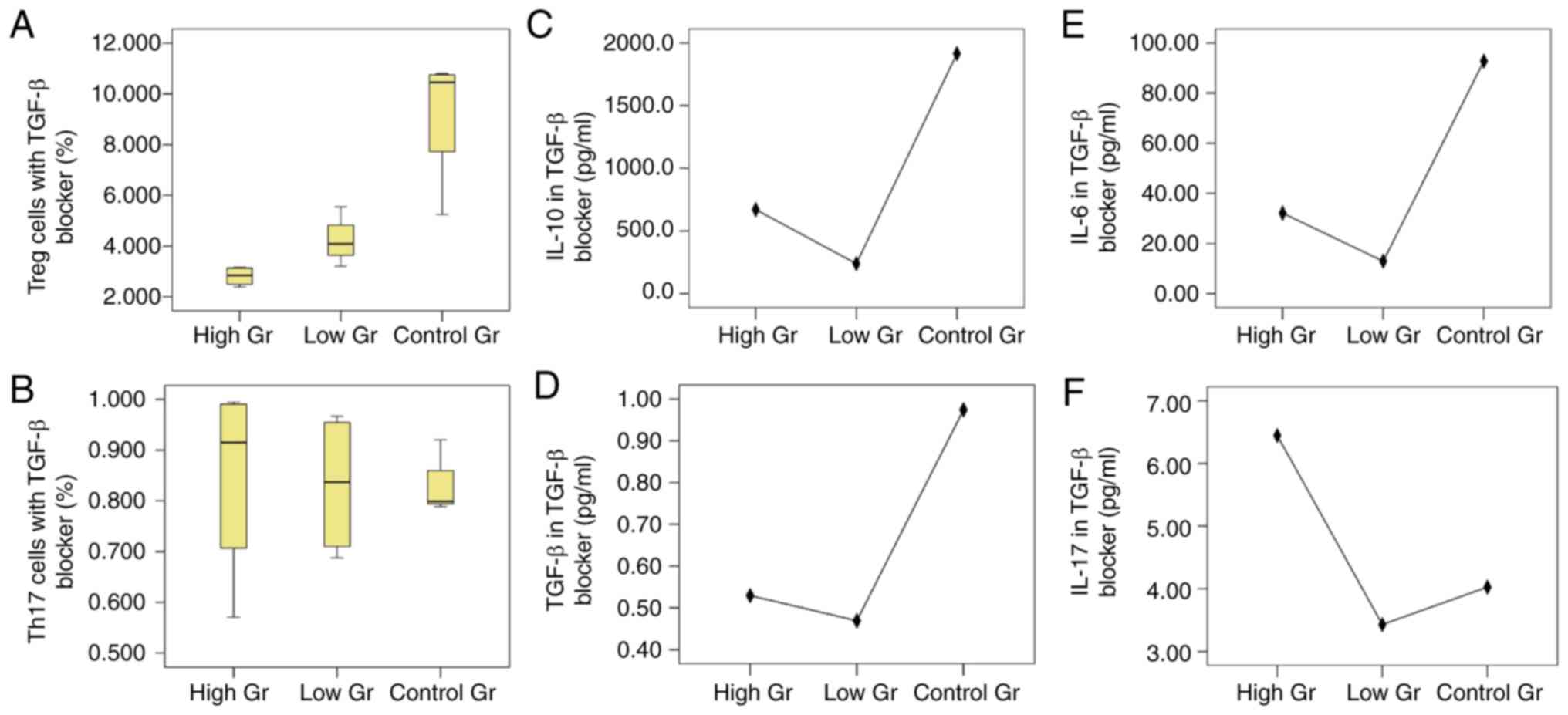
 | Figure 1.Treg/Th17 cells and cytokines in
different concentrations of BM-MSCs co-cultured with
CD4+ T lymphocytes. (A) Proportions of Treg cells in
each group, which showed that BM-MSCs in 2×Gr had the strongest
effect on promoting proliferation of Treg cells. (B) Proportions of
Th17 cells in each group, in which the proportion of 1×Gr was the
highest and the proportion of 4×Gr was the lowest. Levels of: (C)
IL-10, P=0.021; (D) TGF-β, P=0.041; (E) IL-6, P=0.995; and (F)
IL-17, P=0.760. Treg, regulatory T; Th17, T helper 17; BM-MSCs,
bone marrow mesenchymal stem cells; IL, interleukin; TGF,
transforming growth factor. |
There were statistically significant differences in
IL-10 levels between the groups (P=0.021). IL-10 levels in 0.5×Gr
was significantly lower compared with the other groups (P<0.05),
except 4×Gr (P=0.111), although no statistical difference was
observed in comparisons between the other groups. TGF-β levels were
also significantly different amongst the groups (P=0.041). The
level of TGF-β in 0.5×Gr was lower compared with 1×Gr (P=0.044).
TGF-β levels were the lowest in 4×Gr, but the difference was only
significant when compared with 1×Gr (P=0.037). IL-6 and IL-17 were
not detected in 0.5×Gr, and there was no significant difference
observed between the levels of these cytokines in any of the groups
(P=0.385 and P=0.997, respectively).
Effect of different immunosuppressants
on BM-MSC-mediated regulation of the Treg/Th17 balance
In the high tacrolimus concentration group, low
tacrolimus concentration group and control group, the median
proportions of Treg cells were 1.83% (1.36 and 2.50%), 2.98% (2.36
and 3.18%) and 10.45% (6.48 and 10.78%), respectively, and there
was a statistically significant difference amongst the three groups
(P=0.010). As shown in Fig. 2, the
median proportions of Th17 cells in the high tacrolimus
concentration group, low tacrolimus concentration group and the
blank control group was 1.30% (0.76 and 1.56%), 1.06% (0.69 and
1.16%) and 0.80% (0.79 and 0.89%), respectively, and there was no
significant difference amongst the three groups (P=0.333).
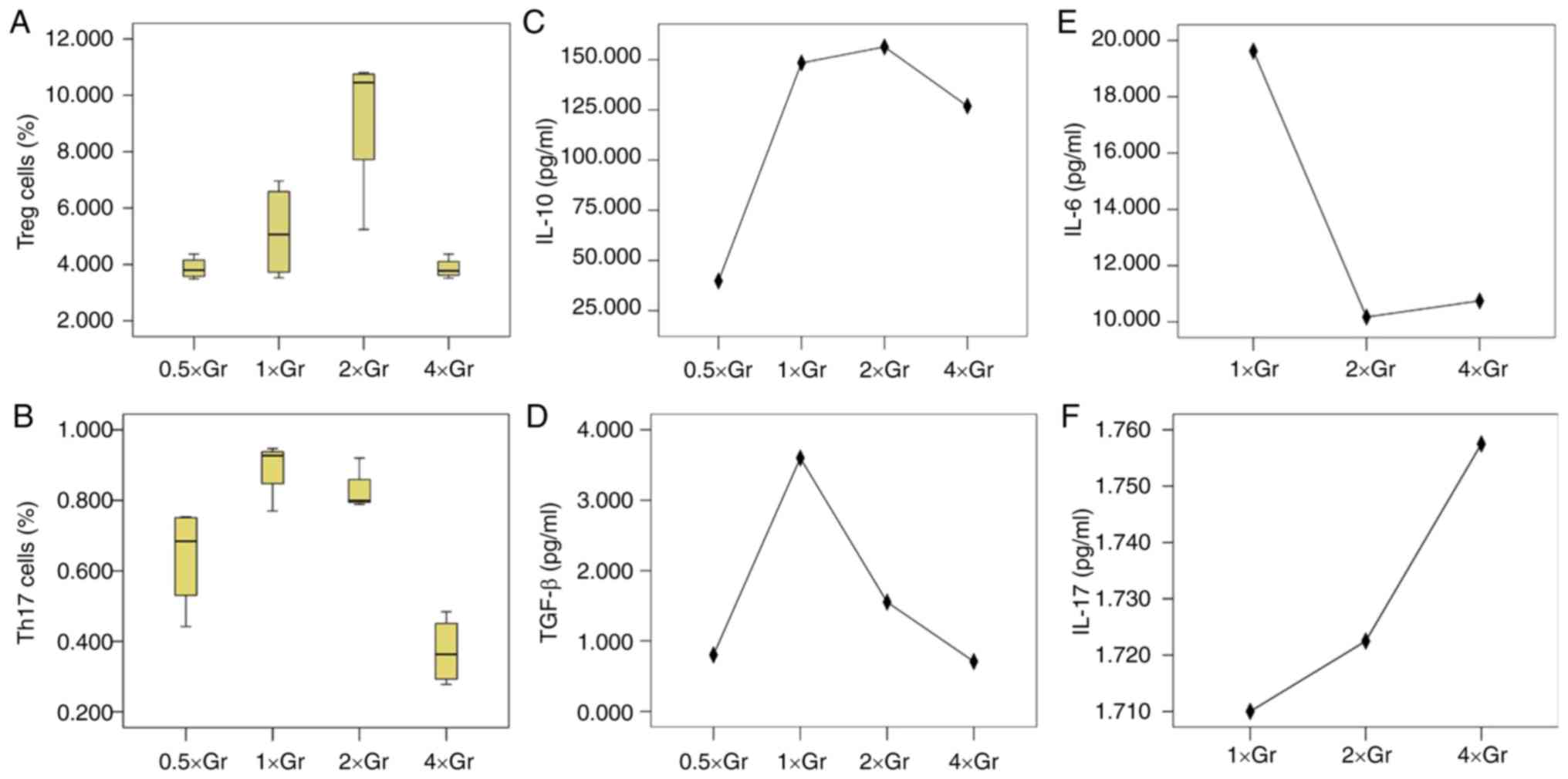
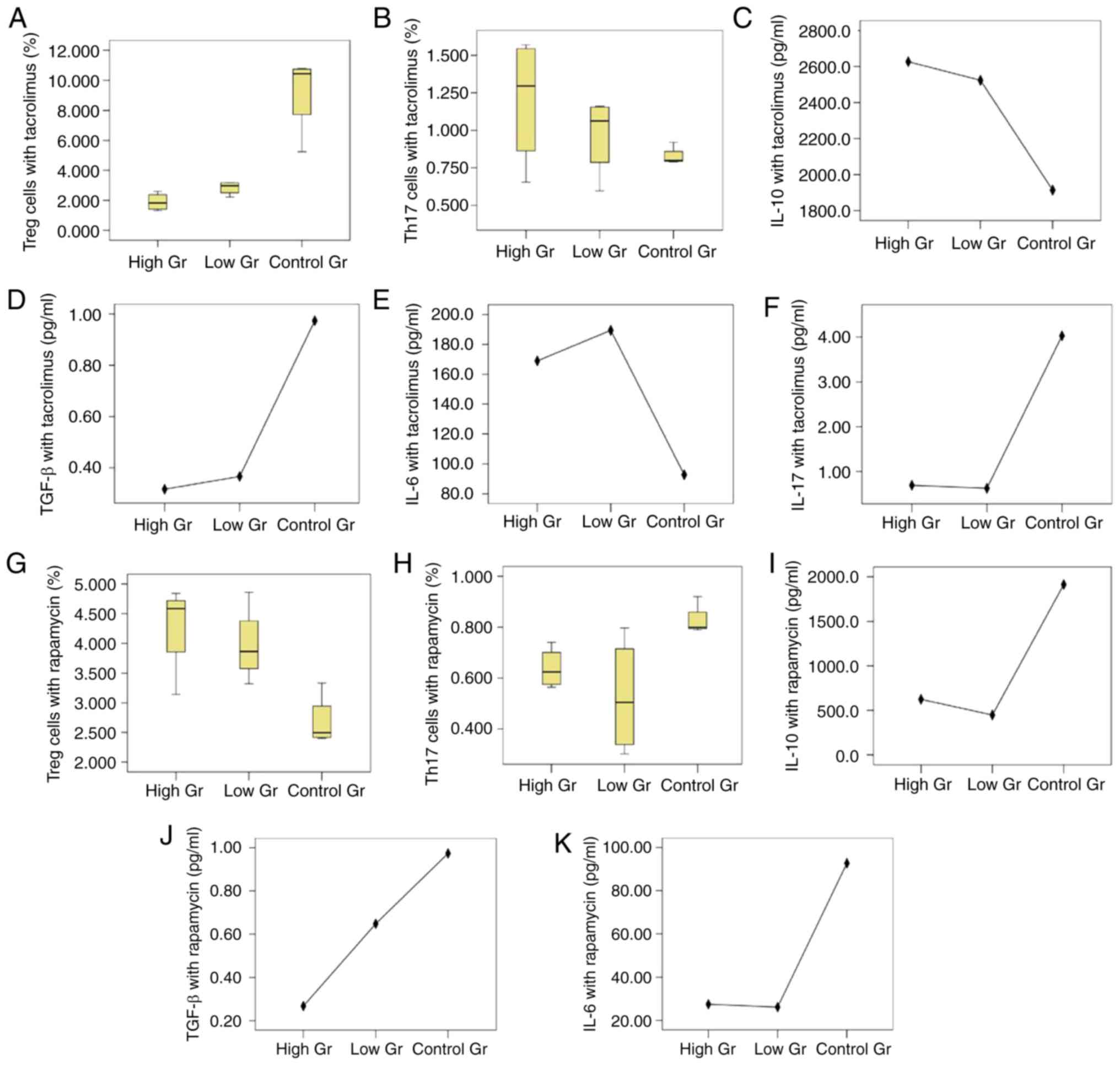 | Figure 2.Treg/Th17 cells and cytokines
co-cultured by BM-MSCs and CD4+ T lymphocytes with
different immunosuppressive agents. Proportions of cultured (A)
Treg cells and (B) Th17 cells under the action of tacrolimus,
respectively. The proportion of Treg cells in the high tacrolimus
group and the low tacrolimus group were lower compared with the
control group (P=0.016 and P=0.018, respectively), but there was no
statistical difference between the high and low group (P=0.055).
The proportions of Th17 cells show no significant difference
(P=0.333). Expression of (C) IL-10 (P=0.058), (D) TGF-β (P=0.035),
(E) IL-6 (P=0.001) and (F) IL-17 (P=0.024) under the action of
tacrolimus, respectively. Proportions of (G) Treg cells and (H)
Th17 cells under the action of rapamycin, respectively. The
proportions of Treg cells in high and low rapamycin concentration
groups were higher than that in the blank control group (P=0.006,
P=0.018) respectively, but the difference between the high and low
concentration groups was not statistically significant (P=0.507).
The proportions of Th17 cells in the group with high and low
concentrations of rapamycin were lower than that in the blank
control group (P=0.039, P=0.018), but the difference between the
groups with high and low concentrations was not statistically
significant (P=0.768). Levels of (I) IL-10 (P<0.001), (J) TGF-β
(P=0.218) and (K) IL-6 (P=0.024) with rapamycin treatment,
respectively. IL-17 wasn't detected in the rapamycin groups. Treg,
regulatory T; Th17, T helper 17; BM-MSCs, bone marrow mesenchymal
stem cells; IL, interleukin; TGF, transforming growth factor. |
The levels of IL-10 in the high tacrolimus
concentration group, low tacrolimus concentration group and blank
control group were not significantly different (P=0.058), but the
differences in the levels of TGF-β were statistically significant
(P=0.035). TGF-β levels in the high and low tacrolimus
concentration group were lower compared with the blank control
group (P=0.020 and P=0.028, respectively), the difference between
the high and low concentration groups was not significant
(P=0.835). The levels of IL-6 and IL-17 in the high and low
tacrolimus concentration group and the blank control group was
statistically significant (P=0.001 and P=0.024, respectively). In
both the high and low tacrolimus concentration groups, IL-6 levels
were significantly increased compared with the blank control group
(P<0.05). IL-17 levels in the high and low tacrolimus
concentration groups were both significantly decreased compared
with the blank control group (P<0.05), and the difference
between the high and low concentration group was not significant
(P=0.241 and P=0.845).
The proportion of Treg cells in the high rapamycin
concentration group, low rapamycin concentration group and the
blank control group was 4.29±0.77, 3.98±0.64 and 2.68±0.44%,
respectively, and the differences between the three groups were
statistically significant (P=0.013). The median proportions of Th17
cells in the high rapamycin concentration group, low rapamycin
concentration group and the blank control group was 0.624% (0.570
and 0.721%), 0.505% (0.320 and 0.756%) and 0.799% (0.792 and
0.890%), respectively, and the differences between the three groups
were statistically significant (P=0.037).
The differences amongst the IL-10 levels in the high
rapamycin concentration group, low rapamycin concentration group
and the blank control group was statistically significant
(P<0.001). IL-10 levels in the high and low rapamycin
concentration groups were lower compared with the blank control
group (both P<0.001), but the difference between the high
concentration and low concentration groups were not statistically
significant (P=0.342). There was no statistically significant
difference in the TGF-β levels amongst the three groups (P=0.218),
but the difference in the level of IL-6 was significant (P=0.024).
IL-6 levels in the high and low concentration rapamycin groups were
lower compared with the blank control group (P=0.027 and P=0.012,
respectively), but the difference between the high and low
concentration groups was not statistically significant
(P=0.768).
The Treg/Th17 balance regulated by BM-MSCs when
treated with tacrolimus or rapamycin showed that both the
proportions of Treg/Th17 cells and their associated cytokines,
excluding TGF-β, were statistically different as shown in Tables I and II. The proportion of Treg cells in
co-cultures treated with rapamycin treatment were higher compared
with the tacrolimus group, whereas the proportion of Th17 cells,
IL-10 and IL-6 levels were lower compared with the tacrolimus
group.
 | Table I.Treg/Th17 balance under high
concentration of immunosuppressants. |
Table I.
Treg/Th17 balance under high
concentration of immunosuppressants.
| Subject | Tacrolimus Gr | Rapamycin Gr | P-value |
|---|
| Treg cells (%) | 1.90±0.60 | 4.29±0.77 | 0.003 |
| Th17 (%) | 1.30 (0.76,
1.56) | 0.62 (0.57,
0.72) | 0.043 |
| IL-10 (pg/ml) | 2,560.865
(2,158.730, 3,163.318) | 683.775
(464.335, 726.020) | 0.021 |
| TGF-β (pg/ml) | 0.316±0.283 | 0.267±0.195 | 0.786 |
| IL-6 (pg/ml) | 167.745 (140.938,
198.168) | 27.640 (23.063,
31.648) | 0.021 |
 | Table II.Treg/Th17 balance under low
concentration of immunosuppressants. |
Table II.
Treg/Th17 balance under low
concentration of immunosuppressants.
| Subject | Tacrolimus Gr | Rapamycin Gr | P-value |
|---|
| Treg cells (%) |
2.84±0.45 | 3.98±0.64 | 0.027 |
| Th17 cells (%) |
0.97±0.26 | 0.53±0.23 | 0.044 |
| IL-10 (pg/ml) |
2,524.180±448.853 |
448.853±148.364 | <0.001 |
| TGF-β (pg/ml) |
0.366±0.301 | 0.648±0.798 | 0.533 |
| IL-6 (pg/ml) | 191.925 (168.505,
207.763) | 24.590 (20.583,
33.180) | 0.021 |
Effect of TGF-β blocker on BM-MSC
mediated regulation of the Treg/Th17 balance
The proportion of Treg cells in the high
concentration of TGF-β blocker, low concentration of TGF-β blocker
and blank control groups were 2.81±0.38, 4.23±0.97 and 9.24±2.68%,
respectively, and there was a statistically significant difference
amongst the three groups (P=0.001). The proportion of Th17 cells in
the high concentration, low concentration and blank control groups
were 0.85±0.20, 0.83±0.14 and 0.83±0.06%, respectively, and the
difference amongst the three groups was not statistically
significant (P=0.976; Fig. 3).
The levels of IL-10 in the high concentration
blocking group, low concentration blocking group and the blank
control group was significantly different (P<0.001). However,
there was no significant difference in TGF-β levels amongst the
three groups. IL-6 levels in the low concentration blocking group
and the high concentration blocking group were significantly lower
compared with the control group (P<0.001), and the difference
between the high concentration blocking group and the low
concentration blocking group was not significant (P=0.062). The
differences in the levels of IL-17 amongst the three groups was not
significant.
Effect of IL-2 blocker on BM-MSC
mediated regulation of the Treg/Th17 balance
The proportions of Treg cells in the high
concentration blocking group, low concentration blocking group and
blank control groups was 1.95±2.21, 1.66±1.51 and 9.24±2.68%,
respectively, and the differences between the three groups was
significant (P=0.001). The proportions of Th17 cells in the three
groups were 0.74±0.16, 0.99±0.05 and 0.83±0.06%, respectively and
the difference between the groups was significant (P=0.022;
Fig. 4).
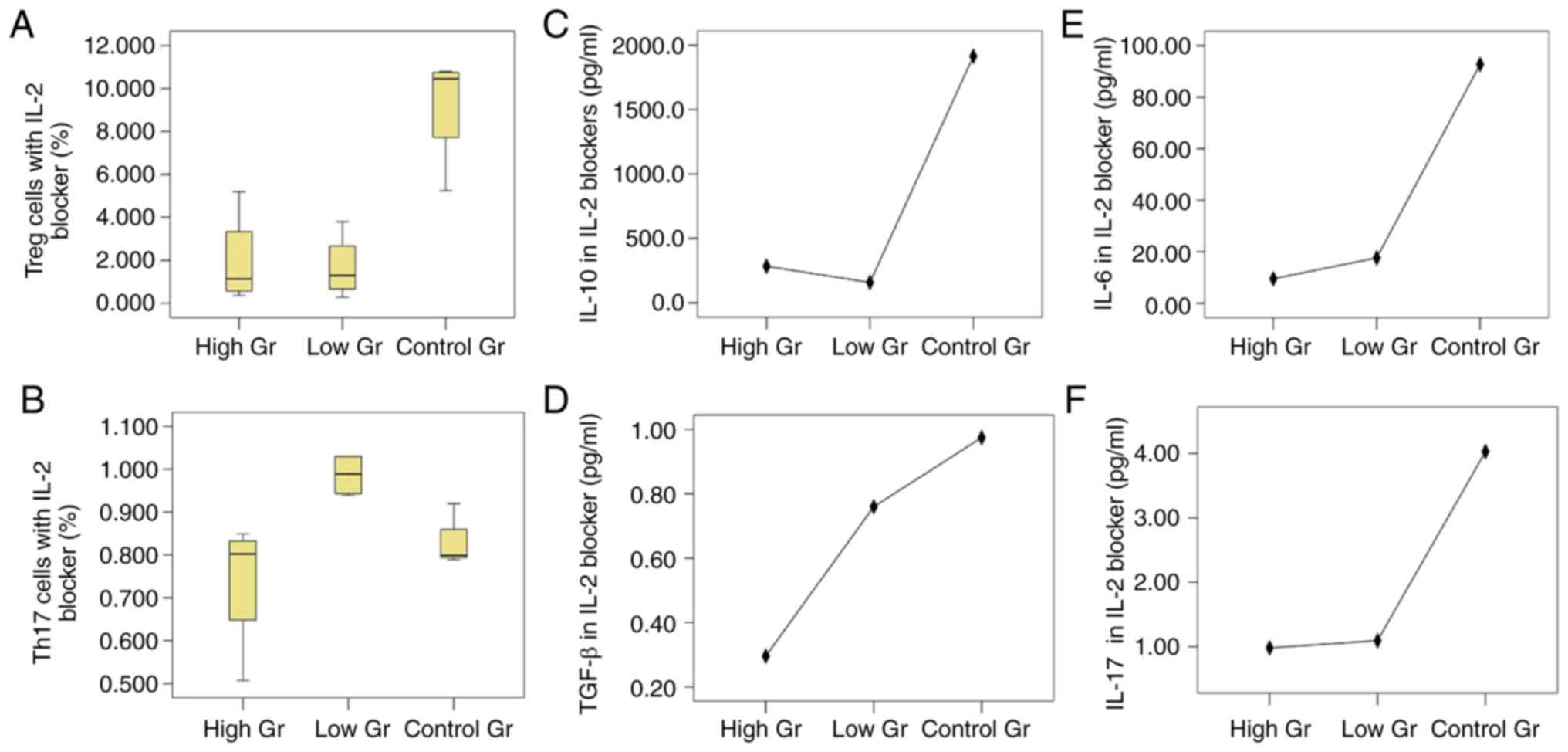 | Figure 4.Proportions of Treg/Th17 cells and
cytokines after co-culture of BM-MSCs and CD4+ T
lymphocytes after blocking IL-2. Proportions of (A) Treg cells and
(B) Th17 cells under the action of an IL-2 blocker. The proportions
of Treg cells in the high and low concentration blocking groups
were both lower than that in the blank control group (P=0.003,
P=0.002), but there was no difference in the proportions of Treg
cells between the groups with different concentrations of blocking
agents (P=0.981). The proportion of Th17 cells in the high
concentration blocking group was lower than that in the low
concentration blocking group (P=0.019), however compared with the
blank control group the difference was not significant (P=0.482).
There was no difference between the low concentration blocking
group and the control group (P=0.054). Expression of (C) IL-10
(P=0.007), (D) TGF-β (P=0.059), (E) IL-6 (P=0.011) and (F) IL-17
(P=0.039) of IL-2 blocking groups at different concentrations,
respectively. Treg, regulatory T; Th17, T helper 17; BM-MSCs, bone
marrow mesenchymal stem cells; IL, interleukin; TGF, transforming
growth factor. |
The levels of IL-10 in the three groups were
significantly different. The IL-10 levels in the low concentration
blocking group was lower compared with the control group (P=0.005)
and no significant difference was observed when compared with the
high concentration blocking group (P=0.350). There was no
significant difference in the TGF-β levels amongst the three
groups. IL-6 in the high concentration blocking group was
significantly higher compared with the control group (P=0.008), but
was not significantly different compared with the low concentration
blocking group (P=0.202). IL-17 levels were significantly different
in the high concentration blocking group, low concentration
blocking group and the blank control group, with IL-17 levels being
lower in the high and low concentration groups compared with the
control group (P=0.031 and P=0.024, respectively), but the
difference between the high and low concentration groups was not
significant (P=0.922).
Discussion
Previous studies have reported that the effect of
MSCs on the Treg/Th17 balance primarily results in promotion of the
proliferation and transformation of Treg cells, inhibition of the
proliferation of Th17 cells and secretion of cytokines (9,10).
Liu et al (9) demonstrated
that BM-MSCs significantly increased the proportion of Treg cells,
induced the differentiation of CD4+CD25−
cells into Treg cells and increased the expression of Foxp3 in a
co-culture of BM-MSCs and T cells in vitro. A decrease in
the number of Th17 cells was also observed in the co-culture of
MSCs and T cells in rats (10).
However, Rozenberg et al (11) found that MSCs promoted the response
of Th17 cells and increased the proportion of Th17 cells in a
co-culture of human MSCs and peripheral blood monocytes.
MSCs regulate the Treg/Th17 balance through various
mechanisms. MSCs can induce the differentiation of CD4+
T lymphocytes into
CD4+CD25+Foxp3+Treg cells via
toll-like receptors and the MSC-mediated increase in proliferation
was absent when toll-like receptor genes were knocked out (12). In the inflammatory environment
in vivo, MSCs exert their inhibitory function on T cell
activation and proliferation via the secretion of IL-10,
hemagglutinin-1 and anti-inflammatory cytokines (13). In addition, MSCs can also promote
the proliferation and differentiation of Treg cells by secreting
anti-inflammatory cytokines, such as TGF-β and prostaglandin
(14). The proportion of Th17
cells is decreased by blocking the IL-10 signaling pathways, which
is possible via inhibition of cytokine signaling 3, signal
transduction and transcriptional activation factor 3 and
retinoid-related orphan nuclear receptor (15).
In the present study, it was demonstrated that the
concentration of BM-MSCs had a significant influence on the
Treg/Th17 balance, where BM-MSCs were able shift the balance
towards both cell types dependent on the concentration of BM-MSCs
added, but there was no positive association between the
concentration of BM-MSCs and the proportion of Treg/Th17 cells.
When the concentration of BM-MSCs in the co-culture was high, the
proportions of Treg cells and Th17 cells were significantly
decreased. However, changes to the levels of relevant cytokines
were not observed and did not appear to be associated with changes
to the proportions of Treg/Th17 cells, and this may be due to
secretion of TGF-β and IL-10 by MSCs.
The most commonly used immunosuppressants include
calcineurin inhibitors and mTOR, and they exert different effects
on Treg and Th17 cells. In a rat model of liver transplantation, Xu
et al (16) found that the
proportion of Treg cells in peripheral blood mononuclear cells in
the rapamycin treatment group was higher compared with the group
treated with tacrolimus. Hajkova et al (17) reported that the inhibitory effects
of BM-MSCs on CD4+RORγt+Th17 were
significantly reduced after treatment with tacrolimus. The addition
of rapamycin significantly reduced the expression of IL-17, whereas
rapamycin significantly increased the expression of Foxp3 in the
co-culture of MSCs and CD4+ T cells. In the present
study, the proportions of Treg cells treated with different
immunosuppressants resulted in opposite changes; the proportion of
Treg cells decreased when treated with tacrolimus but increased
when treated with rapamycin. Conversely, the proportion of Th17
cells notably decreased when treated with rapamycin, whereas a
significant effect on the proportion of Th17 cells was not observed
when treated with tacrolimus. However, there was no difference in
the proportions of Treg cells amongst the cells treated with
various concentrations of either tacrolimus or rapamycin.
Cytokines serve an important role in the regulation
of the Treg/Th17 balance mediated by MSCs. The TGF-β receptor is
expressed on the surface of T cells and TGF-β is an important
cytokine involved in the differentiation and proliferation of
CD4+ T cells into Treg cells. MSCs can secrete TGF-β and
increase the proportion of Treg cells, and TGF-β can amplify the
inducing effect of MSCs on the differentiation of other cells into
Treg cells (18). If the
expression levels of Foxp3 in Treg cells are reduced, MSCs lose
their ability to increase the proportion of Treg following the
addition of a TGF-β receptor antagonist in a co-culture of MSCs and
CD4+ T cells (19).
Treg cells are highly dependent on IL-2. Specific blocking of the
IL-2 receptor in a co-culture of adipose-derived MSCs and
CD4+ T lymphocytes, resulted in the inhibition of IL-2
uptake from the culture medium and this reduced the ability of MSCs
to promote differentiation of Treg cells (20). When excessive exogenous IL-2 was
added to CD4+ T cells, a dose-related decrease in the
proportion of CD4+IL-17+T cells was observed,
whereas the proportion of CD4+IL-17+T cells
was increased following the addition of an IL-2 blocker (21). The present study found that
blocking of TGF-β and IL-2 resulted in a reduced proportion of Treg
cells, and the effect of IL-2 blockers was more prominent.
Similarly, the expression of IL-17 significantly decreased when
cells were treated with an IL-2 blocker. Thus, IL-2 serves a key
role in the regulatory effect of MSCs on the Treg/Th17 balance.
The present study had a number of limitations.
Firstly, the concentration range of immunosuppressants used in the
present study was similar to the concentrations used in the clinic,
therefore, a very narrow range of concentrations was used. This may
partly explain the non-significant differences between different
concentrations of immunosuppressants. Second, the differences in
MSC concentrations between groups were relatively small, resulting
in non-significant results.
In conclusion, BM-MSCs can promote the proliferation
of both Treg and Th17 cells, but increased concentrations of
BM-MSCs can inhibit the proliferation of both of these cells. This
effect was notably influenced by the concentration of MSCs and the
types of immunosuppressants added. In the cell culture, MSCs
themselves secrete certain cytokines, which may serve as a
potential mechanism to influence the immune environment. The future
direction of studies from our lab will focus on how to regulate the
balance of Treg/Th17 in favor of immune tolerance.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Nature Science Foundation of China (grant. no. 81570592) and the
Nature Science Foundation of Tianjin (Tianjin, China; grant. no.
17JCYBJC27500).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WG designed the study. KW performed the experiments,
analyzed the data and wrote the manuscript. YJS and ZLS analyzed
the data and revised the manuscript. BW, CLZ and WL performed the
experiments. All authors contributed to the interpretation of the
study.
Ethics approval and consent to
participate
The present study was approved by Ethics Committee
of Tianjin First Center Hospital (Tianjin, China) and was performed
in accordance with the principles of 3R and those described in
Experimental Animal Welfare Ethics Review Guide of China (GB/T
35892-2018).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mohammadpour H, Pourfathollah AA, Zarif MN
and Tahoori MT: TNF-α modulates the immunosuppressive effects of
MSCs on dendritic cells and T cells. Int Immunopharmacol.
28:1009–1017. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Romano M, Fanelli G, Tan N, Nova-Lamperti
E, McGregor R, Lechler RI, Lombardi G and Scottà C: Expanded
regulatory T cells induce alternatively activated monocytes with a
reduced capacity to expand T helper-17 cells. Front Immunol.
9:16252018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Özdemir AT, Özgül Özdemir RB, Kırmaz C,
Sarıboyacı AE, Ünal Halbutoğlları ZS, Özel C and Karaöz E: The
paracrine immunomodulatory interactions between the human dental
pulp derived mesenchymal stem cells and CD4 T cell subsets. Cell
Immunol. 310:108–115. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Heidt S, Segundo DS, Chadha R and Wood KJ:
The impact of Th17 cells on transplant rejection and the induction
of tolerance. Curr Opin Organ Transplant. 15:456–461. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sun Z, Li T, Wen H, Wang H, Ji W and Ma W:
Immunological effect induced by mesenchymal stem cells in a rat
liver transplantation model. Exp Ther Med. 10:401–406. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gharibi T, Ahmadi M, Seyfizadeh N,
Jadidi-Niaragh F and Yousefi M: Immunomodulatory characteristics of
mesenchymal stem cells and their role in the treatment of multiple
sclerosis. Cell Immunol. 293:113–121. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim N and Cho SG: Overcoming
immunoregulatory plasticity of mesenchymal stem cells for
accelerated clinical applications. Int J Hematol. 103:129–137.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhou Y, Yang X, Zhang H and Jiang J: The
roles of T helper type 17/regulatory T cells in acute rejection
after liver transplantation in rats. Transplantation. 99:1126–1131.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu X, Ren S, Qu X, Ge C, Cheng K and Zhao
RC: Mesenchymal stem cells inhibit Th17 cells differentiation via
IFN-ү-mediated SOCS3 activation. Immunol Res. 61:219–229. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tang J, Yang R, Lv L, Yao A, Pu L, Yin A,
Li X, Yu Y, Nyberg SL and Wang X: Transforming growth
factor-β-expressing mesenchymal stem cells induce local tolerance
in a rat liver transplantation model of acute rejection. Stem
Cells. 34:2681–2692. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rozenberg A, Rezk A, Boivin MN, Darlington
PJ, Nyirenda M, Li R, Jalili F, Winer R, Artsy EA, Uccelli A, et
al: Human mesenchymal stem cells impact Th17 and Th1 responses
through a prostaglandin E2 and myeloid-dependent mechanism. Stem
Cells Transl Med. 5:1506–1514. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rashedi I, Gómez-Aristizábal A, Wang XH,
Viswanathan S and Keating A: TLR3 or TLR4 activation enhances
mesenchymal stromal cell-mediated treg induction via notch
signaling. Stem Cells. 35:265–275. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sioud M, Mobergslien A, Boudabous A and
Fløisand Y: Evidence for the involvement of galectin-3 in
mesenchymal stem cell suppression of allogeneic T-cell
proliferation. Scand. J Immunol. 71:267–274. 2010.
|
|
14
|
Chen W, Huang Y, Han J, Yu L, Li Y, Lu Z,
Li H, Liu Z, Shi C, Duan F and Xiao Y: Immunomodulatory effects of
mesenchymal stromal cells-derived exosome. Immunol Res. 64:831–840.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Qu X, Liu X, Cheng K, Yang R and Zhao RC:
Mesenchymal stem cells inhibit Th17 cell differentiation by IL-10
secretion. Exp Hematol. 40:761–770. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xu G, Wang L, Chen W, Xue F, Bai X, Liang
L, Shen X, Zhang M, Xia D and Liang T: Rapamycin and tacrolimus
differentially modulate acute graft-versus-host disease in rats
after liver transplantation. Liver Transpl. 16:357–363. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hajkova M, Hermankova B, Javorkova E,
Bohacova P, Zajicova A, Holan V and Krulova M: Mesenchymal stem
cells attenuate the adverse effects of immunosuppressive drugs on
distinct T cell subopulations. Stem Cell Rev Rep. 13:104–115. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang JX, Zhang N, Wang HW, Gao P, Yang QP
and Wen QP: CXCR4 receptor overexpression in mesenchymal stem cells
facilitates treatment of acute lung injury in rats. J Biol Chem.
290:1994–2006. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tang RJ, Shen SN, Zhao XY, Nie YZ, Xu YJ,
Ren J, Lv MM, Hou YY and Wang TT: Mesenchymal stem cells-regulated
Treg cells suppress colitis-associated colorectal cancer. Stem Cell
Res Ther. 6:712015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Engela AU, Hoogduijn MJ, Boer K, Litjens
NH, Betjes MG, Weimar W and Baan CC: Human adipose-tissue derived
mesenchymal stem cells induce functional de-novo regulatory T cells
with methylated FOXP3 gene DNA. Clin Exp Immunol. 173:343–354.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Laurence A, Tato CM, Davidson TS, Kanno Y,
Chen Z, Yao Z, Blank RB, Meylan F, Siegel R, Hennighausen L, et al:
Interleukin-2 signaling via STAT5 constrains T helper 17 cell
generation. Immunity. 26:371–381. 2007. View Article : Google Scholar : PubMed/NCBI
|