Introduction
The stratum corneum (SC) is the outermost layer of
the epidermis and is required for barrier function to retain
moisture in the skin and protect the skin from external invasion
(1,2). If the barrier integrity or function
is disrupted, water in the dermis and epidermis will be lost, the
balance of the skin microecology will be disrupted (3), and hostile factors from the external
environment will easily invade the skin, leading to skin ageing and
the occurrence of various skin diseases (1,2,4).
Numerous functions of the skin barrier, including
structural, regulatory, hygroscopic and signalling functions, rely
on epidermal proteins (5,6). Keratins, cytoskeletal proteins of the
skin, are responsible for constituting stable keratin cytoskeletons
that maintain stable intercellular adhesion and cell rigidity
(6,7). Filaggrin (FLG) and FLG2 are critical
for bundling keratins to form dense keratin filaments and attain
flat squames in the cornified layer (8). Caspase-14 is involved in processing
FLG precursors to produce natural moisturizing factors (NMFs)
(9). During cornification,
loricrin (LOR), S100 proteins, involucrin, the late cornified
envelope (CE) family of proteins and hornerin (HRNR) are
cross-linked by transglutaminase (TGM) to form a strong,
indissoluble CE, which functions as a barrier for the skin against
the external environment (2,8). At
sites of attachment to the hemidesmosome and desmosome, the
junction plakoglobin (JUP), plectin, desmoplakin (DSP), desmoglein1
(DSG1), desmocollin1 (DSC1) and corneodesmosin (CDSN) proteins are
responsible for maintaining the mechanical integrity of the
epidermal barrier by binding the intermediate filaments (IFs) to
the cornified cells (10–13). Abnormalities of these skin
barrier-related proteins can lead to disruptions in skin barrier
function and have been verified to be associated with multiple skin
diseases, including atopic dermatitis (AD) (1,2),
ichthyosis (8) and bullous disease
(14).
The present study describes a non-invasive,
quantitative method to analyse epidermal proteins in healthy
individuals using liquid chromatography-tandem mass spectrometry
(LC-MS/MS) analysis, in combination with the most convenient
sampling method: tape stripping. LC-MS/MS is a high-throughput
quantitative technique that can be used to analyse large numbers of
proteins in human tissues (15).
In the present study, LC-MS/MS analysis was performed on an EASY
1200 nanoflow liquid chromatography (EASY-nLC™ 1200) instrument
coupled to a Q Exactive™ HF-X mass spectrometer. Q Exactive HF-X is
the most recently developed MS instrument in the benchtop Orbitrap
series, with a novel peak selection algorithm and a bright ion
source that can efficiently capture MS/MS data above 40 Hz at a
resolution of 7500 (16). MaxLFQ
was also used, a novel intensity determination and normalization
procedure for label-free quantification based on the intensities of
the precursor ion signals. In addition, the label-free
quantification method was coupled with accurate quantitative
standard statistical methods to quantify thousands of proteins
without any labelling steps, and reduce undesirable biases
associated with the additional steps (17).
Materials and methods
Study participants
Skin samples were collected from two healthy Chinese
females (aged 25–35 years with a mean age of 29 years) from Anhui
Medical University on September 2, 2017. The study was performed
between September 2017 and January 2018. Individuals whose history
indicated tape allergies, skin diseases or other systemic diseases
involving the skin were excluded from the study. No topical
emollient or other cosmetics were used 24 h before the experiment.
This study was conducted in accordance with the recommendations of
the Medical Ethics Committee of Anhui Medical University, and
written informed consent was obtained from all included
subjects.
Materials
Sodium dodecyl sulfate (SDS), sodium phosphate, 67%
ethanol, dithiothreitol, iodoacetamide, ammonium bicarbonate,
trifluoroacetic acid (TFA; ≥99.0% purity), and mass acetonitrile
(ACN; Sigma-Aldrich; Merck KGaA). Sequencing-grade modified trypsin
was obtained from Promega Corporation. 3M™ Empore™ C18 47 mm
extraction discs, Model 2215, Pierce™ C18 tips, a 10 µl bed, a
Thermomixer unit (MS-100), CentriVap, rotor, cold trap, vacuum
pump, glass bottle, translucent solvent-absorbing trap,
ammonia-absorbing trap stuffing and vacuum tube kit (Thermo Fisher
Scientific, Inc.). A 10 K 1.5 ml ultrafiltration device (flat base)
was obtained from Pall Corporation. The Q Exactive HF-X instrument
and the EASY-nLC 1200 system were obtained from Thermo Fisher
Scientific, Inc. Unless stated otherwise, all materials were
purchased from Thermo Fisher Scientific, Inc.
Sample preparation
The volar forearm skin was cleaned by gentle
swabbing with a sterile cotton ball, after which 3M medical
adhesive tape was used to remove the skin layers from the volar
forearm area. All skin samples were sequentially collected from the
same site. The procedure was conducted by the same technician on
all volunteers during the study to minimize variations.
Protein extraction, digestion and
clean up
Each tape was transferred (adhesive side towards the
centre) to a sterile 15 ml plastic tube containing a 2% SDS
solution in 0.1 mol/l sodium phosphate (pH 7.8; buffer A). The
tubes were incubated at room temperature for 2 days. Then, cells
that were dissociated from the tape accumulated at the bottom of
the tubes. Afterwards, the cells were removed by pipetting, rinsed
twice with 600 µl of buffer A solution, centrifuged at 8,000 × g
for 3 min at 4°C, and then resuspended in 300 µl of buffer A
solution. Next, 15.8 µl of dithiothreitol was mixed into the cell
suspension. After incubation at 37°C for 2 h, 35 µl of
iodoacetamide was added. After incubation at 25°C in the dark for
40 min, 900 µl of 67% ethanol was added to precipitate the
proteins, and then the protein pellets were washed twice with 67%
ethanol. After centrifugation at 10,000 × g for 5 min at 4°C, the
protein was digested at 37°C in 300 µl of ammonium bicarbonate/10%
ACN by adding 20 mg of trypsin every 24 h until the enzymatic
digestion was completed at ~72 h. The digest was centrifuged at
8,000 × g for 2 min at 4°C, 10% TFA was added to the supernatant,
and the solution was dried by centrifuging at 8,000 × g for 20 min
at 4°C. Then, the residue was re-dissolved, desalted and
concentrated to obtain the peptide mixture.
LC-MS/MS analysis
The resulting peptide mixture was subjected to
LC-MS/MS analysis on an EASY-nLC 1200 system coupled to a Q
Exactive HF-X mass spectrometer (Thermo Fisher Scientific, Inc.);
each peptide was analysed via LC-MS/MS in three replicates. The
samples were loaded onto a C18 µ-precolumn and capillary analytical
C18 column at a flow rate of 0.3 µl/min with a pressure of 860 bar
(12,470 psi). The gradient of 80% ACN/ 0.2% TFA increased from 5 to
100% over a period of 60 min. Eluted peptides were analysed using
the Q Exactive HF-X mass spectrometer with a nanoelectrospray
ionization source and calibrated using tuned instrument control
software. The mass spectrometer was operated in positive
ionization. The tuned spray voltage was set to 2 kV, the maximum
spray current was set to 50, the S-Lens RF level was set to 60, and
the capillary temperature was 275°C. In full MS scans, the
resolution was set to 60,000 at a range of 350–1500 m/z, and the
AGC target was 3e6 with a maximum IT of 20 ms. In subsequent scans,
the fragments were analysed at a resolution of 15,000, and the AGC
target was set to 5e4 with an IT of 45 msec. An isolation window of
1.6 m/z and one microscan were used to collect suitable tandem mass
spectra; the scan range was set to 200–2,000 m/z. The AGC target
value for fragment spectra was set to 1e3, and the intensity
threshold was maintained at 2.2e4.
Data analysis
The raw data generated from the LC-MS/MS analysis
and protein identification and quantification were processed using
Proteome Discoverer™ software 2.2 (Thermo Fisher Scientific, Inc.).
The data were searched against the Human Universal Protein Resource
(UniProt; version June 2017) (18)
to match with known proteins. MaxQuant v1.5.8.3 software (19) was used to conduct the label-free
database search with the uniqueness degree of ‘razor plus unique
peptides’. The MaxLFQ algorithm (17) was adopted to obtain the final
results for each sample, and the LFQ intensities were determined as
the full peak volume. To obtain the minimal overall proteome
variation, the protein quantities were determined through a global
normalization procedure after summing up the intensities with
normalization factors as free variables (17).
Results
In the present study, three replicates of each
peptide were analysed by LC-MS/MS and the MaxLFQ algorithm. Using
this method, 1,157 proteins included in UniProt were quantified. A
total of 50 skin barrier-related proteins were detected in all skin
samples with no significant difference in LFQ intensities. After
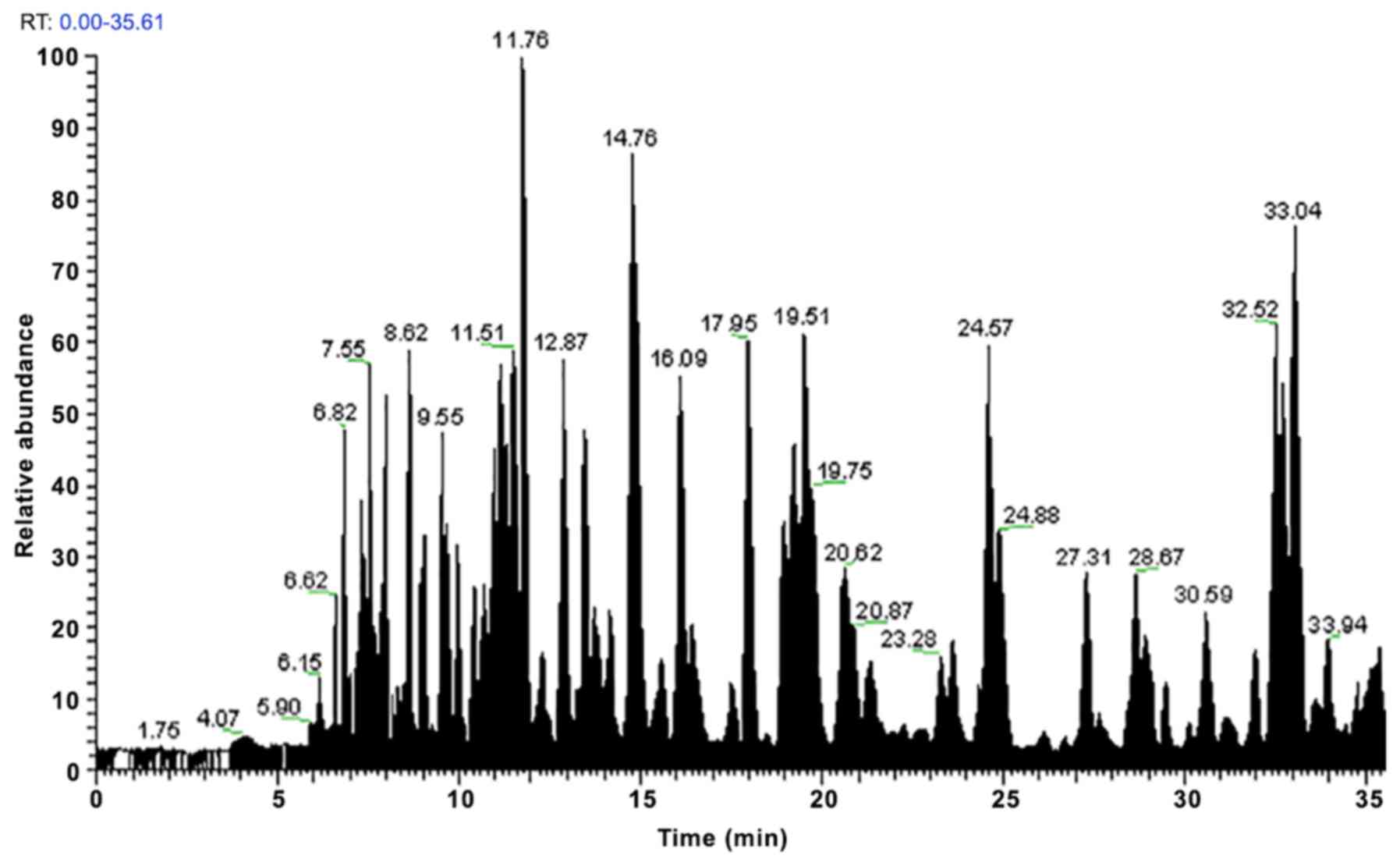
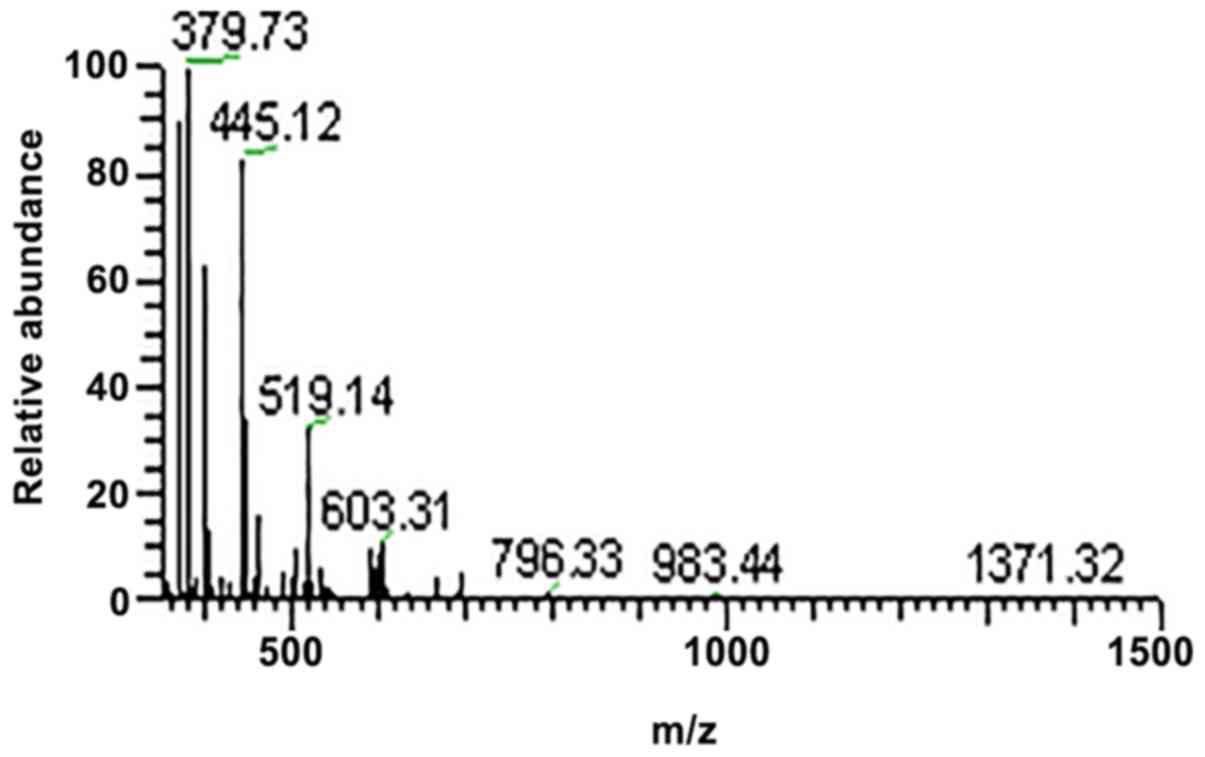
the peptides were subjected to LC-MS/MS analysis, the protein peaks
were obtained (Figs. 1–4). The observed abundances of the
proteins are depicted as log2 median LFQ intensities, which are the
median LFQ intensity values of the three replicates of each sample
(Table I). These proteins were
then divided into 12 groups according to their specific properties
(Table I).
 | Table I.Log2 median LFO intensities of 50
skin-barrier-related proteins detected in the normal forearm
skin. |
Table I.
Log2 median LFO intensities of 50
skin-barrier-related proteins detected in the normal forearm
skin.
|
|
|
| LFQ intensities
(medians) |
|---|
|
|
|
|
|
|---|
| Majority protein
IDs | Protein names | Gene names | Sample 1 | Sample 2 |
|---|
| Keratins |
|
|
|
|
|
P13645 | Keratin, type I
cytoskeletal 10 | KRT10 | 36.95459 | 36.93528 |
|
P02533 | Keratin, type I
cytoskeletal 14 | KRT14 | 31.31441 | 31.5455 |
|
P08779 | Keratin, type I
cytoskeletal 16 | KRT16 | 27.96714 | 28.47433 |
|
Q04695 | Keratin, type I
cytoskeletal 17 | KRT17 | 31.9048 | 32.24141 |
|
P35527 | Keratin, type I
cytoskeletal 9 | KRT9 | 32.05689 | 32.9607 |
|
Q9C075 | Keratin, type I
cytoskeletal 23 | KRT23 | 26.70504 | 26.67815 |
|
Q15323 | Keratin, type I
cuticular Ha1 | KRT31 | 27.02739 | 26.21795 |
|
Q14525 | Keratin, type I
cuticular Ha3-II | KRT33B | 24.78502 | 24.83408 |
|
O76011 | Keratin, type I
cuticular Ha4 | KRT34 | 23.76223 | 24.07802 |
|
P04264 | Keratin, type II
cytoskeletal 1 | KRT1 | 36.64541 | 36.80581 |
|
P35908 | Keratin, type II
cytoskeletal 2 epidermal | KRT2 | 37.13996 | 36.82632 |
|
P13647 | Keratin, type II
cytoskeletal 5 | KRT5 | 31.75963 | 32.1306 |
|
P02538 | Keratin, type II
cytoskeletal 6A | KRT6A | 26.15018 | 26.35316 |
|
P04259 | Keratin, type II
cytoskeletal 6B | KRT6B | 29.9709 | 30.36661 |
|
Q7Z794 | Keratin, type II
cytoskeletal 1b | KRT77 | 30.76232 | 30.73876 |
|
Q8N1N4 | Keratin, type II
cytoskeletal 78 | KRT78 | 29.05447 | 28.91809 |
|
Q6KB66 | Keratin, type II
cytoskeletal 80 | KRT80 | 28.45615 | 28.4225 |
|
O43790 | Keratin, type II
cuticular Hb6 | KRT86 | 27.69677 | 26.73221 |
| CE
constituents |
|
|
|
|
|
Q5D862 | Filaggrin | FLG | 29.40947 | 29.98427 |
|
P20930 | Filaggrin-2 | FLG2 | 24.93454 | 27.0202 |
|
P23490 | Loricrin | LOR | 26.81913 | 27.16087 |
|
Q86YZ3 | Hornerin | HRNR | 27.98104 | 28.781 |
|
Q5T750 | Skin-specific
protein 32 | XP32 | 29.76257 | 29.93143 |
| CE processing
enzymes |
|
|
|
|
|
P22735 | Transglutaminase
1 | TGM1 | 27.61105 | 26.87912 |
|
Q08188 | Transglutaminase
3 | TGM3 | 27.36745 | 27.56856 |
|
O75342 | Arachidonate
12-lipoxygenase, 12R-type | ALOX12B | 27.79241 | 27.72338 |
|
Q9BYJ1 | Hydroperoxide
isomerase ALOXE3 | ALOXE3 | 25.4928 | 25.24318 |
| Calcium binding
proteins |
|
|
|
|
|
Q9HCY8 | Protein
S100-A14 | S100A14 | 25.99766 | 25.62522 |
|
Q96FQ6 | Protein
S100-A16 | S100A16 | 25.70376 | 25.82092 |
| Enzymes
contributing to |
|
|
|
|
| NMFs
generation |
|
|
|
|
|
P31944 | Caspase-14 | CASP14 | 25.40636 | 25.44815 |
|
Q13867 | Bleomycin
hydrolase | BLMH | 25.61336 | 25.63619 |
|
P05089 | Arginase-1 | ARG1 | 25.52211 | 25.33279 |
|
P42357 | Histidine
ammonia-lyase | HAL | 26.91957 | 24.17463 |
| Protease
inhibitors |
|
|
|
|
|
Q96P63 | Serpin B12 | SERPINB12 | 27.14705 | 26.9812 |
|
A8K2U0 |
α-2-macroglobulin-like protein 1 | A2ML1 | 25.62439 | 24.16 |
| Cornedesmosome |
|
|
|
|
| constituents |
|
P14923 | Junction
plakoglobin | JUP | 24.98385 | 25.92189 |
|
Q15149 | Plectin | PLEC | 23.68239 | 22.36937 |
|
P15924 | Desmoplakin | DSP | 26.95593 | 27.4182 |
|
Q02413 | Desmoglein-1 | DSG1 | 29.14523 | 29.43571 |
|
Q08554 | Desmocollin-1 | DSC1 | 27.99108 | 28.31514 |
|
Q15517 | Corneodesmosin | CDSN | 25.82845 | 25.97169 |
| Annexin |
|
|
|
|
|
P07355;A6NMY6 | Annexin A2 | ANXA2 | 27.51893 | 27.104 |
| Substance
metabolism |
|
|
|
|
|
proteins/enzymes |
|
|
|
|
|
P04406 |
Glyceraldehyde-3-phosphate | GAPDH | 25.89973 | 24.53011 |
|
| dehydrogenase |
|
|
|
|
P25311 |
Zinc-α-2-glycoprotein | AZGP1 | 25.35336 | 25.00945 |
| Signal
transduction |
|
|
|
|
|
proteins/enzymes |
|
|
|
|
|
Q96QA5 | Gasdermin-A | GSDMA | 25.79793 | 26.26551 |
|
Q5T749 | Keratinocyte
proline-rich protein | KPRP | 30.88103 | 30.98854 |
|
Q16610 | Extracellular
matrix protein 1 | ECM1 | 24.89957 | 24.52139 |
| Proteins involved
in |
|
|
|
|
| REDOX
reactions |
|
|
|
|
|
P10599 | Thioredoxin | TXN | 26.88925 | 26.86361 |
|
P04040 | Catalase | CAT | 25.53703 | 25.25785 |
| Actin binding
proteins |
|
|
|
|
|
Q09666 | Neuroblast
differentiation-associated protein AHNAK | AHNAK | 24.43657 | 24.1404 |
Keratins
A total of 18 keratins were detected in the volar
forearm skin: K9, K10, K14, K16, K17, K23, K31, K33B, K34 (type I),
K1, K2, K5, K6A, K6B, K77, K78, K80 and K86 (type II). The LFQ
intensities of K1, K2 and K10 were relatively higher than those of
the other keratins (Table I).
CE constituents and processing
enzymes
The proteins that constitute the CE, including FLG,
FLG2, LOR, HRNR and XP32, were detected in this study. Four enzymes
related to CE processing were also detected, including TGM1, TGM3,
arachidonate 12-lipoxygenase (ALOX12B) and the hydroperoxide
isomerase ALOXE3 (ALOXE3).
Calcium-binding proteins
In this study, the expression of two calcium-binding
proteins was identified (S100A14 and S100A16).
Enzymes contributing to NMF
generation
The expression of bleomycin hydrolase, caspase-14,
arginase1 and histidine ammonia lyase was detected; these
contribute to the generation of NMFs.
Protease inhibitors
Two protease inhibitors (serpin B12 and
α-2-macroglobulin-like protein 1) were observed in the SC obtained
from the volunteers.
Proteins related to SC cohesion
In the present study, 6 proteins were detected,
plectin, JUP, DSP, DSG1, DSC1 and CDSN, that are responsible for
reinforcing the cohesion of the SC.
Annexin
Annexin A2 was detected in this study.
Substance metabolism regulator
The expression of GAPDH and zinc-α-2-glycoprotein
(ZAG), which participate in regulating substance metabolism, were
also detected.
Signal transduction proteins
Gasdermin-A, keratinocyte proline-rich protein
(KPRP) and extracellular matrix protein 1 (ECM1) were identified in
this study.
Antioxidant related
proteins/enzymes
The expression of thioredoxin and catalase was
observed, these are both related to antioxidant activity.
Actin-binding protein
The expression of the neuroblast
differentiation-associated protein AHNAK (AHNAK) was also
detected.
Discussion
As a barrier between the external and internal
environment of the human body, the major function of our epidermis
is to protect us from the hostile external invasion, such as
ultraviolet radiation (UVR) and air pollutants (4,20,21).
The main cell type in the epidermis is the keratinocyte, which
expresses cytoskeletal proteins called keratins. The keratin family
is a primary subclass of the IF superfamily, contains 54 proteins
and is subdivided into 28 type I (K9-K40) and 26 type II (K1-K8 and
K71-K86) IF proteins. Numerous members of the keratin family are
widely expressed in the epithelium, so alterations in these
keratins are predicted to be the underlying causes of several
epithelial diseases (22–24). The expression of 9 type I and 9
type II keratins were detected in the volar forearm skin (Table I). These proteins have various
functions, including stabilizing cytoskeletal elements, modulating
cellular metabolism, regulating cellular differentiation and
proliferation, as well as mediating inflammatory pathways (5–7,25).
The keratin pair K5/K14 is expressed in the basal layer, and
mutations in K5 or K14 cause 75% of the basal subtypes of
epidermolysis bullosa simplex (EBS) (14,26,27)
and a rare keratin disease known as Dowling-Degos disease, as well
as its variant named Galli-Galli disease (14,28).
In the initial stage of terminal differentiation, the keratin pair
K1/K10 replaces the K5/K14 pair in the suprabasal layer. One of the
earliest proteins expressed during cornification, the K1/K10 pair
is a significant scaffold protein that sequentially directs the
deposition and cross-linking of CE proteins (7,24).
If the epidermal barrier is disrupted, the K1/K10 pair is consumed
to repair the injured tissue (29,30).
In addition, mutations in K1/K10 lead to ichthyosis, epidermolytic
hyperkeratosis and epidermolytic palmoplantar keratoderma (14,23).
The hyperproliferation-related keratins, K6a, K6b, K16 and K17,
which have specialized roles in the inflammatory response and wound
healing, are biomarkers of psoriasis (31,32)
and cancers (33,34). When the epidermis is injured,
nuclear factor erythroid-derived 2-related factor 2 promotes the
proliferation of human keratinocytes by upregulating the expression
of K6, K16 and K17 (31,32). The expression of K9 was also
detected, which plays important roles in the response to stress and
contributes to enhancing mechanical strength (35).
In the SC, FLG and keratins interact with each other
to form a dense keratin fibre tract that serves as a strong
supportive scaffold for the construction of the CE, which is the
basis of the defensive epidermal barrier (8). In the present study, the expression
of FLG and FLG2 was detected (Table
I). FLG bundles keratin IFs to attain a flattened shape of the
cell during the terminal differentiation of the epidermis and plays
an important role in SC hydration. The dysfunction of FLG can lead
to AD (1,2,8,36)
and ichthyosis vulgaris (37).
FLG2 is essential for maintaining intercellular adhesion in the
cornified layers and provides proper integrity and mechanical
strength to the SC. Mutations in FLG2 can cause peeling skin
syndrome type A, which is characterized by a superficial detachment
of the epidermal cornified layers (38). The expression of caspase-14 and
bleomycin hydrolase (Table I),
which can hydrolyse FLG monomers into free amino acids in the
cornified cell, was also detected. Then, NMFs, an important
component required for SC hydration, are produced. The high
concentration of these hydrolysed amino acids helps to retain the
moisture and elasticity of the cornified layer. Therefore,
abnormalities in caspase-14 and bleomycin hydrolase may affect the
decomposition of FLG in the SC, leading to reduced levels of NMFs
and the subsequent loss of barrier moisture function (8,9,36).
This study also detected two other enzymes, arginase-1 and
histidine ammonia lyase (Table I),
which also contribute to the production of NMFs, urea and urocanic
acid (36).
The CE is further reinforced by a series of
structural proteins, including LOR, S100 proteins and HRNR, which
are cross-linked by TGMs. LOR, a major component of the CE,
constitutes 70% of the total protein mass (8). The expression of LOR was detected in
the SC of healthy subjects. Mutations in LOR may disrupt
keratinocyte differentiation, affect the formation and maturation
of CE, and aggravate skin barrier disorders, such as LOR
keratoderma, palmoplantar keratoderma and psoriasis (39). S100 proteins belong to the
calcium-binding protein family and are characterized by their
EF-hand calcium-binding domains, which play an important role in
keratinocyte differentiation and function as antimicrobial peptides
in the epidermis that protect the skin from microbial invasion
(40). Researchers have found that
the expression level of S100A in skin lesions of psoriasis patients
is higher than that of healthy individuals and is directly
proportional to the severity of psoriasis (41). In the present study, the expression
of HRNR was detected in the volar forearm skin. HRNR, FLG and FLG-2
share amino acid homology outside the S100 domain and thus have
similar structures and numerous common functions in barrier
formation and maintenance. Similar to FLG expression, HRNR
expression was reduced in the epidermis of patients with AD, which
might contribute to the impaired epidermal barrier in patients with
AD (42,43).
In the present study, the expression of TGM1, TGM3
and two lipoxygenases (ALOX12B and ALOXE3) were detected in the
healthy epidermis (Table I). TGMs
provide a direct link between the proteins and lipid components of
the CE and esterify ceramides to increase the hydrophobicity of the
CE. Therefore, the dysfunction of TGM1 may result in an
inappropriate protein scaffold for deposition of the lipid barrier.
TGM3 is expressed in terminally differentiated keratinocytes and is
involved in the cornification process (8). Notably, ALOX12B and ALOXE3 oxidize
the linoleoyl moiety in omega-hydroxy ceramides, which are crucial
for producing the intermolecular organization necessary to maintain
skin barrier function (44).
Some proteins responsible for SC cohesion were also
identified, including JUP, plectin, DSP, DSG1, DSC1 and CDSN
(Table I). As a major component of
the desmosome, JUP is responsible for cell-cell adhesion (45). Plectin plays a vital role in
binding to IFs and protecting cells from osmotic stress (46). At sites of attachment to the
hemidesmosome, the keratin filaments of basal keratinocytes are
less tightly bundled in the absence of plectin, which may result in
the occurrence of EBS (10,46,47).
DSP contributes to maintaining the mechanical integrity of the
epidermal barrier by linking the IFs to the plasma membrane at
sites of attachment to the desmosome. The absence of DSP in the
epidermis can lead to the collapse of the keratin cytoskeleton and
weaken the intercellular adhesion (11). By analogy to DSP, DSG1, DSC1 and
CDSN are cross-linked to the CE to form corneodesmosomes, which
bind to cornified cells and further reinforce the barrier function
(12). The lack of DSG1 can cause
pemphigus vulgaris, indicating important roles for DSG1 in
maintaining intercellular adhesion and epidermal integrity
(13).
Serpin B12, a member of the intracellular serine
protease inhibitors, was highly expressed in the normal forearm
skin in this study. It plays a cytoprotective role in skin barrier
function and has been shown to be a therapeutic target for multiple
disease processes (48). Another
wide-range protease inhibitor, α-2-macroglobulin-like protein 1,
may be implicated in maintaining the homeostasis of the epidermal
barrier as well as the immune defence process (49). As such, α-2-macroglobulin-like
protein 1 is considered a target antigen of paraneoplastic
pemphigus (50).
In this study, the expression of annexin A2 was
observed, this protein functions in regulating fibroblast
proliferation and migration. It is hypothesized that Annexin A2 is
involved in skin keloid formation and may be a potential target for
therapeutic interventions of keloid lesions (51).
The expression of GAPDH and ZAG was also detected;
these are involved in substance metabolism. GAPDH not only
participants in glycolytic metabolism but also plays a critical
role in apoptosis and multiple cellular functions (52). Recently, research has shown that
GAPDH may be a prognostic marker and therapeutic target for
patients with cutaneous melanoma (53). As an adipokine, ZAG can modulate
lipid metabolism, immune responses and skin barrier function in AD,
and is expected to be a biomarker and therapeutic target of AD
(54).
ECM1 consists of a signal peptide and four
functional domains, suggesting that this protein has signal
transduction functions (55).
Another two signal transduction proteins, Gasdermin-A and KRRP,
were also detected in this study. Gasdermin-A has multiple
functions, including forming pores in the cell membrane,
transmitting inflammatory signals and inducing apoptosis and
inflammation (56). The definite
function of KRRP is not yet fully understood. Recently, Suga et
al (57) found that a decrease
in KPRP can lead to dysfunction of the skin barrier in AD, which
revealed that KPRP may enhance the immune responses in AD and could
be a new target for therapeutic interventions of AD.
In this study, the expression of thioredoxin and
catalase were also identified, which are involved in the
antioxidant system. The skin is constantly exposed to oxidative
stress generated by external factors including UVR, which may lead
to premature senescence (4).
Thioredoxin plays a pivotal role in antioxidant and antiapoptotic
defence in the human epidermis (58,59).
Catalase is one of the most important antioxidant enzymes in the
process of skin ageing, and its decrease can lead to accelerated
ageing of human skin (60). The
expression of AHNAK was also detected, which is downregulated in
melanoma cells and may be a prognostic marker of melanoma (61).
In summary, the present study explored a
non-invasive and proteome-wide method for the quantification of
skin proteins. These results provided an objective metric for
future studies on monitoring changes in skin protein levels in the
context of skin ageing and skin diseases. Compared to the commonly
invasive skin biopsy and traditional methods of protein
identification, the tape stripping method combined with LC-MS/MS
analysis and the MaxLFQ algorithm used in the present study has the
advantages of simple application, no surgical trauma, no extra
labelling steps, no requirement of a reference standard, high
throughput, high sensitivity and high accuracy. These properties
may allow this approach to be used in future studies of skin
diseases related to barrier destruction by monitoring changes in
the levels of epidermal proteins, which may provide insight into
the diagnosis, prognosis and development of new targets for
therapeutic intervention of these skin diseases in the future. As a
methodological exploration, the present study still had some
limitations. First, the small sample size was not representative
enough, requiring more detailed studies in the future. Second,
there were inevitably errors in the process of sample collection
and processing, although everything was done to minimize the
effects of human factors.
Acknowledgements
Not applicable.
Funding
The current study was supported by a grant from the
Science & Technology Action Plans for the Prevention and
Treatment of Major Diseases sponsored by National Health and Family
Planning Commission of the People's Republic of China (grant no.
2017ZX-01E-002).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
All authors participated in the design and
interpretation of the studies and review of the paper. JZ performed
the experiments. ML and JZ performed the data analysis. ML, YW and
CX interpreted and collected the data. JM, SX and XW performed the
experiments and revised the manuscript. SY, JG and XZ designed and
guided the study. The paper was written by ML. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was conducted in accordance with
the recommendations of the Medical Ethics Committee of Anhui
Medical University (reference no. PJ2016-03-02), and written
informed consent was obtained from all included subjects.
Patient consent for publication
Consent for publication was obtained from all of the
patients.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Egawa G and Kabashima K: Multifactorial
skin barrier deficiency and atopic dermatitis: Essential topics to
prevent the atopic march. J Allergy Clin Immunol. 138:350–358.e1.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Segre JA: Epidermal barrier formation and
recovery in skin disorders. J Clin Invest. 116:1150–1158. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhai W, Huang Y, Zhang X, Fei W, Chang Y,
Cheng S, Zhou Y, Gao J, Tang X, Zhang X and Yang S: Profile of the
skin microbiota in a healthy Chinese population. J Dermatol.
45:1289–1300. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bocheva G, Slominski RM and Slominski AT:
Neuroendocrine aspects of skin aging. Int J Mol Sci. 20:E27982019.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim S and Coulombe PA: Intermediate
filament scaffolds fulfill mechanical, organizational, and
signaling functions in the cytoplasm. Genes Dev. 21:1581–1597.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Magin TM, Vijayaraj P and Leube RE:
Structural and regulatory functions of keratins. Exp Cell Res.
313:2021–2032. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Homberg M and Magin TM: Beyond
expectations: Novel insights into epidermal keratin function and
regulation. Int Rev Cell Mol Biol. 311:265–306. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Candi E, Schmidt R and Melino G: The
cornified envelope: A model of cell death in the skin. Nat Rev Mol
Cell Biol. 6:328–340. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hvid M, Johansen C, Deleuran B, Kemp K,
Deleuran M and Vestergaard C: Regulation of caspase 14 expression
in keratinocytes by inflammatory cytokines-a possible link between
reduced skin barrier function and inflammation. Exp Dermatol.
20:633–636. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Osmanagic-Myers S, Gregor M, Walko G,
Burgstaller G, Reipert S and Wiche G: Plectin-controlled keratin
cytoarchitecture affects MAP kinases involved in cellular stress
response and migration. J Cell Biol. 174:557–568. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vasioukhin V, Bowers E, Bauer C,
Degenstein L and Fuchs E: Desmoplakin is essential in epidermal
sheet formation. Nat Cell Biol. 3:1076–1085. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kitajima Y: Regulation and impairments of
dynamic desmosome and corneodesmosome remodeling. Eur J Dermatol.
Apr 30–2013.(Epub ahead of print).
|
|
13
|
Koga H, Tsuruta D, Ohyama B, Ishii N,
Hamada T, Ohata C, Furumura M and Hashimoto T: Desmoglein 3, its
pathogenecity and a possibility for therapeutic target in pemphigus
vulgaris. Expert Opin Ther Targets. 17:293–306. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Szeverenyi I, Cassidy AJ, Chung CW, et al:
The Human Intermediate Filament Database: comprehensive information
on a gene family involved in many human diseases. Hum Mutat.
29:351–360. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang B, Wang J, Wang X, Zhu J, Liu Q, Shi
Z, Chambers MC, Zimmerman LJ, Shaddox KF, Kim S, et al:
Proteogenomic characterization of human colon and rectal cancer.
Nature. 513:382–387. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kelstrup CD, Bekker-Jensen DB, Arrey TN,
Hogrebe A, Harder A and Olsen JV: Performance evaluation of the Q
exactive HF-X for shotgun proteomics. J Proteome Res. 17:727–738.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cox J, Hein MY, Luber CA, Paron I, Nagaraj
N and Mann M: Accurate proteome-wide label-free quantification by
delayed normalization and maximal peptide ratio extraction, termed
MaxLFQ. Mol Cell Proteomics. 13:2513–2526. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
The UniProt Consortium: UniProt: a
worldwide hub of protein knowledge. Nucleic Acids Res. 47:D506–515.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cox J and Mann M: MaxQuant enables high
peptide identification rates, individualized p.p.b.-range mass
accuracies and proteome-wide protein quantification. Nat
Biotechnol. 26:1367–1372. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Slominski AT, Zmijewski MA, Skobowiat C,
Zbytek B, Slominski RM and Steketee JD: Sensing the environment:
Regulation of local and global homeostasis by the skin's
neuroendocrine system. Adv Anat Embryol Cell Biol. 212(v, vii):
1–115. 2012. View Article : Google Scholar
|
|
21
|
Slominski AT, Zmijewski MA, Plonka PM,
Szaflarski JP and Paus R: How UV light touches the brain and
endocrine system through skin, and why. Endocrinology.
159:1992–2007. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pan X, Hobbs RP and Coulombe PA: The
expanding significance of keratin intermediate filaments in normal
and diseased epithelia. Curr Opin Cell Biol. 25:47–56. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Toivola DM, Boor P, Alam C and Strnad P:
Keratins in health and disease. Curr Opin Cell Biol. 32:73–81.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fuchs E: Keratins and the skin. Annu Rev
Cell Dev Biol. 11:123–153. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Arul S, Dayalan H, Jegadeesan M and
Damodharan P: Induction of differentiation in psoriatic
keratinocytes by propylthiouracil and fructose. BBA Clin. 6:82–86.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bolling MC, Lemmink HH, Jansen GH and
Jonkman MF: Mutations in KRT5 and KRT14 cause epidermolysis bullosa
simplex in 75% of the patients. Br J Dermatol. 164:637–644.
2011.PubMed/NCBI
|
|
27
|
Haines RL and Lane EB: Keratins and
disease at a glance. J Cell Sci. 125:3923–3928. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hanneken S, Rütten A, Pasternack SM,
Eigelshoven S, El Shabrawi-Caelen L, Wenzel J, Braun-Falco M,
Ruzicka T, Nöthen MM, Kruse R and Betz RC: Systematic mutation
screening of KRT5 supports the hypothesis that Galli-Galli disease
is a variant of Dowling-Degos disease. Br J Dermatol. 163:197–200.
2010.PubMed/NCBI
|
|
29
|
Kim S and Wong P: A keratin cytoskeletal
protein regulates protein synthesis and epithelial cell growth.
Nature. 441:362–365. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Reichelt J and Magin TM:
Hyperproliferation, induction of c-Myc and 14-3-3 sigma, but no
cell fragility in keratin-10-null mice. J Cell Sci. 115:2639–2650.
2002.PubMed/NCBI
|
|
31
|
Yang L, Fan X, Cui T, Dang E and Wang G:
Nrf2 promotes keratinocyte proliferation in psoriasis through
up-regulation of Keratin 6, Keratin 16 and Keratin 17. J Invest
Dermatol. 137:2168–2176. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jin L and Wang G: Keratin 17: A critical
player in the pathogenesis of psoriasis. Med Res Rev. 34:438–454.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Stoler A, Duvic M and Fuchs E: Unusual
patterns of keratin expression in the overlying epidermis of
patients with dermatofibromas: Biochemical alterations in the
epidermis as a consequence of dermal tumors. J Invest Dermatol.
93:728–738. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yoshikawa K, Katagata Y and Kondo S:
Relative amounts of keratin 17 are higher than those of keratin 16
in hair-follicle-derived tumors in comparison with nonfollicular
epithelial skin tumors. J Invest Dermatol. 104:396–400. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Swensson O, Langbein L, McMillan JR,
Stevens HP, Leigh IM, McLean WH, Lane EB and Eady RA: Specialized
keratin expression pattern in human ridged skin as an adaptation to
high physical stress. Br J Dermatol. 139:767–775. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
McAleer MA, Jakasa I, Raj N, O'Donnell
CPF, Lane ME, Rawlings AV, Voegeli R, McLean WHI, Kezic S and
Irvine AD: Early-life regional and temporal variation in
filaggrin-derived natural moisturizing factor, filaggrin-processing
enzyme activity, corneocyte phenotypes and plasmin activity:
Implications for atopic dermatitis. Br J Dermatol. 179:431–441.
2018.PubMed/NCBI
|
|
37
|
Thyssen JP, Godoy-Gijon E and Elias PM:
Ichthyosis vulgaris: The filaggrin mutation disease. Br J Dermatol.
168:1155–1166. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mohamad J, Sarig O, Godsel LM, Peled A,
Malchin N, Bochner R, Vodo D, Rabinowitz T, Pavlovsky M, Taiber S,
et al: Filaggrin 2 deficiency results in abnormal cell-cell
adhesion in the cornified cell layers and causes peeling skin
syndrome type A. J Invest Dermatol. 138:1736–1743. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kim BE, Howell MD, Guttman-Yassky E,
Gilleaudeau PM, Cardinale IR, Boguniewicz M, Krueger JG and Leung
DY: TNF-α downregulates filaggrin and loricrin through c-Jun
N-terminal kinase: Role for TNF-α antagonists to improve skin
barrier. J Invest Dermatol. 131:1272–1279. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Donato R: Functional roles of S100
proteins, calcium-binding proteins of the EF-hand type. Biochim
Biophys Acta. 1450:191–231. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lee Y, Jang S, Min JK, Lee K, Sohn KC, Lim
JS, Im M, Lee HE, Seo YJ, Kim CD and Lee JH: S100A8 and S100A9 are
messengers in the crosstalk between epidermis and dermis modulating
a psoriatic milieu in human skin. Biochem Biophys Res Commun.
423:647–653. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wu Z, Meyer-Hoffert U, Reithmayer K, Paus
R, Hansmann B, He Y, Bartels J, Gläser R, Harder J and Schröder JM:
Highly complex peptide aggregates of the S100 fused-type protein
hornerin are present in human skin. J Invest Dermatol.
129:1446–1458. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Henry J, Hsu CY, Haftek M, Nachat R, de
Koning HD, Gardinal-Galera I, Hitomi K, Balica S, Jean-Decoster C,
Schmitt AM, et al: Hornerin is a component of the epidermal
cornified cell envelopes. FASEB J. 25:1567–1576. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Muñoz-Garcia A, Thomas CP, Keeney DS,
Zheng Y and Brash AR: The importance of the lipoxygenase-hepoxilin
pathway in the mammalian epidermal barrier. Biochim Biophys Acta.
1841:401–408. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Pigors M, Kiritsi D, Krümpelmann S, Wagner
N, He Y, Podda M, Kohlhase J, Hausser I, Bruckner-Tuderman L and
Has C: Lack of plakoglobin leads to lethal congenital epidermolysis
bullosa: A novel clinico-genetic entity. Hum Mol Genet.
20:1811–1819. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wiche G: Role of plectin in cytoskeleton
organization and dynamics. J Cell Sci. 111:2477–2486.
1998.PubMed/NCBI
|
|
47
|
Winter L and Wiche G: The many faces of
plectin and plectinopathies: Pathology and mechanisms. Acta
Neuropathol. 125:77–93. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Niehaus JZ, Good M, Jackson LE, Ozolek JA,
Silverman GA and Luke CJ: Human SERPINB12 is an abundant
intracellular serpin expressed in most surface and glandular
epithelia. J Histochem Cytochem. 63:854–865. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Galliano MF, Toulza E, Gallinaro H, Jonca
N, Ishida-Yamamoto A, Serre G and Guerrin M: A novel protease
inhibitor of the alpha2-macroglobulin family expressed in the human
epidermis. J Biol Chem. 281:5780–5789. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Schepens I, Jaunin F, Begre N, Läderach U,
Marcus K, Hashimoto T, Favre B and Borradori L: The protease
inhibitor alpha-2-macroglobulin-like-1 is the p170 antigen
recognized by paraneoplastic pemphigus autoantibodies in human.
PLoS One. 5:e122502010. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kim SH, Jung SH, Chung H, Jo DI, Kim CK,
Park SH, Won KJ, Jeon HS and Kim B: Annexin A2 participates in
human skin keloid formation by inhibiting fibroblast proliferation.
Arch Dermatol Res. 306:347–357. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Tristan C, Shahani N, Sedlak TW and Sawa
A: The diverse functions of GAPDH: Views from different subcellular
compartments. Cell Signal. 23:317–323. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ramos D, Pellín-Carcelén A, Agustí J,
Murgui A, Jordá E, Pellín A and Monteagudo C: Deregulation of
glyceraldehyde-3-phosphate dehydrogenase expression during tumor
progression of human cutaneous melanoma. Anticancer Res.
35:439–444. 2015.PubMed/NCBI
|
|
54
|
Noh JY, U Shin J, Kim JH, Kim SH, Kim BM,
Kim YH, Park S, Kim TG, Shin KO, Park K and Lee KH: ZAG regulates
the skin barrier and immunity in atopic dermatitis. J Invest
Dermatol. 139:1648–1657.e7. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Chan I: The role of extracellular matrix
protein 1 in human skin. Clin Exp Dermatol. 29:52–56. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Feng S, Fox D and Man SM: Mechanisms of
gasdermin family members in inflammasome signaling and cell death.
J Mol Biol. 430:3068–3080. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Suga H, Oka T, Sugaya M, Sato Y, Ishii T,
Nishida H, Ishikawa S, Fukayama M and Sato S: Keratinocyte
proline-rich protein deficiency in atopic dermatitis leads to
barrier disruption. J Invest Dermatol. 139:1867–1875.e7. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Schallreuter KU, Pittelkow MR and Wood JM:
Free radical reduction by thioredoxin reductase at the surface of
normal and vitiliginous human keratinocytes. J Invest Dermatol.
87:728–732. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Schallreuter KU and Wood JM: Thioredoxin
reductase-its role in epidermal redox status. J Photochem Photobiol
B. 64:179–184. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Shin MH, Lee SR, Kim MK, Shin CY, Lee DH
and Chung JH: Activation of peroxisome proliferator-activated
receptor alpha improves aged and UV-irradiated skin by catalase
induction. PLoS One. 11:e01626282016. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Sheppard HM, Feisst V, Chen J, Print C and
Dunbar PR: AHNAK is downregulated in melanoma, predicts poor
outcome, and may be required for the expression of functional
cadherin-1. Melanoma Res. 26:108–116. 2016. View Article : Google Scholar : PubMed/NCBI
|