Introduction
Pathological angiogenesis occurs in several parts of
the eye, including retina, choroid and cornea. It is a major cause
of vision loss in numerous ocular diseases, such as diabetic
retinopathy, age-related macular degeneration and keratitis.
Corneal neovascularization (CoNV) usually occurs in the
inflammatory or infectious ocular surface diseases (1,2). It
is characterized by the invasion of the capillaries from the
pericorneal limbal vascular plexus into avascular cornea tissue.
CoNV can lead to corneal scarring, edema and inflammation, which
eventually affects visual acuity and worsens the prognosis of
subsequent penetrating keratoplasty (3,4). A
balance exists between the angiogenic factors and anti-angiogenic
factors in the cornea (5,6). CoNV will occur when the balance is
disturbed (7,8). CoNV is associated with increased
expression of angiogenic factors and decreased expression of
anti-angiogenic factors (9).
Numerous growth factors and cytokines have been
reported to be involved in the angiogenic process. Among them,
vascular endothelial growth factor (VEGF) is a critical angiogenic
factor. VEGF is upregulated in the inflamed and vascularized
corneas (10). VEGF is a secreted
growth factor induced by hypoxia or inflammatory stimulation and
plays an important role in endothelial angiogenic functions,
including cell proliferation, migration and new tube formation
(11,12). VEGF usually exerts its biological
effects by binding to and activating its major receptor, VEGF
receptor 2 (VEGFR2) (13–15). Thus, inhibition of VEGFR-2
signaling is a promising strategy for treating CoNV.
SKLB1002, derived from quinazoline, is a small
molecule that displays potent and specific inhibition of VEGFR2
tyrosine kinase activity. It can inhibit VEGF-induced
phosphorylation of VEGFR2 kinase and the downstream protein kinases
(16,17). A previous study has demonstrated
that SKLB1002 inhibits angiogenesis and may be a potential drug
candidate for anti-cancer therapy (18). The present study investigated the
role of SKLB1002 endothelial angiogenic function in vitro
and ocular angiogenesis in vivo. The results revealed that
SKLB1002 can significantly inhibit CoNV induced by alkali-burn
in vivo and inhibit endothelial angiogenic functions in
vitro.
Materials and methods
Corneal alkali-burn mouse model
In total, 40 male ICR mice (age, 12 weeks; weight
30±2 g) were chosen to build CoNV model, and were purchased from
Nanjing Qinglongshan Experimental Animal Center. The mice were
raised under standard conditions (temperature, 22±2°C; humidity,
50±5%) with a controlled 12-h light/dark cycle and had free access
to water and standard laboratory chow. The mice were anesthetized
by intraperitoneal injection of chloral hydrate at a dose of 350
mg/kg. No signs of peritonitis were observed during the experiment.
Corneal alkali-burn was performed by applying a 2.5 mm diameter
filter paper soaked with 1 mol/l NaOH on the corneal center for 25
sec. After the filter paper was removed, the eye was rinsed with
the sterilized saline for 1 min. An eyedrop of SKLB1002 (0.05
mg/ml) or sodium carboxymethyl cellulose (CMC-Na; 0.5%) was used on
the surface of cornea 3 times each day. Corneal neovascularization
was observed using a slit lamp. The animals were euthanized by
cervical dislocation.
Histopathological analysis
Hematoxylin and eosin (H&E) staining was
performed to detect the histopathological change of cornea. The
mice were treated with SKLB1002, CMC-Na (0.5%), or PBS 3 times per
day. The eyeballs were collected and fixed in 4% paraformaldehyde
(Beyotime Institute of Biotechnology) at 4°C for 24 h. Then, the
eyeballs were dehydrated by immersion in a series of increased
concentrations of alcohol, embedded in paraffin wax and sectioned
at 5-µm thickness. The paraffin sections were deparaffinized by
xylene for 30 min and rehydrated by an alcohol gradient. After
washing with ddH2O, the sections were stained with
hematoxylin at room temperature for 10 min. Then, the sections were
washed with ddH2O and stained with eosin at room
temperature for 1 min. After washing with ddH2O, the
sections were dehydrated using alcohol and mounted using resinene.
The images were captured using a light microscope at magnification,
×10.
Cell culture
Human umbilical vein endothelial cells (HUVECs) were
obtained from Lonza Group, Ltd. (cc-2159) and cultured in
Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 10% fetal bovine serum (FBS;
Gibco; Thermo Fisher Scientific, Inc.) at 37°C with 5%
CO2 in a humidified atmosphere.
Cell viability assay
MTT assay was performed to detect cell viability.
HUVECs were seeded onto 96-well plate (3,000-4,000/well) and
treated with different concentrations of SKLB1002 for 48 h. HUVECs
were also treated with p38 inhibitor SB203580 (10 µM; cat. no.
S1863; Beyotime Institute of Biotechnology), ERK inhibitor U0126
(10 µM; cat. no. S1901; Beyotime Institute of Biotechnology) or JNK
inhibitor SP600125 (10 µM; cat. no. S1876; Beyotime Institute of
Biotechnology) for 1 h, and incubated with VEGF (10 ng/ml), VEGF +
SKLB1002 or left untreated at 37°C for 48 h. Then, MTT (5 mg/ml,
Beyotime Institute of Biotechnology) was added at 37°C for 4 h.
After removing the medium, the crystals were dissolved with
isopropanol and determined at 570 nm wavelength using a microplate
reader.
Cell proliferation assay
Ki67 staining was performed to determine cell
proliferation. In brief, HUVECs were seeded onto 24-well plate.
Following the required treatment, they were washed with PBS buffer,
fixed in 4% paraformaldehyde (Beyotime Institute of Biotechnology)
at room temperature for 15 min and blocked in 5% BSA (Biofroxx
GmbH) at 37°C for 0.5 h. Ki67 antibody (1:200; Abcam; cat. no.
ab16667) was added to each well overnight at 4°C. The plate was
placed at room temperature to rewarm for 1 h and incubated with the
secondary antibody for 3 h. The nuclei were stained with DAPI
(1:1,000; Biosharp) at room temperature for 10 min. The plate was
imaged using a fluorescence microscope.
EdU incorporation assay
After the required treatment, the cells were
cultured with EdU medium (50 µM, 300 µl/well; Guangzhou RiboBio
Co., Ltd.) at 37°C for 4 h. Cells were fixed with 4%
paraformaldehyde (Beyotime Institute of Biotechnology) at room
temperature for 30 min and incubated with 0.5% Triton X-100 for 10
min. After washing with PBS for three times, cells were incubated
with Apollo Dye solution (Guangzhou RiboBio Co., Ltd.; cat. no.
C10310) at 37°C for 1 h in the dark. Then, the cells were incubated
with 0.5% Triton X-100 at room temperature for 15 min. Cell nuclei
were stained by Hoechst 33342 at room temperature for 30 min in the
dark. The images were captured using a fluorescent microscope at
magnification, ×40.
Flow cytometry
HUVECs (5×105 cells/well in 6-well
plates) were incubated with or without SKLB1002 at 37°C for 24 h.
They were harvested using a 0.05% trypsin solution, washed twice
with PBS and centrifuged at 1,200 × g at 4°C for 5 min. Then, cell
suspension was stained with fluorescein isothiocyanate-labeled
Annexin V (BD Pharmingen; Becton, Dickinson and Company) and
counterstained with propidium iodide (PI; BD Pharmingen; Becton,
Dickinson and Company) at room temperature for 10 min in the dark.
Finally, the percentage of apoptotic HUVECs were determined using a
flow cytometer (cytoFLEX; Beckman Coulter, Inc.) and analyzed using
CytExpert 2.3 (Beckman Coulter, Inc.).
Scratch wound healing assay
HUVECs were seeded onto a 6-well plate. After they
reached >90% confluence, a 10 µl pipette tip was used to make a
straight line in the middle of confluent monolayer. The floating
cell debris was washed with PBS buffer and the injured cell
monolayers were cultured in serum-free medium. The injured area was
observed and the images captured at different time points (0, 24
and 48 h).
Transwell migration assay
HUVECs were seeded onto the upper Transwell inserts
(Corning, Inc.) at 2×104/well and allowed for migrating
through the hole for 10 h. Meanwhile, serum-free media was added
into the upper insert and the complete media (10% FBS in DMEM) was
put into the lower chamber as the chemoattractant. These
non-migrated cells were removed by cotton swabs. The migrated cells
were fixed with methyl alcohol at room temperature for 15 min and
stained with 0.5% crystal violet solution at room temperature for
30 min. Finally, the stained cells were counted under a light
microscope and images were captured at magnification, ×20.
Tube formation assay
The tube formation assay was performed to detect the
angiogenic ability of HUVECs. The 24-well plate was frozen in
advance, thawed Matrigel (BD Biosciences; Becton, Dickinson and
Company) was added onto the bottom of wells and incubated at 37°C
for 1 h to solidify. After the required treatment, HUVECs were
cultured onto these wells at 1×105/well. The tube
formation was observed by a light microscope and the tube length
was calculated using ImageJ 1.52p software (National Institutes of
Health).
Protein extraction and western blot
analysis
After the required treatment, HUVECs were collected
and lysed in RIPA lysis buffer (Beyotime Institute of
Biotechnology). Following centrifugation at 10,000 × g at 4°C for
15 min, the supernatant was collected and the concentration of
protein was determined using a BCA Protein Assay kit (Thermo Fisher
Scientific, Inc.). The extracted protein (30 µg/lane) was separated
on 10% SDS-PAGE and transferred onto the PVDF membranes (EMD
Millipore). After blocking with 5% non-fat milk at room temperature
for 0.5 h, the membranes were incubated with ERK1/2 (1:1,000,
9102), p-ERK1/2 (1:1,000; 4370), JNK (1:1,000; 9252), p-JNK
(1:1,000; 9251), p38 (1:1,000; 9212), p-p38 (1:1,000; 9215), or
GAPDH (1:1,000; 2118; all from Cell Signaling Technology, Inc.)
overnight at 4°C. After washing with TBST (containing 0.05% Tween)
for 3 times, the PVDF membranes were incubated with the
HRP-conjugated secondary antibody (1:1,000; Beyotime Institute of
Biotechnology) at room temperature for 3 h. The results of blots
were visualized using an ECL detection system (Nanjing KeyGen
Biotech Co., Ltd.) and the densitometry was measured using ImageJ
1.52p software (National Institutes of Health).
Statistical analysis
All experiments were repeated at least 3 times. All
quantitative data were presented as mean ± standard error of the
mean. Statistical significance was calculated using unpaired or
paired Student's t test or one-way ANOVA followed by Bonferroni
post hoc test. P<0.05 was considered to indicate a statistically
significant difference.
Results
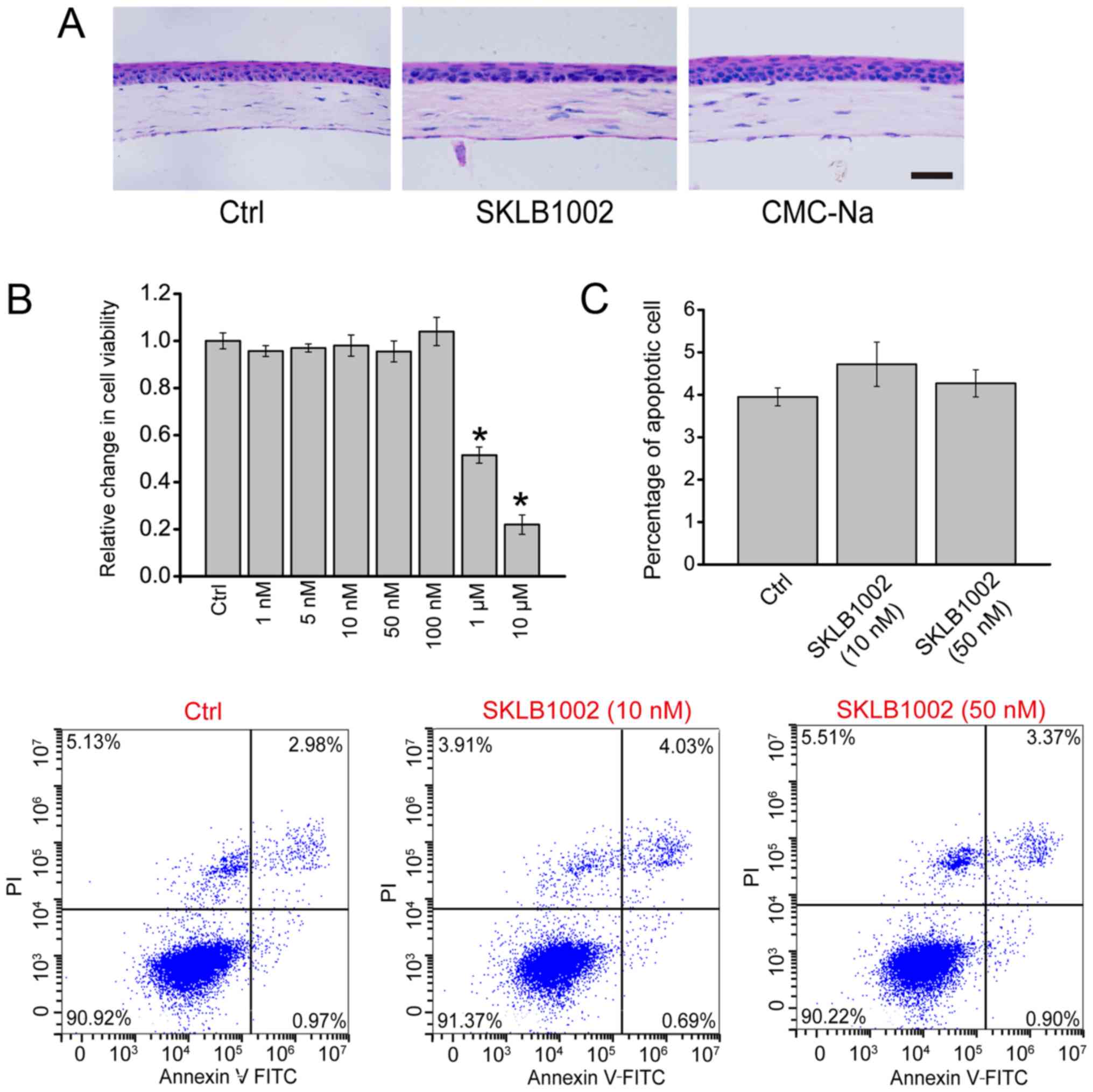
SKLB1002 administration has no obvious
cytotoxicity in vitro and tissue toxicity in vivo
Corneal tissues were administered SKLB1002 (0.05
mg/ml), CMC-Na solution (0.5%), or PBS (Control) for 7 days. The
histological change of the cornea was observed using H&E
staining. Compared with the control group, no obvious morphologic
change was observed after the administration of SKLB1002 or CMC-Na
(Fig. 1A). MTT assay was performed
to determine whether SKLB1002 had cytotoxicity in vitro. The
result demonstrated that SKLB1002 had no obvious cytotoxicity on
HUVECs ranging from 1 to 100 nm (Fig.
1B). Flow cytometry assays through Annexin V-FITC/PI double
labeling revealed that compared with the control group, SKLB1002
administration did not lead to an increased percentage of apoptotic
HUVECs (Fig. 1C). Collectively,
these results show that SKLB1002 administration has no obvious
cytotoxicity in vitro and tissue toxicity in
vivo.
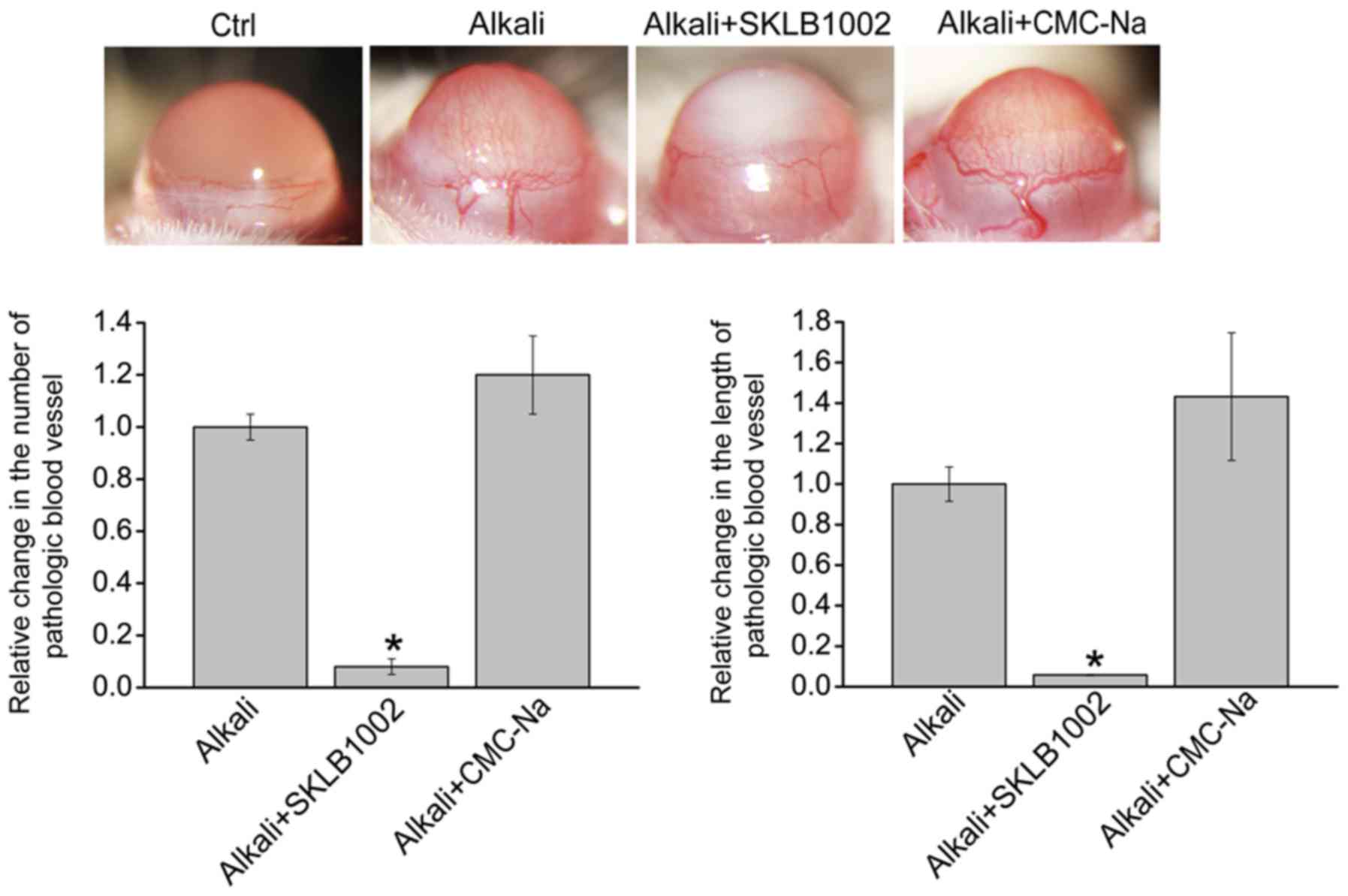
Topical application of SKLB1002
eyedrop inhibits CoNV
To determine whether SKLB1002 administration plays
an inhibitory role in CoNV, a CoNV model was first constructed
through alkali-burn injury and then the injured corneas were
treated with SKLB1002 eyedrops (0.05 mg/ml) or CMC-Na solution
(0.5%) 3 times per day. The results demonstrated that new corneal
blood vessels appeared at 1 day and peaked at 7 days after
alkali-burn injury. The image of the anterior segment was taken
using a slit lamp at 7 days after alkali-burn injury. There was no
neovascularization in normal cornea (Ctrl group). Furthermore,
alkali-burn injury induced an increased number and length of
pathological corneal blood vessels (Alkali group). Compared with
Alkali group, the number and the length of new corneal blood
vessels was significantly reduced after the administration of
SKLB1002 (Alkali + SKLB1002 group), but not CMC-Na (Alkali + CMC-Na
group; Fig. 2). Collectively, the
above-mentioned results suggest that SKLB1002 administration can
suppress CoNV in vivo.
SKLB1002 administration suppresses
endothelial angiogenic function in vitro
To determine the effect of SKLB1002 administration
on HUVEC viability, HUVECs were pre-treated with SKLB1002 and then
treated with or without VEGF (10 ng/ml). VEGF treatment
significantly increased the viability of HUVECs. However,
pre-treatment with SKLB1002 (10 or 50 nM) significantly reduced
VEGF-induced increase in HUVEC viability (Fig. 3A). EdU incorporation assay and Ki67
immunofluorescence staining demonstrated that pre-treatment with
SKLB1002 significantly decreased the proliferation ability of
HUVECs (Fig. 3B and C). Transwell
migration assay and scratch wound healing assay demonstrated that
pre-treatment of SKLB1002 significantly reduced the migration
ability of HUVECs (Fig. 3D and E).
Matrigel tube formation assay demonstrated that VEGF-mediated tube
formation ability was interrupted after the administration of
SKLB1002 (Fig. 3F).
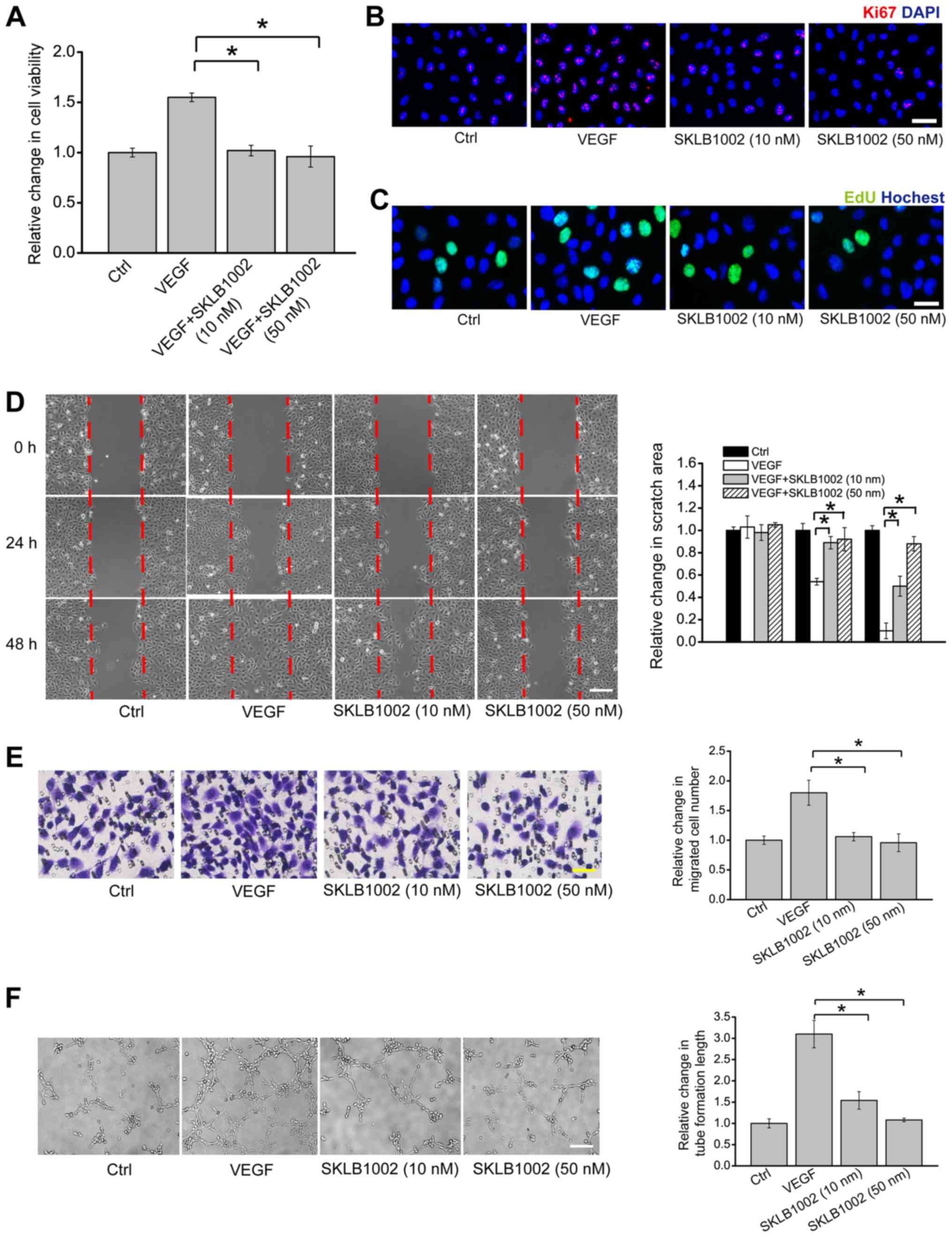 | Figure 3.SKLB1002 administration suppresses
endothelial angiogenic function in vitro. (A-C) HUVECs were
cultured with VEGF (10 ng/ml), VEGF plus SKLB1002 (10 or 50 nM), or
left untreated (Ctrl) for 24 h. Cell viability was determined by
(A) MTT assay. Cell proliferation was detected by (B) Ki67
immunofluorescence staining and (C) EdU incorporation staining.
HUVECs were cultured with VEGF (10 ng/ml), VEGF plus SKLB1002 (10
or 50 nM), or left untreated (Ctrl). (D) Scratch wound healing
assay (n=3; scale bar, 100 µm) and (E) Transwell assay (n=3; scale
bar, 50 µm) were performed to detect the migration of HUVECs.
Quantification of migration was expressed as (D) the relative
change of scratch area or (E) relative change of migrated cell
number. HUVECs were seeded onto the Matrigel matrix and cultured at
37°C for 6 h. (F) The length of tube-like structures was captured
by a light microscope and calculated using Image J software (n=3;
scale bar, 50 µm). All data were from ≥3 independent experiments.
The significant difference (*P<0.05) was evaluated by one-way
ANOVA followed by Bonferroni post hoc test. HUVECs, human umbilical
vein endothelial cells; VEGF, vascular endothelial growth factor;
Ctrl, control. |
SKLB1002 serves its anti-angiogenic
function via the mitogen-activated protein kinase (MAPK) signaling
pathway
VEGF and VEGFR-2 usually serve their roles through
activation of MAPK signaling. Western blotting was performed to
investigate whether SKLB1002 administration could affect the
activation of the MAPK signaling pathway. VEGF treatment led to
increased expression levels of phosphorylated ERK1/2, JNK and p38.
The increased expression of these proteins was markedly interrupted
after SKLB1002 administration (Fig.
4A). It was thus concluded that SKLB1002 played its
anti-angiogenic role in endothelial cells through inactivation of
MAPK signaling as demonstrated in Fig.
4B. To further verify whether SKLB1002 performed its
anti-angiogenic role in endothelial cells through MAPK signaling,
HUVECs were treated with p38 inhibitor (SB203580), ERK inhibitor
(U0126), JNK inhibitor (SP600125), or SKLB1002 and then incubated
with or without VEGF. The results demonstrated that SKLB1002
administration decreased the viability of HUVECs (Fig. 4C) and reduced the migration and
tube formation ability of HUVECs (Fig.
4D and E), showing similar effects as the combined effects of
SB203580, U0126 and SP600125 administration on endothelial
angiogenic functions. These results provided additional evidence
that SKLB1002 plays its anti-angiogenic role in endothelial cells
through the inactivation of MAPK signaling.
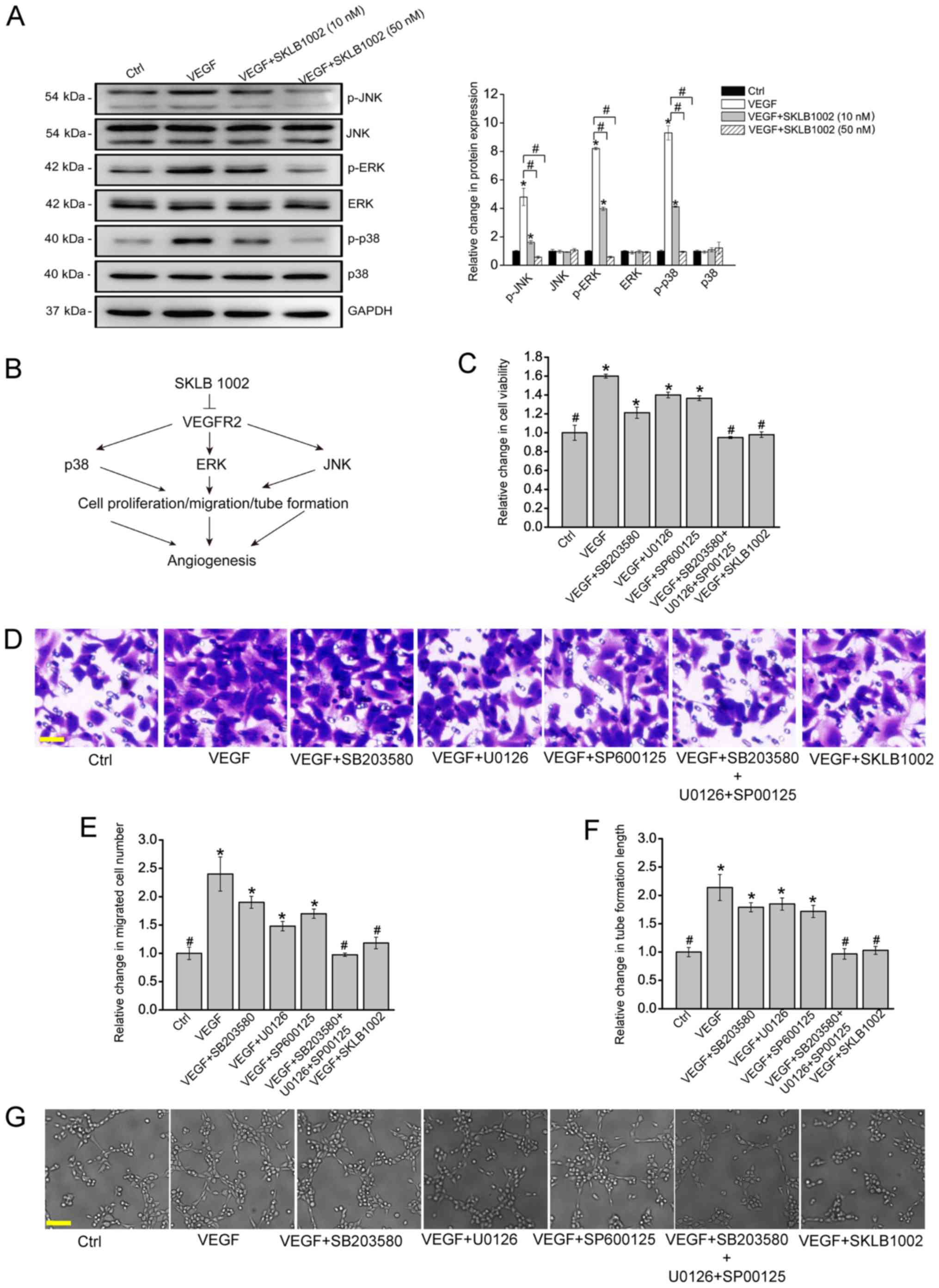 | Figure 4.SKLB1002 performs its anti-angiogenic
role via the MAPK signaling pathway. (A) HUVECs were incubated with
or without SKLB1002 (10 or 50 nM) plus VEGF (10 ng/ml) or left
untreated (Ctrl) for 24 h. HUVECs were collected and lysed. The
expression levels of total ERK1/2, JNK, p38 and the phosphorylated
forms of these proteins (p-ERK1/2, p-JNK and p-p38) were detected
by western blotting. GAPDH was used as the internal control. (A)
Representative immunoblots along with the densitometric
quantitative results are shown. *P<0.05 vs. Ctrl group (P=0.013,
0.028, 0.008, 0.016, 0.0052 and 0.018, respectively).
#P<0.05 vs. the marked groups. (B) A schematic image
showing the potential mechanism of SKLB1002 in the regulation of
endothelial angiogenic functions. HUVECs were pretreated with or
without SB203580, U0126, or SP600125 and then incubated with VEGF
(10 ng/ml), VEGF plus SKLB1002 (50 nM), or left untreated (Ctrl).
Cell viability was determined by (C) MTT assay (n=3; *P=0.017,
0.031, 0.023 and 0.027, respectively; #P=0.017, 0.022
and 0.026, respectively). (D) Transwell assay and (E)
quantification analysis was performed to determine the migration of
HUVECs (n=3; scale bar, 50 µm; *P=0.005, 0.013, 0.022 and 0.015,
respectively; #P=0.005, 0.008 and 0.014, respectively).
HUVECs were seeded onto the Matrigel matrix and cultured at 37°C
for 6 h. (F and G) The length of tube-like structures was captured
by a light microscope and calculated using Image J software (n=3;
scale bar, 50 µm; *P=0.006, 0.017, 0.012 and 0.019, respectively;
#P=0.006, 0.008 and 0.011, respectively). *P<0.05 vs.
Ctrl group; #P<0.05 vs. VEGF group. All data were
from ≥3 independent experiments. The significant difference was
evaluated by one-way ANOVA followed by Bonferroni post hoc test.
MAPK, mitogen-activated protein kinase; HUVECs, human umbilical
vein endothelial cells; VEGF, vascular endothelial growth factor;
Ctrl, control; p, phosphorylated. |
Discussion
CoNV is characterized by the invasion of the new
blood vessels into the cornea and is a major cause of blindness
worldwide (19–21). However, the current therapeutic
options have demonstrated limited or transitory results (22). The present study investigated the
effects of SKLB1002 administration on the progression of CoNV.
SKLB1002 administration can inhibit the progression of CoNV in
vivo and regulate endothelial angiogenic functions in
vitro.
Under normal condition, the transparency of the
cornea is a prerequisite for vision (23). Pathological factors, such as
infection, chemical burns, hypoxia and inflammatory molecules, can
interrupt the balance between angiogenic and anti-angiogenic
factors in the cornea. VEGF has been reported as a major angiogenic
factor during CoNV (24). The VEGF
level in the vascularized cornea is significantly higher compared
with the normal cornea (1,10,25).
Anti-VEGF agents such as bevacizumab have been used for treating
ocular angiogenesis, including CoNV. However, they require
intravitreal injection and a relatively frequent dosing regimen
(4-6 weeks) (26,27). In experimental models of
neovascularization, anti-VEGF treatment became less effective at
blocking vessel growth and regressing vessels as the
neovascularization develops over time (8,28,29).
In addition, subconjunctival injection of an anti-VEGF drug may
lead to hemorrhage and damage patient compliance. Thus, there
remains a requirement to search for novel therapies for CoNV.
SKLB1002 is a novel potent inhibitor of VEGF
receptor 2 signaling. It has no significant toxicity on mouse
corneal structure in vivo and endothelial cell viability
in vitro. An alkali-burn corneal angiogenesis model was used
to investigate the role of SKLB1002 in anti-angiogenic effects.
Eyedrops were used to deliver SKLB1002 onto the ocular surface,
which have great advantage over the traditional subconjunctival
injection because it is noninvasive and easily accepted by the
patients. However, tear screening and nasolacrimal duct drainage
may affect the bioavailability of SKLB1002 (30,31).
To overcome this flaw, CMC-Na was used as the solvent of the
eyedrops to increase the retention time of SKLB1002. CMC-Na is a
hydrosoluble biopolymer derived from cellulose (18,32).
CMC-Na has been widely used due to its high viscosity, non-toxicity
and non-allergenicity (16). In
addition, a previous study reported that CMC-Na can be used as a
drug solvent to treat dry eye (17). Mouse eyes were injured through
incubation with 1 mol/l NaOH to induce CoNV. After 7 days of alkali
burn, the images of the anterior segment showed that the number and
length of CoNV was attenuated in SKLB1002-administrated group.
Angiogenesis is a complicated process comprising the
participation of multifarious cells and factors. Endothelial cells
are the key regulators in the angiogenic cascade (33,34).
Once the balance between angiogenic factors and anti-angiogenic
factors is disturbed, endothelial cells are activated to
proliferate, migrate and form the tubes (35–37).
The present study demonstrated that SKLB1002 administration can
inhibit the VEGF-induced proliferation, migration and tube
formation ability of HUVECs, suggesting a critical role of SKLB1002
in inhibiting endothelial angiogenic functions.
The molecular mechanism of SKLB1002 in
anti-angiogenic effects was also investigated. VEGF-VEGFR-2
signaling has been reported to serve their roles in angiogenesis
through the activation of MAPK signaling (38,39).
MAPKs comprise the ERK1/2, JNK1/2/3 and p38 isoforms (40,41).
ERK1/2 is involved in the regulation of HUVEC proliferation
(42). VEGF-induced p38 change can
affect cell migration (43,44).
JNK plays important roles in the cell proliferation and apoptotic
responses to cellular stresses (45). The present study demonstrated that
SKLB1002 decreased the phosphorylation level of ERK1/2, JNK and
p38. Given that ERK1/2, JNK and p38 have been demonstrated to be
the critical regulators of cell proliferation, migration and tube
formation (46), it is not
surprising that SKLB1002-regulated MAPK signaling pathway is
involved in the regulation of the angiogenic cascade.
In conclusion, the present study demonstrated that
SKLB1002, a small-molecule inhibitor of VEGFR-2, exhibited
anti-angiogenic effects on CoNV by blocking the MAPK signaling
pathway. SKLB1002 administration can inhibit endothelial cell
proliferation, migration and tube formation in vitro. Thus,
selective inhibition of VEGFR-2 through SKLB1002 administration is
a promising therapy for ocular angiogenesis. Angiogenesis can also
contribute to the pathogenesis of other diseases, such as
malignant, inflammatory, infectious and immune disorders.
Anti-angiogenesis by SKLB1002 administration may offer new
therapeutic opportunities for these disorders.
Acknowledgements
The authors would like to thank Dr Yao Jin and Dr
Xiu-Miao Li (Nanjing Medical University, China) for the helpful
statistical discussion and technical assistance for western
blotting.
Funding
The present study was supported by the grants from
the National Natural Science Foundation of China (grant nos.
81470594, 81570859, 81870679 and 81800858), grants from the Medical
Science and Technology Development Project Fund of Nanjing (grant
no. ZKX1705), innovation team Project Fund of Jiangsu Province
(grant no. CXTDB2017010) and the Science and Technology Development
Plan Project Fund of Nanjing (grant no. 201716007).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
BY and QJ were the major contributors to the
experimental design. QZ and ST established the animal models. CL,
JL, XL and JY performed the western blot analysis. QZ and ST
performed the cell culture. BY was involved in writing the
manuscript and the analysis and interpretation of data. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
All experimental procedures adhered to the
principles stated in the Guide for the Care and Use of Laboratory
Animals (updated 2011; National Institutes of Health, Bethesda, MD,
USA) and were approved by the Animal Care and the Use Committee of
Nanjing Medical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ellenberg D, Azar DT, Hallak JA, Tobaigy
F, Han KY, Jain S, Zhou Z and Chang JH: Novel aspects of corneal
angiogenic and lymphangiogenic privilege. Prog Retin Eye Res.
29:208–248. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang SX and Ma JX: Ocular
neovascularization: Implication of endogenous angiogenic inhibitors
and potential therapy. Prog Retin Eye Res. 26:1–37. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Qazi Y, Wong G, Monson B, Stringham J and
Ambati BK: Corneal transparency: Genesis, maintenance and
dysfunction. Brain Res Bull. 81:198–210. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fu YC and Xin ZM: Inhibited corneal
neovascularization in rabbits following corneal alkali burn by
double-target interference for VEGF and HIF-1α. Biosci Rep.
39(pii): BSR201805522019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Qazi Y, Maddula S and Ambati BK: Mediators
of ocular angiogenesis. J Genet. 88:495–515. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Maddula S, Davis DK, Maddula S, Burrow MK
and Ambati BK: Horizons in therapy for corneal angiogenesis.
Ophthalmology. 118:591–599. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sene A, Chin-Yee D and Apte RS: Seeing
through VEGF: Innate and adaptive immunity in pathological
angiogenesis in the eye. Trends Mol Med. 21:43–51. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Roshandel D, Eslani M, Baradaran-Rafii A,
Cheung AY, Kurji K, Jabbehdari S, Maiz A, Jalali S, Djalilian AR
and Holland EJ: Current and emerging therapies for corneal
neovascularization. Ocul Surf. 16:398–414. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bock F, Maruyama K, Regenfuss B, Hos D,
Steven P, Heindl LM and Cursiefen C: Novel anti(lymph)angiogenic
treatment strategies for corneal and ocular surface diseases. Prog
Retin Eye Res. 34:89–124. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Poulaki V, Mitsiades N, Kruse FE, Radetzky
S, Iliaki E, Kirchhof B and Joussen AM: Activin a in the regulation
of corneal neovascularization and vascular endothelial growth
factor expression. Am J Pathol. 164:1293–1302. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Apte RS, Chen DS and Ferrara N: VEGF in
signaling and disease: Beyond discovery and development. Cell.
176:1248–1264. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Eilken HM and Adams RH: Dynamics of
endothelial cell behavior in sprouting angiogenesis. Curr Opin Cell
Biol. 22:617–625. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hoeben A, Landuyt B, Highley MS, Wildiers
H, Van Oosterom AT and De Bruijn EA: Vascular endothelial growth
factor and angiogenesis. Pharmacol Rev. 56:549–580. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ferrara N, Gerber HP and LeCouter J: The
biology of VEGF and its receptors. Nat Med. 9:669–676. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Varricchi G, Loffredo S, Galdiero MR and
Marone G, Cristinziano L, Granata F and Marone G: Innate effector
cells in angiogenesis and lymphangiogenesis. Curr Opin Immunol.
53:152–160. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shen G, Li Y, Du T, Shi G, Dai L, Chen X,
Zheng R, Li W, Su X, Zhang S, et al: SKLB1002, a novel inhibitor of
VEGF receptor 2 signaling, induces vascular normalization to
improve systemically administered chemotherapy efficacy. Neoplasma.
59:486–493. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li WW, Chen JJ, Zheng RL, Zhang WQ, Cao
ZX, Yang LL, Qing XY, Zhou LX, Yang L, Yu LD, et al: Taking
quinazoline as a general support-Nog to design potent and selective
kinase inhibitors: application to FMS-like tyrosine kinase 3. Chem
Med Chem. 5:513–516. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang S, Cao Z, Tian H, Shen G, Ma Y, Xie
H, Liu Y, Zhao C, Deng S, Yang Y, et al: SKLB1002, a novel potent
inhibitor of VEGF receptor 2 signaling, inhibits angiogenesis and
tumor growth in vivo. Clin Cancer Res. 17:4439–4450. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bignami F, Lorusso A, Rama P and Ferrari
G: Growth inhibition of formed corneal neovascularization following
Fosaprepitant treatment. Acta Ophthalmol. 95:e641–e648. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Skobe M and Dana R: Blocking the path of
lymphatic vessels. Nat Med. 15:993–994. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tolentino MJ: Current molecular
understanding and future treatment strategies for pathologic ocular
neovascularization. Curr Mol Med. 9:973–981. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu S, Romano V, Steger B, Kaye SB, Hamill
KJ and Willoughby CE: Gene-based antiangiogenic applications for
corneal neovascularization. Surv Ophthalmol. 63:193–213. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhong W, Montana M, Santosa SM, Isjwara
ID, Huang YH, Han KY, O'Neil C, Wang A, Cortina MS, de la Cruz J,
et al: Angiogenesis and lymphangiogenesis in corneal
transplantation-A review. Surv Ophthalmol. 63:453–479. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nominato LF, Dias AC, Dias LC, Fantucci
MZ, Mendes da Silva LEC, Murashima AA and Rocha EM: Prevention of
corneal neovascularization by adenovirus encoding human vascular
endothelial growth factor soluble receptor (s-VEGFR1) in lacrimal
gland. Invest Ophthalmol Vis Sci. 59:6036–6044. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee JE, Kim KL, Kim D, Yeo Y, Han H, Kim
MG, Kim SH, Kim H, Jeong JH and Suh W: Apatinib-loaded
nanoparticles suppress vascular endothelial growth factor-induced
angiogenesis and experimental corneal neovascularization. Int J
Nanomedicine. 12:4813–4822. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chang JH, Garg NK, Lunde E, Han KY, Jain S
and Azar DT: Corneal neovascularization: An anti-VEGF therapy
review. Surv Ophthalmol. 57:415–429. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Friedman M, Azrad-Lebovitz T, Morzaev D,
Zahavi A, Marianayagam NJ, Nicholson JD, Brookman M, Michowiz S,
Hochhauser E and Goldenberg-Cohen N: Protective effect of TLR4
ablation against corneal neovascularization following chemical burn
in a mouse model. Curr Eye Res. 44:505–513. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
van Wijngaarden P, Coster DJ and Williams
KA: Inhibitors of ocular neovascularization: Promises and potential
problems. JAMA. 293:1509–1513. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Menzel-Severing J: Emerging techniques to
treat corneal neovascularisation. Eye (Lond). 26:2–12. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chang CY, Wang MC, Miyagawa T, Chen ZY,
Lin FH, Chen KH, Liu GS and Tseng CL: Preparation of
arginine-glycine-aspartic acid-modified biopolymeric nanoparticles
containing epigalloccatechin-3-gallate for targeting vascular
endothelial cells to inhibit corneal neovascularization. Int J
Nanomedicine. 12:279–294. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Urtti A: Challenges and obstacles of
ocular pharmacokinetics and drug delivery. Adv Drug Deliv Rev.
58:1131–1135. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kisielewska J, Ligeza J and Klein A: The
effect of tyrosine kinase inhibitors, tyrphostins: AG1024 and
SU1498, on autocrine growth of prostate cancer cells (DU145). Folia
Histochem Cytobiol. 46:185–191. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
De Bock K, Georgiadou M and Carmeliet P:
Role of endothelial cell metabolism in vessel sprouting. Cell
Metab. 18:634–647. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Siemerink MJ, Augustin AJ and Schlingemann
RO: Mechanisms of ocular angiogenesis and its molecular mediators.
Dev Ophthalmol. 46:4–20. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Eelen G, de Zeeuw P, Simons M and
Carmeliet P: Endothelial cell metabolism in normal and diseased
vasculature. Circ Res. 116:1231–1244. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Shi W, Liu J, Li M, Gao H and Wang T:
Expression of MMP, HPSE, and FAP in stroma promoted corneal
neovascularization induced by different etiological factors. Curr
Eye Res. 35:967–977. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Adams RH and Alitalo K: Molecular
regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell
Biol. 8:464–478. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Olsson AK, Dimberg A, Kreuger J and
Claesson-Welsh L: VEGF receptor signalling-in control of vascular
function. Nat Rev Mol Cell Biol. 7:359–371. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cross MJ and Claesson-Welsh L: FGF and
VEGF function in angiogenesis: Signalling pathways, biological
responses and therapeutic inhibition. Trends Pharmacol Sci.
22:201–207. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cargnello M and Roux PP: Activation and
function of the MAPKs and their substrates, the MAPK-activated
protein kinases. Microbiol Mol Biol Rev. 75:50–83. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Arthur JS and Ley SC: Mitogen-activated
protein kinases in innate immunity. Nat Rev Immunol. 13:679–692.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Simons M, Gordon E and Claesson-Welsh L:
Mechanisms and regulation of endothelial VEGF receptor signalling.
Nat Rev Mol Cell Biol. 17:611–625. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
del Barco Barrantes I and Nebreda AR:
Roles of p38 MAPKs in invasion and metastasis. Biochem Soc Trans.
40:79–84. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Penn JS, Madan A, Caldwell RB, Bartoli M,
Caldwell RW and Hartnett ME: Vascular endothelial growth factor in
eye disease. Prog Retin Eye Res. 27:331–371. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Sehgal V and Ram PT: Network Motifs in JNK
Signaling. Genes Cancer. 4:409–413. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kim EK and Choi EJ: Pathological roles of
MAPK signaling pathways in human diseases. Biochim Biophys Acta.
1802:396–405. 2010. View Article : Google Scholar : PubMed/NCBI
|