Introduction
Stress urinary incontinence (SUI), a type of female
pelvic floor dysfunction, is a clinically common disease in
gynecology with an increasing incidence rate of 38–49.2% globally
(1). This condition refers to the
spontaneous spilling of urine when abdominal pressure increases
during bladder detrusor relaxation (2). SUI occurs primarily in postpartum and
postmenopausal women, and can seriously affects women's quality of
life, and physical and mental health (3). It also causes a large social and
economic burden (4,5). A type of physiotherapy known as
pelvic electrical stimulation (PES) has demonstrated good clinical
effects in patients with mild-to-moderate symptoms (6–8).
However, the specific molecular mechanism underlying this treatment
remains unclear.
Studies have demonstrated that pudendal nerve (PN)
damage induces SUI by causing denervation of the pelvic floor
muscles (9–11). At the same time, electrical
stimulation (ES) has been demonstrated to promote the repair of the
central and peripheral nervous system following injury (12–14).
These studies indicated that the therapeutic effect of PES in SUI
may be achieved by promoting the repair of damaged PNs.
The Wnt/β-catenin signaling pathway is associated
with development of the nervous system, stem cell proliferation and
differentiation, and axon guidance (15). Studies of neurodegenerative
diseases have demonstrated that the Wnt/β-catenin signaling pathway
regulates the proliferation and differentiation of neural stem
cells and their precursor cells in the spinal cord (16,17).
In addition, animal models of spinal cord injury have indicated
that ES influences the proliferation and directional migration of
nerve cells via the Wnt/β-catenin signaling pathway (18–20).
In the field of oncology, it has been demonstrated that inhibition
of the Wnt signaling pathway can lead to inhibition of cell
proliferation and cell cycle, as well as the expression levels of
β-catenin, which are associated with the activation and shutdown of
Wnt signaling that regulates expression levels of downstream target
genes, such as cyclin D1, C-myc, E-cadherin and Lgr5, at the
transcriptional level, thereby influencing the progression of
tumors (21–25).
The present study simulated nerve injury by
constructing a cyclic mechanical stretching (CMS) injury model in
rat dorsal root ganglion (DRG) cells. Cells were subjected to
fixed-parameter ES, and changes in the cell cycle and levels of
cell activity, apoptosis and Wnt/β-catenin signaling
pathway-related proteins were detected. Finally, the inhibitor
XAV939 was used to inhibit the Wnt/β-catenin signaling pathway and
changes in DRG cell proliferation activity were detected. The
present study aimed to provide a basis for further studies to
clarify the specific mechanisms of ES in the treatment of SUI and
discover new potential targets.
Materials and methods
Cell resource and groups
DRG cell line was purchased from Zhen Shanghai and
Shanghai Industrial Co., Ltd. (cat. no. HZ-C644) and maintained in
DMEM (Jenom Biomedical Technology Co., Ltd.) containing 15% fetal
bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml
penicillin G and 100 µg/ml streptomycin. DRG cells were divided
into four groups: Control (DRG cells received no treatment), ES
(DRG cells treated with ES only), injury (CMS; DRG cells treated
with CMS only) and treatment group (CMS + ES; DRG cells treated
with CMS, followed by treatment with ES immediately).
Establishment of DRG cell injury
model
DRG cells were cultured on culture plates precoated
with rat tail collagen (Sigma-Aldrich; Merck KGaA). When cell
density reached 80–90%, the culture plates were transferred onto
mechanical loading culture dishes. CMS was performed using a
four-point bending device (Chengdu Power Technology Co., Ltd),
which stresses cells by bending the cell plate up and down. The
instrument mainframe was used to adjust the CMS parameters and the
control power system was used to perform the mechanical stretching.
A previous study determined the appropriate parameters to be 5,333
µ strain (4 mm), 8 h and 1 Hz, which were used in the present
experiment (26). All cells
received treatments under the identical environmental conditions.
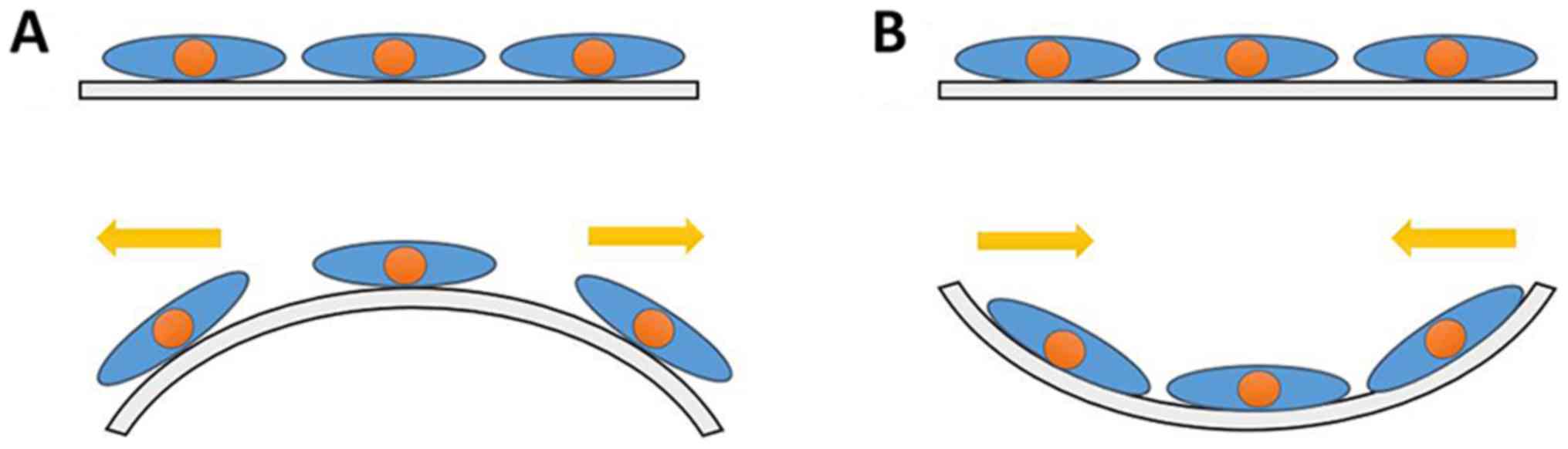
The principle of cell mechanics loading is presented in Fig. 1.
ES model
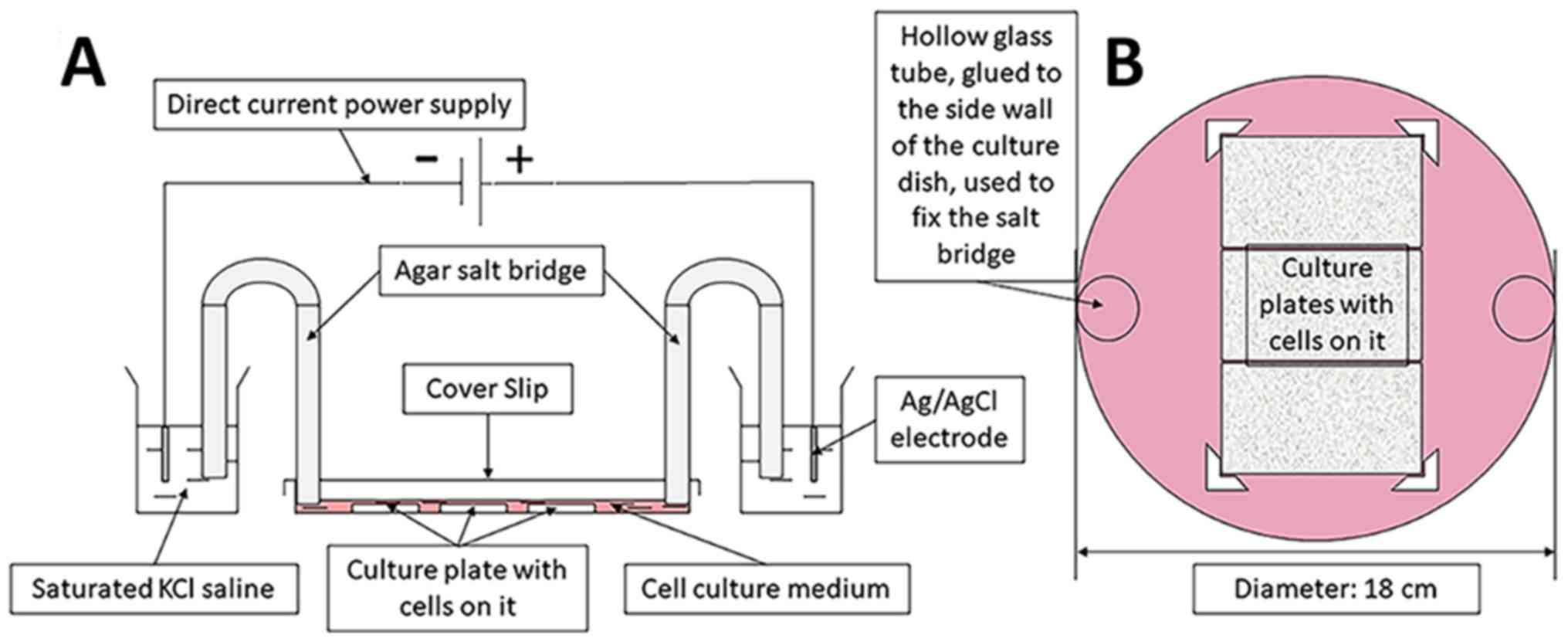
The ES device was constructed with reference to the
method described by Song et al (27) and our device was proven to be
stable after continuous improvement. The unit included a direct
current power supply, a conducting device and a trending circular
petri dish (Fig. 2). The electric
field strength was set using a DC power source (model no. 3303A;
Topward Electric Instruments Co., Ltd.). The culture plates were
placed in a circular culture dish (diameter, 18 cm) filled with
DMEM containing 15% FBS, 100 U/ml penicillin G and 100 µg/ml
streptomycin, and fixed by internal small baffle. A total of three
cell culture plates could be placed together in a circular culture
dish for ES. The electric circuit was formed of a DC power source,
positive and negative electrodes, a wire, an Ag/AgCl electrode, a
saturated KCl electrolyte, an agarose bridge and a culture dish
(filled with DMEM, supplemented with 15% FBS, 100 U/ml penicillin G
and 100 µg/ml streptomycin). The ES parameters were set to 100
mV/mm and 1 h, in accordance with a previous study (28).
Cell proliferation analysis
A Cell Counting Kit-8 (CCK-8; cat. no. C0037;
Beyotime Institute of Biotechnology) was used to detect cell
viability, according to the manufacturer's protocol. Following
treatment with CMS or ES, DRG cells were collected and adjusted to
2,000,000 cells/ml using a cell counting instrument. The cell
suspension (100 µl/well) was pipetted into a 96-well plate and
incubated in 5% CO2 at 37°C for 2 h. CCK-8 solution (10
µl/well) was added to each well and incubated in 5% CO2
at 37°C for 1 h. Finally, the optical density (OD) was measured at
450 nm using a microplate reader (Victor 3; PerkinElmer, Inc.).
A 5-ethynyl-2′-deoxyuridine (EdU)-594 cell
proliferation assay kit (cat. no. C0078; Beyotime Institute of
Biotechnology) was used to detect cell proliferation activity.
Following the treatment of each group, pre-warmed 10 µmol/l EdU
solution (2 ml/plate) was added to each of the plates, which were
incubated for 2 h in 5% CO2 at 37°C. The EdU solution
was then removed and replaced with staining fixative solution (cat.
no. P0098; Beyotime Institute of Biotechnology) (1 ml/plate). The
cells were fixed at room temperature for 15 min, then washed three
times with washing solution. Next, the cells were incubated with
permeabilization solution (cat. no. P0097; Beyotime Institute of
Biotechnology) (1 ml/plate) for 15 min at room temperature. The
Click Reaction Buffer Solution (CuS04: Azide 594: Click Additive
Solution=430:20:1:50) was configured according to the
manufacturer's instructions. After being washed twice, 0.5 ml of
Click Reaction Buffer Solution was added to each of the culture
plates, which were then incubated at room temperature for 30 min in
the dark. Finally, Hoechst 33342 was used for nuclear staining; 1
ml 1X Hoechst 33342 staining solution was added to each of the
culture plates, which were then incubated at room temperature for
10 min in the dark. Images were captured using an upright
fluorescence microscope (IX51; magnification, ×200; Olympus
Corporation).
Cell cycle detection
Cell cycle analysis was performed using the cell
cycle and apoptosis detection kit (cat. no. C1052; Beyotime
Institute of Biotechnology). DRG cells were digested using 0.25%
trypsin and washed twice with PBS following the treatment of each
group. The cells were resuspended with 75% cold ethanol and then
fixed overnight at 4°C with 75% ethanol. The next day, cells were
washed with cold PBS and dispersed to avoid cell agglomeration.
Propidium iodide (PI) staining solution [0.5 ml staining buffer, 25
µl PI (20X), 10 µl RNase A (50X)] was added to the cell pellet (0.5
ml/tube). After being mixed, the cells were incubated at 37°C for
0.5 h in the dark. Finally, flow cytometry (NovoCyte; Agilent
Technologies, Inc.) was used to detect the cell cycle distribution
of each group.
Cell apoptosis detection
Apoptotic cells were quantified using an Annexin
V/PI double staining kit (cat. no. BB-4101-2; BestBio Science)
according to the manufacturer's instructions, and detected using a
FACSCalibur flow cytometer (BD Biosciences). The apoptotic rate was
analyzed using FlowJo software (v.7.6.1; BD Biosciences). Cells
that stained positive for Annexin V and negative for PI were early
apoptotic, whereas those that were positive for both were
identified as late apoptotic cells. The apoptotic rates were
expressed as the percentage of the total cell population.
Western blotting
Intracellular proteins from the DRG cells were
extracted using RIPA lysis buffer (Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol, for 30 min on ice. Then
the cell debris was removed by centrifugation at 12,000 × g for 5
min at 4°C. The protein concentrations were determined using a
bicinchoninic acid (BCA) protein assay kit (cat. no. P0010;
Beyotime Institute of Biotechnology). Each group (30 µg/lane,
volume/lane was kept the same by using loading buffer) was
separated by 10% SDS-PAGE gel electrophoresis and transferred to
PVDF membranes. Following blocking with 5% skimmed milk powder for
1 h at room temperature, primary antibodies for β-catenin (1:1,000;
cat. no. ab32572; Abcam), glycogen synthase kinase (GSK)-3β
(1,1,000; cat. no. 12456S; Cell Signaling Technology, Inc.), Bax
(1,2000; cat. no. ab182733; Abcam), Bcl-2 (1:1,000; cat. no.
ab32124; Abcam), C-myc (1;1,000; cat. no. ab32072; Abcam) and
β-actin (1:1,000; cat. no. GB11001; Wuhan Servicebio Technology
Co., Ltd.) were added prior to incubation overnight at 4°C. The
next day, bands were washed using TBS containing 0.05% Tween and
incubated with fluorescently labeled secondary antibody
(IRDye800CW; goat anti-mouse/rabbit; 1:10,000; cat. nos. 926-32280
and 926-32211; LI-COR Biosciences) at room temperature for 1 h.
Protein bands were scanned using an Odyssey far infrared imaging
system (LI-COR Biosciences) and analyzed using Quantity One
software v.4.6.2 (Bio-Rad Laboratories, Inc.). β-actin was used as
the internal control.
XAV939 treatment
XAV939, an inhibitor of the Wnt signaling pathway,
was purchased from Selleck Chemicals (cat. no. S1180). The 10
mmol/l storage solution was diluted to 1 µmol/l working solution
with DMEM, supplemented with 15% FBS, 100 U/ml penicillin G and 100
µg/ml streptomycin. Cells were pre-treated with 1 µmol/l XAV939 at
room temperature for ~5 min prior to being treated with CMS or ES
immediately to determine cell proliferation as previously
described.
Statistical analysis
Statistical analyses were performed using GraphPad
Prism (version 7.0; GraphPad Software, Inc.), and data are
presented as the mean ± standard deviation. Groups were compared
using one-way analysis of variance. Differences between two groups
were determined using Student's t-test and multiple means were
compared using Tukey's test. P<0.05 was considered to indicate a
statistically significant difference. Each experiment was repeated
at least three times.
Results
ES promotes the viability of injured
DRG cells
CCK-8 assays were used to detect cell viability
under different intervention conditions. DRG cells in the ES group
underwent ES (100 mV/mm; 1 h), in accordance with a previous study
(28). Cells in the CMS group were
subjected to CMS [1 Hz; 8 h; 5,333 µ (4 mm)] (26). In the CMS + ES group, DRG cells
were treated with CMS, and then immediately placed in the ES device
for ES treatment. The OD values at 450 nm of the control, ES, CMS
and CMS + ES groups were 1.21±0.12, 1.64±0.15, 0.97±0.07 and
1.27±0.12, respectively. Compared with the control group, the cell
activity of the ES group was significantly increased whereas the
cell activity of the CMS group was significantly decreased
(P<0.001). In the CMS + ES group, the cell viability was
significantly increased compared with that in the CMS group
(P<0.001; Fig. 3A).
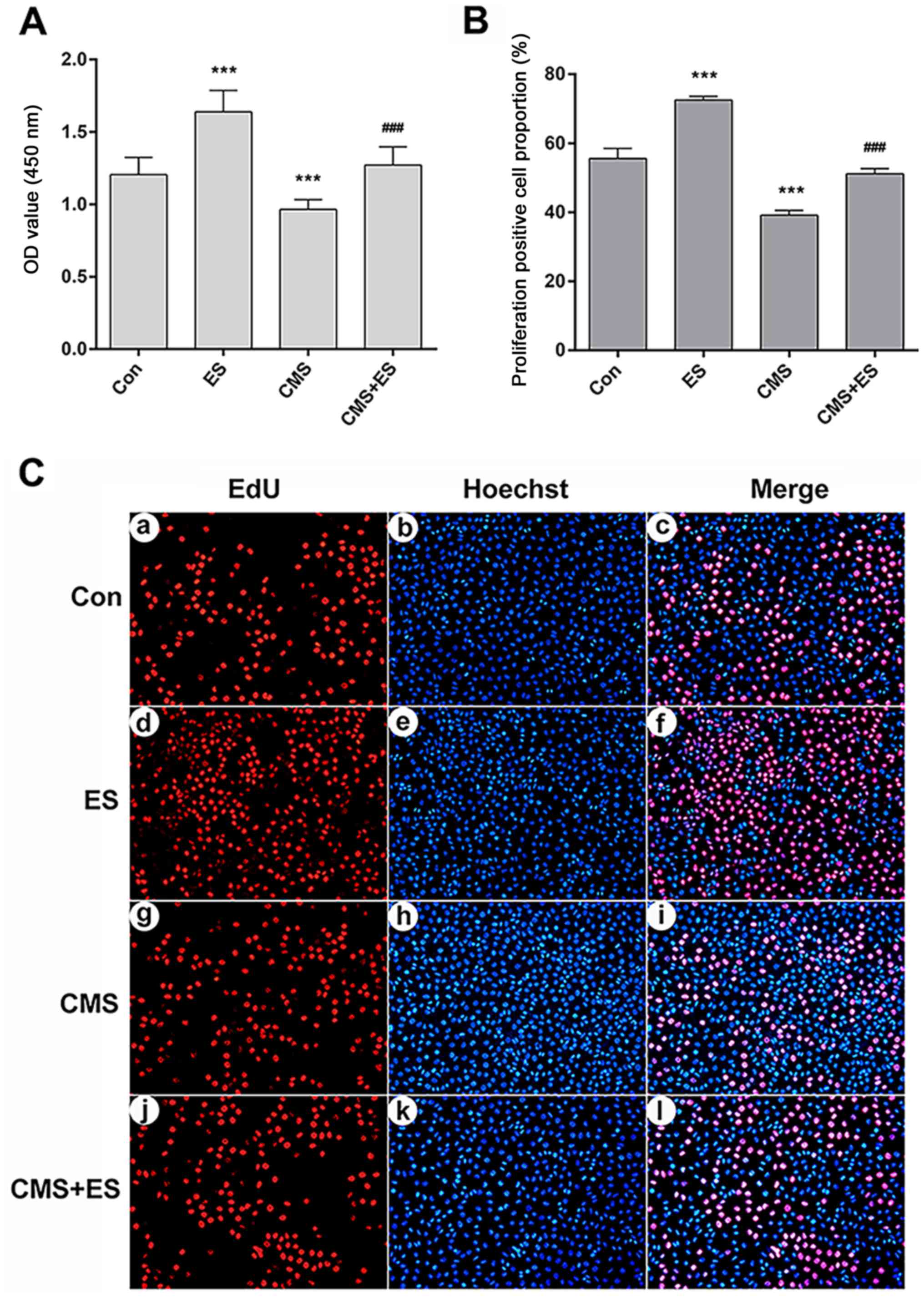 | Figure 3.Influences of ES on the viability of
injured DRG cells. (A) Cell viability analyzed using the OD value.
(B) Quantitative analysis of the proportion of EdU-positive
proliferating DRG cells. (C) EdU staining (magnifcation, ×200). Red
fluorescence represents proliferation-positive cells (a, d, g, j
represent the control, ES, CMS and CMS + ES groups respectively),
and blue fluorescence represents the nuclei of all cells in view
(b, e, h, k represent the control, ES, CMS and CMS + ES groups
respectively). Pink cells in the merged images are
proliferation-positive cells (c, f, i, l represent the control, ES,
CMS and CMS + ES groups respectively). Each set of experiments was
repeated three times. Data are expressed as the mean ± standard
deviation. ***P<0.001 vs. Con; ###P<0.001 vs. CMS
group. ES, electrical stimulation; DRG, dorsal root ganglion; Con,
control; CMS, cyclic mechanical stress; OD, optical density; EdU,
5-ethynyl-2′-deoxyuridine. |
The proliferation of DRG cells was detected using
EdU-594 cell proliferation assay kit. EdU is a novel thymidine
analogue that replaces thymidine in newly synthesized DNA during
DNA synthesis. The Azide Alexa Fluor 594 probe covalently reacts
with the acetylene group of EdU, which ensures direct and accurate
detection of proliferating cells. Hoechst 33342 was used for
nuclear staining. Following treatment, healthy DRG cells would show
a normal blue nucleus under a fluorescence microscope, whereas the
nuclei of apoptotic cells would be stained white. Both blue and red
fluorescence showed the nucleus, where blue represented all DRG
cells in the field of view, and red represented the proliferating
cells (Fig. 3C). A total of three
fields of view were randomly selected from each group of cells, and
the positive rates of cell proliferation were then counted. The
proliferating cell proportion of the control, ES, CMS and CMS + ES
groups were 55.55±2.96, 72.51±1.10, 39.17±1.34 and 51.13±1.54%,
respectively. The proportion of positive proliferating DRG cells
was significantly increased, whereas this proportion in the CMS
group was significantly decreased compared with the control group
(P<0.001). Furthermore, the rate of cell proliferation in the
CMS + ES group was higher than that of the CMS group (P<0.001;
Fig. 3B).
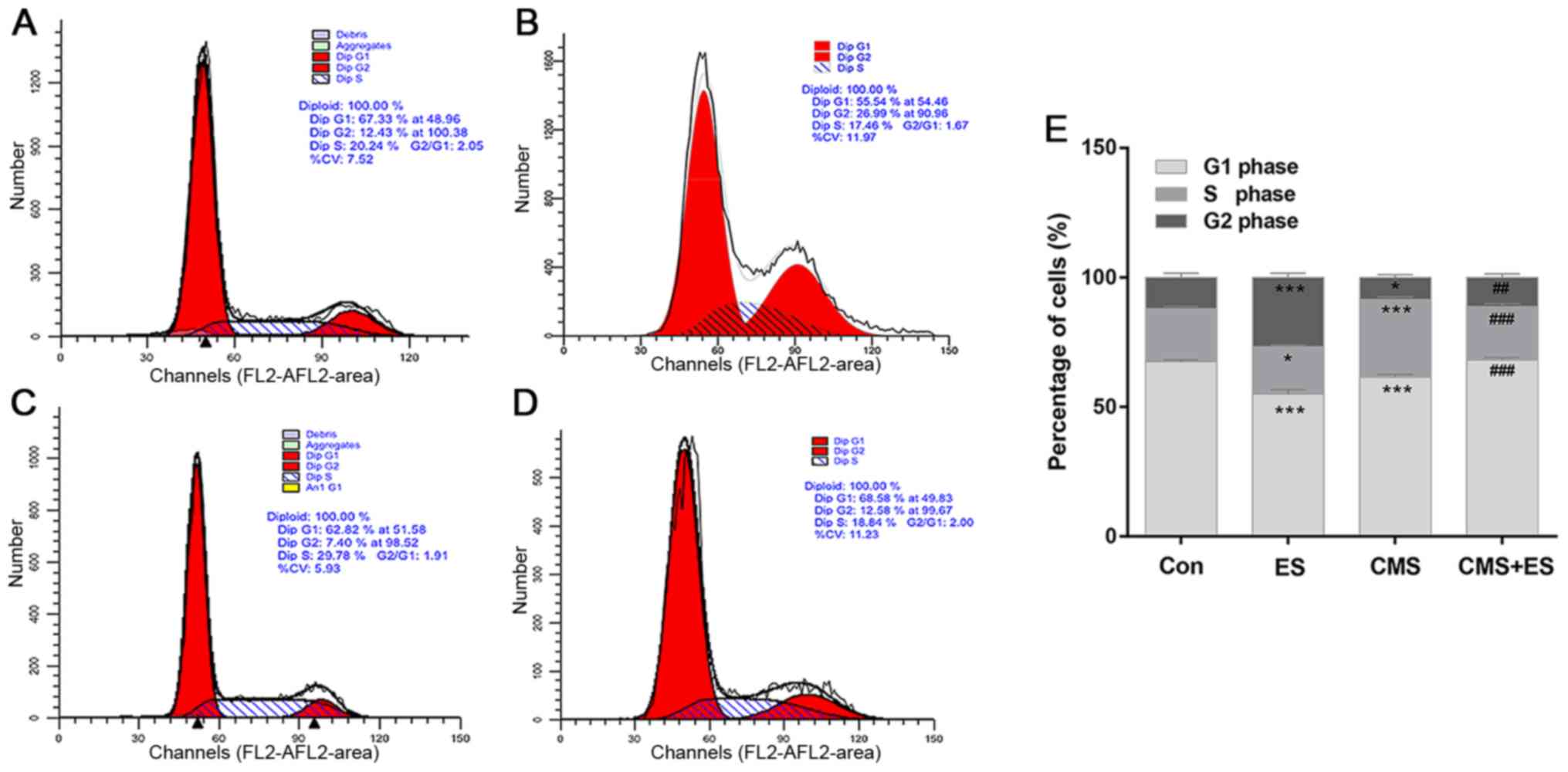
ES reverses CMS-induced cell cycle
arrest and decreases DRG cell apoptosis
Flow cytometry was used to detect cell cycle and
cell apoptosis. The present study demonstrated that the number of
DRG cells in the G2 phase in the ES group was significantly
increased compared with the control group (P<0.001). The number
of cells in the S phase of the CMS group was significantly higher
than that of the control group (P<0.001). Furthermore, the
number of cells in the S phase in the CMS + ES group was
significantly lower than that in the CMS group (P<0.001), and
the number of cells in the G2 phase was significantly increased
(P<0.01; Fig. 4 and Table I). These changes indicated that ES
has significant roles in promoting the progression of the cell
cycle in normal DRG cells. In addition, CMS arrested DRG cells in
the S phase, which impeded cell cycle progression. However, the
results of the present study indicate that this process may be
reversed following ES treatment.
 | Table I.Effects of ES on cell cycle of DRG
cells. |
Table I.
Effects of ES on cell cycle of DRG
cells.
| Group | G0/G1 phase, % | S phase, % | G2 phase, % |
|---|
| Con | 67.47±0.76 | 20.40±0.96 | 12.13±1.71 |
| ES |
55.01±1.69b |
18.24±0.68a |
26.75±1.65b |
| CMS |
61.45±1.07b |
30.02±0.99b |
8.53±1.08a |
| CMS + ES |
68.12±1.00d |
19.58±1.03d |
12.30±0.55c |
| F-value | 107.50 | 134.90 | 146.20 |
| P-value | <0.001 | <0.001 | <0.001 |
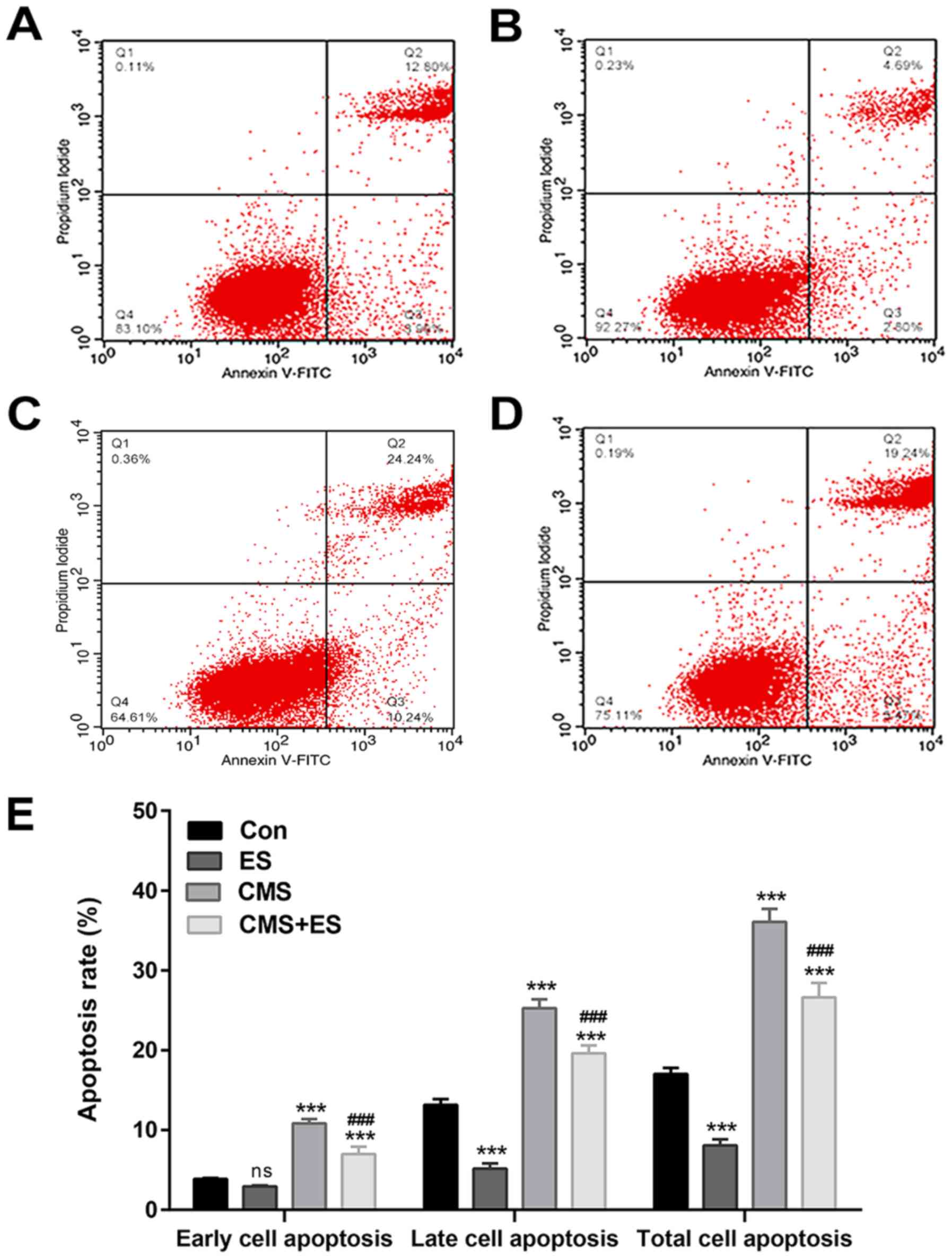
Cells that stained positive for Annexin V and
negative for PI (Annexin V-FITC+/PI−) were
early apoptotic, whereas those that stained positive for both
(Annexin V-FITC+/PI+) were late apoptotic.
The present study demonstrated that the apoptosis rate of the CMS
group was significantly higher than that of the control group
(P<0.001), whereas the total apoptotic rate of the ES group was
significantly lower compared with the control group (P<0.001).
Moreover, the apoptosis rate of DRG cells in the CMS + ES group was
decreased compared with the CMS group (P<0.001; Fig. 5 and Table II). These results were consistent
with the results of the cell viability and cell cycle findings in
the present study, confirming the promotive effects of ES on DRG
cells.
 | Table II.Effects of ES on cell apoptosis of
DRG cells. |
Table II.
Effects of ES on cell apoptosis of
DRG cells.
| Group | Annexin V+/PI-Early
apoptosis, % | Annexin V+/PI+Late
apoptosis, % | Total apoptosis,
% |
|---|
| Con |
3.860±0.110 | 13.140±0.730 | 17.010±0.780 |
| ES |
2.920±0.130a |
5.170±0.640b |
8.090±0.740b |
| CMS |
10.837±0.524b |
25.263±1.132b |
36.100±1.610b |
| CMS + ES |
6.980±0.940b,c |
19.640±0.960b,c |
26.620±1.820b,c |
| F-value | 128.800 |
285.600 |
247.900 |
| P-value |
<0.001 |
<0.001 |
<0.001 |
Effect of ES on the expression levels
of Wnt/β-catenin pathway-associated proteins in injured DRG
cells
The expression levels of β-catenin, Bcl-2 and C-myc
increased following ES treatment (P<0.05 and P<0.001), and
the expression levels of these three proteins in the CMS group were
significantly decreased (P<0.001) in comparison with those in
the control group. The present study demonstrated that the
expression levels of β-catenin, Bcl-2 and C-myc in the CMS + ES
group were also increased compared with those in the CMS group, and
that these differences were statistically significant (P<0.05
and P<0.001; Fig. 6A, B, E and
F). In contrast, the expression levels of GSK-3β and Bax in the
ES group were lower than those in the control group (P<0.05),
and the expression levels of these two proteins in the CMS group
were significantly increased (P<0.001). Furthermore, the
expression levels of GSK-3β and Bax in the CMS + ES group were
decreased compared with those in the CMS group (P<0.05; Fig. 6A, C and D).
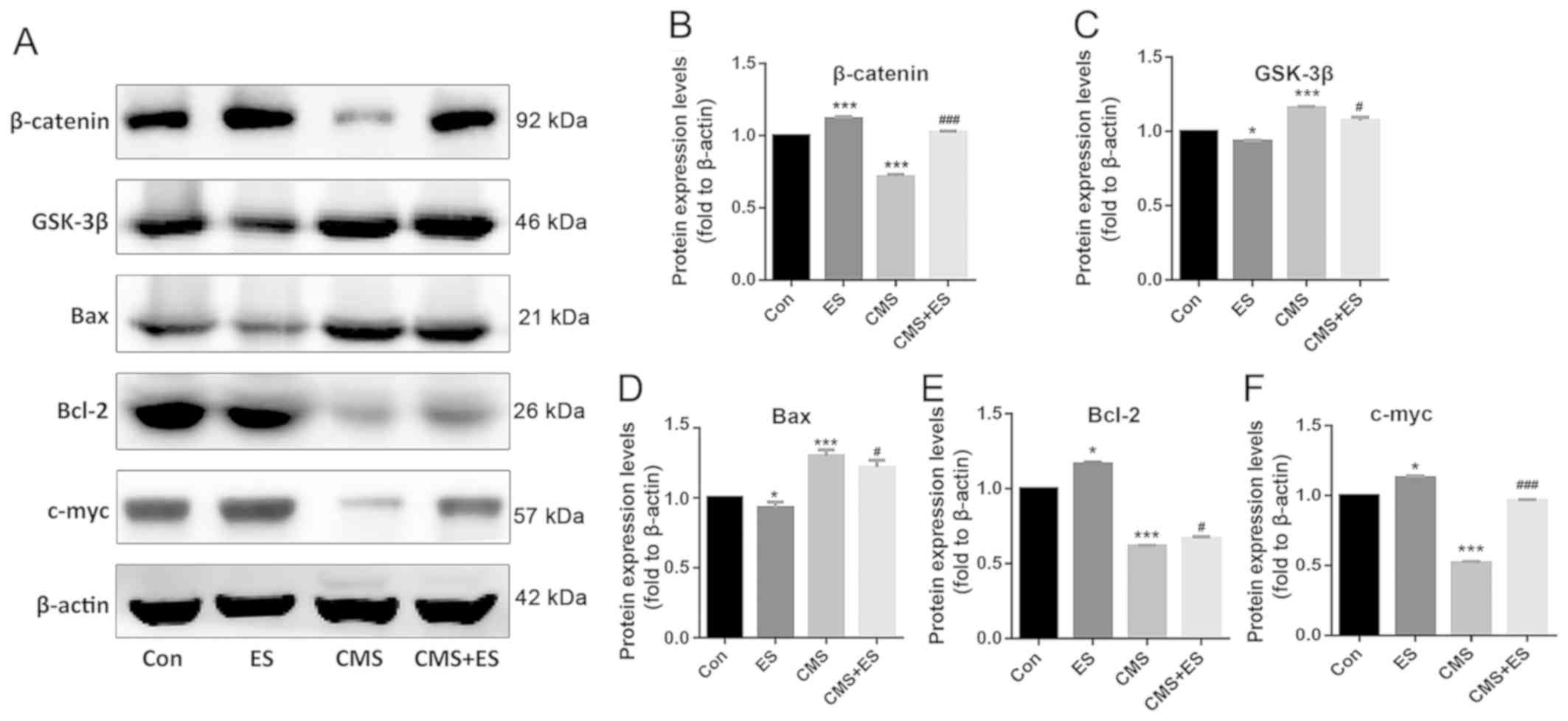 | Figure 6.Effect of ES on the expression levels
of Wnt/β-catenin pathway-related proteins in injured DRG cells. (A)
Representative blots of β-catenin, Bax, Bcl-2 and C-myc.
Quantitative analysis of protein expression levels of (B)
β-catenin, (C) GSK-3β, (D) Bax, (E) Bcl-2 and (F) C-myc. Each set
of experiments was repeated three times. Data are expressed as the
mean ± standard deviation. *P<0.05, ***P<0.001 vs. Con;
#P<0.05, ###P<0.001 vs. CMS group. ES,
electrical stimulation; Con, control; CMS, cyclic mechanical
stretching; GSK-3β, glycogen synthase kinase-3β. |
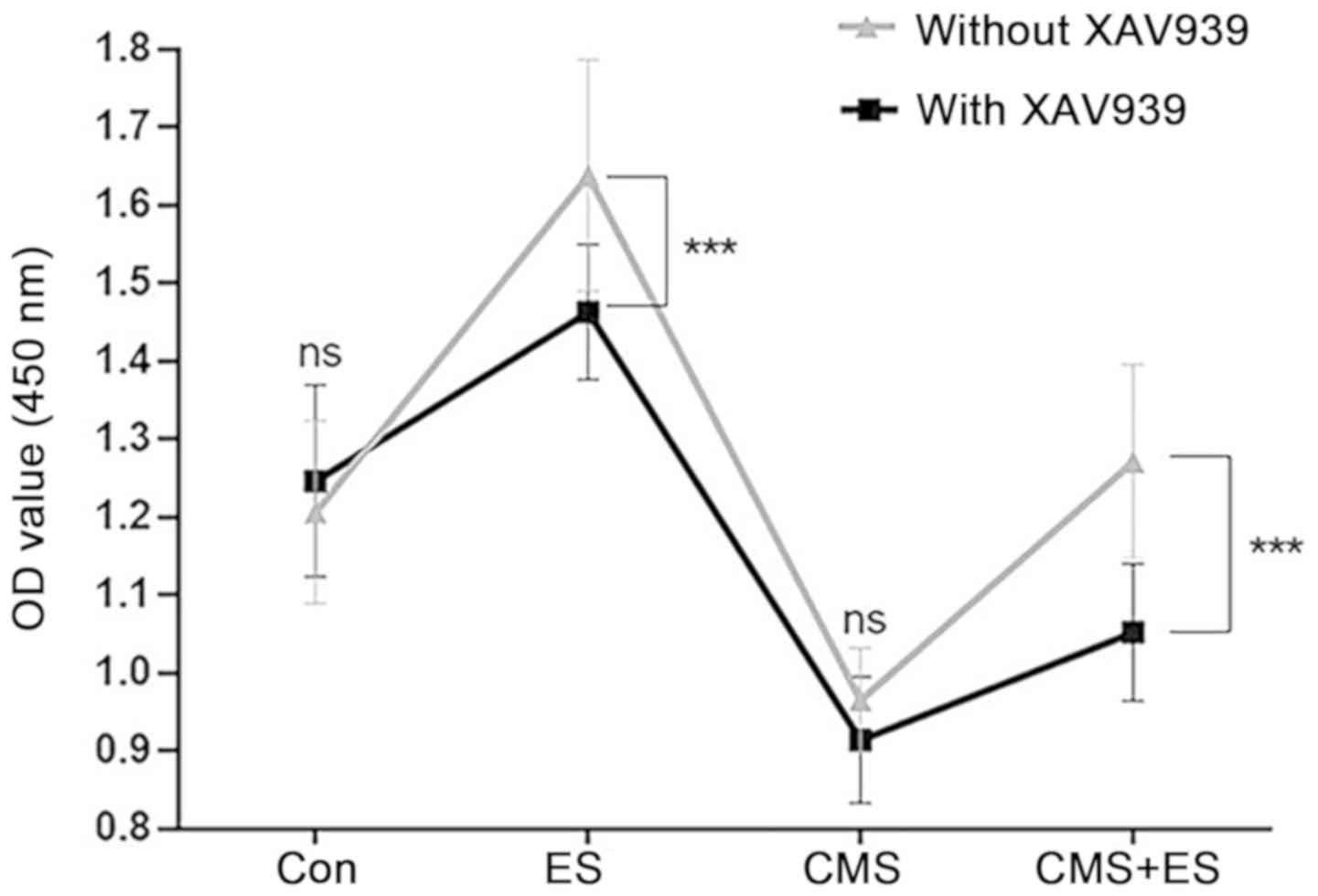
Wnt/β-catenin pathway is potentially
involved in the influence of ES over injured DRG cells
It was hypothesized that the Wnt/β-catenin signaling
pathway was involved in the process by which ES affects DRG cells
because of the detected protein changes associated with the
Wnt/β-catenin pathway. The present study used the Wnt/β-catenin
pathway inhibitor XAV939 at a concentration of 1 µmol/l, which did
not affect DRG cell viability by itself (Fig. 7). The present study demonstrated
that DRG cells in the ES group showed a significant decrease in
activity following administration of XAV939, and similar changes
were observed in the CMS + ES group (P<0.001). However,
following exposure to XAV939, the cell activity in the CMS + ES
group was still slightly increased compared with the CMS group
(P<0.001), indicating that other pathways may regulate the
effects of ES on DRG cells, although this requires verification in
future studies (Fig. 7).
Discussion
With the increasing trend towards an aging
population, the incidence of SUI is increasing by 4–10% per year
globally (1). The multi-center
large sample survey conducted by Zhu et al (3) demonstrated that the overall incidence
of urinary incontinence is 30.9%, of which SUI accounts for 61%,
among 19,024 female subjects. Therefore, research regarding the
etiology and treatment of SUI may have important applications.
Clinically, PES treatment has a significant effect on patients with
mild-to-moderate SUI symptoms: Its cure rate is 30–60%, and its
improvement rate is 60–90% (6–8). To
the best of our knowledge, however, the mechanism of PES in the
treatment for SUI has not previously been identified. Stretching
injury of pelvic floor nerves, muscles and connective tissue caused
by pregnancy and vaginal delivery are major risk factors for SUI
(29). Delancey (11) proposed that PN injury causes
denervation of pelvic floor muscles. Previous studies of pelvic
floor electromyography of patients with SUI, nerve conduction
velocity, pelvic floor muscle pathology and neurofibrillary
immunohistochemical staining have also demonstrated that pelvic
denervation is present in patients with SUI (9,30,31).
These studies indicate that PN injury is important in the
pathogenesis of SUI. In studies of neurological diseases, ES has
been demonstrated to promote synaptic repair, nerve cell growth and
regeneration (12–14) Therefore, it was hypothesized that
the therapeutic effect of PES in the treatment of SUI was achieved
by promoting the repair of injured PNs.
Culturing nerve cells in vitro provides a
basis for the study of nerve cells at the molecular and cellular
levels. Nerve cells in DRGs are widely used in neuroscience
research, particularly in the study of peripheral nerve injury and
peripheral neuropathy (32–34).
Therefore, the present study used DRG cells. In addition, it has
been demonstrated that PN injury in SUI is primarily caused by
nerve traction injury during pregnancy and childbirth (29,35,36).
In order to simulate this phenomenon at the cellular level, DRG
cells were subjected to CMS. A number of studies have constructed
cell traction injury models by mechanical stretching or deformation
by applying pressure to cells (37,38).
Our previous studies have also constructed a fibroblast model by
CMS successfully, which demonstrated that CMS can simulate traction
injury in fibroblasts (39–41).
In addition, our previous study subjected DRG cells to CMS and
demonstrated that the DRG injury could be best simulated under the
parameters of 5,333 µ strain (4 mm), 8 h and 1 Hz (26), which was used in the present study.
There are two main methods of applying ES to cells: Direct ES and
indirect ES (42). The salt bridge
system used in the present study could directly stimulate DRG cells
in order to simulate pudendal nerve electrical stimulation, which
is a direct ES method clinically.
The present study established CMS and ES models in
DRG cells. The present study demonstrated that ES of 100 mV/mm for
1 h increased the proliferative activity of untreated DRG cells. In
addition, CMS of 5,333 µ strain for 8 h significantly decreased
cell proliferation activity, which was attenuated by ES of 100
mV/mm for 1 h. These results demonstrated that ES may alleviate
damage to DRG cells caused by mechanical force. The present study
also measured changes in the cell cycle distribution and apoptosis
of DRG cells. The cell cycle is regulated by two phase transition
points, G1/S and G2/M. In the present study, the proportion of
cells in the G2 phase significantly decreased in the CMS group,
whereas the proportion of cells in the S phase and the apoptosis
rate increased. Combined with the detection of cell activity, the
present study demonstrated that CMS blocked the DRG cell cycle in
the S phase, blocked cell mitosis and ultimately led to cell
apoptosis. ES decreased the proportion of cells in the S phase,
increased the proportion of cells in the G2 phase, advanced cell
cycle progression and decreased the rate of apoptosis.
A number of studies have confirmed that the
Wnt/β-catenin classical signaling pathway is closely associated
with the nervous system, and is involved in the processes of
proliferation and differentiation of neural stem cells and axonal
formation (43–45). Previous studies have demonstrated
that ES treatment alters the expression levels of Wnt
pathway-associated factors (18)
and regulates directional migration of neural cells (19). The present study therefore
investigated the expression levels of these proteins. The present
study demonstrated that CMS increased the expression level of
GSK-3β protein, decreased the expression levels of β-catenin
protein and inhibited the Wnt/β-catenin pathway, whereas ES
decreased GSK-3β, increased β-catenin, and further activated the
Wnt/β-catenin signaling pathway. This indicated that both CMS and
ES have effects on the classical Wnt/β-catenin pathway.
C-myc protein is an important downstream molecule of
the Wnt/β-catenin signaling pathway and is consistent with the
expression level of β-catenin (46). In addition, the Bcl-2 family plays
an important role in apoptosis. Bcl-2, an anti-apoptotic gene,
plays an antagonistic role with Bax, a pro-apoptotic gene, in the
apoptosis process (47). The
present study demonstrated that in the CMS group, the expression
levels of Bcl-2 protein decreased and the expression levels of Bax
protein increased, inducing the apoptosis of DRG cells. Conversely,
the expression levels of Bcl-2 protein increased and the expression
levels of Bax protein decreased when DRG cells were subjected to
ES. These results indicated that the Wnt/β-catenin signaling
pathway may be involved in the process by which ES promotes the
cell viability of DRG cells.
The present study used XAV939, an inhibitor of Wnt,
to investigate the role of Wnt/β-catenin signaling. The present
study demonstrated that the cell proliferation activity promoted by
ES was partly inhibited following administration of XAV939. This
indicated that the Wnt signaling pathway may be involved in the
activation of DRG cells by ES. Further research is required to
identify other pathways that may be involved in this process.
Meanwhile, it was previously demonstrated that the expression
levels of caspase-3 in injured DRG cells treated with ES were
significantly decreased (48).
Caspase is a classical apoptosis-associated factor. Future studies
will investigate how it participates in the regulation of ES and
CMS on DRG cells.
Although PES is a clinically effective means of SUI
treatment, little is currently known regarding its molecular
mechanisms. The present study demonstrated the protective effect of
ES on DRG cells with CMS-induced injury at the cellular level. ES
may promote the proliferative activity of DRG cells, reverse S
phase arrest and limit the CMS-induced increase in the rate of
apoptosis. Furthermore, both CMS and ES exerted effects on the
classical Wnt/β-catenin pathway, and also affected the expression
levels of downstream proteins, which indicated that the
Wnt/β-catenin signaling pathway may be involved in the response of
DRG cells to CMS and ES. The present study therefore provides a
theoretical basis to further investigate the specific mechanism of
ES in the treatment of SUI.
Acknowledgements
The authors would like to thank Dr Lijuan Gu, Dr
Lina Zhou and Mrs. Yingxia Jin of the Central Laboratory of Renmin
Hospital of Wuhan University for their experimental and technical
support and discussions of the manuscript.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81771562) and the
Chinese Women's Pelvic Floor Dysfunction Prevention Project of
Chinese Preventive Medicine Association (grant no. 201817092).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
MH performed the majority of the experiments and
drafted the manuscript; SH, GH, YC and QC helped perform the
experiments and analyze the data; and MH and LH conceived the
study, supervised the experiments and edited the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Reynolds WS, Dmochowski RR and Penson DF:
Epidemiology of stress urinary incontinence in women. Curr Urol
Rep. 5:370–376. 2011. View Article : Google Scholar
|
|
2
|
Fultz NH and Herzog AR: Self-reported
social and emotional impact of urinary incontinence. J Am Geriatr
Soc. 49:892–899. 2010. View Article : Google Scholar
|
|
3
|
Zhu L, Lang JH, Liu CY, Han SM, Huang JS
and Li XM: The epidemiological study of women with urinary
incontinence and risk factors for stress urinary incontinence in
China. Menopause. 107 (Suppl):S2362009.
|
|
4
|
Chong EC, Khan AA and Anger JT: The
financial burden of stress urinary incontinence among Women in the
United States. Curr Urol Rep. 12:358–362. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wilson L, Brown JS, Shin GP, Luc KO and
Subak LL: Annual direct cost of urinary incontinence. Obstetrics
Gynecol. 98:398–406. 2001. View Article : Google Scholar
|
|
6
|
Jeyaseelan SM and Oldham JA: Electrical
stimulation as a treatment for stress incontinence. Br J Nurs.
9:10012000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jeyaseelan SM, Haslam EJ, Winstanley J,
Roe BH and Oldham JA: An evaluation of a new pattern of electrical
stimulation as a treatment for urinary stress incontinence: A
randomized, double-blind, controlled trial. Clin Rehabil.
14:631–640. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Indrekvam S, Sandvik H and Hunskaar S: A
Norwegian national cohort of 3198 women treated with home-managed
electrical stimulation for urinary incontinence-effectiveness and
treatment results. Scand J Urol Nephrol. 35:32–39. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhu L, Lang J and Chen J and Chen J: Study
on nerve fiber density in anterior vaginal epithelium for stress
urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct.
15:272–275. 2004.PubMed/NCBI
|
|
10
|
Yoshida S, Koyama M, Kimura T, Murakami G,
Niikura H, Takenaka A and Murata Y: A clinicoanatomical study of
the novel nerve fibers linked to stress urinary incontinence: The
first morphological description of a nerve descending properly
along the anterior vaginal wall. Clin Anat. 20:300–306. 2010.
View Article : Google Scholar
|
|
11
|
Delancey JO: Anatomy and biomechanics of
genital prolapse. Clin Obstetrics Gynecol. 136:897–909. 1993.
View Article : Google Scholar
|
|
12
|
Liu Y, Grumbles RM and Thomas CK:
Electrical stimulation of embryonic neurons for 1 hour improves
axon regeneration and the number of reinnervated muscles that
function. J Neuropathol Exp Neurol. 72:697–707. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gordon T, Udina E, Verge VM and de Chaves
EI: Brief electrical stimulation accelerates axon regeneration in
the peripheral nervous system and promotes sensory axon
regeneration in the central nervous system. Motor Control.
13:412–441. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Boggio PS, Valasek CA, Campanhã C, Giglio
AC, Baptista NI, Lapenta OM and Fregni F: Non-invasive brain
stimulation to assess and modulate neuroplasticity in Alzheimer's
disease. Neuropsychol Rehabil. 21:703–716. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ille F and Sommer L: Wnt signaling:
Multiple functions in neural development. Cell Mol Life Sci.
62:1100–1108. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
David MD, Cantí C and Herreros J: Wnt-3a
and Wnt-3 differently stimulate proliferation and neurogenesis of
spinal neural precursors and promote neurite outgrowth by canonical
signaling. J Neurosci Res. 88:3011–3023. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Suh HI, Min J, Choi KH, Kim SW, Kim KS and
Jeon SR: Axonal regeneration effects of Wnt3a-secreting fibroblast
transplantation in spinal cord-injured rats. Acta Neurochir.
153:1003–1010. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wu Y, Collier L, Qin W, Creasey G, Bauman
WA, Jarvis J and Cardozo C: Electrical stimulation modulates Wnt
signaling and regulates genes for the motor endplate and calcium
binding in muscle of rats with spinal cord transection. BMC
Neurosci. 14:812013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu J, Zhu B, Zhang G, Wang J, Tian W, Ju
G, Wei X and Song B: Electric signals regulate directional
migration of ventral midbrain derived dopaminergic neural
progenitor cells via Wnt/GSK3β signaling. Exp Neurol. 263:113–121.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang K, Guo J, Ge Z and Zhang J:
Nanosecond pulsed electric fields (nsPEFs) regulate phenotypes of
chondrocytes through Wnt/β-catenin signaling pathway. Sci Rep.
4:58362014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hunter T and Pines J: Cyclins and cancer
II: Cyclin D and CDK inhibitors come of age. Cell. 79:573–582.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cancer Genome Atlas Network, . Muzny DM,
Bainbridge MN, Chang K, Dinh HH, Drummond JA, Fowler G, Kovar CL,
Lewis LR, Morgan MB, et al: Comprehensive molecular
characterization of human colon and rectal cancer. Nature.
487:330–337. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang H, Wang H, Makki MS, Wen J, Dai Y,
Shi Q, Liu Q, Zhou X and Wang J: Overexpression of β-catenin and
cyclinD1 predicts a poor prognosis in ovarian serous carcinomas.
Int J Clin Exp Pathol. 7:264–271. 2013.PubMed/NCBI
|
|
24
|
Lei ZJ, Wang J, Xiao HL, Guo Y, Wang T, Li
Q, Liu L, Luo X, Fan LL, Lin L, et al: Lysine-specific demethylase
1 promotes the stemness and chemoresistance of Lgr5+ liver cancer
initiating cells by suppressing negative regulators of β-catenin
signaling. Oncogene. 34:32142015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wu S, Guo Z, Hopkins CD, Wei N, Chu E,
Wipf P and Schmitz JC: Bis-cyclopropane analog of disorazole C1is a
microtubule-destabilizing agent active in ABCB1-overexpressing
human colon cancer cells. Oncotarget. 6:40866–40879. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhou M, Hu M, He S, Li B, Liu C, Min J and
Hong L: Effects of rsc96 schwann cell-derived exosomes on
proliferation, senescence, and apoptosis of dorsal root ganglion
cells in vitro. Med Sci Monit. 24:7841–7849. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Song B, Gu Y, Pu J, Reid B, Zhao Z and
Zhao M: Application of direct current electric fields to cells and
tissues in vitro and modulation of wound electric field in vivo.
Nat Protoc. 2:1479–1489. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hu M, Hong L, Liu C, Hong S, He S, Zhou M,
Huang G and Chen Q: Electrical stimulation enhances neuronal cell
activity mediated by Schwann-cell-derived exosomes. Sci Rep.
9:42062019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Clark MH, Scott M, Vogt V and Benson JT:
Monitoring pudendal nerve function during labor. Obstet Gynecol.
97:637–639. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bakas P, Liapis A, Karandreas A and
Creatsas G: Pudendal nerve terminal motor latency in women with
genuine stress incontinence and prolapse. Gynecol Obstet Invest.
51:187–190. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Weidner AC, Barber MD, Visco AG, Bump RC
and Sanders DB: Pelvic muscle electromyography of levator ani and
external anal sphincter in nulliparous women and women with pelvic
floor dysfunction. Am J Obstet Gynecol. 183:1390–1401. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wood MD and Willits RK: Applied electric
field enhances DRG Neurite growth: Influence of stimulation media,
surface coating and growth supplements. J Neural Eng. 6:460032009.
View Article : Google Scholar
|
|
33
|
Stewart AL, Anderson RB, Kobayashi K and
Young HM: Effects of NGF, NT-3 and GDNF family members on neurite
outgrowth and migration from pelvic ganglia from embryonic and
newborn mice. BMC Dev Biol. 8:732008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang W, Hu Y, Newman EA and Mulholland
MW: Serum-free culture of rat postnatal neurons derived from the
dorsal motor nucleus of the vagus. J Neurosci Meth. 150:1–7. 2006.
View Article : Google Scholar
|
|
35
|
Lien KC, Morgan DM, Delancey JO and
Ashton-Miller JA: Pudendal nerve stretch during vaginal birth: A 3D
computer simulation. Am J Obstet Gynecol. 192:1669–1676. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Efrat H, Asnat G, Ronen G, Joseph L and
David G: Pregnancy, labor and delivery: The pelvic floor injury.
Harefuah. 143:525–529, 547, 548. 2004.PubMed/NCBI
|
|
37
|
Ellis EF, Mckinney JS, Willoughby KA,
Liang S and Povlishock JT: A new model for rapid stretch-induced
injury of cells in culture: Characterization of the model using
astrocytes. J Neurotrauma. 12:325–339. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Morrison B III, Meaney DF and McIntosh TK:
mechanical characterization of an in vitro device designed to
quantitatively injure living brain tissue. Ann Biomed Eng.
26:381–390. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hu M, Hong L, Hong S, Min J, Zhao Y, Yang
Q, Zhang Q, Tang J and Li Y: Mechanical stress influences the
viability and morphology of human parametrial ligament fibroblasts.
Mol Med Rep. 15:853–858. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li BS, Guo WJ, Hong L, Liu YD, Liu C, Hong
SS, Wu DB and Min J: Role of mechanical strain-activated PI3K/Akt
signaling pathway in pelvic organ prolapse. Mol Med Rep.
14:243–253. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hong S, Li H, Wu D, Li B, Liu C, Guo W,
Min J, Hu M, Zhao Y and Yang Q: Oxidative damage to human
parametrial ligament fibroblasts induced by mechanical stress. Mol
Med Rep. 12:5342–5348. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Balint R, Cassidy NJ and Cartmell SH:
Electrical stimulation: A novel tool for tissue engineering. Tissue
Eng Part B Rev. 19:48–57. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Moon RT, Kohn AD, De Ferrari GV and Kaykas
A: WNT and beta-catenin signalling: Diseases and therapies. Nat Rev
Genet. 5:691–701. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Clevers H: Wnt/beta-catenin signaling in
development and disease. Cell. 127:469–480. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Inestrosa NC and Toledo EM: The role of
Wnt signaling in neuronal dysfunction in Alzheimer's Disease. Mol
Neurodegener. 3:92008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Koehler A, Schlupf J, Schneider M, Kraft
B, Winter C and Kashef J: Loss of Xenopus cadherin-11 leads to
increased Wnt/β-catenin signaling and up-regulation of target genes
c-myc and cyclin D1 in neural crest. Dev Biol. 383:132–145. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lei K, Nimnual A, Zong WX, Kennedy NJ,
Flavell RA, Thompson CB, Bar-Sagi D and Davis RJ: The Bax subfamily
of Bcl2-related proteins is essential for apoptotic signal
transduction by c-Jun NH(2)-terminal kinase. Mol Cell Biol.
22:4929–4942. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Huang G, Hong L, Hu M, Zhou M, Chen Q and
Lu D: Protective role of electrical stimulation in mechanical
damage of rat dorsal root ganglion cells. J Chin Practical
Diagnosis Ther. 7:646–649. 2019.(In Chinese).
|