Introduction
Pancreatic encephalopathy (PE) is a potentially
fatal neurological syndrome resulting from acute pancreatic
disease, with typical symptoms, including inflammatory or
autoimmune reactions, idiopathic nervous system lesions and a
neurovegetative state (1). PE is
an uncommon complication of severe acute pancreatitis (SAP).
Neurological signs can have an acute onset, with convulsions,
amaurosis, paresis and dysarthria, or there can be a progressive
onset with behavioral changes, psychomotor agitation, space and
time disorientation, visual or auditory hallucinations, and
affected consciousness that can lead to coma (2). Important elements involved in the
complicated evolution of SAP include cell factors, including
cytokines such as tumor necrosis factor (TNF)-α, and interleukin
(IL)-1β, −6 and −10 (3). In
pancreatitis, granulocytes, monocytes and lymphocytes are recruited
by local inflammatory processes caused by the release of
proinflammatory and anti-inflammatory cytokines and chemokines
(4). During pancreatic injury,
atrophic acinar cells activate macrophages and granulocytes to
release several inflammatory factors, such as IL-1, IL-6 and TNF-α
(5). These proinflammatory factors
can activate pancreatic stellate cells to accelerate the
development of pancreatitis (6).
SAP complications, including respiratory insufficiency and hypoxia,
can cause abnormal metabolism and cerebral edema (7). The above factors, as part of the
multiple organ failure that can occur during PE, lead to a
potentially fatal neurological syndrome. The neurological lesions
associated with PE probably result from oxidative stress, cytokine
storm and enzyme-derived damage (8), but the detailed pathophysiology
mechanisms remain to be elucidated.
Adler et al (9) found that caerulein can cause severe
pancreatitis during pancreatic necrosis. Caerulein, a molecular
ortholog of the intestinal hormone cholecystokinin, is derived from
the skin of Litorea caerulea (10). Caerulein promotes the procession of
PE via the disruption of collagen and causes pancreatic fibrosis,
initiating edema and exposing neurons to peripheral proinflammatory
factors, including TNF-α and IL-1β, which in turn trigger local
neuroinflammation, neurodegeneration and demyelination (11). Caerulein also leads to inflammatory
cell infiltration, pancreatic edema and acinar cell vacuolization
that are comparable to acute pancreatitis in humans. Indeed,
caerulein exposure is often used to simulate PE in vitro or
in vivo to explore the pathogenic mechanisms of PE (12).
The NF-κB family are a group of structurally related
transcription factors that consist of various combinations of NF-κB
and Rel proteins (13). NF-κB
signaling regulates the expression and release of proinflammatory
factors such as TNF-α, IL-1β and IL-6 (14). Transcriptional activity is
modulated by the IκB kinase (IKK) complex that regulates the
transcriptional activity of NF-κB, as well as IκBα-inhibited NF-κB
phosphorylation (15). The IKK
subunit IKKβ is a critical regulator of inflammation via the
TNF-induced IκB phosphorylation/degradation pathway and so may
regulate the neuroinflammatory response and ensuing nerve cell
damage during PE.
Soluble TNF receptors (TNFRs) generated from the
cleavage of the TNFR extracellular domain can be used to inhibit
TNF-α signaling during inflammatory responses (16). Etanercept is a 934-amino acid (~150
kDa) recombinant dimeric fusion protein consisting of human
immunoglobulin G linked via Fc (the constant region) and the
extracellular ligand-binding domains of human soluble TNFR2
(17). The dimeric structure of
etanercept increases the TNF-binding affinity ~50-fold, which can
enhance TNF-neutralizing capacity ~1,000-fold over monomeric TNFR2
(18,19).
During acute pancreatitis (AP) progression, local
inflammatory cell responses to common proinflammatory cytokines,
including TNF-α, could further enhance the inflammatory response by
upregulating the expression of IKKβ and subsequent activation of
NF-κB signaling (20). These data
suggest that etanercept may suppress damaging neuroinflammation
during PE by disrupting the TNF-α/NF-κB signaling pathway, but this
has not been demonstrated directly. Therefore, the aim of the
current study was to assess the effect of etanercept on
neuroinflammation and nerve cell protection in a caerulein-induced
PE model.
Materials and methods
Animals and groups
The Jinan University Laboratory Animal Welfare and
Ethics Committee charter approved all procedures involving rats.
All protocols also conformed to the guidelines of Animal Use and
Care of the National Institutes of Health (21). In vivo experiments were
conducted on 8-week-old male Sprague–Dawley (SD) rats (weight,
250–300 g) purchased from the Southern Medical University animal
center (Guangzhou, Guangdong). Rats were housed in rectangular
polypropylene cages under a controlled temperature (20±1°C), with a
humidity of 50–60% and a 12-h light/dark cycle with ad
libitum access to food and water. The mortality rate of the
animals during the modeling period was 58%; animals were euthanized
when they reached a humane endpoint (22). Rats were anesthetized or euthanized
with intraperitoneal (IP) injection of pentobarbital sodium (50 or
120 mg/kg, respectively) based on previously described methods
(23,24). To confirm the vital signs of
experimental animals, the vital signs (including spontaneous
breathing and pupil size) were observed on an hourly basis. No
spontaneous breathing, heartbeat, dilated pupils, and direct and
indirect light reflection of bilateral pupils were used to indicate
that the animals had succumbed. Finally, the surviving experimental
animals were euthanized by IP injection of pentobarbital sodium and
then the tissue samples were quickly collected. Dead animals and
euthanized animals were placed in waste bags, temporarily stored in
freezers, and finally recycled and disposed of by the University
Experimental Animal Center. In the process of modeling, the main
causes of death in experimental animals were considered as brain
edema, cerebral hernia and shock. All the surgical interventions
were performed under sterile conditions and strictly complied with
the requirements of NC3Rs guidelines (25).
A total of 30 SD rats were randomly separated into
three groups (n=10/group): The sham group was the control group,
and was subjected to neck skin incision and closure, followed by
injection with an equivalent volume of saline. The Caerulein group
was the PE model group (n=10) and received the same surgical
procedure, but were injected with 50 µg/kg caerulein (26) through the jugular vein. The
Caerulein + EP group was the PE model + EP group and received the
same surgical procedure, but was treated with 50 µg/kg caerulein
and 10 mg/kg etanercept (Immunex Corporation) (27) through the jugular vein. All animals
were sacrificed at 6 h after injection according to the previous
literature (28) for histochemical
and biochemical analyses.
Cell culture and treatment
Rat hippocampal H19–7/IGF-IR cells (29) were obtained from the American Type
Culture Collection (cat. no. CRL-2526™) and cultured in Dulbecco's
modified Eagle's medium (DMEM; Invitrogen; Thermo Fisher
Scientific, Inc.) supplemented with 0.2 mg/ml G418, 0.001 mg/ml
puromycin (Invitrogen; Thermo Fisher Scientific, Inc.) and 10%
fetal calf serum (Invitrogen; Thermo Fisher Scientific, Inc.). The
culture medium was replaced every 2 days. H19–7/IGF-IR cells were
seeded at 2×105/well on 6-well plates coated with 0.015
mg/ml poly-L-lysine and cultured in a humidified incubator at 34°C
under a 5% CO2 atmosphere for 3–5 days. Cells were then
treated with caerulein (10 nmol/ml) alone or in combination with 1,
10 or 100 µmol/ml etanercept according to previous studies
(30,31). At 6 h after administration, cells
were washed with PBS and collected for further investigation.
Histological analysis
Pathogenic changes in the hippocampus, and the
expression levels of various proinflammatory and neurotrophic
factors were examined by hematoxylin and eosin (Η&Ε) staining
and immunohistochemical staining, respectively. Briefly, rats were
euthanized by IP injection of pentobarbital sodium (120 mg/kg) and
perfused transcardially with 100 ml physiological saline. The
hippocampus was isolated and immersed in 0.1 M PBS (pH 7.4) with 4%
paraformaldehyde and 30% sucrose overnight at 4°C. Tissues were
then embedded in optimal cutting temperature compound (Sakura
Finetek) and stored at −80°C. Horizontal and coronal sections (5
µm) were prepared for immunohistochemical and Η&Ε staining for
5 min at room temperature. Each section was washed with PBS plus
0.1% Triton X-100 for 7 min and then blocked for 1 h in 10% bovine
serum albumin (BSA; Sigma-Aldrich; Merck KGaA) at room temperature.
Sections were incubated overnight at 4°C with the following primary
antibodies (all from Abcam): Anti-IL-1β (1:1,000; cat. no. ab9722);
TNF-α (1:1,000; cat. no. ab6671); IL-6 (1:1,000; cat. no. ab9324);
and hypoxia-inducible factor 1 α (HIF-1α; 1:1,000; cat. no.
ab8366). Following washing in PBS, sections were incubated in
secondary antibodies: Anti-mouse IgG H&L [(horseradish
peroxidase; HRP) 1:2,000; cat. no. ab205719; Abcam)] and
anti-Rabbit IgG H&L [(HRP); 1:2,000; cat. no. ab6721; Abcam],
with 2% BSA for 1 h at room temperature and then washed again in
PBS. Immunolabeling was revealed using DAB as a chromogen for light
microscopy. Η&Ε staining was conducted according to the
manufacturer's instructions of Η&Ε staining kit (cat. no.
C0105; Beyotime Institute of Biotechnology). All sections were
viewed under an Olympus BX51 microscope (Olympus Corporation;
magnification, ×100) and six slices were randomly selected for each
group.
RNA extraction, cDNA synthesis and
reverse transcription-quantitative (RT-q)PCR
Total RNA was extracted from rat hippocampal
H19–7/IGF-IR cells using TRIzol® reagent (Invitrogen;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
instructions. A Reverse Transcriptase Reagent kit (Takara Bio,
Inc.; cat. no. RR037A) was used to synthesize cDNA according to the
manufacturer's instructions. SYBR Green Master mix kits (Takara
Bio, Inc.; cat. no. RR820A) were used to assess the efficiency of
RT-qPCR. Reactions were conducted in 200 µl thin-walled reaction
tubes using an ABI Prism 7500 Sequence Detection System (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The reaction mixture
contained 2X SYBR Green Master mix (10 µl), sense and antisense
primers (0.8 µl, 2.5 µM of each) and cDNA (5 µl, 10 ng). The
thermocycling included initial denaturation at 95°C for 60 sec,
followed by 40 cycles of denaturation at 95°C for 5 sec and
annealing/extension at 60°C for 30 sec. GAPDH was used as the
internal control gene. Relative gene expression was calculated
using the 2−ΔΔCq method (32). The primers used for amplification
were: IL-1β forward, 5′-CCAGGATGAGGACCCAAGCA-3′ and reverse,
5′-TCCCGACCATTGCTGTTTCC-3′; TNF-α forward,
5′-AAGCCCGTAGCCCACGTCGTA-3′ and reverse,
5′-GCCCGCAATCCAGGCCACTAC-3′; IL-6 forward,
5′-GATTTACATAAAATAGTCCTTCCTACC-3′ and reverse,
5′-GGTTTGCCGAGTAGATCTCAAAGTG-3′; HIF1-α forward,
5′-ACCTATGACCTGCTTGGTGCTGAT-3′ and reverse,
5′-CAGTTTCTGTGTCGTTGCTGCCAA-3′; β-actin forward,
5′-ACCATTGGCAATGAGCGGT-3′ and reverse,
5′-GTCTTTGCGGATGTCCACGT-3′.
ELISA
Blood samples (1 ml) were collected and centrifuged
at 1,000 × g for 15 min at 4°C to extract serum. The samples were
stored at −80°C before the detection. Blood samples (three rat
blood samples in each group) were used to detect the expression of
amylase (cat. no. E-EL-R2544c; Elabscience), TNF-α (cat. no.
ab100785; Abcam) and IL-6 (cat. no. ab100772; Abcam) according to
the manufacturer's protocols.
Western blot assay
Western blotting was used to detect protein
expression and phosphorylation status. H19-7/IGF-IR cells in 6-well
plates (2×105 cells/well) treated as described were
submerged in ice-cold homogenization buffer containing 50 mM
Tris-HCl (pH 7.5), 0.5% Triton X-100, and phosphatase/protease
inhibitor cocktail (Roche Diagnostics), and centrifuged (10,000 ×
g, 20 min, 4°C). The supernatants were transferred into new tubes
and total protein concentration was determined using bicinchoninic
acid protein assay kit (cat. no. 23227; Thermo Fisher Scientific,
Inc.). Then, 30 µg of protein were separated by 10% SDS-PAGE and
transferred to nitrocellulose membranes (Bio-Rad Laboratories,
Inc.). The membranes were The membranes were blocked using 5% milk
(cat. no. MA0097-400ML; Dalian Meilunbio Co., Ltd.) at 37°C for 1
h. Then, incubated with species-specific primary antibodies,
including rabbit anti IL-1β (1:1,000; cat. no. ab9722; Abcam),
TNF-α (1:1,000; cat. no. ab6671; Abcam), IL-6 (1:1,000; cat. no.
ab9324; Abcam), HIF1-α (1:1,000; cat. no. ab179483; Abcam),
phosphorylated (p)-NF-κB (1:1,000; cat. no. 3033; Cell Signaling
Technology, Inc.), NF-κB (1:1,000; cat. no. 8242; Cell Signaling
Technology, Inc.), Anti-β-actin (1:1,000; cat. no. ab115777;
Abcam), p-IKKβ (1:1,000; cat. no. 2697; Cell Signaling Technology,
Inc.) and IKKβ (1:1,000; cat. no. 2678; Cell Signaling Technology,
Inc.) overnight at 4°C. Membranes were then incubated with
species-specific horseradish peroxidase conjugated secondary
antibodies goat anti-mouse (1:2,000; Abcam; cat. no. ab6789) and
goat anti-rabbit (1:2,000; Abcam; cat. no. ab6721). Protein bands
were visualized using an ECL kit (Pierce; Thermo Fisher Scientific,
Inc.) according to the manufacturer's instructions and exposed to
X-ray films. β-actin was used as a protein loading control.
Detection of reactive oxygen species
(ROS) level
Cellular ROS level was measured fluorometrically
using the ROS sensitive dye 2′-7′-dichlorodihydrofluorescein
diacetate (DCFH-DA) as described by Chen et al (33). Briefly, H19-7/IGF-IR cells treated
as indicated were collected, centrifuged at 500 × g for 5 min at
room temperature, resuspended in PBS with DCFH-DA (10 µM), and
incubated at room temperature for 30 min in darkness. ROS levels
were measured by flow cytometry using a BD FACSCalibur flow
cytometer (BD Biosciences) with excitation set at 488 nm and
emission at 530 nm and analyze by Flowjo V10 software (34).
Statistical analyses
All data are expressed as mean ± standard deviation
of at least three independent experiments. Student's t-test and
one-way ANOVA followed by Bonferroni's post hoc test were used to
compare treatment group means. P<0.05 was considered to indicate
a statistically significant difference. All statistical
calculations were performed using SPSS 21.0 (IBM Corp.).
Results
Effects of etanercept treatment on
proinflammatory cytokine release in caerulein-induced PE in
vitro
To investigate the role of etanercept in PE
pathogenesis in vitro, the present study employed a PE model
with caerulein (10 nmol/l) administration using rat hippocampal
H19-7/IGF-IR cells treated with different concentrations of
etanercept (1, 10 and 100 µmol/ml). At 6 h after etanercept
administration in caerulein-induced H19-7/IGF-IR cells, the
proinflammatory cytokines TNF-α, IL-1β and IL-6 were examined by
immunoblotting and RT-qPCR analysis. The effects of etanercept on
the expression of the neuroprotective factor HIF-1α were also
examined. It was determined that the mRNA levels of TNF-α, IL-1β
and IL-6 were significantly reduced in caerulein-induced
H19-7/IGF-IR cells following etanercept treatment (Fig. 1A-C). By contrast, HIF-1α mRNA
expression levels were significantly increased in caerulein-induced
H19-7/IGF-IR cells following etanercept treatment (Fig. 1D). It was found that etanercept
effectively altered the mRNA levels of TNF-α, IL-1β, IL-6 and HIF-α
in a dose-dependent manner in caerulein-induced H19-7/IGF-IR cells.
The protein levels of proinflammatory cytokines were confirmed by
western blot analysis. It was identified that the protein
expression levels of TNF-α, IL-1β and IL-6 were attenuated, whereas
those of HIF-1α were enhanced (Fig.
1E). The effects of etanercept administration on protein
expression levels were dose-dependent. Taken together, these data
indicated that etanercept can contribute to the activation of
HIF-1α and inhibit the activation of proinflammatory factors,
including TNF-α, IL-1β and IL-6; thus, it may work as an
anti-inflammatory factor in clinic disease treatment.
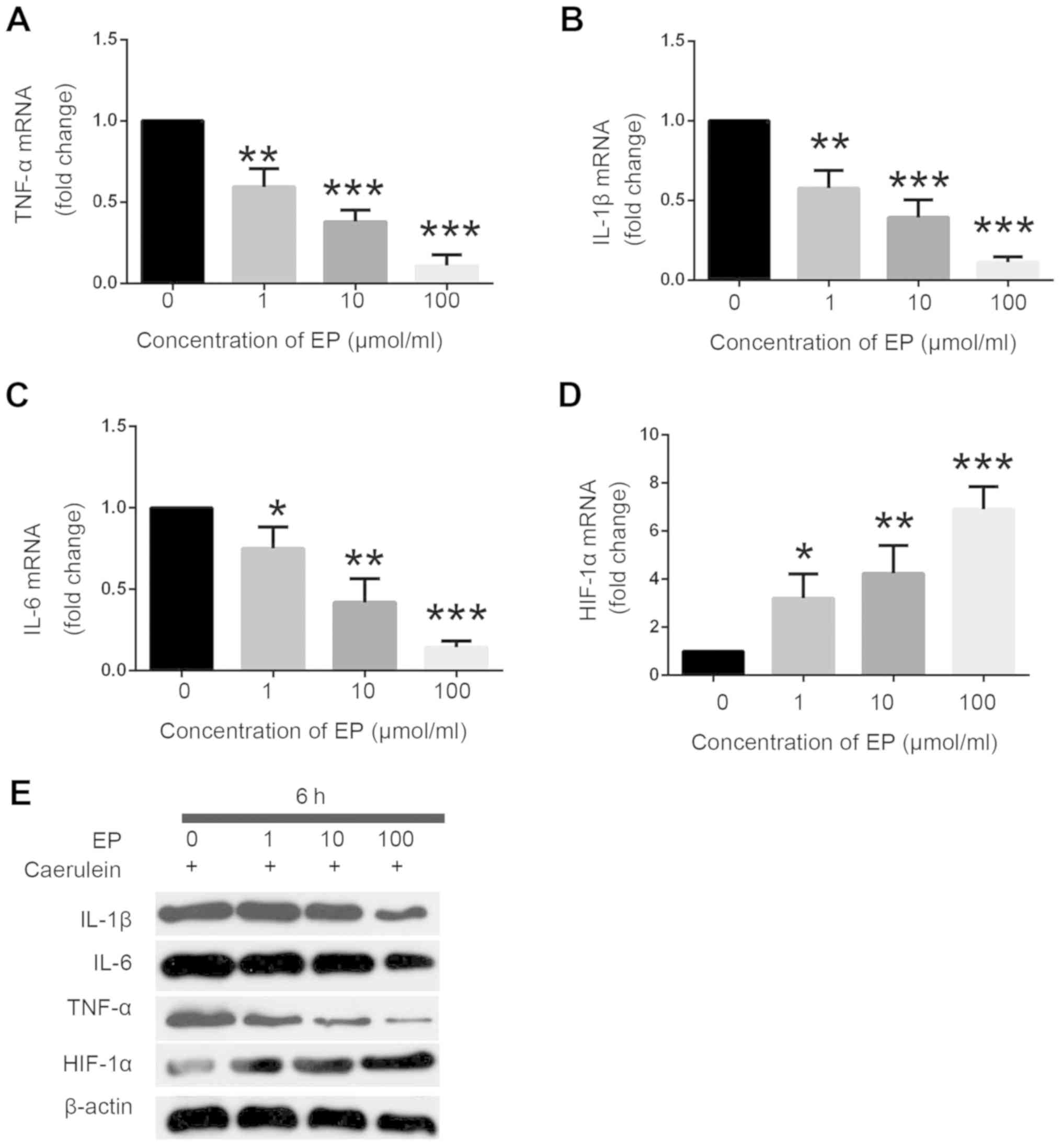 | Figure 1.Etanercept attenuates
caerulein-induced decreases in proinflammatory cytokine release.
Etanercept (1, 10, or 100 µmol/ml) dose-dependently downregulated
mRNA expression levels of (A) TNF-α, (B) IL-1β and (C) IL-6 in a
caerulein-induced (10 nmol/l) model of pancreatic encephalopathy in
H19-7/IGF-IR cells, as measured by RT-qPCR at 6 h after etanercept
treatment. (D) Etanercept (1, 10, or 100 µmol/ml) dose-dependently
enhanced HIF-1α mRNA expression in caerulein-induced (10 nmol/l)
H19-7/IGF-IR cells, as measured by RT-qPCR at 6 h after etanercept
treatment. N=3; *P<0.05, **P<0.01, ***P<0.001 vs. group
without Etanercept. (E) Etanercept (1, 10, or 100 µmol/ml)
dose-dependently downregulated protein expression levels of IL-1β,
IL-6, and TNF-α, and upregulated HIF-1α protein expression, as
determined via western blotting in caerulein-induced (10 nmol/l)
H19-7/IGF-IR cells at 6 h after etanercept treatment. Data are
presented as mean ± standard deviation. Experiments were repeated
three times for reproducibility. TNF, tumor necrosis factor;
RT-qPCR, reverse transcription-quantitative PCR; IL, interleukin;
HIF-1α, hypoxia-inducible factor 1 α; EP, etanercept. |
Effects of etanercept treatment on ROS
in caerulein-induced PE in vitro
Oxidative stress, and ensuing accumulation of DNA
lesions and damage to other macromolecules may contribute to PE
pathogenesis (35). To investigate
whether etanercept can suppress caerulein-induced oxidative stress
in H19-7/IGF-IR cells, DCFH-DA fluorescence was compared among
cultures treated with 10 nmol/ml caerulein alone or combined with
1, 10, or 100 µmol/ml etanercept by flow cytometry (Fig. S1A-D). Flow cytometry showed the
peak shifted to the left with the increased concentration of
etanercept. Indeed, etanercept dose-dependently reduced
caerulein-induced ROS accumulation in H19-7/IGF-IR cells (Fig. S1E).
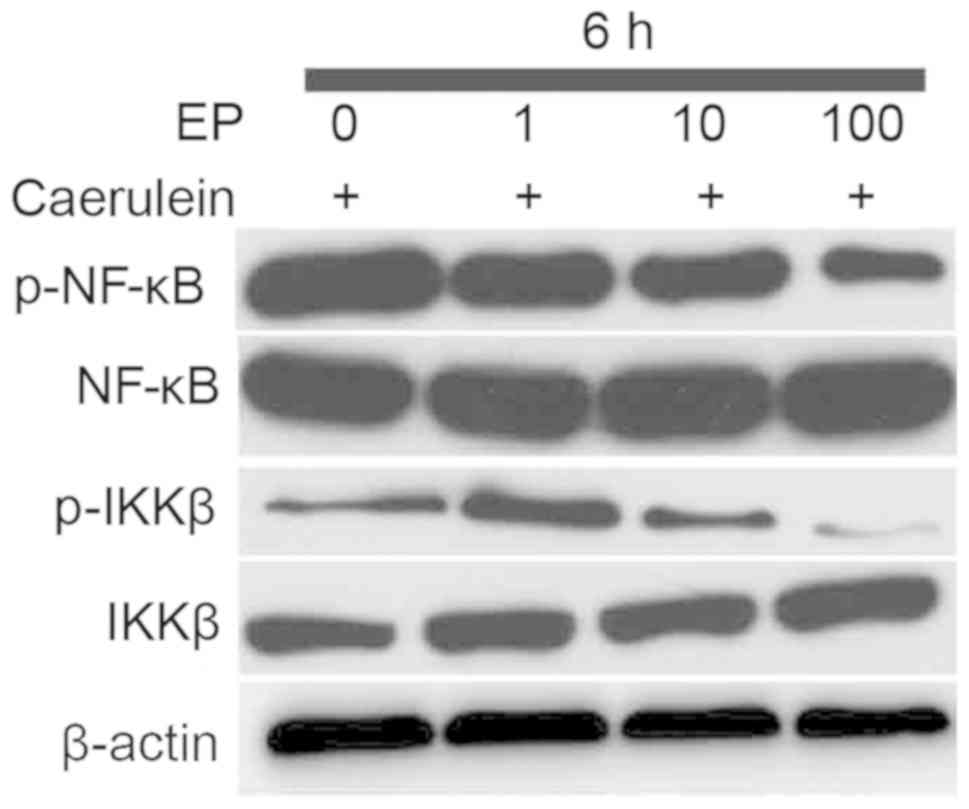
Effects of etanercept treatment on the
NF-κB signaling pathway in caerulein-induced PE in vitro
NF-κB is a master regulator of inflammatory cytokine
expression, and contributes to the progression of neuroinflammation
and ensuing neuronal damage (36).
Therefore, it was examined whether etanercept altered
caerulein-induced phosphorylation (activation) of NF-κB and the
upstream effector IKKβ. At 6 h after treatment with etanercept,
western blot analysis indicated downregulated expression of p-IKKβ
and p-NF-κB in caerulein-induced H19-7/IGF-IR cells (Fig. 2). As expected, the phosphorylation
of p-IKKβ and p-NF-κB reduced in line with the increased
concentration of etanercept. These findings identified the dominant
role of etanercept in negatively regulating the NF-κB signaling
pathway in caerulein-induced H19-7/IGF-IR cells in
vitro.
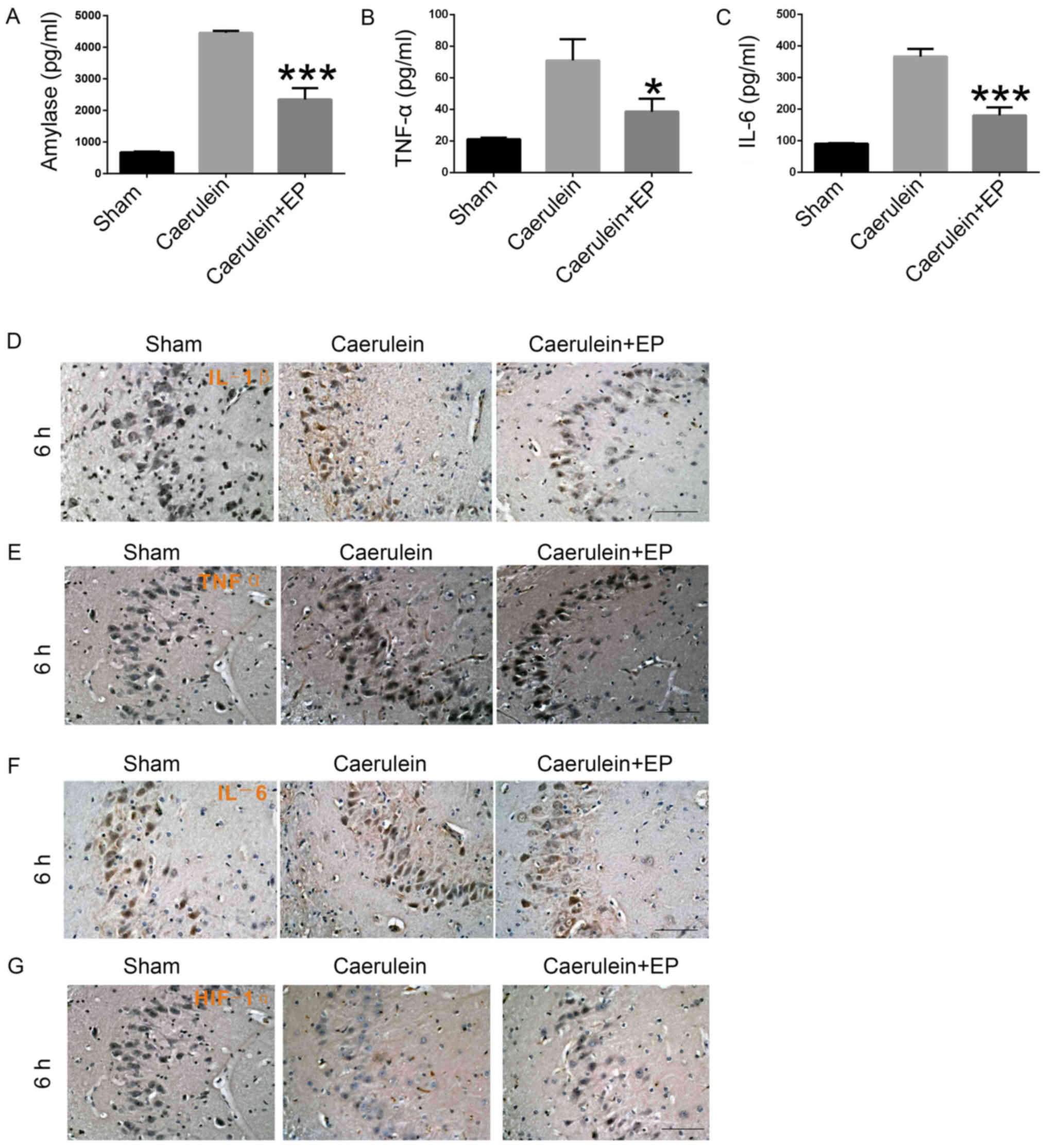
Effects of etanercept treatment on
hippocampus cell inflammatory factor expression in
caerulein-induced PE in vivo
Rats were injected with caerulein (50 µg/kg) alone
or combined with etanercept (10 mg/kg). At 6 h after injection,
hippocampal inflammation and damage were detected via ELISA and
histochemical analysis. ELISAs revealed decreased expression levels
of amylase, TNF-α and IL-6 in the Caerulein + EP group compared
with the Caerulein group (Fig.
3A-C). Furthermore, immunohistochemical analysis demonstrated
elevated expression levels of IL-1β (Fig. 3D), TNF-α (Fig. 3E) and IL-6 (Fig. 3F) in hippocampal neurons in the
Caerulein group, which were reversed by cotreatment with etanercept
in the Caerulein + EP group. However, etanercept enhanced
expression of HIF-1α in hippocampus cells in the Caerulein + EP
group compared with the Caerulein group (Fig. 3G). Therefore, etanercept can
inhibit caerulein-induced PE progression and decrease the release
of inflammatory factors in hippocampus cells.
Discussion
Inflammatory and autoimmune processes are critical
to the pathogenesis and progression of AP, but it is not fully
understood how the progression of AP leads to PE. PE is among the
most serious complications of AP (37), characterized by neurodegeneration
and psychiatric manifestations including orientation disorders,
trance, excitement and hallucinations (38). The mortality rate of AP with mental
and neurological symptoms can reach 67–100%, as reported in China
(39). Thus, models allowing for
elucidation of PE pathogenesis are essential for improving
treatment strategies. The current study demonstrated that
etanercept had an important role in caerulein-triggered immune
reactions and TNF-α signaling for PE-associated neural damage and
that it provides a potential therapeutic treatment for
anti-inflammatory disease.
Previous studies have shown that proinflammatory
cytokines can cause neurovegetative states and depressive symptoms
(40,41). The elevated activity of
proinflammatory cytokines and associated signaling pathways in
inflammatory disorders may aggravate depression and decrease the
response to conventional antidepressant medications (42). In AP, acinar cell injury initiates
local inflammatory and immune responses, and accelerates the
release of proinflammatory cytokines, including TNF-α and IL-6
(43). The activated cytokine
cascades can accelerate systemic inflammatory responses and
multisystem failure independently from AP development (44). Though these processes, patients
with AP may experience changes in consciousness and eventual PE
(45).
Clinical studies have reported associations between
serum TNF-α and the severity of disease such as multiple sclerosis
(46), acquired immunodeficiency
syndrome (AIDS) (47) and malaria
(48), as well as associations
between serum proinflammatory cytokine concentrations and central
nervous system damage. For instance, patients with systemic lupus
erythematosus exhibiting neural sequela (49) and patients with AIDS with
associated encephalopathy exhibit markedly elevated serum and
cerebrospinal fluid IL-6 levels (50). In addition, TNF-α and IL-6
expression levels are notably increased in patients with severe
head trauma (45). Local oxidative
stress appears to be a major final common pathway for neuronal
damage in these disorders. Using flow cytometry analysis, the
current study demonstrated that etanercept could prevent the
accumulation of ROS in caerulein-induced PE.
TNF-α is hypothesized to be directly or indirectly
responsible for the majority of inflammatory and destructive events
in rheumatoid arthritis (RA) (51). For instance, TNF-α controls the
expression of IKKβ in esophageal epithelia, regulates the
activation of human endothelial cells (52,53)
and stimulates neoangiogenesis (54,55),
which leads to an influx of inflammatory cells into the rheumatoid
joint. On the other hand, ensuing local immune reactions increase
the production of enzymes responsible for the degradation of
cartilage, such as matrix metalloproteinases (56), and activate osteoclasts, thereby
enhancing bone resorption. In addition, TNF-α induces the
production of proinflammatory cytokines, including IL-1 and IL-6
(57,58), and increases prostaglandin E2
production (59). TNF-α and IL-6
serve as important early markers for AP and the severity of its
complications (60,61). Patients with severe depression also
exhibit enhanced levels of proinflammatory cytokines in peripheral
blood (62). These cytokines can
enter the brain and contribute to the development of depression by
affecting indolamine 2,3-dioxygenase and neurotransmitter
metabolism (63).
The TNF-α inhibitor etanercept is approved
clinically for the treatment of plaque psoriasis, RA, psoriatic
arthritis and axial spondyloarthritis (64). Etanercept can alleviate arthritic
signs and symptoms, thus reducing disease-associated disability and
promoting health-associated quality of life. Further, these
benefits appear to be maintained during long-term treatment
(43). Similarly, arthritic
symptoms were significantly improved by etanercept compared to a
placebo, according to subjective and objective assessments of
disease activity and physical function (65). These benefits may be further
augmented by combination therapy using methotrexate. The efficacy
of etanercept against RA is also rapid and can be observed as early
as 1 week after initiating therapy (66). Its interaction with the immune
system by associating with anxiety and the activation of auditory
evoked potentials, which may increase the levels of proinflammatory
cytokines such as TNF-α and IL-6 (67). Injection of IL-1α could block the
function of IL-1β and significantly reduce the expression of NF-κB
in the medial prefrontal cortex, thus significantly improving
depressive behaviors in rats (68). Previous studies have shown that
etanercept can be used in the treatment of serum-positive RA and
Stevens-Johnson syndrome/toxic epidermolysis triggered by
carbamazepine, and play a key role in cardiovascular safety in
patients with RA (69–71). However, there are few cases of
clinical treatment of PE with etanercept.
In the current study, etanercept was used to
evaluate the role of TNF-α-mediated inflammation in
caerulein-induced PE. The results demonstrated that etanercept can
act as an anti-inflammatory and protective agent against
PE-associated neural dysfunction in the suppression of TNF-α, IL-1β
and IL-6 expression, in vitro and in vivo. In
addition, inhibition of TNF-α led to the attenuation of NF-κB
signaling and amelioration of oxidative stress. However, etanercept
promoted the induction of HIF-1α, which is released by mononuclear
cells and/or neutrophils, and consequently reduces leukocyte
migration at the site of the inflammatory response (72). In order to confirm the hypothesis
of the present study, it is necessary to consider more literature
on the scoring indicators of PE, such as the blood-brain barrier
score (73); this will be
performed in future studies. Taken together, etanercept effectively
improves caerulein-induced PE by suppressing inflammatory
responses, and could act as a safe and effective therapeutic option
in the development of therapeutic strategies for the treatment of
PE-related disease.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by Guangdong Natural
Science Foundation (grant no. 2015A030313834) and Guangdong
Department of Education Foundation (grant no. 2017JD094).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
XW designed the study. YL, HL, GZ and YX were
involved in data acquisition and conducted the experiments. GJ, BL
and CL analyzed the data. YL drafted the article. XW was involved
in the final approval of the submitted manuscript. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
The Jinan University Laboratory Animal Welfare and
Ethics Committee charter approved all procedures involving rats.
All protocols also conformed to the guidelines of Animal Use and
Care of the National Institutes of Health.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Colmant HJ and Noltenius H: Pancreatic
encephalopathy. Med Klin. 72:2146–2154. 1977.(In German).
PubMed/NCBI
|
|
2
|
Zhang XP and Tian H: Pathogenesis of
pancreatic encephalopathy in severe acute pancreatitis.
Hepatobiliary Pancreat Dis Int. 6:134–140. 2007.PubMed/NCBI
|
|
3
|
Mentula P and Leppäniemi A: Position
paper: Timely interventions in severe acute pancreatitis are
crucial for survival. World J Emerg Surg. 9:152014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zheng L, Xue J, Jaffee EM and Habtezion A:
Role of immune cells and immune-based therapies in pancreatitis and
pancreatic ductal adenocarcinoma. Gastroenterology. 144:1230–1240.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Al Mofleh IA: Severe acute pancreatitis:
Pathogenetic aspects and prognostic factors. World journal of
gastroenterology. 14:675–684. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang H, Neuhöfer P, Song L, Rabe B,
Lesina M, Kurkowski MU, Treiber M, Wartmann T, Regnér S, Thorlacius
H, et al: IL-6 trans-signaling promotes pancreatitis-associated
lung injury and lethality. J Clin Invest. 123:1019–1031. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang X, Zhuang X, Wei R, Wang C, Xue X and
Mao L: Protective effects of Acanthopanax vs. Ulinastatin against
severe acute pancreatitis-induced brain injury in rats. Int
Immunopharmacol. 24:285–298. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ohkubo T, Shiojiri T and Matsunaga T:
Severe diffuse white matter lesions in a patient with pancreatic
encephalopathy. J Neurol. 251:476–478. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Adler G, Hahn C, Kern HF and Rao KN:
Cerulein-induced pancreatitis in rats: Increased lysosomal enzyme
activity and autophagocytosis. Digestion. 32:10–18. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yin T, Peeters R, Liu Y, Feng Y, Zhang X,
Jiang Y, Yu J, Dymarkowski S, Himmelreich U, Oyen R and Ni Y:
Visualization, quantification and characterization of
caerulein-induced acute pancreatitis in rats by 3.0T clinical MRI,
biochemistry and histomorphology. Theranostics. 7:285–294. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Marrache F, Tu SP, Bhagat G, Pendyala S,
Osterreicher CH, Gordon S, Ramanathan V, Penz-Osterreicher M, Betz
KS, Song Z and Wang TC: Overexpression of interleukin-1beta in the
murine pancreas results in chronic pancreatitis. Gastroenterology.
135:1277–1287. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Terao K, Wake H, Adachi N, Liu K,
Teshigawara K, Takahashi H, Mori S and Nishibori M: Histidine-rich
glycoprotein suppresses hyperinflammatory responses of lung in a
severe acute pancreatitis mouse model. Pancreas. 47:1156–1164.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hoesel B and Schmid JA: The complexity of
NF-κB signaling in inflammation and cancer. Mol Cancer. 12:862013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cildir G, Low KC and Tergaonkar V:
Noncanonical NF-κB signaling in health and disease. Trends Mol Med.
22:414–429. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hayden MS and Ghosh S: NF-κB in
immunobiology. Cell Res. 21:223–244. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cui X, Chang L, Li Y, Lv Q, Wang F, Lin Y,
Li W, Meade JD, Walden JC and Liang P: Trivalent soluble TNF
receptor, a potent TNF-alpha antagonist for the treatment
collagen-induced arthritis. Sci Rep. 8:73272018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhao S, Mysler E and Moots RJ: Etanercept
for the treatment of rheumatoid arthritis. Immunotherapy.
10:433–445. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kovar FM, Marsik C, Cvitko T, Wagner OF,
Jilma B and Endler G: The tumor necrosis factor alpha-308 G/A
polymorphism does not influence inflammation and coagulation
response in human endotoxemia. Shock. 27:238–241. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sheng YQ, Li F and Qin ZH: TNF receptor 2
makes tumor necrosis factor a friend of tumors. Front Immunol.
9:11702018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Muniraj T, Gajendran M, Thiruvengadam S,
Raghuram K, Rao S and Devaraj P: Acute pancreatitis. Dis Mon.
58:98–144. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
National Research Council Committee for
the Update of the Guide for the C, Use of Laboratory A. The
National Academies Collection, . Reports funded by National
Institutes of Health. Th, editor: Guide for the Care and Use of
Laboratory Animals (Washington (DC)). National Academies Press (US)
National Academy of Sciences. 2011.
|
|
22
|
How to determine humane endpoints for
research animals. Lab Anim (NY). 45:192016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sano Y, Ito S, Yoneda M, Nagasawa K,
Matsuura N, Yamada Y, Uchinaka A, Bando YK, Murohara T and Nagata
K: Effects of various types of anesthesia on hemodynamics, cardiac
function, and glucose and lipid metabolism in rats. Am J Physiol
Heart Circ Physiol. 311:H1360–H1366. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pierozan P, Jernerén F, Ransome Y and
Karlsson O: The choice of euthanasia method affects metabolic serum
biomarkers. Basic Clin Pharmacol Toxicol. 121:113–118. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
NC3Rs Reporting Guidelines Working Group,
: Animal research: Reporting in vivo experiments: The ARRIVE
guidelines. J Physiol. 588:2519–2521. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Orabi AI, Shah AU, Ahmad MU, Choo-Wing R,
Parness J, Jain D, Bhandari V and Husain SZ: Dantrolene mitigates
caerulein-induced pancreatitis in vivo in mice. Am J Physiol
Gastrointest Liver Physiol. 299:G196–G204. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jeong YK, Lee S, Lim JW and Kim H:
Docosahexaenoic acid inhibits cerulein-induced acute pancreatitis
in rats. Nutrients. 9:E7442017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Huang HL, Tang GD, Liang ZH, Qin MB, Wang
XM, Chang RJ and Qin HP: Role of Wnt/β-catenin pathway agonist
SKL2001 in Caerulein-induced acute pancreatitis. Can J Physiol
Pharmacol. 97:15–22. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Morrione A, Navarro M, Romano G, Dews M,
Reiss K, Valentinis B, Belletti B and Baserga R: The role of the
insulin receptor substrate-1 in the differentiation of rat
hippocampal neuronal cells. Oncogene. 20:4842–4852. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
DiMagno MJ, Williams JA, Hao Y, Ernst SA
and Owyang C: Endothelial nitric oxide synthase is protective in
the initiation of caerulein-induced acute pancreatitis in mice. Am
J Physiol Gastrointest Liver Physiol. 287:G80–G87. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ye J, Jiang R, Cui M, Zhu B, Sun L, Wang
Y, Zohaib A, Dong Q, Ruan X, Song Y, et al: Etanercept reduces
neuroinflammation and lethality in mouse model of Japanese
encephalitis. J Infect Dis. 210:875–889. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen X, Duan Y, Riley AM, Welch MA, White
FA, Grant MB and Obukhov AG: Long-term diabetic microenvironment
augments the decay rate of capsaicin-induced currents in mouse
dorsal root ganglion neurons. Molecules. 24:7752019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fernandez R and Maecker H:
Cytokine-stimulated Phosphoflow of PBMC using CyTOF mass cytometry.
Bio Protoc. 5:e14962015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shi Q, Liao KS, Zhao KL, Wang WX, Zuo T,
Deng WH, Chen C, Yu J, Guo WY, He XB, et al: Hydrogen-rich saline
attenuates acute renal injury in sodium taurocholate-induced severe
acute pancreatitis by inhibiting ROS and NF-κB pathway. 2015.
6850432015.PubMed/NCBI
|
|
36
|
Karin M and Ben-Neriah Y: Phosphorylation
meets ubiquitination: The control of NF-[kappa]B activity. Ann Rev
Immunol. 18:621–663. 2000. View Article : Google Scholar
|
|
37
|
Pitchumoni CS, Agarwal N and Jain NK:
systemic complications of acute-pancreatitis. Am J Gastroenterol.
83:597–606. 1988.PubMed/NCBI
|
|
38
|
Sharma V, Sharma R, Rana SS and Bhasin DK:
Pancreatic encephalopathy: An unusual cause of asterixis. JOP.
15:383–384. 2014.PubMed/NCBI
|
|
39
|
Zhuan L and Zhaoshen L: Progress in
Pancreatic Encephalopathy. Pancreatology. 4:248–250. 2003.
|
|
40
|
Parlar A, Arslan SO, Doğan MF, Çam SA,
Yalçin A, Elibol E, Özer MK, Üçkardeş F and Kara H: The exogenous
administration of CB2 specific agonist, GW405833, inhibits
inflammation by reducing cytokine production and oxidative stress.
Exp Ther Med. 16:4900–4908. 2018.PubMed/NCBI
|
|
41
|
Dantzer R, O'Connor JC, Freund GG, Johnson
RW and Kelley KW: From inflammation to sickness and depression:
When the immune system subjugates the brain. Nat Rev Neurosci.
9:46–57. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Song C and Wang H: Cytokines mediated
inflammation and decreased neurogenesis in animal models of
depression. Prog Neuropsychopharmacol Biol Psychiatry. 35:760–768.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Habtezion A: Inflammation in acute and
chronic pancreatitis. Curr Opin Gastroenterol. 31:395–399. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Liu Y and Li Y, Chen KL, Zhou B, Lv ZY,
Zhou ZG and Li Y: Knockdown of Myeloid differentiation factor 88
attenuates lipopolysaccharide-induced inflammatory response in
pancreatic ductal cells. Pancreas. 45:755–760. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yan EB, Hellewell SC, Bellander BM,
Agyapomaa DA and Morganti-Kossmann MC: Post-traumatic hypoxia
exacerbates neurological deficit, neuroinflammation and cerebral
metabolism in rats with diffuse traumatic brain injury. J
Neuroinflamm. 8:1472011. View Article : Google Scholar
|
|
46
|
Farrokhi M, Etemadifar M, Jafary Alavi MS,
Zarkesh-Esfahani SH, Behjati M, Rezaei A and Amani-Beni A:
TNF-alpha production by peripheral blood monocytes in multiple
sclerosis patients and healthy controls. Immunol Invest.
44:590–601. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Gallitano SM, McDermott L, Brar K and
Lowenstein E: Use of tumor necrosis factor (TNF) inhibitors in
patients with HIV/AIDS. J Am Acad Dermatol. 74:974–980. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kinra P and Dutta V: Serum TNF alpha
levels: A prognostic marker for assessment of severity of malaria.
Trop Biomed. 30:645–653. 2013.PubMed/NCBI
|
|
49
|
Yoshio T, Okamoto H, Kurasawa K, Dei Y,
Hirohata S and Minota S: IL-6, IL-8, IP-10, MCP-1 and G-CSF are
significantly increased in cerebrospinal fluid but not in sera of
patients with central neuropsychiatric lupus erythematosus. Lupus.
25:997–1003. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Witwer KW, Sarbanes SL, Liu J and Clements
JE: A plasma microRNA signature of acute lentiviral infection:
Biomarkers of central nervous system disease. AIDS. 25:2057–2067.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Paleolog EM, Young S, Stark AC, McCloskey
RV, Feldmann M and Maini RN: Modulation of angiogenic vascular
endothelial growth factor by tumor necrosis factor alpha and
interleukin-1 in rheumatoid arthritis. Arthritis Rheum.
41:1258–1265. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Tétreault MP, Weinblatt D, Ciolino JD,
Klein-Szanto AJ, Sackey BK, Twyman-Saint Victor C, Karakasheva T,
Teal V and Katz JP: Esophageal expression of active IκB Kinase-β in
mice up-regulates tumor necrosis factor and granulocyte-macrophage
colony-stimulating factor, promoting inflammation and angiogenesis.
Gastroenterology. 150:1609–1619.e11. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Pober JS, Gimbrone MA Jr, Lapierre LA,
Mendrick DL, Fiers W, Rothlein R and Springer TA: Overlapping
patterns of activation of human endothelial cells by interleukin 1,
tumor necrosis factor, and immune interferon. J Immunol.
137:1893–1896. 1986.PubMed/NCBI
|
|
54
|
Lupia E, Montrucchio G, Battaglia E,
Modena V and Camussi G: Role of tumor necrosis factor-alpha and
platelet-activating factor in neoangiogenesis induced by synovial
fluids of patients with rheumatoid arthritis. Eur J Immunol.
26:1690–1694. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Strieter RM, Kunkel SL, Showell HJ, Remick
DG, Phan SH, Ward PA and Marks RM: Endothelial cell gene expression
of a neutrophil chemotactic factor by TNF-alpha, LPS, and IL-1
beta. Science. 243:1467–1469. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Sui Y, Lee JH, DiMicco MA, Vanderploeg EJ,
Blake SM, Hung HH, Plaas AH, James IE, Song XY, Lark MW and
Grodzinsky AJ: Mechanical injury potentiates proteoglycan
catabolism induced by interleukin-6 with soluble interleukin-6
receptor and tumor necrosis factor alpha in immature bovine and
adult human articular cartilage. Arthritis Rheum. 60:2985–2996.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Yuan JT, Wang L, Lin YJ, Chen JH and Hu
JH: Differences of plasma IL-1 and TNF-α in healthy Chinese
population. Open Med (Wars). 10:306–310. 2015.PubMed/NCBI
|
|
58
|
Ishitsuka Y, Kawachi Y, Maruyama H,
Taguchi S, Fujisawa Y, Furuta J, Nakamura Y, Ishii Y and Otsuka F:
Pituitary tumor transforming gene 1 induces tumor necrosis factor-α
production from keratinocytes: Implication for involvement in the
pathophysiology of psoriasis. J Invest Dermatol. 133:2566–2575.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Dayer JM, Beutler B and Cerami A:
Cachectin/tumor necrosis factor stimulates collagenase and
prostaglandin E2 production by human synovial cells and dermal
fibroblasts. J Exp Med. 162:2163–2168. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Fisic E, Poropat G, Bilic-Zulle L, Licul
V, Milic S and Stimac D: The role of IL-6, 8, and 10, sTNFr, CRP,
and pancreatic elastase in the prediction of systemic complications
in patients with acute pancreatitis. Gastroenterol Res Pract.
2013:2826452013. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Xue D, Zhang W, Zhang Y, Wang H, Zheng B
and Shi X: Adjusting effects of baicalin for nuclear Factor-kappa B
and tumor necrosis factor-alpha on rats with caerulein-induced
acute pancreatitis. Mediat Inflamm. 2006:262952006. View Article : Google Scholar
|
|
62
|
Otsuka Y, Kamata K, Minaga K, Takenaka M,
Watanabe T and Kudo M: Acute pancreatitis with disturbed
consciousness caused by hyperparathyroidism. Intern Med.
57:3075–3078. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Miller AH, Maletic V and Raison CL:
Inflammation and its discontents: The role of cytokines in the
pathophysiology of major depression. Biol Psychiat. 65:732–741.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Scott LJ: Etanercept: A review of its use
in autoimmune inflammatory diseases. Drugs. 74:1379–1410. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Hobbs K, Deodhar A, Wang B, Bitman B,
Nussbaum J, Chung J and Collier DH: Randomized, double-blind,
placebo-controlled study to evaluate the efficacy and safety of
etanercept in patients with moderately active rheumatoid arthritis
despite DMARD therapy. Springerplus. 4:1132015. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Mease PJ, Gladman DD, Collier DH, Ritchlin
CT, Helliwell PS, Liu L, Kricorian G and Chung JB: Etanercept and
methotrexate as monotherapy or in combination for psoriatic
arthritis: Primary results from a randomized, controlled phase III
trial. Arthritis Rheumatol. 71:1112–1124. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Soyalp M, Yalcin M, Oter V and Ozgonul A:
Investigation of procalcitonin, IL-6, oxidative stress index (OSI)
plasma and tissue levels in experimental mild and severe
pancreatitis in rats. Bratisl Lek Listy. 118:137–141.
2017.PubMed/NCBI
|
|
68
|
Horowitz MA, Wertz J, Zhu D, Cattaneo A,
Musaelyan K, Nikkheslat N, Thuret S, Pariante CM and Zunszain PA:
Antidepressant compounds can be both pro- and anti-inflammatory in
human hippocampal cells. Int J Neuropsychopharmacol. 18:pyu0762014.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Giles JT, Sattar N, Gabriel S, Ridker PM,
Gay S, Warne C, Musselman D, Brockwell L, Shittu E, Klearman M and
Fleming TR: cardiovascular safety of tocilizumab versus etanercept
in rheumatoid arthritis: A randomized controlled trial. Arthritis
Rheumatol. 72:31–40. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Smith KM, Amin S, Jones LK Jr, Lachance
DH, Flanagan EP, Jentoft ME and Kantarci OH: Glial fibrillary
acidic protein (GFAP) autoimmunity in the setting of seropositive
rheumatoid arthritis treated with etanercept. Neurologist.
24:152–154. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Pham CH, Gillenwater TJ, Nagengast E,
McCullough MC, Peng DH and Garner WL: Combination therapy:
Etanercept and intravenous immunoglobulin for the acute treatment
of Stevens-Johnson syndrome/toxic epidermal necrolysis. Burns.
45:1634–1638. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Yang J, He F, Meng Q, Sun Y, Wang W and
Wang C: Inhibiting HIF-1α decreases expression of TNF-α and
caspase-3 in specific brain regions exposed kainic acid-induced
status epilepticus. Cell Physiol Biochem. 38:75–82. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Genet GF, Bentzer P, Hansen MB, Ostrowski
SR and Johansson PI: Effects of propranolol and clonidine on brain
edema, blood-brain barrier permeability, and endothelial glycocalyx
disruption after fluid percussion brain injury in the rat. J Trauma
Acute Care Surg. 84:89–96. 2018. View Article : Google Scholar : PubMed/NCBI
|