Introduction
Spinal cord injury (SCI) results from neurological
damage in the spinal cord and leads to serious impairment of
sensorimotor functions, along with other side effects (1), such as paraplegia and tetraplegia
(2,3). Patients with SCI suffer from pain
(4). There has been substantial
research exploring pathophysiological changes that occur post-SCI
(5). However, it is not clear how
to most effectively promote spinal cord repair following damage.
The systemic inflammatory response has received substantial
attention as a major factor contributing to the development of
SCI-induced immunological dysfunction (6,7).
Moreover, the role of inflammatory response in SCI remains unclear.
Therefore, it is important to further clarify the molecular
mechanisms of SCI in order to develop novel therapeutic
strategies.
MicroRNAs (miRNAs/miRs) are a class of non-coding
RNA that regulate gene expression through translational cleavage or
repression (8) at the
post-transcriptional level (9).
miRNAs directly bind to the 3′untranslated region (3′UTR) of mRNAs,
resulting in translational repression or mRNA degradation (10). Mounting evidence has revealed that
miRNAs are involved in various biological processes (11–13),
including cell growth, cell apoptosis and cell differentiation
(11,14–19).
Altered expression of various miRNAs following traumatic SCI has
been observed in adult rats (20).
miR-138-5p, a miRNA that has been studied in a variety of tumors
(21–23), has also been found to play an
important role in Parkinson's disease (24). However, so far, the role of
miR-138-5p in SCI has not been reported.
Sirtuin 1 (SIRT1) is a NAD+-dependent deacetylase
important in regulating cell apoptosis (25). Studies have also indicated that
SIRT1 plays critical roles in the regulation of inflammatory
responses (26–29). Notably, potential binding sites
between SIRT1 and miR-138-5p were detected in the present study via
bioinformatics software analysis. Thus, it was hypothesized that
they may interact and in doing so affect SCI progression. As the
expression or regulatory mechanisms of miR-138-5p in modulating SCI
remain largely unexplored, the purpose of the present study was to
determine the role of miR-138-5p in SCI by evaluating its
expression and molecular mechanisms.
Materials and methods
Clinical samples
A total of 18 serum specimens from patients with SCI
(age range, 27–58 years; 12 male patients and 6 female patients)
and 18 serum specimens from healthy controls (age range, 25–60
years; 12 male patients and 6 female patients) were collected at
the First People's Hospital of Lianyungang between June 2015 and
November 2017. Serum samples were isolated from blood samples by
centrifugation at 4°C at 1,000 × g for 15 min. Patients with SCI
were diagnosed according to American Spinal Injury Association
(30). Written informed consent
was obtained from each patient. The present study was approved by
the Ethics Committee of the First People's Hospital of
Lianyungang.
Animals
A total of 20 adult male Sprague-Dawley rats (6–8
weeks, 200–300 g) were obtained from the Animal Center of Nanjing
Medical University, housed in a standard animal room (23±1°C,
relative humidity 40–60%, under a 12:12-h light/dark cycle) with
free access to standard rodent chow and water. No animals exhibited
any neurological disorder prior to the SCI induction protocol. All
protocols of animal experiments were performed according to the
guidelines for Institutional Animal Care and Use of Laboratory
Animals by the National Institutes of Health (31). The present study was approved by
the Animal Ethics Committee of the First People's Hospital of
Lianyungang.
Establishment of the rat model of
SCI
Animals in were randomly divided into two groups:
The control group (n=10) and the SCI group (n=10). In the control
group, the surgical area was exposed without SCI induction. In the
SCI model group, rats were shaved and treated aseptically to
construct the SCI model as described previously (32). Briefly, all rats were anesthetized
via intraperitoneal injection of pentobarbital (30 mg/kg). After
anesthesia, the rat skin was shaved, opened and cleaned with
betadine carefully. Then, a 20-mm midline incision was made to
expose the vertebral column in the thoracic region. After a
thoracic-level (T8-T11) midline skin incision, the paravertebral
muscle was dissected and a laminectomy of T10 was undertaken in
order to expose the dorsal cord surface without disrupting the
dura. Subsequently, SCI was induced by dropping a 10 g rod from a
height of 5.0 cm onto the T10 level of the spinal cord. Finally,
the incision was sutured and all rats were allowed to recover from
anesthesia in warm boxes. At 12 h after SCI induction, animals were
anesthetized with pentobarbital (40 mg/kg) through intraperitoneal
injection and sacrificed via cervical dislocation, following which
specimens were obtained.
Cell lines and cell culture
PC12 cells were obtained from American Type Culture
Collection, cultured in DMEM (Gibco; Thermo Fisher Scientific,
Inc.) supplemented with 10% FBS (Gibco; Thermo Fisher Scientific,
Inc.) and 1% penicillin/streptomycin, and maintained at 37°C in a
humidified atmosphere with 5% CO2.
PC12 cells (1×106 cell/ml) were
transfected with 100 nM miR-138-5p inhibitor
(5′-CGGCCUGATTCACAACACCAGCT-3′; Shanghai GenePharma Co., Ltd.), 100
nM inhibitor control (5′-CAGUACUUUUGUGUAGUACAA-3′; Shanghai
GenePharma Co., Ltd.), 0.2 µM control-small interfering (si)RNA
(cat. no. Sc-36869; Santa Cruz Biotechnology, Inc.), 0.2 µM
SIRT1-siRNA (cat. no. Sc-40986; Santa Cruz Biotechnology, Inc.) or
100 nM miR-138-5p inhibitor + 0.2 µM SIRT1-siRNA using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol. The
efficiency of cell transfection was evaluated via reverse
transcription-quantitative PCR (RT-qPCR) and western blot analysis
at 48 h after transfection.
An in vitro cell model of SCI in PC12 cells
was established according to a previous study (33). In brief, PC12 cells were subjected
to lipopolysaccharide (LPS; 100 ng/ml) for 4 h at 37°C. Control
cells were left untreated.
miRNA target analysis and
dual-luciferase reporter assay
TargetScan (version 7.1; www.targetscan.org/vert_71) was used to predict the
potential targets of miR-138-5p. The results showed that SIRT1 was
a potential target of miR-138-5p. In order to investigate the
direct target binding sites between miR-138-5p and SIRT1, the
wild-type 3′UTR (WT-SIRT1) and mutant 3′UTR (MUT-SIRT1) of SIRT1
were cloned into a pMIR-RB-Report™ dual luciferase reporter gene
plasmid vector (Guangzhou RiboBio Co., Ltd.) according to the
manufacturer's protocols; a QuikChange Site-Directed Mutagenesis
kit (Stratagene; Agilent Technologies, Inc.) was used according to
the manufacturer's instructions to point-mutate the miR-138-5p
binding domain in the 3′UTR of SIRT1. PC12 cells (5×104
cells/well) were co-transfected with 1 ng reporter vector
containing WT-SIRT1 or MUT-SIRT1, as well as 50 nM miR-138-5p mimic
(5′-AGCUGGUGUUGUGAAUCAGGCCG-3′; Shanghai GenePharma Co., Ltd.) or
50 nM mimic control (5′-CAGUACUUUUGUGUAGUACAA-3′; Shanghai
GenePharma Co., Ltd.) using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. The luciferase activity was analyzed at 48
h after co-transfection using a dual-luciferase reporter assay
system (Promega Corporation), according to the manufacturer's
protocol. Firefly luciferase activities were normalized to
Renilla luciferase activities. The experiment was performed
at least three times.
RNA extraction and RT-qPCR
Total RNA was isolated from rat spinal cord tissues
or PC12 cells using TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) according to the manufacturer's protocols.
Spinal cord specimens isolated from control and SCI model rats were
divided into three equal segments to detect gene expression.
NanoDrop™ ND-1000 spectrophotometer (NanoDrop Technologies; Thermo
Fisher Scientific, Inc.) was used to measure the RNA concentrations
at 260 and 280 nm. A cDNA Synthesis Kit (Invitrogen; Thermo Fisher
Scientific, Inc.) was used to perform RT, according to the
manufacturer's protocol. qPCR was performed using a Prism 7000
Real-Time PCR system with Power SYBR Green Master mix (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The amplification
conditions were as follows: 35 cycles of denaturation at 94°C for
60 sec, annealing at 60°C for 60 sec and chain extension at 72°C
for 1 min, followed by a final extension step at 72°C for 10 min.
The expression levels of miR-138-5p and SIRT1 were normalized to
the expression levels of the control genes U6 and GAPDH,
respectively. Primer sequences (Sangon Biotech Co., Ltd.) were as
follows: miR-138-5p forward, 5′-AGCTGGTGTTGTGAATCAGGCCG-3′ and
reverse, 5′-TGGTGTCGTGGAGTCG-3′; SIRT1 forward,
5′-AATCCAGTCATTAAAGGTCTACAA-3′ and reverse,
5′-TAGGACCATTACTGCCAGAGG-3′; U6 forward,
5′-GCTTCGGCAGCACATATACTAAAAT-3′ and reverse,
5′-CGCTTCACGAATTTGCGTGTCAT-3′; GAPDH forward,
5′-CTTTGGTATCGTGGAAGGACTC-3′ and reverse,
5′-GTAGAGGCAGGGATGATGTTCT-3′. The relative expression levels of
genes were calculated using the 2−∆ΔCq method (34).
Western blot analysis
Tissues and cells were treated with RIPA buffer
(Beyotime Institute of Biotechnology) and centrifuged at 1,000 × g
for 30 min at 4°C to extract the total protein. Protein
concentration was determined with a bicinchoninic acid protein
assay kit (Pierce; Thermo Fisher Scientific, Inc.). Then, equal
quantities of protein were separated by 10% SDS-PAGE, followed by
transferring onto PVDF membranes. The membranes were blocked with
5% skimmed milk for 1 h at room temperature and incubated overnight
at 4°C with anti-SIRT1 (1:1,000; cat. no. 9475), anti-PTEN
(1:1,000; cat. no. 9188), anti-phosphorylated (p)-AKT (1:1,000;
cat. no. 4060), AKT (1:1,000; cat. no. 9272) and anti-β-actin
(1:1,000; cat. no. 4970; all Cell Signaling Technology, Inc.)
antibodies. After five washes in PBS-0.1% Tween 20, the membranes
were incubated with horseradish peroxidase-conjugated goat
anti-rabbit secondary antibody (1:2,000; cat. no. 7074) for 1 h at
37°C. Finally, the protein bands were detected using
chemiluminescent ECL reagent (EMD Millipore). Protein expression
levels were quantified using Image Lab Software (v.6.0; Bio-Rad
Laboratories, Inc.).
MTT assay
In order to determine the viability of PC12 cells,
cells were seeded (10,000 cells/well) into 96-well plates (BD
Biosciences) and cultured for 24 h at 37°C. Then, the culture
medium was removed, and miR-138-5p inhibitor, inhibitor control or
miR-138-5p inhibitor + SIRT1-siRNA were subsequently transfected
into the cells for 48 h at 37°C as previously described. PC12 cells
were then subjected to LPS (100 ng/ml) treatment for 4 h.
Subsequently, MTT solution (10 µl) was added to the medium and
cultivated at 37°C for 4 h according to the manufacturer's
instructions. DMSO (100 µl; Nanjing KeyGen Biotech Co., Ltd.) to
dissolve the formazan crystals. The 96-well plates were placed in a
multifunctional plate reader (VICTOR3™; PerkinElmer, Inc.) to
measure the absorbance at 490 nm.
ELISA
After transfection, PC12 cells were treated with LPS
(100 ng/ml) for 4 h. Then the cells were collected and centrifuged
for measurement of tumor necrosis factor-α (TNF-α; cat. no.
430207), interleukin (IL)-1β (cat. no. 437007) and IL-6 (cat. no.
430507) secretion using ELISA kits according to the manufacturer's
instructions (BioLegend, Inc.). The absorbance was measured at 450
nm using a microplate reader (Model 550; Bio-Rad Laboratories,
Inc.) and the levels were calculated using standard curves.
Flow cytometric assay
To determine cell apoptosis, PC12 cells were
transfected with miR-138-5p inhibitor, inhibitor control or
miR-138-5p inhibitor + SIRT1-siRNA for 48 h as aforementioned, and
then treated with LPS (100 ng/ml) for 4 h at 37°C. After
treatments, the PC12 cells (1×106 cells/well) were
trypsinized, washed with PBS, and stained with Annexin V-FITC and
propidium iodide (PI) for 30 min at 37°C. An Annexin V-FITC/PI cell
apoptosis detection kit (Beyotime Institute of Biotechnology) was
used to detect the early and late apoptosis of PC12 cells via flow
cytometry using a FACSCalibur flow cytometer (BD Biosciences) and
FlowJo software (version 7.6.1; FlowJo LLC).
Statistical analysis
Experiments were repeated at least three times. Data
were expressed as the mean ± SD and all statistical analyses were
conducted using SPSS 18.0 software (SPSS, Inc.). Differences
between groups were determined by two-tailed Student's t-test or
one-way ANOVA followed by Tukey's post hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
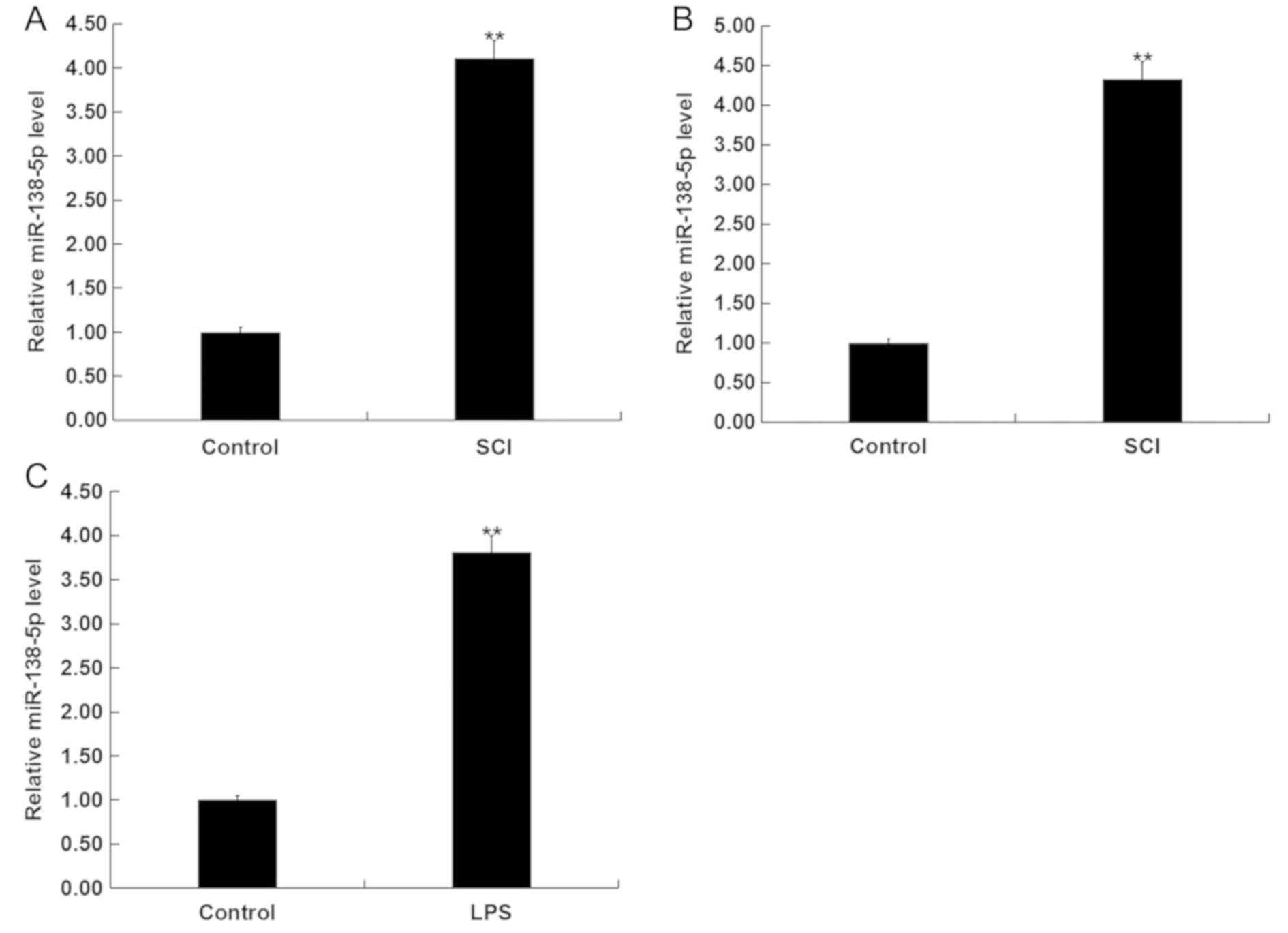
Expression of miR-138-5p is increased
in SCI tissues and in vitro SCI cell models
miR-138-5p belongs to the miR-138 family that has
previously been shown to play an important role in various cancers
(21–23). The levels of miR-138-5p in the
blood of patients with SCI and the spinal tissues of SCI rats were
assessed by RT-qPCR. It was found that the levels of miR-138-5p
were upregulated in the blood of patients with SCI compared with
healthy controls, and in the spinal cord tissues of the SCI rats
compared with the control group (Fig.
1A and B). Next, RT-qPCR was used to measure the levels of
miR-138-5p in an in vitro SCI cell model compared with
normal control cells (Fig. 1C). It
was demonstrated that in LPS-induced PC12 cells, the expression of
miR-138-5p was significantly upregulated compared with the control
group. These results indicated that miR-138-5p may be involved in
the progression of SCI.
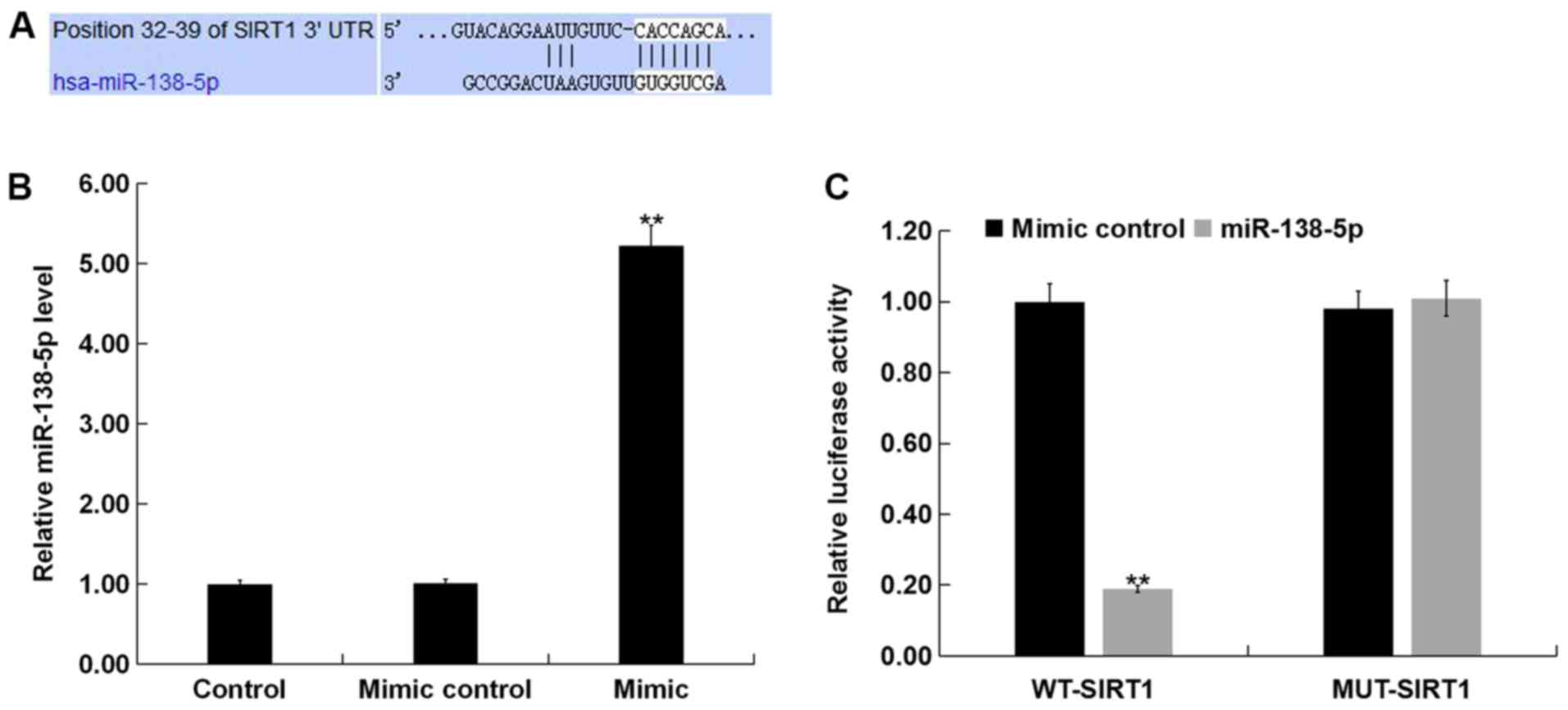
SIRT1 is a direct target of
miR-138-5p
To analyze the molecular mechanisms underlying the
role of miR-138-5p in PC12 cells, potential targets were predicted
using TargetScan. A binding region of miR-138-5p in the 3′UTR of
SIRT1 was predicted by, suggesting that SIRT1 was a potential
target of miR-138-5p (Fig. 2A). A
luciferase reporter assay was performed to validate this
prediction. It was demonstrated that miR-138-5p mimic significantly
enhanced miR-138-5p levels in PC12 cells (Fig. 2B). Subsequently, PC12 cells were
co-transfected with luciferase vector plasmids containing the 3′UTR
of SIRT1, along with miR-138-5p mimic or mimic control for 48 h.
The results showed that the luciferase activity of luciferase
vectors harboring WT-SIRT1 were significantly reduced in the
miR-138-5p mimic co-transfection group, whereas no significant
inhibition was found in the MUT-SIRT1 + miR-138-5p mimic
co-transfection group (Fig. 2C)
These results indicated that SIRT1 was a direct target of
miR-138-5p.
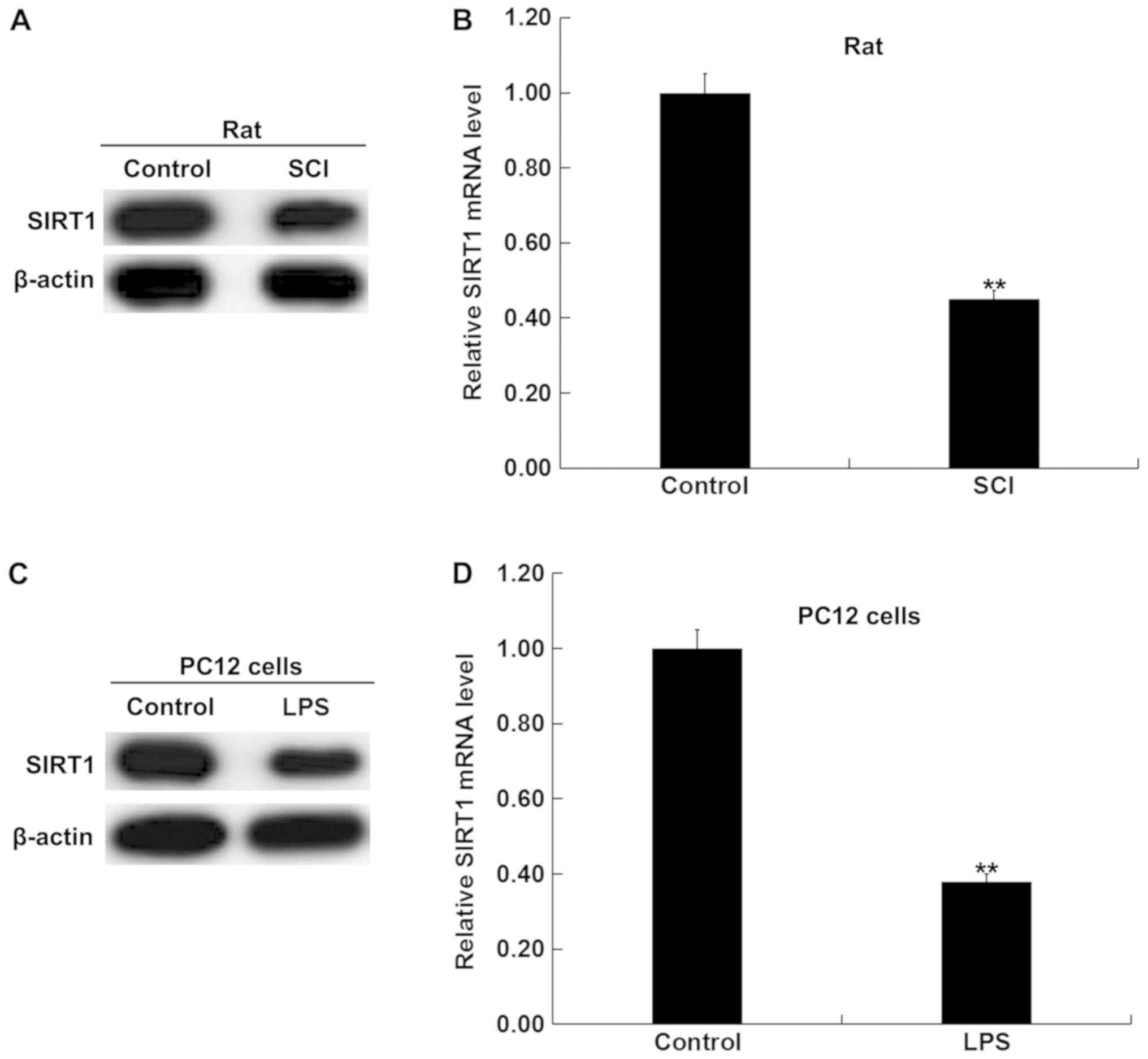
Expression of SIRT1 in SCI rat and in
vitro SCI cell models is reduced
Based on these observations, it was hypothesized
that SIRT1 may be involved in the effects of miR-138-5p in SCI. To
test this hypothesis, western blot and RT-qPCR analyses were
performed to detect the protein and mRNA expression levels of SIRT1
in the spinal cord tissues of SCI rats and LPS-treated PC12 cells,
and it was revealed that SIRT1 expression was downregulated in SCI
model spinal tissues compared with the control group (Fig. 3A and B). Additionally, compared
with the untreated PC12 cells, SIRT1 expression was significantly
downregulated in LPS-treated PC12 cells (Fig. 3C and D).
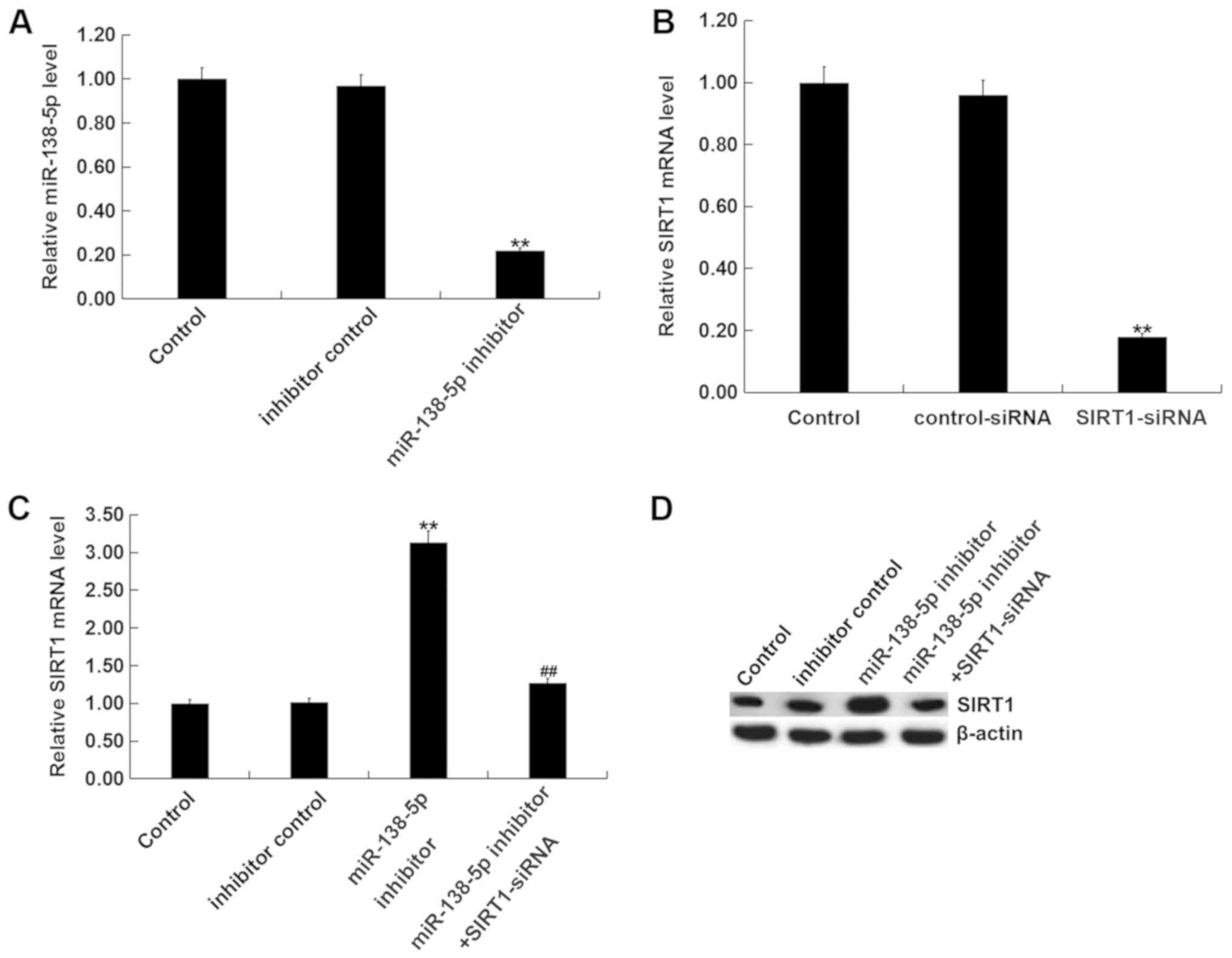
Downregulation of miR-138-5p results
in SIRT1 accumulation
In order to evaluate whether miR-138-5p can
interfere with SIRT1 expression in an SCI model in vitro,
PC12 cells were transfected with control-siRNA, SIRT1-siRNA,
miR-138-5p inhibitor, inhibitor control, or SIRT1-siRNA +
miR-138-5p inhibitor for 48 h. As presented in Fig. 4A, miR-138-5p levels were
significantly decreased in PC12 cells transfected with miR-138-5p
inhibitor compared with the inhibitor control. Additionally,
SIRT1-siRNA significantly reduced the mRNA levels of SIRT1 in PC12
cells (Fig. 4B). Compared with the
control group, the mRNA and protein levels of SIRT1 were
significantly enhanced by miR-138-5p inhibitor; this increase was
reversed by SIRT1-siRNA (Fig. 4C and
D). The findings indicated that miR-138-5p negatively regulates
SIRT1 expression in PC12 cells.
Inhibition of miR-138-5p attenuates
inflammatory injury in the SCI model in vitro
In order to evaluate the function of miR-138-5p in
an in vitro SCI model, miR-138-5p inhibitor, inhibitor
control, or SIRT1-siRNA + miR-138-5p inhibitor were transfected
into PC12 cells for 48 h. Then, the PC12 cells were subjected to
100 ng/ml LPS for 4 h. Subsequently, the viability of cells was
assessed using an MTT assay. The results indicated that cell
viability was significantly decreased in the LPS treatment group
compared with the control group (Fig.
5A). However, compared with LPS treatment alone, miR-138-5p
inhibitor significantly promoted PC12 cell viability, which was
significantly reversed by SIRT1-siRNA (Fig. 5A). Then, the apoptosis of cells was
analyzed via flow cytometry. Compared with the control group, LPS
treatment significantly enhanced PC12 cell apoptosis; however,
miR-138-5p inhibitor significantly reduced the effect of LPS on
apoptosis (Fig. 5B and C). This in
turn was reversed by SIRT1-siRNA co-transfection. Additionally,
inflammatory factors were detected via ELISA. The results
demonstrated that the levels of TNF-α, IL-1β and IL-6 were
significantly upregulated in the LPS treatment group compared with
the control group. Inhibition of miR-138-5p significantly
attenuated the expression of inflammatory factors compared with LPS
treatment alone; this reduction was significantly attenuated by
SIRT1 silencing (Fig. 5D-F).
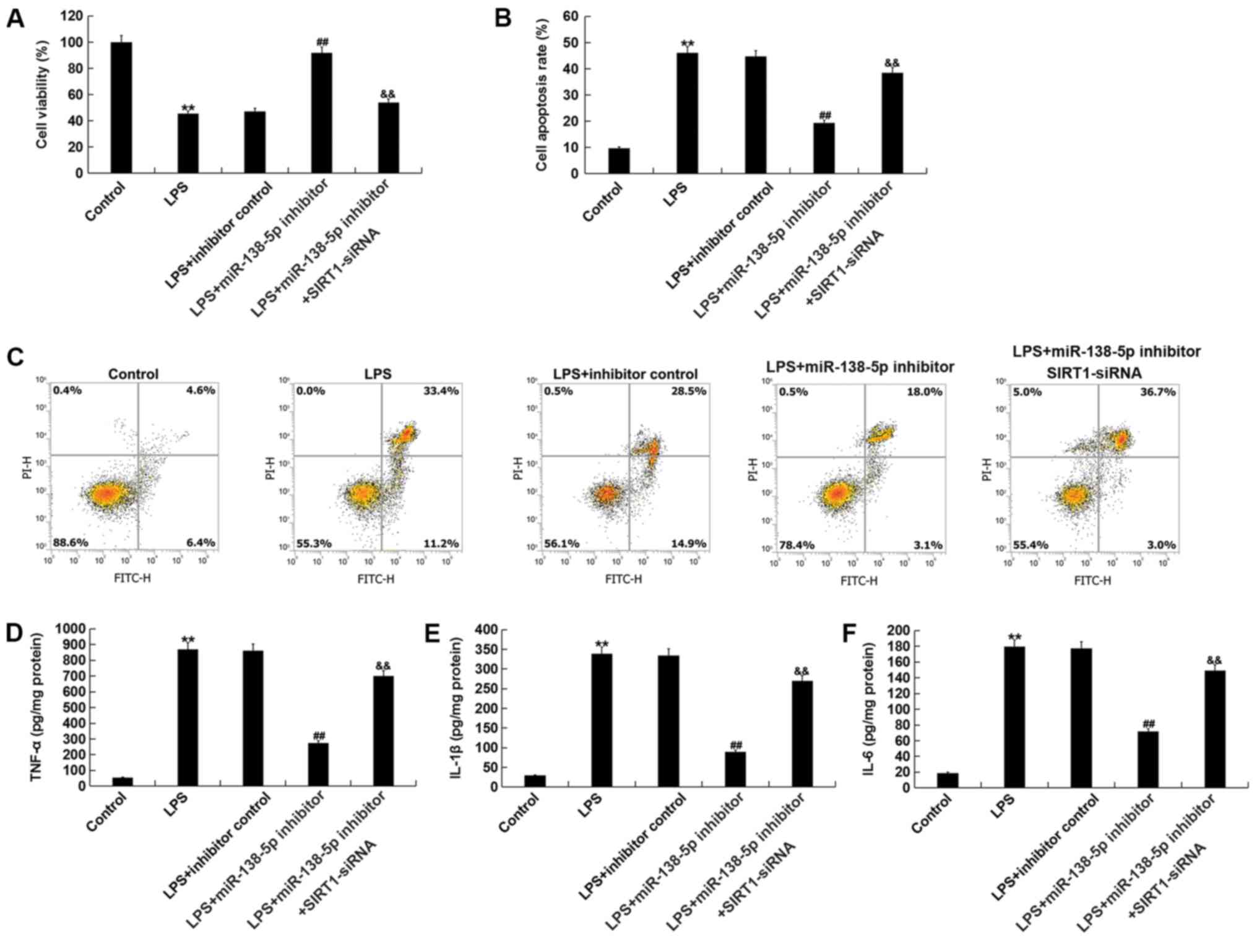 | Figure 5.Downregulation of miR-138-5p or SIRT1
knockdown alter cell viability, apoptosis and the expression of
inflammatory factors. PC12 cells were transfected with inhibitor
control, miR-138-5p inhibitor or miR-138-5p inhibitor + SIRT1-siRNA
for 48 h; then, these cells were subjected to LPS treatment (100
ng/ml) for 4 h. (A) Cell viability was measured by MTT assays. (B)
Histogram of the percentage of early and late apoptotic cells. (C)
Flow cytometric analysis was conducted to evaluate cell apoptosis
using Annexin V/PI double staining. Inflammatory factors including
(D) TNF-α, (E) IL-1β and (F) IL-6 were detected via ELISA. Data are
presented as the mean ± SD. The experiments were performed in
triplicate. **P<0.01 vs. Control; ##P<0.01 vs.
LPS; &&P<0.01 vs. LPS + inhibitor.
miR-138-5p, microRNA-138-5p; SIRT1, sirtuin 1; siRNA, small
interfering RNA; LPS, lipopolysaccharide; IL, interleukin; TNF,
tumor necrosis factor; PI, propidium iodide. |
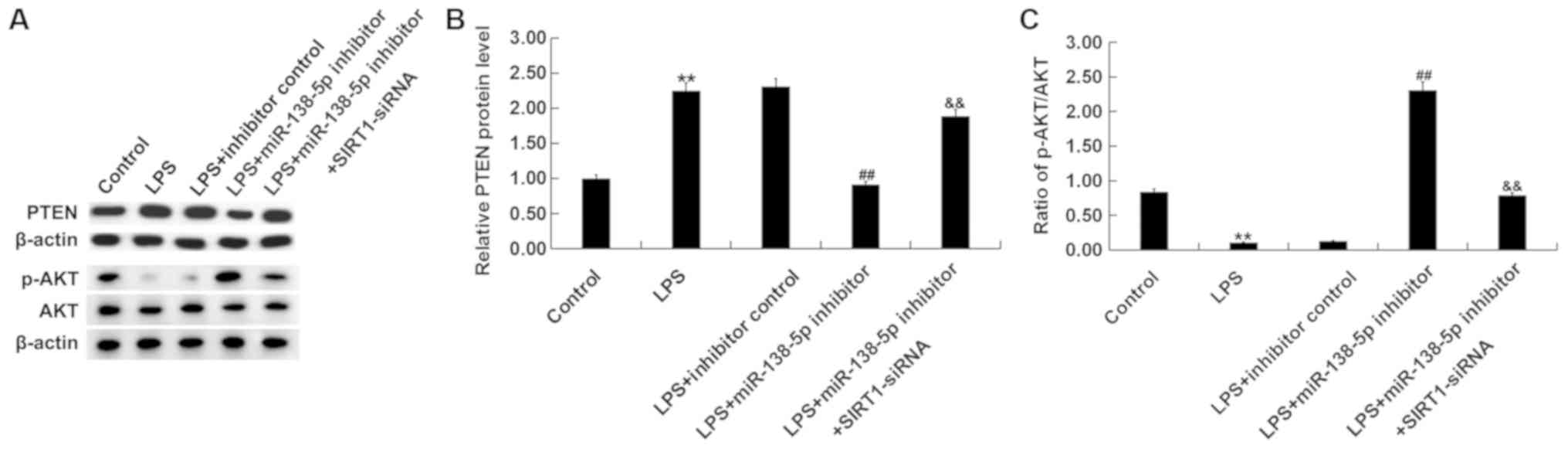
Role of miR-138-5p in the PTEN/AKT
pathway in an in vitro SCI model
To further investigate the molecular mechanisms of
miR-138-5p in SCI, after treatments, the protein levels of PTEN,
AKT and p-AKT were measured in PC12 cells via western blotting.
Results indicated that compared with the control group, LPS
treatment significantly increased PTEN expression (Fig. 6A and B) and decreased AKT
phosphorylation (Fig. 6A and C).
miR-138-5p inhibitor significantly reversed these effects, which
was attenuated by SIRT1 silencing. These results suggested that
miR-138-5p modulated the PTEN/AKT signaling pathway by targeting
SIRT1 in in vitro SCI cell model.
Discussion
There has been substantial focus into the importance
of miRNAs. Several studies have reported that miRNAs altered the
response to SCI by regulating the expression of various key factors
in cell growth and apoptosis (35–37).
SCI refers to primary mechanical damage in the spinal cord
exacerbated by subsequent biological processes, including
inflammation, apoptosis and altered gene expression (38). However, an association between
miR-138-5p and the proliferation and apoptosis of SCI cells has not
previously been identified, to the best of our knowledge. The
biological function and underlying mechanisms of miR-138-5p in SCI
model rats and cells remain to be further explored.
In the present study, damage was induced at T10 to
generate a rodent SCI model. An SCI in vitro cell model was
also established by subjecting PC12 cells to LPS exposure for 4 h.
Then, RT-qPCR was performed to detect the expression of miR-138-5p
in patients with SCI, rats and LPS-induced cells. The results
indicated that miR-138-5p was significantly upregulated in SCI. In
addition, it was further identified that SIRT1 was a potential
target of miR-138-5p. SIRT1 has been implicated as a target of
miR-138-5p in various studies (24,39,40).
For example, overexpression of miR-138-5p has been reported to
suppress manganese-induced autophagy by targeting SIRT1 in SH-SY5Y
cells (24). miR-138-5p enhances
TNF-α-induced apoptosis in human intervertebral disc degeneration
by targeting SIRT1 (39).
miR-138-5p has also been found to prevent autophagy in pancreatic
cancer by targeting SIRT1 (40).
The present study again demonstrated that SIRT1 was
a direct target of miR-138-5p, and further investigated the
expression and roles of miR-138-5p and SIRT1 in SCI. In order to
further explore the relationship between miR-138-5p and SIRT1, the
expression of SIRT1 was detected in an SCI rat model and in
vitro cell model. The present study demonstrated high
expression of miR-138-5p and low expression of SIRT1 in SCI tissues
and cells, suggesting a relationship between the expression levels
of miR-138-5p and SIRT1 in the development of SCI. Additionally, it
was found that the expression of SIRT1 in PC12 cells was negatively
regulated by miR-138-5p. To explore the underlying mechanisms of
miR-138-5p in SCI, PC12 cells were transfected with inhibitor
control, miR-138-5p inhibitor or SIRT1-siRNA + miR-138-5p inhibitor
for 48 h, then the cells were subjected to 100 ng/ml LPS for 4 h.
The present findings showed that knockdown of miR-138-5p
upregulated SIRT1 and further reduced the apoptosis of SCI model
cells.
Results from a previous study indicated that
overexpression of proinflammatory factors can promote apoptosis and
further aggravate SCI (41). In
the present study, the levels of the proinflammatory factors IL-1β,
TNF-α and IL-6 were detected via ELISA. The results demonstrated
that inhibition of miR-138-5p significantly attenuated the
expression of inflammatory factors compared with the inhibitor
control group.
Finally, the expression of PTEN and p-AKT was
investigated via western blotting. The results indicated that
compared with the control group, LPS treatment significantly
increased PTEN protein expression and decreased AKT
phosphorylation. miR-138-5p inhibitor significantly decreased PTEN
protein expression and increased p-AKT protein expression; these
changes were attenuated by SIRT1 silencing. Of note, all effects of
miR-138-5p inhibitor on LPS-induced PC12 cells were significantly
reversed by co-transfection with SIRT1-siRNA. These results
suggested that miR-138-5p modulated the PTEN/AKT signaling pathway
by targeting SIRT1 in an in vitro model of SCI. Thus, it is
hypothesized miR-138-5p inhibitor may suppress SCI-associated
biological process by inhibiting the PTEN/AKT signaling pathway,
highlighting it as a potential therapeutic target in SCI.
In conclusion, to the best of our knowledge, this
was the first study to investigate the relationship between
miR-138-5p and SIRT1 following SCI. Further study into miRNAs in
SCI is urgently required to develop effective and safe therapeutic
strategies for patients with SCI, and to improve the prognosis of
SCI. However, the present study is a preliminary study into the
role of miR-138-5p in SCI, with further experiments required. For
example, the expression of SIRT1, PTEN, p-AKT and AKT in patients
with SCI should be detected in order to validate the conclusions of
the present study in humans. Additionally, time course studies were
not performed for either in vivo or in vitro
experiments in the present study; this will be explored in future
studies.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JC contributed to designing the study, collecting,
analyzing and interpreting the data, and preparing the manuscript.
RQ contributed to collecting and analyzing the data, and preparing
the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Written informed consent was obtained from each
patient. The human study was approved by the Ethics Committee of
the First People's Hospital of Lianyungang. The animal study was
approved by the Animal Ethics Committee of the First People's
Hospital of Lianyungang.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Silva NA, Sousa N, Reis RL and Salgado AJ:
From basics to clinical: A comprehensive review on spinal cord
injury. Prog Neurobiol. 114:25–57. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Brommer B, Engel O, Kopp MA, Watzlawick R,
Müller S, Prüss H, Chen Y, DeVivo MJ, Finkenstaedt FW, Dirnagl U,
et al: Spinal cord injury-induced immune deficiency syndrome
enhances infection susceptibility dependent on lesion level. Brain.
139:692–707. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siddall PJ, McIndoe L, Austin P and
Wrigley PJ: The impact of pain on spiritual well-being in people
with a spinal cord injury. Spinal Cord. 55:105–111. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ahmed MM, King KC, Pearce SM, Ramsey MA,
Miranpuri GS and Resnick DK: Novel targets for spinal cord injury
related neuropathic pain. Ann Neurosci. 18:162–167. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hooshmand MJ, Galvan MD, Partida E and
Anderson AJ: Characterization of recovery, repair, and inflammatory
processes following contusion spinal cord injury in old female
rats: Is age a limitation? Immun Ageing. 11:152014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Garshick E, Stolzmann KL, Gagnon DR, Morse
LR and Brown R: Systemic inflammation and reduced pulmonary
function in chronic spinal cord injury. PM R. 3:433–439. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gris D, Hamilton EF and Weaver LC: The
systemic inflammatory response after spinal cord injury damages
lungs and kidneys. Exp Neurol. 211:259–270. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pillai RS: MicroRNA function: Multiple
mechanisms for a tiny RNA? RNA. 11:1753–1761. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hammond SM: An overview of microRNAs. Adv
Drug Deliv Rev. 87:3–14. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shukla GC, Singh J and Barik S: MicroRNAs:
Processing, maturation, target recognition and regulatory
functions. Mol Cell Pharmacol. 3:83–92. 2011.PubMed/NCBI
|
|
11
|
Calin GA and Croce CM: MicroRNA signatures
in human cancers. Nat Rev Cancer. 6:857–866. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Feng W and Feng Y: MicroRNAs in neural
cell development and brain diseases. Sci China Life Sci.
54:1103–1112. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bian S and Sun T: Functions of noncoding
RNAs in neural development and neurological diseases. Mol
Neurobiol. 44:359–373. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Papagiannakopoulos T and Kosik KS:
MicroRNAs: Regulators of oncogenesis and stemness. BMC Med.
6:152008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Blandino G, Fazi F, Donzelli S, Kedmi M,
Sas-Chen A, Muti P, Strano S and Yarden Y: Tumor suppressor
microRNAs: A novel non-coding alliance against cancer. FEBS Lett.
588:2639–2652. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Palanichamy JK and Rao DS: miRNA
dysregulation in cancer: Towards a mechanistic understanding. Front
Genet. 5:542014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang Y, Kim S and Kim IM: Regulation of
metastasis by microRNAs in ovarian cancer. Front Oncol. 4:1432014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Othman N and Nagoor NH: The role of
microRNAs in the regulation of apoptosis in lung cancer and its
application in cancer treatment. Biomed Res Int. 2014:3180302014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Han C, Yu Z, Duan Z and Kan Q: Role of
microRNA-1 in human cancer and its therapeutic potentials. Biomed
Res Int. 2014:4283712014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu NK, Wang XF, Lu QB and Xu XM: Altered
microRNA expression following traumatic spinal cord injury. Exp
Neurol. 219:424–429. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Roberto GM, Lira RC, Delsin LE, Vieira GM,
Silva MO, Hakime RG, Yamashita ME, Engel EE, Scrideli CA, Tone LG
and Brassesco MS: microRNA-138-5p as a worse prognosis biomarker in
pediatric, adolescent, and young adult osteosarcoma. Pathol Oncol
Res. Mar 12–2019.doi: 10.1007/s12253-019-00633-0. [Epub ahead of
print].
|
|
22
|
He Z, Ruan X, Liu X, Zheng J, Liu Y, Liu
L, Ma J, Shao L, Wang D, Shen S, et al:
FUS/circ_002136/miR-138-5p/SOX13 feedback loop regulates
angiogenesis in Glioma. J Exp Clin Cancer Res. 38:652019.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhu D, Gu L, Li Z, Jin W, Lu Q and Ren T:
MiR-138-5p suppresses lung adenocarcinoma cell
epithelial-mesenchymal transition, proliferation and metastasis by
targeting ZEB2. Pathol Res Pract. 215:861–872. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ma J, Zhang Y, Ji H, Chen L, Chen T, Guo
C, Zhang S, Jia J and Niu P: Overexpression of miR-138-5p
suppresses MnCl2 -induced autophagy by targeting SIRT1 in SH-SY5Y
cells. Environ Toxicol. 34:539–547. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nogueiras R, Habegger KM, Chaudhary N,
Finan B, Banks AS, Dietrich MO, Horvath TL, Sinclair DA, Pfluger PT
and Tschöp MH: Sirtuin 1 and sirtuin 3: Physiological modulators of
metabolism. Physiol Rev. 92:1479–1514. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
da Cunha MSB and Arruda SF:
Tucum-do-Cerrado (Bactris setosa Mart.) may promote anti-aging
effect by upregulating SIRT1-Nrf2 pathway and attenuating oxidative
stress and inflammation. Nutrients. 9:E12432017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rada P, Pardo V, Mobasher MA,
García-Martínez I, Ruiz L, González-Rodríguez Á, Sanchez-Ramos C,
Muntané J, Alemany S, James LP, et al: SIRT1 controls acetaminophen
hepatotoxicity by modulating inflammation and oxidative stress.
Antioxid Redox Signal. 28:1187–1208. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chan SH, Hung CH, Shih JY, Chu PM, Cheng
YH, Lin HC and Tsai KL: SIRT1 inhibition causes oxidative stress
and inflammation in patients with coronary artery disease. Redox
Biol. 13:301–309. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cheng YY, Kao CL, Ma HI, Hung CH, Wang CT,
Liu DH, Chen PY and Tsai KL: SIRT1-related inhibition of
pro-inflammatory responses and oxidative stress are involved in the
mechanism of nonspecific low back pain relief after exercise
through modulation of Toll-like receptor 4. J Biochem. 158:299–308.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Roberts TT, Leonard GR and Cepela DJ:
Classifications in brief: American spinal injury association (ASIA)
impairment scale. Clin Orthop Relat Res. 475:1499–1504. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bayne K: Revised guide for the care and
use of laboratory animals available. American Physiological
Society. Physiologist. 39:199208–199211. 1996.
|
|
32
|
Xin DQ, Hu ZM, Huo HJ, Yang XJ, Han D,
Xing WH, Zhao Y and Qiu QH: Schisandrin B attenuates the
inflammatory response, oxidative stress and apoptosis induced by
traumatic spinal cord injury via inhibition of p53 signaling in
adult rats. Mol Med Rep. 16:533–538. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tan Y, Yu L, Zhang C, Chen K, Lu J and Tan
L: miRNA-146a attenuates inflammation in an in vitro spinal cord
injury model via inhibition of TLR4 signaling. Exp Ther Med.
16:3703–3709. 2018.PubMed/NCBI
|
|
34
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang C, Pan Y, Cheng B, Chen J and Bai B:
Identification of conserved and novel microRNAs in cerebral
ischemia-reperfusion injury of rat using deep sequencing. J Mol
Neurosci. 54:671–683. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wan G, An Y, Tao J, Wang Y, Zhou Q, Yang R
and Liang Q: MicroRNA-129-5p alleviates spinal cord injury in mice
via suppressing the apoptosis and inflammatory response through
HMGB1/TLR4/NF-κB pathway. Biosci Rep. 40:BSR201933152020.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sun F, Li SG, Zhang HW, Hua FW, Sun GZ and
Huang Z: MiRNA-411 attenuates inflammatory damage and apoptosis
following spinal cord injury. Eur Rev Med Pharmacol Sci.
24:491–498. 2020.PubMed/NCBI
|
|
38
|
Weishaupt N, Silasi G, Colbourne F and
Fouad K: Secondary damage in the spinal cord after motor cortex
injury in rats. J Neurotrauma. 27:1387–1397. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang B, Wang D, Yan T and Yuan H:
MiR-138-5p promotes TNF-α-induced apoptosis in human intervertebral
disc degeneration by targeting SIRT1 through PTEN/PI3K/Akt
signaling. Exp Cell Res. 345:199–205. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tian S, Guo X, Yu C, Sun C and Jiang J:
miR-138-5p suppresses autophagy in pancreatic cancer by targeting
SIRT1. Oncotarget. 8:11071–11082. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bank M, Stein A, Sison C, Glazer A, Jassal
N, McCarthy D, Shatzer M, Hahn B, Chugh R, Davies P and Bloom O:
Elevated circulating levels of the pro-inflammatory cytokine
macrophage migration inhibitory factor in individuals with acute
spinal cord injury. Arch Phys Med Rehabil. 96:633–644. 2015.
View Article : Google Scholar : PubMed/NCBI
|