Introduction
Diabetes mellitus (DM) belongs to a group of chronic
metabolic diseases characterized by chronic hyperglycemia and
disturbance of fat and protein metabolism, which occur as a result
of insulin deficiency and/or resistance, thus leading to elevated
blood glucose levels and abnormal fat and protein metabolism
(1,2). Long-term hyperglycemia can result in
microvascular and macrovascular damage, particularly cardiovascular
disease (CVD) complications, which increase the morbidity and
mortality rates of patients with diabetes (3). Furthermore, atherosclerosis is a major
risk factor for DM-related CVD (4),
and it is accelerated in type 1 and type 2 DM (5). Previous studies have shown that high
glucose (HG)-induced migration and proliferation of vascular smooth
muscle cells (VSMCs) are associated with the progression of
atherosclerosis in DM (4,6).
MicroRNAs (miRNAs/miRs) are endogenous non-coding
RNAs, 20–24 nucleotides in length, which have a variety of
important regulatory roles in cells (7). miRNAs inhibit translation or degrade
mRNA by interacting with the 3′-untranslated region (3′-UTR) of a
target mRNA (7,8). Moreover, via this interaction miRNAs
modulate several pathological and physiological pathways in human
diseases, including CVDs, diabetes and cancer (9). Each miRNA has the potential to
regulate multiple genes in biological processes, including
development and metabolism (8,9). Thus,
miRNA dysregulation affects several pathological pathways in DM
(9–11).
The aims of the present study were to assess the
change in the miRNA expression profile in VSMCs following HG
exposure, and to investigate the effect of dysregulated miRNA
expression on VSMC proliferation and migration.
Materials and methods
Cell culture
VSMCs were isolated from thoracic aortic explants
from male Sprague Dawley (SD) rats (age, 6 weeks; weight 200±20 g)
as previously described (12). The
SD rats were purchased from Zhejiang Academy of Medical Science.
The explants were cultured in low glucose DMEM (Thermo Fisher
Scientific, Inc.) supplemented with 10% FBS (Thermo Fisher
Scientific, Inc.) and 1% antibiotic-antimycotic (Thermo Fisher
Scientific, Inc.), and maintained at 37°C in a humidified
atmosphere containing 5% CO2 and 95% air. After 10 days,
cells that had migrated from the aortic explants were collected and
sub-cultured. Experiments were conducted from cell passages three
to six. 293T cells were purchased from the American Type Culture
Collection, and were maintained in high glucose DMEM containing 10%
FBS and 1% antibiotic-antimycotic at 37°C in a humidified
atmosphere containing 5% CO2.
miRNA sequencing
VSMCs, normally cultured with 5.6 mM glucose, were
incubated with 22.2 mM glucose (HG; Sigma-Aldrich; Merck KGaA) at
37°C for 48 h. Total RNA was subsequently extracted from the
HG-exposed and control VSMCs using TRIzol® reagent
(Thermo Fisher Scientific, Inc.). Then, ~1 µg of the total RNA was
used to prepare a small RNA library using a NEBNext Small RNA
Library Prep Set (New England Biolabs, Inc.) according to the
manufacturer's instructions. Single-end sequencing (50 bp) was
performed using an Illumina HiSeq2500 system (Illumina, Inc.)
according to the manufacturer's protocol. Raw reads were checked
for contaminants and potential sequencing issues using FastQC
(https://www.bioinformatics.babraham.ac.uk/projects/fastqc/;
version 0.11.2). Low-quality reads, contaminating 5′ adapters and
homopolymers were filtered. Reads were trimmed for 3′ adapters, and
reads with a length of <10 and >34 nucleotide (nt) were
discarded. Clean reads were processed using the Basic Local
Alignment Search Tool (https://blast.ncbi.nlm.nih.gov/Blast.cgi; version
2.2.18). The Rfam10.1 database (https://rfam.org/) was used to annotate the RNA
sequences and to align the sequences against miRNA
precursors/mature miRNAs in the miRBase database (http://www.mirbase.org/; version 20.0) for the
identification of known miRNAs. The read counts of each known miRNA
were then normalized to the total counts of mapped sequence reads,
and are presented as counts per million mapped reads. miRNAs with
fold-changes >1.5 were selected as candidate miRNAs.
miRNA verification: Reverse
transcription-quantitative PCR (RT-qPCR)
For further assessment of miRNA expression, miRNAs
from the VSMCs in the aortic media were extracted using mirVana
miRNA isolation kit (Thermo Fisher Scientific, Inc.). cDNA was
synthesized using the PrimeScript RT reagent kit (Takara Bio, Inc.)
at 42°C for 1 h according to the manufacturer's instructions with
specific rno-miR-125a RT primers
(5′-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACAGGGAC-3′;
Guangzhou RiboBio Co., Ltd.). qPCR was performed using the SYBR
Premix Ex Taq kit (Takara Bio, Inc.) under the following
conditions: Initial denaturation at 95°C for 30 sec, followed by 40
cycles at 95°C for 5 sec and at 60°C for 30 sec in an ABI Prism
7900 system (Applied Biosystems; Thermo Fisher Scientific, Inc.).
The following sequences were used: rno-miR-125a forward,
5′-GCCGGGAGTGTCCAATTTCCCAGA-3′ and reverse,
5′-CAGTGCAGGGTCCGAGGTAT-3′. U6 forward, 5′-CTCGCTTCGGCAGCACA-3′ and
reverse 5′-AACGCTTCACGAATTTGCGT-3′. Relative miRNA expression was
quantified using the 2−ΔΔCq method (13) and normalized to U6 expression.
miRNA, small interfering (si) RNA and
plasmid transfection
siRNA against 3-hydroxy-3-methyglutaryl-coA
reductase (HMGCR) and scrambled control (ctrl) siRNA were purchased
from Shanghai GenePharma Co., Ltd. (cat. no. A10001). miR-125a
mimic (sense, 5′-UCCCUGAGACCCUUUAACCUGUGA-3′ and antisense,
5′-UCACAGGUUAAAGGGUCUCAGGGA-3′), miRNA mimic negative control
(miR-NC, sense, 5′-UUUGUACUACACAAAAGUACUG-3′ and antisense,
5′-CAGUACUUUUGUGUAGUACAAA-3′), inhibitor
(5′-UCACAGGUUAAAGGGUCUCAGGGA-3′) and miRNA inhibitor negative
control (miR-NC, 5′-CAGUACUUUUGUGUAGUACAAA-3′) were purchased from
Guangzhou RiboBio Co., Ltd. siRNA (100 nM) and plasmid (1 µg/ml)
transfections were performed using Lipofectamine 3000 (Thermo
Fisher Scientific, Inc.). miRNA mimic (50 nM) and miRNA inhibitor
(50 nM) were transfected into VSMCs cultured under HG or normal
conditions (5.6 nM glucose) by using Lipofectamine®
RNAiMAX (Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. Western blotting and RT-qPCR analysis
were used to detect the effects of gene silencing and miR-125a
expression in VSMCs at 24 h after transfection (Fig. S1).
VSMC proliferation assay
VSMCs were cultured in different concentrations of
glucose (5.6, 11.1, 22.2 and 44.5 mM) at 37°C in a humidified
atmosphere containing 5% CO2 for 72 h and cell
proliferation was detected with a Cell Counting Kit-8 (CCK-8) assay
(Dojindo Molecular Technologies, Inc.). In addition, after 24 h
transfection with HMGCR siRNA or miR-125a mimic, VSMCs were plated
at a density of ~5,000 cells/well in 96-well plates and maintained
in HG-culture medium (22.2 mM) for 72 h. Cell proliferation was
also determined using the CCK-8 assay at 37°C for 3 h and the
absorbance was measured at 450 nm according to the manufacturer's
instructions.
Wound healing assay
VSMCs were plated in 6-well plates at a density of
5×105 cells per well and cultured in different
concentrations of glucose (5.6, 11.1, 22.2 and 44.5 mM). At 90%
confluency, cells were exposed to mitomycin C (10 mg/ml;
Sigma-Aldrich; Merck KGaA), a potent cell proliferation inhibitor,
at 37°C for 2 h. The confluent cell monolayer was subsequently
scratched using a 200 µl pipette tip. After wounding, the medium
was replaced with fresh serum-free medium. Serial images were
obtained immediately after wounding (0 h) and after 48 h by light
microscopy (magnification, ×20). VSMC migration was determined by
calculating the initial wound area and the wound area after 48 h,
the percent wound size (the wound area at 48 h relative to the
initial wound area) was analyzed by ImageJ software (version 1.46r;
National Institutes of Health).
Dual-luciferase assay
The 3′-UTR of HMGCR carrying the predicted
miR-125a-binding site (http://www.targetscan.org/vert_72/; version 7.2) was
amplified and subcloned into the pmirGLO vector (Promega
Corporation) to generate the wild-type (WT) HMGCR dual-luciferase
expression vector (HMGCR-WT). The rapid mutation kit (New England
Biolabs, Inc.; cat. no. E0554S) was used to generate a HMGCR mutant
(Mut), which was also cloned into the pmirGLO vector (HMGCR-Mut).
293T cells were co-transfected with HMGCR-Mut or HMGCR-WT, and
miR-125a or the control mimics using Lipofectamine® 3000
(Thermo Fisher Scientific, Inc.). After 48 h, the activities of
firefly and Renilla luciferase were determined using a
Dual-Luciferase Reporter assay system (Promega Corporation). The
results were shown as the ratio of Renilla to firefly
luciferase activity.
Western blot analysis
Aortic media samples from SD rats or VSMCs were
lysed with RIPA lysis buffer (Beyotime Institute of Biotechnology)
and 1% protease inhibitors. Bicinchoninic acid assay kit (Thermo
Fisher Scientific, Inc.) was used to quantify the protein samples.
Western blot analysis was carried out according to standard
procedures. Proteins (30 µg/lane) were separated via SDS-PAGE on a
10% gel and then transferred to a 0.22-µm PVDF membrane. The
primary antibodies used included anti-HMGCR (1:1,000; cat. no.
ab174830; Abcam), anti-farnesyl diphosphate synthase (FDPS; 1:1000;
cat. no. ab189874; Abcam), anti-squalene synthase (SQS; 1:1,000;
cat. no. ab195046; Abcam), anti-geranylgeranyltransferase type I
(GGTase-I; 1:1,000; cat. no. ab122122; Abcam) and anti-GAPDH
(1:3,000; cat. no. ET1601-4; Huabio) were incubated at room
temperature for 3 h. A horseradish peroxidase-conjugated goat
anti-rabbit IgG H&L (1:3,000; cat. no. ab7090; Abcam) was used
as the secondary antibody and was incubated at room temperature for
1 h. Protein bands were visualized using enhanced chemiluminescence
and a ChemiScope Western Blot Imaging system (Clinx Science
Instruments Co., Ltd.). The gray-scale value was analyzed using
ImageJ software (version 1.46r; National Institutes of Health) for
densitometry analysis.
Animal experiments
A total of 60 male SD rats (age, 6-week old; weight,
200±20 g) were purchased from the Shanghai Laboratory Animal
Center. In total, 24 rats were used for the control group and 36
rats for the DM group. Animals had free access to food and water,
and were housed in a pathogen-free environment with normoxic
atmosphere (temperature, 20–25°C). The animal experiments were
reviewed and approved by The Institutional Animal Care and Use
Committee of Zhejiang University. DM was induced by daily
intraperitoneal injection of 50 mg/kg streptozotocin (STZ;
Sigma-Aldrich; Merck KGaA) for 5 days after a 4-h fast. Control
rats received an intraperitoneal injection of vehicle (0.1 M
citrate buffer; pH 4.5; 0.1 ml). Fasting plasma glucose (FPG)
levels were measured in 0.05 ml venous blood drawn from the caudal
vein using a glucometer (ACCU-CHECK Active kit; Roche Diagnostics).
At 3 days after the last STZ injection, rats with FPG level
>16.7 mM were considered to have been successful were
established as DM models (14). The
12 rats failed to induce DM were not used for subsequent
experiments.
Blood lipid analysis
At the start and then 5, 10 and 20 weeks after STZ
injection, serum total cholesterol (cat. no. 05168538190), high-
and low-density lipoprotein cholesterol (cat. nos. 07528582190 and
07005768190), triglyceride concentration (cat. no. 05171407190) and
FPG (cat. no. 05168791190) in 3 ml blood samples were collected
from the inferior vena cava of the anesthetized animal following
overnight fasting conditions, and were determined using
commercially available kits (Sigma-Aldrich; Merck KGaA). Fasting
serum insulin (FINS) was determined by chemiluminometry (15). The following equation: (FINS in mU/l
× FPG in mmol/l)/22.5, was used to assess the homeostasis model of
insulin resistance, while the homeostasis model of β-cell function
was quantified as follows: (FINS in mU/l × 20)/(FPG in mmol/l-3.5)
(15).
Histological analysis
The aorta was dissected in situ from the
ascending aorta to the iliac bifurcation. Peripheral fat was
removed under an anatomical microscope and the aorta was
subsequently fixed using 10% neutral formalin for 24 h at room
temperature and embedded in paraffin at room temperature for 6 h.
Embedded specimens were cut into 5 µm sections, and subjected to
standard hematoxylin and eosin staining (hematoxylin, 10 min;
eosin, 30 sec; room temperature) to determine the media thickness
(MT) and media cross-sectional area (MCSA), which is the area
between the internal and external elastic lamina. Morphometric
analysis was performed using Image-Pro Plus software (version 6.0)
(12).
Statistical analysis
The differences between two groups were compared
using the Student's t-test. One-way ANOVA followed by Bonferroni
post-hoc test was used to determine significant differences between
multiple groups. All the experiments were repeated ≥3 times, and
data are presented as the mean ± SD. P<0.05 was considered to
indicate a statistically significant difference.
Results
HG-induced proliferation and migration
of VSMCs is consistent with decreased miR-125a expression
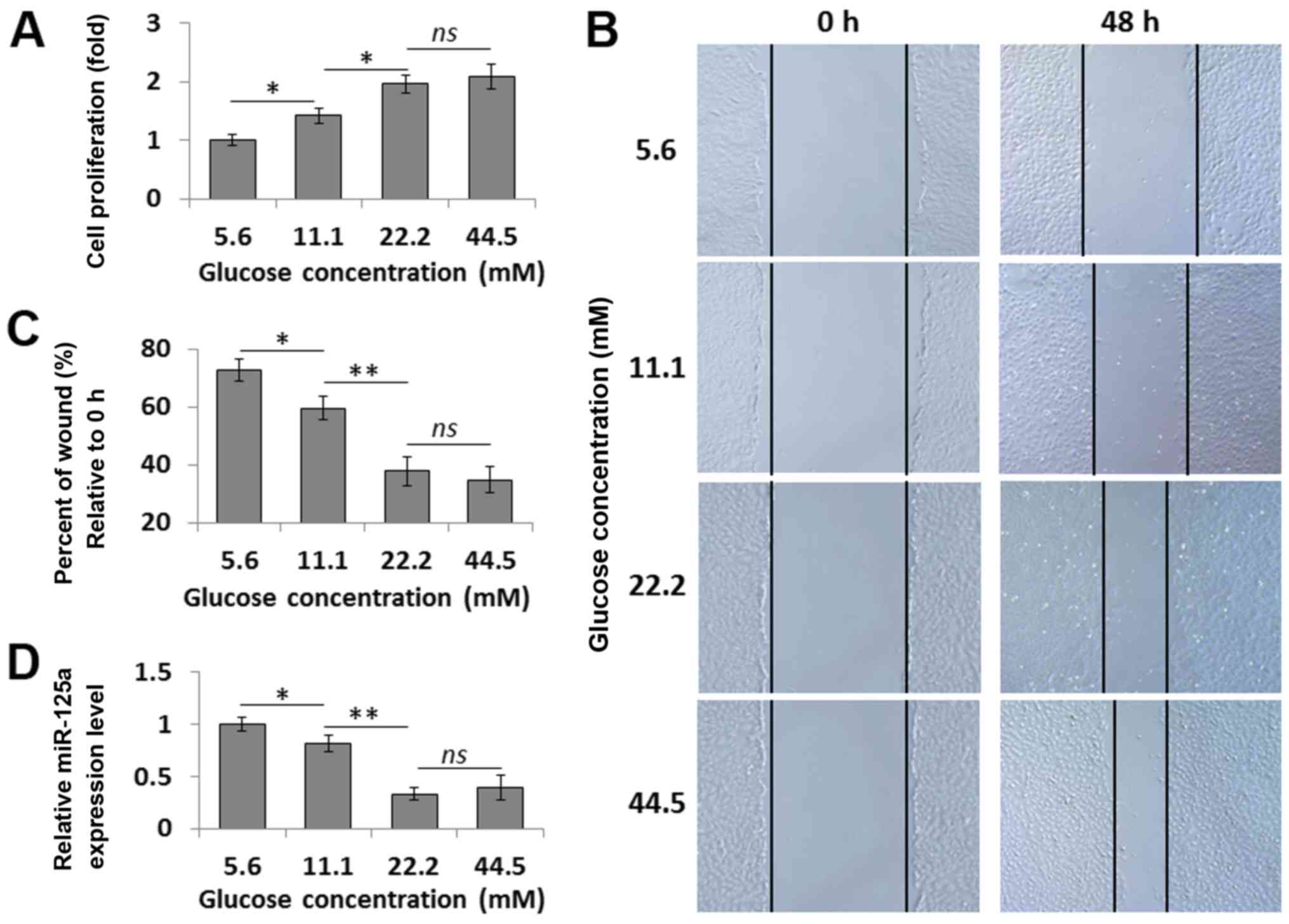
CCK-8 assay results indicated that VSMCs cultured in
different concentrations of glucose (5.6, 11.1, 22.2 and 44.5 mM)
had increased proliferation (Fig.
1A) and migration (Fig. 1B and
C) in a dose-dependent manner. To determine whether HG exposure
altered miRNA expression in VSMCs, the miRNA expression profile of
VSMCs exposed to HG (22.2 mM glucose) for 48 h was compared with
VSMCs cultured under normal conditions (5.6 mM glucose). It was
found that a total of 42 miRNAs were differentially expressed
(fold-change, >1.5) between the two groups. Of the 42 miRNAs, 30
miRNAs were upregulated, including rno-miR-431, rno-miR-182,
rno-miR-708-5p, rno-miR-495 and rno-miR-377-3p, and 12 miRNAs were
downregulated, including rno-miR-125a-5p, rno-miR-331-3p,
rno-miR-450b-5p, rno-miR-101a-3p and rno-miR-125b-5p, in VSMCs
exposed to HG (Table SI). Among
the differentially expressed miRNAs, miR-125a exhibited the largest
change. Furthermore, it was demonstrated that glucose stimulation
decreased the expression of miR-125a in a dose-dependent manner in
VSMCs (Fig. 1D).
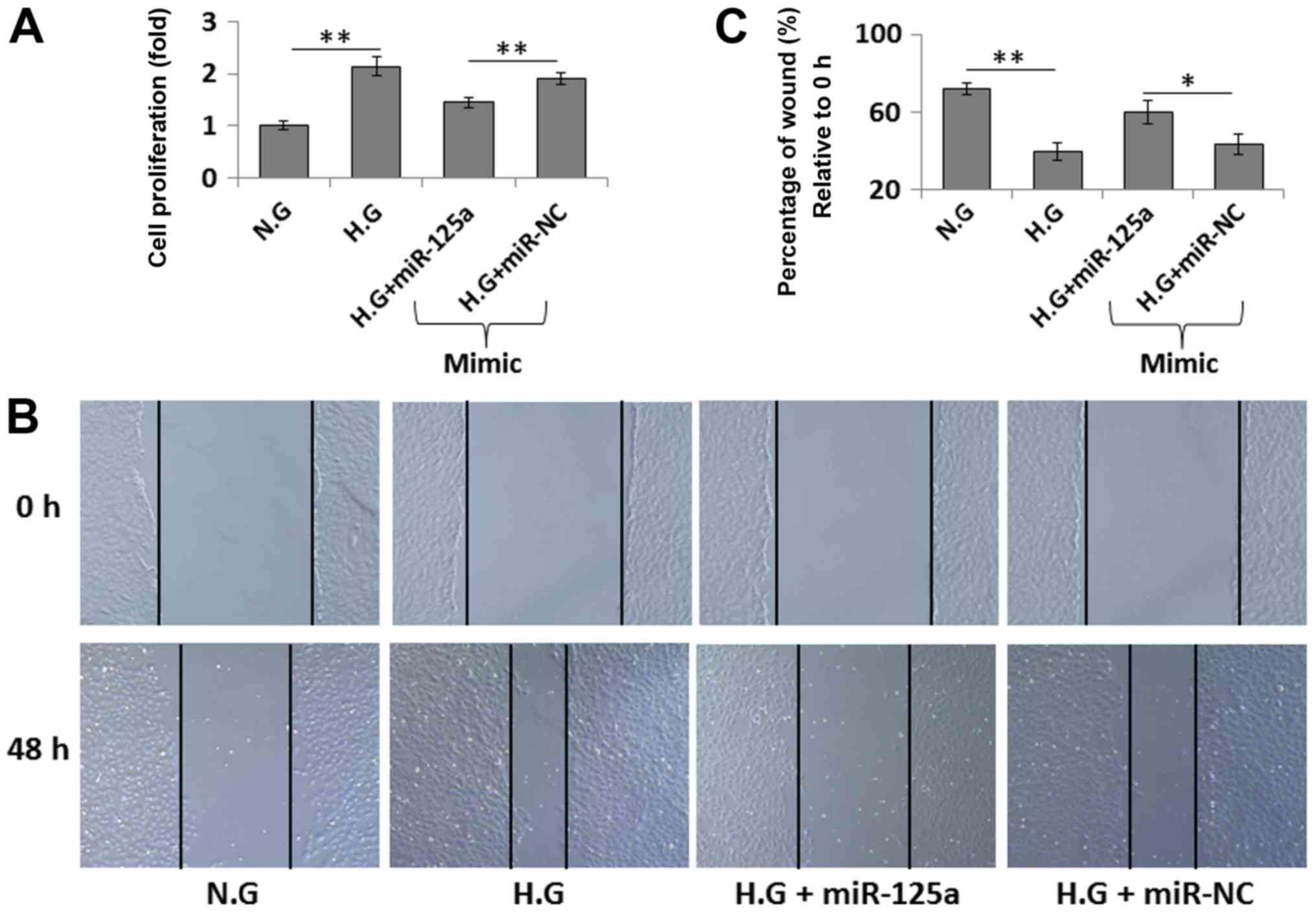
miR-125a abrogates HG-induced VSMC
migration and proliferation
To investigate whether HG-induced VSMC proliferation
and migration were dependent on decreased miR-125 expression, a
miR-125a mimic was used to overexpress miR-125a in VSMCs. It was
found that exposure to 22.2 mM glucose significantly increased VSMC
proliferation compared with the control (Fig. 2A). Moreover, this increased
proliferation was partially suppressed following miR-125a mimic
transfection, and was not affected by miR-NC transfection. In
addition, wound healing assay results indicated that HG-treated
VSMCs exhibited increased migration, while transfection with a
miR-125a mimic reduced the migration of HG-treated VSMCs, which was
determined by the percent wound size (the wound area at 48 h
relative to the initial wound area; Fig. 2B and C).
HMGCR is a direct target of
miR-125a
HMGCR was predicted as a candidate target of
miR-125a using TargetScan software (http://www.targetscan.org/vert_72/; version 7.2), and
it was identified that the HMGCR 3′-UTR had a potential binding
site for miR-125a (Fig. 3A). To
investigate this, HMGCR-WT and HMGCR-Mut were subcloned into the
pmirGLO vectors, and the resulting plasmids were co-transfected
with a miR-125a mimic or miR-NC. Compared with the miR-NC, the
miR-125a mimic significantly reduced the luciferase activity of
HMGCR-WT. Transfection with the HMGCR-Mut abrogated the inhibitory
effect of miR-125a (Fig. 3B).
Moreover, transfection with a miR-125a mimic and miR-125a inhibitor
significantly reduced and increased HMGCR protein expression levels
in VSMCs, respectively (Fig. 3C).
Therefore, the present results indicated that HMGCR may be a direct
target of miR-125a.
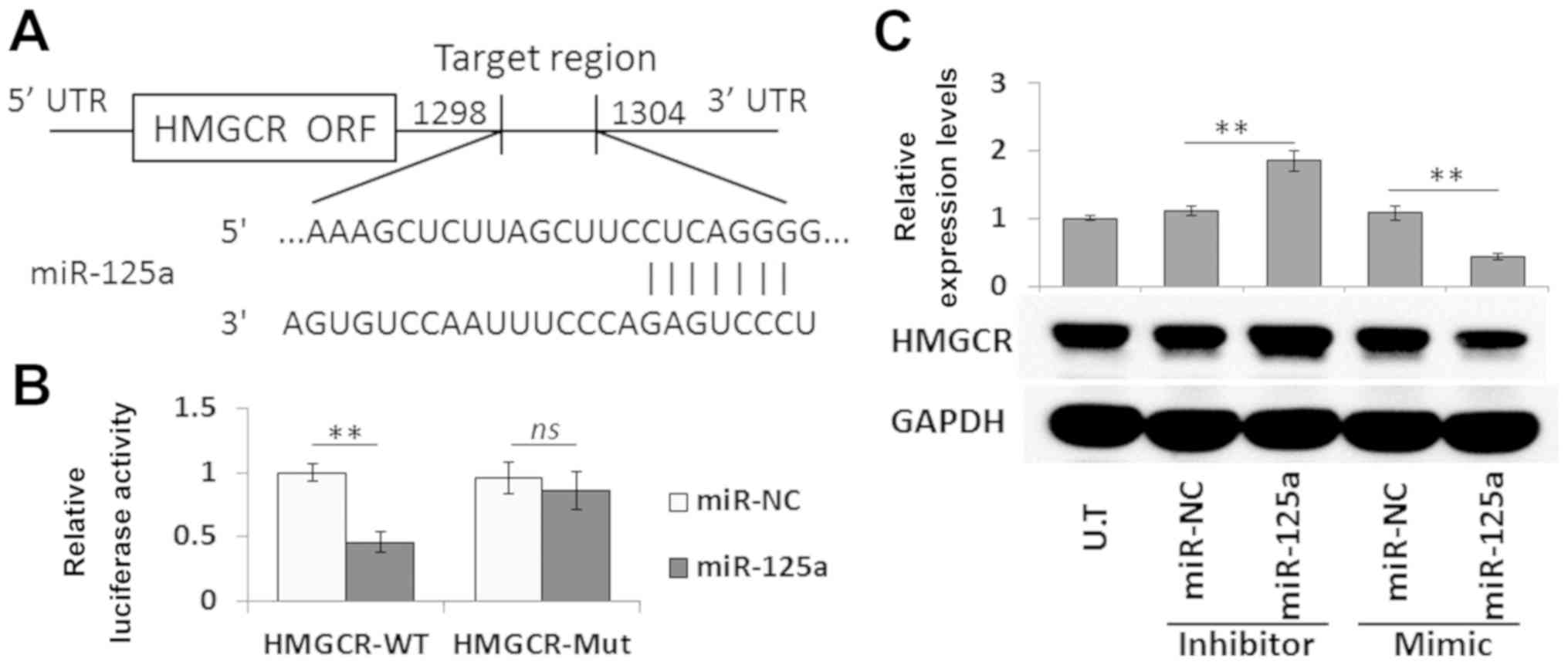 | Figure 3.Validation of HMGCR as a direct
target of miR-125a. (A) Target site of miR-125a in the HMGCR
3′-UTR. (B) Dual-luciferase activity of the WT and Mut HMGCR 3′-UTR
reporter in the presence of miR-125a or miR-NC. (C) Western blot
analysis of HMGCR expression in VSMCs transfected with a miR-125a
mimic, miR-125a inhibitor or miR-NC. Data are presented as the mean
± SD. n=3. **P<0.01; ns, not significant; HMGCR,
3-hydroxy-3-methylglutaryl-coenzyme A reductase; miR, microRNA; WT,
wild-type; Mut, mutant; 3′-UTR, 3′-untranslated region; NC,
negative control; VSMC, vascular smooth muscle cell; UT, untreated
VSMCs. |
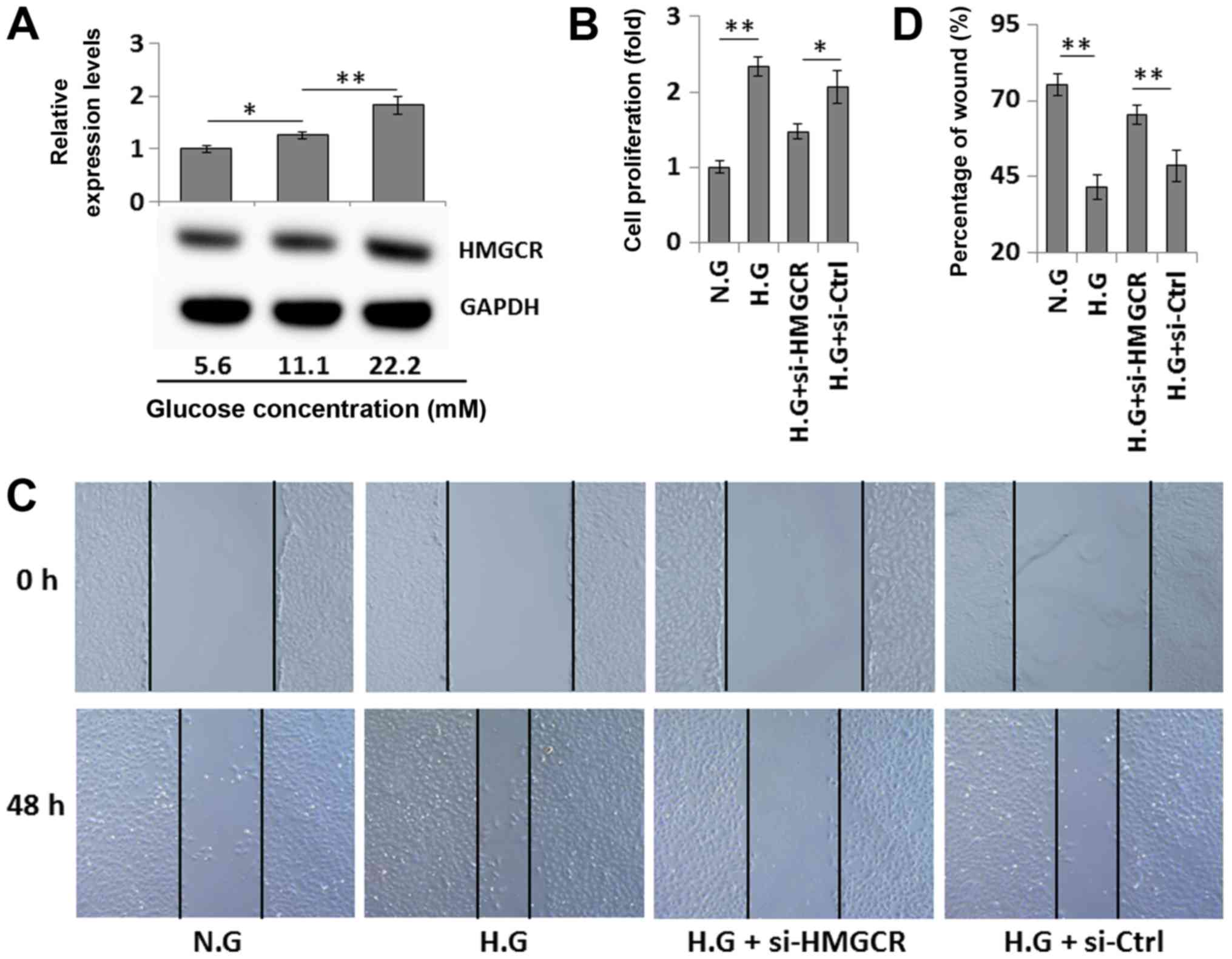
HMGCR downregulation suppresses
HG-induced VSMC proliferation and migration
The effects of HMGCR on HG-induced cell
proliferation and migration were evaluated by knocking down HMGCR
expression in VSMCs, using an HMGCR-specific siRNA. It was found
that the expression of HMGCR in VSMCs was upregulated in a
dose-dependent manner following HG exposure (Fig. 4A). Furthermore, CCK-8 and wound
healing assays demonstrated that HG-induced proliferation and
migration of VSMCs were significantly repressed by transfection
with si-HMGCR, compared with transfection with si-Ctrl (Fig. 4B-D). Collectively, the present
results suggested that HG-induced VSMC proliferation and migration
may be due to increased HMGCR expression, and decreased miR-125a
expression.
miR-125a regulates the mevalonate
signaling pathway via HMGCR in VSMCs
HMGCR is one of the key enzymes in the mevalonate
pathway, which is associated with development of atherosclerosis
(10). To further determine the
mechanistic role of miR-125a in the progression of atherosclerosis,
the effect of miR-125a on the mevalonate signaling pathway was
evaluated. Key enzymes in this pathway, including FDPS and GGTase-I
were upregulated, while SQS was downregulated, in VSMCs exposed to
HG, but the dysregulation of these enzymes could be significantly
reversed following transfection with the miR-125a mimic (Fig. 5A). However, miR-125a inhibition
could upregulate the FDPS and GGTase-I levels and downregulate the
SQS levels in VSMCs, even under normal culture conditions (Fig. 5B). Therefore, the present results
indicated that miR-125a may be involved in HG-induced dysregulation
of the mevalonate signaling pathway by targeting HMGCR.
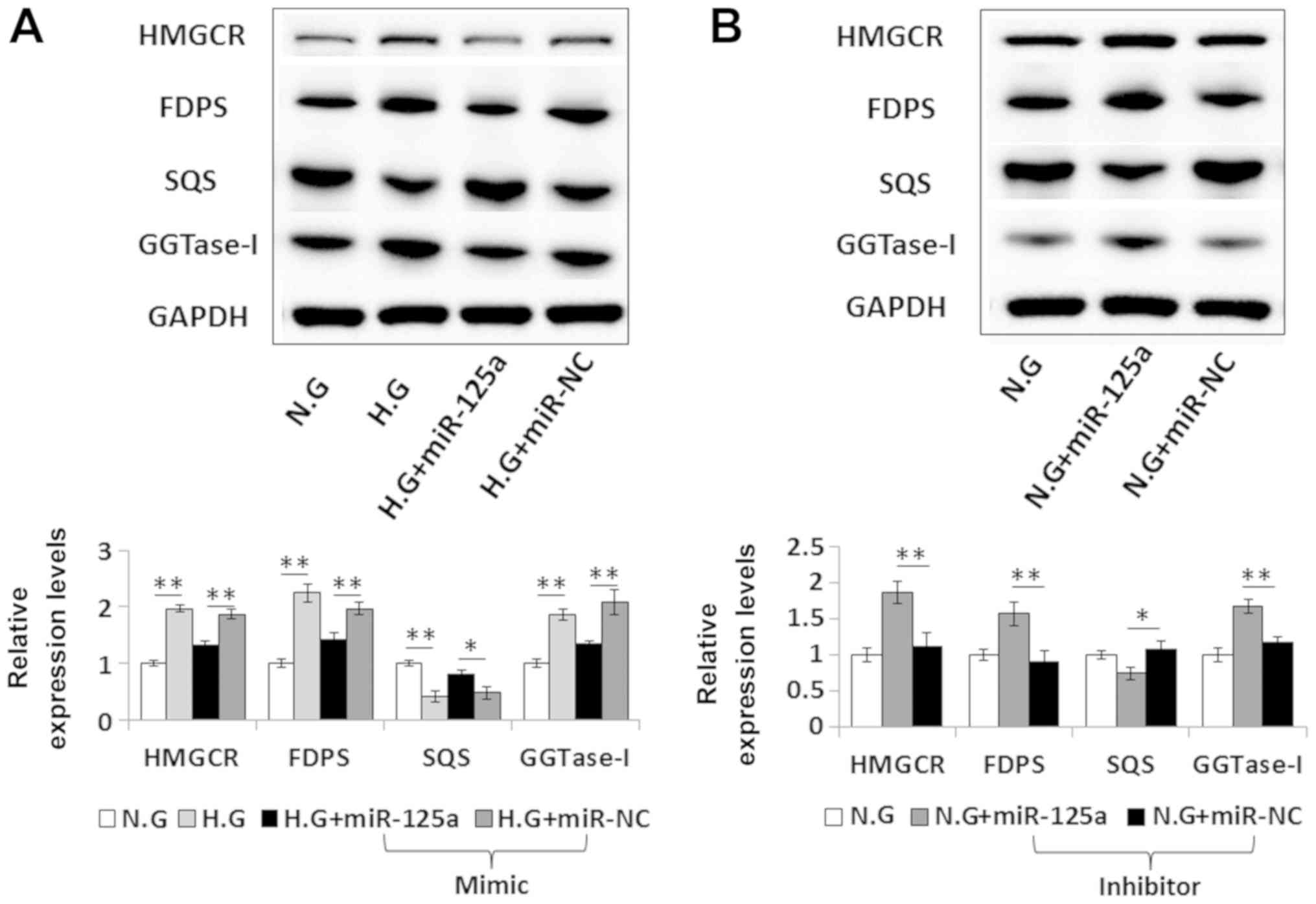 | Figure 5.miR-125a regulates the expression
levels of key enzymes in the mevalonate signaling pathway. (A)
Protein expression levels of HMGCR, FDPS, SQS and GGTase-I were
measured by western blotting. miR-125a mimic transfection reversed
HG-induced dysregulation of these proteins in VSMCs. (B) miR-125a
inhibitor transfection induced the activation of the mevalonate
signaling pathway in VSMCs cultured under normal conditions (5.6 nM
glucose). Data are presented as the mean ± SD. n=3. *P<0.05,
**P<0.01. ns, not significant; miR, microRNA; HMGCR,
3-hydroxy-3-methylglutaryl-coenzyme A reductase; FDPS, farnesyl
diphosphate synthase; SQS, squalene synthase; GGTase-I,
geranylgeranyltransferase type I; H.G, high glucose; N.G, normal
glucose; VSMC, vascular smooth muscle cell; NC, negative
control. |
miR-125a-mediated regulation of the
mevalonate signaling pathway contributes to HG-induced
atherosclerosis
To further investigate the effect of miR-125a on the
mevalonate signaling pathway in vivo, a rat STZ-induced
model of DM was established. It was demonstrated that blood glucose
levels gradually increased over 1 week in the animal model.
Moreover, the elevation of blood glucose was consistent with the
development of aortic atherosclerosis (Table I), which was determined by increased
MT and MCSA levels in the thoracic aorta in experimental rats
compared with age-matched controls (Fig. 6A). The expression levels of
miR-125a, HMGCR, FDPS, SQS and GGTase-I in the aortic media were
subsequently analyzed. It was found that miR-125a expression was
gradually downregulated, and the protein expression levels of
HMGCR, FDPS, SQS and GGTase-I were dysregulated in the aortic media
of the experimental rats compared with age-matched controls after 5
weeks (Fig. 6B and C). Thus,
miR-125a-mediated dysregulation of the mevalonate signaling pathway
may be involved in hyperglycemia-associated atherosclerosis.
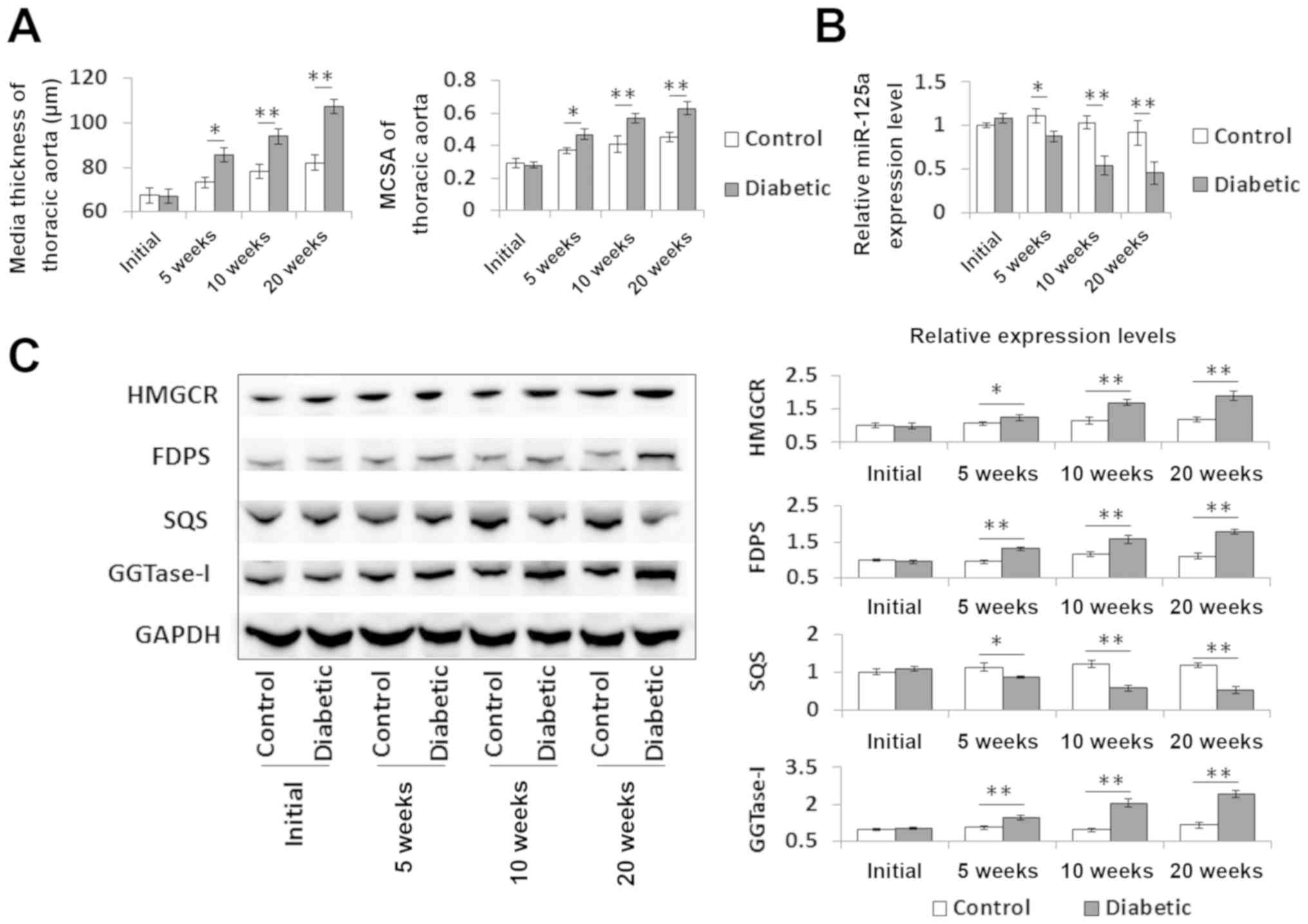 | Figure 6.High glucose-induced atherosclerosis
is associated with miR-125a-mediated dysregulation of the
mevalonate signaling pathway. (A) Media thickness and MCSA of the
thoracic aorta from Sprague Dawley rats at time 0, and 5, 10 and 20
weeks after streptozotocin injection. (B) miR-125a expression in
the aortic media. (C) Protein expression levels of HMGCR, FDPS, SQS
and GGTase-I in the aortic media were measured by western blotting.
Data are presented as the mean ± SD. n=6. *P<0.05, **P<0.01.
ns, not significant; MCSA, media cross-sectional area; HMGCR,
3-hydroxy-3-methylglutaryl-coenzyme A reductase; FDPS, farnesyl
diphosphate synthase; SQS, squalene synthase; GGTase-I,
geranylgeranyltransferase type I; miR, microRNA. |
 | Table I.Biochemical characteristics of rat
model. |
Table I.
Biochemical characteristics of rat
model.
| Weeks | FPG (mM) | TC (mM) | LDL-C (mM) | Insulin (IU/l) | HOMA-IR | HOMA-B |
|---|
| Control rats |
| Initial
(n=6) | 6.71±0.29 | 2.93±0.10 | 1.48±0.03 | 5.79±0.63 | 1.72±0.24 | 36.08±4.19 |
|
5 (n=6) | 6.32±0.26 | 2.79±0.11 | 1.32±0.07 | 7.02±0.84 | 1.91±0.27 | 39.02±3.01 |
| 10
(n=6) | 7.40±0.58 | 2.53±0.07 | 1.63±0.09 | 6.61±0.90 | 2.01±0.42 | 33.98±3.15 |
| 20
(n=6) | 7.84±0.84 | 2.83±0.14 | 1.61±0.12 | 6.99±0.74 | 2.36±0.12 | 41.65±4.43 |
| Diabetic rats |
| Initial
(n=6) | 7.27±0.31 | 2.91±0.16 | 1.47±0.16 | 5.87±0.76 | 1.90±0.40 | 31.14±3.36 |
|
5 (n=6) |
20.22±0.43b |
3.97±0.15a |
1.70±0.04a | 8.72±0.93 |
7.84±1.18b |
10.43±2.02b |
| 10
(n=6) |
24.67±2.91b |
4.37±0.09b |
2.03±0.07b |
4.65±0.70a |
5.10±1.10b |
4.39±0.54b |
| 20
(n=6) |
27.08±3.43b |
4.93±0.14b |
2.17±0.12b |
2.13±1.07b | 2.56±0.82 |
1.81±0.22b |
Discussion
VSMCs maintain the physiological structure of blood
vessels and play a key role in the development of atherosclerosis
(10). Moreover, VSMCs are able to
switch phenotypes by undergoing differentiation or
dedifferentiation, which is closely related to the formation of
atherosclerosis (10). Pathological
states, such as hyperglycemia in DM, may cause VSMCs to enter a
synthetic state, which is characterized by excessive proliferation,
migration and extracellular matrix secretion (4,9). In
atherosclerosis, the VSMC phenotype contributes to a series of
pathological processes relevant to CVD, including plaque formation
(10). Therefore, the inhibition of
hyperglycemia-induced vascular dysfunction may provide a novel
strategy for the treatment of DM-associated CVD (12). The present results suggested that
HG-induced VSMC proliferation and migration were associated with
decreased miR-125a expression and dysregulated expression of
mevalonate pathway-related enzymes. Moreover, miR-125a upregulation
abrogated HG-induced VSMC proliferation and migration by targeting
HMGCR, and modulating the mevalonate signaling pathway.
miRNAs play important roles in physiological and
pathological conditions (16,17).
Furthermore, previous studies have shown that several miRNAs are
involved in the regulation of cardiovascular physiological and
pathological processes, and are closely related to VSMCs (18–23).
miR-21 promotes neointima formation following vascular injury by
increasing VSMC proliferation, which occurs via the inhibition of
phosphatase and subsequent activation of the PI3K/Akt signaling
pathway (20). Moreover, miR-221,
miR-222, miR-26a and miR-138 promote VSMC proliferation or
migration by inhibiting cyclin-dependent kinase inhibitor 1B,
cyclin-dependent kinase inhibitor 1C, SMAD family member 1 and
sirtuin 1, respectively (21–23).
However, it has been revealed that miR-143/145 and miR-133 decrease
VSCM proliferation and promote the synthetic phenotype (24,25).
miR-24 and miR-145 also decrease VSMC proliferation and migration
by targeting HMGB1 and Rho-associated coiled-coil containing
protein kinase 1 (ROCK1). In addition, ROCK1 promotes the
proliferation and migration of VSMCs by acting on Kruppel like
factor 4 via miR-92a (26,27).
The present study used bioinformatics analysis and
RT-qPCR to demonstrate that miR-125a may modulate HG-induced
proliferation and migration of VSCMs. miR-125a is an endogenous
non-coding miRNA with regulatory functions that are conserved from
nematodes to humans (28).
Moreover, miR-125a serves important roles in several cellular
processes, including cell differentiation, hematopoiesis,
organogenesis, cell proliferation, lipid metabolism and apoptosis,
by targeting transcription factors, matrix-metalloproteases and
growth factors (28,29). The tissue expression level of
miR-125a is typically upregulated during differentiation, but is
often downregulated in disease states, including diabetic
retinopathy and breast cancer (30). Therefore, dysregulated miR-125a
expression may be involved in the progression of diseases and can
serve as a potential therapeutic target (31,32).
The present results suggested that miR-125a expression was
downregulated in VSMCs exposed to HG and in aortic media from rats
with STZ-induced DM. Furthermore, it was found that miR-125a mimic
transfection reversed HG-induced VSMC proliferation and migration.
Therefore, it was speculated that decreased miR-125a expression may
contribute to the VSMC phenotypic switch in atherosclerosis.
To further investigate the role of miR-125a in
HG-induced VSMC proliferation and migration, potential targets of
miR-125a were identified using miRNA target prediction software. It
was found that HMGCR was a target gene of miR-125a, and a
luciferase reporter assay was subsequently used to assess this
observation. Moreover, miR-125a-mediated regulation of HMGCR was
further demonstrated by western blot analysis. It was demonstrated
that the expression of HMGCR in VSMCs was decreased following
transfection with a miR-125a mimic, but increased following
transfection with a miR-125a inhibitor. Furthermore, the
downregulation of HMGCR expression abrogated HG-induced
proliferation and migration of VSCMs, similarly to the effects
observed following miR-125a mimic transfection. Collectively, the
present results suggested that HMGCR is a functional target gene of
miR-125a in VSMCs, and that miR-125a may decrease the progression
of atherosclerosis by targeting HMGCR.
HMGCR is an important enzyme in the mevalonate
signaling pathway, which is a metabolic pathway that synthesizes
isoprene pyrophosphate and dimethylallyl pyrophosphate from acetyl
coenzyme A (33). The end-product
of this pathway is an activated isoprene unit that serves as
precursor of steroids, terpenoids and other biological molecules
(33,34). Previous studies (12,35,36)
have revealed that the mevalonate signaling pathway serves an
important role in the cardiovascular system. It has been shown that
the expression levels of key enzymes in the mevalonate pathway,
including HMGCR, FDPS, SQS and GGTase-1, are significantly
dysregulated in aortas from spontaneously hypertensive rats or mice
with STZ-induced DM, and are associated with aortic structural
remodeling or atherosclerosis development, respectively (10,35).
The present results indicated that dysregulated expression levels
of key enzymes in the mevalonate signaling pathway may accelerate
atherosclerosis in DM. Moreover, the dysregulated expression levels
of HMGCR, FDPS, SQS and GGTase-I were associated with gradually
downregulated miR-125a expression in the aortic media from rats
with STZ-induced DM, or in cultured VSMCs exposed to HG. Thus,
miR-125a may be involved in the HG-induced dysregulation of the
mevalonate signaling pathway. However, it was identified that
HG-induced activation of the mevalonate signaling pathway in VSMCs
was suppressed following miR-125a mimic transfection.
In conclusion, it was found that downregulation of
miR-125a expression was associated with HG-induced VSMC
proliferation and migration. Furthermore, upregulation of miR-125a
abrogated these effects by directly targeting HMGCR and suppressing
the activation of the mevalonate signaling pathway. Therefore,
miR-125a-mediated regulation of this pathway may serve as a
potential therapeutic strategy for DM-related atherosclerosis.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was supported by National Natural Science
Funds (grant nos. 81500616 and 81701365).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DY and GL performed the research and drafted the
manuscript. AL, FD and GC participated in the in vitro
studies and performed the statistical analysis. WX established the
rat model and performed the in vivo studies. YL and SH
conceived the idea of the study, and participated in its design and
coordination. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The animal experiments were approved by the Animal
Experimental Ethics Committee, The First Affiliated Hospital of
Zhejiang University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Atkinson MA, Eisenbarth GS and Michels AW:
Type 1 diabetes. Lancet. 383:69–82. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chatterjee S, Khunti K and Davies MJ: Type
2 diabetes. Lancet. 389:2239–2251. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gilbert RE and Krum H: Heart failure in
diabetes: Effects of anti-hyperglycaemic drug therapy. Lancet.
385:2107–2117. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Katakami N: Mechanism of Development of
atherosclerosis and cardiovascular disease in diabetes mellitus. J
Atheroscler Thromb. 25:27–39. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Low Wang CC, Hess CN, Hiatt WR and
Goldfine AB: Clinical update: Cardiovascular disease in diabetes
mellitus: Atherosclerotic cardiovascular disease and heart failure
in type 2 diabetes mellitus-mechanisms, management, and clinical
considerations. Circulation. 133:2459–2502. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shi L, Ji Y, Jiang X, Zhou L, Xu Y, Li Y,
Jiang W, Meng P and Liu X: Liraglutide attenuates high
glucose-induced abnormal cell migration, proliferation, and
apoptosis of vascular smooth muscle cells by activating the GLP-1
receptor, and inhibiting ERK1/2 and PI3K/Akt signaling pathways.
Cardiovasc Diabetol. 14:182015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Carthew RW and Sontheimer EJ: Origins and
mechanisms of miRNAs and siRNAs. Cell. 136:642e552009. View Article : Google Scholar
|
|
8
|
He L and Hannon GJ: MicroRNAs: Small RNAs
with a big role in gene regulation. Nat Rev Genet. 5:522–531. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ding Y, Sun X and Shan PF: MicroRNAs and
cardiovascular disease in diabetes mellitus. Biomed Res Int.
2017:40803642017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rudijanto A: The role of vascular smooth
muscle cells on the pathogenesis of atherosclerosis. Acta Med
Indones. 39:86–93. 2007.PubMed/NCBI
|
|
11
|
Chait A and Bornfeldt KE: Diabetes and
atherosclerosis: Is there a role for hyperglycemia? J Lipid Res. 50
(Suppl):S335–S339. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen GP, Zhang XQ, Wu T, Li L, Han J and
Du CQ: Alteration of mevalonate pathway in proliferated vascular
smooth muscle from diabetic mice: Possible role in
high-glucose-induced atherogenic process. J Diabetes Res.
2015:3792872015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Masiello P, Broca C, Gross R, Roye M,
Manteghetti M, Hillaire-Buys D, Novelli M and Ribes G: Experimental
NIDDM: Development of a new model in adult rats administered
streptozotocin and nicotinamide. Diabetes. 47:224–229. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Matthews DR, Hosker JP, Rudenski AS,
Naylor BA, Treacher DF and Turner RC: Homeostasis model assessment:
Insulin resistance and beta-cell function from fasting plasma
glucose and insulin concentrations in man. Diabetologia.
28:412–419. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hwang HW and Mendell JT: MicroRNAs in cell
proliferation, cell death, and tumorigenesis. Br J Cancer.
96:776–780. 2007.PubMed/NCBI
|
|
17
|
Ye D, Zhang T, Lou G and Liu Y: Role of
miR-223 in the pathophysiology of liver diseases. Exp Mol Med.
50:1282018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Maegdefessel L, Rayner KJ and Leeper NJ:
MicroRNA regulation of vascular smooth muscle function and
phenotype: Early career committee contribution. Arterioscler Thromb
Vasc Biol. 35:2–6. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Santovito D, Egea V and Weber C: Small but
smart: MicroRNAs orchestrate atherosclerosis development and
progression. Biochim Biophys Acta 1861 (12 Pt B). 2075–2086.
2016.
|
|
20
|
Ji R, Cheng Y, Yue J, Yang J, Liu X, Chen
H, Dean DB and Zhang C: MicroRNA expression signature and
antisense-mediated depletion reveal an essential role of MicroRNA
in vascular neointimal lesion formation. Circ Res. 100:1579–1588.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu X, Cheng Y, Zhang S, Lin Y, Yang J and
Zhang C: A necessary role of miR-221 and miR-222 in vascular smooth
muscle cell proliferation and neointimal hyperplasia. Circ Res.
104:476–487. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Leeper NJ, Raiesdana A, Kojima Y, Chun HJ,
Azuma J, Maegdefessel L, Kundu RK, Quertermous T, Tsao PS and Spin
JM: MicroRNA-26a is a novel regulator of vascular smooth muscle
cell function. J Cell Physiol. 226:1035–1043. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xu J, Li L, Yun HF and Han YS: MiR-138
promotes smooth muscle cells proliferation and migration in db/db
mice through down-regulation of SIRT1. Biochem Biophys Res Commun.
463:1159–1164. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cordes KR, Sheehy NT, White MP, Berry EC,
Morton SU, Muth AN, Lee TH, Miano JM, Ivey KN and Srivastava D:
miR-145 and miR-143 regulate smooth muscle cell fate and
plasticity. Nature. 460:705–710. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Torella D, Iaconetti C, Catalucci D,
Ellison GM, Leone A, Waring CD, Bochicchio A, Vicinanza C, Aquila
I, Curcio A, et al: MicroRNA-133 controls vascular smooth muscle
cell phenotypic switch in vitro and vascular remodeling in vivo.
Circ Res. 109:880–893. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang J, Chen L, Ding J, Fan Z, Li S, Wu H,
Zhang J, Yang C, Wang H, Zeng P and Yang J: MicroRNA-24 inhibits
high glucose-induced vascular smooth muscle cell proliferation and
migration by targeting HMGB1. Gene. 586:268–273. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen M, Zhang Y, Li W and Yang J:
MicroRNA-145 alleviates high glucose-induced proliferation and
migration of vascular smooth muscle cells through targeting ROCK1.
Biomed Pharmacother. 99:81–86. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bi Q, Tang S, Xia L, Du R, Fan R, Gao L,
Jin J, Liang S, Chen Z, Xu G, et al: Ectopic expression of MiR-125a
inhibits the proliferation and metastasis of hepatocellular
carcinoma by targeting MMP11 and VEGF. PLoS One. 7:e401692012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sun YM, Lin KY and Chen YQ: Diverse
functions of miR-125 family in different cell contexts. J Hematol
Oncol. 6:62013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang Y, Zhang D, Lv J, Wang S and Zhang
Q: MiR-125a-5p suppresses bladder cancer progression through
targeting FUT4. Biomed Pharmacother. 108:1039–1047. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cai M, Chen Q, Shen J, Lv C and Cai L:
Epigenetic silenced miR-125a-5p could be self-activated through
targeting Suv39H1 in gastric cancer. J Cell Mol Med. 22:4721–4731.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yan L, Yu MC, Gao GL, Liang HW, Zhou XY,
Zhu ZT, Zhang CY, Wang YB and Chen X: MiR-125a-5p functions as a
tumour suppressor in breast cancer by downregulating BAP1. J Cell
Biochem. 119:8773–8783. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Goldstein JL and Brown MS: Regulation of
the mevalonate pathway. Nature. 343:425–430. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Buhaescu I and Izzedine H: Mevalonate
pathway: A review of clinical and therapeutical implications. Clin
Biochem. 40:575–584. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Han J, Jiang DM, Du CQ and Hu SJ:
Alteration of enzyme expressions in mevalonate pathway: Possible
role for cardiovascular remodeling in spontaneously hypertensive
rats. Circ J. 75:1409–1417. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen GP, Zhang XQ, Wu T, Han J and Ye D:
Inhibition of farnesyl pyrophosphate synthase attenuates high
glucose-induced vascular smooth muscle cells proliferation. Mol Med
Rep. 15:3153–3160. 2017. View Article : Google Scholar : PubMed/NCBI
|