Introduction
Renal cell carcinoma is one of the most common types
of kidney tumor originating from the renal tubular epithelium and
has the highest incidence rate of cancer types found in the urinary
system (1). According to cancer
statistics in the United States, in 2018 there were 65,340 new
cases of renal cell carcinoma, which accounted for 43.46% of the
total number of urinary cancers diagnosed; of these cases, 14,970
resulted in death, accounting for 45.13% of the total number of
urinary cancer deaths (2). Amongst
adult malignant tumors, the incidence of renal cell carcinoma is
~3% (1), and ~30% of patients with
renal cell carcinoma present with metastasis at the time of
diagnosis (3). Surgical resection
remains an effective treatment option for renal cell carcinoma, as
the cancer cells are usually resistant to chemical drug treatment
(4), which is the main
contributing factor to the short survival time of patients. It has
been discovered that certain factors are related to the tolerance
of tumors to chemotherapeutic agents; for example, the regulation
of drug uptake and elimination by renal cell carcinoma cells is
mediated through membrane translocation-related proteins, such as
P-glycoprotein (P-gp) and multidrug resistance-associated proteins
(5).
MicroRNAs (miRNAs/miRs) are a class of non-coding
RNAs that have no open reading frame in their sequences and
therefore do not encode proteins (6). The abnormal expression of miRNAs has
been closely associated with numerous types of tumour (7); they have been found to serve
important roles in the development and progression of tumors,
further to regulating cell migration, proliferation,
differentiation and apoptosis by controlling the functions of
oncogenes and tumor suppressor genes (7,8). Of
note, one study observed that multiple miRNAs are abnormally
expressed in renal cell carcinoma (9), whilst another study found that miRNAs
were highly stable in the serum, easy to detect and not easily
degraded (10). These findings
provided a theoretical and methodological basis for studying the
function of miRNAs as biomarkers of renal cell carcinoma. In fact,
one study suggested that miR-133b may be used as a tumor suppressor
gene to regulate cell growth in types of cancer (11,12).
For example, the expression levels of miR-133b were found to be
increased in lung cancer, which prevented lung cancer cells from
proliferating, whilst promoting cell apoptosis (11). Similarly, a previous study
demonstrated that miR-133b can inhibit the proliferation, migration
and invasion of esophageal cancer cells (12).
The ERKs, including ERK1 and ERK2, are involved in
the transmission of extracellular signals intracellularly (13). Upon activation by phosphorylation,
theERK protein translocates into the nucleus from the cytoplasm,
where it transmits signals into the nucleus to participate in
various biological reactions (13). The ERK protein is considered to be
the convergence point of multiple signaling pathways that are
involved in various biological functions, such as cell development,
colonization, apoptosis and malignant transformation, amongst
others (13). One previous study
reported that miR-133b shortened the latency of cervical cancer and
promoted the occurrence and metastasis through activating the ERK
and AKT signaling pathways in mice (14); however, to the best of our
knowledge, there are no studies on the relationship between
miR-133b and ERK in renal cell carcinoma. In addition, the ERK
signaling pathway is closely associated with the chemosensitivity
of cells, for instance, the inactivation of the ERK signaling
pathway was observed to increase the chemosensitivity of
osteosarcoma U2OS cells to cisplatin (15). Thus, in the present study, the
expression levels of miR-133b in renal cell carcinoma were
determined and its effect on cell proliferation, invasion and
chemosensitivity, further its potential mechanisms were
investigated.
Materials and methods
Patient studies
The present study was approved by the Research
Ethics Committee of School of Medicine, Shandong University and was
conducted under the Declaration of Helsinki principles. Informed
written consent was obtained from all participating patients. A
total of 60 patients with renal cell carcinoma (43 male patients;
17 female patients; age, 27–73 years; mean age, 55.14±10.45 years)
at the Shandong Provincial Hospital Affiliated to Shandong
University were recruited from June 2017 to July 2018. All patients
had comprehensive clinical pathological data and did not receive
chemotherapy, radiation therapy or immunotherapy before surgery.
The patients' renal cell carcinoma and adjacent healthy tissue
specimens were collected and the specimens were subsequently stored
in liquid nitrogen or 4% paraformaldehyde solution within 10 min of
being removed, then stored at −80°C. Samples were confirmed to be
either renal cell carcinoma tissue or healthy tissue by
histopathological diagnosis.
Cell collection, culture and
treatment
In total, four human renal cell carcinoma cell
lines, ACHN, Caki-1, A-498 and 786-O, and the 293 cell line were
obtained from the American Type Culture Collection. In further
experiments, 786-O cells were used. All cells were cultured in
RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% FBS (Sigma-Aldrich; Merck KGaA), 100 U/ml
penicillin and 100 mg/ml streptomycin, and maintained in a 5%
CO2 incubator (Thermo Fisher Scientific, Inc.) at
37°C.
The 786-O cells were collected for experiments upon
reaching the logarithmic growth phase and were divided into 23
groups: i) The control group, which contained untreated cells; ii)
the negative control (NC) group, which contained cells transfected
with the miR-133b mimic NC; iii) the miR-133b mimic (miR-133b)
group, which contained cells transfected with the miR-133b mimic;
iv-viii) the (DDP; MedChemExpress) groups, which received 0, 2.5,
5, 10 or 20 µg/ml DDP; ix-xiii) the docetaxel (DXT; MedChemExpress)
groups, which received 0, 5, 10, 20 or 40 µg/ml DXT; xiv-xviii) the
doxorubicin (ADR; MedChemExpress) groups, which received 0, 0.25,
0.5, 1 or 2 µg/ml DXT; xix) the miR-133b + DDP group, which
contained cells transfected with the miR-133b mimic and treated
with 5 µg/ml DDP; xx) the miR-133b + DXT group, which contained
cells transfected with miR-155p mimic and treated with 20 µg/ml
DXT; xxi) the miR-133b + ADR group, which contained cells
transfected with miR-133b mimic and treated with 0.5 µg/ml ADR;
xxii) the U0126 group, which contained cells treated with the ERK
pathway signal transduction inhibitor, U0216 (20 µmol/; Selleck
Chemicals); and xxiii) the miR-133b + LM22B-10 group, which
contained cells transfected with the miR-133b mimic and treated
with the ERK activator LM22B-10 (5 mmol/l; cat. no. HY-104047;
MedChemExpress).
Cell transfection
The 786-O cells were digested and passaged with
0.25% trypsin (Invitrogen; Thermo Fisher Scientific, Inc.), and
2×105 cells in the exponential growth phase were plated
into 6-well plates. Following 24 h of incubation at 37°C with 5%
CO2, cell growth was observed using an inverted
fluorescent microscope (Olympus Corporation) under ×200
magnification. Upon reaching 30–50% confluence, the miR-133b mimic
(5′-UUUGGUCCCCUUCAACCAGCUA-3′) and the miR-133b mimic NC
(5′-UUUGGUAAAAUUCAACCAGCUA-3′; Guangzhou RiboBio Co., Ltd.) were
transfected into the cells at a concentration of 20 nmol/l to
construct transiently overexpressing miR-133b cell lines using
Lipofectamine® 2000 reagent (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol. After
24 h, the proof of successful transfection was analyzed using
reverse transcription-quantitative PCR (RT-qPCR).
RT-qPCR
Total RNA was extracted from cells using the
MagMAX™mirVana™ Total RNA Isolation kit (cat. no. A27828;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol. Total RNA was reverse transcribed into cDNA at 42°C for 1
h then 90°C for 5 min with a high-capacity RNA to cDNA™ kit (cat.
no. 4387406; Thermo Fisher Scientific, Inc.). qPCR was subsequently
performed using SYBR-Green qPCR Master Mix (MedChemExpress) and 2
µl cDNA as a template. The following primer pairs (Guangzhou
RiboBio Co., Ltd.) were used for the qPCR: miR-133b forward,
5′-AAAGGACCCCAACAACCAGCAA-3′ and reverse,
5′-TTGCTGGTTGTTGGGGTCCTTT-3′; and U6 forward,
5′-CTCGCTTCGGCAGCACATATACT-3′ and reverse,
5′-ACGCTTCACGAATTTGCGTGTC-3′. The following thermocycling
conditions were used for the PCR: Initial denaturation at 95°C for
2 min; and 40 cycles of 95°C for 15 sec and 60°C for 1 min.
Expression levels of miR-133b were quantified using the
2−∆∆Cq method (16) and
normalized to the internal reference gene U6 gene.
MTT assay for cell proliferation
The 786-O cells were digested using trypsinase and
1×104 cells/ml were plated into 96-well culture plates
and incubated with 5% CO2 at 37°C. At 24, 48 and 72 h,
20 µl MTT reagent (5 mg/ml) was added and then cells were further
cultured for 4 h in a 37°C incubator. The medium was discarded and
200 µl DMSO (Sigma-Aldrich; Merck KGaA) was added. The absorbance
[optical density (OD) value] of each well was measured at a
wavelength of 490 nm using a spectrophotometer (Bio-Rad
Laboratories, Inc.).
Wound healing assay
The 786-O cells in the exponential growth phase were
digested with 0.25% trypsin into a single cell suspension and
adjusted to a concentration of 3×105 cells/ml. A total
of 1 ml cell suspension was added to each well of the 6-well plate.
Upon cells reaching confluence, a 10-µl pipette tip was used to
create a single scratch on the cell monolayer in a sterile
environment. Cells were then washed with PBS to remove the cell
debris. Subsequently, RPMI-1640 medium was added and cells were
incubated at 37°C and 5% CO2. Cells were visualized
using an inverted fluorescent microscope (Olympus Corporation) and
the wells were imaged at 0 and 24 h. The degree of wound healing
was analyzed by ImageJ software (version 6.0, National Institutes
of Health).
Matrigel assay
A total of 2×105 786-O cells/ml were
collected with 0.25% trypsin-EDTA solution and plated in the upper
chambers of a Transwell plate in 100 µl RPMI-1640 DMEM (Gibco;
Thermo Fisher Scientific, Inc.). Transwell membranes were precoated
with 50 µl Matrigel for 30 min at 37°C. A total of 600 µl complete
RPMI-1640 medium supplemented with 10% FBS was plated in the lower
chambers. Following incubation for 24 h at 37°C, the invasive cells
were fixed in 4% paraformaldehyde for 30 min at 37°C and then
stained with 1% crystal violet (cat. no. G1062; Beijing Solarbio
Science & Technology Co., Ltd.) for 20 min at 37°C. Stained
cells were counted in nine randomly selected fields using an
optical fluorescent microscope under ×400 magnification (Olympus
Corporation). The number of invasive cells was subsequently
calculated by ImageJ (version 6.0; National Institutes of
Health).
Flow cytometric analysis of
apoptosis
The 786-Ocells in the logarithmic phase were
trypsinized and were collected by centrifugation at 999 × g for 5
min at 4°C. Cells were subsequently thoroughly washed twice with
pre-cooled sterile PBS at 4°C. A total of 1×106 cells/ml
were resuspended in 250 µl 1X binding buffer, of which 195 µl was
subsequently added to 5 µl Annexin V-FITC and mixed for 3 min.
Then, 10 µl propidium iodide (PI) solution (20 µg/ml) was added and
mixed. Following incubation for 10 min at room temperature in the
dark, 1X binding buffer (400 µl) was added and gently mixed.
Apoptotic cells were subsequently analyzed using a Gallios flow
cytometer (Beckman Coulter, Inc.) and BD CellQuest™ software
(version 5.1; BD Biosciences). The Q4 data reflected the apoptotic
rate in this study.
Detection of cellular activity using
the MTS assay
The 786-O cells in the logarithmic growth phase were
digested with 0.25% trypsin and seeded into 96-well plates at a
cell density of 3×105 cells/ml. Following treatment for
48 h at 37°C, 20 µl MTS reagent (Promega Corporation) was added to
each well and incubated at 37°C for 3 h. A microplate reader was
used to record the OD value at 490 nm. The cells that received
treatments were set as the experimental groups and 0 µg/ml drug
with same volume saline treatmentwas used as the control group.
Distilled water was used as the blank group. The cell survival rate
(%) was calculated using the following formula:
(OD490experimental group-OD490blank
group)/(OD490control group-OD490blank
group) ×100. The experiment was repeated in triplicate.
Detection of chemosensitivity using
the MTT assay
The 786-O cells were digested by trypsinase, and a
total of 1×104/ml cells were subsequently plated into
96-well plates and incubated as described above. Different doses of
DDP, DXT and ADR were administrated to evaluate the IC50 of each
drug. Following 48 h of culture, 20 µl 5 mg/ml MTT was added to
each well and incubated at 37°C for 4 h. The IC50 was analyzed by
SPSS 19.0 (SPSS, Inc.). After adherence, the miR-133b groups were
treated with 5 µg/ml DDP, 20 µg/ml DXT or 0.5 µg/ml ADR,
respectively. Following 24, 48 and 72 h of culture, 20 µl 5 mg/ml
MTT was added to each well of all groups and incubated at 37°C for
4 h. The supernatant was discarded and 200 µl DMSO (Sigma-Aldrich;
Merck KGaA) was added. The OD value was measured at 490 nm using a
spectrophotometer (Bio-Rad Laboratories, Inc.). The proliferative
ability of the cells in each group following treatment with the
different drugs was evaluated to determine the effect of the
overexpression of miR-133b on drug chemosensitivity.
Western blotting
The expression levels of proliferating cell nuclear
antigen (PCNA), matrix metalloproteinase (MMP)-2, MMP-9, Bcl-2,
Bax, ATP-binding cassette subfamily G2 (ABCG2), P-gp,
phosphorylated (p)-ERK1/2 and ERK1/2 were analyzed using western
blotting. Total protein was extracted from 786-O cells with a total
protein extraction kit (cat. no. BC3640-50T; Beijing Solarbio
Science & Technology Co., Ltd.), according to the
manufacturer's protocol. Total protein was quantified using a
bicinchoninic acid assay kit (cat. no. 23225; Pierce; Thermo Fisher
Scientific, Inc.) and 40 µg protein/lane was separated via 10%
SDS-PAGE (Bio-Rad Laboratories, Inc.). The separated proteins were
subsequently transferred onto a PVDF membrane (EMD Millipore) and
blocked with 5% skim milk at 25°C for 1 h. The membranes were
incubated overnight at 4°C with the following primary antibodies
diluted in 5% BSA (Sigma-Aldrich; Merck KGaA): Anti-MMP-2 (1:1,000;
cat. no. ab37150; Abcam); anti-MMP-9 (1:1,000; cat. no. ab38898;
Abcam); anti-p-ERK1/2 (1:1,000; cat. no. ab176640; Abcam);
anti-ERK1/2 (1:1,000; cat. no. ab17942; Abcam); anti-PCNA (1:1,000;
cat. no. ab152112; Abcam); anti-Bax (1:1,000; cat. no. ab53154;
Abcam); anti-Bcl-2 (1:1,000; cat. no. ab59348; Abcam); anti-ABCG2
(1:1,000; cat. no. ab63907; Abcam); anti-P-gp (1:1,000; cat. no.
ab129450; Abcam); and anti-β-actin (1:1,000; cat. no. ab8227;
Abcam). Following the primary antibody incubation, the membranes
were washed thrice with TBS-0.01% Tween-20 (TBST) for 10 min each
and then incubated with a horseradish peroxidase-conjugated goat
anti-rabbit IgG secondary antibody (1:2,000; cat. no. ab6721;
Abcam) for 1 h at room temperature. The membranes were washed
thrice with TBST. Protein bands were visualized using an ECL
chemiluminescence reagent (Hanbio Biotechnology Co., Ltd.). Protein
expression was quantified using ImageJ software (version 6;
National Institutes of Health) and normalized to β-actin.
Statistical analysis
Statistical analysis was performed using SPSS
version 19.0 software (IBM Corp.). The experiments were repeated
three times and data are expressed as the mean ± SD. Statistical
differences between two groups were determined using a Student's
t-test, whereas the statistical differences between multiple groups
were analyzed using a one-way ANOVA followed by Tukey's test for
multiple comparisons. P<0.05 was considered to indicate a
statistically significant difference.
Results
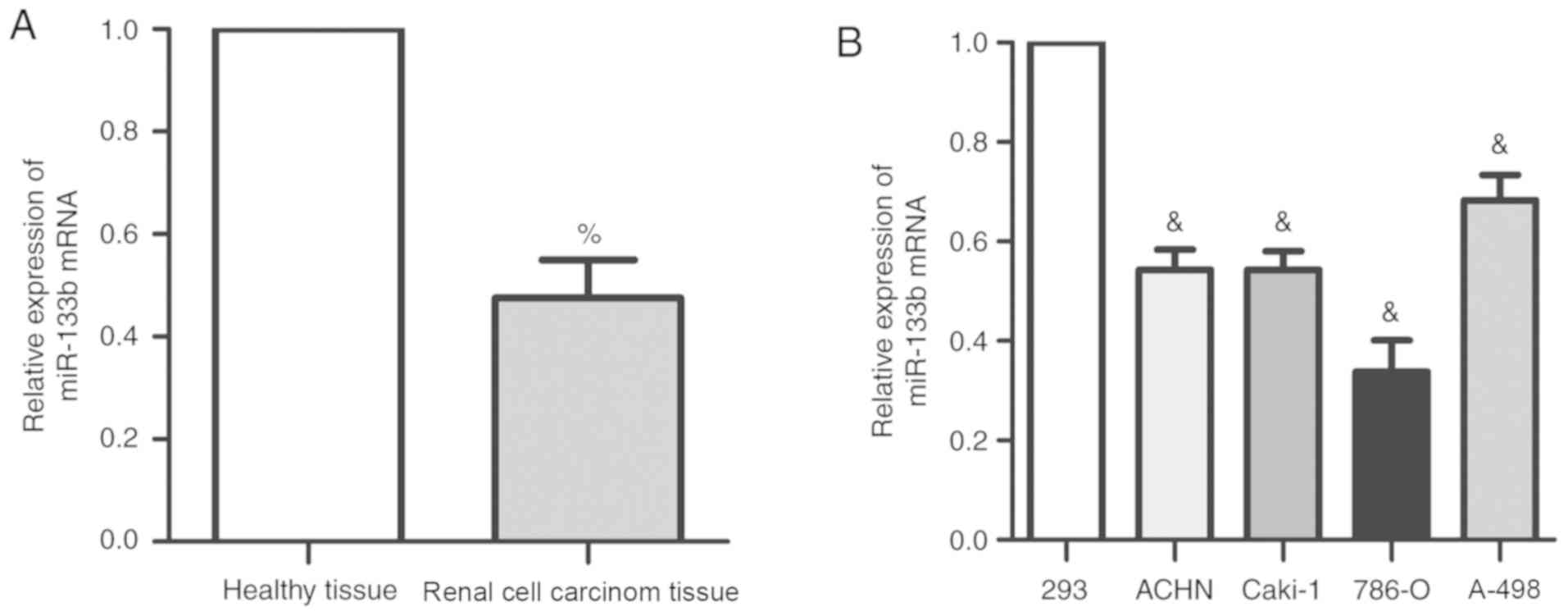
miR-133b expression is decreased in
renal cell carcinoma tissues and cells
The expression levels of miR-133b were detected
using RT-qPCR in 60 cases of renal cell carcinoma and adjacent
healthy tissues. The expression levels of endogenous miR-133b in
renal cell carcinoma were significantly decreased compared with the
adjacent healthy tissues (Fig.
1A). Similarly, miR-133b expression was significantly decreased
in all four renal cell carcinoma cell lines compared with 293
cells, with the most significant difference being observed in 786-O
cells (P<0.05). Therefore, 786-O cells were used for subsequent
experiments. These findings suggested that miR-133b expression may
be decreased in both renal cell carcinoma tissues and renal
carcinoma cell lines.
Overexpression of miR-133b inhibits
the proliferation and invasion of renal cell carcinoma cells
The efficiency of miR-133b transfection in 786-O
cells was quantified using RT-qPCR (Fig. 2A); there was no significant
difference in the relative expression levels of miR-133b between
the control group and the NC group (P>0.05), whereas the
expression levels of miR-133b mRNA were significantly increased in
the miR-133b group when compared to NC group (P<0.05).
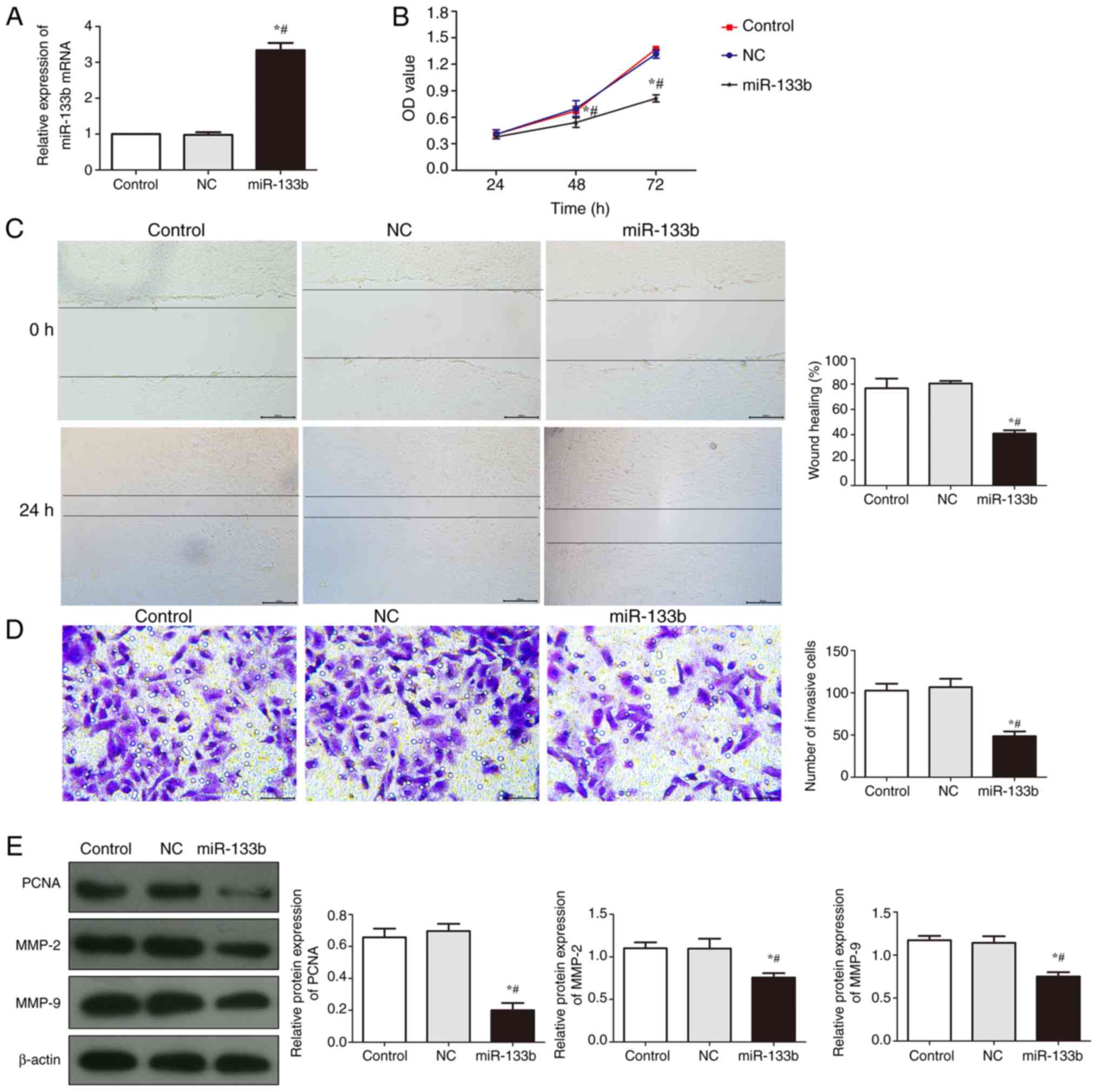 | Figure 2.Overexpression of miR-133b inhibits
proliferation and invasion in 786-O cells. (A) Transfection
efficiency of miR-133 was determined using reverse
transcription-quantitative PCR. (B) Cell proliferation was detected
using an MTT assay following miR-133b overexpression. (C) Wound
healing assay was used to investigate cell migration following
miR-133b overexpression, scale bar, 200 µm. (D) Matrigel assay was
used to investigate the number of invasive cells following miR-133b
overexpression, scale bar, 50 µm. (E) Western blotting was used to
analyze the expression levels of PCNA, MMP-2 and MMP-9 following
miR-133b overexpression. *P<0.05 vs. control;
#P<0.05 vs. NC. miR, microRNA; NC, negative control;
PCNA, proliferating cell nuclear antigen; MMP, matrix
metalloproteinase; OD, optical density. |
Following 24, 48 and 72 h of cell culture, an MTT
assay was used to analyze the proliferation of cells in each group
(Fig. 2B). Compared with the
control group, there was no significant difference observed in the
proliferative rate in the NC group (P>0.05); however, there was
significant difference in the proliferative rate of the miR-133b
group (P<0.05), in which the proliferative rate of the cells in
the miR-133b group was significantly reduced compared with cells in
the NC group. These findings suggested that the overexpression of
miR-133b may reduce the proliferative ability of renal carcinoma
cells.
A wound healing and Matrigel assay were used to
determine the migratory and invasive ability, respectively, of
786-O cells (Fig. 2C and D). The
wound healing rate and the number of invasive cells in the miR-133b
group were significantly reduced compared with the control and NC
groups (P<0.05), which indicated that the overexpression of
miR-133b may inhibit the migratory and invasive ability of renal
carcinoma cells.
The expression levels of PCNA, MMP-2 and MMP-9 were
analyzed using western blotting (Fig.
2E). The expression levels of PCNA, MMP-2 and MMP-9 in the
miR-133b group were significantly reduced compared with the control
and NC groups (P<0.05). These results suggested that the
overexpression of miR-133b may inhibit the expression of
proliferation and invasion-related proteins.
Overexpression of miR-133b induces
cells apoptosis and enhances drug sensitivity
The apoptotic rate of renal carcinoma cells was
detected using flow cytometry (Fig.
3A). Compared with the control and NC groups, the apoptotic
rate of the miR-133b group was significantly increased (P<0.05),
suggesting that the overexpression of miR-133b may promote the
apoptosis of renal cell carcinoma cells.
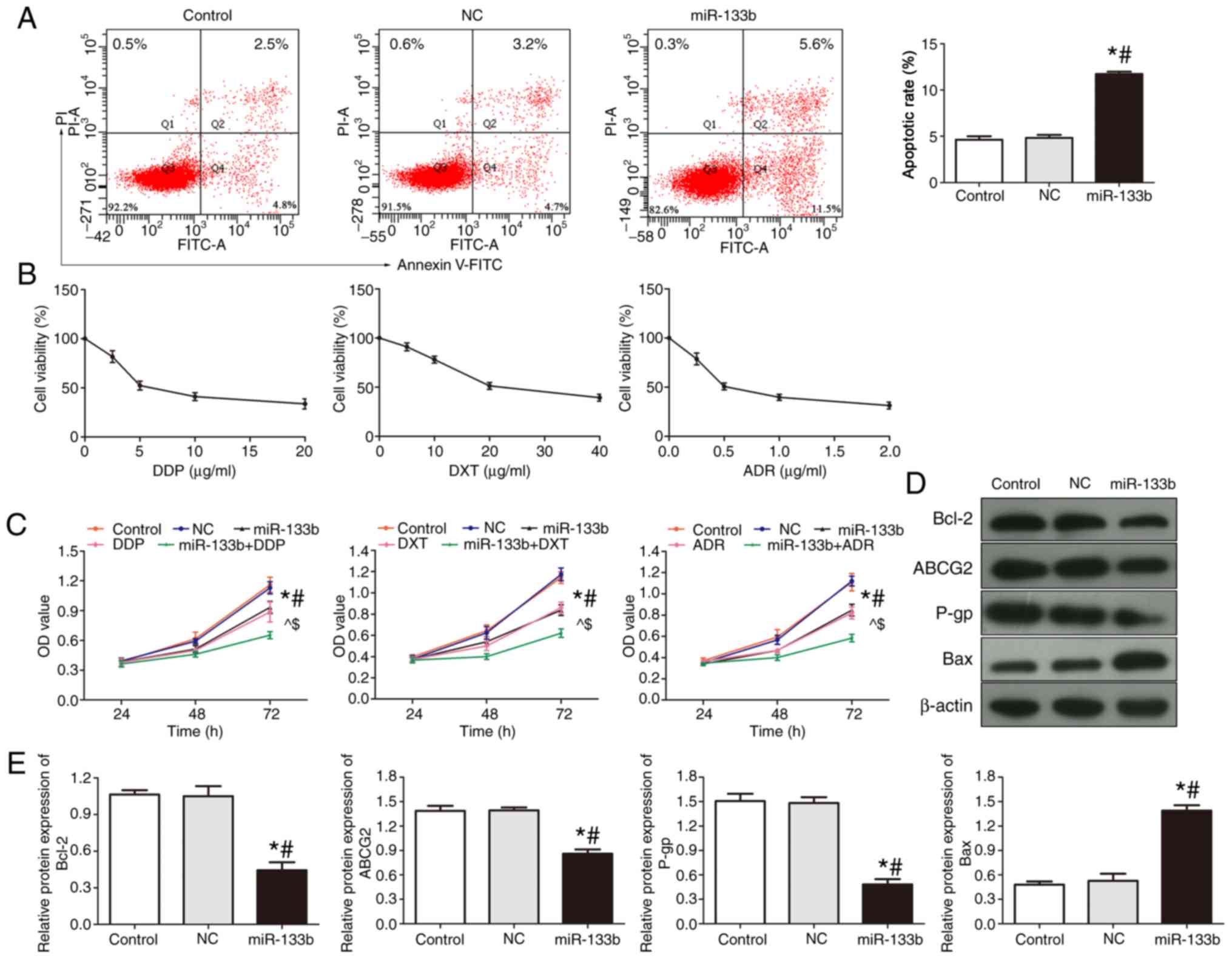 | Figure 3.Overexpression of miR-133b induces
apoptosis of 786-O cells and enhances drug sensitivity. (A) Flow
cytometric analysis of apoptosis was performed following miR-133b
overexpression. (B) MTS assay was used to investigate cell
viability following the treatment of cells with increasing
concentrations of DDP, DXT and ADR. (C) Proliferation of 786-O
cells was investigated following the treatment of DDP, DXT or ADR
and/or miR-133b overexpression. (D) Western blotting was used to
investigate the protein expression levels of Bcl-2, Bax, ABCG2 and
P-gp in cells following miR-133b overexpression. (E)
Semi-quantitative analysis of protein expression levels. *P<0.05
vs. control; #P<0.05 vs. NC; ^P<0.05
vs. miR-133b; $P<0.05 vs. DDP/DXT/ADR. miR, microRNA;
DDP, cisplatin; DXT, docetaxel; ADR, doxorubicin; ABCG2,
ATP-binding cassette subfamily G2; P-gp, P-glycoprotein; NC,
negative control; PI, propidium iodide; OD, optical density. |
Cellular viability was subsequently analyzed using a
MTT assay; following treatment with a range of doses of DDP, DXT
and ADR for 48 h, the cell activity was observed to decrease in a
dose-dependent manner with all three drugs (Fig. 3B). Drug concentration values close
to each drug's half-maximal inhibitory concentration value (DDP, 5
µg/ml; DXT, 20 µg/ml; ADR, 0.5 µg/ml) were used in the subsequent
experiments.
The effects of overexpressing miR-133b on the drug
sensitivity of 786-O cells to DDP, DXT and ADR were subsequently
investigated using an MTT assay (Fig.
3C). The cell proliferative ability of the miR-133b, DDP, DXT,
ADR and the miR-133b + DDP/DXT/ADR groups was significantly
decreased compared with the control and NC groups, and this effect
was found to be time-dependent (P<0.05; Fig. 3C). The proliferative ability of the
cells in the miR-133b + DDP/DXT/ADR group was inhibited to a
greater level compared with the miR-133b, DDP/DXT/ADR groups,
respectively. These results suggested that the overexpression of
miR-133b may increase the sensitivity of renal carcinoma cells to
these three drugs.
The expression levels of Bcl-2, Bax, ABCG2 and P-gp
in each group were detected using western blotting (Fig. 3D and E). Compared with the control
and NC groups, the protein expression levels of Bax in the miR-133b
group were significantly increased, whilst the protein expression
levels of ABCG2, P-gp and Bcl-2 were significantly decreased
(Fig. 3D and E). These findings
indicated that the overexpression of miR-133b may decrease the
expression levels of drug resistance-related proteins ABCG2 and
P-gp, and the anti-apoptotic protein Bcl-2, whilst increasing the
levels of Bax.
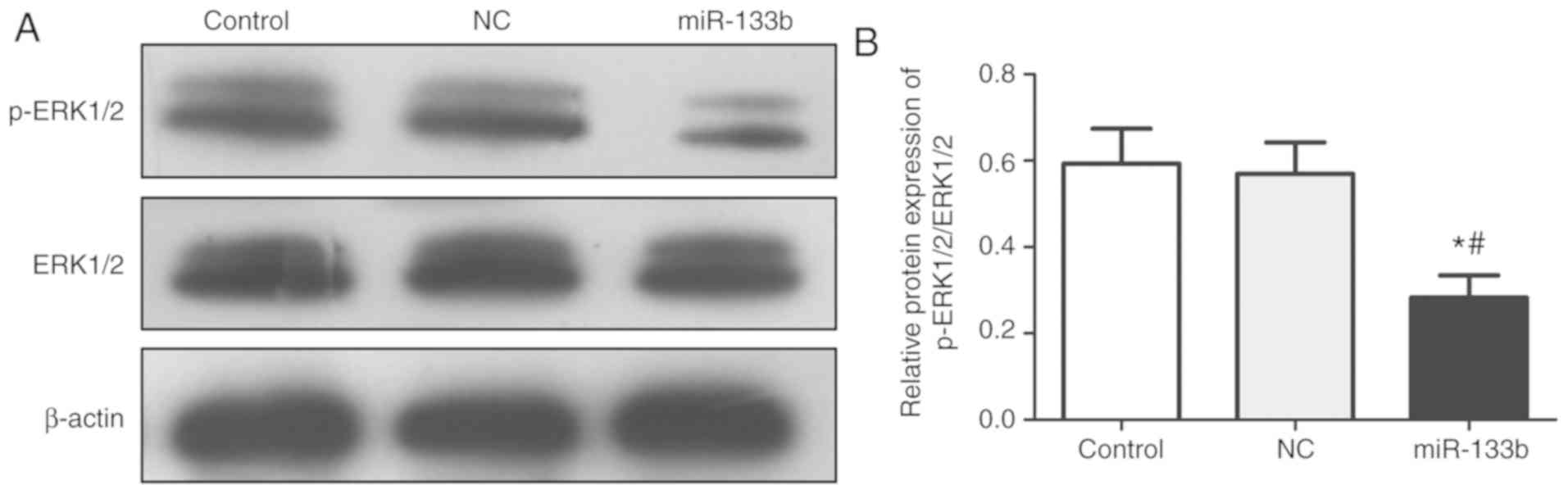
Overexpression of miR-133b inhibits
the activity of the ERK signaling pathway
The expression levels of p-ERK1/2 and ERK1/2 in each
group were analyzed (Fig. 4).
Compared with the control and NC groups, the protein expression
levels of p-ERK1/2 in the miR-133b group were significantly
decreased, whilst the protein expression levels of total ERK1/2
were not significantly different. Overall, the data suggested that
the overexpression of miR-133b may inhibit ERK1/2 phosphorylation
levels and reduce the activity of the ERK signaling pathway.
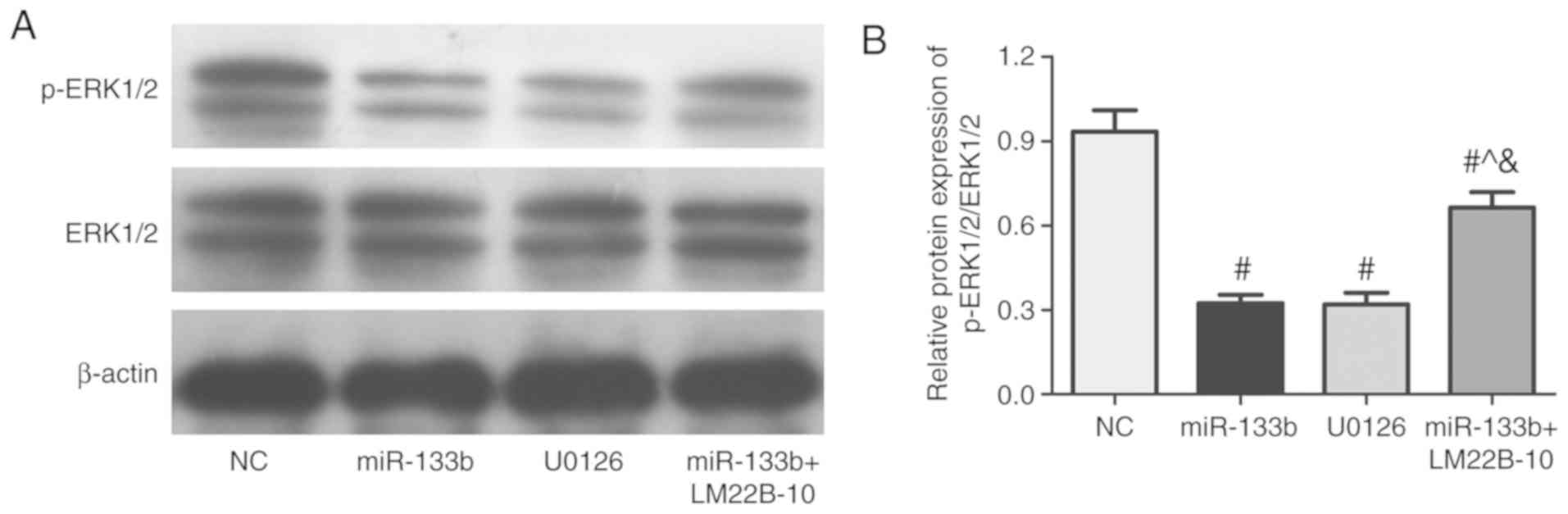
Effects of the miR-133b-mediated ERK
signaling pathway on the occurrence and development of renal cell
carcinoma
To further investigate whether miR-133b has a role
in the occurrence and development of renal cell carcinoma through
the ERK signaling pathway, the ERK pathway signal transduction
inhibitor, U0126, was used, in addition to the signaling pathway
inhibitor, LM22B-10, which was used in cells also overexpressing
miR-133b. Compared with the NC group, the proliferative, migratory
and invasive abilities of cells in the miR-133b and U0126 group
were significantly decreased (P<0.05, Fig. 5A-C). Compared with miR-133b group,
the proliferative, migratory and invasive abilities of cells were
significantly increased in miR-133b + LM22B-10 group (P<0.05;
Fig. 5A-C). The protein expression
levels of PCNA, MMP-2 and MMP-9 in the miR-133b and U0126 group
were significantly decreased contrasted to control group, were
decreased in miR-133b + LM22B-10 group compared with the miR-133b
group (P<0.05; Fig. 5D). The
apoptotic rate and the sensitivity of 786-O cells to DDP, DXT and
ADR were significantly increased in the miR-133b and U0126 group
compared with miR-133b + LM22B-10 group (P<0.05; Fig. 6A and B). Compared with miR-133b
group and U0126 group, the expression levels of Bcl-2, ABCG2 and
P-gp protein were significantly increased (Fig. 6C and D). Furthermore, the
phosphorylation of ERK1/2 in different groups was observed
(Fig. 7). Compared with NC group,
the expression of p-ERK1/2/ERK1/2 was significantly decreased in
miR-133b and U0126 group (P<0.05). But compared with the
miR-133b group or U0126 group, the expression of p-ERK1/2/ERK1/2
was significantly increased in miR-133b + LM22B-10 group
(P<0.05). These results suggested that the overexpression of
miR-133b combined with LM22B-10 significantly reversed or weakened
the anticancer effects of miR-133b overexpression.
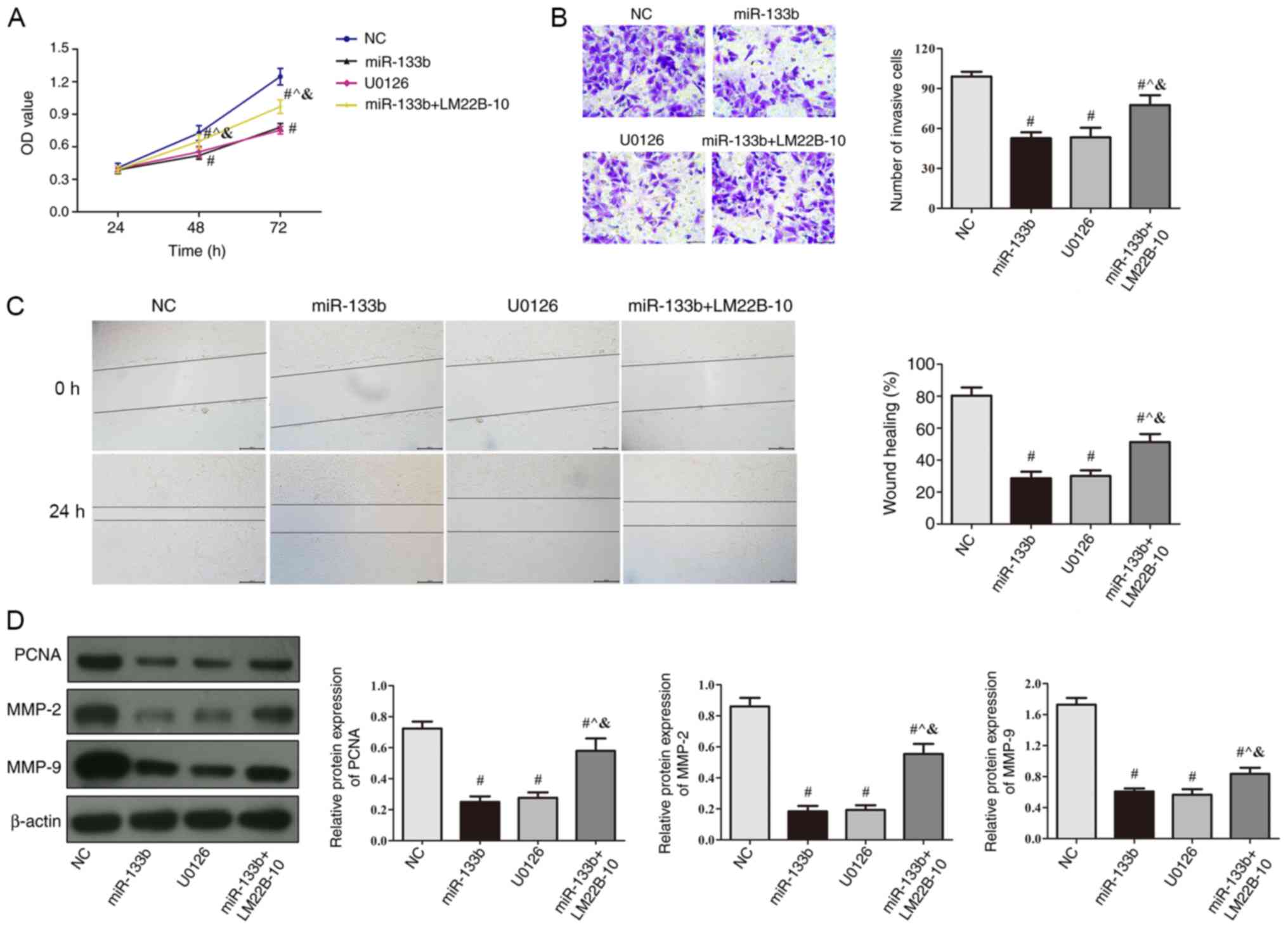 | Figure 5.Overexpression of miR-133b inhibits
786-O cell proliferation and invasion by inhibiting the ERK
signaling pathway. (A) Cell proliferation was determined in cells
treated with U0126 (ERK pathway signal transduction inhibitor) or
transfected with miR-133b mimic with or without LM22B-10 (ERK
activator) treatment using an MTT assay. (B) Matrigel assays were
used to determine the number of invasive cells in cells treated
with U0126 or transfected with miR-133b mimic with or without
LM22B-10 treatment, scale bar, 50 µm. (C) Wound healing assays in
cells treated with U0126 or transfected with miR-133b mimic with or
without LM22B-10 treatment were used to detect cellular migration,
scale bar, 200 µm. (D) Western blotting was used to detect PCNA,
MMP-2 and MMP-9 protein expression levels in cells treated with
U0126 or transfected with miR-133b mimic with or without LM22B-10
treatment. #P<0.05 vs. NC; ^P<0.05 vs.
miR-133b; &P<0.05 vs. U0126. miR, microRNA; NC,
negative control; OD, optical density; PCNA, proliferating cell
nuclear antigen; MMP, matrix metalloproteinase. |
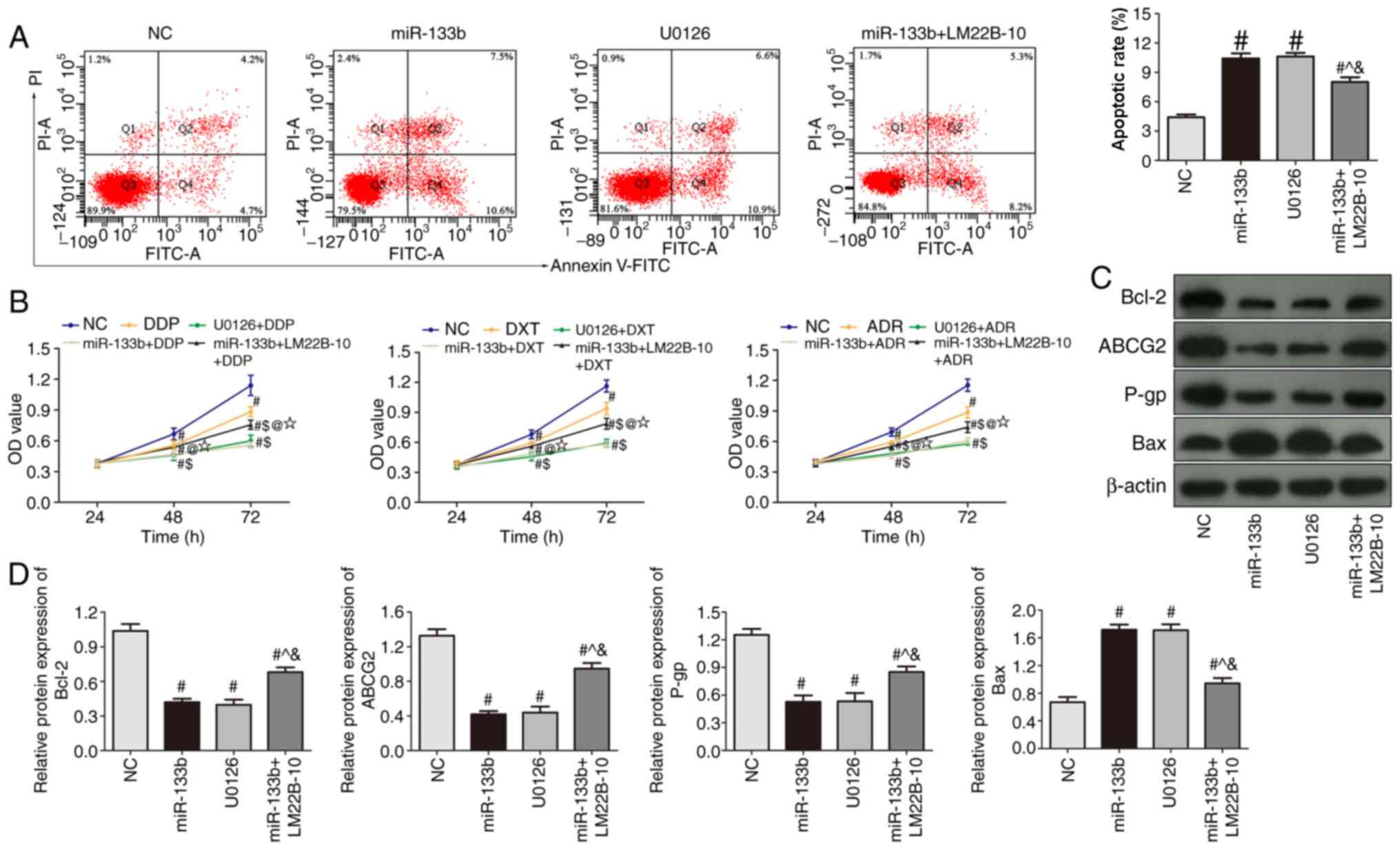 | Figure 6.Overexpression of miR-133b induces the
apoptosis of 786-O cells and enhances drug sensitivity through
inhibiting the ERK signaling pathway. (A) Flow cytometric analysis
of apoptosis in cells treated with U0126 or transfected with
miR-133b mimic with or without LM22B-10 treatment. (B) Changes in
the proliferation rate of 786-O cells treated with DDP, DXT or ADR
alone, or in combination with U0126 or miR-133b overexpression with
or without LM22B-10 treatment. (C) Western blotting was used to
detect the protein expression levels of Bcl-2, Bax, ABCG2 and P-gp
in cells treated with U0126 or transfected with miR-133b mimic with
or without LM22B-10 treatment. (D) Semi-quantitative analysis of
protein expression levels. #P<0.05 vs. NC;
^P<0.05 vs. miR-133b; &P<0.05 vs.
U0126; $P<0.05 vs. DDP/DXT/ADR; @P<0.05
vs. miR-133b group + DDP/DXT/ADR; ⋆P<0.05 vs. U0126 +
DDP/DXT/ADR. miR, microRNA; DDP, cisplatin; DXT, docetaxel; ADR,
doxorubicin; UO126, ERK pathway signal transduction inhibitor;
LM22B-10, ERK activator; NC, negative control; PI, propidium
iodide; ABCG2, ATP-binding cassette subfamily G2; P-gp,
p-glycoprotein; OD, optical density. |
Discussion
Due to the current lack of distinct symptoms and
signs in early stage renal cell carcinoma, the majority of clinical
cases present with middle-to-late stage cancer upon diagnosis;
unfortunately, the therapeutic treatment of advanced renal cell
carcinoma is poor and the survival rate is low (17). Surgical resection remains an
effective treatment option for local, early stage renal cell
carcinoma; however, >1/3 of patients will eventually develop
metastatic disease (2).
Immunotherapy has also been observed to moderately prolong the
survival time of patients in circumstances when chemotherapy and
radiation therapy are not effective, and it has even been found to
promote tumor regression and long-term survival in a small number
of patients (18). Therefore, it
is an urgent requirement to identify appropriate tumor biomarkers
for the early diagnosis, treatment and prognosis of renal cell
carcinoma (19). The present study
found miR-133b expression in renal cell carcinoma was significantly
lower compared with adjacent healthy tissues. Moreover, miR-133b
expression in four types of renal cancer cells was lower compare
with human embryonic kidney cells.
miRNAs are abnormally expressed in all cancer cells,
and it has been reported that this abnormal expression can regulate
the activity of signaling pathways in cancer cells (7). Thus, the regulation of miRNAs over
target genes may prevent certain diseases in humans. Conversely,
changes in the expression levels of miRNAs has also been reported
to lead to cancer occurrence (20); therefore, investigating the
expression levels of miRNAs in tumor tissues may provide a
theoretical basis for an improved understanding of cancer types
(20). In the present study, the
overexpression of miR-133b significantly decreased cell
proliferation, and the migratory and invasive ability of 786-O
cells, whilst increasing the rate of apoptosis. Concurrently, the
protein expression levels of PCNA, MMP-2 and MMP-9, which are
related to cell proliferation and invasion, were significantly
decreased (11). These results
preliminarily suggested a potential inhibitory effect of miR-133b
in renal cell carcinoma.
ABCG2 is a member of the ABC transporter family; it
has been found that ABCG2 can affect the concentration of
intracellular chemotherapeutic drugs, in addition to inhibiting the
activity of certain chemotherapeutic drugs or affecting their
metabolites, which overall results in a poor response to
chemotherapeutic regimens (21).
Similar to P-gp, ABCG2 can actively pump chemotherapeutic drugs
with different chemical structures and target sites out of cells,
which subsequently induces tumor resistance to various anticancer
drugs and reduces the sensitivity of cancer cells to
chemotherapeutic drugs (22,23).
To investigate the effect of miR-133b on chemosensitivity in renal
cell carcinoma, miR-133b was overexpressed and three
chemotherapeutic drugs, DDP, DXT and ADR, were used to treat the
cells. The results indicated that miR-133b overexpression increased
the sensitivity of the 786-O cell line to these drugs. In addition,
overexpression of miR-133b could decrease the expression levels of
ABCG2 and P-gp. Concurrently, miR-133b overexpression reduced the
expression levels of the anti-apoptotic protein, Bcl-2, and
increased the expression levels of the pro-apoptotic protein, Bax.
These findings further suggested that the anticancer effect of
miR-133b may be related to the induced enhanced chemosensitivity of
renal cell carcinoma cells and the increased apoptotic rate of the
cancer cells.
The activation of ERK by phosphorylation is a
prerequisite for the function of the ERK signaling pathway
(14,15). The present results demonstrated
that the phosphorylation level of ERK was significantly reduced
following the treatment with U0126 or miR-133b mimic. Bcl-2 and Bax
are proteins involved in mitochondrial apoptosis (24), and P-gp, MMP-2 and MMP-9 are
mitogen-activated protein kinase/ERK signaling pathway-related
factors (24,25). The present study observed that
U0126 or miR-133b mimic treatment could also increase the protein
expression levels of Bax and decrease the protein expression levels
of MMP-2, MMP-9, P-gp and Bcl-2. However, the overexpression of
miR-133b combined with LM22B-10 treatment was discovered to reverse
or weaken the anticancer effects of miR-133b overexpression, which
suggested that miR-133b may serve a role as a tumor suppressor gene
through mediating the expression levels of ERK in renal cell
carcinoma.
In conclusion, the findings from the present study
indicated that miR-133b is expressed at low levels in renal cell
carcinoma tissues and cells. The overexpression of miR-133b was
found to inhibit the proliferation, migration and invasion of renal
cell carcinoma cells, in addition to inducing apoptosis and
enhancing the sensitivity of renal cell carcinoma cells to
chemotherapeutic drugs; these functions may be related to the
induction of ERK1/2 dephosphorylation, and thus the inhibition of
the ERK signaling pathway. Since ERK is the convergence point of
multiple signaling pathways, whether miR-133b affects the
expression of genes upstream of ERK requires further study.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XY designed the study and drafted the manuscript. YM
and XLL performed the experiments. XLL and SLG performed the
statistical analysis. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Research
Ethics Committee of School of Medicine, Shandong University and was
conducted under the Declaration of Helsinki principles. Informed
written consent was obtained from all participating patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
White NM and Yousef GM: MicroRNAs:
Exploring a new dimension in the pathogenesis of kidney cancer. BMC
Med. 8:652010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen C, Xue S, Zhang J, Chen W, Gong D and
Zheng J, Ma J, Xue W, Chen Y, Zhai W and Zheng J:
DNA-methylation-mediated repression of miR-766-3p promotes cell
proliferation via targeting SF2 expression in renal cell carcinoma.
Int J Cancer. 141:1867–1878. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhai W, Li S, Zhang J, Chen Y, Ma J, Kong
W, Gong D, Zheng J, Xue W and Xu Y: Sunitinib-suppressed miR-452-5p
facilitates renal cancer cell invasion and metastasis through
modulating SMAD4/SMAD7 signals. Mol Cancer. 17:1572018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yan L, Ding B, Liu H, Zhang Y, Zeng J, Hu
J, Yao W, Yu G, An R, Chen Z, et al: Inhibition of SMYD2 suppresses
tumor progression by down-regulating microRNA-125b and attenuates
multi-drug resistance in renal cell carcinoma. Theranostics.
9:8377–8391. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Palanichamy JK and Rao DS: miRNA
dysregulation in cancer: Towards a mechanistic understanding. Front
Genet. 5:542004.
|
|
9
|
Juan D, Alexe G, Antes T, Liu H,
Madabhushi A, Delisi C, Ganesan S, Bhanot G and Liou LS:
Identification of a microRNA panel for clear-cell kidney cancer.
Urology. 75:835–841. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Redova M, Poprach A, Nekvindova J, Iliev
R, Radova L, Lakomy R, Svoboda M, Vyzula R and Slaby O: Circulating
miR-378and miR-451 in serum are potential biomarkers for renal cell
carcinoma. J Transl Med. 10:552012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhen Y, Liu J, Huang Y, Wang Y, Li W and
Wu J: miR-133b inhibits cell growth, migration, and invasion by
targeting MMP9 in non-small cell lung cancer. Oncol Res.
25:1109–1116. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zeng W, Zhu JF, Liu JY, Li YL, Dong X,
Huang H and Shan L: miR-133b inhibits cell proliferation, migration
and invasion of esophageal squamous cell carcinoma by targeting
EGFR. Biomed Pharmacother. 111:476–484. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Roskoski R Jr: ERK1/2 MAP kinases:
Structure, function, and regulation. Pharmacol Res. 66:105–143.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Qin W, Dong P, Ma C, Mitchelson K, Deng T,
Zhang L, Sun Y, Feng X, Ding Y, Lu X, et al: MicroRNA-133b is a key
promoter of cervical carcinoma development through the activation
of the ERK and AKT1 pathways. Oncogene. 31:4067–4075. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li S, Cui Z and Meng X: Knockdown of
PARP-1 inhibits proliferation and ERK signals, increasing drug
sensitivity in osteosarcoma U2OS cells. Oncol Res. 24:279–286.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak JK and Schmittgen TD: Analysis of
relative gene expression data using quantitative PCR and the
2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rasmussen F: Metastatic renal cell cancer.
Cancer Imaging. 13:374–380. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gardner CS, Ensor JE, Ahrar K, Huang SY,
Sabir SH, Tannir NM, Lewis VO and Tam AL: Cryoablation of bone
metastases from renal cell carcinoma for local tumor control. J
Bone Joint Surg Am. 99:1916–1926. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Romero-Laorden N, Doger B, Hernandez M,
Hernandez C, Rodriguez-Moreno JF and Garcia-Donas J: Predictive
biomarker candidates to delineate efficacy of antiangiogenic
treatment in renal cell carcinoma. Clin Transl Oncol. 18:1–8. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rupaimoole R, Calin GA, Lopez-Berestein G
and Sood AK: miRNA deregulation in cancer cells and the tumor
microenvironment. Cancer Discov. 6:235–246. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bram EE, Stark M, Raz S and Assaraf YG:
Chemotherapeutic drug-induced ABCG2 promoter demethylation as a
novel mechanism of acquired multidrug resistance. Neoplasia.
11:1359–1370. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
To KK, Poon DC, Wei Y, Wang F, Lin G and
Fu LW: Vatalanib sensitizes ABCB1 and ABCG2-overexpressing
multidrug resistant colon cancer cells to chemotherapy under
hypoxia. Biochem Pharmacol. 97:27–37. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Reustle A, Fisel P, Renner O, Büttner F,
Winter S, Rausch S, Kruck S, Nies AT, Hennenlotter J, Scharpf M, et
al: Characterization of the breast cancer resistance protein
(BCRP/ABCG2) in clear cell renal cell carcinoma. Int J Cancer.
143:3181–3193. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sun Y, Wang C, Meng Q, Liu Z, Huo X, Sun
P, Sun H, Ma X, Peng J and Liu K: Targeting P-glycoprotein and
SORCIN: Dihydromyricetin strengthens anti-proliferative efficiency
of adriamycin via MAPK/ERK and Ca2+-mediated apoptosis
pathways in MCF-7/ADR and K562/ADR. J Cell Physiol. 233:3066–3079.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Han CK, Tien YC, Jine-Yuan Hsieh D, Ho TJ,
Lai CH, Yeh YL, Hsuan Day C, Shen CY, Hsu HH, Lin JY and Huang CY:
Attenuation of the LPS-induced, ERK-mediated upregulation of
fibrosis-related factors FGF-2, uPA, MMP-2, and MMP-9 by Carthamus
tinctorius L in cardiomyoblasts. Environ Toxicol. 32:754–763. 2017.
View Article : Google Scholar : PubMed/NCBI
|