The current SARS-CoV-2 pandemic challenges the
health systems across the world to an unprecedented degree
(1); the scientific community was
put to a test in order to evaluate the risk factors associated with
the viral response, since the most sensitive patients in respect to
the respiratory response to the pathogen are those in a
prerequisite unbalanced health condition.
Obesity is one of the most important conditions that
increases exponentially the mortality risk of the SARS-CoV-2
patients (2); the Center for
Disease Control and Prevention (CDC) determined the most important
groups with higher risk for severe illness, noting that, besides
obesity, asthma, chronic lung disease, diabetes, serious heart
conditions, chronic kidney disease, severe obesity, age 65 years
and older, people in nursing homes or long-term care facilities,
immune-compromised, and liver disease patients are the most
vulnerable to the complications. Examining the respective list we
can conclude that most of the high risk group patients have
conditions associated frequently or consequences of obesity
(diabetes, heart conditions, asthma, etc.) (3–5).
The strong correlation between obesity and the
complications of viral infections was previously pointed out, for
the influenza virus as well as for the previous corona-viruses
causing wide spread infections (SARS, MERS). This association is
important when analyzing results, since there is an increased
genetic similarity between SARS-CoV-2 (determining COVID-19),
SARS-CoV (80%) and MERS-CoV (50%) (6). Studies show that obese patients are
at higher risk of hospital admission regardless of their viral
status and also, obese patients are at higher risk of
hospitalization compared to normo-ponderal ones, when affected by
influenza (7,8).
Literature data state that there is a direct
metabolic link between the state of inflammation (such as the one
associated with diabetes and metabolic syndrome) and the ‘cytokine
storm’ contributing to the respiratory decline of COVID-19
patients. The entry of the virus to the cell is mediated by the
ACE2 ectoenzyme located at the cell surface in the lungs and by the
serine protease TMPRSS2; thus, the correlation with the
renin-angiotensin system is obvious. Indeed, ACE1 activity is
increased and ACE2 inhibited; while angiotensin II activates
angiotensin receptors 1 and 2 (AT1R, AT2R) mediating a
pro-inflammatory response, and a consequent increase of vascular
permeability. Importantly, this metabolic imbalance is aggravated
by pre-existing diabetes or hypertension, usual consequences of
obesity (9,10). Moreover, some drugs frequently used
for treatment of obese patients complications (such as
antihypertensives, statins, thiazolidinediones) have the tendency
to up-regulate ACE2, thus increasing the viral up-take (11–13).
Previous preclinical studies in an animal model showed that the
association of diabetes with MERS-CoV dampened the ability of the
body to engage an effective immune response and induced a poor
recovery after illness (14).
The disease burden of obesity is obvious in
hospitalized COVID-19 patients and has strong relationship with the
development of obese-related major and lethal complications.
Obesogenic comorbidities (15,16)
have become major health problems in hospitalized patients
including increased insulin resistance (17–20)
and type 2 diabetes (21), fatty
liver diseases (22,23), vascular inflammation and coronary
heart disease (24,25), immune diseases (26), risk of cerebral ischemia and brain
injury (27), atherosclerotic
vascular disease and myocardial infarction (28) as well as cancers (29). Adipose tissue and adipocytes
produce leptin and other molecules that affect the cardiovascular
function. The production of these substances is perpetrated through
distinct endocrine, autocrine and paracrine mechanisms and believed
to lead to cytokine-mediated inflammatory changes in the liver and
systemic inflammation and atherosclerosis (30–33).
The pulmonary tissue is the most sensitive human
tissue in intensive care unit (ICU) COVID-19 patients. Systemic
hypoxia due to reduced pulmonary functions, increased adipokines
and cytokines, gut (60) and
pulmonary (61) microbiome
alterations (62),
cardiopulmonary, vascular and epithelial complications due to
chronic obstructive pulmonary disease (63) are cofactors associated with
critical illness among hospitalized patients with COVID-19 disease
reported in Zhanjiang province (64) and Jiangsu province, China (65) as well as in New York City, USA
(66). Petrilli et al
pointed out that, in COVID-19 ICU patients, the highest risks are
exhibited by those aged ≥75 years, body mass index (BMI) >40 and
heart failure. Moreover, strong critical illness parameters were
admission oxygen saturation <88%, d-dimer >2,500, ferritin
>2.500 as well as C-reactive protein (CRP) >200.
Aging seems to present with a great variety of
patterns and unique sets of obesity and age-related disease. Among
older adults, independent of their BMI, blood pressure and blood
lipid concentrations (67),
decline in immune function is observed (known as immune-senescence)
leading to increased susceptibility and exhibiting more serious
complications as compared to younger individuals; reflecting the
deterioration of function in both the acquired and innate immune
systems (20,45,68).
In elderly, most cells produce cytokines/chemokines/adipokines and
soluble mediators of inflammation due to inflammation-related gene
expression by ROS induced lipid oxidation-derived products and
formation of lipid droplets within the monocytes/macrophages
(69). Ageing is also associated
with a multi-factorial decrease of T cell function and number,
T-cell subset composition and functional capacity, fewer naive T
cells, more memory cells in the circulation, thymic involution and
decreased thymic output and naive T cells as well as increased
memory cells in the circulation (70). Furthermore, modifications of
immunoglobulin levels, micronutrient deficiencies (71) and biological dysfunctions including
lymphocyte proliferation and cytokine production, thus increasing
inflammation, as well as hospitalization and death have been
documented (72).
The World Health Organization (WHO) has
characterized both the COVID-19 outbreak and obesity ‘epidemic’ as
international public health emergencies. Global clinical and
epidemiological observations confirm that CoVs can cause more
severe symptoms and complications in people with obesity-related
conditions. Indeed, Wu et al (4) established the correlation between
obesity-induced immune deficiency and COVID-19 adverse
outcomes.
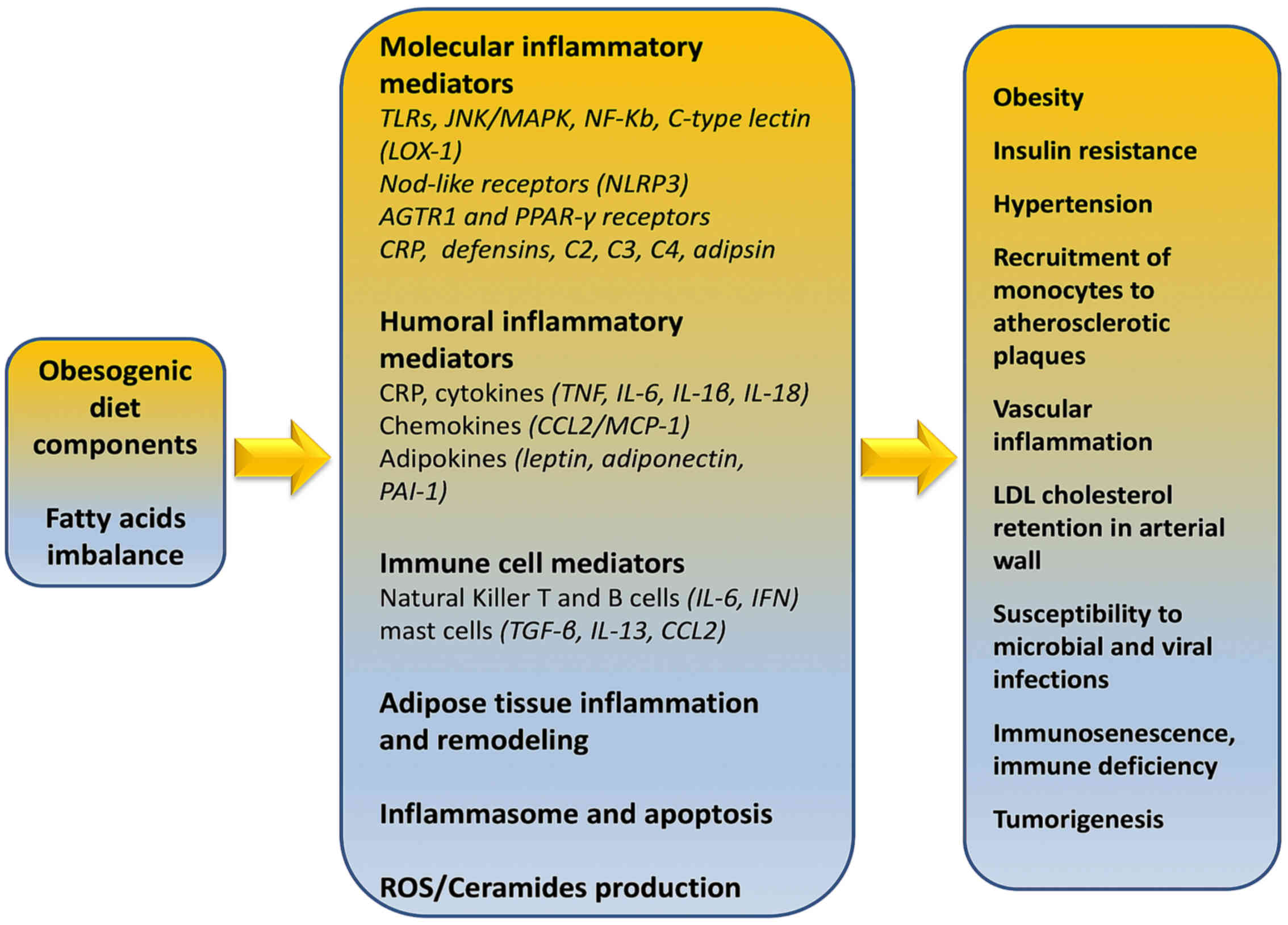
Immunologically, obesity is characterized as a
chronic sub-clinical inflammatory morbid entity which can impact
the immune responses to infectious diseases through direct,
indirect and epigenetic (73,74)
mechanisms. Evans et al (75) described various fat
tissue-associated cytokines (adipokines) that are produced and
released in proportion to the amount of visceral adipose tissue in
the body. Serum amyloid-A is an adipokine secreted by adipocytes,
that can act directly on macrophages to increase their production
of inflammatory cytokines such as tumor necrosis factor (TNF)-α,
interleukin (IL)-1, and IL-6, and resistin (22,23,75).
Indeed, Alam et al (76)
reported in detail that the majority of respective adipokines are
inflammatory mediators such as IL-8, PAI-1, MCP-1, IL-6, IL-1Ra,
TNF-α, sTNFRII, and IL-18.
In addition, IL-8, IL-10, interferon gamma (IFN-γ)
and inducible protein 10 (IP-10 or CXCL10) have been shown to be
associated with excessive body weight (77). Obesity-induced adipokine production
such as leptin /adiponectin ratio increases insulin resistance in
type 2 diabetes, resulting in inability to feel and detect satiety
leptin in the arcuate nucleus of mediobasal hypothalamus (78). Moreover, adverse effects are
evident, despite high energy stores, on hunger, food energy use,
physical exercise and energy balance as well as on
hippocampus-mediated deficit in learning and memory functions
(79). Furthermore, the prolonged
IFN responses during persistent chronic inflammation and
obesogenesis comprise reciprocal causality between virus
susceptibility and obesity (80).
Additional epigenetic signatures in obesity are likewise altered
including methylation and/or histone acetylation levels in genes
involved in specific and general metabolic processes, altering
thus, the metabolic phenotype of the offspring (81–83).
Although no specific therapy exists to block the effects of these
factors, recognizing the high risk and anticipating
inflammation-associated complications of adipokine release is an
important part of optimal patient management.
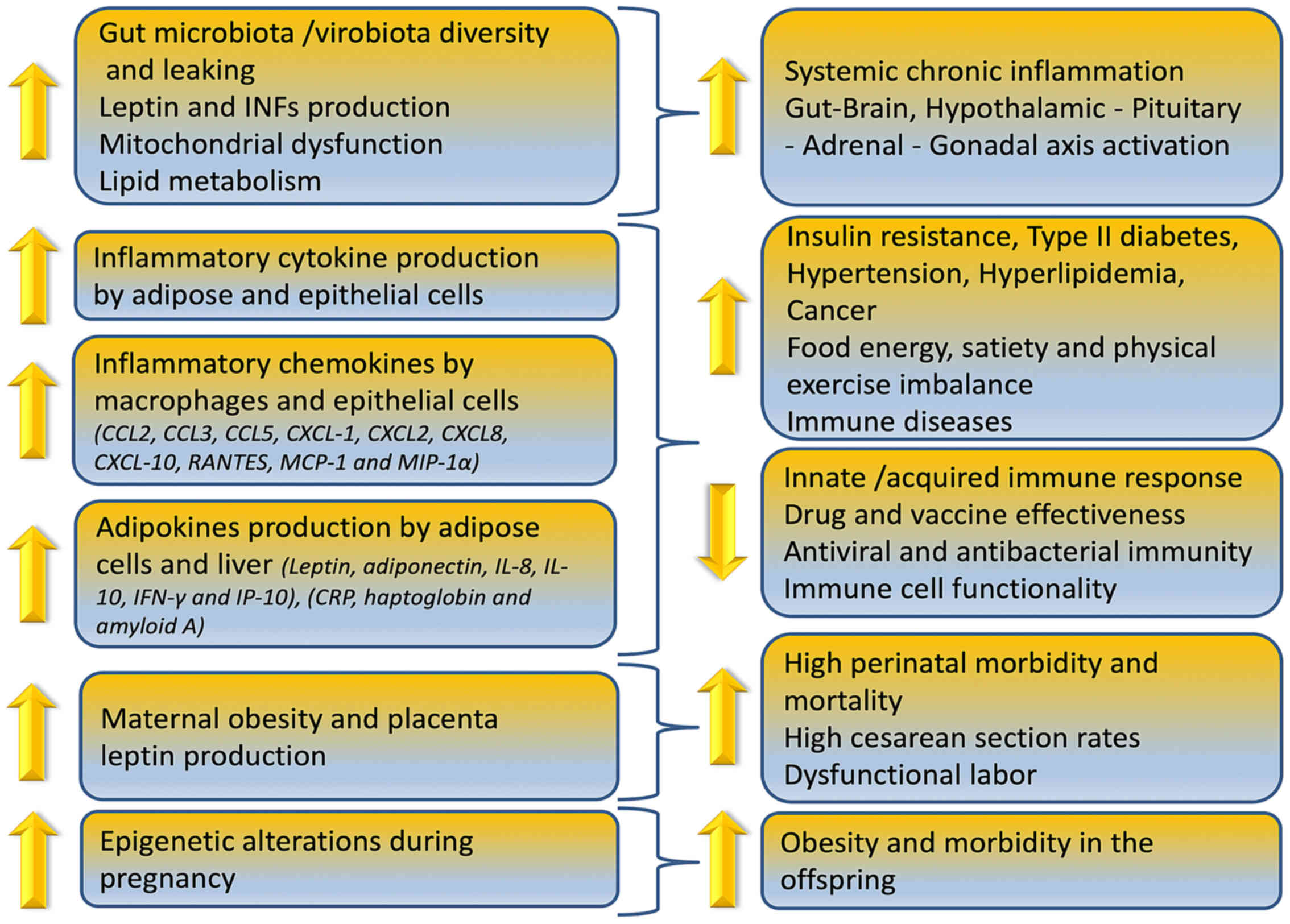
Obesity can reduce immune cell functionality, induce
gut microbiome/virome imbalance, inflammatory cytokine phenotype
and increase antiviral, antimicrobial and anticoagulant resistance
as depicted in Fig. 1. In
overweight children, anti-tetanus IgG antibodies were significantly
lower compared to normal weight controls due to the chronic low
grade inflammation expressed by the higher levels of IL-6 (84). Similarly, researchers reported in a
2019 study that reduction or elimination of food in
overweight/obese adults can lead to a decreased IgG concentration
over time (85).
The mechanisms involved in increased risk for higher
COVID-19 prevalence and mortality in obese are correlated with
specific fat-resident regulatory T cells (86) (Treg) and particularly promotion of
TH17 (T-cell sub-lineage)-biased immunity (87,88).
Indeed, these processes are partly dependent on increased IL-6, as
well as IL-23/IL-17, other inflammatory obesity-associated plasma
cytokine expression such as TNF-α, transforming growth factor
(TGF), pro-inflammatory cytokine macrophage migration inhibitory
factor and macrophage inflammatory protein-1α (89). Furthermore, in this mechanism
increased CRP levels (90) and
disrupted tight junctions in pulmonary epithelia (91) have also been implicated.
Additionally, Ahmed and Gaffen (92) argued that ‘obesity selectively
promotes expansion of the Th17 T-cell lineage, exacerbating immune
diseases in specific organs in obese individuals, such as brain and
gut, according to the results of new experimental and human
studies. Th17 cells are also associated with autoimmune disease
such as multiple sclerosis, rheumatoid arthritis, and psoriasis
(93), but also
glomerulonephritis, asthma, and pandemic H1N1 influenza virus
(94).
On April 15, 2020, the Centers for Disease Control
and Prevention (CDC), based on currently available information and
clinical expertise, advised that older adults and people of any age
who have serious underlying medical conditions, including obesity
with a BMI of 40 or above, might be at higher risk for
complications and severe illness from COVID-19. In this report,
emphasis is given on ascertainment that severe obesity increases
the risk of acute respiratory distress syndrome (ARDS) (3).
Obesity, increased food intake, nutrient/energy
imbalance affect in a bidirectional way the immune deficiency,
especially in vulnerable populations. Patients with type 2 diabetes
and those with metabolic syndrome (pre-diabetes) may be up to ten
times more likely to die when they become ill with COVID-19
(9). The mortality rate for young
people who had to be admitted to a hospital with severe respiratory
illness due to COVID-19 was approximately 2%, but this increased to
14% for the most vulnerable such as COVID-19 patients with obesity
and related co-morbidity (97).
For autoimmune diseases, such as systemic lupus erythematosus,
systemic vasculitis and rheumatoid arthritis, treatment may include
drugs including methotrexate and hydroxychloroquine (Plaquenil)
that suppress the immune system to treat the disease and symptoms
making immune-compromised patients more vulnerable to COVID-19
infection (98).
Foods may have calories, but do not contain
vitamins, minerals, antioxidants etc., indicating that increased
calorie intake, in relation to nutrient intake, is the main reason
behind the immunodeficiency today. For example, even short-term
severe vitamin D deficiency (99)
may directly promote hypertension and impacts on renin-angiotensin
system components that could contribute to target-organ damage
(100). Nutrient deficiencies are
highly prevalent in the United States (and elsewhere), increasing
much of the consequent risk of premature aging, chronic disease and
COVID-19 prevalence and mortality due to acceleration of the risk
associated with aging (101).
Lipotoxicity is a condition, induced by the
aggregation of intermediate lipids to non-fatty tissue, which leads
to cell malfunction and death (106). Tissues that are usually affected
include kidneys, liver, heart, and skeletal muscle. Lipotoxicity is
believed to play a role in heart failure, obesity and diabetes and
is estimated to affect approximately 25% of the adult American
population (20,45,107,108).
Under physiological conditions, there is a balance
between cellular lipid production and their oxidation or transport.
However, this balance might be disrupted when the cells are
lipotoxic, that is, when an imbalance between the amount of
cellular lipids produced and the amount consumed is evident. Thus,
upon fatty acids entry to cells, conversion to various types of
storage lipids is initiated. Triacylglycerol (TG), consisting of a
glycerin molecule bound to three fatty acids, is established as the
safest type of intracellular lipid storage. Apart from TG, fatty
acids can be converted to fatty acyl-CoAs, ceramides and
diacylglycerol. Importantly, these molecules were shown to induce
lipotoxicity when found in high concentrations and can inflict
damage to cells (109).
It is not well-established whether lipotoxicity is
related to genetic or non-genetic causes, but it is not considered
to be a single-gene disease. Opinions which argue that obesity can
either attenuate or facilitate lipotoxicity exist. Indisputably, a
high-fat diet increases the risk for this pathological condition.
Importantly, individuals with high number of lipotoxic cells appear
to be resistant to both insulin and leptin (110).
Initially the term oxidative stress was defined as
the imbalance between pro-oxidants and antioxidants in favor of the
former (111). Over the years,
this definition is based on new data, and oxidative stress is now
considered a disorder of the redox signaling (112).
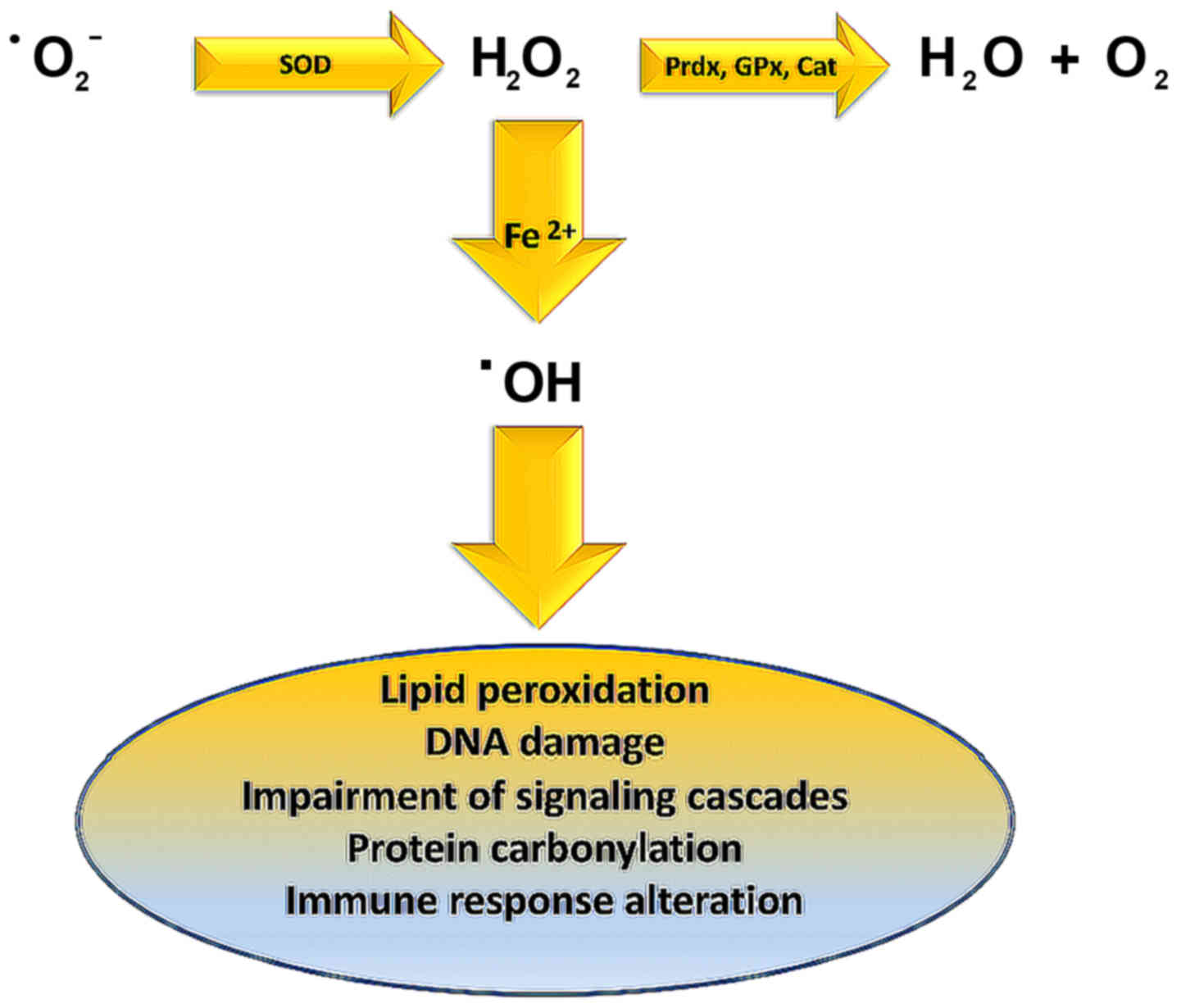
Lipid peroxidation is caused by hydroxyl radical
(OH·) derived from elevated superoxide anion levels
(−Ο2·) (Fig. 3). In
turn, hydroxyl radical causes the formation of LOOH. Finally, a
variety of reactive lipid aldehydes (RLA) such as malondialdehyde
(MDA) and 4-hydroxynonenal (4-HNE) are created, with the ability to
modify proteins through protein carbonylation (113,114). Normally phase I and phase II
enzymes would detoxify RLA products, but in individuals exhibiting
metabolic syndrome, RLAs accumulate causing damage to cells and
their membranes. These alterations have been associated with
inflammation and various pathologies including obesity,
neurodegenerative disease, asthma, cardiovascular desease (CVD),
type II diabetes, and cancer (115–124).
Oxidative stress, as mentioned above, leads to
increased free fatty acids concentration in β-type pancreatic
cells, enhanced insulin secretion, type II diabetes and cell death,
through the exit of cytochrome c from the mitochondria and the
activation of caspase 3 (113).
One strategy for protection against these detrimental effects has
been proposed in a study by Zhu et al (125) in which the effects of perilipin 5
were examined. Perilipin 5 activates the PI3K/Akt path as well as
ERK signaling which activates NRF2β-type pancreatic cells.
Subsequently, NRF2 enters the nucleus and as a transcriptional
factor activates the antioxidant response element (ARE) that
enhances the transcription of down-stream antioxidant enzymes. As a
result, β-type pancreatic cells exhibit increased glutathione
levels and decreased reactive oxygen species levels. The above
signaling pathway thus, enhances β-type pancreatic cell protection,
defends them from apoptosis and facilitates the execution of the
normal function of these cells.
Clinical reports of patients infected with
SARS-CoV-2 show that several parameters associated with infection
as well as the severity of the disease (i.e., older age,
hypertension, diabetes, cardiovascular disease) are correlated to
viral invasion induced ACE2 down-regulation. Importantly,
angiotensin converting enzyme-2 (ACE2) receptors mediate the entry
of SARS-CoV, NL63 and SARS-COV-2 into the cell (136), particularly in type 2 pneumocytes
and macrophages, as well as angiotensin II degradation to
angiotensin 1–7 via the ACE→Angiotensin II→Angiotensin II receptor
type 1 or AT1 receptor axis (137). Structural, allelic or genetic
variations in the SARS-CoV-2 spike protein and genetic or allelic
variations of the host receptor ACE2 including rs73635825 (S19P)
and rs143936283 (E329G) lead to the cross-species transmission of
the virus. The specific modifications have been found to
significantly contribute to the susceptibility and/or resistance
against the viral infection (138). Thus, the resulting ACE2
deficiency upon viral invasion may enhance the dys-regulation
between the ‘adverse’ ACE→Angiotensin II→AT1 receptor axis and the
‘protective’ ACE2→Angiotensin 1–7→Mas receptor signaling. In the
lungs, decreased angiotensin 1–7 and G-protein coupled Mas receptor
binding, enhances the severity of vasoconstriction, fibrosis,
inflammation, thrombosis and pulmonary damage including edema and
permeability triggered by local angiotensin II hyperactivity
unopposed by angiotensin 1–7 (139).
Although the association between obesity and heart
disease is well-established, underlying pathophysiological
processes remain elusive, and it is unclear whether cardiomyopathy
in obese patients is due to increased deposition of adipose tissue
itself or due to the effects of obesity-associated comorbidities,
including hypertension, metabolic syndrome, and diabetes. In
obesity, the heart undergoes structural remodeling and functional
alterations leading to obesity-associated cardiomyopathy due to
interstitial fibrosis, cardiomyocyte hypertrophy, and cardiac
steatosis (140) Oxidative
stress, natriuretic peptides, endothelin-1, advanced glycation end
products, induction of TGFβ, increased mean arterial pressure in
elderly through the activation of the renin-angiotensin-aldosterone
system (RAAS) (141), Rho-kinase
signaling, leptin-mediated actions, up-regulation of matricellular
proteins (such as thrombospondin 1), are molecular mechanisms
associated with these processes especially in obese adults
(142). Furthermore, the sleep
apnea/obesity hypoventilation syndrome, as well as respective
coexisting neurohormonal and metabolic alterations, diabetes
(143), insulin resistance and
long-term inflammatory adipocyte-derived factors directly influence
the pro-inflammatory signaling in the heart (144).
The above data are very important as it seems that
lipotoxicity and obesity are directly related to viral infections
as well as to the challenges in combating this infection, as shown
by the increased need of severely obese patients for ventilator
support. The fact that in the western world general population
exhibits much higher rates of obesity argues that these countries
need to be prepared as regarding intensive care units (ICU) and
ventilator devices. Finally, it is important for obese individuals
to take all the preventive actions proposed by the WHO to minimize
the chances of becoming infected with COVID-19 (145,146).
It is important to note that RAAS and ACE2 are
expressed in adipose tissue and that angiotensin II can be released
from fat tissue during periods of increased sympathetic nervous
system activity with complex interactions linking central RAAS with
adipose tissue RAAS (147).
Indeed, it has been suggested in cardiovascular
disease patients that the aforesaid obese may reflect the
biological properties of adipose tissue (153). Indeed, not all obese people
present inflammation; thus, the terms of metabolically healthy and
unhealthy obesity are used with metabolically unhealthy obesity
being linked to increased visceral/abdominal fat (76).
Obesity was rarely mentioned in early clinical
reports evaluating the clinical risk factors for SARS-CoV-2
infection. Novel data from a single center retrospective study
confirm that obesity had a high frequency among patients admitted
in intensive care for SARS-CoV-2 requiring invasive mechanical
ventilation and that disease severity increased with BMI (154). Indeed, a very recent report on a
large sample of patients younger than 60 years tested positive for
COVID-19, correlated higher BMIs values with increased probability
for admission to critical care (155).
Importantly, overweight, pre-pregnancy obese and
obese pregnant women are at increased risk for both morbidity and
mortality from CMV (156),
including all recorded influenza pandemics, 1918 (157), 1957 (158), and 2009 (159), Varicella Zoster (160), Listeria monocytogenes (161), malaria (162), as well as SARS (163). Moreover, the highest mortality
risk for these pregnant women was correlated to acute
cardiopulmonary conditions presenting in the second and third
trimesters (164). Furthermore,
the above mentioned pathological conditions were associated with
adverse pregnancy outcomes, including preterm birth and fetal death
(165).
Maternal burden of coronavirus infection may have
significant implications for neonatal immune ontogeny, as high
maternal viremia has been associated with significantly lower
CD4+ T-cell count in uninfected progeny. Increased
levels of pro-inflammatory cytokines produced by placental cells
can negatively affect infant innate cytokine responses in early
life, pregnancy outcomes and facilitate mother-to-child
transmission of coronavirus in the infant. Indeed, increased
placenta leptin production affects uterine contractility playing a
role in the dysfunctional labor process associated with maternal
obesity, and the resultant high cesarean section rates (166).
Pregnancy immune phenotype may be correlated with
the SARS-COV-2 prevalence in pregnant women. Both xenobiotics and
enveloped viruses such as SARS-COV-2 induce immune-suppression
(167) characterized by
significant increases in blood phagocytes, placental DCs and
immature monocyte-derived DCs, decreases in the number and activity
of NK and T cells (168), and
inhibition of TH1 responses from maternal-fetal TH2 cytokine
cocktail especially IL-1β+IL-6+TNF-α +α-defensins (169) shifting toward Th2 immunity
(170), inefficacy of
adaptive/inflammatory immunity in the later stages of pregnancy as
well as membrane permeabilization to SARS-COV-2 entry (171).
Obesity is a medical condition with complex
pathophysiology, comprising various mechanisms, which now emerges
as a significant risk factor for COVID-19. Targeted epidemiological
studies specifically oriented in order to reveal the impact of
obesity in COVID-19 severity and mortality rates are needed in
order to determine specific therapeutic strategies for obese
patients.
Not applicable.
No funding was received.
Not applicable.
Conceptualization: DP, DM, DK, DAS, AT; writing
(original draft preparation): DP, DM, KT, FT; writing (review and
editing): DP, DM, KT, FT, DN, DK, DAS, AT; figure preparation: DP,
DM, FT; supervision: DM and AT; all authors have read and agreed to
the published version of the manuscript.
Not applicable.
Not applicable.
DAS is the Editor-in-Chief for the journal, but had
no personal involvement in the reviewing process, or any influence
in terms of adjudicating on the final decision, for this article.
The other authors declare that they have no competing
interests.
|
1
|
Docea AO, Tsatsakis A, Albulescu D,
Cristea O, Zlatian O, Vinceti M, Moschos SA, Tsoukalas D, Goumenou
M, Drakoulis N, et al: A new threat from an old enemy: Re emergence
of coronavirus (Review). Int J Mol Med. 45:1631–1643.
2020.PubMed/NCBI
|
|
2
|
Goumenou M, Sarigiannis D, Tsatsakis A,
Anesti O, Docea AO, Petrakis D, Tsoukalas D, Kostoff R, Rakitskii
V, Spandidos DA, et al: COVID 19 in Northern Italy: An integrative
overview of factors possibly influencing the sharp increase of the
outbreak (Review). Mol Med Rep. 22:20–32. 2020.PubMed/NCBI
|
|
3
|
Centers for Disease Control and Prevention
(CDC): Coronavirus Disease 2019 (COVID-19), . People who are at
higher risk for severe illness. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/groups-at-higher-risk.htmlApril
15–2020
|
|
4
|
Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S,
Huang H, Zhang L, Zhou X, Du C, et al: Risk factors associated with
acute respiratory distress syndrome and death in patients with
coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern
Med. Mar 13–2020.(Epub ahead of print). View Article : Google Scholar
|
|
5
|
Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z,
Xiang J, Wang Y, Song B, Gu X, et al: Clinical course and risk
factors for mortality of adult inpatients with COVID-19 in Wuhan,
China: A retrospective cohort study. Lancet. 395:1054–1062. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Muniyappa R and Gubbi S: COVID-19
pandemic, coronaviruses, and diabetes mellitus. Am J Physiol
Endocrinol Metab. 318:E736–E741. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Moser JS, Galindo-Fraga A, Ortiz-Hernández
AA, Gu W, Hunsberger S, Galán-Herrera JF, Guerrero ML,
Ruiz-Palacios GM and Beigel JH; La Red ILI 002 Study Group, :
Underweight, overweight, and obesity as independent risk factors
for hospitalization in adults and children from influenza and other
respiratory viruses. Influenza Other Respir Viruses. 13:3–9. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yuen KS, Ye ZW, Fung SY, Chan CP and Jin
DY: SARS-CoV-2 and COVID-19: The most important research questions.
Cell Biosci. 10:402020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bornstein SR, Dalan R, Hopkins D, Mingrone
G and Boehm BO: Endocrine and metabolic link to coronavirus
infection. Nat Rev Endocrinol. Apri 2–2020.(Epub ahead of print).
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Drucker DJ: Coronavirus infections and
type 2 diabetes-shared pathways with therapeutic implications.
Endocr Rev. 41:bnaa0112020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang W, Xu YZ, Liu B, Wu R, Yang YY, Xiao
XQ and Zhang X: Pioglitazone upregulates angiotensin converting
enzyme 2 expression in insulin-sensitive tissues in rats with
high-fat diet-induced nonalcoholic steatohepatitis.
ScientificWorldJournal. 2014:6034092014.PubMed/NCBI
|
|
12
|
Tikoo K, Patel G, Kumar S, Karpe PA,
Sanghavi M, Malek V and Srinivasan K: Tissue specific up regulation
of ACE2 in rabbit model of atherosclerosis by atorvastatin: Role of
epigenetic histone modifications. Biochem Pharmacol. 93:343–351.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Romaní-Pérez M, Outeiriño-Iglesias V, Moya
CM, Santisteban P, González-Matías LC, Vigo E and Mallo F:
Activation of the GLP-1 receptor by liraglutide increases ACE2
expression, reversing right ventricle hypertrophy, and improving
the production of SP-A and SP-B in the lungs of type 1 diabetes
rats. Endocrinology. 156:3559–3569. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kulcsar KA, Coleman CM, Beck SE and
Frieman MB: Comorbid diabetes results in immune dysregulation and
enhanced disease severity following MERS-CoV infection. JCI
Insight. 4:1317742019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Petrakis D, Vassilopoulou L, Mamoulakis C,
Psycharakis C, Anifantaki A, Sifakis S, Docea AO, Tsiaoussis J,
Makrigiannakis A and Tsatsakis AM: Endocrine disruptors leading to
obesity and related diseases. Int J Environ Res Public Health.
14:E12822017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vassilopoulou L, Psycharakis C, Petrakis
D, Tsiaoussis J and Tsatsakis AM: Obesity, persistent organic
pollutants and related health problems. Adv Exp Med Biol.
960:81–110. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bastard JP, Maachi M, Lagathu C, Kim MJ,
Caron M, Vidal H, Capeau J and Feve B: Recent advances in the
relationship between obesity, inflammation, and insulin resistance.
Eur Cytokine Netw. 17:4–12. 2006.PubMed/NCBI
|
|
18
|
Baltzis D, Meimeti E, Grammatikopoulou MG,
Roustit M, Mavrogonatou E, Kletsas D, Efraimidou S, Manes C,
Nikolouzakis TK, Tsiaoussis J, et al: Assessment of telomerase
activity in leukocytes of type 2 diabetes mellitus patients having
or not foot ulcer: Possible correlation with other clinical
parameters. Exp Ther Med. 15:3420–3424. 2018.PubMed/NCBI
|
|
19
|
Engin AB, Tsatsakis AM, Tsoukalas D and
Engin A: Do flavanols-rich natural products relieve obesity-related
insulin resistance? Food Chem Toxicol. 112:157–167. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ungurianu A, Margină D, Grădinaru D,
Băcanu C, Ilie M, Tsitsimpikou C, Tsarouhas K, Spandidos DA and
Tsatsakis AM: Lipoprotein redox status evaluation as a marker of
cardiovascular disease risk in patients with inflammatory disease.
Mol Med Rep. 15:256–262. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zimmet P, Alberti KG and Shaw J: Global
and societal implications of the diabetes epidemic. Nature.
414:782–787. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tilg H: The role of cytokines in
non-alcoholic fatty liver disease. Dig Dis. 28:179–185. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tilg H and Moschen AR: Adipocytokines:
Mediators linking adipose tissue, inflammation and immunity. Nat
Rev Immunol. 6:772–783. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gomes F, Telo DF, Souza HP, Nicolau JC,
Halpern A and Serrano CV Jr: Obesity and coronary artery disease:
role of vascular inflammation. Arq Bras Cardiol. 94:255–266.
2010.(In English, Portuguese, Spanish). PubMed/NCBI
|
|
25
|
Ungurianu A, Şeremet O, Gagniuc E, Olaru
OT, Guţu C, Grǎdinaru D, Ionescu-Tȋrgovişte C, Marginǎ D and
Dǎnciulescu-Miulescu R: Preclinical and clinical results regarding
the effects of a plant-based antidiabetic formulation versus well
established antidiabetic molecules. Pharmacol Res. 150:1045222019.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Procaccini C, Carbone F, Galgani M, La
Rocca C, De Rosa V, Cassano S and Matarese G: Obesity and
susceptibility to autoimmune diseases. Expert Rev Clin Immunol.
7:287–294. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Denes A, Thornton P, Rothwell NJ and Allan
SM: Inflammation and brain injury: Acute cerebral ischaemia,
peripheral and central inflammation. Brain Behav Immun. 24:708–723.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ohman MK, Wright AP, Wickenheiser KJ, Luo
W and Eitzman DT: Visceral adipose tissue and atherosclerosis. Curr
Vasc Pharmacol. 7:169–179. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wolin KY, Carson K and Colditz GA: Obesity
and cancer. Oncologist. 15:556–565. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mangge H, Almer G, Truschnig-Wilders M,
Schmidt A, Gasser R and Fuchs D: Inflammation, adiponectin, obesity
and cardiovascular risk. Curr Med Chem. 17:4511–4520. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tsoukalas D, Alegakis A, Fragkiadaki P,
Papakonstantinou E, Nikitovic D, Karataraki A, Nosyrev AE,
Papadakis EG, Spandidos DA, Drakoulis N, et al: Application of
metabolomics: Focus on the quantification of organic acids in
healthy adults. Int J Mol Med. 40:112–120. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tsoukalas D, Alegakis AK, Fragkiadaki P,
Papakonstantinou E, Tsilimidos G, Geraci F, Sarandi E, Nikitovic D,
Spandidos DA and Tsatsakis A: Application of metabolomics part II:
Focus on fatty acids and their metabolites in healthy adults. Int J
Mol Med. 43:233–242. 2019.PubMed/NCBI
|
|
33
|
Tsoukalas D, Fragoulakis V, Sarandi E,
Docea AO, Papakonstaninou E, Tsilimidos G, Anamaterou C,
Fragkiadaki P, Aschner M, Tsatsakis A, et al: Targeted metabolomic
analysis of serum fatty acids for the prediction of autoimmune
diseases. Front Mol Biosci. 6:1202019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Smethers AD and Rolls BJ: Dietary
management of obesity: Cornerstones of healthy eating patterns. Med
Clin North Am. 102:107–124. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pérez-Escamilla R, Obbagy JE, Altman JM,
Essery EV, McGrane MM, Wong YP, Spahn JM and Williams CL: Dietary
energy density and body weight in adults and children: A systematic
review. J Acad Nutr Diet. 112:671–684. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Arango-Angarita A, Rodríguez-Ramírez S,
Serra-Majem L and Shamah-Levy T: Dietary energy density and its
association with overweight or obesity in adolescents: A systematic
review of observational studies. Nutrients. 10:E16122018.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Patterson E, Wärnberg J, Poortvliet E,
Kearney JM and Sjöström M: Dietary energy density as a marker of
dietary quality in Swedish children and adolescents: The European
Youth Heart Study. Eur J Clin Nutr. 64:356–363. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Rouhani MH, Haghighatdoost F, Surkan PJ
and Azadbakht L: Associations between dietary energy density and
obesity: A systematic review and meta-analysis of observational
studies. Nutrition. 32:1037–1047. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sassi F, Tamone C and D'Amelio P: Vitamin
D: Nutrient, hormone, and immunomodulator. Nutrients. 10:E16562018.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lee GY and Han SN: The role of vitamin E
in immunity. Nutrients. 10:E16142018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Avery JC and Hoffmann PR: Selenium,
selenoproteins, and immunity. Nutrients. 10:E12032018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Manzel A, Muller DN, Hafler DA, Erdman SE,
Linker RA and Kleinewietfeld M: Role of ‘Western diet’ in
inflammatory autoimmune diseases. Curr Allergy Asthma Rep.
14:4042014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Papandreou C, Schiza SE, Tzatzarakis MN,
Kavalakis M, Hatzis CM, Tsatsakis AM, Kafatos AG, Siafakas NM and
Tzanakis NE: Effect of Mediterranean diet on lipid peroxidation
marker TBARS in obese patients with OSAHS under CPAP treatment: a
randomised trial. Sleep Breath. 16:873–879. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Tsarouhas A, Soufla G, Tsarouhas K,
Katonis P, Pasku D, Vakis A, Tsatsakis AM and Spandidos DA:
Molecular profile of major growth factors in lumbar intervertebral
disc herniation: Correlation with patient clinical and
epidemiological characteristics. Mol Med Rep. 15:2195–2203. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Gradinaru D, Margina D, Borsa C, Ionescu
C, Ilie M, Costache M, Dinischiotu A and Prada GI: Adiponectin:
Possible link between metabolic stress and oxidative stress in the
elderly. Aging Clin Exp Res. 29:621–629. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Gaforio JJ, Visioli F,
Alarcón-de-la-Lastra C, Castañer O, Delgado-Rodríguez M, Fitó M,
Hernández AF, Huertas JR, Martínez-González MA, Menendez JA, et al:
Virgin olive oil and health: Summary of the III International
Conference on Virgin Olive Oil and Health Consensus Report, JAEN
(Spain) 2018. Nutrients. 11:E20392019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Margina D, Ilie M and Gradinaru D:
Quercetin and epigallocatechin gallate induce in vitro a
dose-dependent stiffening and hyperpolarizing effect on the cell
membrane of human mononuclear blood cells. Int J Mol Sci.
13:4839–4859. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Bischoff SC, Barbara G, Buurman W,
Ockhuizen T, Schulzke JD, Serino M, Tilg H, Watson A and Wells JM:
Intestinal permeability - a new target for disease prevention and
therapy. BMC Gastroenterol. 14:1892014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Timmermans S, Bogie JF, Vanmierlo T,
Lütjohann D, Stinissen P, Hellings N and Hendriks JJ: High fat diet
exacerbates neuroinflammation in an animal model of multiple
sclerosis by activation of the renin angiotensin system. J
Neuroimmune Pharmacol. 9:209–217. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Jhun JY, Yoon BY, Park MK, Oh HJ, Byun JK,
Lee SY, Min JK, Park SH, Kim HY and Cho ML: Obesity aggravates the
joint inflammation in a collagen-induced arthritis model through
deviation to Th17 differentiation. Exp Mol Med. 44:424–431. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Paik J, Fierce Y, Treuting PM, Brabb T and
Maggio-Price L: High-fat diet-induced obesity exacerbates
inflammatory bowel disease in genetically susceptible Mdr1a-/- male
mice. J Nutr. 143:1240–1247. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Klack K, Bonfa E and Borba Neto EF: Diet
and nutritional aspects in systemic lupus erythematosus. Rev Bras
Reumatol. 52:384–408. 2012.PubMed/NCBI
|
|
53
|
De Rosa V, Procaccini C, La Cava A,
Chieffi P, Nicoletti GF, Fontana S, Zappacosta S and Matarese G:
Leptin neutralization interferes with pathogenic T cell
autoreactivity in autoimmune encephalomyelitis. J Clin Invest.
116:447–455. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Cipolletta D, Feuerer M, Li A, Kamei N,
Lee J, Shoelson SE, Benoist C and Mathis D: PPAR-γ is a major
driver of the accumulation and phenotype of adipose tissue Treg
cells. Nature. 486:549–553. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Grimes CA, Bolhuis DP, He FJ and Nowson
CA: Dietary sodium intake and overweight and obesity in children
and adults: A protocol for a systematic review and meta-analysis.
Syst Rev. 5:72016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Savica V, Bellinghieri G and Kopple JD:
The effect of nutrition on blood pressure. Annu Rev Nutr.
30:365–401. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Kleinewietfeld M, Manzel A, Titze J,
Kvakan H, Yosef N, Linker RA, Muller DN and Hafler DA: Sodium
chloride drives autoimmune disease by the induction of pathogenic
TH17 cells. Nature. 496:518–522. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Kino T, Takatori H, Manoli I, Wang Y,
Tiulpakov A, Blackman MR, Su YA, Chrousos GP, DeCherney AH and
Segars JH: Brx mediates the response of lymphocytes to osmotic
stress through the activation of NFAT5. Sci Signal. 2:ra52009.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Woehrle T, Yip L, Manohar M, Sumi Y, Yao
Y, Chen Y and Junger WG: Hypertonic stress regulates T cell
function via pannexin-1 hemichannels and P2X receptors. J Leukoc
Biol. 88:1181–1189. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Musso G, Gambino R and Cassader M:
Interactions between gut microbiota and host metabolism
predisposing to obesity and diabetes. Annu Rev Med. 62:361–380.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Sze MA, Dimitriu PA, Hayashi S, Elliott
WM, McDonough JE, Gosselink JV, Cooper J, Sin DD, Mohn WW and Hogg
JC: The lung tissue microbiome in chronic obstructive pulmonary
disease. Am J Respir Crit Care Med. 185:1073–1080. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Greenblum S, Turnbaugh PJ and Borenstein
E: Metagenomic systems biology of the human gut microbiome reveals
topological shifts associated with obesity and inflammatory bowel
disease. Proc Natl Acad Sci USA. 109:594–599. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Tkacova R: Systemic inflammation in
chronic obstructive pulmonary disease: May adipose tissue play a
role? Review of the literature and future perspectives. Mediators
Inflamm. 2010:5859892010. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Jiang F, Deng L, Zhang L, Cai Y, Cheung CW
and Xia Z: Review of the clinical characteristics of coronavirus
disease 2019 (COVID-19). J Gen Intern Med. Mar 4–2020.(Epub ahead
of print). View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Huang R, Zhu L, Xue L, Liu L, Yan X, Wang
J, Zhang B, Xu T, Ji F, Zhao Y, Cheng J, et al: Clinical findings
of patients with Coronavirus Disease 2019 in Jiangsu Province,
China: A Retrospective, Multi-Center Study. SSRN. http://dx.doi.org/10.2139/ssrn.3548785
View Article : Google Scholar
|
|
66
|
Petrilli CM, Jones SA, Yang JJ,
Rajagopalan H, O'Donnell LF, Chernyak Y, Tobin K, Cerfolio RJ,
Francois F and Horwitz LI: Factors associated with hospitalization
and critical illness among 4,103 patients with COVID-19 disease in
New York City. medRxiv. https://doi.org/10.1101/2020.04.08.20057794
View Article : Google Scholar
|
|
67
|
Miles EA, Rees D, Banerjee T, Cazzola R,
Lewis S, Wood R, Oates R, Tallant A, Cestaro B, Yaqoob P, et al:
Age-related increases in circulating inflammatory markers in men
are independent of BMI, blood pressure and blood lipid
concentrations. Atherosclerosis. 196:298–305. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Crooke SN, Ovsyannikova IG, Poland GA and
Kennedy RB: Immunosenescence: A systems-level overview of immune
cell biology and strategies for improving vaccine responses. Exp
Gerontol. 124:1106322019. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Leonarduzzi G, Gamba P, Gargiulo S, Biasi
F and Poli G: Inflammation-related gene expression by lipid
oxidation-derived products in the progression of atherosclerosis.
Free Radic Biol Med. 52:19–34. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Berzins SP, Uldrich AP, Sutherland JS,
Gill J, Miller JF, Godfrey DI and Boyd RL: Thymic regeneration:
Teaching an old immune system new tricks. Trends Mol Med.
8:469–476. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Maggini S, Pierre A and Calder PC: Immune
function and micronutrient requirements change over the life
course. Nutrients. 10:E15312018. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Salanitro AH, Ritchie CS, Hovater M, Roth
DL, Sawyer P, Locher JL, Bodner E, Brown CJ and Allman RM:
Inflammatory biomarkers as predictors of hospitalization and death
in community-dwelling older adults. Arch Gerontol Geriatr.
54:e387–e391. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Wadhwa PD, Buss C, Entringer S and Swanson
JM: Developmental origins of health and disease: Brief history of
the approach and current focus on epigenetic mechanisms. Semin
Reprod Med. 27:358–368. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Jirtle RL and Skinner MK: Environmental
epigenomics and disease susceptibility. Nat Rev Genet. 8:253–262.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Evans AC, Papachristou GI and Whitcomb DC:
Obesity and the risk of severe acute pancreatitis. Minerva
Gastroenterol Dietol. 56:169–179. 2010.PubMed/NCBI
|
|
76
|
Alam I, Ng TP and Larbi A: Does
inflammation determine whether obesity is metabolically healthy or
unhealthy? The aging perspective. Mediators Inflamm.
2012:4564562012. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Sharabiani MT, Vermeulen R, Scoccianti C,
Hosnijeh FS, Minelli L, Sacerdote C, Palli D, Krogh V, Tumino R,
Chiodini P, et al: Immunologic profile of excessive body weight.
Biomarkers. 16:243–251. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Elias CF, Aschkenasi C, Lee C, Kelly J,
Ahima RS, Bjorbaek C, Flier JS, Saper CB and Elmquist JK: Leptin
differentially regulates NPY and POMC neurons projecting to the
lateral hypothalamic area. Neuron. 23:775–786. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Forny-Germano L, De Felice FG and Vieira
MNDN: The role of leptin and adiponectin in obesity-associated
cognitive decline and Alzheimer's disease. Front Neurosci.
12:10272019. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Honce R and Schultz-Cherry S: Impact of
obesity on influenza A virus pathogenesis, immune response, and
evolution. Front Immunol. 10:10712019. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Katsikantami I, Sifakis S, Tzatzarakis MN,
Vakonaki E, Kalantzi OI, Tsatsakis AM and Rizos AK: A global
assessment of phthalates burden and related links to health
effects. Environ Int. 97:212–236. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Burgio E, Lopomo A and Migliore L: Obesity
and diabetes: From genetics to epigenetics. Mol Biol Rep.
42:799–818. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Katsarou MS, Karathanasopoulou A,
Andrianopoulou A, Desiniotis V, Tzinis E, Dimitrakis E, Lagiou M,
Charmandari E, Aschner M, Tsatsakis AM, et al: Beta 1, Beta 2 and
Beta 3 Adrenergic receptor gene polymorphisms in a Southeastern
European population. Front Genet. 9:5602018. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Eliakim A, Schwindt C, Zaldivar F, Casali
P and Cooper DM: Reduced tetanus antibody titers in overweight
children. Autoimmunity. 39:137–141. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Neuendorf R, Corn J, Hanes D and Bradley
R: Impact of food immunoglobulin G-based elimination diet on
subsequent food immunoglobulin G and quality of life in
overweight/obese adults. J Altern Complement Med. 25:241–248. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Poutahidis T, Kleinewietfeld M, Smillie C,
Levkovich T, Perrotta A, Bhela S, Varian BJ, Ibrahim YM, Lakritz
JR, Kearney SM, et al: Microbial reprogramming inhibits Western
diet-associated obesity. PLoS One. 8:e685962013. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Winer S, Paltser G, Chan Y, Tsui H,
Engleman E, Winer D and Dosch HM: Obesity predisposes to Th17 bias.
Eur J Immunol. 39:2629–2635. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Skalny AV, Rink L, Ajsuvakova OP, Aschner
M, Gritsenko VA, Alekseenko SI, Svistunov AA, Petrakis D, Spandidos
DA, Aaseth J, et al: Zinc and respiratory tract infections:
Perspectives for COVID-19 (Review). Int J Mol Med. Apr
14–2020.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Sumarac-Dumanovic M, Stevanovic D, Ljubic
A, Jorga J, Simic M, Stamenkovic-Pejkovic D, Starcevic V, Trajkovic
V and Micic D: Increased activity of interleukin-23/interleukin-17
proinflammatory axis in obese women. Int J Obes. 33:151–156. 2009.
View Article : Google Scholar
|
|
90
|
Kahn SE, Zinman B, Haffner SM, O'Neill MC,
Kravitz BG, Yu D, Freed MI, Herman WH, Holman RR, Jones NP, et al
ADOPT Study Group, : Obesity is a major determinant of the
association of C-reactive protein levels and the metabolic syndrome
in type 2 diabetes. Diabetes. 55:2357–2364. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Wittekindt OH: Tight junctions in
pulmonary epithelia during lung inflammation. Pflugers Arch.
469:135–147. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Ahmed M and Gaffen SL: IL-17 in obesity
and adipogenesis. Cytokine Growth Factor Rev. 21:449–453. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Zambrano-Zaragoza JF, Romo-Martínez EJ,
Durán-Avelar MJ, García-Magallanes N and Vibanco-Pérez N: Th17
cells in autoimmune and infectious diseases. Int J Inflamm.
2014:6515032014. View Article : Google Scholar
|
|
94
|
Bermejo-Martin JF, Ortiz de Lejarazu R,
Pumarola T, Rello J, Almansa R, Ramírez P, Martin-Loeches I,
Varillas D, Gallegos MC, Serón C, et al: Th1 and Th17
hypercytokinemia as early host response signature in severe
pandemic influenza. Crit Care. 13:R2012009. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Strissel KJ, DeFuria J, Shaul ME, Bennett
G, Greenberg AS and Obin MS: T-cell recruitment and Th1
polarization in adipose tissue during diet-induced obesity in
C57BL/6 mice. Obesity (Silver Spring). 18:1918–1925. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Zhou Y, Fu B, Zheng X, Wang D, Zhao C, Qi
Y, Sun R, Tian Z, Xu X and Wei H: Pathogenic T cells and
inflammatory monocytes incite inflammatory storm in severe COVID-19
patients. Natl Sci Rev. doi:10.1093/nsr/nwaa041.
|
|
97
|
Fang L, Karakiulakis G and Roth M: Are
patients with hypertension and diabetes mellitus at increased risk
for COVID-19 infection? Lancet Respir Med. 8:e212020. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Favalli EG, Ingegnoli F, De Lucia O,
Cincinelli G, Cimaz R and Caporali R: COVID-19 infection and
rheumatoid arthritis: Faraway, so close! Autoimmun Rev.
19(102523)2020.
|
|
99
|
Andersen LB, Przybyl L, Haase N, von
Versen-Höynck F, Qadri F, Jørgensen JS, Sorensen GL, Fruekilde P,
Poglitsch M, Szijarto I, et al: Vitamin D depletion aggravates
hypertension and target-organ damage. J Am Heart Assoc.
4:e0014172015. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Santos RA, Ferreira AJ, Verano-Braga T and
Bader M: Angiotensin-converting enzyme 2, angiotensin-(1–7) and
Mas: New players of the renin-angiotensin system. J Endocrinol.
216:R1–R17. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Ames BN: Prolonging healthy aging:
Longevity vitamins and proteins. Proc Natl Acad Sci USA.
115:10836–10844. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Costa C, Tsatsakis A, Mamoulakis C,
Teodoro M, Briguglio G, Caruso E, Tsoukalas D, Margina D, Dardiotis
E, Kouretas D, et al: Current evidence on the effect of dietary
polyphenols intake on chronic diseases. Food Chem Toxicol.
110:286–299. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Hernandez JP, Mota LC and Baldwin WS:
Activation of CAR and PXR by dietary, environmental and
occupational chemicals alters drug metabolism, intermediary
metabolism, and cell proliferation. Curr Pharmacogenomics Person
Med. 7:81–105. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Krzyżowska M, Wincenciak M, Winnicka A,
Baranowski A, Jaszczak K, Zimny J and Niemiałtowski M: The effect
of multigenerational diet containing genetically modified triticale
on immune system in mice. Pol J Vet Sci. 13:423–430.
2010.PubMed/NCBI
|
|
105
|
Lack G: Clinical risk assessment of GM
foods. Toxicol Lett. 127:337–340. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
van Herpen NA and Schrauwen-Hinderling VB:
Lipid accumulation in non-adipose tissue and lipotoxicity. Physiol
Behav. 94:231–241. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Li C, Ford ES, Mokdad AH, Balluz LS, Brown
DW and Giles WH: Clustering of cardiovascular disease risk factors
and health-related quality of life among US adults. Value Health.
11:689–699. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Garbarino J and Sturley SL: Saturated with
fat: New perspectives on lipotoxicity. Curr Opin Clin Nutr Metab
Care. 12:110–116. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Bosma M, Kersten S, Hesselink MK and
Schrauwen P: Re-evaluating lipotoxic triggers in skeletal muscle:
Relating intramyocellular lipid metabolism to insulin sensitivity.
Prog Lipid Res. 51:36–49. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Unger RH and Scherer PE: Gluttony, sloth
and the metabolic syndrome: A roadmap to lipotoxicity. Trends
Endocrinol Metab. 21:345–352. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Sies H: Oxidative Stress. (1st). Academic
Press. 1985.
|
|
112
|
Jones DP: Redefining oxidative stress.
Antioxid Redox Signal. 8:1865–1879. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Benedetti A, Comporti M and Esterbauer H:
Identification of 4-hydroxynonenal as a cytotoxic product
originating from the peroxidation of liver microsomal lipids.
Biochim Biophys Acta. 620:281–296. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Hauck AK and Bernlohr DA: Oxidative stress
and lipotoxicity. J Lipid Res. 57:1976–1986. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Barrera G, Pizzimenti S, Ciamporcero ES,
Daga M, Ullio C, Arcaro A, Cetrangolo GP, Ferretti C, Dianzani C,
Lepore A and Gentile F: Role of 4-hydroxynonenal-protein adducts in
human diseases. Antioxid Redox Signal. 22:1681–1702. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Grimsrud PA, Picklo MJ Sr, Griffin TJ and
Bernlohr DA: Carbonylation of adipose proteins in obesity and
insulin resistance: Identification of adipocyte fatty acid-binding
protein as a cellular target of 4-hydroxynonenal. Mol Cell
Proteomics. 6:624–637. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Wagner TM, Mullally JE and Fitzpatrick FA:
Reactive lipid species from cyclooxygenase-2 inactivate tumor
suppressor LKB1/STK11: Cyclopentenone prostaglandins and
4-hydroxy-2-nonenal covalently modify and inhibit the AMP-kinase
kinase that modulates cellular energy homeostasis and protein
translation. J Biol Chem. 281:2598–2604. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Barreiro E, del Puerto-Nevado L,
Puig-Vilanova E, Pérez-Rial S, Sánchez F, Martínez-Galán L, Rivera
S, Gea J, González-Mangado N and Peces-Barba G: Cigarette
smoke-induced oxidative stress in skeletal muscles of mice. Respir
Physiol Neurobiol. 182:9–17. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Castegna A, Aksenov M, Aksenova M,
Thongboonkerd V, Klein JB, Pierce WM, Booze R, Markesbery WR and
Butterfield DA: Proteomic identification of oxidatively modified
proteins in Alzheimer's disease brain. Part I: Creatine kinase BB,
glutamine synthase, and ubiquitin carboxy-terminal hydrolase L-1.
Free Radic Biol Med. 33:562–571. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Dalle-Donne I, Rossi R, Giustarini D,
Milzani A and Colombo R: Protein carbonyl groups as biomarkers of
oxidative stress. Clin Chim Acta. 329:23–38. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Pirinccioglu AG, Gökalp D, Pirinccioglu M,
Kizil G and Kizil M: Malondialdehyde (MDA) and protein carbonyl
(PCO) levels as biomarkers of oxidative stress in subjects with
familial hypercholesterolemia. Clin Biochem. 43:1220–1224. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Leonarduzzi G, Chiarpotto E, Biasi F and
Poli G: 4-Hydroxynonenal and cholesterol oxidation products in
atherosclerosis. Mol Nutr Food Res. 49:1044–1049. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Zarkovic K: 4-hydroxynonenal and
neurodegenerative diseases. Mol Aspects Med. 24:293–303. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Kassir R: Risk of COVID-19 for patients
with obesity. Obes Rev. Apr 13–2020.(Epub ahead of print).
View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Zhu Y, Ren C, Zhang M and Zhong Y:
Perilipin 5 reduces oxidative damage associated with lipotoxicity
by activating the PI3K/ERK-mediated Nrf2-ARE signaling pathway in
INS-1 pancreatic β-cells. Front Endocrinol (Lausanne). 11:1662020.
View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Misumi I, Starmer J, Uchimura T, Beck MA,
Magnuson T and Whitmire JK: Obesity expands a distinct population
of T cells in adipose tissue and increases vulnerability to
infection. Cell Rep. 27:514–524.e5. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Bourgeois C, Gorwood J, Barrail-Tran A,
Lagathu C, Capeau J, Desjardins D, Le Grand R, Damouche A, Béréziat
V and Lambotte O: Specific biological features of adipose tissue,
and their impact on HIV persistence. Front Microbiol. 10:28372019.
View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Ryan PM and Caplice NM: Is adipose tissue
a reservoir for viral spread, immune activation and cytokine
amplification in COVID-19. Obesity (Silver Spring). Apr
21–2020.(Epub ahead of print). View Article : Google Scholar
|
|
129
|
Venkata C, Sampathkumar P and Afessa B:
Hospitalized patients with 2009 H1N1 influenza infection: The Mayo
Clinic experience. Mayo Clin Proc. 85:798–805. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Louie JK, Acosta M, Winter K, Jean C,
Gavali S, Schechter R, Vugia D, Harriman K, Matyas B, Glaser CA, et
al California Pandemic (H1N1) Working Group, : Factors associated
with death or hospitalization due to pandemic 2009 influenza
A(H1N1) infection in California. JAMA. 302:1896–1902. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Thompson DL, Jungk J, Hancock E, Smelser
C, Landen M, Nichols M, Selvage D, Baumbach J and Sewell M: Risk
factors for 2009 pandemic influenza A (H1N1)-related
hospitalization and death among racial/ethnic groups in New Mexico.
Am J Public Health. 101:1776–1784. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Dietz W and Santos-Burgoa C: Obesity and
its implications for COVID-19 mortality. Obesity (Silver Spring).
Apr 1–2020.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Sun Y, Wang Q, Yang G, Lin C, Zhang Y and
Yang P: Weight and prognosis for influenza A(H1N1)pdm09 infection
during the pandemic period between 2009 and 2011: A systematic
review of observational studies with meta-analysis. Infect Dis
(Lond). 48:813–822. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Liu M, He P, Liu HG, Wang XJ, Li FJ, Chen
S, Lin J, Chen P, Liu JH and Li CH: Clinical characteristics of 30
medical workers infected with new coronavirus pneumonia. Zhonghua
Jie He He Hu Xi Za Zhi. 43(E016)2020.(In Chinese).
|
|
135
|
Peng YD, Meng K, Guan HQ, Leng L, Zhu RR,
Wang BY, He MA, Cheng LX, Huang K and Zeng QT: Clinical
characteristics and outcomes of 112 cardiovascular disease patients
infected by 2019-nCoV. Zhonghua Xin Xue Guan Bing Za Zhi.
48(E004)2020.(In Chinese).
|
|
136
|
Verdecchia P, Cavallini C, Spanevello A
and Angeli F: The pivotal link between ACE2 deficiency and
SARS-CoV-2 infection. Eur J Intern Med. Apr 20–2020.(Epub ahead of
print). View Article : Google Scholar
|
|
137
|
Hoffmann M, Kleine-Weber H, Schroeder S,
Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH,
Nitsche A, et al: SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2
and is blocked by a clinically proven protease inhibitor. Cell.
181:271–280.e8. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Hussain M, Jabeen N, Raza F, Shabbir S,
Baig AA, Amanullah A and Aziz B: Structural variations in human
ACE2 may influence its binding with SARS-CoV-2 spike protein. J Med
Virol. Apr 6–2020.(Epub ahead of print). View Article : Google Scholar
|
|
139
|
Santos RA and Ferreira AJ:
Angiotensin-(1–7) and the renin-angiotensin system. Curr Opin
Nephrol Hypertens. 16:122–128. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Cavalera M, Wang J and Frangogiannis NG:
Obesity, metabolic dysfunction, and cardiac fibrosis:
Pathophysiological pathways, molecular mechanisms, and therapeutic
opportunities. Transl Res. 164:323–335. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Senador D, Kanakamedala K, Irigoyen MC,
Morris M and Elased KM: Cardiovascular and autonomic phenotype of
db/db diabetic mice. Exp Physiol. 94:648–658. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Alpert MA, Omran J, Mehra A and Ardhanari
S: Impact of obesity and weight loss on cardiac performance and
morphology in adults. Prog Cardiovasc Dis. 56:391–400. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Touyz RM, Savoia C, He Y, Endemann D, Pu
Q, Ko EA, Deciuceis C, Montezano A and Schiffrin EL: Increased
inflammatory biomarkers in hypertensive type 2 diabetic patients:
Improvement after angiotensin II type 1 receptor blockade. J Am Soc
Hypertens. 1:189–199. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Lumeng CN and Saltiel AR: Inflammatory
links between obesity and metabolic disease. J Clin Invest.
121:2111–2117. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
World Health Organization (WHO), . Advice
on the use of masks in the context of COVID-19: interim guidance.
6–April;2020.WHO. (Geneva). 2020.
|
|
146
|
World Health Organization (WHO), .
Rational use of personal protective equipment for coronavirus
disease (COVID-19) and considerations during severe shortages:
interim guidance. 6–April;2020.WHO. (Geneva). 2020.
|
|
147
|
Marcus Y, Shefer G and Stern N: Adipose
tissue renin-angiotensin-aldosterone system (RAAS) and progression
of insulin resistance. Mol Cell Endocrinol. 378:1–14. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Jose RJ and Manuel A: Does COVID-19
disprove the obesity paradox in ARDS? Obesity (Silver Spring). Apr
15–2020.(Epub ahead of print). PubMed/NCBI
|
|
149
|
O'Brien JM Jr, Phillips GS, Ali NA,
Lucarelli M, Marsh CB and Lemeshow S: Body mass index is
independently associated with hospital mortality in mechanically
ventilated adults with acute lung injury. Crit Care Med.
34:738–744. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Fernandez-Bustamante A and Repine J:
Adipose-lung cell crosstalk in the obesity-ARDS paradox. J Pulm
Respir Med. 3:1442013.
|
|
151
|
Nie W, Zhang Y, Jee SH, Jung KJ, Li B and
Xiu Q: Obesity survival paradox in pneumonia: A meta-analysis. BMC
Med. 12:612014. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Stefan N, Birkenfeld AL, Schulze MB and
Ludwig DS: Obesity and impaired metabolic health in patients with
COVID-19. Nat Rev Endocrinol. Apr 23–2020.(Epub ahead of print).
View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Akoumianakis I, Akawi N and Antoniades C:
Exploring the crosstalk between adipose tissue and the
cardiovascular system. Korean Circ J. 47:670–685. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Simonnet A, Chetboun M, Poissy J, Raverdy
V, Noulette J, Duhamel A, Labreuche J, Mathieu D, Pattou F and
Jourdain M; Lille Intensive Care COVID-19 and Obesity study Group,
: High prevalence of obesity in severe acute respiratory syndrome
coronavirus-2 (SARS-CoV-2) requiring invasive mechanical
ventilation. Obesity (Silver Spring). Apr 9–2020.(Epub ahead of
print). View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Lighter J, Phillips M, Hochman S, Sterling
S, Johnson D, Francois F and Stachel A: Obesity in patients younger
than 60 years is a risk factor for Covid-19 hospital admission.
Clin Infect Dis ciaa. 415:2020.
|
|
156
|
Lederman MM: Cell-mediated immunity and
pregnancy. Chest. 86 (Suppl 3):6S–9S. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Harris JW: Influenza occurring in pregnant
women: a statistical study of thirteen hundred and fifty cases.
JAMA. 72:978–980. 1919. View Article : Google Scholar
|
|
158
|
Greenberg M, Jacobziner H, Pakter J and
Weisl BA: Maternal mortality in the epidemic of Asian influenza,
New York City, 1957. Am J Obstet Gynecol. 76:897–902. 1958.
View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Klein SL, Passaretti C, Anker M, Olukoya P
and Pekosz A: The impact of sex, gender and pregnancy on 2009 H1N1
disease. Biol Sex Differ. 1:52010. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Harger JH, Ernest JM, Thurnau GR, Moawad
A, Momirova V, Landon MB, Paul R, Miodovnik M, Dombrowski M, Sibai
B, et al; National Institute of Child Health and Human Development,
Network of Maternal-Fetal Medicine Units, . Risk factors and
outcome of varicella-zoster virus pneumonia in pregnant women. J
Infect Dis. 185:422–427. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
161
|
Braden CR: Listeriosis. Pediatr Infect Dis
J. 22:745–746. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Shulman CE and Dorman EK: Importance and
prevention of malaria in pregnancy. Trans R Soc Trop Med Hyg.
97:30–35. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Stockman LJ, Lowther SA, Coy K, Saw J and
Parashar UD: SARS during pregnancy, United States. Emerg Infect
Dis. 10:1689–1690. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Neuzil KM, Reed GW, Mitchel EF, Simonsen L
and Griffin MR: Impact of influenza on acute cardiopulmonary
hospitalizations in pregnant women. Am J Epidemiol. 148:1094–1102.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Irving WL, James DK, Stephenson T, Laing
P, Jameson C, Oxford JS, Chakraverty P, Brown DW, Boon AC and
Zambon MC: Influenza virus infection in the second and third
trimesters of pregnancy: A clinical and seroepidemiological study.
BJOG. 107:1282–1289. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Moynihan AT, Hehir MP, Glavey SV, Smith TJ
and Morrison JJ: Inhibitory effect of leptin on human uterine
contractility in vitro. Am J Obstet Gynecol. 195:504–509. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Mor G and Cardenas I: The immune system in
pregnancy: A unique complexity. Am J Reprod Immunol. 63:425–433.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Kraus TA, Engel SM, Sperling RS, Kellerman
L, Lo Y, Wallenstein S, Escribese MM, Garrido JL, Singh T, Loubeau
M, et al: Characterizing the pregnancy immune phenotype: Results of
the viral immunity and pregnancy (VIP) study. J Clin Immunol.
32:300–311. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Rodríguez-García M, Oliva H, Climent N,
García F, Gatell JM and Gallart T: Human immature monocyte-derived
dendritic cells produce and secrete alpha-defensins 1–3. J Leukoc
Biol. 82:1143–1146. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Wegmann TG, Lin H, Guilbert L and Mosmann
TR: Bidirectional cytokine interactions in the maternal-fetal
relationship: Is successful pregnancy a TH2 phenomenon? Immunol
Today. 14:353–356. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
White SH, Wimley WC and Selsted ME:
Structure, function, and membrane integration of defensins. Curr
Opin Struct Biol. 5:521–527. 1995. View Article : Google Scholar : PubMed/NCBI
|