Introduction
Sevoflurane is the most commonly used inhalant
anesthetic in pediatric surgery (1); however, it exerts neurotoxic effects
on the developing brain (2).
Clinical studies have reported that children with long-time or
multiple exposures to general anesthesia and surgery before 3–4
years of age may have an increased risk of learning and memory
disability (1,3). In addition, numerous studies have
reported that the use of inhalant anesthetics such as sevoflurane
can cause cognitive impairment, brain structural changes,
neuroinflammation, apoptosis and synaptic deficits in animal or
cell models (4–8). Since millions of neonates and
children worldwide require surgery under anesthesia each year
(9), exploring the mechanisms
underlying sevoflurane-induced neurotoxicity in the developing
brain and finding an approach to alleviate the postoperative
neurocognitive disorder in children are not only of general
scientific interest, but may also have substantial impact on public
health.
Apolipoprotein E (ApoE), a 35-kDa glycoprotein, is a
type of lipoprotein component important in controlling lipoprotein
metabolism and cholesterol homeostasis (10). Previous studies have suggested that
ApoE may serve a protective role in neuronal activity and injury
repair in the central nervous system (10,11).
However, specific neurotoxic ApoE fragments, such as an 18 kDa
N-terminal fragment, have been reported to be directly associated
with the formation of amyloid-β plaques, tau hyperphosphorylation,
neurofibrillary triangle formation, and the initiation of
neuroinflammation and neurodegeneration in patients with
Alzheimer's disease (AD) (11–13).
In addition, several studies have also demonstrated that tau
protein hyperphosphorylation and neuroinflammation enhancement may
be the main reason for anesthesia-induced cognitive impairment in
young mice (5,6,14).
However, whether ApoE and its fragments contribute to tau
phosphorylation and neuroinflammation following general anesthesia
remains to be investigated.
Coenzyme Q10 (CoQ10), a strong antioxidant and
mitochondrial energy storage agent, has neuroprotective, energy
conversion, anti-inflammatory and anti-oxidative effects (15,16).
Xu et al (17) reported
that an intraperitoneal injection of CoQ10 prior to anesthesia
reduced sevoflurane-induced mitochondrial dysfunction and cognitive
deficiency in 6-day-old mice. However, the specific underlying
mechanism remains unknown.
The present study aimed to reveal the role of ApoE
in the pathogenesis of tau protein hyperphosphorylation and
neuroinflammation induced by sevoflurane anesthesia, as well as the
protective mechanism of CoQ10 in an anesthetic sevoflurane
treatment model of primary mouse hippocampal neurons.
Materials and methods
Primary neuron culture with serum free
medium and treatment
All experimental protocols in the present study were
approved by the Animal Experimental Ethics Committee of Tianjin
Medical University General Hospital (Tianjin, China; approval no.
2018-X6-11). Pregnant C57BL/6J mice (2-month old, weight 20–25 g,
n=5) were purchased from Beijing Huafukang Biotechnology Co., Ltd.
and were housed under a 12-h light/dark cycle with food and water
provided ad libitum. The room temperature was 22–24°C and the
humidity 40–50%. The mice were decapitated on gestation day 15, and
the embryos were removed and soaked in 75% ethanol for 1 min. The
hippocampal tissue of the fetal mice was removed intact, repeatedly
cut into fragments with scissors and transferred to a petri dish
containing DMEM (Gibco; Thermo Fisher Scientific, Inc.). Next,
0.25% trypsin was added to the petri dish for digestion in a 37°C
thermostat for 30 min. The tissue fragments and digestive juices
were transferred to a centrifuge tube, and an equivalent volume of
digestive stop fluid (DMEM+10% FBS; Gibco; Thermo Fisher
Scientific, Inc.) was added and mixed gently for 15 min.
Subsequently, the mixture was strained through a 60-µm sieve. After
1 min, the supernatant containing a single-cell suspension was
collected and mixed with a digestive stop fluid (DMEM+10% FBS;
Gibco; Thermo Fisher Scientific, Inc.). Cells were counted under a
microscope to ensure a density of 7.0×106 cells/ml.
Then, 1.5 ml of cell and digestive stop fluid mixture was
inoculated into each well of a 6-well plate. After 4 h of culture,
neuron elongation was observed using serum free medium
(Neurobasal-A+2% B27; Engreen Biosystem Ltd.) in place of digestive
stop fluid. Neuron culture medium was replaced every 3 days.
Neurons cultured for 5 days were used in the
experiments. The neurons were randomly assigned into the following
groups: i) Control; ii) sevoflurane; iii) control+corn oil (the
vehicle of CoQ10); iv) sevoflurane+corn oil; v) control+CoQ10; and
vi) control+CoQ10. Each dish of neurons in the corn oil groups was
incubated with neuron culture medium mixed with 500 µl corn oil
overnight on day 4 of cell culture. For neurons in the CoQ10 group,
500 µg CoQ10 (dissolved in corn oil at 1.5 mg/ml) was added to each
dish on day 4 and incubated overnight at 37°C. Neurons in the
sevoflurane group were placed in a sealed resin box (20×15×7 cm)
and treated with 21% O2, 5% CO2 and 4.1%
sevoflurane (2X minimum alveolar concentration) for 4 h in a 37°C
incubator as described by Lu et al (5). The control groups were treated with
21% O2 and 5% CO2 for 4 h under the same
conditions. A variable anesthetic gas monitor (Vamos; Dräger
Medical AG & Co. KgaA) was used to continuously monitor the
concentration of O2, CO2 and sevoflurane
during anesthesia or control treatment.
Cell Counting Kit-8 (CCK-8) cell
viability assay
CCK-8 was used according to the manufacturer's
protocol. When sevoflurane or control treatment was finished, the
original culture medium in each well was replaced with 90 µl serum
free medium and 10 µl CCK8 reagent (cat. no. CK04; Dojindo
Molecular Technologies, Inc.) was added immediately, followed by
incubation at 37°C in the dark for 2 h. The absorbance value at 450
nm was measured with a VERSAMax microplate reader (Molecular
Devices, LLC) to determine the cell viability. The viability of the
control group was considered as 100%, and the results of the other
groups were calculated as the percentage of the control group.
Primary neuron harvesting
The neurons in different groups were collected for
biochemical tests immediately after anesthesia or control
treatment. The culture medium was removed, and the petri dishes
were washed three times with PBS. Then, 1 ml RIPA buffer (Beijing
Solarbio Science & Technology Co., Ltd.) was added, and the
neurons were scraped out carefully from the bottom of the petri
dish with a cell curette. Finally, the lysates were collected and
then centrifuged at 12,000 × g for 10 min at 4°C.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA extraction was performed by the RNA
TRIzol® isolation reagent kit (Invitrogen; Thermo Fisher
Scientific, Inc.). Total RNA concentration was determined using a
spectrophotometer. A total of 500 ng mRNA in each group was reverse
transcribed into cDNA with the All-in-One First-Strand cDNA
Synthesis kit (cat. no. AORT-0050; GeneCopoeia, Inc.), and
amplified by PCR with SYBR® Green qPCR Master Mix
(MedChemExpress LLC.) using an iQ™5 system (Bio-Rad Laboratories,
Inc.) under the following conditions: 5 min at 95°C and 40 cycles
of 15 s at 95°C and 60°C for 1 min. Relative mRNA levels were
calculated by the 2−∆∆Cq method (18). The primer sequences were as
follows: GAPDH forward, 5′-TGTAGACCATGTAGTTGAGGTCA-3′ and reverse,
5′-AGGTCGGTGTGAACGGATTTG-3′; and ApoE forward,
5′-GACCCAGCAAATACGCCTG-3′ and reverse,
5′-CATGTCTTCCACTATTGGCTCG-3′.
ELISA
The levels of total ApoE, inflammatory factors
interleukin (IL)-1β, IL-6 and tumor necrosis factor (TNF)-α,
superoxidase dismutase (SOD)1 and ATPase in the neurons of each
group were determined using mouse ELISA kits (ApoE, cat. no.
E-EL-M0135; IL1-β, cat. no. E-EL-M0037c; IL-6, cat. no.
E-EL-M0044c; TNF-α, cat. no. E-EL-M0049c; SOD1, cat. no.
E-EL-M2398c; and ATPase, cat. no. E-EL-M0813c; all from Elabscience
Biotechnology, Inc.). Briefly, 100 µl standard or neuron protein
was added to each well and incubated at 37°C for 90 min. Then, 100
µl specific antibody was added to each well and incubated at 37°C
for an additional 60 min. Subsequently, 100 µl enzyme conjugate was
added to each well and incubated at 37°C for 30 min. After five
washes, 90 µl substrate solution was added for 15 min, followed by
50 µl terminating solution to stop the reaction. Finally, a
fluorescence reader was used to measure the optical density (OD) of
each well at 450 nm.
Western blot analysis
Western blotting was used to detect the expression
of full-length ApoE, ApoE fragments, Tau5, AT8 and PHF1 in neurons.
Total protein was quantified using the a bicinchoninic acid protein
assay kit (Pierce; Thermo Fisher Scientific, Inc.).
Neuron protein (50 µg) per well was separated by
4–20% SDS-PAGE and transferred to a nitrocellulose membrane
(Bio-Rad Laboratories, Inc.). The membrane was blocked in 5%
skimmed milk powder in TBS for 2 h at 37°C and incubated overnight
at 4°C with primary antibodies against the following proteins:
Full-length ApoE and ApoE fragments (1:4,000; cat. no. 178479;
EMD/Merck KGaA), Tau5 (1:1,000; cat. no. ABN454; EMD Millipore),
AT8 (1:2,000; cat. no. MN1020; Thermo Fisher Scientific, Inc.),
PHF1 (1:1,000; cat. no. 3Ab184951; Abcam) and β-actin (1:2,000;
cat. no. A5060; Sigma-Aldrich; Merck KGaA). After rinsing with TBS,
a goat anti-mouse IgG (H+L) secondary antibody (1:5,000; cat. no.
31430; Invitrogen; Thermo Fisher Scientific, Inc.) was added and
incubated at 37°C for 1 h. The membrane was washed in TBS+0.2%
Tween-20 prior to adding the ECL reagent (cat. no. 34577;
Invitrogen; Thermo Fisher Scientific, Inc.). Then, the membrane was
scanned and photographed using a quantitative Gel Quantity One
system (FluorChem FC3, ProteinSimple Inc.). The expression level of
the target protein was calculated as the ratio of the integral
optical density of the target band to the β-actin band. For
comparison across experimental conditions, 100% of protein level
changes refer to the control levels for the purpose of comparison
to the experimental conditions.
Immunocytochemistry
Slides (2×2 cm) were pre-coated in the culture dish
prior to neuron culture. Following sevoflurane or control
treatment, the culture medium in the dishes was removed, and the
dishes were washed three times with pre-cooled PBS. Each well was
fixed with 1 ml 4% paraformaldehyde for 30 min at 37°C, washed five
times and treated with 0.3% Triton for 30 min at 37°C. The neurons
were blocked for 1 h in 10% goat serum (Gibco; Thermo Fisher
Scientific, Inc.) at 37°C. After removing any excess liquid,
anti-AT8 (1:1,000; cat. no. MN1020; Thermo Fisher Scientific, Inc.)
and anti-MAP2 (1:1,000; cat. no. EPR19691; Abcam) antibodies were
added to each well. The slides were placed in a wet box with PBS
and incubated overnight at 4°C. After washing three times with PBS,
Alexa Fluor® 488 goat anti-mouse IgG (1:500; cat. no.
A-11001; Thermo Fisher Scientific, Inc.) and Alexa
Fluor® 594 goat anti-rabbit IgG (1:500; cat. no.
A-11012; Thermo Fisher Scientific, Inc.) secondary antibodies were
added to the slides and incubated for 1 h at room temperature in
the dark. After washing the slides three times with PBS, the slides
were incubated with DAPI (cat. no. 104139; Abcam) in a humidified
dark chamber for 10 min at 37°C and subsequently washed five times.
Images (magnification, ×40) were observed and imaged captured under
a fluorescence microscope.
Statistical analysis
The data are presented as the mean ± standard
deviation. The number of samples was four per group in the RT-qPCR,
western blotting, ELISA and immunohistochemistry assays.
Statistical analysis was performed using GraphPad Prism software
version 5.0 (GraphPad Software, Inc.) and SPSS statistical software
version 21.0 (IBM Corp.). Unpaired Student's t-test (if the values
followed a Gaussian distribution) or Mann-Whitney test (if the
values did not follow a Gaussian distribution) was used to analyze
the difference between two groups in the biochemistry data.
P<0.05 was considered to indicate a statistically significant
difference.
Results
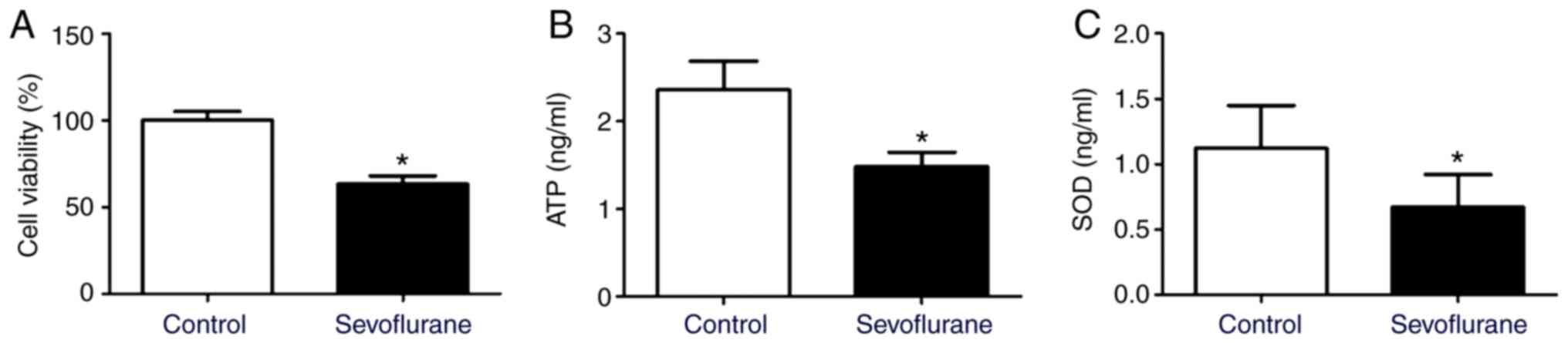
Sevoflurane anesthesia reduces cell
viability, ATP level and SOD contents in mouse hippocampal
neurons
Compared with the control group, the levels of ATP
and SOD, as well as the cell viability decreased significantly in
neurons after sevoflurane treatment (Fig. 1).
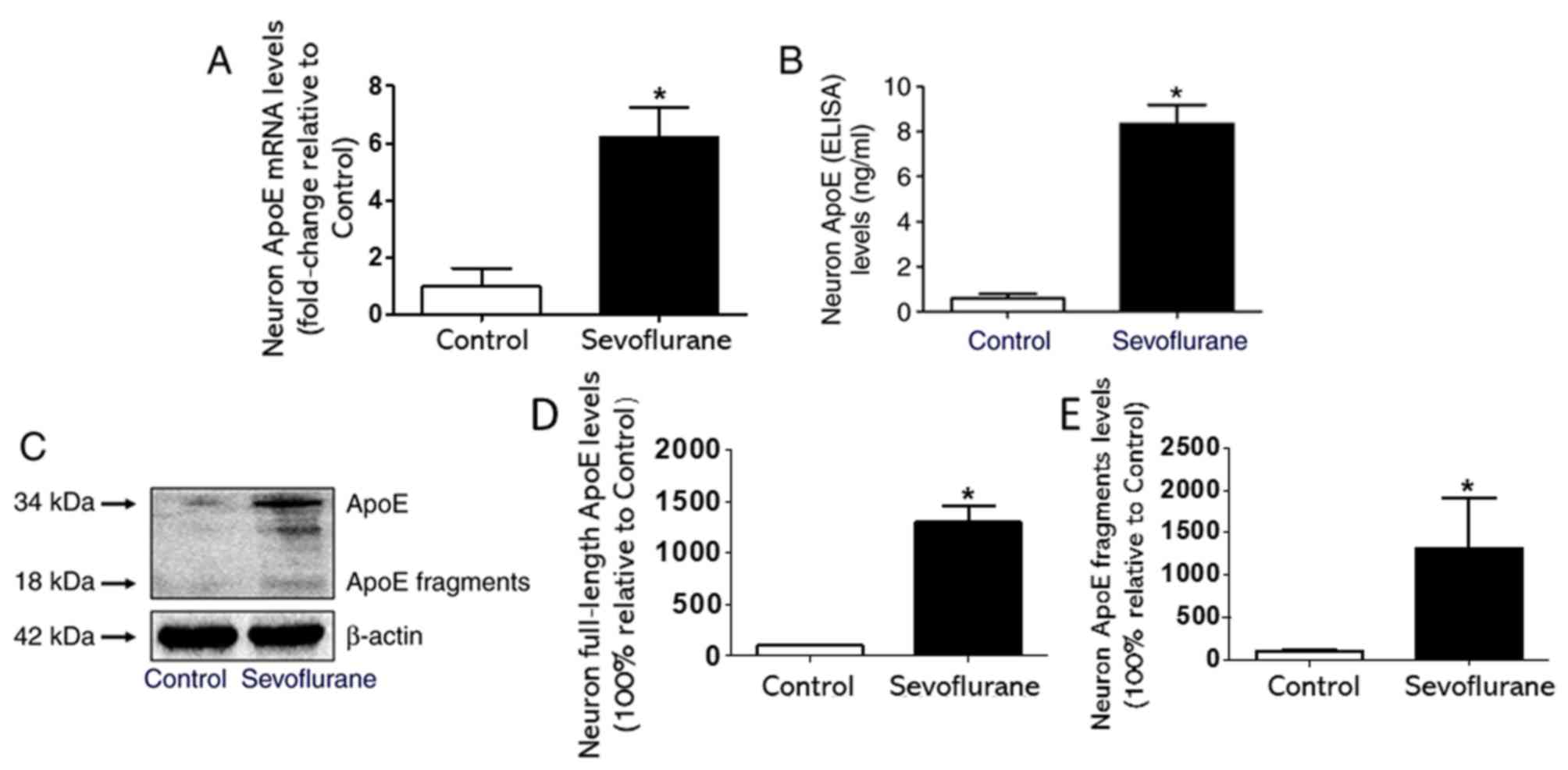
Sevoflurane anesthesia increases the
expression of ApoE and its toxic fragments in mouse hippocampal
neurons
The western blotting results revealed that compared
with the control group, the expression levels of full-length ApoE
and its 18-kDa toxic fragments increased significantly in the
sevoflurane group (Fig. 2C-E). In
addition, compared with the neurons in the control group, the
neurons treated with 4.1% sevoflurane for 4 h expressed higher
levels of ApoE mRNA (Fig. 2A). The
ELISA results indicated that the sevoflurane group expressed higher
levels of total ApoE compared with the control group (Fig. 2B).
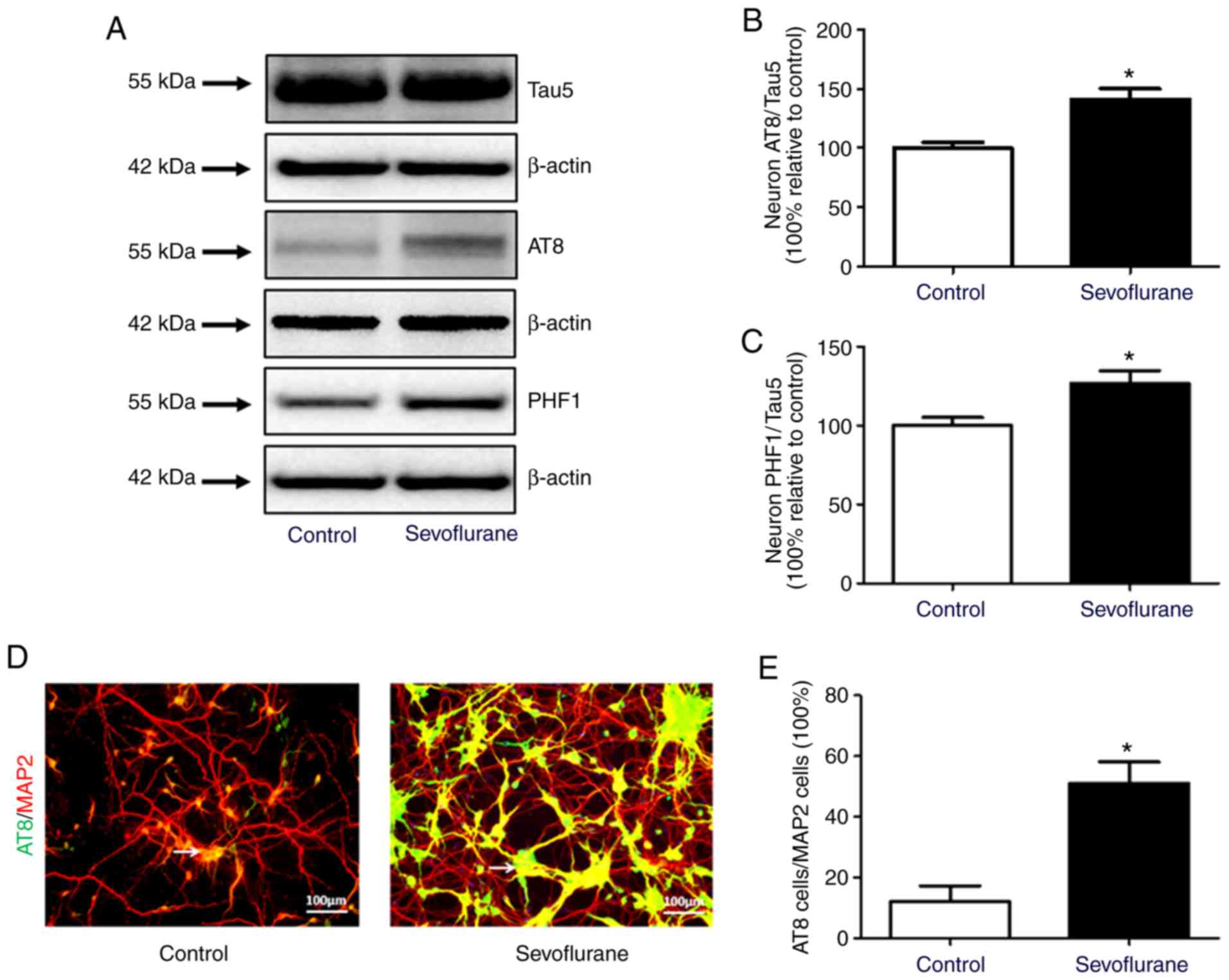
Sevoflurane anesthesia increases tau
phosphorylation in mouse hippocampal neurons
The levels of total tau (Tau5) and phosphorylated
tau (AT8 and PHF1) in neurons were compared between the control and
sevoflurane groups by western blotting. The results demonstrated
that the ratios of AT8 to Tau5 and PHF1 to Tau5 increased
significantly after sevoflurane treatment (Fig. 3A-C). The immunofluorescence results
also demonstrated that the ratio of AT8-positive to MAP2-positive
neurons in sevoflurane group was significantly higher compared with
the control group (Fig. 3).
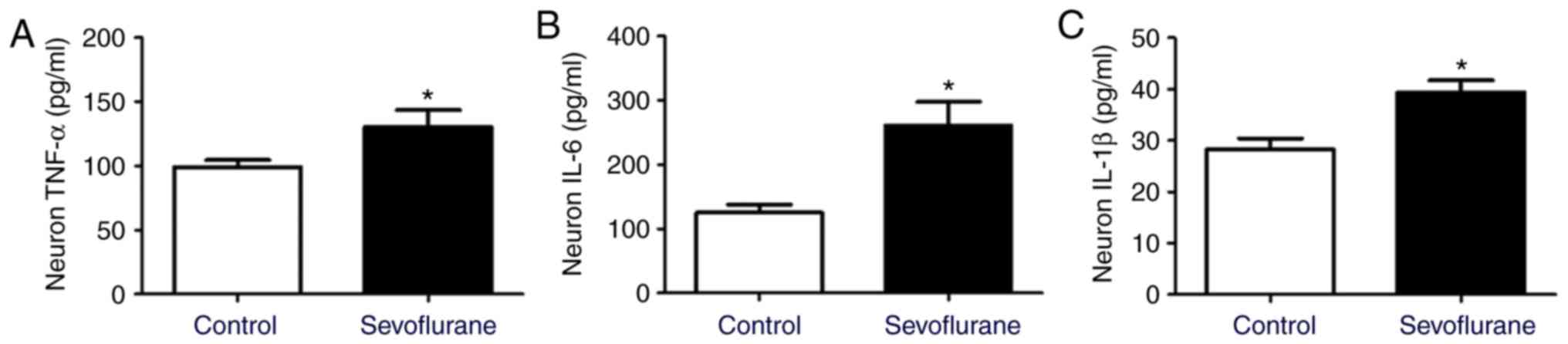
Sevoflurane anesthesia induces
neuroinflammation in mouse hippocampal neurons
ELISA was used to detect the expression of
inflammatory factors in neurons. The results demonstrated that the
levels of TNF-α, IL-6 and IL-1β in primary neurons were increased
in the sevoflurane group compared with the control group (Fig. 4).
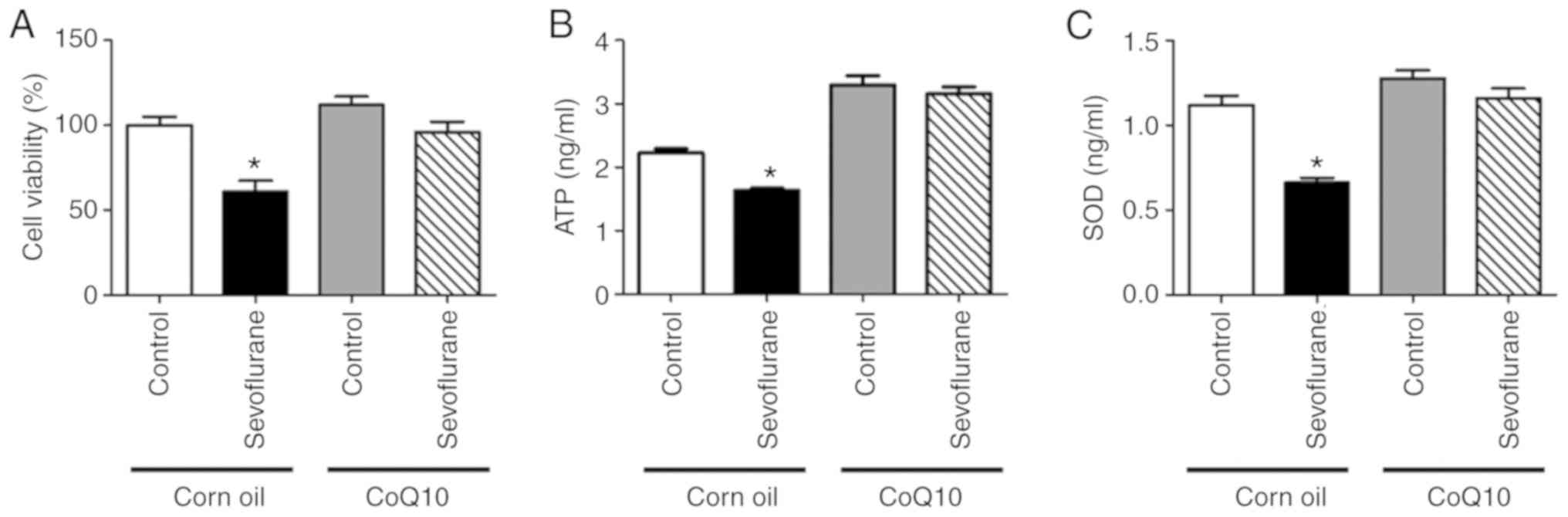
CoQ10 alleviates sevoflurane
anesthesia-induced ATP, SOD and cell viability decrease in mouse
hippocampal neurons
The contents of ATP and SOD were significantly
decreased in the sevoflurane+corn oil group neurons compared with
the control+corn oil group neurons (Fig. 5B and C). In addition, cell
viability in the sevoflurane+corn oil group neurons was reduced
compared with the control+corn oil group neurons (Fig. 5A). However, no significant
differences were observed in ATP levels, SOD expression or cell
viability between neurons in the sevoflurane+CoQ10 and the
control+CoQ10 groups (Fig. 5).
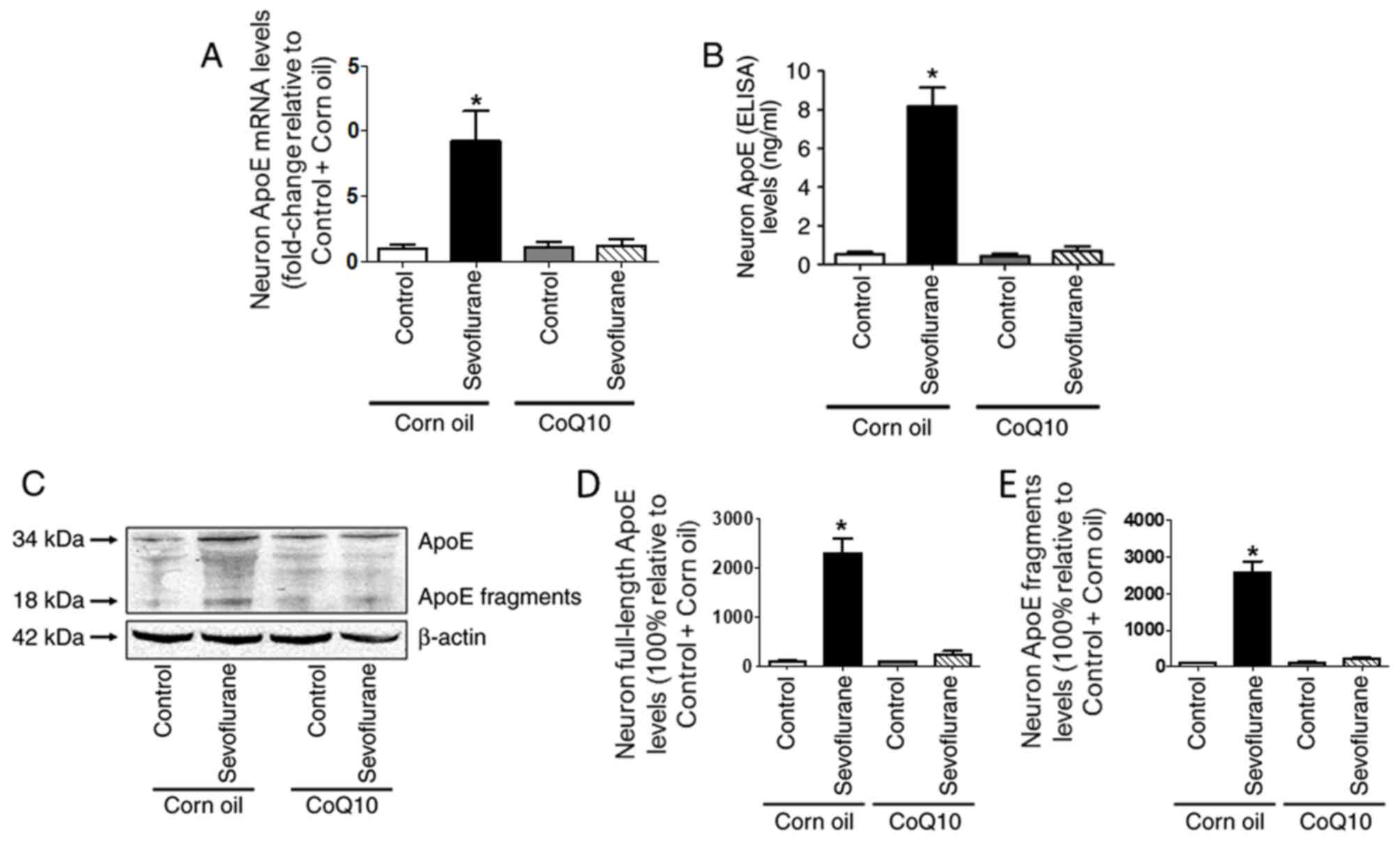
CoQ10 mitigates sevoflurane-induced
ApoE expression in mouse hippocampal neurons
The RT-qPCR and ELISA results demonstrated that ApoE
gene and protein levels were significantly higher in the
sevoflurane+corn oil group compared with those in the control+corn
oil group (Fig. 6A and B). In
addition, the western blotting results indicated that the
expression of full-length ApoE and its toxic fragments were
increased in the sevoflurane+corn oil group compared with the
control+corn oil group (Fig.
6C-E). However, after CoQ10 treatment, no significant
differences were observed in ApoE protein or mRNA levels between
the control and sevoflurane groups (Fig. 6).
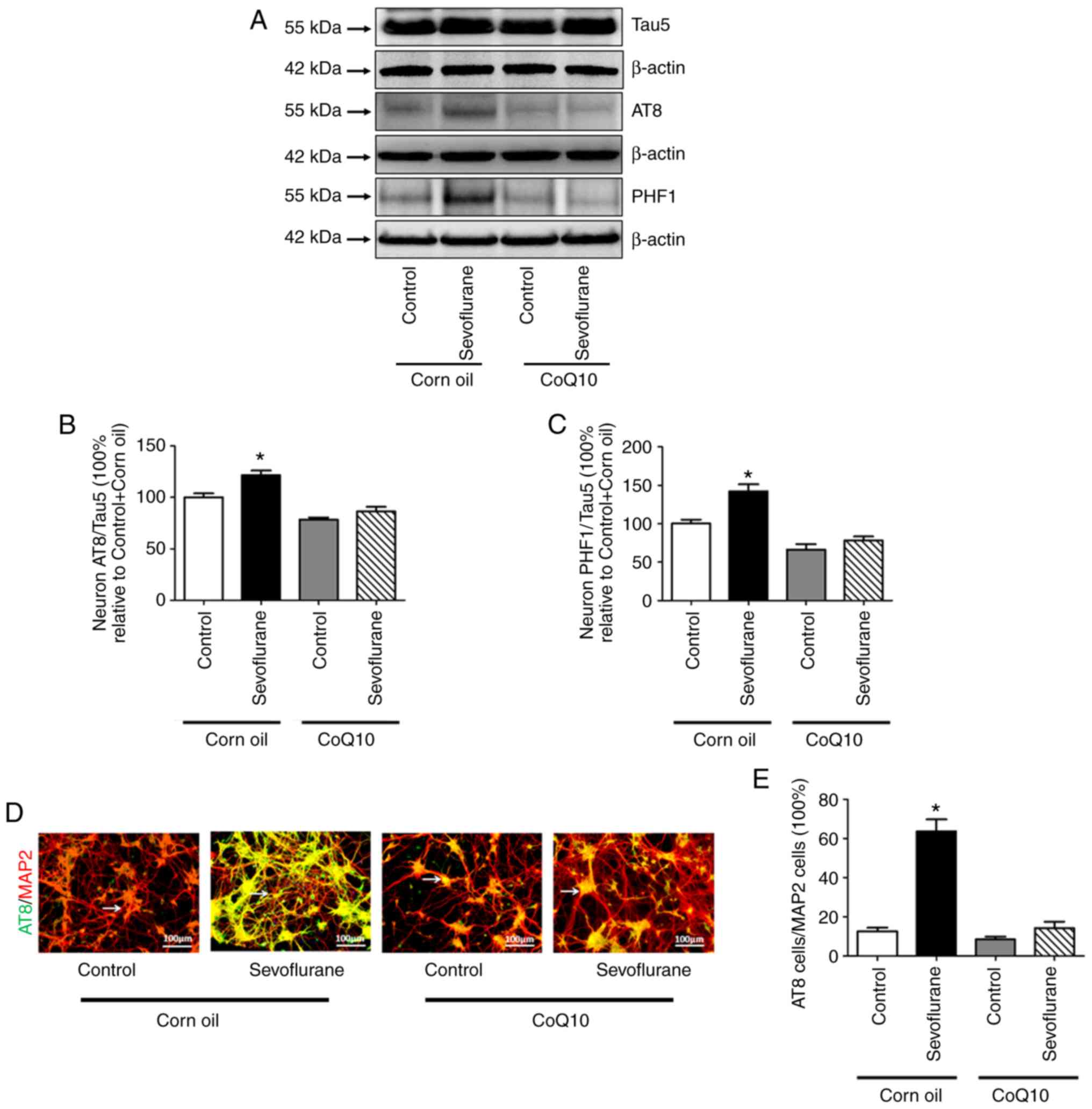
CoQ10 decreases sevoflurane-induced
tau phosphorylation in mouse hippocampal neurons
According to the western blotting results, no
significant differences were observed in the expression level of
Tau5 between the control+corn oil and the sevoflurane+corn oil
groups, whereas the ratios of AT8 to Tau5 and PHF1 to Tau5
increased significantly after sevoflurane treatment compared with
the control group. The immunofluorescence results demonstrated that
the ratio of AT8-positive to MAP2-positive neurons in
sevoflurane+corn oil group was significantly higher compared with
the control+corn oil group. (Fig. 7D
and E). No significant differences were observed in the above
indices between the control+CoQ10 group and the sevoflurane+CoQ10
group (Fig. 7).
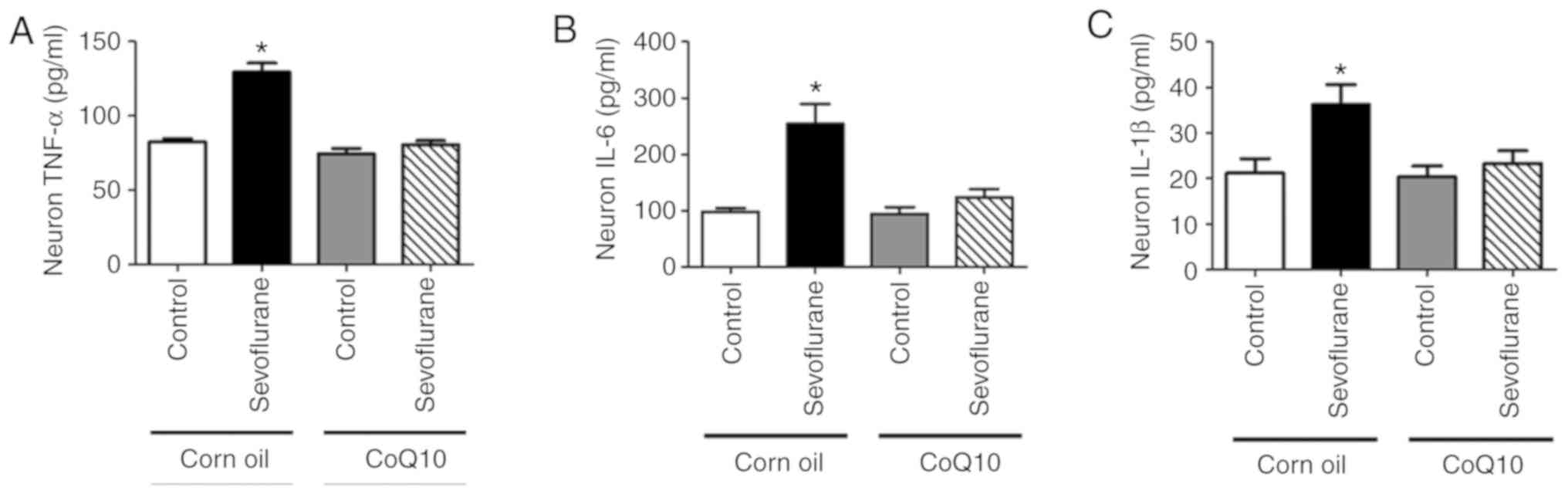
CoQ10 improves sevoflurane-induced
neuroinflammation in mouse hippocampal neurons
The ELISA results demonstrated that the levels of
TNF-α, IL-6 and IL-1β were increased in the sevoflurane+corn oil
group neurons compared with the control+corn oil group neurons
(Fig. 8). After CoQ10 treatment,
no significant differences were observed in inflammatory factor
secretion between the control+CoQ10 and sevoflurane+CoQ10 groups
(Fig. 8).
Discussion
Sevoflurane is the most commonly used inhalant
anesthetic in pediatric surgery (1). However, studies have reported that
repeated or long-term use of sevoflurane during induction and
maintenance of general anesthesia in children may increase the risk
of learning disability, thus greatly limiting the clinical
application of sevoflurane (19,20).
Lu et al (5)
demonstrated that 4.1% sevoflurane exposure for 4 h affected the
phosphorylation and neuroinflammatory activity of tau protein in
primary neurons cultured for 5 days, but the specific mechanism
remained unclear. In the present study, the cell model of Lu et
al (5) was used to investigate
the effects of high-concentration sevoflurane treatment on mouse
hippocampal neurons. The results demonstrated that the contents of
ATP and SOD, as well as cell viability decreased, whereas the
levels of tau phosphorylation and inflammatory factors in the
primary neurons were significantly increased after 4 h of
sevoflurane treatment compared with those in the control group.
ApoE is a polymorphic protein involved in the
transformation and metabolism of lipoproteins, and its gene can
regulate multiple biological functions including lipid metabolism
regulation and immune regulation (21). At present, the study of ApoE and
its gene polymorphism is one of the hot topics in medical research
(22). ApoE is considered to be a
risk factor for AD (23).
Generally, ApoE is expressed mainly by astrocytes in the brain, but
in the case of injury or oxidative stress, neurons also activate
the expression of ApoE to promote their repair (24,25).
When ApoE is hydrolyzed into neurotoxic protein fragments, they
enter the cytoplasm, causing tau protein lesions and mitochondrial
damage, further leading to neurodegeneration (12). Since γ-aminobutyric acid neurons
are mainly located in the hippocampus and are responsible for
cognitive functions such as learning and memory, which is
particularly sensitive to the toxicity of ApoE fragments, the early
symptoms of AD include cognitive impairment (13). A previous study has observed that
repeated inhalation of sevoflurane can lead to oxidative stress in
the developing brain, thus affecting cognitive functions (6). It was also observed that repeated
anesthesia with 2.6% sevoflurane not only reduced the volume of the
hippocampus, but also increased the expression of the ApoE gene in
the CA1 and CA3 regions of the hippocampus (26). In the present study, western
blotting, ELISA and RT-qPCR indicated that immature neurons
expressed a small amount of ApoE and its fragments in the control
group, whereas ApoE and its fragment expression significantly
increased following treatment with sevoflurane. In addition,
sevoflurane treatment decreased the ATP and SOD levels, but
increased the phosphorylated tau (AT8 and PHF1) and inflammatory
factor (TNF-α, IL-6 and IL-1β) levels compared with those in the
control group. These data indicated that sevoflurane treatment not
only caused energy deficiency and oxidative stress, but also led to
tau hyperphosphorylation and neuroinflammation; all of these
processes may be associated with the enhancement of ApoE
expression.
CoQ10 is an endogenous lipid-soluble antioxidant
that is mainly present in the mitochondrial membrane (16). CoQ10 is an important cofactor in
the mitochondrial electron transport chain; it exerts
neuroprotective, energy conversion, anti-inflammatory and
antioxidative effects (15). In
particular, CoQ10 can improve bioenergy metabolism and
mitochondrial function, increase brain energy level and improve the
brain function of rodents (27). A
preliminary experiment confirmed that the treatment dose of 500
µg/well reversed the sevoflurane neurotoxicity (data not shown).
Therefore, 500 µg/well was chosen as the treatment. In the present
study, hippocampal neurons were pre-treated with 1.5 mg/ml CoQ10
(dissolved in corn oil) for 4 h before inhalation of oxygen and
sevoflurane in the sevoflurane+CoQ10 and Control+CoQ10 groups; the
results demonstrated that sevoflurane significantly increased ApoE,
ApoE fragment and phosphorylated tau levels, and decreased the ATP
and SOD levels in the sevoflurane+corn oil group, but not in the
sevoflurane+CoQ10 group. These results suggested that CoQ10
increased the ATP level, but decreased the oxidative stress level
of neurons, thus alleviating the high levels of ApoE and ApoE
fragments produced by sevoflurane stimulation and mitigating the
neuroinflammation caused by the sevoflurane-induced tau
hyperphosphorylation in the neurons.
However, the present study has several limitations.
Firstly, it was confirmed that the primary neurons cultured for 5
days were in their infancy; however, whether these neurons
accurately represented the 6 day-old mouse sevoflurane anesthesia
model was uncertain. Secondly, the role of ApoE in the neurons of
ApoE-knockdown or -knockout mice was not addressed. Lastly, only
ELISA was used to detect the inflammation-related indicators, such
as TNF-α, IL-6 and IL-1β; the protein levels of these indicators
should be assessed by western blotting.
In summary, the present study demonstrated that
sevoflurane treatment impaired mouse hippocampal neurons, which may
be associated with expression of ApoE and its toxic fragments.
CoQ10 improved energy replenishment and inhibited oxidative stress,
which may lead to a decrease in ApoE and phosphorylated tau protein
expression, thus mitigating the sevoflurane-induced
neuroinflammation in mouse hippocampal neurons.
Acknowledgements
Not applicable.
Funding
This study was supported by the Tianjin Natural
Science Foundation (grant no. 18JCZDJC35100) and the Science and
Technology Development Fund of Tianjin Education Commission for
Higher Education (grant no. 2017KJ194).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MY, YoY and YaY conceived and designed the study.
MY, KX NL, YW and YaY performed the experiments. MY and YaY wrote
the manuscript. KX and YoY reviewed and edited the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
All experimental protocols in the present study were
approved by the Animal Experimental Ethics Committee of Tianjin
Medical University General Hospital (Tianjin, China; approval no.
2018-X6-11).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Flick RP, Katusic SK, Colligan RC, Wilder
RT, Voigt RG, Olson MD, Sprung J, Weaver AL, Schroeder DR and
Warner DO: Cognitive and behavioral outcomes after early exposure
to anesthesia and surgery. Pediatrics. 128:e1053–e1061. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhou R, Li X, Li L and Zhang H:
Theaflavins alleviate sevoflurane-induced neurocytotoxicity via
Nrf2 signaling pathway. Int J Neurosci. 130:1–8. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wilder RT, Flick RP, Sprung J, Katusic SK,
Barbaresi WJ, Mickelson C, Gleich SJ, Schroeder DR, Weaver AL and
Warner DO: Early exposure to anesthesia and learning disabilities
in a population-based birth cohort. Anesthesiology. 110:796–804.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Raper J, Alvarado MC, Murphy KL and Baxter
MG: Multiple anesthetic exposure in infant monkeys alters emotional
reactivity to an acute stressor. Anesthesiology. 123:1084–1092.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lu H, Liufu N, Dong Y, Xu G, Zhang Y, Shu
L, Soriano SG, Zheng H, Yu B and Xie Z: Sevoflurane acts on
ubiquitination-proteasome pathway to reduce postsynaptic density 95
protein levels in young mice. Anesthesiology. 127:961–975. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tao G, Zhang J, Zhang L, Dong Y, Yu B,
Crosby G, Culley DJ, Zhang Y and Xie Z: Sevoflurane induces tau
phosphorylation and glycogen synthase kinase 3β activation in young
mice. Anesthesiology. 121:510–527. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zheng H, Dong Y, Xu Z, Crosby G, Culley
DJ, Zhang Y and Xie Z: Sevoflurane anesthesia in pregnant mice
induces neurotoxicity in fetal and offspring mice. Anesthesiology.
118:516–526. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhou H, Li S and Wang G: Euxanthone
ameliorates sevoflurane-induced neurotoxicity in neonatal mice. J
Mol Neurosci. 68:275–286. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
DiMaggio C, Sun LS, Kakavouli A, Byrne MW
and Li G: A retrospective cohort study of the association of
anesthesia and hernia repair surgery with behavioral and
developmental disorders in young children. J Neurosurg Anesthesiol.
21:286–291. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fuentes D, Fernández N, García Y, García
T, Morales AR and Menéndez R: Age-related changes in the behavior
of apolipoprotein E knockout mice. Behav Sci (Basel). 8:E332018.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rohn TT, Catlin LW, Coonse KG and Habig
JW: Identification of an amino-terminal fragment of apolipoprotein
E4 that localizes to neurofibrillary tangles of the Alzheimer's
disease brain. Brain Res. 1475:106–115. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Munoz SS, Garner B and Ooi L:
Understanding the role of ApoE fragments in Alzheimer's disease.
Neurochem Res. 44:1297–1305. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang C, Najm R, Xu Q, Jeong DE, Walker D,
Balestra ME, Yoon SY, Yuan H, Li G, Miller ZA, et al: Gain of toxic
apolipoprotein E4 effects in human iPSC-derived neurons is
ameliorated by a small-molecule structure corrector. Nat Med.
24:647–657. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shen X, Dong Y, Xu Z, Wang H, Miao C,
Soriano SG, Sun D, Baxter MG, Zhang Y and Xie Z: Selective
anesthesia-induced neuroinflammation in developing mouse brain and
cognitive impairment. Anesthesiology. 118:502–515. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Turunen M, Olsson J and Dallner G:
Metabolism and function of coenzyme Q. Biochim Biophys Acta.
1660:171–199. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Stefely JA and Pagliarini DJ: Biochemistry
of mitochondrial coenzyme Q biosynthesis. Trends Biochem Sci.
42:824–843. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xu G, Lu H, Dong Y, Shapoval D, Soriano
SG, Liu X, Zhang Y and Xie Z: Coenzyme Q10 reduces
sevoflurane-induced cognitive deficiency in young mice. Br J
Anaesth. 119:481–491. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee JR and Loepke AW: Does pediatric
anesthesia cause brain damage?-Addressing parental and provider
concerns in light of compelling animal studies and seemingly
ambivalent human data. Korean J Anesthesiol. 71:255–273. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sun L: Early childhood general anaesthesia
exposure and neurocognitive development. Br J Anaesth. 105 (Suppl
1):i61–i68. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Elliott DA, Tsoi K, Holinkova S, Chan SL,
Kim WS, Halliday GM, Rye KA and Garner B: Isoform-specific
proteolysis of apolipoprotein-E in the brain. Neurobiol Aging.
32:257–271. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
van der Kant R, Goldstein LSB and
Ossenkoppele R: Amyloid-β-independent regulators of tau pathology
in Alzheimer disease. Nat Rev Neurosci. 21:21–35. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dai J, Johnson ECB, Dammer EB, Duong DM,
Gearing M, Lah JJ, Levey AI, Wingo TS and Seyfried NT: Effects of
APOE genotype on brain proteomic network and cell type changes in
Alzheimer's disease. Front Mol Neurosci. 11:4542018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Brecht WJ, Harris FM, Chang S, Tesseur I,
Yu GQ, Xu Q, Dee Fish J, Wyss-Coray T, Buttini M, Mucke L, et al:
Neuron-specific apolipoprotein e4 proteolysis is associated with
increased tau phosphorylation in brains of transgenic mice. J
Neurosci. 24:2527–2534. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Morrow JA, Segall ML, Lund-Katz S,
Phillips MC, Knapp M, Rupp B and Weisgraber KH: Differences in
stability among the human apolipoprotein E isoforms determined by
the amino-terminal domain. Biochemistry. 39:11657–11666. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jiang J, Tang C, Ren J, Zhang C, Dong L
and Zhu Z: Effect of multiple neonatal sevoflurane exposures on
hippocampal apolipoprotein E levels and learning and memory
abilities. Pediatr Neonatol. 59:154–160. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mohammadi-Bardbori A, Najibi A,
Amirzadegan N, Gharibi R, Dashti A, Omidi M, Saeedi A,
Ghafarian-Bahreman A and Niknahad H: Coenzyme Q10 remarkably
improves the bio-energetic function of rat liver mitochondria
treated with statins. Eur J Pharmacol. 762:270–274. 2015.
View Article : Google Scholar : PubMed/NCBI
|