Introduction
Familial glucocorticoid deficiency (FGD) was
reported for the first time by Shepard et al (1), in 1959. The study described a case of
two sisters who presented adrenal insufficiency, cortisol hormone
deficiency and normal levels of aldosterone, renin, skin
hyperpigmentation, muscle weakness and other performances. In
addition, the serum electrolytes and blood pressure were normal and
the cortisol (COR) levels were not affected by the treatment with
adrenocorticotropic hormone (ACTH). Hitherto, the identified
FGD-related genes were the following: Melanocortin 2 receptor/ACTH
receptor (MC2R) (2),
MC2R accessory protein (MRAP) (3), nicotinamide nucleotide
transhydrogenase (NNT) (4),
the minichromosome maintenance-deficient 4 homolog gene
(MCM4) (5), thioredoxin
reductase (TXNRD2) (6),
steroid hormone acute regulatory protein (STAR) (7) and cytochrome P450 family 11 subfamily
A polypeptide 1 (CYP11A1) (8). Of these, MC2R and MRAP
accounted for 50% of all the mutations.
The STAR gene encodes a steroid acute
regulatory protein that serves a key function in the steroid
synthesis. In this process, cholesterol is presented to the
cytochrome P450scc encoded by the CYP11A1 gene, catalyzing
the cholesterol to pregnenolone, which regulates the supply of
substrate cholesterol from the mitochondrial outer membrane to the
underlying mitochondrial membrane. The gene mutation effectuates as
early onset and causes severe steroid hormone deficiency,
hypoglycemia, loss of salt, dysplasia and adrenal lipid deposition
(9,10), usually within a few months after
birth.
Some non-classical mutations can retain certain
features, resulting in FGD clinical manifestations, such as that of
the two sibling patients described in this article. Interestingly,
they demonstrated the clinical diagnosis as primary adrenal
insufficiency (PAI) but with normal gonadal function and adrenal
hyperplasia. The c.533T>A (p. Leu178Gln) and c.737A>G (p.
Asp246Gly) mutations in the STAR gene were obtained from the
parents and confirmed by the second-generation sub-whole exon gene
sequencing.
Case report
Patient 1
A normal female 34-year-old proband was the
first-born child of non-consanguineous parents. The patient was
admitted to The First Affiliated Hospital of Zhengzhou University
in March 2017 because of skin pigmentation experienced for 32
years, low blood pressure for 6 years and intermittent nausea and
vomiting for 6 months. The patient was born in 1982 after an
uneventful pregnancy. At the age of two, the patient suffered from
upper respiratory tract infection several times and skin
hyperpigmentation gradually started to appear. Pubertal development
started at the age of 11, menarche was presented at 13 and
currently (in 2018), the patient has a 10-year-old son. The patient
was diagnosed with ‘bipolar affective disorder’ 4 years ago and was
treated with oral medication such as lithium carbonate, lorazepam,
sertraline and buspirone. The karyotype of the patient was 46, XX
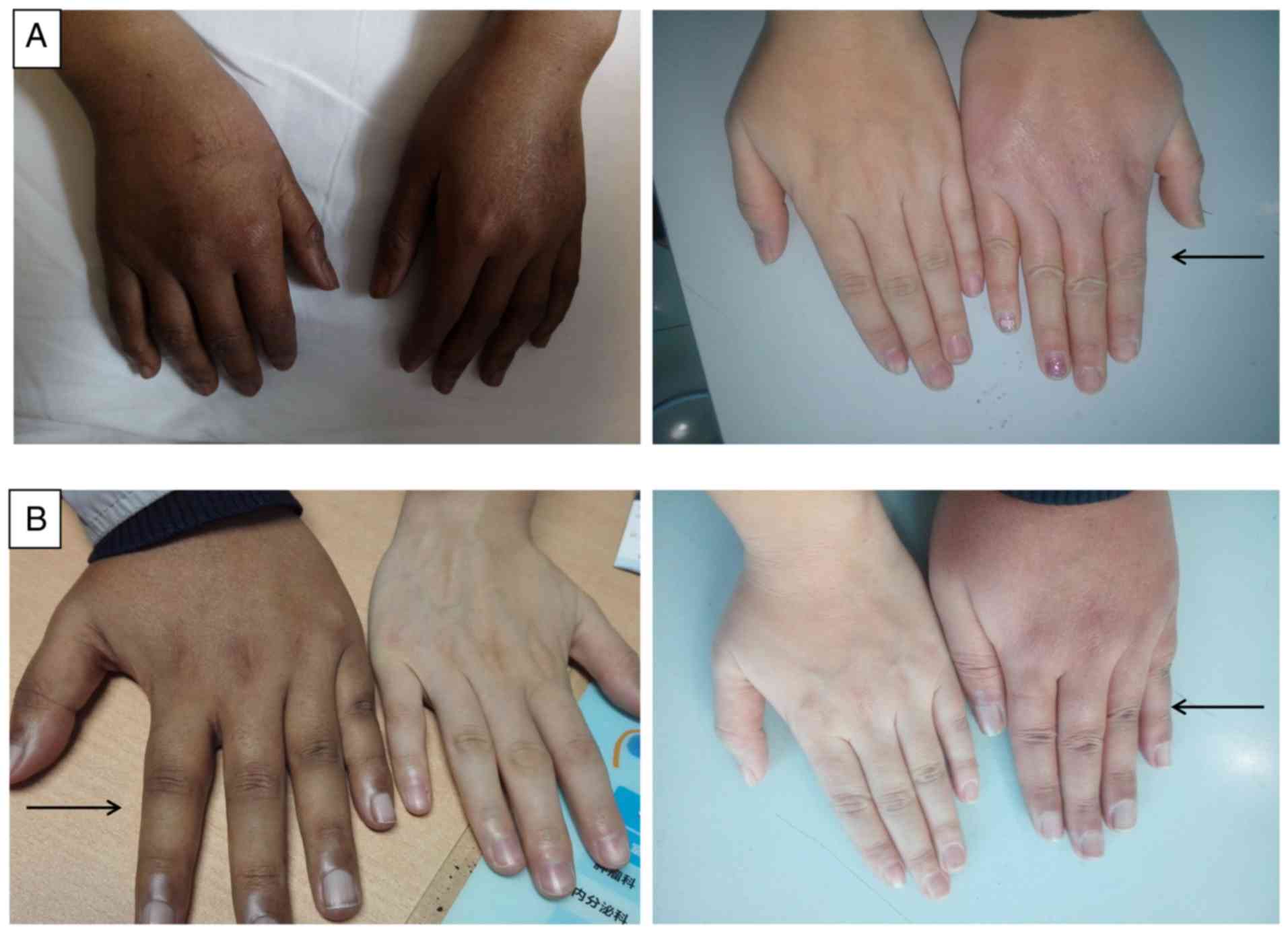
with skin hyperpigmentation (Fig.
1A); however, the breasts and vulva were normal. Blood pressure
was 80/40 mmHg, fasting blood glucose was 3.9 (normal range:
3.6–6.1) mmol/l, serum sodium was 126 mmol/l (normal range: 135–155
mmol/l), 17-hydroxyprogesterone (17-OHP) was 0.31 ng/ml,
dehydroepiandrosterone (DHEA) was <15 (normal range: 35–430)
µg/dl, normal serum gonadotropin concentrations for follicle
stimulating hormone (FSH) and luteinizing hormone (LH) were 5.36
and 5.83 mIU/ml, respectively, the lying position
renin-angiotensin-aldosterone system (RAAS) showed that renin
activity was >26.68 (normal range: 0.15–2.33) ng/ml.h,
angiotensin II was 232.0 (normal range: 25–80) pg/ml and
aldosterone was 153.0 (normal range: 30–160) pg/ml. ACTH-COR rhythm
is listed in Table I. The 24-h
urine-free cortisol (UFC) was 83 (normal range: 73–372) nmol/l. The
cortisol levels did not respond to the stimulation with ACTH (250
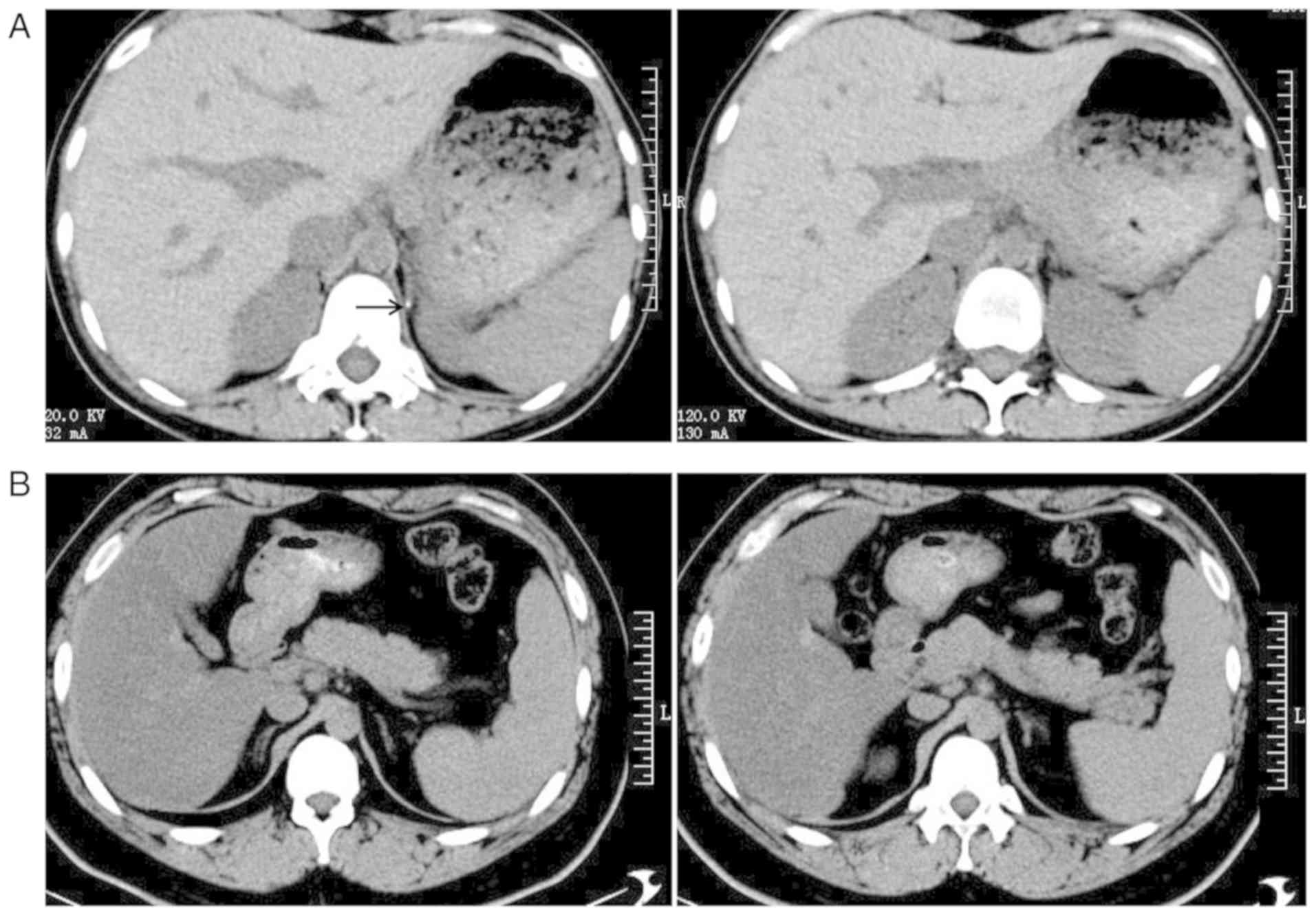
µg intravenous injection in 1 min) (Table II). Computed tomography (CT)
revealed bilateral adrenal glands without hyperplasia and the left
side presented calcification (Fig.
2A).
 | Table I.ACTH-COR rhythm of the two
patients. |
Table I.
ACTH-COR rhythm of the two
patients.
| A, ACTH (pg/ml) |
|---|
|
|---|
| Patient | 8:00 | 16:00 | 00:00 |
|---|
| Patient 1 | >1,250 | >1,250 | >1,250 |
| Patient 2 | >1,250 | >1,250 | – |
|
| B, COR
(ng/ml) |
|
| Patient | 8:00 | 16:00 | 00:00 |
|
| Patient 1 | 201 | 189 | 151 |
| Patient 2 | 312 | 186 | – |
 | Table II.ACTH stimulation test of patient
1. |
Table II.
ACTH stimulation test of patient
1.
| Variable | 0 min | 30 min | 60 min |
|---|
| COR (ng/ml) | 246 | 231 | 263 |
| 17-OHP (ng/ml) | 0.31 | 1.82 | 2.05 |
| DHEA (µg/dl) | <15 | 21.7 | 20.8 |
| Androstenedione
(ng/ml) | <0.3 | 0.6 | 0.5 |
Patient 2
The younger brother of patient 1 was born in 1992
after an uneventful pregnancy. The patient displayed skin
hyperpigmentation from childhood and occasional fatigue. The
karyotype of the patient was 46, XY. Pubertal development started
at the age of 14 years. Skin hyperpigmentation (Fig. 1B) by the Tanner staging of testis
was 4, and the patient was unmarried. Normal blood glucose, serum
electrolytes, serum gonadotropin concentrations: FSH 6.12 (normal
range: 0.95–11.95) mIU/ml and LH 4.95 (normal range: 1.14–8.75)
mIU/ml, DHEA was 58.2 (normal range: 80–560) µg/dl and lying
position RAAS: Renin activity was 22.95 ng/ml.h, angiotensin II was
73.27 pg/ml, aldosterone was 151.7 pg/ml. ACTH-COR rhythm is listed
in Table I; the 24-h UFC was 134
nmol/l. CT did not reveal any hyperplasia in the bilateral adrenal
glands (Fig. 2B).
Genomic DNA was extracted from the peripheral blood
of the two patients and their familial relatives (parents and son
of the proband) after written informed consent (the patients agreed
to blood sampling, gene testing, publication of their data and
figures). Sub-whole exon gene sequencing was performed using Exon
Chip Capture (Agilent Technologies, Inc.) and high-throughput
sequencing (Beijing Jinzhun Gene Technology Co., Ltd). Sequences of
the mutations were aligned against that in the National Center for
Biotechnology Information (NCBI) database. The data interpretation
was according to the guidelines of the American College of Medical
Genetics and Genomics (ACMG) and variations were named according to
the recommendations of the Human Genome Variation Society (HGVS;
www.hgvs.org/mutnomen). The protein was
predicted by Polyphen2, Sorting Intolerant From Tolerant (SIFT) and
Protein Variation Effect Analyzer (PROVEAN) software and
SWISS-MODEL was used to construct the protein model. Alterations in
the protein structure and amino acid hydrogen bond were analyzed
before and after the mutation.
The patients were treated with oral administration
of 20 mg hydrocortisone (HC) in the morning and 10 mg in the
evening. Indicators such as skin pigmentation, appetite, blood
pressure, ACTH, serum cortisol, serum electrolytes and blood
glucose were monitored regularly and the HC dose was changed
according to the symptoms and the results of testing.
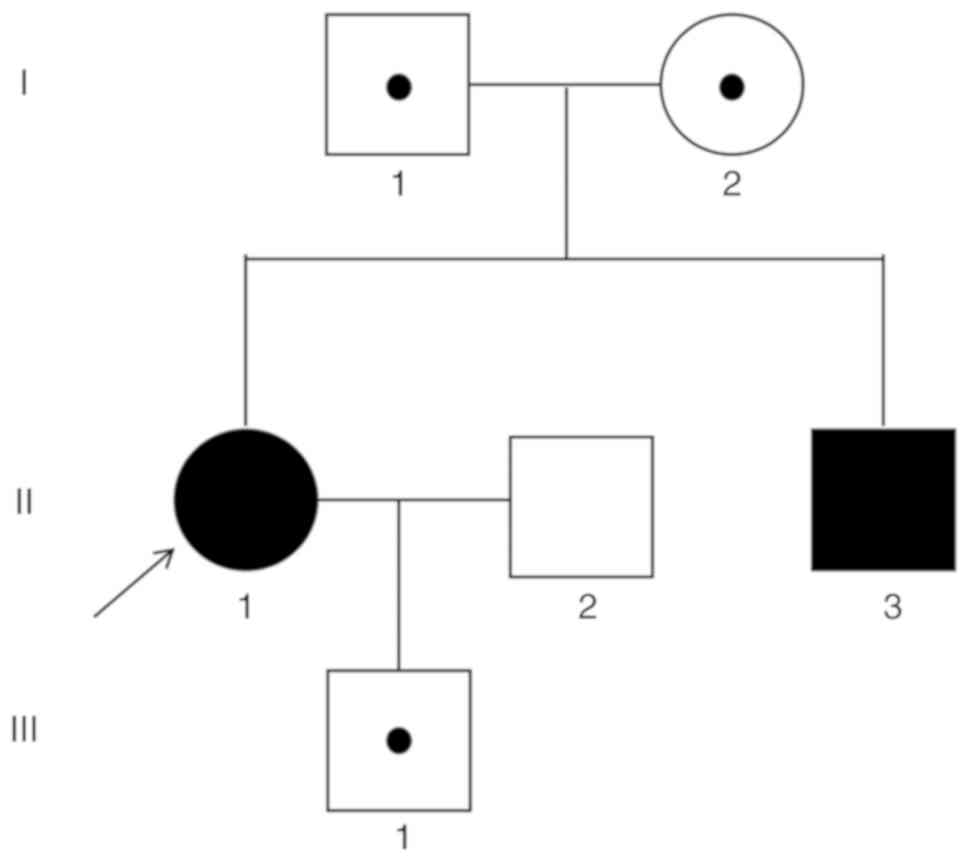
Two heterozygote mutations c.533T>A (p.
Leu178Gln) and c.737A>G (p. Asp246Gly) were found on the
STAR gene in the two patients; one was passed from each
parent, respectively, and the son of the proband carried one of the
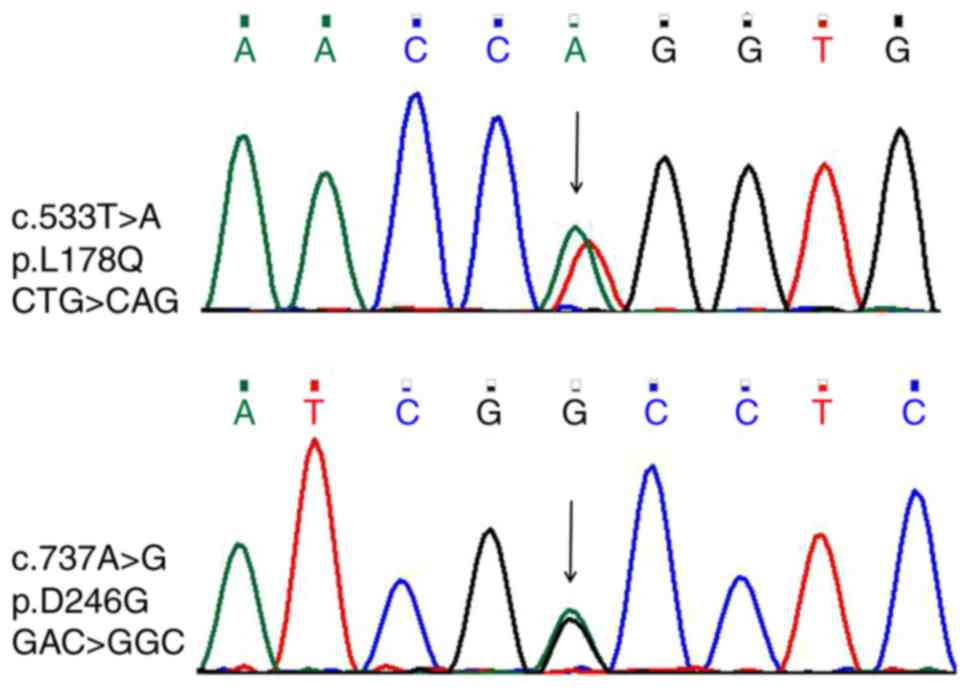
mutations (Fig. 3). The sequencing
of the peak pattern revealed an additional peak that was further
verified by the first generation of gene sequencing (Fig. 4). The amino acid sequence
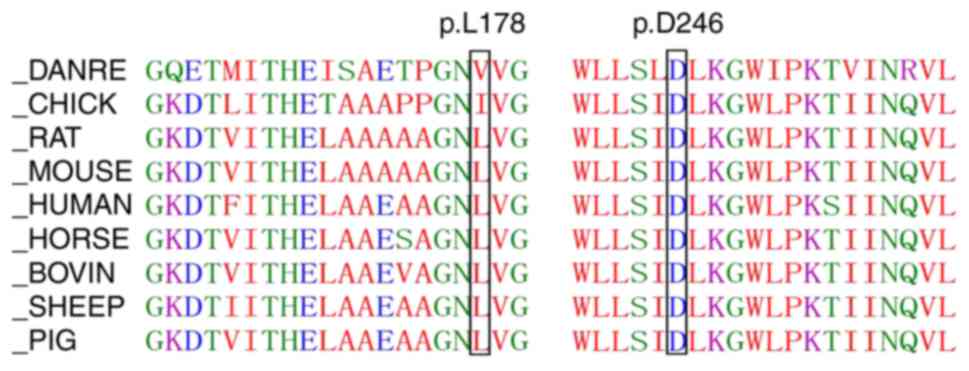
alignments revealed that positions 178 and 246 were highly
conserved across species (Fig. 5).
Polyphen2 (genetics.bwh.harvard.edu/pph2), SIFT (sift.bii.a-star.edu.sg) and PROVEAN (provean.jcvi.org/genome_submit_2.php?species=human)
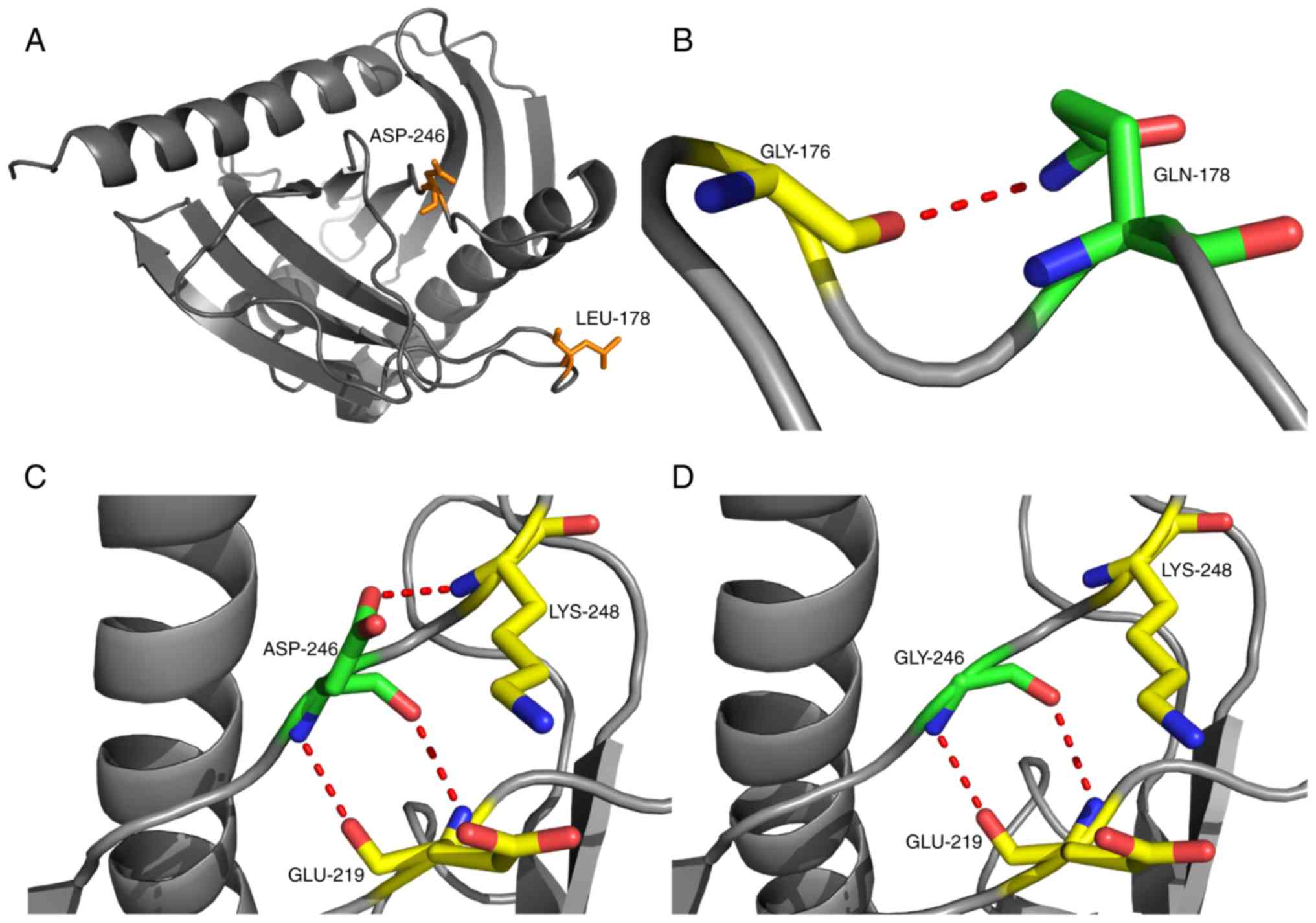
software predicted the mutations as detrimental (Table III). The computational model used
in this study was modified by introducing the missense mutations
using the Swiss-Model program (Fig.
6). The tertiary structure before and after the mutation did
not alter; however, Gln178 formed a new hydrogen bond with Gly176
and the hydrogen bond between Asp246 and Lys248 disappeared after
mutation. The skin hyperpigmentation (Fig. 1) and the general state of the two
patients was improved after three months of treatment. They also
gained weight. The serum cortisol, serum electrolytes and blood
glucose were normal, cortisol increased and ACTH decreased
significantly in the proband, while the ACTH level in patient 2 was
high in the short-term.
 | Table III.Results predicted by Polyphen2, SIFT
and PROVEAN software. |
Table III.
Results predicted by Polyphen2, SIFT
and PROVEAN software.
|
| Polyphen2 | SIFT | PROVEAN |
|---|
|
|
|
|
|
|---|
| Variant | HumVar score | Prediction | Score | Prediction | Score (cutoff:
−2.5) | Prediction |
|---|
| Leu178Gln | 0.897 (sensitivity,
0.70; specificity, 0.90) | Probably
damaging | 0 | Damaging | −3.340 | Deleterious |
| Asp246Gly | 0.989 (sensitivity,
0.52; specificity, 0.95) | Probably
damaging | 0 | Damaging | −5.204 | Deleterious |
Discussion
The patients included in the present case were
siblings, who presented skin hyperpigmentation throughout the body,
particularly in hands, lips and nipples. The gonadal development
was normal, but laboratory tests suggested glucocorticoid
deficiency and mild lack of mineralocorticoid, indicating
hyponatremia and hypotension in the proband. The cortisol level was
not affected by the stimulation with ACTH and the clinical
diagnosis was PAI; however, the adrenal glands did not show any
hyperplasia. Gene analysis revealed two novel compound heterozygote
mutations c.533T>A (p. Leu178Gln) and c.737A>G (p. Asp246Gly)
in the STAR gene, which were passed onto the patients, one
from each parent, respectively.
In 1994, Clark et al (11) cloned the complementary DNA for a
30-kDa mouse mitochondrial protein that appeared to be rapidly
inducible, cycloheximide-sensitive mediator of the acute
steroidogenic response, and termed it STAR. STAR is produced as a
285-amino-acid protein with an N-terminal signal sequence that
targets the molecule to the interior of the mitochondria, while the
C-terminal STAR-related lipid transfer domain is essential for its
function and forms a pocket that might bind cholesterol (12,13).
All steroids are derived from pregnenolone, a product of the
cholesterol side chain cleavage reaction catalyzed by CYP11A
(14) and the supply of substrate
cholesterol from the outer mitochondrial membrane to this inner
mitochondrial membrane enzyme is regulated by STAR. The transfer of
cholesterol to P450scc constitutes the rate-limiting step in
steroidogenesis. The mutations in the STAR gene are
primarily concentrated in the C-terminal of the protein encoded by
exons 5, 6 and 7, causing congenital lipoid adrenal hyperplasia
(CLAH), which is the rarest but most severe form of CAH (15). It results from a general loss of
all steroid production and is presented as primary adrenal
insufficiency or Addison's disease. The symptoms include low body
weight, severe dehydration, skin hyperpigmentation, respiratory
distress and vomiting in patients.
However, in 2006, Baker et al (16) reported that patients with STAR
disorder show a late presentation and normal male genitalia, thus
are defined as having a new disorder, ‘non-classic LCAH’ (NCLAH).
This phenomenon represented a new cause of non-autoimmune Addison's
disease (primary adrenal failure). In 2009, Metherell et al
(7) screened FGD patients from 80
families and revealed homozygous STAR mutations in five families.
In addition, the results demonstrated that specific mutations in
STAR cause NCLAH masquerading as FGD and present a phenotype
indistinguishable from that of FGD. The partial loss-of-function
due to STAR mutations was deemed as a cause of type 3 FGD with ≥10
cases of the mutations reported (17).
The study by Baker et al (16) described the FGD-like phenotypes in
three patients with mutations, V187M and R188C, in adjacent codons.
The structural modeling of both residues encompassed within the
cholesterol-binding pocket of STAR suggested that the R188C
mutation would prevent the formation of a salt bridge typically
localized between residues E169 and R188 but could result in a weak
bond between residues T167 and R188 that may sufficiently preserve
the binding pocket to transport the cholesterol. The functional
analysis of both V187M and R188C mutants revealed that >20%
cholesterol binding activity was retained. This residual activity
may be ascribed to the cortisol deficiency and mild hyperreninemia
in the 2-year-old patients, thereby resembling the patients with
FGD. Thus, in the patients of the present study, the mutations
Leu178Gln and Asp246Gly, also within the cholesterol-binding pocket
of STAR, may exhibit similar mechanisms. The statistical analysis
of 10 cases of mutations in the study by Flück et al
demonstrated a 3–5% retention of the cholesterol binding activity
and the maximum age of onset was 58 years (17). The low levels of STAR-independent
steroidogenesis and a complete loss of steroidogenesis due to
cellular destruction by accumulated lipids formed the two-hit model
of LCAH (18). Interestingly, the
adrenals of both patients in the present case revealed no
hyperplasia and patient 1 had unilateral calcification.
Furthermore, a previous study had already speculated that the
cirrhotic end stage of previous fat deposition resembled the
imaging changes observed during progression from steatosis
hepatitis to liver cirrhosis, although a precise mechanism has yet
to be elucidated (7).
In summary, two compound heterozygous mutations in
the STAR gene in two related patients (sister and brother)
with isolated glucocorticoid deficiency were reported. Moreover,
lack of mineralocorticoid and normal gonadal function was observed.
The proband was preparing for her second child; however, proband
and her brother's adrenal function may be further affected after
several years, thereby necessitating regular monitoring.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YL, RB, XZ and JX acquired, analyzed and interpreted
the data. YL drafted the manuscript, figures and table, and revised
the manuscript. RB drafted the manuscript. ZW made substantial
contributions to the conception and design of the study, and
critically revised the manuscript for important intellectual
content. XL made substantial contributions to the conception and
design of the study, and approved the final version of the
manuscript. All authors approved the final version of the
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Scientific
Research and Clinical Trials Ethics Committee of the First
Affiliated Hospital of Zhengzhou University (approval no.
2019-005). Written informed consent was obtained from the
participants and the parent of the son of the proband.
Patient consent for publication
Written informed consent for the publication of the
data presented in the present study was obtained from the
participants and the parent of the son of the proband.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Shepard TH, Landing BH and Mason DG:
Familial Addison's disease; case reports of two sisters with
corticoid deficiency unassociated with hypoaldosteronism. AMA J Dis
Child. 97:154–162. 1959. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Clark AJ, McLoughlin L and Grossman A:
Familial glucocorticoid deficiency associated with point mutation
in the adrenocorticotropin receptor. Lancet. 341:461–462. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Metherell LA, Chapple JP, Cooray S, David
A, Becker C, Rüschendorf F, Naville D, Begeot M, Khoo B, Nürnberg
P, et al: Mutations in MRAP, encoding a new interacting partner of
the ACTH receptor, cause familial glucocorticoid deficiency type 2.
Nat Genet. 37:166–170. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Meimaridou E, Kowalczyk J, Guasti L,
Hughes CR, Wagner F, Frommolt P, Nürnberg P, Mann NP, Banerjee R,
Saka HN, et al: Mutations in NNT encoding nicotinamide nucleotide
transhydrogenase cause familial glucocorticoid deficiency. Nat
Genet. 44:740–742. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hughes CR, Guasti L, Meimaridou E, Chuang
CH, Schimenti JC, King PJ, Costigan C, Clark AJ and Metherell LA:
MCM4 mutation causes adrenal failure, short stature and natural
killer cell deficiency in humans. J Clin Invest. 122:814–820. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Prasad R, Chan LF, Hughes CR, Kaski JP,
Kowalczyk JC, Savage MO, Peters CJ, Nathwani N, Clark AJ, Storr HL
and Metherell LA: Thioredoxin Reductase 2 (TXNRD2) mutation
associated with familial glucocorticoid deficiency (FGD). J Clin
Endocrinol Metab. 99:E1556–E1563. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Metherell LA, Naville D, Halaby G, Begeot
M, Huebner A, Nürnberg G, Nürnberg P, Green J, Tomlinson JW, Krone
NP, et al: Nonclassic lipoid congenital adrenal hyperplasia
masquerading as familial glucocorticoid deficiency. J Clin
Endocrinol Metab. 94:3865–3871. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Flück CE, Tajima T, Pandey AV, Arlt W,
Okuhara K, Verge CF, Jabs EW, Mendonça BB, Fujieda K and Miller WL:
Mutant P450 oxidoreductase causes disordered steroidogenesis with
and without Antley-Bixler syndrome. Nat Genet. 36:228–230. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bose HS, Pescovitz OH and Miller WL:
Spontaneous feminization in a 46, XX female patient with congenital
lipoid adrenal hyperplasia due to a homozygous frameshift mutation
in the steroidogenic acute regulatory protein. J Clin Endocrinol
Metab. 82:1511–1515. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fujieda K, Tajima T, Nakae J, Sageshima S,
Tachibana K, Suwa S, Sugawara T and Strauss JF III: Spontaneous
puberty in 46,XX subjects with congenital lipoid adrenal
hyperplasia. Ovarian steroidogenesis is spared to some extent
despite inactivating mutations in the steroidogenic acute
regulatory protein (StAR) gene. J Clin Invest. 99:1265–1271. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Clark BJ, Wells J, King SR and Stocco DM:
The purification, cloning, and expression of a novel luteinizing
hormone-induced mitochondrial protein in MA-10 mouse Leydig tumor
cells. Characterization of the steroidogenic acute regulatory
protein (StAR). J Biol Chem. 269:28314–28322. 1994.PubMed/NCBI
|
|
12
|
King SR, Ronen-Fuhrmann T, Timberg R,
Clark BJ, Orly J and Stocco DM: Steroid production after in vitro
transcription, translation, and mitochondrial processing of protein
products of complementary deoxyribonucleic acid for steroidogenic
acute regulatory protein. Endocrinology. 136:5165–5176. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tsujishita Y and Hurley JH: Structure and
lipid transport mechanism of a StAR-related domain. Nat Struct
Biol. 7:408–414. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Stocco DM and Clark BJ: Regulation of the
acute production of steroids in steroidogenic cells. Endocr Rev.
17:221–244. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bhangoo A, Anhalt H, Ten S and King SR:
Phenotypic variations in lipoid congenital adrenal hyperplasia.
Pediatr Endocrinol Rev. 3:258–271. 2006.PubMed/NCBI
|
|
16
|
Baker BY, Lin L, Kim CJ, Raza J, Smith CP,
Miller WL and Achermann JC: Nonclassic congenital lipoid adrenal
hyperplasia: A new disorder of the steroidogenic acute regulatory
protein with very late presentation and normal male genitalia. J
Clin Endocrinol Metab. 91:4781–4785. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Flück CE, Pandey AV, Dick B, Camats N,
Fernández-Cancio M, Clemente M, Gussinyé M, Carrascosa A, Mullis PE
and Audi L: Characterization of novel StAR (steroidogenic acute
regulatory protein) mutations causing non-classic lipoid adrenal
hyperplasia. PLoS One. 6:e201782011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Miller WL: Mitochondrial specificity of
the early steps in steroidogenesis. J Steroid Biochem Mol Biol.
55:607–616. 1995. View Article : Google Scholar : PubMed/NCBI
|