Introduction
Colon cancer is one of the most frequent malignant
tumors and the second leading cause of tumor-related mortality in
the United States (1,2). The incidence of colon cancer is
increasing annually, and the disease seriously threatens the
physical and mental health of patients (3). In recent years, a large number of
clinical studies have reported that metastatic recurrence is the
primary cause of the prognosis of colon cancer (4–6). In
the early stages, the 5-year survival rate of colon cancer is
>90%; however, when colon cancer is diagnosed with local lymph
node metastasis, the 5-year survival rate decreases to 65%
(7). Hence, it is of great
clinical significance to explore the inhibition of tumor
metastasis.
Tumor metastasis is a sequential process of
interaction among tumor cells, host cells and the tissue
microenvironment (8).
Epithelial-mesenchymal transition (EMT), which plays a key role in
tumor metastasis (9,10), is characterized by the deficiency
of epithelial phenotypes, loss of cell polarity, reduced contact
with surrounding cells and matrix, and enhanced cell migration and
invasion in the presence of interstitial phenotype (11). Furthermore, EMT participates in
nearly all physiological and pathological processes, such as the
differentiation of various tissues and organs, repair of tissue
damage, tissue fibrosis, tumor occurrence and metastasis (12–14).
MicroRNAs (miRNAs/miRs), a class of small, 18- to
28-nucleotide-long, noncoding RNA molecules, are involved in the
progression of tumors (15,16).
To date, the human genome contains ~1,000 miRNAs, and each miRNA is
expected to interact with dozens or even hundreds of genes via
matching 5′ sequences and 3′ untranslated regions (3′UTRs) of
target mRNAs (17–19). miR-205 is a highly conserved miRNA,
and its homologous chromosomes can be found in different species
(20,21). Homo sapiens (hsa)-miR-205 is
located in the second intron of the LOC642587 site of the first
chromosome (22). Whether miR-205
is an oncogene or a tumor suppressor still remains controversial
(23), though studies have
reported that miR-205 participates in the EMT process of tumor
cells (24–26).
Mouse double minute 4 (MDM4), which was isolated and
identified in 1996, is also known as MDMX, and is an important
upstream regulator of p53 (27).
Following MDM4 phosphorylation, the p53 binding domain of MDM4 can
combine with the transcriptional activation domain of the wild and
mutant p53 proteins to form a MDM4/p53 complex to inhibit the
transcriptional activity of p53 (28,29).
Increasing evidence has shown that MDM4 is abnormally expressed in
a number of tumor tissues such as breast cancer, retinoblastoma,
lung cancer, colon cancer and gastric cancer (30–33).
Accordingly, the inhibition of abnormal expression of MDM4 has
attracted increasing attention of researchers in the field of
anti-tumor mechanisms.
The current study determined the expression of
miR-205 and MDM4 in colon cancer tissues, adjacent normal tissues,
and colon and colorectal cancer cell lines. In addition, the
correlation between the expression of miR-205 and MDM4 colon cancer
tissue was studied. A binding site for miR-205 in the 3′UTR of MDM4
was identified by TargetScan prediction software, and the role of
miR-205 in the migration, invasion and EMT process of tumor cells
gene was explored by targeting MDM4.
Materials and methods
Tissue source
The colon cancer tissues and adjacent normal tissues
(distance from tumor margin, 2 cm) were obtained from 47 patients
with colon cancer who were diagnosed at Beijing Jishuitan Hospital
between March 2011 and March 2016. All patients signed informed
consent and agreed that their tissues would be used for clinical
research. The relationship between miR-205 expression and clinical
characteristics of colon cancer are presented in Table I. Patients were divided into high-
and low-expression groups for analysis of associations with
clinical characteristics based on the mean miR-205 expression value
in tumor tissues. The study was reviewed and approved by the Ethics
Committee of Beijing Jishuitan Hospital (permit no.
J20110104015).
 | Table I.Relationship between miR-205
expression and clinical characteristics of colon cancer. |
Table I.
Relationship between miR-205
expression and clinical characteristics of colon cancer.
|
|
| miR-205
expression |
|
|
|---|
|
|
|
|
|
|
|---|
| Clinicopathological
variable | n | Low | High | χ2 | P-value |
|---|
| All cases | 47 | 24 | 23 |
|
|
| Age |
|
|
| 0.180 | 0.671 |
|
≤65 | 21 | 10 | 11 |
|
|
|
>65 | 26 | 14 | 12 |
|
|
| Sex |
|
|
| 0.216 | 0.642 |
|
Female | 20 | 11 | 9 |
|
|
|
Male | 27 | 13 | 14 |
|
|
| Pathological
grade |
|
|
| 4.381 | 0.036 |
|
I–II | 35 | 21 | 14 |
|
|
|
III | 12 | 3 | 9 |
|
|
| Stage |
|
|
| 7.817 | 0.005 |
|
I–II | 28 | 19 | 9 |
|
|
|
III–IV | 19 | 5 | 14 |
|
|
| Lymph node
metastasis |
|
|
| 4.846 | 0.028 |
|
Positive | 19 | 6 | 13 |
|
|
|
Negative | 28 | 18 | 10 |
|
|
Cell culture and transfection
The human colorectal cancer cell line (HT29), colon
cancer cell lines (HCT116, HCT8, LS174T and SW480) and 293T cells
were purchased from Shanghai Gaining Biotechnology Co., Ltd. HT29,
HCT116, HCT8, LS174T and SW480 cells were maintained in RPMI-1640
Medium (Thermo Fisher Scientific, Inc.) containing 10% fetal bovine
serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) and 100X
penicillin-streptomycin mixed solution (Beijing Leagene Biotech
Co., Ltd.) in an incubator (cat. no. DH-160I; Shanghai SANTN
Instrument Co., Ltd.) with 5% CO2 at 37°C and 95%
humidity. 293T cells were cultured in DMEM (Thermo Fisher
Scientific, Inc.) containing 10% FBS and 100X
penicillin-streptomycin mixed solution and used for luciferase
activity assays.
Human wild-type (WT) and mutant (MT) MDM4 3′UTRs
were cloned downstream of Renilla luciferase in a psiCHECK-2
vector (Hangzhou Hibio Technology Co., Ltd.). Subsequently, miR-205
mimic/control mimic (mimic NC; 30 µmol/l) was co-transfected into
293T cells (3×103 cells/well) using
Lipofectamine® 2000 (Thermo Fisher Scientific, Inc.) for
24 h at 37°C. HCT116 cells (5×105 cells/well) were
transfected with 50 nmol/l miR-205 mimic (cat. no. HmiR0026;
GeneCopoeia, Inc.), mimic NC (cat. no. CmiR0001; GeneCopoeia,
Inc.), miR-205 inhibitor (cat. no. HmiR-AN0307; GeneCopoeia, Inc.),
negative control for inhibitor (IC; cat. no. CmiR-AN0001;
GeneCopoeia, Inc.), control small interfering (si)RNA (siNC; cat.
no. AM4641; Thermo Fisher Scientific, Inc.), MDM4-siRNA (siMDM4;
cat. no. AM16708; Thermo Fisher Scientific, Inc.), miR-205
inhibitor + siMDM4, control + siNC or miR-205 inhibitor + siNC
using Lipofectamine® 2000 (Thermo Fisher Scientific,
Inc.) for 24 h at 37°C. The transfection efficiency was assessed by
western blotting analysis. Subsequent experiments were conducted at
24 h post-transfection.
Reverse transcription-quantitative
(RT-q)PCR
Total RNA was extracted from tissues and cells
(1.3×105 cells/well) using RNAiso Plus (Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocols. A
total of 1 µg of RNA was used to synthesize cDNA using a RevertAid™
cDNA Synthesis kit (Takara Biotechnology Co., Ltd.), according to
the manufacturer's protocol. Subsequently, qPCR was performed using
the SYBR Premix Ex Taq™ II kit (Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol. The following
thermocycling conditions were used for qPCR: Initial denaturation
at 95°C for 10 min; followed by 40 cycles of 94°C for 2 min, 60°C
for 50 sec; a final extension at 60°C for 1 min; and storage at
4°C. The primer sequences are listed in Table II. U6 and GAPDH were used as
internal references. The formula 2−ΔΔCq was used to
calculate relative gene expression (34).
 | Table II.Primer sequences. |
Table II.
Primer sequences.
|
| Sequence
(5′→3′) |
|---|
|
|
|
|---|
| Primer | Forward | Reverse |
|---|
| MDM4 |
GAAAGACCCAAGCCCTCTCT |
GCAGTGTGGGGATATCGTCT |
| miR-205 |
CTCGAGCAGGTGCAAGGACGTGTTG |
GGATCCGTGGCTTAGAAGGCCGGG |
| E-cadherin |
ACGCATTGCCACATACACTC |
GGTGTTCACATCATCGTCCG |
| N-cadherin |
CTTGCCAGAAAACTCCAGGG |
TGTGCCCTCAAATGAAACCG |
| MMP2 |
CAGCCCTGCAAGTTTCCATT |
GTTGCCCAGGAAAGTGAAGG |
| MMP9 |
GAGACTCTACACCCAGGACG |
GAAAGTGAAGGGGAAGACGC |
| Vimentin |
AATAAGATCCTGCTGGCCGA |
GGTGTTTTCGGCTTCCTCTC |
| U6 |
ACACCAAGCAGTCCGAAGAG |
ACAAAATTTCTCACGCCGGT |
| GAPDH |
CCATCTTCCAGGAGCGAGAT |
TGCTGATGATCTTGAGGCTG |
Western blot analysis
Total protein in tissues and cells were extracted
using RIPA lysis buffer (Beyotime Institute of Biotechnology).
Protein quantification was performed using a BCA protein assay kit
(Bio-Rad Laboratories, Inc.). Subsequently, proteins (40 µg) were
separated via 10% SDS-PAGE and transferred to a PVDF membrane. The
membrane was blocked in 5% non-fat milk at room temperature for 2
h. Subsequently, the membrane was incubated overnight at 4°C with
the following primary antibodies: Anti-MDM4 (1:1,200; cat. no.
ab154324; Abcam), anti-E-cadherin (1:800; cat. no. MAB1838; R&D
Systems, Inc.), anti-N-cadherin (1:1,200; cat. no. ab18203; Abcam),
anti-vimentin (1:700; cat. no. AF2105; R&D Systems, Inc.),
anti-matrix metalloproteinase (MMP)2 (1:1,000; cat. no. MA1-772;
Invitrogen; Thermo Fisher Scientific, Inc.), anti-MMP9 (1:800; cat.
no. AF911; R&D Systems, Inc.) and anti-GAPDH (1:800; cat. no.
AF5718; R&D Systems, Inc.). Following primary incubation, the
membranes were incubated with corresponding horseradish
peroxidase-conjugated secondary antibodies for 90 min at room
temperature [rabbit anti-mouse IgG (1:5,000; cat. no. 58802; Cell
Signaling Technology, Inc.); goat anti-mouse IgG (1:8,000; cat. no.
31430; Invitrogen; Thermo Fisher Scientific, Inc.); mouse
anti-rabbit IgG (1:10,000; cat. no. 31464; Invitrogen; Thermo
Fisher Scientific, Inc.)]. Finally, the protein was exposed using
an ECL chemiluminescence kit [Yeasen Biotechnology (Shanghai) Co.,
Ltd.]. Protein expression was quantified using ImageJ software
(version 5.0; Bio-Rad Laboratories, Inc.) with GAPDH as the loading
control.
Bioinformatics prediction
According to the computational analysis performed
using TargetScan software (version 7.2; www.targetscan.org/vert_72) (35), the 3′UTR of MDM4 contained a
predicted binding site for miR-205.
Luciferase activity analysis
At 24 h post-transfection, the 293T cells were lysed
using RIPA lysis buffer. The cell suspension was centrifuged at 500
× g for 5 min at room temperature, the supernatant was placed in
96-well plates and luciferase detection reagent was added (Promega
Corporation). Luciferase activity was detected with a
Nano-Glo® Dual-Luciferase Reporter Assay System (Promega
Corporation). Firefly luciferase activity was normalized to
Renilla luciferase activity.
Cell Counting Kit-8 (CCK-8)
analysis
CCK-8 (Beyotime Institute of Biotechnology) was
carried out to determine the cell viability of HCT116 cells
following the manufacturer's protocol. Cells were transfected for
24 h, re-seeded into 96-well plates (6×103 cells/well)
and incubated for 0, 24, 48 and 72 h at 37°C. Subsequently, CCK-8
reagent (10 µl) was added to the cells and incubated for 4 h at
37°C. The absorbance was then analyzed at 450 nm using a microplate
reader (FilterMax F3/F5; Molecular Devices, LLC).
Transwell analysis
BD Matrigel (Qcbio Science & Technologes Co.,
Ltd.) was added into the upper chambers of Transwell inserts
(96-well inserts; pore size, 0.4 µm; diameter, 4.26 mm) at room
temperature for 25 min, and RPMI-1640 medium was added into the
upper chambers. Subsequently, the Transwell inserts were placed in
the culture plate. RPMI 1640 medium with 15% FBS was placed in the
lower chamber to attract cells. HCT116 cell suspensions
(4×105 cells/well) were cultured in RPMI-1640 medium in
the upper chambers at 37°C for 24 h. The cells were fixed with 4%
paraformaldehyde for 15 min at room temperature, stained with 0.05%
crystal violet (Beijing Solarbio Science & Technology Co.,
Ltd.) for 20 min at room temperature, and washed with PBS three
times. Finally, the cells were observed and photographed under a
fluorescence microscope (magnification, ×200; MF53; Guangzhou
Micro-shot Technology Co., Ltd.).
Wound healing assay
Cells were transfected with 50 nM PBS, miR-205
mimic, mimic NC, siNC or siMDM4 for 0 and 12 h. During the wound
healing assay, cells were serum-starved (0.2% FBS). Following
transfection, HCT116 cells were seeded in 6-well plates
(2×104 cell/well) and cultured in an incubator for 24 h
at 37°C. Following culturing, a 6-µm width scratch was created in
the cells using a pipette tip, and the cells were washed by the
medium 3 times. Cells were observed and photographed under an
inverted microscope (magnification, ×200).
Statistical analysis
All experiments were conducted in triplicate. The
data were shown as the mean ± standard deviation using SPSS
software (version 20; IBM, Corp.). Associations between miR-205
expression and clinicopathological characteristics were analyzed
using χ2 tests. One-way analysis of variance and
Bonferroni's post hoc test were used to evaluate the differences
among groups. The correlation between the miR-205 and MDM4 mRNA
expression was analyzed by Pearson correlation analysis. P<0.05
was considered to indicate a statistically significant
difference.
Results
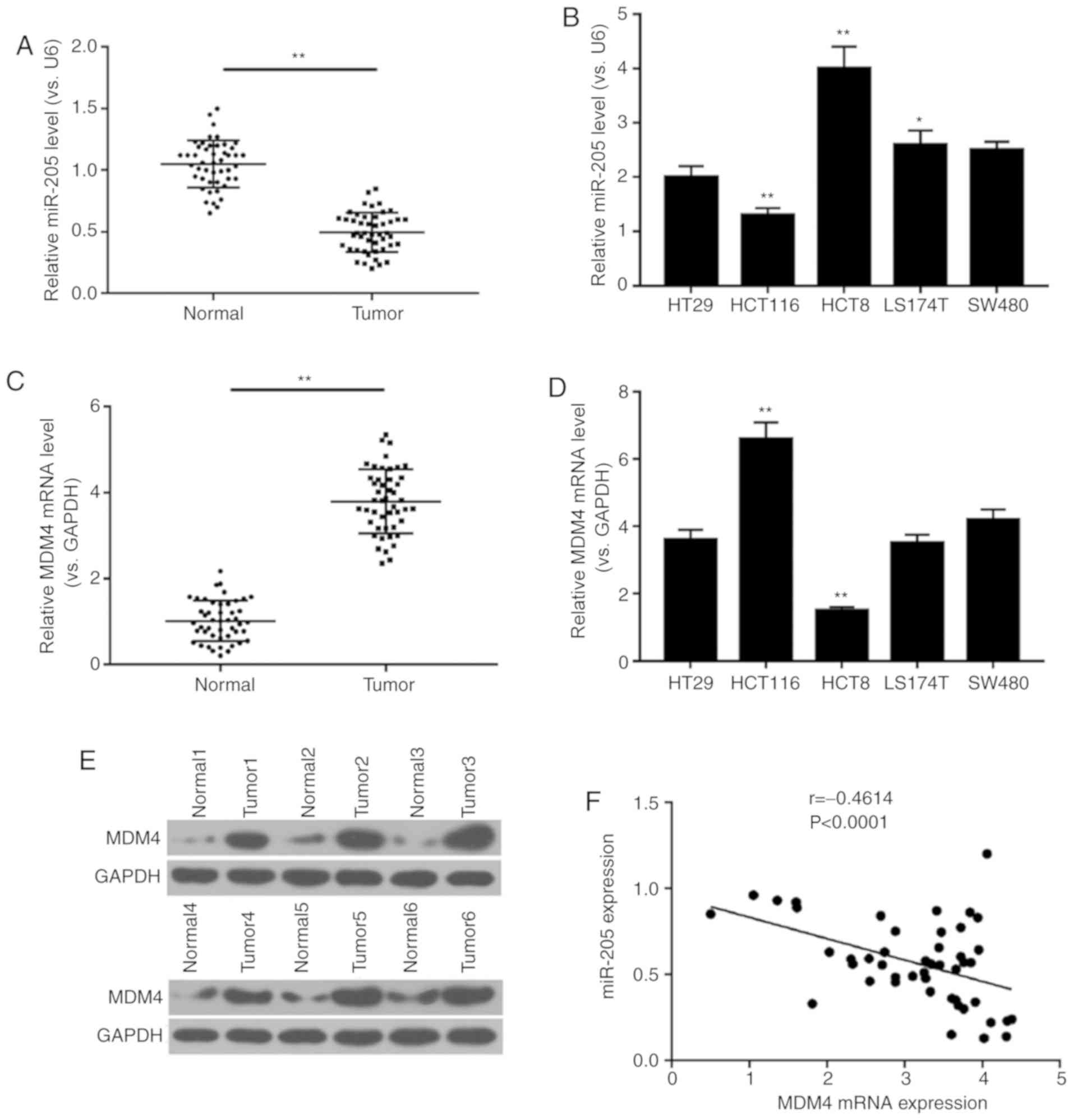
Negative correlation between miR-205
and MDM4 expression in colon cancer tissue
In order to determine the association between
miR-205 and MDM4 in colon cancer tissues and cells, RT-qPCR and
western blot analyses were performed. The results demonstrated that
the expression level of miR-205 in normal tissue was higher
compared with tumor tissue. In addition, miR-205 expression was
determined in different colon tumor cells, and it was found that
miR-205 had the lowest expression in HCT116 cells. However, mRNA
and protein levels of MDM4 in normal tissue were lower compared
with tumor tissue, and mRNA expression of MDM4 was the highest in
HCT116 cells (P<0.01; Fig.
1A-E). Thus, HCT116 cells were selected for subsequent
experiments. In addition, the data revealed a negative correlation
between miR-205 and MDM4 mRNA expression in colon cancer tissue
(r=−0.4614, P<0.0001; Fig. 1F).
As Table I demonstrates, miR-205
expression was closely associated with pathological grade, stage
and lymph node metastasis (P<0.05), and age and sex had no
significant effect.
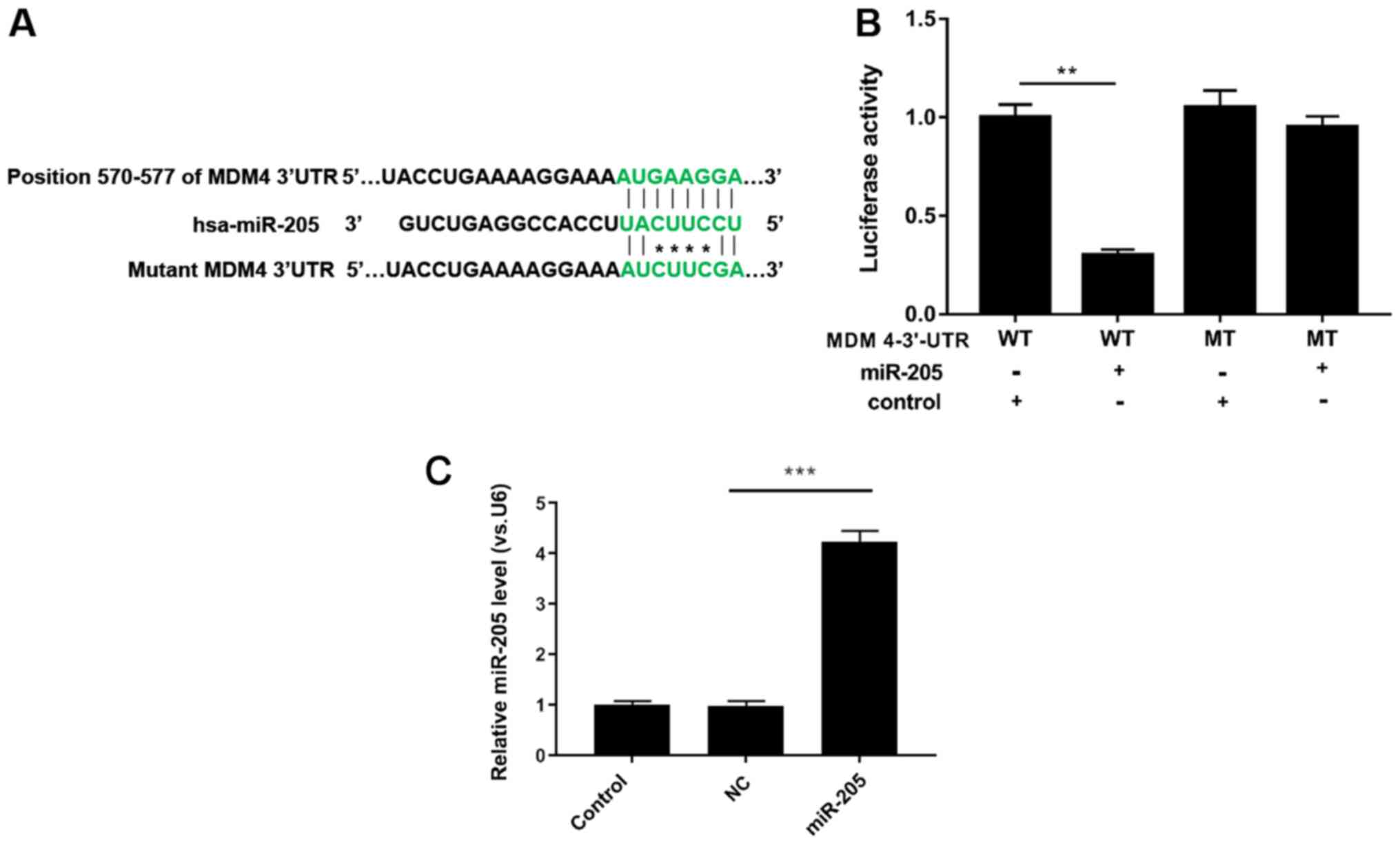
miR-205 silences MDM4 by binding with
its 3′UTR
TargetScan prediction software was used to examine
whether MDM4 is a potential target gene for miR-205, and the
results indicated that there was a single eight-nucleotide
complementary sequence at position 570–577 of the MDM4 3′UTR
(Fig. 2A). Subsequently,
luciferase activity assays demonstrated that when 293T cells were
co-transfected with miR-205 and WT MDM4 3′UTR, the luciferase
activity was significantly reduced. In addition, MT MDM4 3′UTR had
no effect on luciferase activity (P<0.05; Fig. 2B). In addition, RT-qPCR analysis
demonstrated that miR-205 was significantly upregulated in 293T
cells transfected with miR-205 mimic compared with mimic NC
(P<0.001; Fig. 2C). Thus, it
was demonstrated that miR-205 inhibited MDM4 expression by
interacting with its 3′UTR.
miR-205 suppresses cell viability
RT-qPCR, western blot and CCK-8 analyses were
performed to evaluate the expression levels of miR-205 and MDM4,
and the viability of HCT116 cells exposed to PBS, miR-205 mimic,
mimic NC, siNC and siMDM4. miR-205 was markedly upregulated in the
miR-205 mimic group compared with the mimic NC group (P<0.001;
Fig. 3A). As RT-qPCR and western
blot assay results revealed, siMDM4 significantly downregulated the
mRNA and protein level of MDM4 compared with the siNC group. In
comparison with the mimic NC group, miR-205 mimic transfection also
significantly reduced the expression of MDM4 (P<0.01; Fig. 3B-D). In addition, the CCK-8 data
demonstrated that compared with the mimic NC group, the OD value
significantly decreased in the miR-205 mimic group at 48 and 72 h,
and MDM4 silencing in cells reduced the value of OD compared with
the siNC group at 24, 48 and 72 h (P<0.05; Fig. 3E).
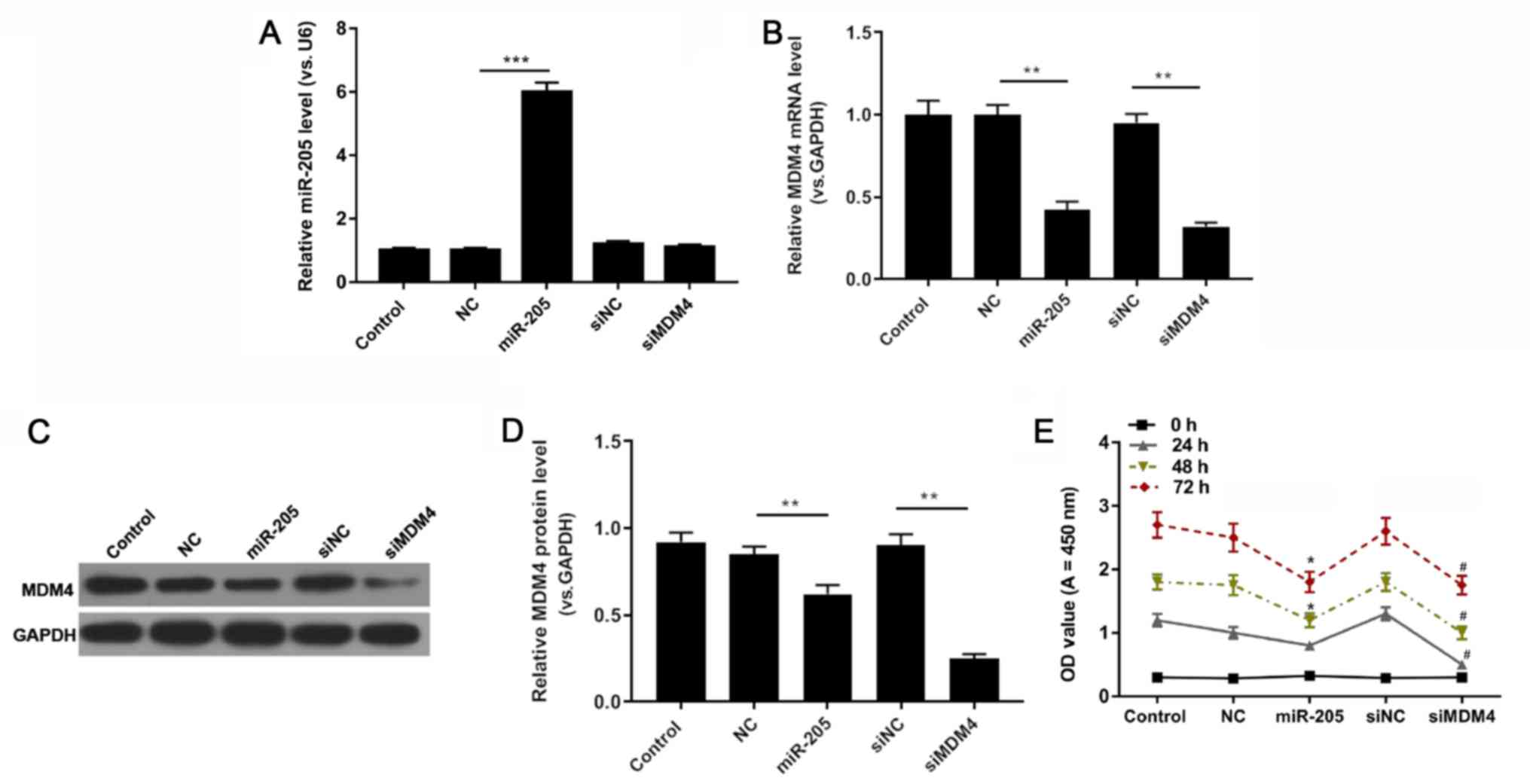 | Figure 3.miR-205 suppresses cell viability.
HCT116 cells were treated with PBS, miR-205, NC, siNC and siMDM4.
(A) miR-205 and (B) MDM4 expression were determined using reverse
transcription-quantitative PCR. **P<0.01 and ***P<0.001, as
indicated. MDM4 protein expression was (C) determined by western
blotting and (D) quantified. **P<0.01, as indicated. (E) Cell
Counting Kit-8 was used to investigate cell viability. *P<0.05
vs. NC and #P<0.05 vs. siNC. miR, microRNA; control,
PBS; miR-205, miR-205 mimic; NC, (mimic) negative control; si,
small interfering RNA; MDM4, mouse double minute 4; OD, optical
density. |
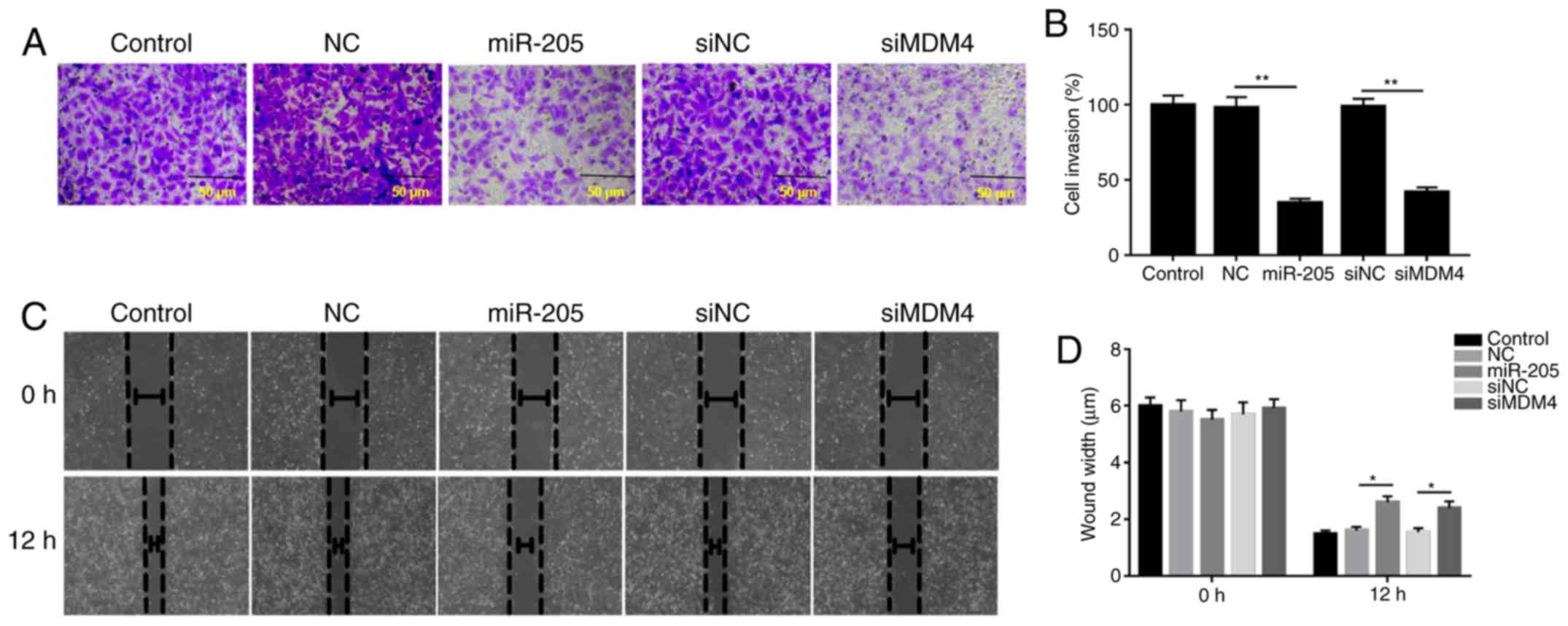
miR-205 suppresses cell invasion and
migration
The invasive and migratory abilities of cells were
explored via Transwell and wound healing assays. The data from
Transwell assays demonstrated that the invasion of cells was
inhibited by siMDM4 compared with siNC group; similarly, miR-205
overexpression significantly decreased cell invasion compared with
the mimic NC group (P<0.05; Fig.
4A). In addition, the wound healing assay results revealed that
the wound width was increased in the miR-205 mimic and siMDM4
groups compared with the mimic NC and siNC groups, respectively.
The wound healing results demonstrated that miR-205 suppressed cell
migration (P<0.05; Fig.
4B).
miR-205 mediates the expression of
EMT-associated factors
In order to further study the molecular mechanism of
miR-205 inhibiting cell invasion and migration, the expression
levels of E-cadherin, N-cadherin, vimentin, MMP-2 and MMP-9 were
measured by RT-qPCR and western blotting. As RT-qPCR and western
blot assays revealed, siMDM4 downregulated the mRNA and protein
levels of MDM4, N-cadherin, vimentin, MMP2 and MMP9 compared with
the siNC group. In comparison with the mimic NC group, miR-205
overexpression also significantly reduced the expression of
N-cadherin, vimentin, MMP2 and MMP9. However, the expression levels
of E-cadherin were upregulated in the siMDM4 and miR-205 mimic
groups compared with the siNC and mimic NC groups (P<0.05;
Fig. 5).
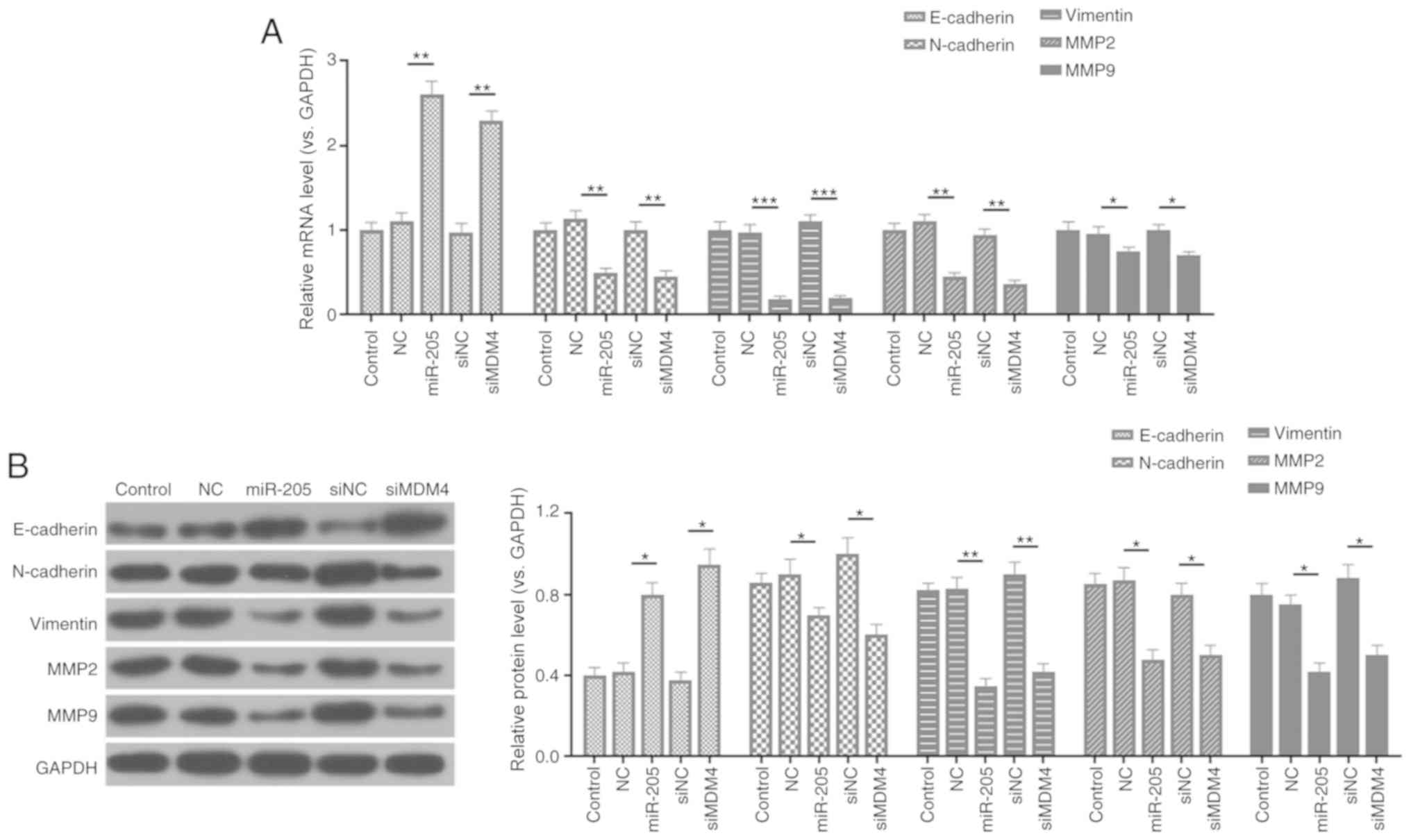 | Figure 5.miR-205 mediates the expressions of
epithelial-mesenchymal transition-associated factors. (A) Reverse
transcription-quantitative PCR was carried out to determine the
mRNA levels of E-cadherin, N-cadherin, vimentin, MMP2, and MMP9.
(B) Protein expressions of E-cadherin, N-cadherin, vimentin, MMP2,
and MMP9 were detected by western blot assay. *P<0.05,
**P<0.01 and ***P<0.001. miR, microRNA; control, PBS;
miR-205, miR-205 mimic; NC, (mimic) negative control; si, small
interfering RNA; MDM4, mouse double minute 4; MMP, matrix
metalloproteinase. |
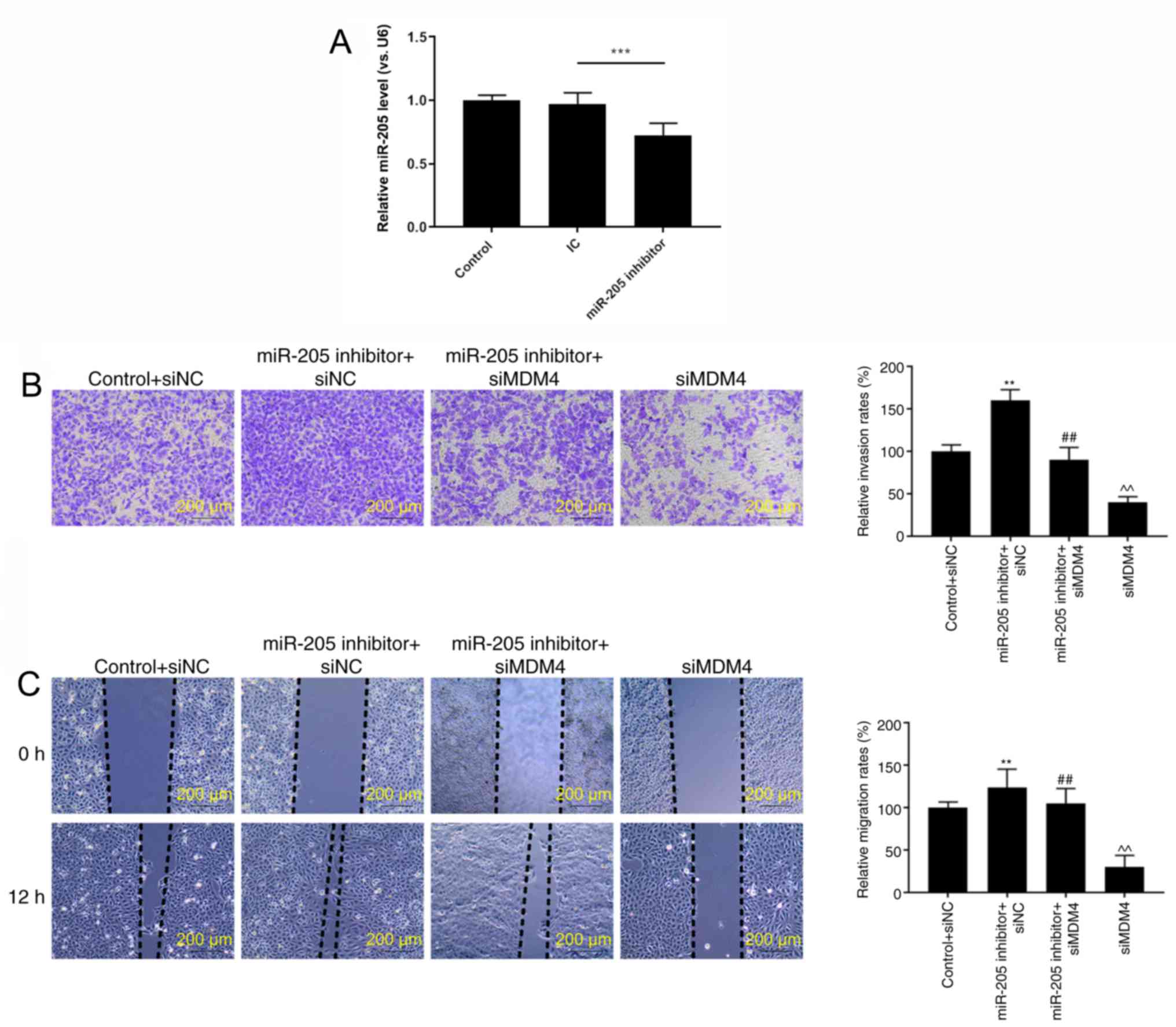
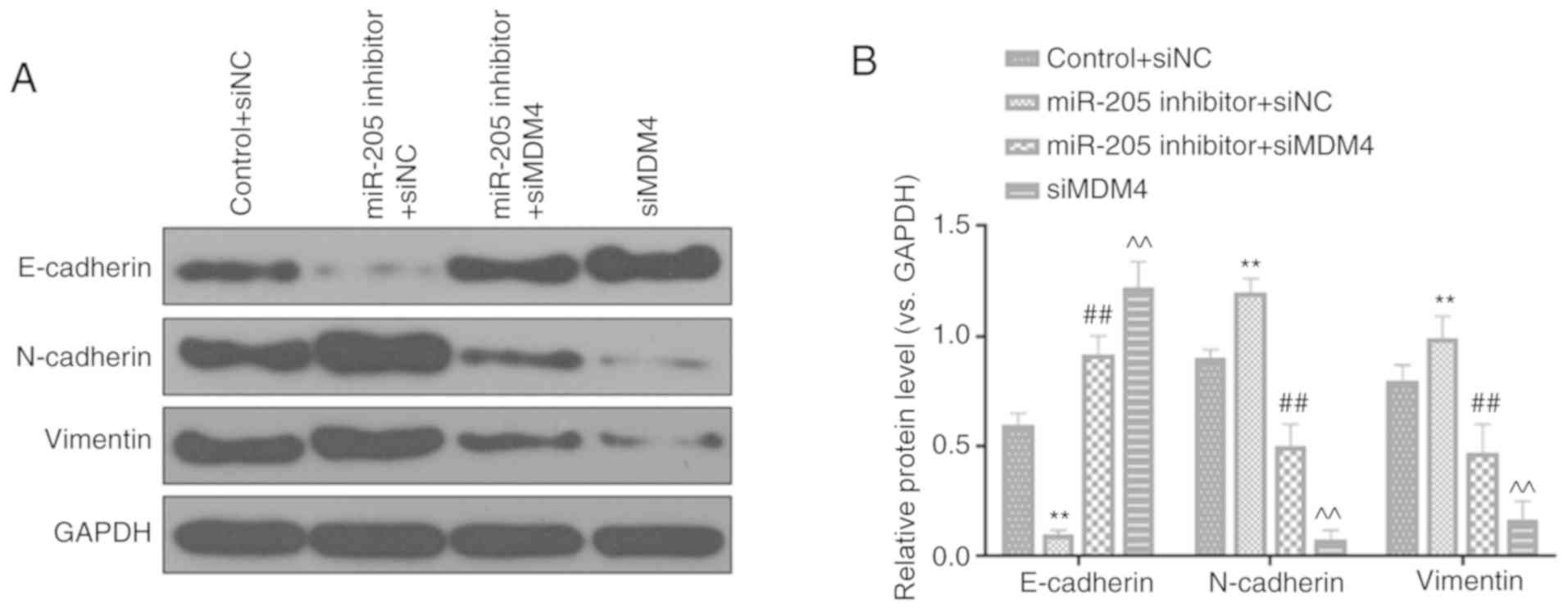
Silencing MDM4 partially reverses the
regulatory effects of miR-205 inhibition on invasion, migration and
EMT
RT-qPCR analysis demonstrated that miR-205 was
significantly downregulated in cells transfected with miR-205
inhibitor compared with the inhibitor control (P<0.001; Fig. 6A). In order to further verify the
effects of miR-205 through MDM4, rescue experiments were performed.
It was identified that miR-205 inhibitor significantly promoted
cell invasion (P<0.01; Fig. 6B)
and migration (P<0.01; Fig.
6C). In addition, silencing MDM4 could partially reversed the
increase effects of miR-205 inhibition on invasion and migration
(P<0.01). Furthermore, the expression of EMT-related proteins
was also observed. miR-205 inhibition significantly inhibited
E-cadherin expression, while increasing N-cadherin and vimentin
levels. Silencing MDM4 could also partially reversed the regulatory
effects of miR-205 inhibition on EMT (P<0.01; Fig. 7A and B).
Discussion
MDM4, which mediates p53-independent activities, is
abnormally expressed in various cancer cells and contributes to the
development of cancer (36–38).
Gilkes et al (39) noted
that MDM4 is overexpressed in human colon tumors; consistent with
these findings, the present results also revealed overexpressed
MDM4 in human colon cancer tissues and cells. In addition, it has
been found that inhibition of the expression of MDM4 can impede the
proliferation and metastasis of tumor cells (40). To some extent, the present study
demonstrated that MDM4 silencing in human colon tumor HCT116 cells
significantly suppressed proliferation, migration and invasion.
A number of miRNAs can regulate cancer cell progress
by targeting MDM4. For example, Jiang et al (41) reported that overexpressed miR-33a
can suppress renal cell cancer growth by inhibiting the expression
of MDM4. miR-766 can increase human colon cancer cell apoptosis
through MDM4 (42). Previous
studies suggested that miR-205 possibly has distinct functions in
different cancers. It is reported that miR-205 is downregulated in
colon, breast and prostate cancers (43–45),
but upregulated in lung, bladder and ovarian cancers (46,47).
The present study also demonstrated the downregulation of miR-205
in human colon tumor tissues and cells. miR-205 also can interact
with the 3′UTR of certain genes, and then mediate the translation
of genes and regulate tumor processes (48–50).
Zhuang et al (51)
demonstrated that miR-205 suppresses human pancreatic cancer
progression by targeting runt-related transcription factor 2. A
previous study indicated that miR-205 downregulates Prospero
homeobox 1 by binding to its 3′UTR, thus further suppressing the
viability and metastasis of human colon cancer cells (44). Thus, the present study investigated
the relationship between miR-205 and MDM4 in colon cancer, and the
data demonstrated that the expression levels of miR-205 and MDM4
were negatively correlated. In addition, the prediction results
indicated that there was a single 8-nucleotide complementary
sequence between hsa-miR-205 and the position 570–577 of MDM4
3′UTR.
miR-205 plays a vital role in the growth, migration
and invasion of tumors (52,53).
Previous studies have confirmed that miR-205 has anti-proliferation
and anti-invasion effects on gastric and cervical tumors (54,55).
As expected, the upregulation of miR-205 in HCT116 cells notably
attenuated cell proliferation, migration and invasion by silencing
the MDM4 gene. Furthermore, EMT-related proteins, including
E-cadherin, N-cadherin, vimentin, MMP2 and MMP9, were detected.
E-cadherin is an important adhesion molecule for maintaining
epithelial cell characteristics. N-cadherin, which plays a key role
in promoting cell movement, is considered as one of the
characteristic molecular markers of mesenchymal cells (56,57).
A recent study observed that the overexpression of miR-205 in
anaplastic thyroid carcinoma predominantly blocks the process of
EMT by targeting zinc finger E-box-binding homeobox 1 gene, which
upregulates E-cadherin expression, and downregulates N-cadherin,
vimentin, MMP2 and MMP9 expression levels (26). Similarly, the results of the
present study demonstrated that miR-205 targeted the MDM4 gene to
suppress EMT, followed by downregulation of N-cadherin, vimentin,
MMP2 and MMP9, and upregulation of E-cadherin.
In conclusion, the present study characterized the
miR-205-MDM4 mechanism in human colon cancer. It found that miR-205
and MDM4 expressions are negatively correlated in human colon
cancer. In addition, miR-205 significantly suppressed the
proliferation, migration, invasion and EMT of human colon cancer
cells by silencing MDM4 gene. Thus, miR-205 could be employed in
the treatment of human colon cancer.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YF made substantial contributions to the study
conception and design. KW performed data acquisition, data analysis
and interpretation. YF drafted the manuscript and critically
revised it for important intellectual content. Both authors gave
final approval to the published version of the study and agreed to
be accountable for all aspects of the work in ensuring that
questions related to the accuracy or integrity of the work are
appropriately investigated and resolved. Both authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The current study was reviewed and approved by the
Ethics Committee of Beijing Jishuitan Hospital (approval no.
J20110104015). All procedures performed in studies involving human
participants were in accordance with the ethical standards of the
institutional research committee and the Declaration of Helsinki.
All patients signed informed consent and agreed that their tissues
would be used for clinical research.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lozano R, Naghavi M, Foreman K, Lim S,
Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, et
al: Global and regional mortality from 235 causes of death for 20
age groups in 1990 and 2010: A systematic analysis for the Global
Burden of Disease Study 2010. Lancet. 380:2095–2128. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Muto Y, Chochi K, Morita D, Oka A and
Rikiyama T: An elderly patient with metastatic colon cancer
achieved long-term survival following single-agent chemotherapy
with S-1. Gan To Kagaku Ryoho. 45:55–57. 2018.(In Japanese).
PubMed/NCBI
|
|
5
|
Sun LY: Essential and interpretation of
Japanese society for cancer of the colon and rectum (JSCCR)
guidelines 2019 for the treatment of colorectal cancer. Zhonghua
Wei Chang Wai Ke Za Zhi. 22:1088–1094. 2019.(In Chinese).
PubMed/NCBI
|
|
6
|
Taieb J, Shi Q, Pederson L, Alberts S,
Wolmark N, Van Cutsem E, de Gramont A, Kerr R, Grothey A, Lonardi
S, et al: Prognosis of microsatellite instability and/or mismatch
repair deficiency stage III colon cancer patients after disease
recurrence following adjuvant treatment: Results of an ACCENT
pooled analysis of seven studies. Ann Oncol. 30:1466–1471. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
O'Connell JB, Maggard MA and Ko CY: Colon
cancer survival rates with the new American joint committee on
cancer sixth edition staging. J Natl Cancer Inst. 96:1420–1425.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fang WG and Tian XX: Identification of a
new pro-invasion factor in tumor microenvironment: Progress in
function and mechanism of extracellular ATP. Beijing Da Xue Xue Bao
Yi Xue Ban. 49:188–195. 2017.(In Chinese). PubMed/NCBI
|
|
9
|
Fan Y, Shen B, Tan M, Mu X, Qin Y, Zhang F
and Liu Y: TGF-β-induced upregulation of malat1 promotes bladder
cancer metastasis by associating with suz12. Clin Cancer Res.
20:1531–1541. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhao Y, Guo Q, Chen J, Hu J, Wang S and
Sun Y: Role of long non-coding RNA HULC in cell proliferation,
apoptosis and tumor metastasis of gastric cancer: A clinical and in
vitro investigation. Oncol Rep. 31:358–364. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Diepenbruck M and Christofori G:
Epithelial-mesenchymal transition (EMT) and metastasis: Yes, no,
maybe? Curr Opin Cell Biol. 43:7–13. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lou Y, Diao L, Cuentas ER, Denning WL,
Chen L, Fan YH, Byers LA, Wang J, Papadimitrakopoulou VA, Behrens
C, et al: Epithelial-mesenchymal transition is associated with a
distinct tumor microenvironment including elevation of inflammatory
signals and multiple immune checkpoints in lung adenocarcinoma.
Clin Cancer Res. 22:3630–3642. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tan TZ, Miow QH, Miki Y, Noda T, Mori S,
Huang RY and Thiery JP: Epithelial-mesenchymal transition spectrum
quantification and its efficacy in deciphering survival and drug
responses of cancer patients. EMBO Mol Med. 6:1279–1293. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhu BB, Wang H, Chi YF, Wang YM, Yao XM,
Liu S, Qiu H, Fang J, Yin PH, Zhang XM and Peng W: Protective
effects of probucol on Ox-LDL-induced epithelial-mesenchymal
transition in human renal proximal tubular epithelial cells via
LOX1/ROS/MAPK signaling. Mol Med Rep. 17:1289–1296. 2018.PubMed/NCBI
|
|
15
|
Esquela-Kerscher A and Slack FJ:
Oncomirs-microRNAs with a role in cancer. Nat Rev Cancer.
6:259–269. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ma S, Deng X, Yang Y, Zhang Q, Zhou T and
Liu Z: The lncRNA LINC00675 regulates cell proliferation,
migration, and invasion by affecting Wnt/β-catenin signaling in
cervical cancer. Biomed Pharmacother. 108:1686–1693. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bentwich I, Avniel A, Karov Y, Aharonov R,
Gilad S, Barad O, Barzilai A, Einat P, Einav U, Meiri E, et al:
Identification of hundreds of conserved and nonconserved human
microRNAs. Nat Genet. 37:766–770. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Berezikov E, Guryev V, van de Belt J,
Wienholds E, Plasterk RH and Cuppen E: Phylogenetic shadowing and
computational identification of human microRNA genes. Cell.
120:21–24. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Stojkovic S, Jurisic M, Kopp CW,
Koppensteiner R, Huber K, Wojta J and Gremmel T: Circulating
microRNAs identify patients at increased risk of in-stent
restenosis after peripheral angioplasty with stent implantation.
Atherosclerosis. 269:197–203. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lim LP, Glasner ME, Yekta S, Burge CB and
Bartel DP: Vertebrate microRNA genes. Science. 299:15402003.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang G, Hou X, Li Y and Zhao M: MiR-205
inhibits cell apoptosis by targeting phosphatase and tensin homolog
deleted on chromosome ten in endometrial cancer Ishikawa cells. BMC
Cancer. 14:4402014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lebanony D, Benjamin H, Gilad S, Ezagouri
M, Dov A, Ashkenazi K, Gefen N, Izraeli S, Rechavi G, Pass H, et
al: Diagnostic assay based on hsa-miR-205 expression distinguishes
squamous from nonsquamous non-small-cell lung carcinoma. J Clin
Oncol. 27:2030–2037. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Qin AY, Zhang XW, Liu L, Yu JP, Li H, Wang
SZ, Ren XB and Cao S: MiR-205 in cancer: An angel or a devil? Eur J
Cell Biol. 92:54–60. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Grelet S, Link LA, Howley B, Obellianne C,
Palanisamy V, Gangaraju VK, Diehl JA and Howe PH: A regulated PNUTS
mRNA to lncRNA splice switch mediates EMT and tumour progression.
Nat Cell Biol. 19:1105–1115. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tellez CS, Juri DE, Do K, Bernauer AM,
Thomas CL, Damiani LA, Tessema M, Leng S and Belinsky SA: EMT and
stem cell-like properties associated with miR-205 and miR-200
epigenetic silencing are early manifestations during
carcinogen-induced transformation of human lung epithelial cells.
Cancer Res. 71:3087–3097. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Vosgha H, Ariana A, Smith RA and Lam AK:
miR-205 targets angiogenesis and EMT concurrently in anaplastic
thyroid carcinoma. Endocr Relat Cancer. 25:323–337. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shvarts A, Steegenga WT, Riteco N, van
Laar T, Dekker P, Bazuine M, van Ham RC, van der Houven van Oordt
W, Hateboer G, van der Eb AJ and Jochemsen AG: MDMX: A novel
p53-binding protein with some functional properties of MDM2. EMBO
J. 15:5349–5357. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Golestanian S, Sharifi A, Popowicz GM,
Azizian H, Foroumadi A, Szwagierczak A, Holak TA and Amanlou M:
Discovery of novel dual inhibitors against Mdm2 and Mdmx proteins
by in silico approaches and binding assay. Life Sci. 145:240–246.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Popowicz GM, Czarna A and Holak TA:
Structure of the human Mdmx protein bound to the p53 tumor
suppressor transactivation domain. Cell Cycle. 7:2441–2443. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gansmo LB, Romundstad P, Birkeland E,
Hveem K, Vatten L, Knappskog S and Lonning PE: MDM4 SNP34091
(rs4245739) and its effect on breast-, colon-, lung-, and prostate
cancer risk. Cancer Med. 4:1901–1907. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hu L, Zhang H, Bergholz J, Sun S and Xiao
ZX: MDM2/MDMX: Master negative regulators for p53 and RB. Mol Cell
Oncol. 3:e11066352016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pedram N, Pouladi N, Feizi MA, Montazeri
V, Sakhinia E and Estiar MA: Analysis of the association between
MDM4 rs4245739 single nucleotide polymorphism and breast cancer
susceptibility. Clin Lab. 62:1303–1308. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Siebring-van Olst E, Blijlevens M, de
Menezes RX, van der Meulen-Muileman IH, Smit EF and van Beusechem
VW: A genome-wide siRNA screen for regulators of tumor suppressor
p53 activity in human non-small cell lung cancer cells identifies
components of the RNA splicing machinery as targets for anticancer
treatment. Mol Oncol. 11:534–551. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Agarwal V, Bell GW, Nam JW and Bartel DP:
Predicting effective microRNA target sites in mammalian mRNAs.
Elife. 4:2015. View Article : Google Scholar
|
|
36
|
Chee SMQ, Wongsantichon J, Siau J, Thean
D, Ferrer F, Robinson RC, Lane DP, Brown CJ and Ghadessy FJ:
Structure-activity studies of Mdm2/Mdm4-binding stapled peptides
comprising non-natural amino acids. PLoS One. 12:e01893792017.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lu Z, Zhang H, Tao Y, Li X and Li G: MDM4
genetic variants predict HPV16-positive tumors of patients with
squamous cell carcinoma of the oropharynx. Oncotarget.
8:86710–86717. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ten Kate FJ, Suzuki L, Dorssers LC,
Dinjens WN, Jones DT, Nieboer D, Doukas M, Van Lanschot JJ,
Wijnhoven BP, Looijenga LH and Biermann K: Pattern of p53 protein
expression is predictive for survival in chemoradiotherapy-naive
esophageal adenocarcinoma. Oncotarget. 8:104123–104135. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gilkes DM, Pan Y, Coppola D, Yeatman T,
Reuther GW and Chen J: Regulation of MDMX expression by mitogenic
signaling. Mol Cell Biol. 28:1999–2010. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
McCubrey JA, Lertpiriyapong K, Fitzgerald
TL, Martelli AM, Cocco L, Rakus D, Gizak A, Libra M, Cervello M,
Montalto G, et al: Roles of TP53 in determining therapeutic
sensitivity, growth, cellular senescence, invasion and metastasis.
Adv Biol Regul. 63:32–48. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Jiang K, Sun F, Zhu J, Luo G, Ban Y and
Zhang P: miR-33a inhibits cell growth in renal cancer by
downregulation of MDM4 expression. Mol Genet Genomic Med.
7:e8332019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chen W, Cai G, Liao Z, Lin K, Li G and Li
Y: miRNA-766 induces apoptosis of human colon cancer cells through
the p53/Bax signaling pathway by MDM4. Exp Ther Med. 17:4100–4108.
2019.PubMed/NCBI
|
|
43
|
Gulei D, Magdo L, Jurj A, Raduly L,
Cojocneanu-Petric R, Moldovan A, Moldovan C, Florea A, Pasca S, Pop
LA, et al: The silent healer: miR-205-5p up-regulation inhibits
epithelial to mesenchymal transition in colon cancer cells by
indirectly up-regulating E-cadherin expression. Cell Death Dis.
9:662018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Nguyen-Vu T, Wang J, Mesmar F,
Mukhopadhyay S, Saxena A, McCollum CW, Gustafsson JÅ, Bondesson M
and Williams C: Estrogen receptor beta reduces colon cancer
metastasis through a novel miR-205-PROX1 mechanism. Oncotarget.
7:42159–42171. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yamada Y, Nishikawa R, Kato M, Okato A,
Arai T, Kojima S, Yamazaki K, Naya Y, Ichikawa T and Seki N:
Regulation of HMGB3 by antitumor miR-205-5p inhibits cancer cell
aggressiveness and is involved in prostate cancer pathogenesis. J
Hum Genet. 63:195–205. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Braicu OL, Budisan L, Buiga R, Jurj A,
Achimas-Cadariu P, Pop LA, Braicu C, Irimie A and Berindan-Neagoe
I: miRNA expression profiling in formalin-fixed paraffin-embedded
endometriosis and ovarian cancer samples. Onco Targets Ther.
10:4225–4238. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Li JH, Sun SS, Li N, Lv P, Xie SY and Wang
PY: MiR-205 as a promising biomarker in the diagnosis and prognosis
of lung cancer. Oncotarget. 8:91938–91949. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wei J, Zhang L, Li J, Zhu S, Tai M, Mason
CW, Chapman JA, Reynolds EA, Weiner CP and Zhou HH: MicroRNA-205
promotes cell invasion by repressing TCF21 in human ovarian cancer.
J Ovarian Res. 10:332017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Xu CG, Yang MF, Fan JX and Wang W: MiR-30a
and miR-205 are downregulated in hypoxia and modulate
radiosensitivity of prostate cancer cells by inhibiting autophagy
via TP53INP1. Eur Rev Med Pharmacol Sci. 20:1501–1508.
2016.PubMed/NCBI
|
|
50
|
Yang G, Zhang P, Lv A, Liu Y and Wang G:
MiR-205 functions as a tumor suppressor via targeting TGF-α in
osteosarcoma. Exp Mol Pathol. 100:160–166. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zhuang L, Guo J, Yao Y and Li Z: miR-205
targets runt-related transcription factor 2 to inhibit human
pancreatic cancer progression. Oncol Lett. 17:843–848.
2019.PubMed/NCBI
|
|
52
|
Chen S, Jin L, Nie S, Han L, Lu N and Zhou
Y: MiR-205 inhibits growth and invasion of neuroblastoma by
targeting cAMP responsive element binding protein 1. Oncol Res.
26:445–455. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Xu C, Li M, Zhang L, Bi Y, Wang P, Li J
and Jiang X: MicroRNA-205 suppresses the invasion and
epithelial-mesenchymal transition of human gastric cancer cells.
Mol Med Rep. 13:4767–4773. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Pang H and Yue X: MiR-205 serves as a
prognostic factor and suppresses proliferation and invasion by
targeting insulin-like growth factor receptor 1 in human cervical
cancer. Tumour Biol. 39:10104283177013082017. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Yin WZ, Li F, Zhang L, Ren XP, Zhang N and
Wen JF: Down-regulation of microRNA-205 promotes gastric cancer
cell proliferation. Eur Rev Med Pharmacol Sci. 18:1027–1032.
2014.PubMed/NCBI
|
|
56
|
Chen T, You Y, Jiang H and Wang ZZ:
Epithelial-mesenchymal transition (EMT): A biological process in
the development, stem cell differentiation, and tumorigenesis. J
Cell Physiol. 232:3261–3272. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Rodriguez-Monterrosas C, Diaz-Aragon R,
Leal-Orta E, Cortes-Reynosa P and Perez Salazar E: Insulin induces
an EMT-like process in mammary epithelial cells MCF10A. J Cell
Biochem. 119:4061–4071. 2018. View Article : Google Scholar : PubMed/NCBI
|