Introduction
Host restrictive factors can inhibit human
immunodeficiency virus-1 (HIV-1) infection at various stages, and
these factors include apolipoprotein B mRNA editing enzyme
catalytic subunit 3G proteins (1,2),
Tripartite motif-containing protein 5 α (3,4),
tetherin/bone marrow stromal cell antigen 2 (5) and sterile α motif and
histidine/aspartic acid domain-containing protein 1 (SAMHD1)
(6–9). SAMHD1 is a newly discovered HIV-1
host restriction factor, which can be degraded by the viral
accessory protein Vpx of HIV-2 and certain simian immunodeficiency
viruses (6–10). However, HIV-1 has lost the Vpx
protein during evolution (9).
Therefore, SAMHD1 is a host restriction factor that cannot be
antagonized by HIV-1.
HIV-1 RNA is reversely transcribed into DNA
following its fusion to target host cells, and this requires an
adequate supply of deoxy-ribonucleoside triphosphate (dNTPs).
SAMHD1 is a diguanosine triphosphate-dependent phosphatase that
primarily hydrolyzes dNTPs into deoxynucleoside and inorganic
triphosphoric acid, thus reducing the nuclear dNTP concentrations
and inhibiting the reverse transcription of HIV-1 (11). There are several in vivo
studies that have investigated SAMHD1 in the peripheral blood of
patients with HIV-1 and analyzed its role in disease progression.
Riveira-Muñoz et al (12)
studied SAMHD1 expression in the CD4+ T cells of
patients with HIV-1, including elite controllers (ECs) with
sustained plasma viral load below the limit of detection in the
absence of antiretroviral treatment and viremic progressors (VPs)
with high level of viral load (>5,000 cp/ml) and notable loss of
CD4+ T cells (<400/µl); the results demonstrated that
higher levels of SAMHD1 were present in ECs compared with the
healthy controls (HCs) and VPs, suggesting that SAMHD1 may serve a
role in controlling viral replication and slowing the rate of
disease progression. However, conflicting results were reported in
HIV-exposed seronegative (HESN) individuals in other studies
(13,14). Therefore, SAMHD1 expression in the
peripheral blood of patients with HIV-1, especially in
CD4+ T cells, should continue to be investigated.
Furthermore, since SAMHD1 restricts the reverse transcription of
HIV-1, it may be interesting to study the association between
SAMHD1 expression and HIV-1 DNA levels in CD4+ T cells.
The present study aimed to investigate the expression of SAMHD1
CD4+ T cells of patients with HIV-1 receiving
antiretroviral therapy (HIV-ARTs), VPs, ECs and HCs, and to study
the associations between SAMHD1 expression and immune activation as
well as the levels of HIV-1 DNA.
Materials and methods
Subjects
HIV-1 positive patients with the mean age of 47.75
years (range, 27–62 years) were recruited from the Second
Affiliated Hospital and Yuying Children's Hospital of Wenzhou
Medical University and the First Affiliated Hospital, School of
Medicine, Zhejiang University (Zhejiang, China) between January and
September 2018. HIV-1 infection was diagnosed based on the positive
results obtained from serological and HIV RNA detection assays. All
subjects were volunteers and provided written informed consent to
participate in the study. This study was approved by the ethics
review boards of The Second Affiliated Hospital and Yuying
Children's Hospital of Wenzhou Medical University and the First
Affiliated Hospital, School of Medicine, Zhejiang University
(Zhejiang, China).
Three groups of patients with HIV-1 were included in
the present study: i) 32 VPs who were ART naive and exhibited
typical disease progression; ii) 26 HIV-ARTs with suppressed HIV-1
replication, whose viral load was maintained <50 copies/µl or
with viral blips <1,000 copies/µl; and iii) 15 ECs with a
sustained plasma viral load below the limit of detection in the
absence of ART (non-consecutive blips of <2,000 copies/mlwere
allowed if present in <20% of viral load determinations)
(12). In addition, 28 HCs were
recruited from community clinics and comprised individuals who had
not suffered from infectious or immune diseases during the previous
month. The four groups were age- and sex-matched, and there were no
significant differences in the mean CD8+ T cell counts
(Table I).
 | Table I.Clinical information of the
subjects. |
Table I.
Clinical information of the
subjects.
| Variable | HCs | VPs | HIV-ARTs | ECs | P-value |
|---|
| Number | 28 | 32 | 26 | 15 |
|
| Male (%) | 17 (60.71%) | 19 (59.38%) | 17 (65.38%) | 4 (80.00%) | 0.37 |
| Age, years | 31.35±14.63 |
38.71±7.63 | 42.66±11.57 | 36.81±15.19 | 0.53 |
| Viral load
(log10) | – |
4.35±1.42 | 1.74±1.01 | – |
<0.01a |
| CD4+ T
cells/µl | 702.66±142.82 |
356.17±188.83 | 517.05±161.20 | 637.58±216.33 | 0.01a |
| CD8+ T
cells/µl | 674.46±228.94 |
1,056.81±523.02 | 835.88±370.36 | 747.88±298.36 | 0.17 |
Detection of SAMHD1 mRNA in the
peripheral blood
Peripheral blood (~5 ml) was collected from the
participants into tubes containing EDTA. Peripheral blood
mononuclear cells (PBMCs) were isolated by a density gradient
centrifugation method (1,000 × g for 20–30 min at room temperature)
using Ficoll-Paque PLUS (GE Healthcare Life Science).
CD4+ T lymphocytes were purified from the PBMCs using
MACS human CD4 microbeads for positive selection (Miltenyi Biotec
GmbH). The purity of CD4+ T lymphocytes was >90%. The
total RNA was extracted from the PBMCs and CD4+ T
lymphocytes using TRIzol® reagent (Thermo Fisher
Scientific, Inc.) and reverse-transcribed into cDNA using a total
volume of 20 µl with the temperature protocol of 30°C for 10 min,
42°C for 45 min, and 95°C for 5 min. The quantitative analysis of
SAMHD1 mRNA was performed using reverse transcription-quantitative
(RT-q) PCR as previously described (15). Briefly, the reaction mixture
included 10 µl SYBR® Green Master Mix (Takara
Biotechnology Co., Ltd.), 0.2 µl each of the forward and reverse
primers, and 2 µl cDNA, increased to a final volume of 20 µl with
RNase-free water. RT-qPCR was performed using a DNA Engine Chromo 4
Real-time qPCR System (Bio-Rad Laboratories, Inc.) and the
thermocycling conditions were as follows: 40 cycles of denaturation
at 95°C for 45 sec, annealing at 62°C for 30 sec and extension at
72°C for 30 sec; the dsDNA was measured at 86°C after each cycle.
GAPDH mRNA was detected as the internal control. The primers used
were as follows: SAMHD1 forward, 5′-AAAACCAGGTTTCACAACTTCTGC-3′ and
reverse, 5′-TGCGGCATACAAACTCTTTCTGT-3′; and GAPDH forward,
5′-TGCACCACCAACTGCTTAGC-3′ and reverse,
5′-GGCATGGACTGTGGTCATGAG-3′. The relative expression levels of
SAMHD1 and GAPDH were calculated using the 2−ΔΔCq method
(16). Each sample was amplified
three times.
Detection of plasma interferon (IFN)-α
levels
The plasma IFN-α levels were detected using a human
IFN-α ELISA kit (cat. no. 41100-1, R&D Systems, Inc.),
according to the manufacturer's instructions. IFN-α concentrations
were determined by comparing the samples with a standard curve.
Quantification of HIV-1 RNA and
DNA
The HIV-1 viral load in the plasma of patients
infected with HIV-1 was quantitatively detected using a
standardized RT-qPCR (Cobas Amplicor HIV-1 Monitor Test; version
1.5; Ultra-sensitive specimen preparation; Roche Diagnostic Systems
Inc) as previously described (17). The detection limit in the plasma
was defined as 50 HIV-1 RNA copies/ml.
The total HIV-1 DNA, including the integrated HIV-1
DNA and episomal two long terminal repeat (2LTR) circles, in the
peripheral blood was detected using a HIV-1 DNA Detection kit
(PCR-Fluorescent Probing; SUPBIO; Guangzhou Hailite Biotechnology
Co., Ltd.). Briefly, the total DNA was isolated from the blood
using a QIAamp DNA Blood Mini Kit (Qiagen GmbH). The 50 µl reaction
system contained 5 µl DNA and 45 µl PCR master mix. The test was
performed using a Light Cycler 1.2 (Roche Diagnostics GmbH). The
amplification conditions were set as follows: i) five cycles of
37°C for 5 min, 95°C for 10 min; ii) 95°C for 15 sec, 65°C for 15
sec and 72°C for 20 sec; iii) 40 cycles of 95°C for 15 sec, 62°C
for 15 sec and 72°C for 20 sec; and iv) 10 cycles of 95°C for 15
sec, 52°C for 15 sec and 72°C for 32 sec. Two standard curves were
calculated according to the volume of blood or PBMC counts. The
results were expressed as copies/ml and copies/106
PBMCs.
Flow cytometry
CD4+, CD8+ and activated
CD4+CD38+ human leukocyte antigen
(HLA)-DR+ T cells in the peripheral blood were measured
by flow cytometry (FACSCantoII; Becton, Dickinson and Company)
using BD FACSCanto II System Software Upgrade (v 3.0); Becton,
Dickinson and Company. The cell counts for
CD3+CD4+, CD3+CD8+ and
activated
CD3+CD4+CD38+HLA-DR+ T
cells were determined by a five-color strategy using
anti-CD3-allophycocyanin, anti-CD4-fluorescein isothiocyanate,
anti-CD8-phycoerythrin-Cy7, anti-CD38-phycoerythrin, and
anti-HLA-DR-Peridinin Chlorophyll Protein Complex-Cy5.5 (Becton,
Dickinson and Company). Cell staining was performed according to
the manufacturer's instructions.
Statistical analysis
Data are presented as the mean ± standard deviation.
The statistical analyses were performed using SPSS 20.0 (IBM
Corp.). One-way analysis of variance with Bonferroni's post hoc
test was used when comparing three or more groups. Correlations
were tested using the Pearson correlation analysis. All tests were
two-tailed. P<0.05 was considered to indicate a statistically
significant difference
Results
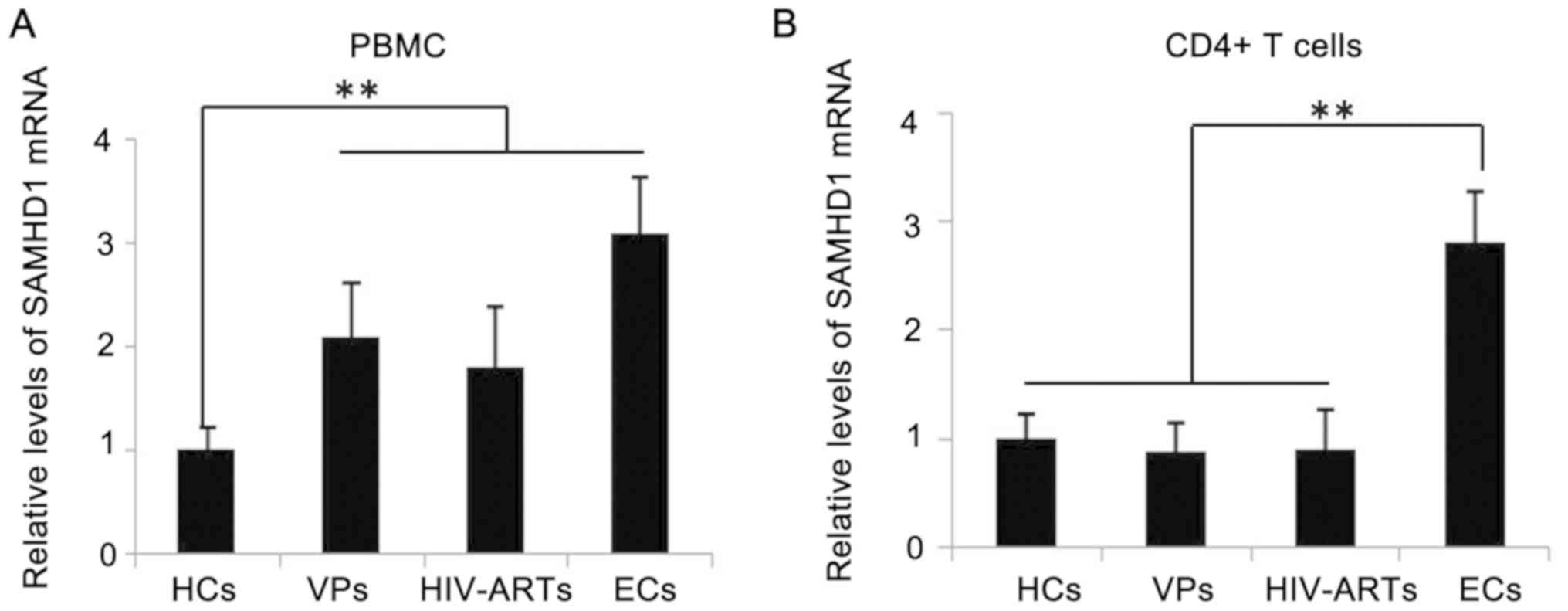
Levels of SAMHD1 increase in the PBMCs
of VPs and HIV-ARTs
SAMHD1 expression was detected in the PBMCs and
CD4+ T cells of patients with HIV-1 and HCs. SAMHD1 mRNA
expression was significantly increased in the PBMCs of VPs,
HIV-ARTs and ECs compared with that in the HCs, with the highest
level exhibited by the EC group; however, no significant
differences were observed in the SAMHD1 levels in the PBMCs between
the three groups of patients with HIV-1 (Fig. 1A). In addition, the SAMHD1 levels
in the CD4+ T cells were significantly elevated in the
ECs compared with those of the VPs, the HIV-ARTs and the HCs, but
no significant differences were observed in CD4+ T-cell
expression of SAMHD1 between the VPs, the HIV-ARTs and the HCs
(Fig. 1B). The relationship
between SAMHD1 levels in CD4+ T cells, the
CD4+ T cell count and the viral load were also analyzed.
No significant correlations were observed between the expression of
SAMHD1 in CD4+ T cells, the CD4+ T-cell count
or the viral load (Table II).
 | Table II.Correlation between SAMHD1 expression
levels in CD4+ T cells and immunological and virological
indexes. |
Table II.
Correlation between SAMHD1 expression
levels in CD4+ T cells and immunological and virological
indexes.
| SAMHD1 mRNA in
CD4+ T cells vs. | R | P-value |
|---|
| CD4+ T
cells, /µl) | 0.064 | 0.683 |
| Viral load,
cp/ml) | −0.306 | 0.107 |
| IFN-α, pg/ml) | −0.218 | 0.164 |
|
CD4+CD38+HLA-DR+
T cells, % | −0.401 | 0.013a |
| HIV-1
DNA/106 PBMC | −0.168 | 0.596 |
| HIV-1 DNA/ml
blood | 0.032 | 0.357 |
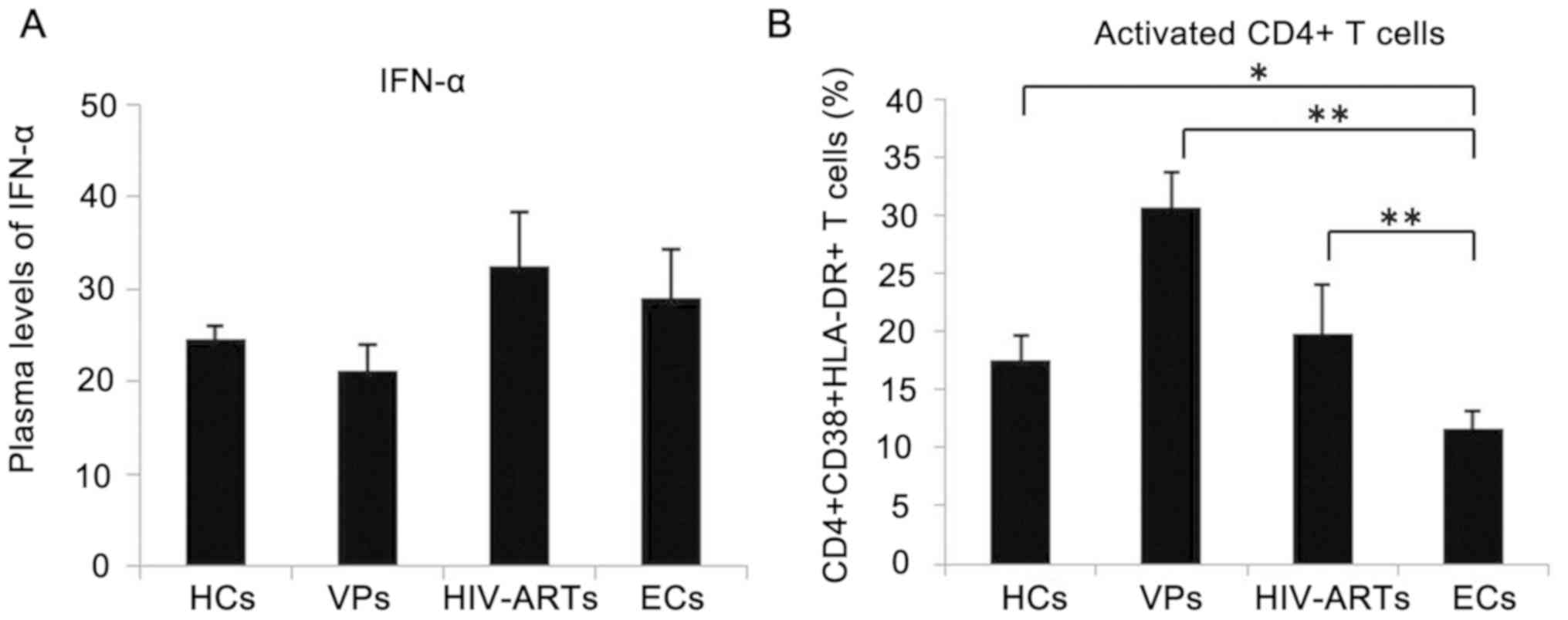
SAMHD1 expression is associated with
the low level of CD4+ T-cell activation
The secretion of IFN-α and the level of T cell
activation are typically increased during a viral infection
(18–20). Considering that SAMHD1 expression
is affected by IFNs and immune activation (21), the concentration of IFN-α in the
plasma and the percentage of activated CD4+ T cells in
patients withHIV-1 and HCs were assessed. No significant
differences were observed in the plasma IFN-α levels between
patients with HIV-1 and the HCs (Fig.
2A). Flow cytometric analysis revealed significantly higher
immune activation of CD4+ T cells in the VPs and the
HIV-ARTs (Fig. 2B) compared with
those in the ECs and the HCs. In addition, the activation of
CD4+ T cells in ECs was lower compared with that in HCs,
VPs and HIV-ARTs (Fig. 2B).
Correlation analysis revealed that SAMHD1 expression was inversely
correlated with the activation of CD4+ T cells; no
correlation was observed between SAMHD1 expression in
CD4+ T cells and plasma IFN-α levels (Table II).
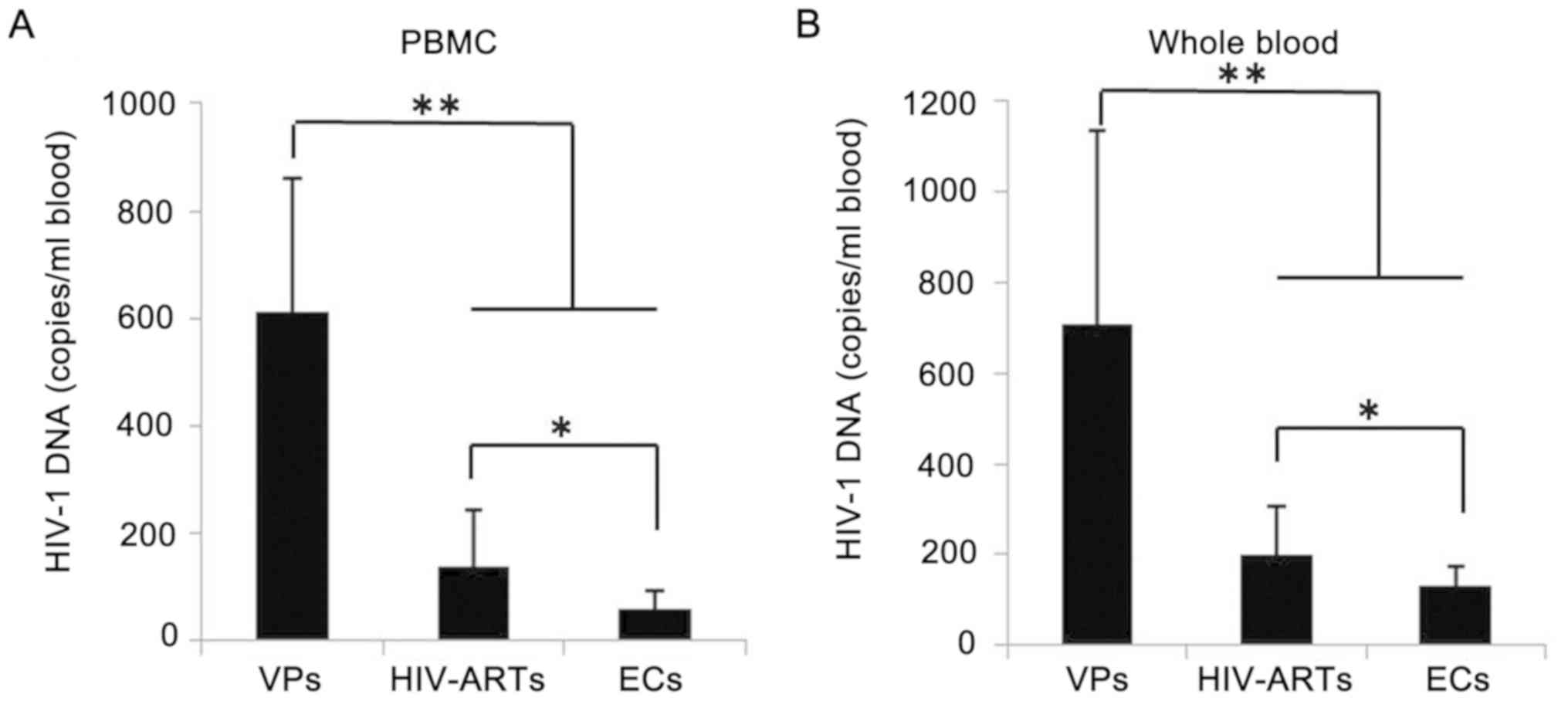
Total HIV-1 DNA levels in ECs are not
correlated with the levels of SAMHD1 expression
Whether the total HIV-1 DNA levels in the peripheral
blood correlated with SAMHD1 expression in CD4+ T cells
was further determined. The total HIV-1 DNA levels in the VPs were
612.86±248.95 copies/106 PBMCs or 708.52±427.30
copies/ml blood, which was significantly higher compared with those
in the HIV-ARTs (137.30±105.04 copies/106 PBMC or
201.76±103.04 copies/ml blood) and in the ECs (61.26±30.69
copies/106 PBMC and 131.26±42.66 copies/ml blood)
(Fig. 3). The total HIV-1 DNA
levels exhibited by the ECs were also lower compared those in the
HIV-ARTs. The total HIV-1 DNA levels did not correlate with SAMHD1
expression in CD4+ T cells (Table II).
Discussion
In addition to myeloid cells, SAMHD1 is also
important for HIV-1 infection of CD4+ T lymphocytes
(22–24). Over expression of SAMHD1 in
CD4+ T cell lines alters the permissibility of HIV-1
infection (22), and higher SAMHD1
expression has been observed in resting CD4+ T cells
that are resistant to HIV-1 infection compared with those in
activated CD4+ T cells (23,24).
In addition, Riveira-Muñoz et al (12) demonstrated elevated
SAMHD1expression in the CD4+ T cells of HIV-1-positive
ECs compared with the HCs and VPs, indicating an important role for
SAMHD1 in HIV-1 resistance. However, inconsistent results have been
reported by other researchers; for example, Gonzalez et al
(13) demonstrated that SAMHD1
expression was higher in the oral and genital mucosa and lower in
the PBMCs of HESN individuals who were resistant to HIV-1compared
with HCs. In addition, Santos et al (14) reported that no significant
differences were observed in the basal SAMHD1 mRNA expression
levels in the CD4+ T cells of HESN individuals compared
with those of HCs and HIV-ARTs. The results of the present study
revealed higher SAMHD1 expression in the CD4+ T cells of
ECs compared with those of HCs, which was consistent with the
findings of the study by Riveira-Muñoz et al (12). In addition, there were no
differences in the SAMHD1 levels of CD4+ T cells between
the HIV-ARTs, VPs and HCs. Notably, the present study demonstrated
higher SAMHD1 expression in the PBMCs of the HIV-ARTs and the VPs
compared with the HCs, but no significant deference was observed in
the CD4+ T cells. One reason for these findings may be
related to the altered PBMC components present in patients with
HIV-1. PBMCs consist of a mixture of different cell types,
including myeloid cells with elevated SAMHD1 expression and
lymphoid cells with low levels of SAMHD1 (22,25).
As a result of the loss of CD4+ T cells, HIV-1 VPs
exhibit a higher percentage of myeloid cells and correspondingly
higher SAMHD1 levels in their PBMCs. Other reasons for the higher
SAMHD1 levels in the PBMCs of patients with HIV-1 need to be
explored in future studies. The use of different subjects (e.g.,
ECs, HESN individuals, and patients receiving ART or not) and the
types of cells investigated (e.g., PBMCs or CD4+ T
cells) may explain the inconsistent results among published
studies.
The expression of SAMHD1 can be induced by IFNs
(15,26); however, it is unclear whether the
in vivo expression of SAMHD1 is affected by plasma IFN
levels. The present study assessed plasma IFN-α levels and the
effects on SAMHD1 expression in CD4+ T cells. No
differences in the plasma IFN-α levels were observed in any of the
four subject groups. In addition, there was no correlation between
IFN-α and SAMHD1 expression levels. Another potential factor
impacting SAMHD1 expression in CD4+ T cells is immune
activation (21). SAMHD1 is highly
expressed in resting CD4+ T cells but is decreased
during CD4+ T cell activation and proliferation
(21,23,24).
Consistent with previous studies (27,28)
indicating that HIV-1 patients have increased CD4+ T
activation, the present study demonstrated that the percentage of
activated CD4+ T cells was increased in the HIV-ARTs and
the VPs, but was decreased in the ECs compared with the HCs. In
addition, SAMHD1 expression was inversely correlated with
CD4+ T-cell activation. SAMHD1 is reported to be an
important negative regulator of the IFN response and immune
activation (29), which may
explain the reverse relationship between SAMHD1 and immune
activation. Another explanation may be that immune activation
induced by HIV-1 infection can increase the proportion of activated
CD4+ T cells with low SAMHD1 expression and decrease the
proportion of resting CD4+ T cells with high SAMHD1
expression (27). The results of
the present study suggested that high SAMHD1 expression correlates
with a low percentage of activated CD4+ T cells, and
therefore, HIV-1 target cells. Thus, the elevated expression of
SAMHD1 in the CD4+ T cells of ECs may restrict HIV-1
infection by inhibiting viral reverse transcription as well as the
extent of immune activation.
The inhibition of reverse transcription by SAMHD1
results in the reduced production of total HIV-1 DNA (30), which contains two components:
Integrated HIV-1 DNA and episomal 2LTR circles, which are transient
by-products of failed HIV-1 DNA integration (31). The total level of HIV-1 DNA has
been demonstrated to be inversely correlated with the time to viral
rebound in patients treated early with interrupted ART (32). The results of the present study
demonstrated lower total HIV-1 DNA levels in the peripheral blood
of the ECs compared with the VPs and the HIV-ARTs; however, no
correlation was observed between SAMHD1 expression and the total
HIV-1 DNA levels. The function of SAMHD1 is affected by
post-transcriptional and post-translational modifications (33). Phosphorylation of SAMHD1 T592 can
abolish the dNTPase activity of SAMHD1 (33). In addition to SAMHD1, the total
HIV-1 DNA levels can be impacted by a variety of other factors. For
example, high HIV-1 RNA production leads to high total HIV-1 DNA
levels, and the number and percentage of activated CD4+
T cells can also impact the total level of HIV-1 DNA (34,35).
Additionally, the total HIV-1 DNA level can be affected by the
formation of episomal 2LTR circles (36). Cytosolic DNA (e.g. double-stranded
HIV-1 DNA, including episomal 2LTR circles) is sensed by the cyclic
GMP-AMP synthase/stimulator of interferon genes cytosolic
DNA-sensing pathway in HIV-1 infected cells, resulting in a
spontaneous IFN response and subsequent immune activation (37). Therefore, reduced production of
HIV-1 DNA by SAMHD1 may limit the magnitude of IFN and effector T
cell responses.
The limitations associated with the present study
were as follows: i) The levels of integrated HIV-1 DNA and episomal
2LTR circles were not investigated separately due to technological
limitations; ii) the number of ECs was small; and iii) the mRNA
rather than protein levels of SAMHD1 in purified CD4+ T
cells from patients with HIV-1 were only investigated due to
limited volume of blood samples. Future studies with an expanded
sample size should be carried out to further explore the expression
of SAMHD1 in patients withHIV-1 exhibiting differential disease
progression as well as the relationship between SAMHD1 and HIV-1
DNA.
In summary, the results of the present study
indicated that SAMHD1 was highly expressed in ECs and may
contribute to low total HIV-1 DNA levels and low immune activation
observed in individuals infected with HIV-1 compared with those in
HCs. However, no correlation was observed between SAMHD1 expression
and the HIV-1 DNA levels. The results of the present study also
suggested that SAMHD1 may restrict HIV-1 infection in vivo
by inhibiting immune activation and thus reducing the number of
HIV-1 target cells instead of directly inhibiting HIV-1 reverse
transcription. This study also emphasized the importance of
reducing the immune activation levels of CD4+ T cells
for patients with HIV-1. To the best of our knowledge, this is the
first in vivo study on the relationship between SAMHD1 and
HIV-1 DNA levels in CD4+ T cells.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Zhejiang
Provincial Natural Science Foundation (grant no. QY20H190002).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JL, CG and CJ contributed to study concept and
design. SH and LJ contributed to the clinical sampling. JL, CG, SH
and CJ performed the experiments, and acquired, analyzed or
interpreted data. JL, CG and CJ drafted and revised the manuscript.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the ethics review boards
of The Second Affiliated Hospital and Yuying Children's Hospital of
Wenzhou Medical University (No. 201700837) and the First Affiliated
Hospital, School of Medicine, Zhejiang University (approval no.
IIT20171207A). Written informed consent to participate was obtained
from all subjects.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Reddy K, Ooms M, Letko M, Garrett N, Simon
V and Ndung'u T: Functional characterization of Vif proteins from
HIV-1 infected patients with different APOBEC3G haplotypes. AIDS.
30:1723–1729. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bertine M, Charpentier C, Visseaux B,
Storto A, Collin G, Larrouy L, Damond F, Matheron S, Brun-Vézinet F
and Descamps D; ANRS CO5 HIV-2 Cohort, : High level of APOBEC3F/3G
editing in HIV-2 DNA vif and pol sequences from
antiretroviral-naive patients. AIDS. 29:779–784. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Merindol N and Berthoux L: Restriction
factors in HIV-1 disease progression. Curr HIV Res. 13:448–461.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nakayama EE and Shioda T: Impact of
TRIM5alpha in vivo. AIDS. 29:1733–1743. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li SX, Barrett BS, Guo K and Santiago ML:
Tetherin/BST-2: Restriction factor or immunomodulator? Curr HIV
Res. 14:235–246. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lahouassa H, Daddacha W, Hofmann H, Ayinde
D, Logue EC, Dragin L, Bloch N, Maudet C, Bertrand M, Gramberg T,
et al: SAMHD1 restricts the replication of human immunodeficiency
virus type 1 by depleting the intracellular pool of deoxynucleoside
triphosphates. Nat Immunol. 13:223–228. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
St Gelais C and Wu L: SAMHD1: A new
insight into HIV-1 restriction in myeloid cells. Retrovirology.
8:552011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jermy A: Viral infection: SAMHD1 cuts the
power to HIV-1. Nat Rev Microbiol. 10:2372012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Laguette N and Benkirane M: How SAMHD1
changes our view of viral restriction. Trends Immunol. 33:26–33.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kang J, Hou J, Zhao K, Yu XF and Du J: HD
domain of SAMHD1 influences Vpx-induced degradation at a
post-interaction step. Biochem Biophys Res Commun. 470:690–696.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Powell RD, Holland PJ, Hollis T and
Perrino FW: Aicardi-Goutieres syndrome gene and HIV-1 restriction
factor SAMHD1 is a dGTP-regulated deoxynucleotide
triphosphohydrolase. J Biol Chem. 286:43596–43600. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Riveira-Muñoz E, Ruiz A, Pauls E,
Permanyer M, Badia R, Mothe B, Crespo M, Clotet B, Brander C,
Ballana E and Esté JA: Increased expression of SAMHD1 in a subset
of HIV-1 elite controllers. J Antimicrob Chemother. 69:3057–3060.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gonzalez SM, Taborda NA, Feria MG, Arcia
D, Aguilar-Jiménez W, Zapata W and Rugeles MT: High expression of
antiviral proteins in mucosa from individuals exhibiting resistance
to human immunodeficiency virus. PLoS One. 10:e01311392015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Santos ÍM, da Rosa EA, Gräf T, Ferreira
LG, Petry A, Cavalheiro F, Reiche EM, Zanetti CR and Pinto AR:
Analysis of immunological, viral, genetic, and environmental
factors that might be associated with decreased susceptibility to
HIV infection in serodiscordant couples in florianopolis, Southern
Brazil. AIDS Res Hum Retroviruses. 31:1116–1125. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jin C, Peng X, Liu F, Cheng L, Lu X, Yao
H, Wu H and Wu N: MicroRNA-181 expression regulates specific
post-transcriptional level of SAMHD1 expression in vitro. Biochem
Biophys Res Commun. 452:760–767. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Khopkar P, Mallav V, Chidrawar S and
Kulkarni S: Comparative evaluation of the Abbott HIV-1 RealTime™
assay with the standard Roche COBAS® Amplicor™ HIV-1
Monitor® Test, v1.5 for determining HIV-1 RNA levels in
plasma specimens from Pune, India. J Virol Methods. 191:82–87.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shmagel KV, Saidakova EV, Shmagel NG,
Korolevskaya LB, Chereshnev VA, Robinson J, Grivel JC, Douek DC,
Margolis L, Anthony DD and Lederman MM: Systemic inflammation and
liver damage in HIV/hepatitis C virus coinfection. HIV Med.
17:581–589. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nguyen TP, Shukla S, Asaad R, Freeman ML,
Lederman MM, Harding CV and Sieg S: Responsiveness to IL-7 but not
to IFN-alpha is diminished in CD4+ T cells from treated HIV
infected patients who experience poor CD4+ T-cell recovery. AIDS.
30:2033–2042. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jin C, Ji S, Xie T, Höxtermann S, Fuchs W,
Lu X, Wu H, Cheng L, Skaletz-Rorowski A, Brockmeyer NH and Wu N:
Severe dyslipidemia and immune activation in HIV patients with
dysglycemia. HIV Clin Trials. 17:189–196. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ruffin N, Brezar V, Ayinde D, Lefebvre C,
Schulze Zur, Wiesch J, van Lunzen J, Bockhorn M, Schwartz O, Hocini
H, et al: Low SAMHD1 expression following T-cell activation and
proliferation renders CD4+ T cells susceptible to HIV-1. AIDS.
29:519–530. 2015.PubMed/NCBI
|
|
22
|
Hrecka K, Hao C, Gierszewska M, Swanson
SK, Kesik-Brodacka M, Srivastava S, Florens L, Washburn MP and
Skowronski J: Vpx relieves inhibition of HIV-1 infection of
macrophages mediated by the SAMHD1 protein. Nature. 474:658–661.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Baldauf HM, Pan X, Erikson E, Schmidt S,
Daddacha W, Burggraf M, Schenkova K, Ambiel I, Wabnitz G, Gramberg
T, et al: SAMHD1 restricts HIV-1 infection in resting CD4(+) T
cells. Nat Med. 18:1682–1687. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wu L: SAMHD1: A new contributor to HIV-1
restriction in resting CD4+ T-cells. Retrovirology. 9:882012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Laguette N, Sobhian B, Casartelli N,
Ringeard M, Chable-Bessia C, Ségéral E, Yatim A, Emiliani S,
Schwartz O and Benkirane M: SAMHD1 is the dendritic- and
myeloid-cell-specific HIV-1 restriction factor counteracted by Vpx.
Nature. 474:654–657. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jin C, Peng X, Liu F, Cheng L, Xie T, Lu
X, Wu H and Wu N: Interferon-induced sterile alpha motif and
histidine/aspartic acid domain-containing protein 1 expression in
astrocytes and microglia is mediated by microRNA-181a. AIDS.
30:2053–2064. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jin C, Zhang F, Wu L, Xie T, Cheng Y, Tang
Z and Wu N: Immune activation and CD127 expression on T lymphocyte
subsets of a Chinese cohort of pediatric AIDS patients with
different viral responses. Curr HIV Res. 10:584–591. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jin C, Cheng L, Hoxtermann S, Höxtermann
S, Xie T, Lu X, Wu H, Skaletz-Rorowski A, Brockmeyer NH and Wu N:
MicroRNA-155 is a biomarker of T-cell activation and immune
dysfunction in HIV-1-infected patients. HIV Med. 18:354–362. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rice GI, Bond J, Asipu A, Brunette RL,
Manfield IW, Carr IM, Fuller JC, Jackson RM, Lamb T, Briggs TA, et
al: Mutations involved in Aicardi-Goutières syndrome implicate
SAMHD1 as regulator of the innate immune response. Nat Genet.
41:829–832. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kim B, Nguyen LA, Daddacha W and
Hollenbaugh JA: Tight interplay among SAMHD1 protein level,
cellular dNTP levels, and HIV-1 proviral DNA synthesis kinetics in
human primary monocyte-derived macrophages. J Biol Chem.
287:21570–21574. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kiselinova M, De Spiegelaere W, Buzon MJ,
Malatinkova E, Lichterfeld M and Vandekerckhove L: Integrated and
total HIV-1 DNA predict ex vivo viral outgrowth. PLoS Pathog.
12:e10054722016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Williams JP, Hurst J, Stöhr W, Robinson N,
Brown H, Fisher M, Kinloch S, Cooper D, Schechter M, Tambussi G, et
al: HIV-1 DNA predicts disease progression and post-treatment
virological control. Elife. 3:e038212014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Welbourn S, Dutta SM, Semmes OJ and
Strebel K: Restriction of virus infection but not catalytic dNTPase
activity is regulated by phosphorylation of SAMHD1. J Virol.
87:11516–11524. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Weiss L, Chevalier MF, Assoumou L, Didier
C, Girard PM, Piketty C, Costagliola D and Rouzioux C; ANRS 116
SALTO Study Group, : T-cell activation positively correlates with
cell-associated HIV-DNA level in viremic patients with primary or
chronic HIV-1 infection. AIDS. 28:1683–1687. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Geretti AM, Arribas JR, Lathouwers E,
Foster GM, Yakoob R, Kinloch S, Hill A, van Delft Y and
Moecklinghoff C: Dynamics of cellular HIV-1 DNA levels over 144
weeks of darunavir/ritonavir monotherapy versus triple therapy in
the MONET trial. HIV Clin Trials. 14:45–50. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cuzin L, Pugliese P, Sauné K, Allavena C,
Ghosn J, Cottalorda J, Rodallec A, Chaix ML, Fafi-Kremer S, Soulié
C, et al: Levels of intracellular HIV-DNA in patients with
suppressive antiretroviral therapy. AIDS. 29:1665–1671. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Maelfait J, Bridgeman A, Benlahrech A,
Cursi C and Rehwinkel J: Restriction by SAMHD1 limits
cGAS/STING-dependent innate and adaptive immune responses to HIV-1.
Cell Rep. 16:1492–1501. 2016. View Article : Google Scholar : PubMed/NCBI
|