Introduction
Each organ contains blood vessels that undergo
neovascularization and vascular remodeling processes for the
organ's growth. To understand the regulation of neovascularization
and vascular remodeling for each organ, it is necessary to regulate
these processes (1). The uterus is
a unique organ; it undergoes extensive neovascularization and
vessel degeneration during the menstrual cycle, differing from
typical neovascularization, which occurs mostly during premenstrual
and postmenopausal stages of life. During early pregnancy, the
embryo rapidly develops in the uterus; the uterus supplies
sufficient oxygen and nutrients for neovascularization and vascular
remodeling until the placenta becomes structurally complete and
functional (2,3).
Furthermore, in early pregnancy, the decidua
supplies a vascular network for the developing embryo before
placentation (4). Decidualization
of the uterine endometrium involves dramatic differentiation of the
uterine tissue, including morphological and functional
transformations (5,6). However, during this process, a lack
of vascularity in the decidua leads to early abortion and
preeclampsia via nutritional deficiency (7). It is fair to say that the uterus
requires profuse vascularity in the decidua to prevent pregnancy
failure. Endometrium formation resulting from decidualization is
termed ‘decidual angiogenesis’, and involves the impressive
development of uterine neovascularization; which includes
angiogenesis, vasculogenesis, arteriogenesis - and vascular
remodeling stimulated by steroid hormones (8–10).
The steroid hormones released from the ovaries
stimulate vascular remodeling and uterine neovascularization, which
are necessary for successful pregnancy. Progesterone and estrogen
are representative hormones which bind to the progesterone receptor
(PR) and estrogen receptor (ER), respectively, and cooperate to
regulate decidua formation during early pregnancy (11,12).
In addition, progesterone promotes decidual angiogenesis during
this period via the vascular endothelial growth factor-A/vascular
endothelial growth factore receptore-2 (VEGF-A/VEGFR-2) system.
Furthermore, progesterone and estrogen further regulate the
induction of angiogenesis in the uterus (1,13).
Angiogenesis, one of the essential processes in
blood vessel formation, represents the development of new branching
vessels from existing vascular networks and is operated by
endothelial cells, the main components of blood vessels (14). The phenomenon is essential for
embryonic growth, wound healing in recovery of adults, and the
menstrual cycle in thickening the uterus (15). Angiogenesis also contributes to
inflammatory disease and tumor growth. In some cases, inappropriate
angiogenesis may result in ischemia (16). Given the variety of functions it
performs, vascular remodeling, which results from angiogenesis of
the uterus, is essential for successful pregnancy. Angiogenesis and
vascular remodeling are thought to be regulated by the cooperative
interaction between several angiogenic factors (17). There are two major angiogenic
factors and their respective responsive receptors: VEGF and the
receptor VEGF-R, and angiopoietin and the Tie receptor, which
regulate both neovascularization, including vasculogenesis and
angiogenesis, and vascular remodeling, including enlargement and
blood network formation, in the uterus (18–20).
The VEGF, one of the major angiogenic factors of
vascular regulator in the endometrium, increases endothelial cells'
proliferation, permeability, and migration (21). Another vascular growth factor,
Angiopoietin-1 (Ang-1), increases the recruitment of endothelial
cells with pericytes and vascular smooth muscle cells to remodel
newly formed blood vessels, stimulating and stabilizing their
maturation (22). Angiopoietin-2
(Ang-2), as an antagonist of Ang-1, plays an important role
alongside VEGF, as a regulator of vascular remodeling, to migrate
and proliferate endothelial cells. The Ang-1/Ang-2 ratio is
inversely associated with blood vessel destabilization, a
prerequisite for new blood vessel formation (23). During angiogenesis, Ang-2 binds to
its receptor named Tie-2, competitively with Ang-1 (24). Recently, many studies have proved
that Ang-2 holds a crucial role in female reproduction (25). Overexpression of Ang-2 in mice
resulted in embryonic fatality in consequence to failure of
angiogenesis (26). Interestingly,
Ang-2 is initially expressed in the ovaries and later, during early
pregnancy, in the uterus and placenta (23).
Previous research has shown that progesterone
governs uterine angiogenesis and vascular remodeling via
VEGF-A/VEGFR-2 signaling, especially in the anti-mesometrial region
(AMR), where the embryo resides during pregnancy (1). However, the functional role of
spatiotemporal-localized Ang-2 expression in the pregnancy uterus
is not yet fully understood. In our study, we hypothesized that
spatiotemporal changes are focused on the mesometrial region (MR)
of the uterus because decidual development and vascular remodeling
are both developed by Ang-2 which is regulated by progesterone
during early pregnancy. To examine the relationship between Ang-2
and progesterone, we underwent in vitro and in vivo
experiments. Consequently, our results supported our hypothesis
that Ang-2 regulated by progesterone is a key regulator of vascular
remodeling in the uterus during pregnancy.
Materials and methods
Mice
C57BL/6 mice aged 8 to 10 weeks were used for this
study and female mice were mated with adult male mice.
Identification of a vaginal plug the following morning was
interpreted as successful mating, and designated 0.5 day post
coitum (dpc). Ang-2+/LacZ mice were transferred and bred
in our pathogen-free animal facilities. The Specific pathogen-free
(SPF) C57BL/6J mice were all given ad libitum access to standard
diet (PMI Lab diet) and water. All animal experiments were
performed following approval from the Institutional Animal Care and
Use Committees (IACUC) of Jeonbuk National University.
Histological analysis
Mice were sacrificed using the cervical dislocation
method on the indicated days. Segments of the uterus containing
implanted embryos were fixed in 4% paraformaldehyde (Biosesang;
cat. no. PC2031) for 4 h, followed by overnight dehydration in 20%
sucrose solution. Dehydrated samples were embedded with tissue
freezing medium (Scigen; cat. no. 4586) and the frozen blocks cut
into 20 µm sections.
Samples were blocked with 5% donkey serum (Jackson
ImmunoResearch; cat. no. 017-000-121) or goat serum (Jackson
ImmunoResearch; cat. no. 005-000-121) in PBST (0.03% Triton X-100
in PBS) and then incubated for 4 h at room temperature (RT) with
the following primary antibodies: anti-CD31 (hamster monoclonal,
Millipore; cat. no. MAB1398Z), anti-Ang-2 (rabbit polyclonal,
Proteintech TM; cat. no. 24613-1-AP), anti-PR (rabbit polyclonal,
Cell signaling; cat. no. 8757), and anti-Tie-2 (mouse monoclonal,
Abcam; cat. no. ab24859). After several washes, the samples were
incubated for 2 h at RT with the following secondary antibodies:
Cy3-conjugated anti-hamster IgG (Jackson ImmunoResearch; cat. no.
127-165-160), and Cy3- or FITC-conjugated anti-rabbit IgG (Jackson
ImmunoResearch; cat. no. 711-165-152 or cat. no. 111-095-003).
Nuclei were stained with 4′,6-diamidino-2-phenylindole (Enzo; cat.
no. BML-AP402). Afterward, the samples were mounted in fluorescent
mounting medium (DAKO; cat. no. S3023).
To examine β-galactosidase activity, the
cryo-sections were incubated with a staining solution [2 mM
magnesium chloride, 5 mM potassium ferricyanide, 5 mM potassium
ferrocyanide and 1 mg/ml
4-chloro-5-bromo-3-indolyl-β-D-galactopyranoside (X-gal) in PBS] at
37°C for 24 h. Immunofluorescent images and β-gal activity were
acquired using a Zeiss LSM510 confocal fluorescence microscope
(Carl Zeiss) and a microscope equipped with a CCD camera (Carl
Zeiss).
Detection of Ang-2 expression by
reverse transcription (RT)-qPCR
Total RNA was extracted from the uterus using
TRIzol® Reagent (Invitrogen; cat. no. 15596018)
according to the manufacturer's instructions. The RNA concentration
was measured using NanoDrop 2000 (Thermo Fisher Scientific, Inc.).
The RNA (2 µg) was reverse transcribed into cDNA using SuperScript
II Reverse Transcriptase (Invitrogen; cat. no. 18064071). RT-qPCR
was carried out using the following conditions: preheating for 5
min at temperature 95°C; and then repeating 32 cycles in
temperature 95°C for 20 sec and 30 sec at 59°C. The primer
sequences were as follows: (1)
Ang-2, Foward; 5′-GGATCTGGGGAGAGAGGAAC-3′, Reverse; 5′-
CTCTGCACCGAGTCATCGTA −3′. (2)
GAPDH, Forward; 5′-ACCACAGTCCATGCCATCAC-3′, Reverse;
5′-TCCACCACCCTGTTGCTGTA-3′. The PCR products were loaded onto a
1.5% agarose gel containing Loading STAR nucleic acid dye (6X,
Dynebio; cat. no. A750), electrophoresed, and photographed using a
Fusion FX7 acquisition system (Vilbert Lourmat). The band was
semi-quantified using Quantity One software (v4.6.2; Bio-Rad
Laboratories, Inc.) with GAPDH as the loading controls.
Cell culture
Human uterine microvascular endothelial cells
(HUtMEC) purchased from Lonza Group, Ltd., cat. no. CC-2564) were
grown in endothelial cell growth medium (EGM-2MV BulletKit; cat.
no. CC-3202) with 5% fetal bovine serum (FBS) and used at passage
3–4 in all the experiments. Cell were incubated at 37 with 5%
CO2. To examine the change of Ang-2 expression in
cultured HUtMEC due to progesterone, the cells were starved for 6 h
and then treated with progesterone and estrogen (10 µM) for 24
h.
Immunocytochemistry (ICC)
analysis
HUtMEC were cultured on glass slides, fixed with
cold acetone, and blocked by 5% FBS in PBST. They were incubated
with anti-Ang-2 (rabbit polyclonal; proteintech TM; cat. no.
24613-1-AP) and anti-VE-cadherin (rabbit polyclonal, Cell
Signaling; cat. no. 2500) at 4°C. Cells were incubated with
anti-rabbit IgG conjugated with Alexa Fluor®546 (Abcam;
cat. no. ab60317) and anti-human Ig G conjugated with Alexa
Fluor®488 (Abcam; cat. no. ab150129). Nuclei were
stained with 4′,6-diamidino-2-phenylindole phenylindole (Enzo; cat.
no. BML-AP402). Afterward, the samples were mounted in fluorescent
mounting medium (DAKO; cat. no. S3023) and immunofluorescent images
were acquired using a Zeiss LSM510 confocal fluorescence microscope
(Carl Zeiss).
Western blot analysis
The uterus tissues and cells were homogenized in
ice-cold RIPA buffer (150 mM NaCl, 1% Triton X-100, 1% Sodium
deoxycholate, 0.1% SDS, 50 mM Tris-HCl pH 7.5, 0 2 mM EDTA pH 8,
Biosesang; cat. no. R2002) on the indicated days. Total protein was
quantified using a bicinchoninic acid assay kit (Thermo Fisher
Scientific, Inc.; cat. no. 23225). Equal amounts of protein (40 µg
per lane) were separated on an 8~12% SDS-PAGE gel by 120 V for 90
min in 1X running buffer and transferred to nitrocellulose (NC)
membranes (EMD Millipore; cat. no. 66485). Each NC membrane was
blocked in Tris-buffered saline/Tween (TBST) with 5% non-fat milk
for 60 min with gently shaking at room temperature (RT) and then
incubated with the designated primary antibodies (1:1,000)
overnight at 4°C. The primary antibodies used were as follows:
anti-angiopoietin-2 (polyclonal, Proteintech; cat. no. 24613-1-AP),
anti-progesterone receptor (rabbit polyclonal, Cell Signaling; cat.
no. 8757), and anti- β-actin antibody (rabbit polyclonal,
Sigma-Aldrich; cat. no. A2066). Each NC membrane was washed three
times in TBST for 10 min per wash and then incubated with the
1:2,000 goat anti-rabbit IgG (Enzo; cat. no. ADI-SAB-300) or goat
anti-mouse IgG (Enzo; cat. no. ADI-SAB-100) in TBST with 5% non-fat
milk for 1 h at RT. The membranes were washed three times in TBST
for 10 min per wash and visualized by horseradish peroxidease (HRP)
substrate (Enzo; cat. no. ADI-SAB-300-J) using a Fusion FX 7
(Vilber). The band was semi-quantified using Quantity One software
(v4.6.2; Bio-Rad Laboratories, Inc.) with β-actin as the loading
controls.
Statistical analysis
Values are presented as mean ± standard deviation
(SD). Significant differences between means were determined by
unpaired Student's t-tests or analysis of variance with one-way and
two-way ANOVA followed by the Student-Newman-Keuls test or
Bonferroni post hoc test. All statistical analysis was performed
using the GraphPad Prism software. P<0.05 was considered to
indicate a statistically significant difference.
Results
Ang-2 expressed in the uterine
endometrium during early pregnancy, mainly in CD31+
blood vessels
We analyzed Ang-2 expression to investigate the role
of Ang-2 in early pregnancy (from 4.5 dpc to 8.5 dpc). Over time,
Ang-2 expression increased remarkably around the embryo and
spatiotemporal area in the MR, focusing in the venous sinus region
(VSR) from implantation until placentation (Fig. 1A). Protein and mRNA expression
levels of Ang-2 also rose in the pregnant uterus (Fig. 1B-D). In the 8.5 dpc uterus, Ang-2
protein expression was 2.7-fold higher that of the 4.5 dpc uterus,
and Ang-2 mRNA expression was 4.2-fold higher that of the estrus
non pregnant mice. We further examined regional Ang-2 expression in
early pregnancy through X-gal staining, using Ang-2-LacZ reporter
(Ang-2+/LacZ) mice at 6.5, 8.5 and 10.5 dpc. Endothelial
cells consisting of blood vessels' lumen in VSR gave off a green
color in the presence of Ang-2 to confirm these results (Fig. 2A). During the post-implantation
period, expressed Ang-2 was localized on the VSR in the MR. These
results indicate that Ang-2 is associated with vascular remodeling
and is mainly expressed in CD31 positive blood vessels in the MR.
In addition, we performed co-staining of CD31 and Ang-2 via
immunofluorescence (IF) method in the 5.5 dpc uterus when the Ang-2
expression initiated to figure out the relationship between
endothelial cells and Ang-2. We observed an overlapped color as a
result of co-stain red (CD31) and green (Ang-2). The result of
stain showed us that Ang-2 was mainly expressed in CD31 positive
blood vessels (Fig. 2B). Vascular
remodeling of blood vessels regulated by angiogenesis factors is
associated with the Tie-2 receptor (27). Therefore, Tie-2 and Ang-2 expressed
in the uterus during early pregnancy were observed. As a result, it
was confirmed that Tie-2 was also expressed in the CD31+
blood vessel that was expressing Ang-2 (Fig. 2C). Through these results, we were
able to predict that Ang-2 could be involved in intravascular
vascular remodeling via the Tie-2 receptor inside the uterus.
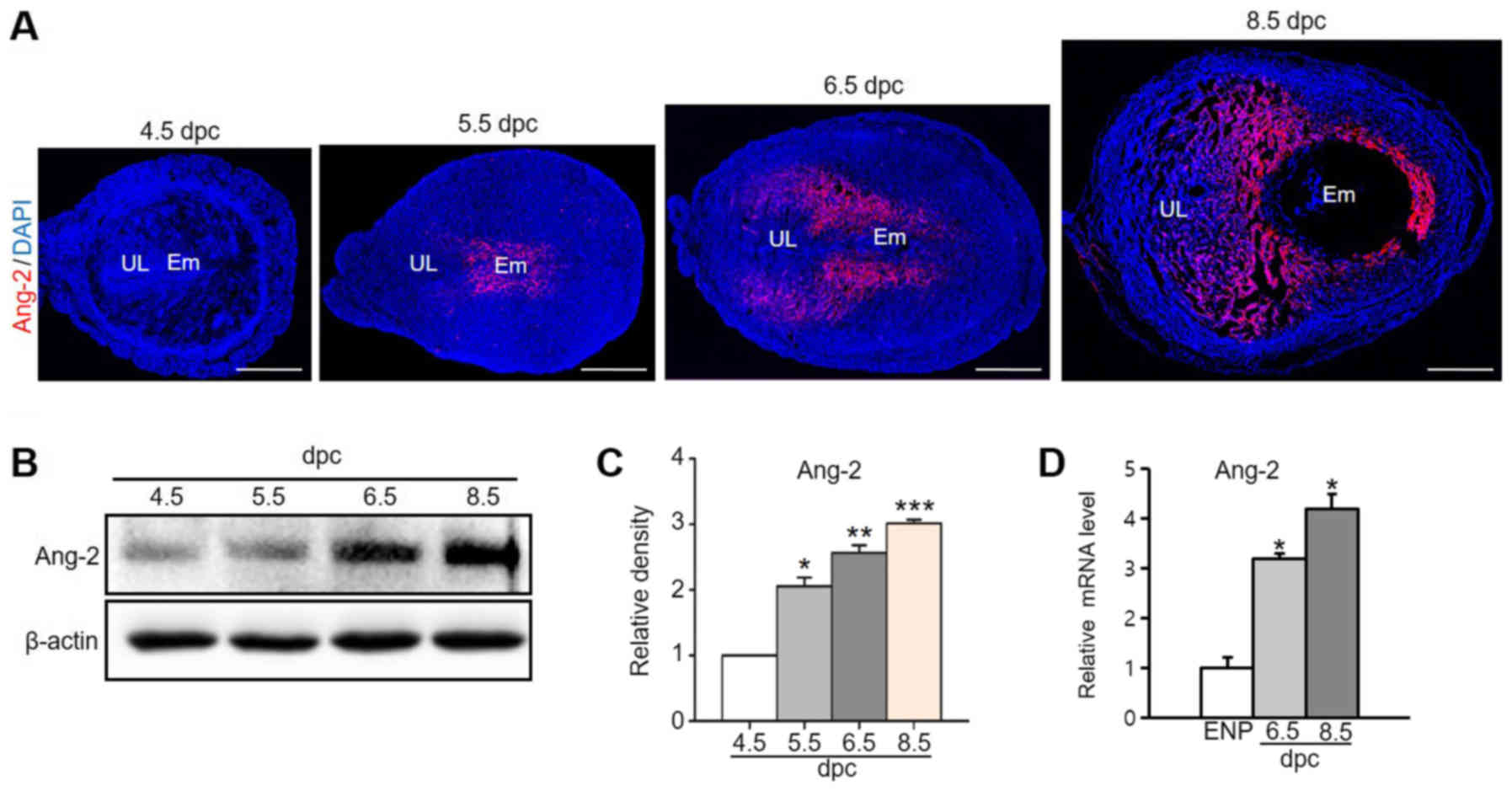 | Figure 1.Ang-2 expression in the uterus during
pregnancy. (A) Image showing Ang-2 expression in mouse uteri during
early pregnancy at 4.5, 5.5, 6.5 and 8.5 dpc. Scale bar, 500 µm.
(B) Protein expression levels and (C) semi-quantitative analysis of
Ang-2 in the uteri at 4.5, 5.5, 6.5 and 8.5 dpc were measured by
western blotting. Loading of similar amounts of protein for each
sample was verified by a similar intensity of β-actin signal. Data
are presented as the mean ± SD from three independent experiments.
*P<0.05, **P<0.01 and ***P<0.001 vs. 4.5 dpc. (D) Ang-2
mRNA expression in the uteri of ENP, and at 6.5 and 8.5 dpc. Data
is presented as relative fold change compared with the levels of
ENP after normalization to GAPDH. n=4 in each group. *P<0.02 vs.
ENP. Ang-2, angiopoietin-2; dpc, days post coitum; UL, uterine
lumen; Em, embryo; ENP, estrous of non-pregnancy. |
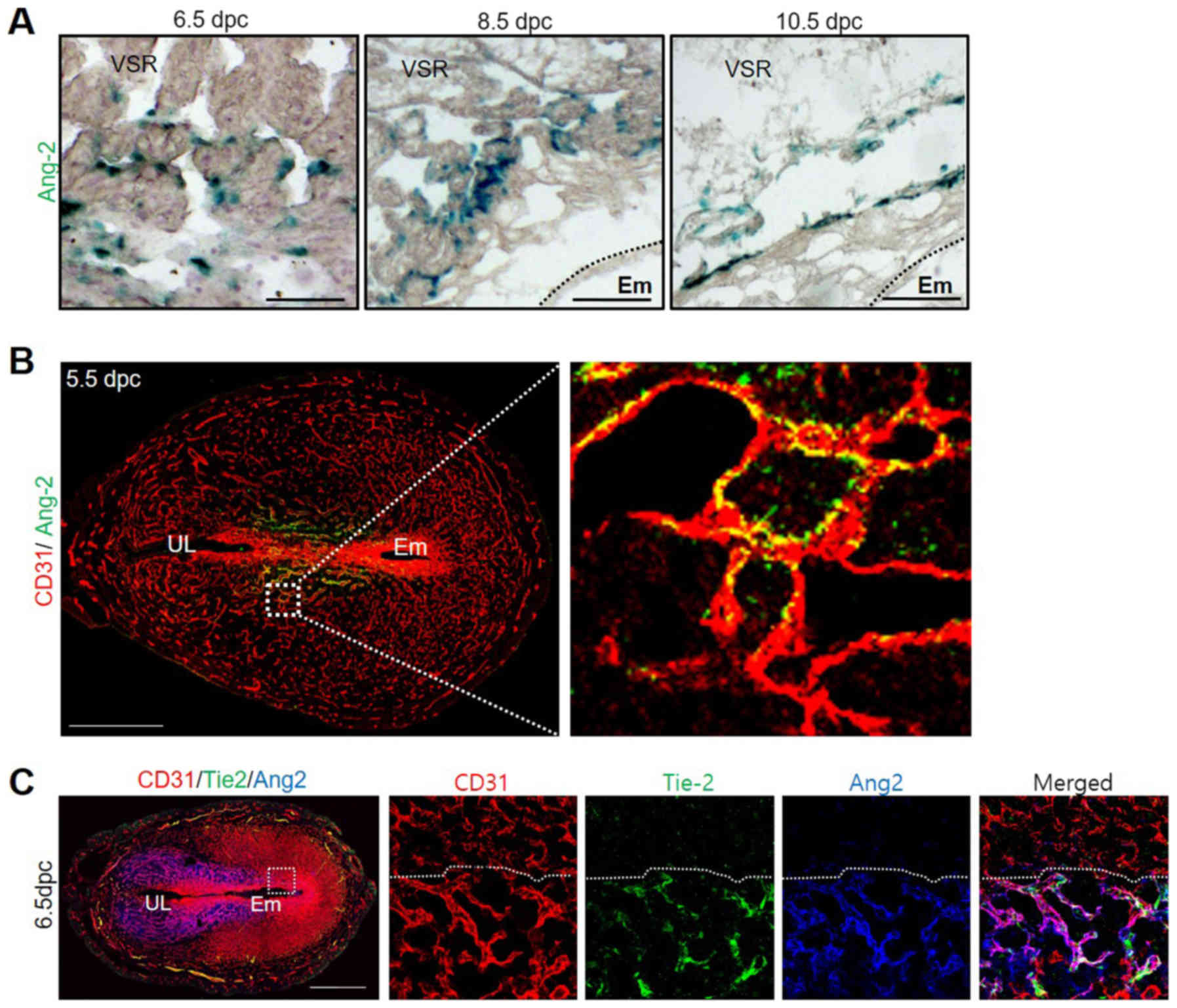 | Figure 2.Ang-2 expression around vascular
endothelial cells. (A) Image showing Ang-2 expression in the VSR of
Ang-2+/LacZ mice at 6.5, 8.5 and 10.5 dpc. The tissues
were counterstained with hematoxylin following X-gal staining.
Scale bar, 500 µm. (B) Image showing CD31+ BVs and
Ang-2+ cells in the uterus at 5.5 dpc. Scale bar, 500
µm. The section in the square is magnified on the right. (C) Image
showing CD31+ BVs and Ang-2+ and
Tie-2+ cells in the uterus at 6.5 dpc. Scale bar, 500
µm. Ang-2, angiopoietin-2; BVs, blood vessels; dpc, days post
coitum; UL, uterine lumen; VSR, venous sinus region; Em,
embryo. |
Expression of Ang-2 is regulated by
progesterone
In previous studies, it was found that vascular
remodeling inside the uterus is regulated by progesterone (1). In addition, vascular remodeling by
progesterone occurs via the PR, which is a receptor for
progesterone. Based on these results, we expected that Ang-2
expressed in the uterus may be related to progesterone. To confirm
this hypothesis, the expression regions of the PR and Ang-2 were
compared. As a result, it was confirmed that PR was also expressed
at the region where Ang-2 was expressed (Fig. 3A). Ang-2 expression with
progesterone treatment in vitro using HUtMEC confirmed the
in vivo data. Progesterone treatment increased Ang-2 levels
in HUtMEC in comparison to untreated cells, the control, by
1.6-fold. However, estrogen treatment was not much different to the
control. In addition, there was no difference between treatment
with both progesterone and estrogen in HUtMEC and treatment with
only progesterone (Fig. 3B and C).
We assumed that progesterone known as a pregnancy hormone mainly
controls Ang-2 in HUtMEC. Moreover, progesterone-treated HUtMEC
displayed higher Ang-2 protein and mRNA expression than untreated
cells (Fig. 3D and E).
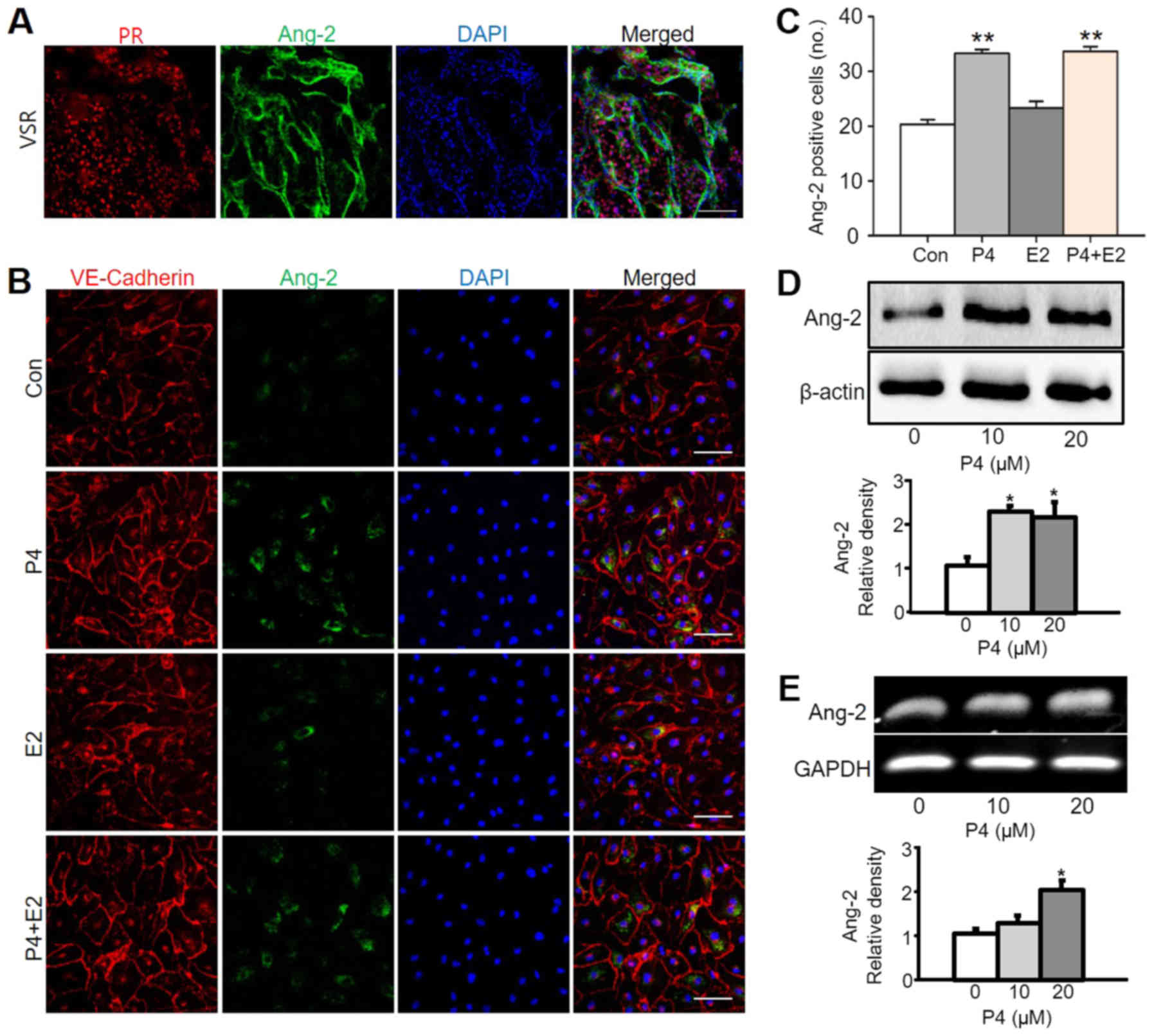 | Figure 3.Ang-2 expression is increased by
progesterone treatment in HUtMECs. (A) Image showing PR+
region and Ang-2+ cells in the uterus at 6.5 days post
coitum. Scale bar, 100 µm. (B) Immunofluorescence staining of
VE-Cadherin and Ang-2 in HUtMECs treated with Con, P4 (10 µM), E2
(10 µM) and P4 + E2 (each 10 µM). Nuclei were counterstained with
DAPI (blue). Scale bar, 100 µm. (C) Comparisons of
Ang-2+ HUtMECs treated with Con, P4, E2, P4 + E2 (10
µM). **P<0.01 vs. Con. (D) Comparisons of Ang-2 protein
expression levels and relative density (lower graph) in HUtMECs
treated with Con and P4 (10 and 20 µM). Loading of similar amounts
of protein for each sample was verified by a similar intensity of
β-actin, and the relative expression levels of Ang-2 after
normalization to β-actin are shown. Three independent experiments
exhibited similar results. *P<0.02 vs. Con. (E)
Semi-quantitative reverse transcription-PCR showing mRNA levels of
Ang-2 in HUtMECs treated with Con and P4 (10 and 20 µM). The lower
graph presents the relative fold change of the levels of Ang-2
after normalization to GAPDH. n=5 in each group. *P<0.02 vs.
Con. Ang-2, angiopoietin-2; Con, control; E2, estrogen; HUtMECs,
human uterine microvascular endothelial cells; P4, progesterone;
PR, progesterone receptor; VSR, venous sinus region. |
Expression of Ang-2 related with PR in
pregnancy uterus
Through previous results, we recognized progesterone
controlled the expression of Ang-2 in HUtMEC, so we assumed that
vascular remodeling operated by Ang-2 regulated its expression in
the presence of the progesterone receptor, which binds to
progesterone, exists in HUtMEC. To test the hypothesis, we
performed western blotting and ICC to discover the progesterone
receptor via progesterone treatment. As a result, it was confirmed
that PR was expressed regardless of the concentration of the
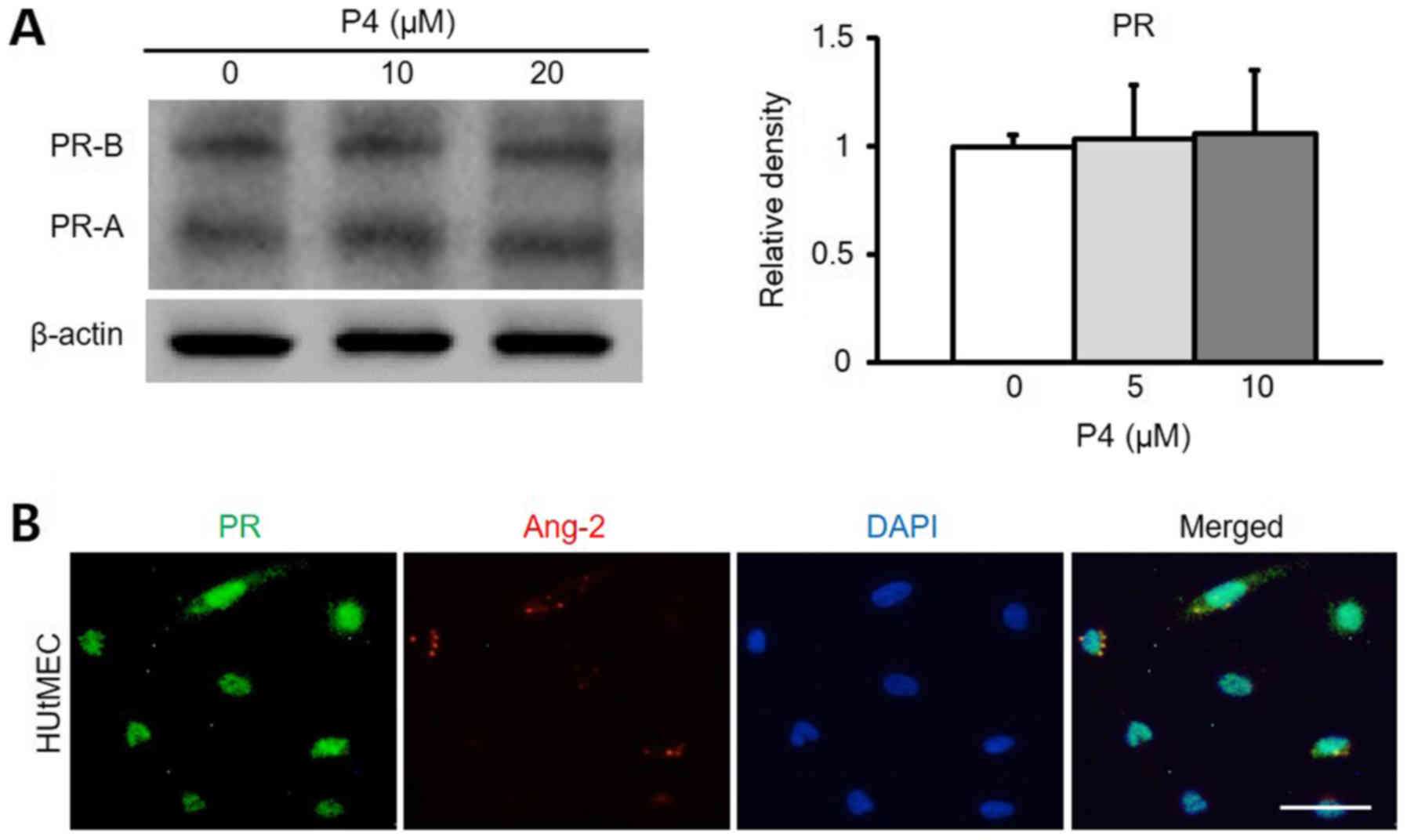
progesterone in HUtMEC (Fig. 4A).
Also, expression of PR and Ang-2 was observed in HUtMEC (Fig. 4B). Therefore, Ang-2 in the uterus
is expressed by progesterone, and the expression of Ang-2 proves
that progesterone is involved via the PR. To clarify the
relationship between progesterone and Ang-2, the function of the
progesterone receptor was blocked using RU486 (8 mg/kg). As a
result, it was confirmed that the remodeling of blood vessels
inside the uterus was significantly reduced by RU486, and indeed,
the expression of Ang-2 was also decreased by RU486 (Fig. 5A and C). Compared to the control
groups, treatment with RU486 dramatically decreased the number of
blood vessels with diameter of over 300 µm. Consequently, the
number of blood vessels diameter between 100–300 µm increased
significantly following RU486 treatment (Fig. 5B). This showed that vascular
remodeling is inhibited by RU486 as a pharmacological blockade of
PR. Moreover, it was confirmed that Ang-2 expressed in vascular
endothelial cells also decreases by the PR blockade (Fig. 5C). Through these results, it was
confirmed that when the function of the PR is blocked using RU486,
the intrauterine vascular remodeling regulated by the progesterone
decreases.
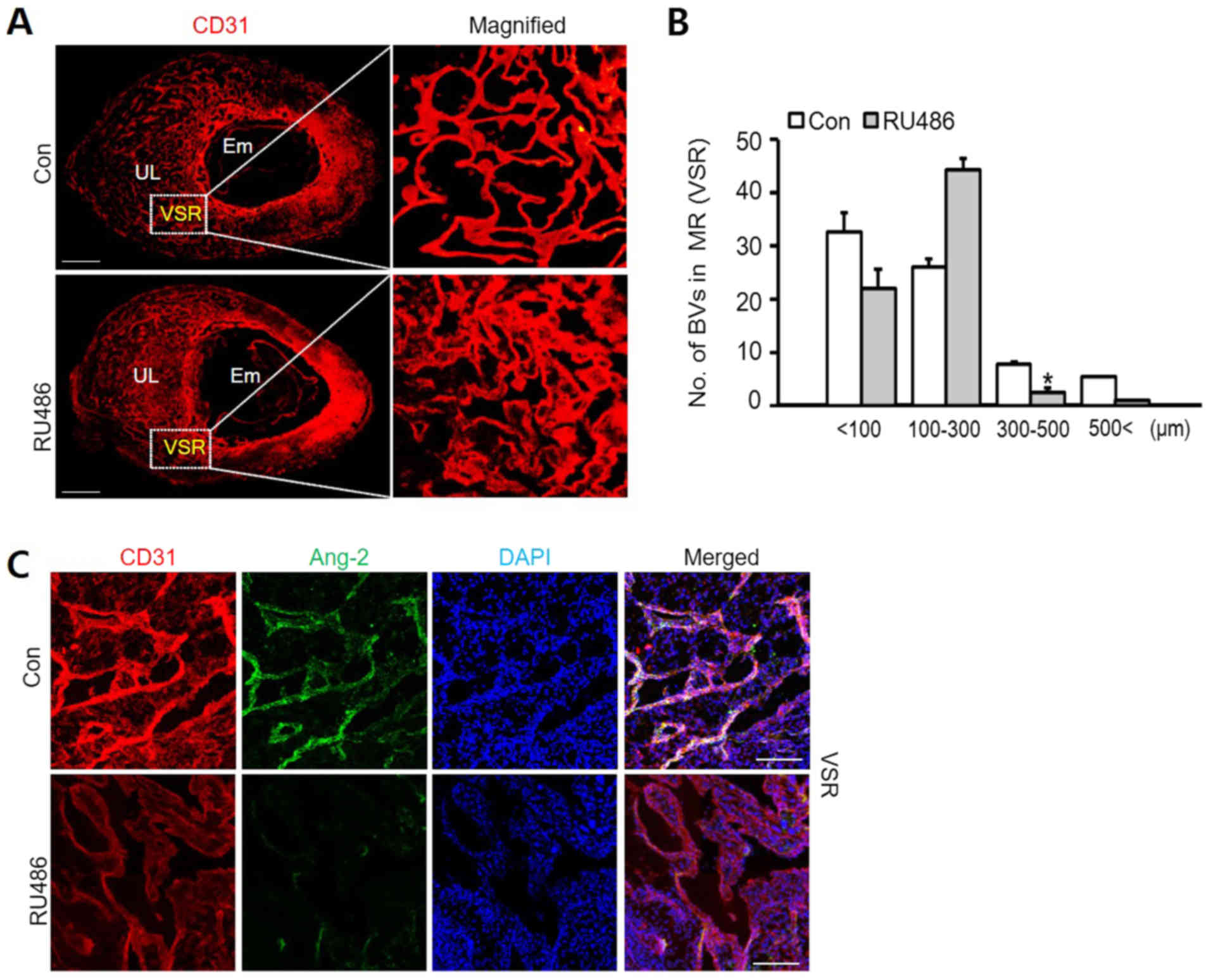 | Figure 5.Vascular remodeling in VSR is
decreased by RU486. (A) Images showing CD31+ BVs in the
uterus at 8.5 dpc following treatment with Con and RU486 (8 mg/kg).
n=4. Scale bar, 500 µm. (B) Number of BVs in MR (VSR) was dependent
on their size, compared with Con and treatment RU486. n=4.
*P<0.02 vs. Con (t-test). (C) Images showing CD31+
BVs and Ang-2 in the VSR at 8.5 dpc after treatment with RU486 (8
mg/kg). n=4. Scale bar, 100 µm. Ang-2, angiopoietin-2; BVs, blood
vessels; Con, control; dpc, days post coitum; UL, uterine lumen;
Em, embryo; MR, mesometrial region; VSR, venous sinus region. |
Discussion
The female uterus experiences cyclic changes from
menstrual periods and pregnancy, times of extraordinary
neovascularization and vascular remodeling (28). Especially during pregnancy, the
maternal body increases the volume of blood towards the uterus to
supply oxygen and nutrients for embryo development. Blood vessels
in the uterus undergo drastic changes to accept the blood from the
mother. In the case of failed transformation, miscarriage and
preeclampsia, which are result from vascularity in the uterus, lead
to an unsuccessful delivery. Therefore, vascular remodeling in
early pregnancy is essential for embryonic growth.
Recently, research has shown that progesterone
regulates uterine angiogenesis and vascular remodeling via
VEGF-A/VEGFR-2 signaling in the AMR during pregnancy (1). However, spatiotemporal vascular
remodeling by progesterone in the MR is not fully understood.
Interestingly, it is known that endothelial cell proliferation is
due to progesterone (29). Thus,
in this study, we focused on spatiotemporal ang-2 expression and
vascular remodeling in the MR including the VSR during early
pregnancy. Furthermore, we investigated the relationship between
Ang-2 expression and progesterone treatment through in vitro
experiments.
Our results demonstrated that Ang-2 steadily
increases after implantation until placentation. Remarkably, Ang-2
was expressed spatiotemporally in the uterus - especially in the
VSR of the MR. In this period, Ang-2 also constantly rose in terms
of the total level of protein and mRNA in uterus. Other studies
indicated that the amount of mRNA of Ang-2 increased during early
pregnancy (23). From this result,
we predicted Ang-2 has an effect on vascular remodeling in VSR
where lots of blood vessels are lined up by endothelial cells. We
found out the correlation between Ang-2 and endothelial cell by
performing an X-gal stain. Based on these results, the treatment of
HUtMEC with progesterone is very different compared to treatment
with progesterone in vitro. Progesterone increased the
expression of Ang-2. Progesterone binds to a PR to activate its
mechanism. As shown in Fig. 4, PR
expressed in the HUtMEC and PR expression was not dose regulated by
progesterone concentration. Expression of PR is not related to
progesterone level but other factors. According to another study,
PR is up-regulated by estrogen at the level of gene expression
(30). It seems that the
concentration of progesterone has no influence on the expression of
PR in HUtMEC.
Progesterone is a biomarker for decidualization of
pregnant uterus (31). Ang-2 was
observed in MR during this period. Therefore, we tested the
relationship between Ang-2 and PR in decidual angiogenesis using PR
blockade named RU486. We injected it intraperitoneally in pregnant
mice to observe vascular remodeling during pregnancy. RU486
inhibited Ang-2 mediated vascular remodeling in the MR. Our results
indicated that vascular remodeling and decidual angiogenesis are
related to signaling pathways that are affected differently by
progesterone and Ang-2 mediated vascular remodeling. Notably,
vascular remodeling induced by Ang-2 expression was not dependent
on VEGF-A-related signaling in the MR. VEGF-A was mainly expressed
in the AMR and was not detected in the VSR during pregnancy
(1). Thus, vascular remodeling
mediated by Ang-2 signaling would be different to VEGF-A/VEGFR-2
signaling in the pregnant uterus.
Taking all of this information into account, we
speculate that the expression of Ang-2 is stimulated by
progesterone which influences spatiotemporally different parts of
the uterus in early pregnancy. Progesterone most likely induces
vascular remodeling via VEGF-A/VEGFR-2 signaling only in the AMR.
In the MR, Ang-2 expression by progesterone affects vascular
remodeling for placentation.
In conclusion, our results provide insight on
previously undefined features of vascular remodeling in the
pregnant uterus by Ang-2-mediated signaling during early pregnancy.
In the post-implantation period, the pregnant uterus undergoes
profound vascular remodeling in response to progesterone for
placentation, which has spatiotemporally different effects on the
AMR and MR. Progesterone-PR-regulated Ang-2 is a key regulator for
placentation and prevention of pregnancy failure through
spatiotemporal vascular remodeling in the MR.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Research Foundation of Korea funded by the Ministry of Science, ICT
and Future Planning (grant no. 2015R1A1A1A05001546 to JWS).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YGP and JC designed and performed the experiments,
analyzed the data, generated the figures and wrote the manuscript.
JWS designed, organized and supervised the project, and wrote the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All experiments were performed following approval
from the Institutional Animal Care and Use Committees (IACUC) of
Jeonbuk National University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
HUtMEC
|
human uterine microvascular
endothelial cell
|
|
Ang-2
|
angiopoietin-2
|
|
PR
|
progesterone receptor
|
|
MR
|
mesometrial region
|
|
AMR
|
anti-mesometrial region
|
|
VSR
|
venous sinus region
|
|
VEGFs
|
vascular endothelial growth
factors
|
|
DPC
|
day post coitum
|
References
|
1
|
Kim M, Park HJ, Seol JW, Jang JY, Cho YS,
Kim KR, Choi Y, Lydon JP, Demayo FJ, Shibuya M, et al: VEGF-A
regulated by progesterone governs uterine angiogenesis and vascular
remodelling during pregnancy. EMBO Mol Med. 5:1415–1430. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cha J, Sun X and Dey SK: Mechanisms of
implantation: Strategies for successful pregnancy. Nat Med.
18:1754–1767. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang H and Dey SK: Roadmap to embryo
implantation: Clues from mouse models. Nat Rev Genet. 7:185–199.
2006. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Smith SD, Choudhury RH, Matos P, Horn JA,
Lye SJ, Dunk CE, Aplin JD, Jones RL and Harris LK: Changes in
vascular extracellular matrix composition during decidual spiral
arteriole remodeling in early human pregnancy. Histol Histopathol.
31:557–571. 2016.PubMed/NCBI
|
|
5
|
Bany BM and Cross JC: Post-implantation
mouse conceptuses produce paracrine signals that regulate the
uterine endometrium undergoing decidualization. Dev Biol.
294:445–456. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jones RL and Critchley HO: Morphological
and functional changes in human endometrium following intrauterine
levonorgestrel delivery. Hum Reprod. 15 (Suppl 3):162–172. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Osol G and Moore LG: Maternal uterine
vascular remodeling during pregnancy. Microcirculation. 21:38–47.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kiepiela P, Smith AN and Rosenberg E:
Retraction notice to ‘Laboratory markers associated with
progression of HIV infection [Best Pract Res Clin Obstet Gynaecol
19: 243–254, 2005]’. Best Pract Res Clin Obstet Gynaecol.
21:8832007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Conneely OM, Mulac-Jericevic B and Lydon
JP: Progesterone-dependent regulation of female reproductive
activity by two distinct progesterone receptor isoforms. Steroids.
68:771–778. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Carson DD, Bagchi I, Dey SK, Enders AC,
Fazleabas AT, Lessey BA and Yoshinaga K: Embryo implantation. Dev
Biol. 223:217–237. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schumacher A, Costa SD and Zenclussen AC:
Endocrine factors modulating immune responses in pregnancy. Front
Immunol. 5:1962014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wetendorf M and DeMayo FJ: The
progesterone receptor regulates implantation, decidualization, and
glandular development via a complex paracrine signaling network.
Mol Cell Endocrinol. 357:108–118. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Groothuis PG, Dassen HH, Romano A and
Punyadeera C: Estrogen and the endometrium: Lessons learned from
gene expression profiling in rodents and human. Hum Reprod Update.
13:405–417. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Saraswati S, Kumar S and Alhaider AA:
α-santalol inhibits the angiogenesis and growth of human prostate
tumor growth by targeting vascular endothelial growth factor
receptor 2-mediated AKT/mTOR/P70S6K signaling pathway. Mol Cancer.
12:1472013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bauer SM, Bauer RJ and Velazquez OC:
Angiogenesis, vasculogenesis, and induction of healing in chronic
wounds. Vasc Endovascular Surg. 39:293–306. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Abbas OL, Borman H, Terzi YK, Terzi A,
Bayraktar N and Yazıcı AC: The Notch pathway is a critical
regulator of angiogenesis in a skin model of ischemia. Vasc Med.
20:205–211. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Koga K, Osuga Y, Tsutsumi O, Yano T,
Yoshino O, Takai Y, Matsumi H, Hiroi H, Kugu K, Momoeda M, et al:
Demonstration of angiogenin in human endometrium and its enhanced
expression in endometrial tissues in the secretory phase and the
decidua. J Clin Endocrinol Metab. 86:5609–5614. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Augustin HG, Koh GY, Thurston G and
Alitalo K: Control of vascular morphogenesis and homeostasis
through the angiopoietin-Tie system. Nat Rev Mol Cell Biol.
10:165–177. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Carmeliet P: Angiogenesis in health and
disease. Nat Med. 9:653–660. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chung AS and Ferrara N: Developmental and
pathological angiogenesis. Annu Rev Cell Dev Biol. 27:563–584.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fan X, Krieg S, Kuo CJ, Wiegand SJ,
Rabinovitch M, Druzin ML, Brenner RM, Giudice LC and Nayak NR: VEGF
blockade inhibits angiogenesis and reepithelialization of
endometrium. FASEB J. 22:3571–3580. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kwak HJ, So JN, Lee SJ, Kim I and Koh GY:
Angiopoietin-1 is an apoptosis survival factor for endothelial
cells. FEBS Lett. 448:249–253. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Matsumoto H, Ma WG, Daikoku T, Zhao X,
Paria BC, Das SK, Trzaskos JM and Dey SK: Cyclooxygenase-2
differentially directs uterine angiogenesis during implantation in
mice. J Biol Chem. 277:29260–29267. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Felcht M, Luck R, Schering A, Seidel P,
Srivastava K, Hu J, Bartol A, Kienast Y, Vettel C, Loos EK, et al:
Angiopoietin-2 differentially regulates angiogenesis through TIE2
and integrin signaling. J Clin Invest. 122:1991–2005. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Geva E, Ginzinger DG, Moore DH II, Ursell
PC and Jaffe RB: In utero angiopoietin-2 gene delivery remodels
placental blood vessel phenotype: A murine model for studying
placental angiogenesis. Mol Hum Reprod. 11:253–260. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tsuzuki T, Okada H, Cho H, Shimoi K,
Miyashiro H, Yasuda K and Kanzaki H: Divergent regulation of
angiopoietin-1, angiopoietin-2, and vascular endothelial growth
factor by hypoxia and female sex steroids in human endometrial
stromal cells. Eur J Obstet Gynecol Reprod Biol. 168:95–101. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Akwii RG, Sajib MS, Zahra FT and Mikelis
CM: Role of Angiopoietin-2 in Vascular Physiology and
Pathophysiology. Cells. 8:4712019. View Article : Google Scholar
|
|
28
|
Cartwright JE, Fraser R, Leslie K, Wallace
AE and James JL: Remodelling at the maternal-fetal interface:
Relevance to human pregnancy disorders. Reproduction. 140:803–813.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Walter LM, Rogers PA and Girling JE: The
role of progesterone in endometrial angiogenesis in pregnant and
ovariectomised mice. Reproduction. 129:765–777. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ing NH and Tornesi MB: Estradiol
up-regulates estrogen receptor and progesterone receptor gene
expression in specific ovine uterine cells. Biol Reprod.
56:1205–1215. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Das A, Mantena SR, Kannan A, Evans DB,
Bagchi MK and Bagchi IC: De novo synthesis of estrogen in pregnant
uterus is critical for stromal decidualization and angiogenesis.
Proc Natl Acad Sci USA. 106:12542–12547. 2009. View Article : Google Scholar : PubMed/NCBI
|