Introduction
Asthma is a common chronic airway inflammatory
disease with two subtypes, eosinophilic asthma and non-eosinophilic
asthma. Neutrophilic asthma (NA) accounts for more than one-half of
non-eosinophilic asthma (1–3).
Corticosteroid treatment is effective for most patients with mild
and moderate asthma. However, patients with NA require a high dose
of corticosteroids for symptom control (4). An in-depth understanding of the
mechanisms underlying NA would provide insight into therapeutic
options for this condition.
Our previous studies (Jiang et al,
unpublished data) demonstrated that Th17 cells were dominant and
promoted neutrophil-mediated airway inflammation through
interleukin (IL)-17 in a mouse model of NA. The elevated IL-6 and
transforming growth factor-β levels in NA model mice
bronchio-alveolar lavage fluid (BALF) were discovered to
participate in the Th17-mediated response through regulating the
expression levels of retinoic acid receptor-related orphan
receptor-γt (RORγt) and suppressor of cytokine signaling 3. In
addition, the increase in Th17 cells and RORγt expression in the
peripheral blood, as well as upregulated sputum IL-17 levels, in
children with NA were also verified. Moreover, the expression
levels of phosphorylated (p)-STAT5 and Bcl-2 in Th17 cells and IL-7
levels in BALF were increased (Jiang et al, unpublished
data). However, the effect of IL-7 on Th17 cells in NA remains
unclear. It is known that IL-7 plays a critical role in
proliferation, survival and differentiation of T lymphocytes.
Indeed, IL-7 activates the JAK/STAT signaling pathway, thereby
promoting T cell survival by upregulating the expression of the
anti-apoptotic protein Bcl-2 (5–8).
Therefore, it was hypothesized that the IL-7/JAK/STAT5 signaling
pathway might also be involved in Th17 cell responses in NA.
Materials and methods
Experimental animals
A total of 12 female C57BL/6 mice (age, 6–8 weeks;
weight, 18–20 g) were purchased from Shanghai SLAC Laboratory
Animal Co., Ltd. and randomly divided into two groups (n=6 in each
group), NA group and normal control (NC) group. Mice were housed
under specific pathogen-free conditions in separate cages at a
relatively stable temperature of 20–24°C and a humidity of 55±10%,
with a 12-h light/dark cycle and free access to food and water. All
experimental animal protocols were approved by The Ethics Committee
of The First Affiliated Hospital of Guangxi Medical University
[2019 (KY-E-035)].
Mouse model of NA
The model used in the present study was established
according to the protocol from Wilson et al (9). Mice were sensitized by airway
delivery of 100 µg ovalbumin (OVA; Grade II & V; Sigma-Aldrich;
Merck KGaA) and 0.1 µg lipopolysaccharide (LPS; Sigma-Aldrich;
Merck KGaA) in a total volume of 50 µl PBS on days 0, 6 and 13. The
OVA + LPS mixture was instilled along the posterior oropharyngeal
wall, and the mixed solution was inhaled into the airway, followed
by a challenge with 1% OVA aerosol for 1 h from day 21 for 3
consecutive days. The NC group received PBS treatment instead of
OVA + LPS for sensitization and challenge.
Measurement of airway
hyper-responsiveness (AHR)
Airway responses to aerosolized methacholine were
measured using a lung function test instrument for mouse
(FinePointe Resistance and Compliance; Data Sciences International;
Harvard Bioscience, Inc.). Mice were anesthetized with 1%
pentobarbital sodium (50 mg/kg body weight) by intraperitoneal
injection, and the trachea was cannulated with a needle, followed
by mechanical ventilation. Airway resistance (R;
cmH2O.s/ml) was measured after aerosolization of 10 µl
PBS and administration of increasing doses of aerosolized
methacholine (3.125, 6.25, 12.5, 25 and 50 mg/ml in 10 µl;
Sigma-Aldrich; Merck KGaA) sequentially. The results are presented
as fold-increase of R (cmH2O.s/ml) above the baseline
and were calculated as follows: [R(response) -
R(baseline)]/R(baseline).
Cell classification of BALF
Mice were sacrificed 24 h after the final
aerosolization. Cervical dislocation was used for euthanasia and
death was confirmed by the onset of rigor mortis, according to The
National Institutes of Health Guide for the Care and Use of
Laboratory Animals. The trachea was exposed, and a 22-gauge needle
was used for endotracheal intubation. The lungs were subjected to
broncho-alveolar lavage twice with 0.5 ml PBS (recovery rate ≥80%)
and the total volume of BALF was 0.8 ml. Total and differential
cell counts from BALF were determined by staining with Diff-Quick
(Beijing Solarbio Science & Technology Co., Ltd.) for 1 min at
room temperature. BALF was centrifuged at 160 × g for 10 min at 4°C
and the supernatants were stored at −20°C for further
experiments.
Histopathology
Lungs were fixed in 4% paraformaldehyde solution for
24 h at room temperature and subjected to gradient alcohol
dehydration and paraffin-embedding, which were cut into 5–7-µm
thick sections. The sections were subsequently stained with
hematoxylin at room temperature for 2–3 min and then with eosin at
room temperature for 30–60 sec. An Olympus CX31 light microscope
(Olympus Corporation) was used to evaluate the general inflammation
and the airway morphology (magnification, ×200).
ELISA
An ELISA kit (cat. no. ELM-IL17-1; RayBiotech Life)
was used to measure the levels of IL-17 in the BALF, according to
the manufacturer's protocol.
Isolation of mononuclear cells from
mouse spleens
Spleens were homogenized and filtered on a 0.054-mm
diameter 300-mesh metal screen. The resulting cell suspension was
centrifuged at 135 × g for 5 min at 4°C. Red blood cell lysis
buffer (3 ml) (Beijing Solarbio Science & Technology Co., Ltd.)
was added to the cell pellet and rested for 5 min at room
temperature after thorough mixing. Subsequently, the reaction was
stopped, and the supernatant discarded after centrifugation at 135
× g for 5 min at 4°C. The cells were washed twice with cold PBS and
centrifuged at 135 × g for 5 min at 4°C, before adjusting the cell
concentration to 1×108 cells/ml. Subsequently, 20 µl
cell suspension were mixed with an equal volume of 2% Trypan Blue,
then visually examined to confirm cell viability (unstained cells
per ml/total cells per ml) of >95%, using an Olympus CX31 light
microscope (Olympus Corporation; magnification, ×200).
Immunomagnetic bead separation of
CD4+ T cells from splenic mononuclear cells
CD4+ T cells were purified using a
magnetic separation kit (Dynal; Thermo Fisher Scientific, Inc.).
Splenic mononuclear cells from mice were mixed with the supplied
antibody (20 µl/107 cells), followed by inactivated
fetal bovine serum (Wisent, Inc.; 20 µl/107 cells). The
reaction was mixed thoroughly and incubated for 20 min at 4°C.
After centrifugation at 211 × g for 5 min at 4°C, the cells were
resuspended in cold PBS (800 µl/107 cells), followed by
magnetic beads and incubation for 15 min at room temperature. The
system was placed in the automated cell selector for 2 min to
collect the supernatant containing purified CD4+ T
cells, then centrifuged at 211 × g for 8 min at 4°C. The cells were
resuspended at a density of 1.5×106 cells/ml, and 100 µl
of this solution was incubated with PerCP-Cy™ 5.5-labeled
anti-mouse CD4 monoclonal antibody (BD Biosciences; cat. no.
550954) at room temperature for 20 min. Finally, the cells were
washed with PBS before fixation with 1% paraformaldehyde for 10–20
min at 4°C. Cell purity was then assessed by flow cytometry (to
determine whether purity was >91%).
Culture of mouse splenic
CD4+ T cells
Anti-CD3 (BD Biosciences; cat. no. 561798) and
anti-CD28 (BD Biosciences; cat. no. 562764) antibodies were coated
in each well on day 1, followed by addition of anti-IFN (BD
Biosciences; cat. no. 551506) and anti-IL-4 (BD Biosciences; cat.
no. 555090). Purified CD4+ T cells were seeded into a
24-well plate at a density of 1.5×106 cell per well in a
1 ml volume. Subsequently, 10 ng/ml IL-7 (PeproTech, Inc.) and 100
µM STAT5 inhibitor (Merck KGaA) were added to the culture, and
incubated for 72 h. The following culture groups were obtained:
Negative control (NC) group, anti-CD3 + anti-CD28 + DMSO; NC + IL-7
group, anti-CD3 + anti-CD28 + IL-7 + DMSO; NA group, anti-CD3 +
anti-CD28 + DMSO; NA + IL-7 group, anti-CD3 + anti-CD28 + IL-7 +
DMSO; NA + IL-7 + STAT5 inhibitor group:, anti-CD3 + anti-CD28 +
IL-7 + STAT5 inhibitor. The cells in each group were detected by a
flow cytometer (FACS Calibur; BD Biosciences) and analyzed using
FlowJo 7.6.5 software (FlowJo LLC).
Flow cytometric analysis
Briefly, cells from the spleen were stimulated in a
complete medium with 50 ng/ml phorbol myristate acetate and 1 µg/ml
ionomycin (both from Sigma-Aldrich; Merck KGaA) at 37°C in 5%
CO2 for 5 h. The cells were washed with PBS and stained
with surface PerCP-Cy™ 5.5 anti-mouse CD4 antibody (cat. no.
550954; clone, RM4-5; BD Biosciences) at room temperature in the
dark for 30 min. Intracellular staining for p-STAT5 and IL-17A was
subsequently performed; briefly, the cells were fixed at 37°C for
10 min using warm BD Phosflow™ Fix Buffer I (cat. no. 557870; BD
Biosciences) and washed with PBS. Subsequently, the cells were
permeabilized for 30 min at 4°C using the BD Phosflow™ Perm Buffer
III (cat. no. 558050; BD Biosciences), washed with PBS and stained
with BD Phosflow™ Alexa Fluor® 488 Anti-p-STAT5 (Y694;
cat. no. 612598; clone, 47/Stat5 (pY694); BD Biosciences), BD
Pharmingen™ phycoerythrin (PE) anti-mouse IL-17A (cat. no. 559502;
clone, TC11-18H1; BD Biosciences) or with its isotypic control
antibody for 50 min at 4°C.
Intracellular staining for IL-17A,
active caspase-3, Ki-67 and Bcl-2 was also performed
Briefly, the cells were fixed and permeabilized for
30 min at 4°C using the CytoFix/CytoPerm kit (cat. no. 554714; BD
Biosciences), washed with PBS and stained with PE anti-mouse IL-17A
(cat. no. 559502; clone, TC11-18H1; BD Biosciences), BD
Transduction Laboratories™ FITC mouse anti-Ki-67 (cat. no. 612472;
clone, 35/Ki-67; BD Biosciences), BD Pharmingen™ FITC rabbit
anti-active caspase-3 (cat. no. 560901; clone, C92-605; BD
Biosciences), BD Pharmingen™ FITC hamster anti-mouse Bcl-2 (cat.
no. 556357; BD Biosciences) or with its isotypic control antibody
for 50 min at 4°C. Live lymphocytes were gated according to forward
and side scatter, then by CD4 expression. BD Pharmingen™ PE-Cy™5
mouse IgG1 isotype control (cat. no. 550618; clone, MOPC-31C; BD
Biosciences) was used as the isotype control. According to this
gating strategy, CD4+ cells were considered helper T
(Th) cells; CD4+IL-17+ cells were defined as
Th17 cells. Ki-67, caspase-3 and Bcl-2 expression in Th17 cells
were measured as described. Cells were detected by a flow cytometer
(FACS Calibur; BD Biosciences) and analyzed using FlowJo 7.6.5
software (FlowJo LLC).
Statistical analysis
Statistical analysis was performed using GraphPad
Prism 6 software (GraphPad Software, Inc.). Normally distributed
parameters are presented as the mean ± SD of three independent
experimental repeats. Statistical differences between two groups
were determined by Student's t-test, and multi-group comparisons
were carried out using one-way ANOVA after homogeneity test of
variances. Tukey's multiple comparisons test was used after one-way
ANOVA. P<0.05 was considered to indicate a statistically
significant difference.
Results
Establishment of a mouse model of
NA
Airway resistance increased significantly in the NA
group after challenge with 25 and 50 mg/ml methacholine, compared
with the NC group (P<0.05; Fig.
1A). The enumeration of total cells collected from BALF
suggested that NA mice developed airway inflammation, and the
numbers of neutrophils and eosinophils significantly increased in
NA, compared with NC (P<0.05; Fig.
1B). Lung histopathology showed intact bronchial lumen and
alveolar structure, aligned airway epithelial cells and no
infiltration of the inflammatory cells in NC. However, disordered
lung structure, widened alveolar septum, broken alveolar wall, and
inflammatory cell infiltration (mainly neutrophils) were observed
around airways and the interstitial pulmonary in NA (Fig. 1C).
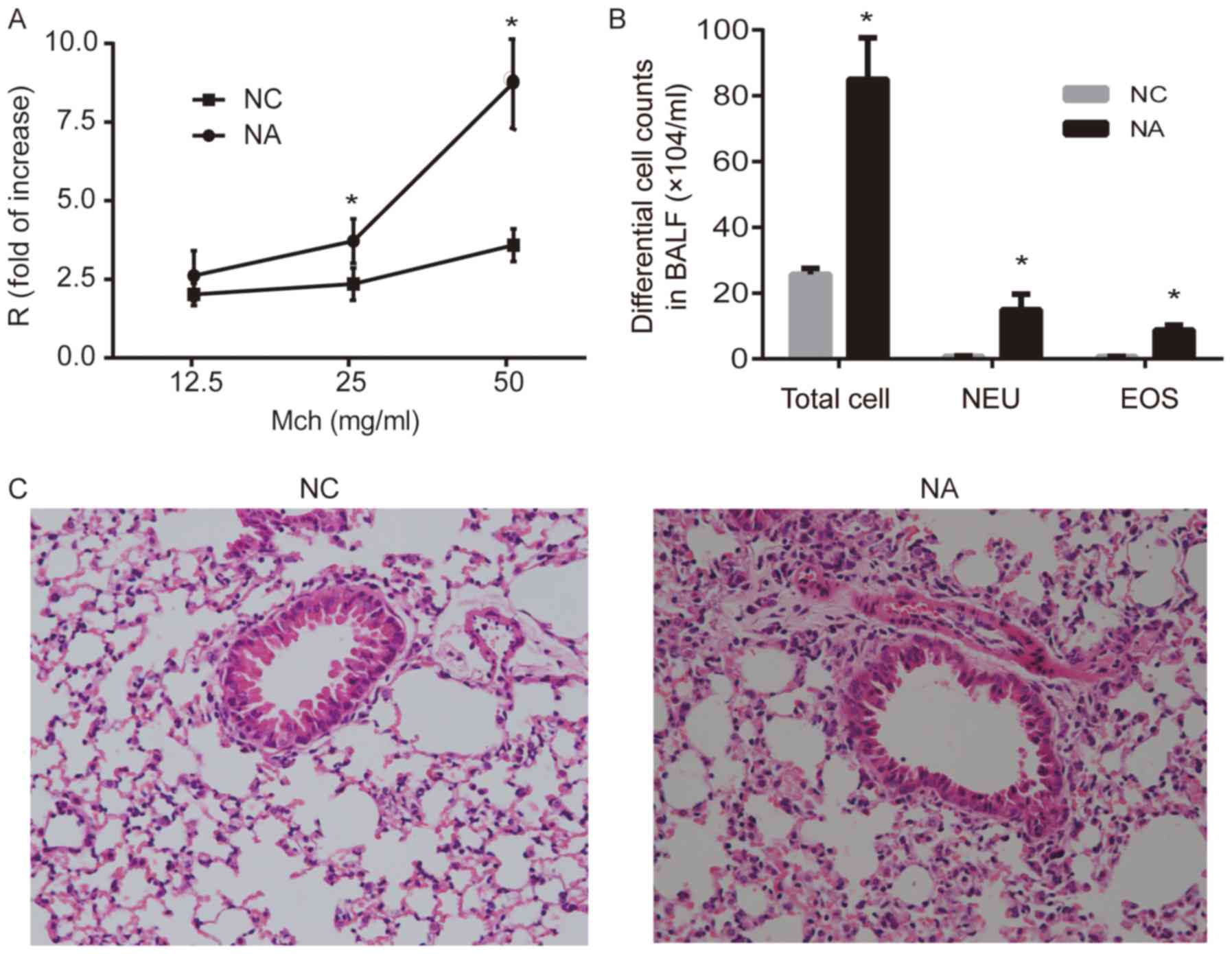 | Figure 1.Airway resistance, differential cell
counts and histopathology of the lung in a mouse model of NA. (A)
Airway resistance increased significantly in NA. (B) Analysis of
total and differential cell counts in BALF. Levels of NEU and EOS
were increased in NA. (C) Representative images of hematoxylin and
eosin-stained lung tissue. Disordered lung structure, widened
alveolar septum, broken alveolar wall and infiltration of
neutrophils around airways and in the interstitial pulmonary tissue
was observed in NA (magnification, ×100). Data are presented as the
mean ± SD and analyzed by Student's t-test. n=6 in each group.
*P<0.05 vs. respective NC. NC, normal control; NA, neutrophilic
asthma; Mch, methacholine; BALF, bronchoalveolar lavage fluid; NEU,
neutrophils; EOS, eosinophils; R, airway resistance. |
Th17 cell expression in a mouse model
of NA
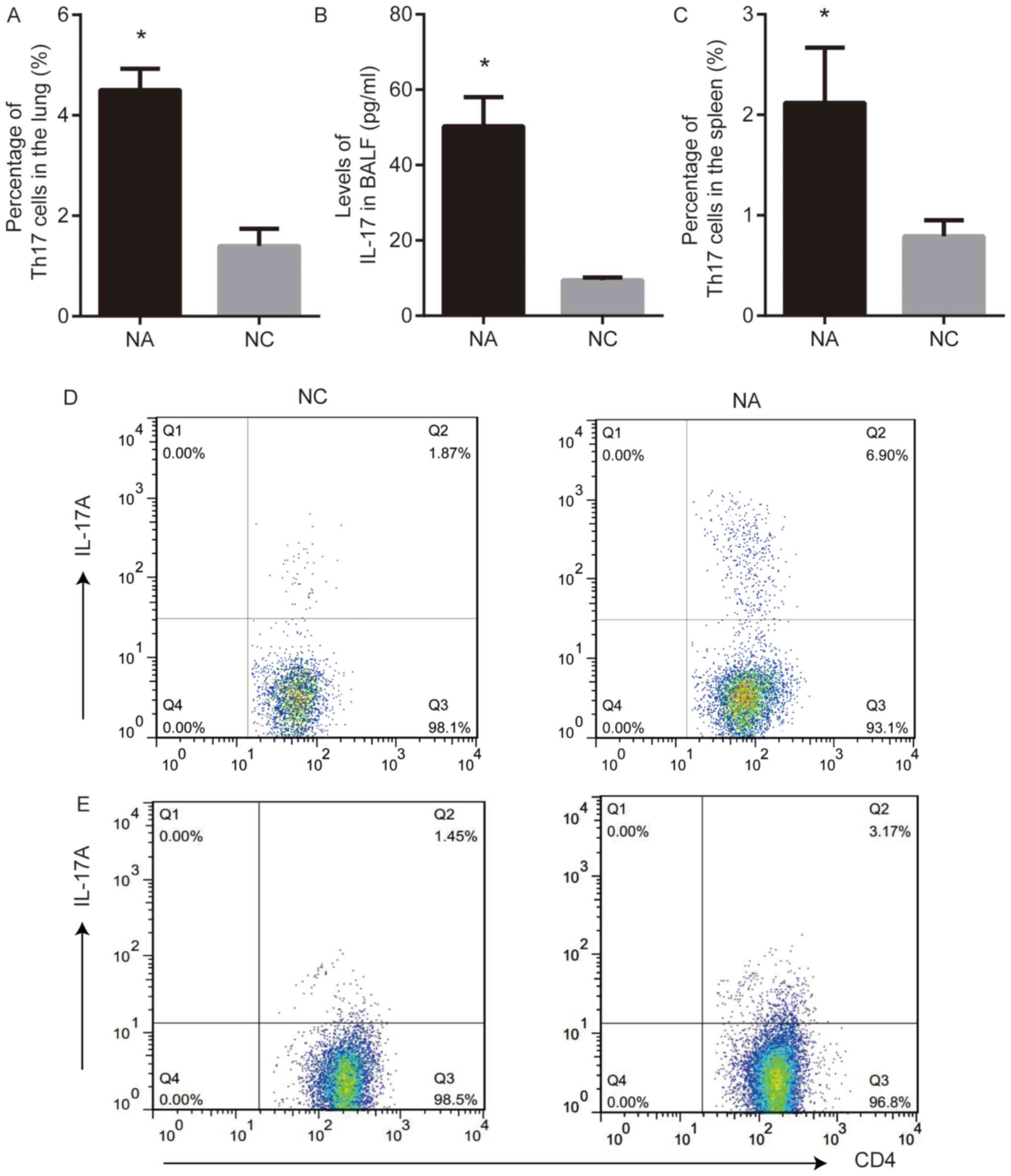
Th17 cells and the levels of IL-17 were analyzed
using flow cytometry and ELISA, respectively. Th17 cell frequency
in the lung and the level of IL-17 in BALF from NA mice were higher
than those in NC (P<0.05; Fig. 2A,
B and D). The frequency of Th17 cells in the spleens from NA
mice was higher than that in NC (P<0.05; Fig. 2C and E).
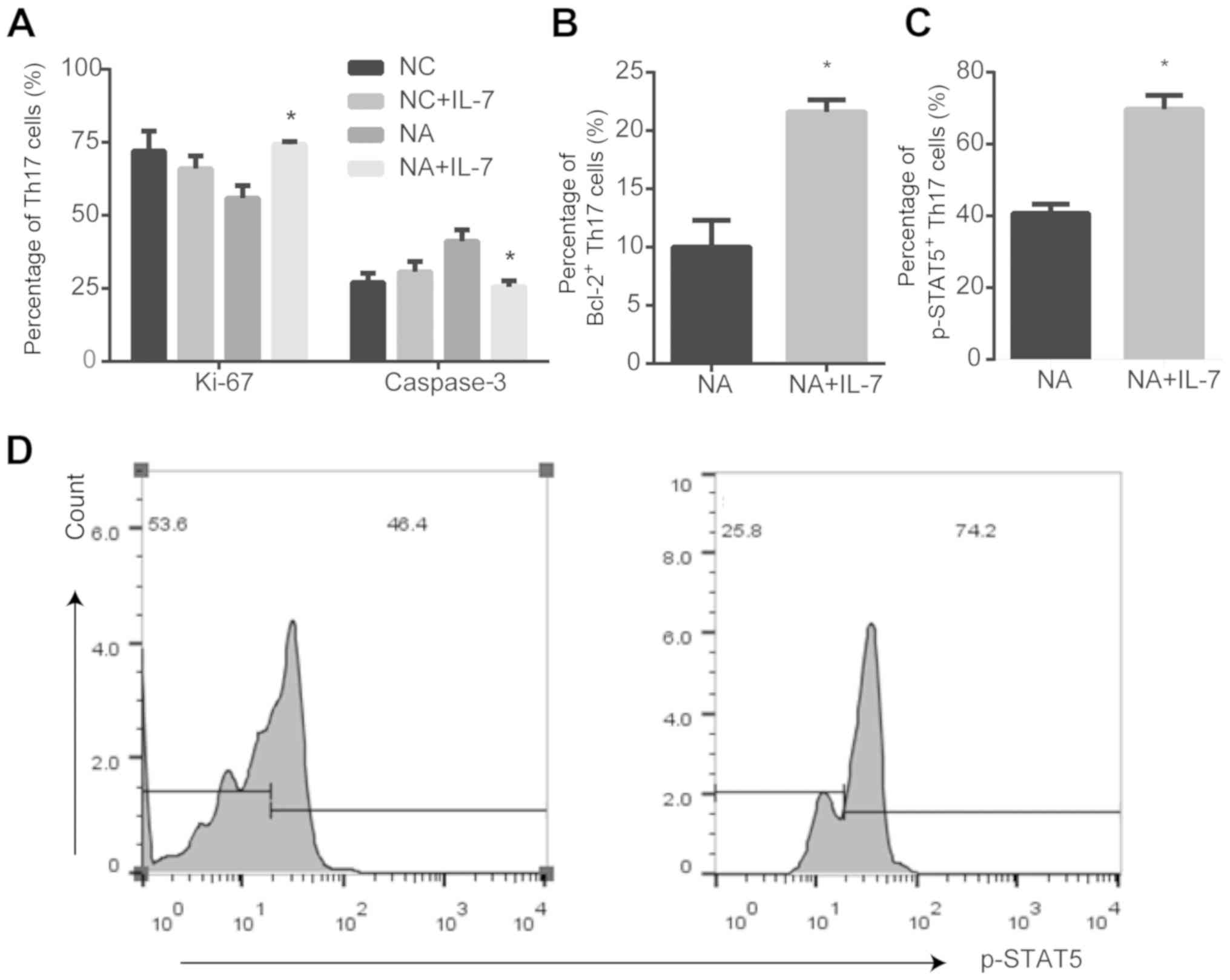
IL-7 affects the expression of
proliferation markers and anti-apoptotic proteins in Th17 cells in
NA mice
In the NC group, there were no differences in Ki-67
and caspase-3 expression in the presence or absence of IL-7
(Fig. S1). IL-7 promoted the
expression of the proliferation marker Ki-67 (P<0.05; Fig. 3A), anti-apoptotic protein Bcl-2
(P<0.05; Fig. 3B) and p-STAT5
(P<0.05; Fig. 3C and D) in Th17
cells from NA mice, compared with untreated cells. However, IL-7
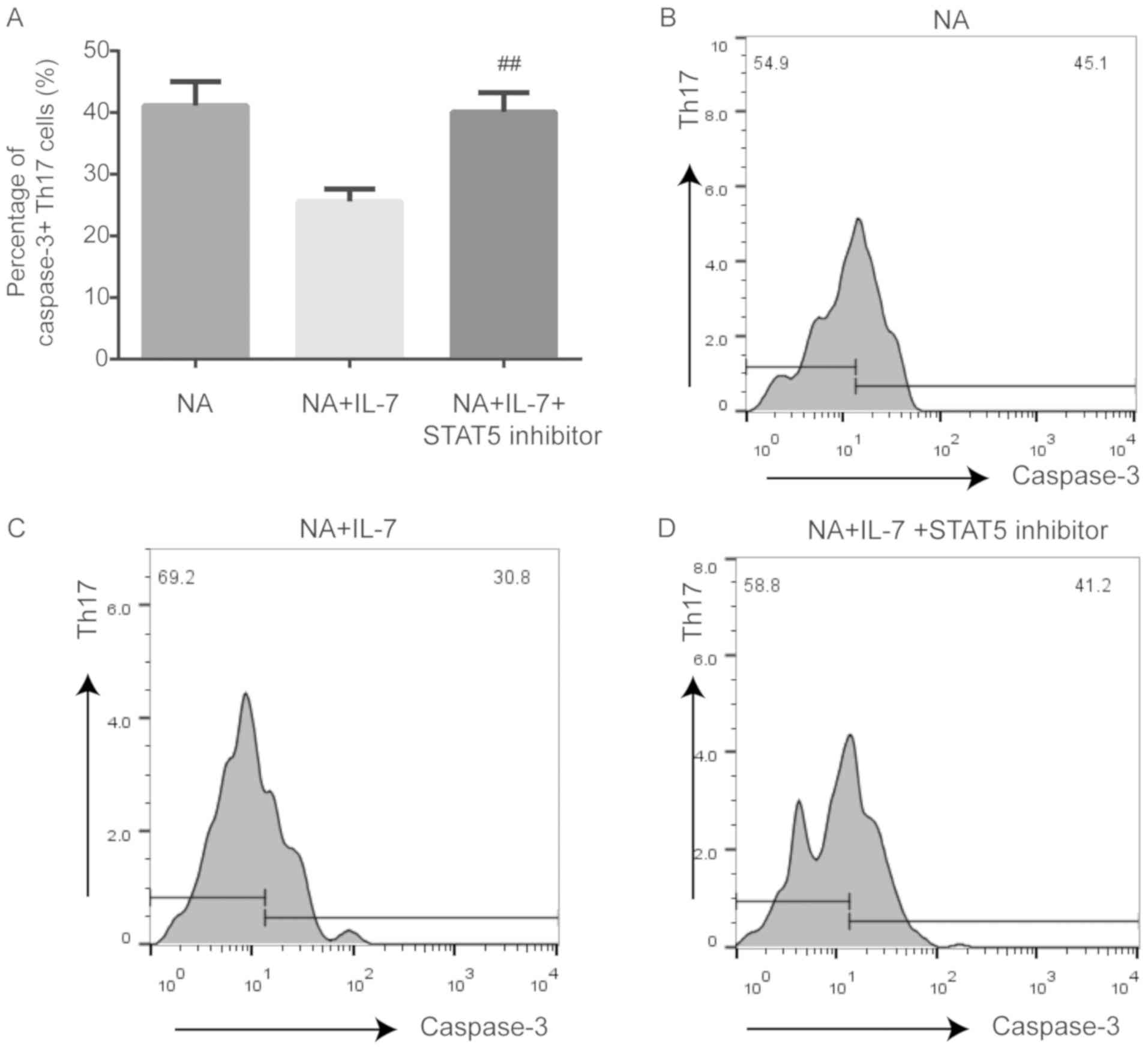
decreased activated caspase-3 expression in NA (P<0.05; Fig. 3A).
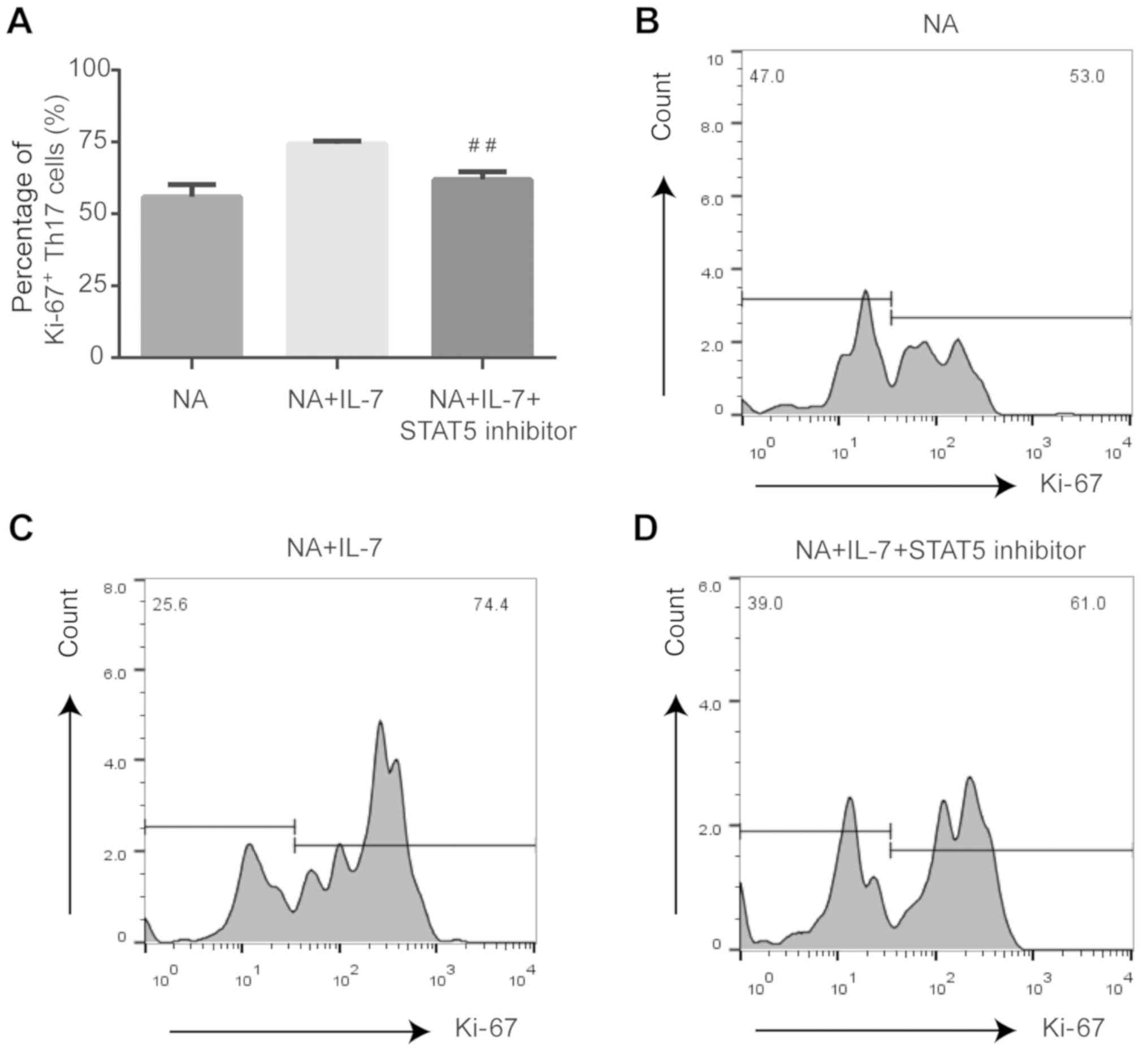
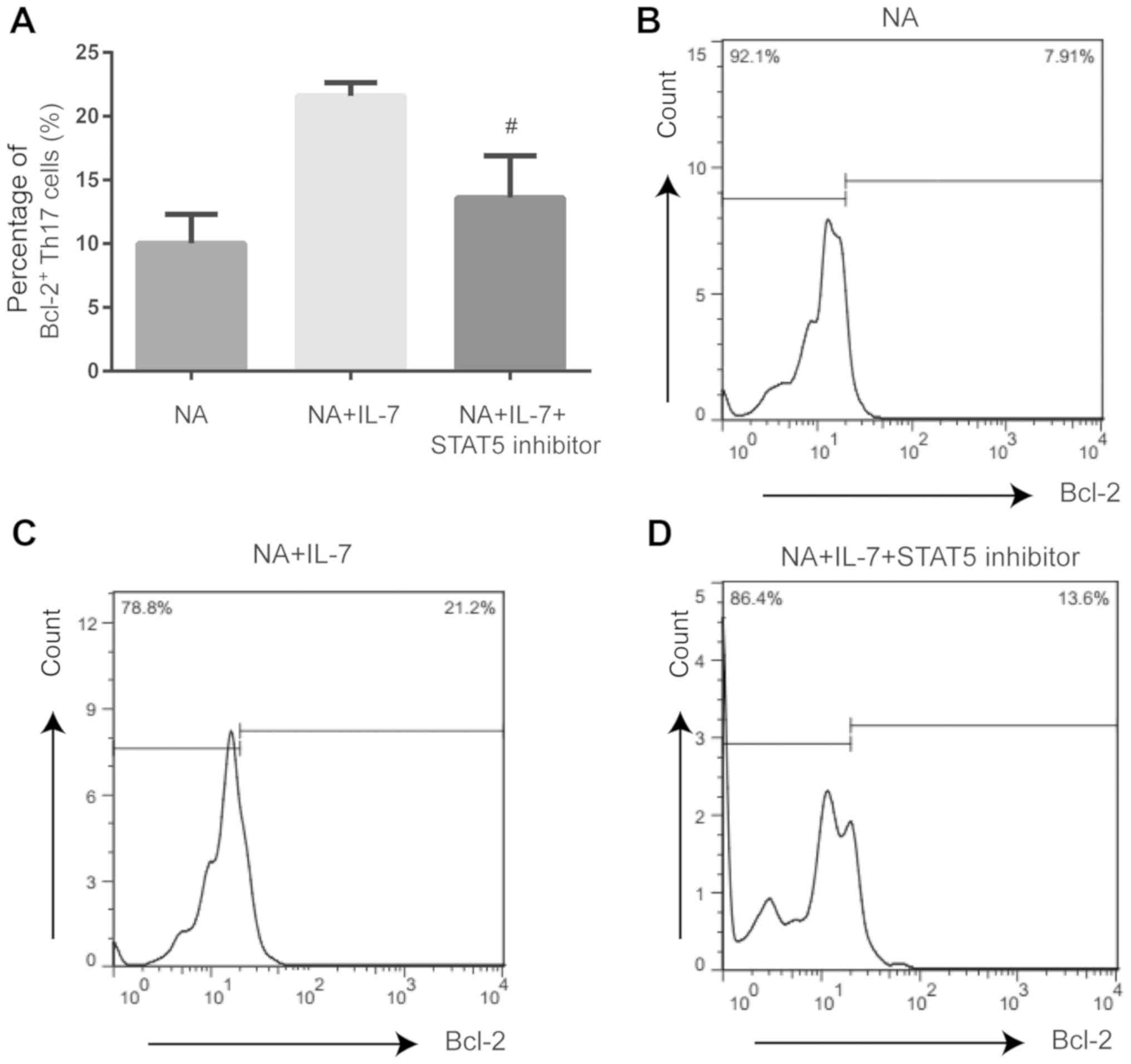
Expression of proliferating and
anti-apoptotic proteins is affected by JAK/STAT pathway inhibition
in Th17 cells from NA mice
Compared with NA mice treated with IL-7, STAT5
inhibition decreased the expression of Ki-67 (P<0.01; Fig. 4) and Bcl-2 (P<0.05; Fig. 5) and increased the expression of
caspase-3 (P<0.01; Fig. 6) in
Th17 cells.
Discussion
NA is associated with neutrophil responses not only
in severe asthmatics but also in patients with mild and moderate
asthma (10). Our previous studies
demonstrated that NA mice displayed increased levels of IL-7 and
Th17 cell immune responses, as well as IL-17 secreted by Th17
cell-mediated neutrophilic airway inflammation (Jiang et al,
unpublished data). However, the mechanisms underlying Th17 cell
responses in NA remain unknown. Our previous study demonstrated
that sputum supernatant from patients with NA inhibited apoptosis
of peripheral blood neutrophils in normal individuals, which
suggested a role for the airway micro-environment in the regulation
of neutrophil airway inflammation (11). Several previous studies
demonstrated that IL-7 induced the expression of the anti-apoptotic
protein Bcl-2 and activated the JAK/STAT signaling pathway, which
contributed to T cell survival (6–8).
Therefore, it was hypothesized that IL-7 was involved in the Th17
responses seen in NA. In the present study, an NA mouse model was
established, in which Th17 cells were affected by IL-7 and
inhibited by suppression of the JAK/STAT pathway. Thus, the
IL-7/JAK/STAT signaling pathway was involved in Th17 cell responses
in this model of NA.
The results from a previous study on the
conventional animal model of asthma sensitized by intraperitoneal
injection were inconsistent with those of NA and did not reflect
the exact state of airway inflammation and AHR (9). Thus, a mouse model of NA might
provide insight into the mechanisms underlying these differences. A
previous study demonstrated that allergic sensitization of the
airway stimulated robust Th17 responses, and that neutrophilia was
required for AHR (9). In the
present study, a mouse model of NA was successfully established by
airway delivery of OVA and LPS, based on the previous study by
Wilson et al (9). NA mice
displayed increased AHR, elevated levels of IL-17 and a high number
of neutrophils in BALF. Moreover, numerous inflammatory cells
infiltrated around the bronchus and blood vessels. In summary, the
current mouse model presented the following features of NA: i)
Presence of AHR; ii) accumulation of inflammatory cells in the
lung, primarily with increased neutrophils; and iii) high number of
neutrophils in BALF.
Accumulating evidence suggests that Th17 cells are
involved in the pathogenesis of asthma (9,12),
including severe forms of asthma that are refractory to treatment
with corticosteroids (13). IL-17
production by Th17 cells is associated with asthma AHR (13), corticosteroid resistance, goblet
cell hypersecretion, airway fibrosis and airway remodeling
(14). In the present study, NA
mice demonstrated strong Th17 responses in the lung and spleen.
Moreover, Th17 cell frequency, the level of IL-17 and AHR increased
in NA mice when compared with the NC, which was was consistent with
a previous study, in which Th17 cells were associated with
neutrophilic airway inflammation (15).
IL-7 promotes airway inflammation by activating and
maintaining eosinophil survival (16). However, the effects of IL-7 on
neutrophils remain unclear. IL-7 is a member of the type I cytokine
receptor family and plays a critical role in proliferation,
survival and differentiation of T lymphocytes (6,17).
Bcl-2 is a crucial anti-apoptotic protein (18), and IL-7 can upregulate Bcl-2
expression (19). Liu et al
(20) demonstrated that IL-7
promoted the survival and inhibited Th17 cell apoptosis in
auto-immune encephalomyelitis mice. Ki-67 is a nuclear protein
associated with cellular proliferation (21), and caspase-3 plays a key role in
the execution phase of cell apoptosis (22). The present study was consistent
with the findings of Liu et al (20), in which IL-7 administration in NA
resulted in increased levels of Ki-67 and Bcl-2, suggesting IL-7 is
likely involved in Th17 cell proliferation. Furthermore,
lymphocytes treated with caspase-3 inhibitor showed reduced
apoptosis (23); IL-7 inhibited
caspase-3 activation and reduced T cell apoptosis (5). In the present study, IL-7
administration reduced caspase-3 expression, which suggested that
IL-7 was involved in inhibiting Th17 cell apoptosis. These data
demonstrated that IL-7 was involved in the response of Th17
cells.
The JAK/STAT signaling pathway is involved in
proliferation, differentiation and survival of immune cells
(24). Previous studies suggested
that the JAK/STAT5 signaling pathway played a role in asthma
pathogenesis, and that JAK inhibition significantly antagonized
p-STAT5 activation in T cells (25). However, only one previous study
suggested that JAK/STAT5 signaling was involved in NA airway
inflammation (26). Moreover, the
JAK/STAT signaling pathway activated by IL-7 might be the mechanism
underlying T cell survival and the development of corticosteroid
resistance in asthma (27). The
present study demonstrated that IL-7 increased the expression of
p-STAT5, and the downstream proteins Ki-67 and Bcl-2 in Th17 cells
from NA mice. Moreover, STAT5 inhibition reversed the effect of
IL-7 on Ki-67, Bcl-2 and caspase-3 expression in Th17 cells. Thus,
IL-7/JAK/STAT5 played a role in the prevalence of Th17 cells in NA
mice.
In conclusion, the present study successfully
established an NA mouse model by airway delivery of OVA in the
presence of LPS. Furthermore, the response of Th17 cells in this
model was identified. IL-7 regulated the expression of the
proliferation marker Ki-67, anti-apoptotic protein Bcl-2 and
pro-apoptotic protein caspase-3, and STAT5 inhibition could reverse
this effect. To the best of the authors' knowledge, this is the
first study on the contribution of IL-7 to Th17 responses in NA. A
limitation of the present study is that the mechanism has not yet
been verified in vivo. When assessing the response to IL-7,
the lack of a positive control lymphocyte population was another
limitation of the present study. Thus, further studies in mice are
essential to determine whether IL-7 and JAK/STAT5 pathway blockade
are a potential therapeutic approach for NA.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by International
Communication of Guangxi Medical University Graduate Education,
Guangxi Natural Science Foundation (grant no. 2018GXNSFAA281256),
Innovation Project of Guangxi Graduate Education (grant no.
YCBZ2019045), Chen Xiaoping Foundation for the Development of
Science and Technology of Hubei Province (grant no. CXPJJH1
1900003-17), Guangxi Health Commission Project (grant no.
Z20190762) and The Education Foundation of Guangxi (grant no.
2017KY0117).
Availability of data and materials
All data generated or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XZ performed the experiments and analyzed the data,
as well as prepared the manuscript. MZ performed the experiments
and interpreted the data, as well as drafted the paper. MJ designed
the study and revised the manuscript for important intellectual
content. GN contributed to the conception of this study and overall
supervision. All authors read and approved the final version.
Ethics approval and consent to
participate
The present study was approved by The Ethics
Committee of The First Affiliated Hospital of Guangxi Medical
University [approval no. 2019(KY-E-035)].
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
NA
|
neutrophilic asthma
|
|
AHR
|
airway hyperresponsiveness
|
|
BALF
|
bronchio-alveolar lavage fluid
|
References
|
1
|
Hekking PP and Bel EH: Developing and
emerging clinical asthma phenotypes. J Allergy Clin Immunol Pract.
2:671–80; quiz 81. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Simpson JL, Scott R, Boyle MJ and Gibson
PG: Inflammatory subtypes in asthma: assessment and identification
using induced sputum. Respirology. 11:54–61. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tsoumakidou M, Papadopouli E, Tzanakis N
and Siafakas NM: Airway inflammation and cellular stress in
noneosinophilic atopic asthma. Chest. 129:1194–1202. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Basyigit I, Yildiz F, Ozkara SK, Boyaci H
and Ilgazli A: Inhaled corticosteroid effects both eosinophilic and
non-eosinophilic inflammation in asthmatic patients. Mediators
Inflamm. 13:285–291. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chetoui N, Boisvert M, Gendron S and
Aoudjit F: Interleukin-7 promotes the survival of human
CD4+ effector/memory T cells by up-regulating Bcl-2
proteins and activating the JAK/STAT signalling pathway.
Immunology. 130:418–426. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fry TJ and Mackall CL: The many faces of
IL-7: from lymphopoiesis to peripheral T cell maintenance. J
Immunol. 174:6571–6576. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zaunders JJ, Levy Y and Seddiki N:
Exploiting differential expression of the IL-7 receptor on memory T
cells to modulate immune responses. Cytokine Growth Factor Rev.
25:391–401. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Read KA, Powell MD, McDonald PW and
Oestreich KJ: IL-2, IL-7, and IL-15: Multistage regulators of
CD4(+) T helper cell differentiation. Exp Hematol. 44:799–808.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wilson RH, Whitehead GS, Nakano H, Free
ME, Kolls JK and Cook DN: Allergic sensitization through the airway
primes Th17-dependent neutrophilia and airway hyperresponsiveness.
Am J Respir Crit Care Med. 180:720–730. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gauthier M, Ray A and Wenzel SE: Evolving
Concepts of Asthma. Am J Respir Crit Care Med. 192:660–668. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Uddin M, Nong G, Ward J, Seumois G, Prince
LR, Wilson SJ, Cornelius V, Dent G and Djukanovic R: Prosurvival
activity for airway neutrophils in severe asthma. Thorax.
65:684–689. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Choy DF, Hart KM, Borthwick LA, Shikotra
A, Nagarkar DR, Siddiqui S, Jia G, Ohri CM, Doran E, et al: TH2 and
TH17 inflammatory pathways are reciprocally regulated in asthma.
Sci Transl Med. 7:301ra1292015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Newcomb DC and Peebles RS Jr:
Th17-mediated inflammation in asthma. Curr Opin Immunol.
25:755–760. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cosmi L, Liotta F and Annunziato F: Th17
regulating lower airway disease. Curr Opin Allergy Clin Immunol.
16:1–6. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kudo M, Melton AC, Chen C, Engler MB,
Huang KE, Ren X, Wang Y, Bernstein X, Li JT, et al: IL-17A produced
by alphabeta T cells drives airway hyper-responsiveness in mice and
enhances mouse and human airway smooth muscle contraction. Nat Med.
18:547–554. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kelly EA, Koziol-White CJ, Clay KJ, Liu
LY, Bates ME, Bertics PJ and Jarjour NN: Potential contribution of
IL-7 to allergen-induced eosinophilic airway inflammation in
asthma. J Immunol. 182:1404–1410. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sportes C, Hakim FT, Memon SA, Zhang H,
Chua KS, Brown MR, Fleisher TA, Krumlauf MC, Babb RR, et al:
Administration of rhIL-7 in humans increases in vivo TCR repertoire
diversity by preferential expansion of naive T cell subsets. J Exp
Med. 205:1701–1714. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kale J, Osterlund EJ and Andrews DW: BCL-2
family proteins: changing partners in the dance towards death. Cell
Death Differ. 25:65–80. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Goodsell DS: The molecular perspective:
Bcl-2 and apoptosis. Oncologist. 7:259–260. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu X, Leung S, Wang C, Tan Z, Wang J, Guo
TB, Fang L, Zhao Y, Wan B, et al: Crucial role of interleukin-7 in
T helper type 17 survival and expansion in autoimmune disease. Nat
Med. 16:191–197. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sun X and Kaufman PD: Ki-67: more than a
proliferation marker. Chromosoma. 127:175–186. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Burgon PG and Megeney LA: Caspase
signaling, a conserved inductive cue for metazoan cell
differentiation. Semin Cell Dev Biol. 11:01–09. 2017.
|
|
23
|
Hotchkiss RS, Coopersmith CM and Karl IE:
Prevention of lymphocyte apoptosis - a potential treatment of
sepsis? Clin Infect Dis. 41 (Suppl 7):S465–S469. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Harrison DA: The Jak/STAT pathway. Cold
Spring Harb Perspect Biol. 4:a0112052012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Howell MD, Fitzsimons C and Smith P:
JAK/STAT inhibitors and other small molecule cytokine antagonists
for the treatment of allergic disease. Ann Allergy Asthma Immunol.
120:367–375. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li RF and Wang GF: JAK/STAT5 signaling
pathway inhibitor ruxolitinib reduces airway inflammation of
neutrophilic asthma in mice model. Eur Rev Med Pharmacol Sci.
22:835–843. 2018.PubMed/NCBI
|
|
27
|
Liu S, Verma M, Michalec L, Liu W, Sripada
A, Rollins D, Good J, Ito Y, Chu H, et al: Steroid resistance of
airway type 2 innate lymphoid cells from patients with severe
asthma: The role of thymic stromal lymphopoietin. J Allergy Clin
Immunol. 141:257–68.e6. 2018. View Article : Google Scholar : PubMed/NCBI
|