Introduction
Respiratory syncytial virus (RSV) infection enhances
the cell-mediated immune responses of type 2 helper T cells and
promotes the progression of allergic inflammation and asthma by
producing thymic stromal lymphopoietin (TSLP), especially long
isoform TSLP (lfTSLP) (1,2). TSLP, an interleukin (IL)-7-like
cytokine first discovered in the supernatant of thymic stromal cell
cultures in mice, derives from the epithelium and acts in the
pathophysiology of autoimmune and allergic diseases, including
atopic dermatitis, allergic conjunctivitis, allergic rhinitis and
asthma (3–5). TSLP exists in two distinct isoforms,
lfTSLP and short isoform TSLP (sfTSLP). lfTSLP has a sequence of
159 amino acids, while sfTSLP relies on a downstream in-frame start
codon to generate a peptide of either 63 or 60 amino acids, which
fully overlaps the amino-acid sequence of lfTSLP in the C-terminal
region (6). sfTSLP, the main
isoform in the steady state, demonstrates protective and
antimicrobial activity (3). In
contrast, lfTSLP overexpression can promote the process of
inflammation. Concerted research has been performed to explore the
expression and related biological functions of these two isoforms
in diseases (3,4,6).
Early-life viral respiratory tract infections can
increase the risk of recurrent wheezing and asthma (7–9).
Respiratory syncytial virus (RSV) is a major causative agent of
lower respiratory infections in the pediatric population,
especially in infants (10). RSV
infection triggers type 2 helper T cell (Th2) immune response
characterized by IL-4, IL-5 and IL-13 production. The resultant
excessive mucus in the respiratory tract enhances the pulmonary
inflammatory response, subsequently leading to asthma (1,2,11).
TSLP produced by epithelial cells in RSV infection can create a
permissive environment for the differentiation of dendritic cells,
T cells and congenital lymphocytes and the subsequent production of
the Th2-type cytokines (2,12). Current research is more focused on
lfTSLP, rarely on sfTSLP (2,12,13).
Activating protein 2 (AP-2)α, a
transcription-suppressor, may reduce TSLP transcriptional activity.
Rs2289276, a single-nucleotide polymorphism (SNP) located in 5′
untranslated region of sfTSLP and the intron 2 of lfTSLP,
potentially changes the affinity of AP-2α between two alleles. The
allele containing AP-2α binding site acts in a protective manner
(13). This means that AP-2α may
participate in the transcriptional regulation of TSLP in a
non-inflammatory state.
The present study aimed to determine the role of
sfTSLP in RSV infection, the association between the two isoforms
of TSLP and the potential transcriptional mechanisms. The results
identified that sfTSLP can be regulated by AP-2α, supporting the
hypothesis AP-2α deficiency in bronchial epithelial cells might
promote sfTSLP and lfTSLP expression in RSV infection.
Materials and methods
Cell lines
African green monkey kidney epithelial cells (Vero),
human bronchial epithelium cells (Beas-2B) and HeLa cells were
provided by the American Type Culture Collection. Cells were
maintained in Dulbecco's modified Eagle's medium (Nanjing KeyGen
Biotech Co., Ltd.), containing 80 U/ml penicillin and 0.08 mg/ml
streptomycin, supplemented with 10% heat-inactivated fetal bovine
serum (FBS, Gibco; Thermo Fisher Scientific, Inc.) at 37°C and in a
humidified atmosphere of 5% CO2.
Reagents
Rabbit anti-GAPDH antibody (cat. no. ab181602),
rabbit anti-transcription factor AP-2α (cat. no. ab52222) and
rabbit anti-TSLP antibody (cat. no. ab47943) were purchased from
Abcam. ProteinTech Group, Inc. provided the goat anti-rabbit IgG
(cat. no. SA00001-2).
RSV preparation and experimental
infection
Human RSV type A (strain A2) was kept in the
laboratory of the Department of Pediatrics, The First Affiliated
Hospital, Nanjing Medical University and had been used in previous
studies (14). As previously
described, the virus was expanded in DMEM containing 2% FBS in Vero
cells. When the cytopathic effect reached 80%, the infected cells
were frozen-thawed in the medium. The RSV was purified by
centrifugation (850 × g, 10 min, 4°C) and then stored at −80°C for
further investigation. The viral titer was determined by 50% tissue
culture infective dose in the Reed-Muench method (15) and was expressed as multiplicities
of infection (MOI). The Beas-2B cells were cultured in DMEM mixed
with 2% FBS for 24 h and then infected with RSV for 48 h before
harvest and assay.
AP-2α overexpression and small
interfering (si)RNA knockdown
Cells (1×105) were seeded in 12-well
plates overnight for transfection. Following the manufacturer's
protocol, transfection was carried out in Beas-2B cells using
Lipofectamine® 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.). The AP-2α expression plasmid was supplied by
Thermo Fisher Scientific, Inc., and the pcDNA3.1-basic vector was
used as a control. The customized siRNA was synthesized by
Guangzhou RiboBio Co., Ltd., with a target sequence of
GAGGAAGATCTTTAAGAGA. The controlled siRNA was also synthesized by
Guangzhou RiboBio Co., Ltd., with a target sequence of
CGUAAACGGCCACAAGUUC. The transfection reaction was carried out in
DMEM without FBS. The expression plasmid (1,000 ng/ml) or siRNA
(100 nM) were transfected into cells. The cell culture medium was
replenished with the extract 16 h after transfection. The cells
were harvested for mRNA analysis 24 h after transfection and for
protein analysis 48 h after transfection.
Reverse transcription-quantitative
(RT-q) PCR
Cells (1×105) were seeded in 12-well
plates and lysed by TRIzol® (Invitrogen; Thermo Fisher
Scientific, Inc.) 24 h after transfection. Total RNA was extracted
and purified according to the manufacturer's protocol. The amount
and purity of RNA were measured using a NanoDrop 2000
Spectrophotometer (Thermo Fisher Scientific, Inc.). RNA (1,000 ng)
was reverse-transcribed into cDNA using the Prime Script RT Master
Mix Perfect Real Time kit (Takara Biotechnology Co., Ltd.). Samples
were measured on a LightCycler 40 II instrument (Roche Diagnostics)
using SYBR Green technology (Takara Biotechnology Co., Ltd.) for
RT-qPCR. The mRNA level was normalized based on the level of GAPDH
mRNA and calculated into a 2−ΔΔCq value (16). DNA amplification was performed as
follows: Initial denaturation at 95°C for 30 sec, followed by 50
cycles of amplification (denaturation at 95°C for 5 sec and
annealing at 60°C for 30 sec). Fluorescence data were collected
during the annealing phase of the amplification. All experiments
were repeated at least three times. The primers used were: AP-2α
sense, 5′-ATTGACCTACAGTGCCCAGC-3′ and antisense,
5′-TCCATGAAAATGCTTTGGAA-3′; sfTSLP sense, 5′-CCGCCTATGAGCAGCCAC-3′
and antisense, 5′-CCTGAGTAGCATTTATCTGAG-3′; lfTSLP sense,
5′-ACCAGTGGGAAGGGCAACC-3′ and antisense,
5′-CATTGTTTGGCTGAAGGCTTGT-3′; GAPDH sense,
5′-ATGACATCAAGAAGGTGGTG-3′ and antisense,
5′-CATACCAGGAAATGAGCTTG-3′.
Western blot assay
Cells were collected on ice 48 h after transfection
and lysed in 200 µl of pre-iced lysis buffer containing 0.1 mM PMSF
(Nanjing KeyGen Biotech Co., Ltd.). Total protein was determined
using a BCA assay. Protein samples (40 µg/lane) were loaded onto
10% precast polyacrylamide gradient gels (Beyotime Institute of
Biotechnology) and transferred to nitrocellulose membranes
(Immobilon; EMD Millipore) for 150 min. To block non-specific
sites, they were then incubated in TBS-T saline (0.25 M Tris-HCl;
pH 7.6, 0.19 M NaCl, 0.1% Tween-20) in 5% dried milk for 2 h at
room temperature. Protein blots were incubated for 12–14 h at 4°C
with primary antibodies against TSLP, AP-2α and GAPDH (1:2,000;
diluted with Primary Antibody Dilution Buffer purchased from
Beyotime Institute of Biotechnology). Then, membranes were
incubated for 1 h at room temperature with a goat anti-rabbit IgG
secondary antibody (1:10,000; diluted with Secondary Antibody
Dilution Buffer purchased from Beyotime Institute of
Biotechnology). Reactive proteins were visualized by enhanced
chemiluminescence (Biosharp Life Sciences) and densitometry was
performed using a Bio-Rad gel imager and Quantity One 1-D version
4.6 software (Bio-Rad Laboratories, Inc.).
Bioinformatics prediction and plasmid
construction
The luciferase reporter gene was cloned at MIUI/XhoI
sites in the pGL3-Basic vector. A series of 5′-flanking region of
sfTSLP were inserted to pGL3-Basic as previously (17). JASPAR (http://jaspar.genereg.net/analysis) (18) was used to predict the AP-2α
transcriptional sites of the promoter 5′-flanking region. The
plasmids containing the core promoter of sfTSLP (pGL-200/+25) and
mutations of the AP-2α binding sites on the sfTSLP core promoter
region were synthesized directly by TSINGKE Biological Technology
Co., Ltd. All the plasmids were verified by Sanger sequencing
(provided by TSINGKE Biological Technology Co., Ltd.).
Transient transfection and luciferase
assay
For characterizing the reporter cell lines,
1×104 HeLa cells were plated in 96-well plates overnight
before transfection for luciferase assay. Transfection mixtures
were prepared in OptiMEM I Reduced Serum Medium (Gibco; Thermo
Fisher Scientific, Inc.) using Lipofectamine® 3000
(Invitrogen; Thermo Fisher Scientific, Inc.). The AP-2α expression
plasmid (100 ng) or siRNA (100 nM) was co-transfected into HeLa
cells together with 100 ng of luciferase reporter plasmids driven
by wild-type or mutated sfTSLP promoter. Then, 4 ng of the pRL-TK
plasmid (Promega Corporation) was co-transfected as a control
plasmid to maintain the total DNA amount. Cells were stimulated 24
h after transfection and lysed in 30 µl of lysis buffer (Promega
Corporation). The Dual Reporter Assay System (Promega Corporation)
and TD-20/20 Turner Designs Luminometer were used for measuring
luciferase activity. Samples were analyzed in triplicate in each
experiment and each experiment was repeated ≥3 times.
Chromatin immunoprecipitation (ChIP)
assay
According to the manufacturer's instructions, 2 µg
of anti-AP-2α, anti-Histone H3 antibody and control IgG antibody
were analyzed in ChIP assay using the ChIP-IT kit (cat. no. 53008;
Active Motif, Inc.) as described previously (14). The products were subjected to PCR
amplification and further amplified by RT-qPCR with the following
promoter-specific primers: sense, 5′-GGGAACGTTGTTAGGGGCA-3′ and
antisense, 5′-GGGGAACACAAGTCGAGAGT-3′. PCR products were resolved
on a 2% agarose gel together with 100 bp DNA ladder (Takara
Biotechnology Co., Ltd.). Input DNA samples from the sonicated
nuclear lysates and DNA samples immunoprecipitated with antibodies
against IgG were used as positive or negative controls. All
experiments were repeated three times.
Statistical analysis
Results are expressed as the mean ± standard
deviation of the mean. Student's t-test and Welch's ANOVA with a
Tamhane post hoc test were performed using SPSS 22.0 (IBM Corp.)
and GraphPad Prism 7 (GraphPad Software, Inc.). P<0.05 was
considered to indicate a statistically significant difference.
Results
RSV infection increases TSLP
expression and decreases AP-2α expression in Beas-2B cells
The present study first examined the effect of RSV
infection on lfTSLP mRNA expression, finding that the mRNA and
protein levels of lfTSLP were significantly increased in Beas-2B
cells following RSV infection for 48 h (Fig. 1). Compared with the uninfected
controls, a 16-fold increase in lfTSLP mRNA expression and a 3-fold
increase in sfTSLP mRNA expression were detected in RSV-treated
cells (MOI=2) for 48 h (Fig. 1A).
It was also revealed that the level of AP-2α mRNA expression was
decreased by 62% (Fig. 1A).
Western blotting also confirmed that lfTSLP expression was
significantly increased after RSV infection (MOI=2; Fig. 1B).
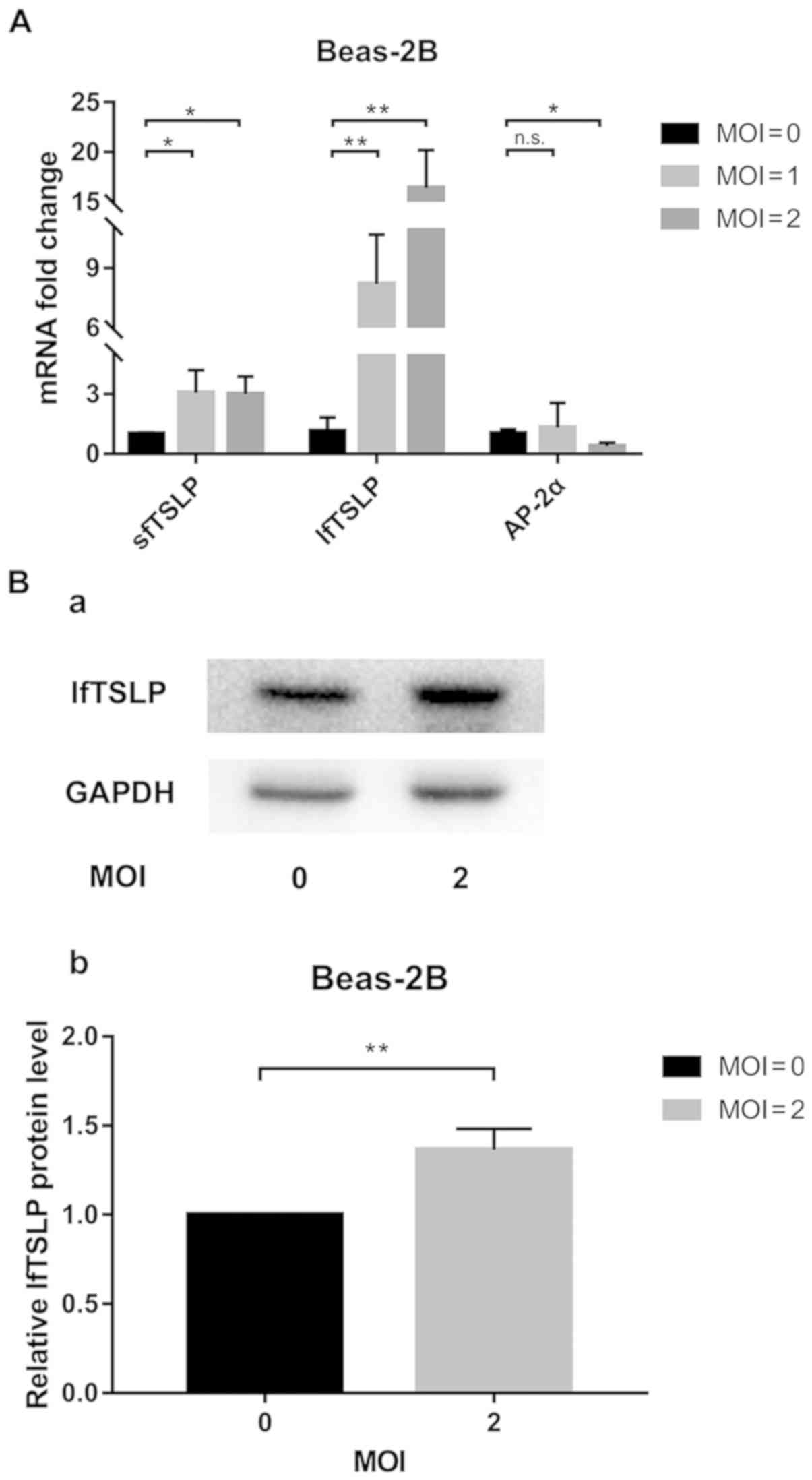 | Figure 1.RSV infection upregulates TSLP
expression in Beas-2B cells. (A) Beas-2B cells were cultured for 48
h in the presence of RSV and lysed in TRIzol®. Following
total mRNA extraction, reverse transcription-quantitative PCR was
performed using specific lfTSLP, sfTSLP and AP-2α primers. (B)
lfTSLP protein released from Beas-2B cells at 48 h of RSV (MOI=2)
treatment was (a) measured by western blotting and (b) quantified.
Representative data from ≥3 independent experiments performed under
similar conditions. *P<0.05, **P<0.01 vs. the corresponding
unstimulated controls; n.s., not significant; RSV, respiratory
syncytial virus; TSLP, thymic stromal lymphopoietin; lf, long
isoform; sf, short isoform; AP-2α, activating protein 2 α; MOI,
multiplicity of infection. |
AP-2α regulates the expression of both
lfTSLP and sfTSLP
The AP-2α overexpression vector and siRNA AP-2α were
transfected into Beas-2B cells to examine the effect of AP-2α on
TSLP expression. The transfection efficacy of the overexpression
vector was validated at the mRNA (Fig.
2A-c) and protein level (Fig. 2B-a
and b). The transfection efficacy of the siRNA AP-2α was
validated at the mRNA (Fig. 2A-c)
and protein level (Fig. 2C-a and
b). Using RT-qPCR, it was found that the mRNA levels of both
lfTSLP and sfTSLP were significantly decreased with the
overexpression of AP-2α (Fig. 2A-a and
b). By contrast, significantly increased mRNA levels of lfTSLP
and sfTSLP were observed in the AP-2α knockdown cells (Fig. 2A-a and b). Western blotting also
confirmed that lfTSLP expression was regulated by AP-2α in the
pulmonary epithelial cells (Fig. 2B
and C).
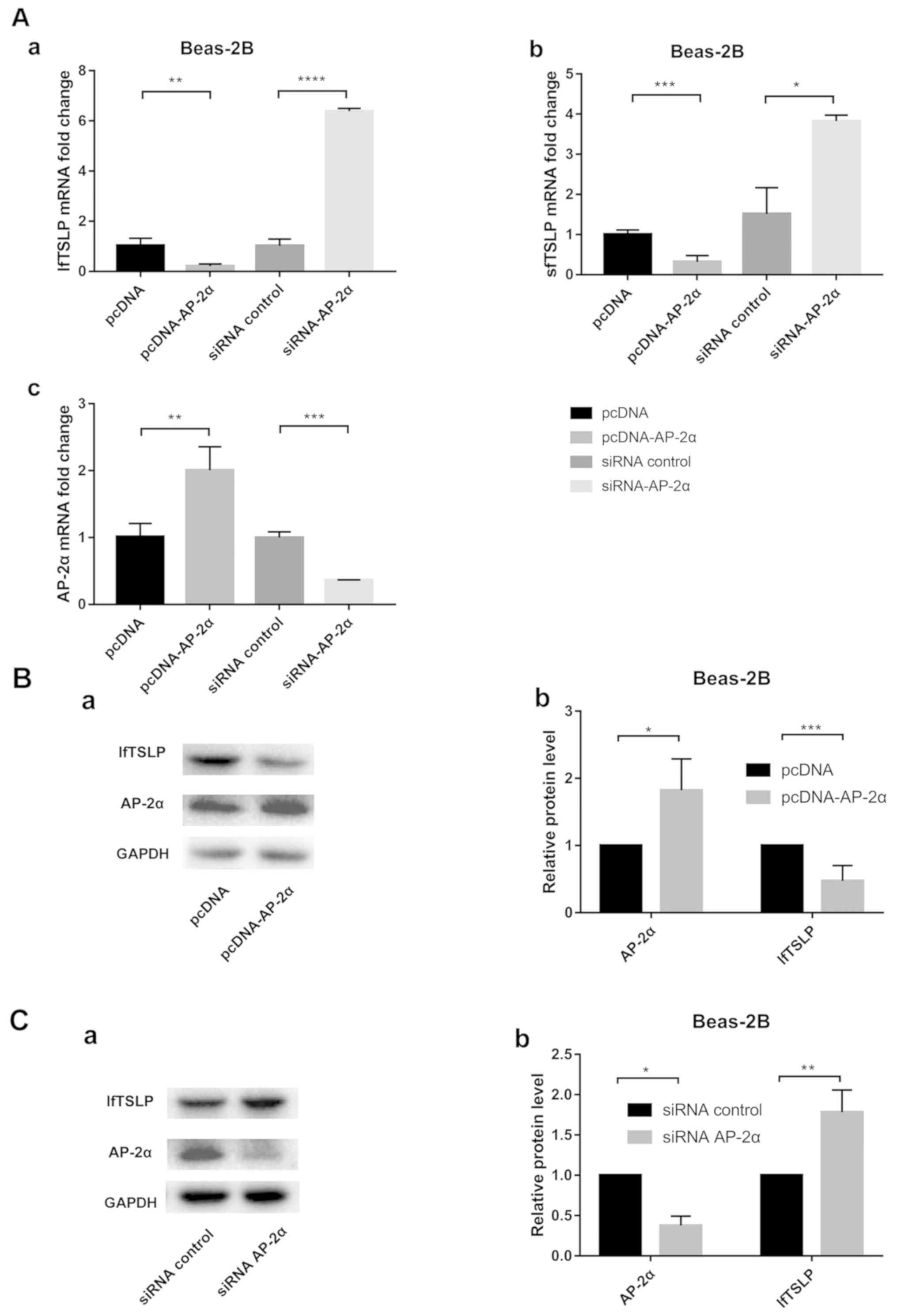 | Figure 2.AP-2α siRNA increases and AP-2α
overexpression decreases lfTSLP and sfTSLP mRNA expression in
Beas-2B cells. (A) Beas-2B cells were transfected with AP-2α
overexpression plasmid (pcDNA-AP-2α) or siRNA-AP-2α for 24 h. (a)
Quantification of lfTSLP mRNA level after transfection. (b)
Quantification of sfTSLP mRNA level after transfection. (c)
Quantification of AP-2α mRNA level after transfection. (Ba)
Following transfection with AP-2α overexpression plasmid
(pcDNA-AP-2α) or control plasmid (pcDNA) for 48 h, the levels of
lfTSLP and AP-2α proteins were detected in Beas-2B cells by western
blotting. (b) Quantification of western blotting. (Ca) Following
transfection with AP-2α-siRNA or siRNA control for 48 h, the levels
of lfTSLP and AP-2α proteins were detected in Beas-2B cells by
western blotting. (b) Quantification of western blotting. Each
experiment was performed in triplicate and significant differences
were determined with the Student's t-test. *P<0.05, **P<0.01,
***P<0.001, ****P<0.0001 vs. the corresponding control. AP-2,
activating protein 2; si, small interfering; lf, long isoform; sf,
short isoform; TSLP, thymic stromal lymphopoietin. |
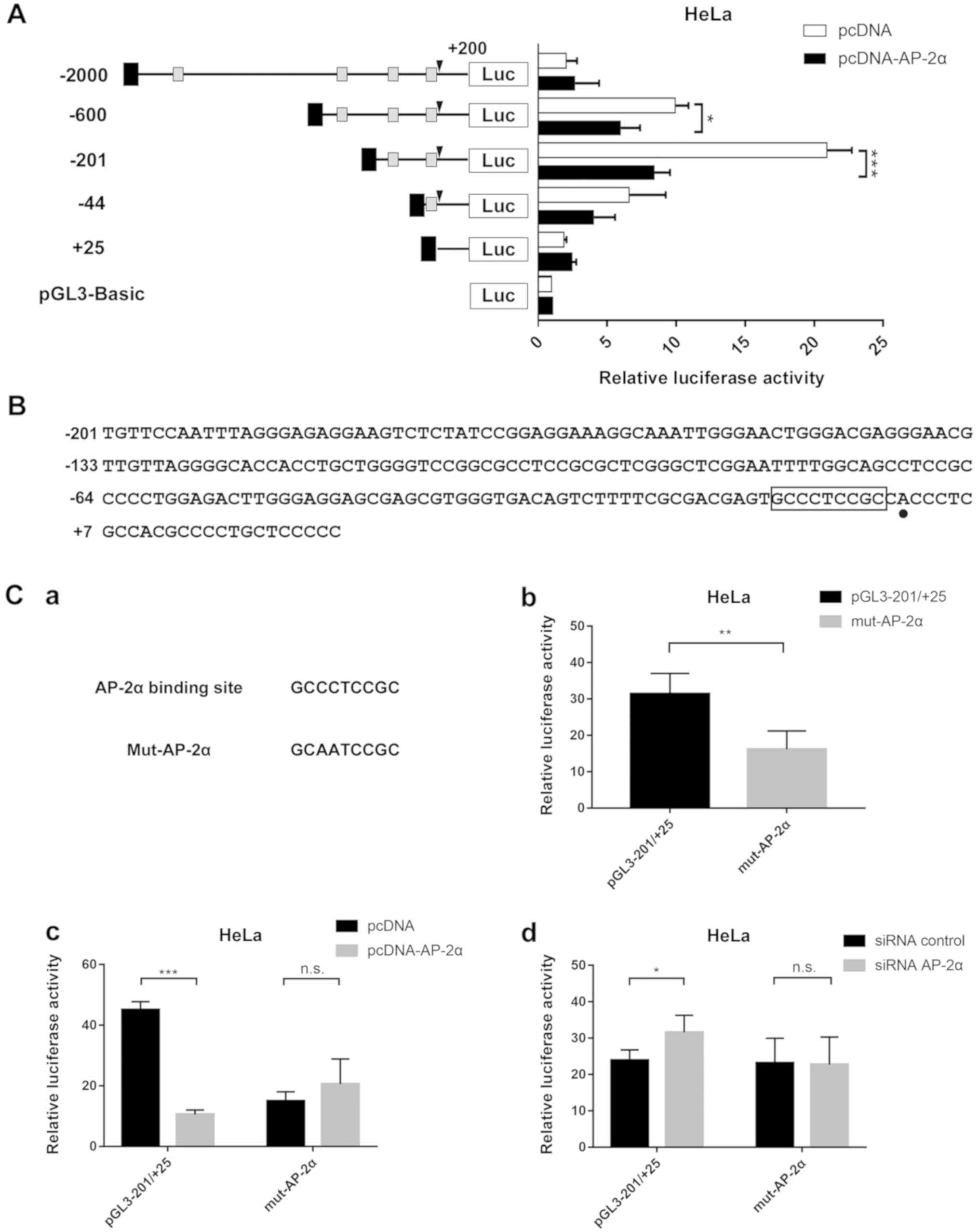
AP-2α regulates sfTSLP transcriptional
promoter activity
To elucidate the specific promoter region
responsible for sfTSLP regulation, JASPAR (http://jaspar.genereg.net/analysis) (18) was used to predict the AP-2α
transcriptional sites of the promoter 5′-flanking region. Several
putative AP-2α binding sites were identified. A series of
5′-flanking regions of sfTSLP were inserted to pGL3-Basic as before
(Fig. S1A). As shown in Fig. 3A, the core promoter region of
sfTSLP was located at −200/+25nt as revealed in our previous
experiment (17). A statistically
significant decrease (~60%) in luciferase activity was observed in
response to Ap-2α overexpression.
To further confirm that the binding site at
−200/+25nt (Fig. 3B) is critical
for sfTSLP modulation, site-directed mutagenesis targeting AP-2α in
the context of the core promoter region was performed (Fig. 3C-a). A 76.5% decrease in the
luciferase activity was observed in wild-type pGL3-200/+25 when
AP-2α was overexpressed (Fig.
3C-c) and a 31.9% increase when AP-2α was knocked down by siRNA
(Fig. 3C-d). By contrast, the
mutant plasmid did not alter the AP-2α-mediated TSLP promoter
activity. The activity of reporter genes was significantly reduced
(by ~50%) after the −10/-2 nt AP-2α site mutation, compared with
that in wild-type pGL3 −200/+25 (Fig.
3C-b). Therefore, the imperfect AP-2α site at −102/-94 nt was
mutated (Fig. S1A) and a 50%
increase in luciferase activity in wild-type was observed (Fig. S1B). A decrease of 25% was observed
after both AP-2α binding sites were mutated (Fig. S1B). The luciferase activity of
plasmid with the mutation of at the two AP-2α sites was
significantly different from that in the mutation of AP-2α site at
−102/-94 nt (P<0.05), but not from that in the mutation of AP-2α
site at −10/-2 nt. This meant that the main binding site was
located at −10/-2 nt. These results pointed to the important
function of AP-2α site at −200/+25 nt in sfTSLP expression.
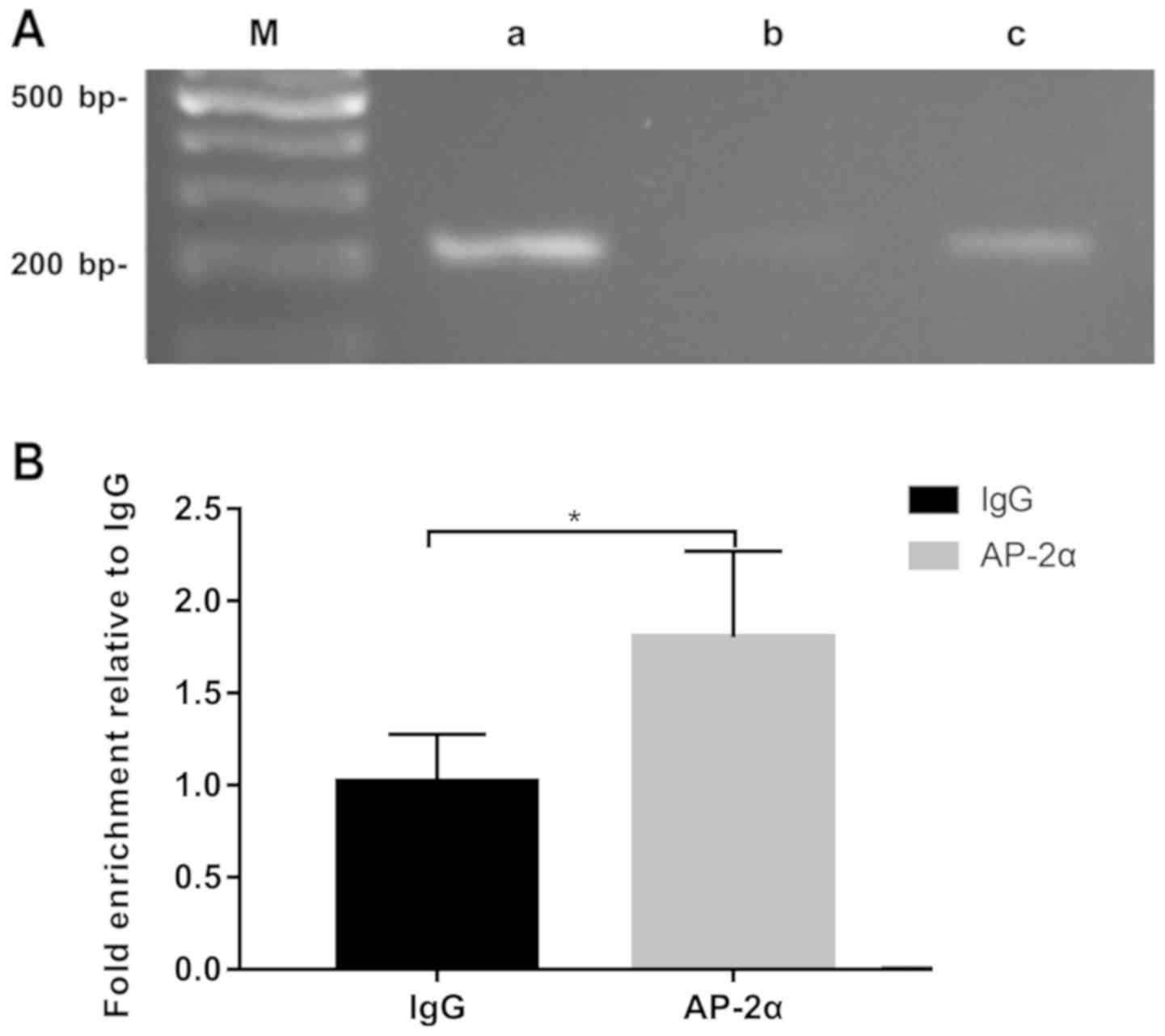
AP-2α directly binds to the sfTSLP
promoter in vivo
ChIP assays were performed in Beas-2B cells to
determine whether AP-2α could directly or indirectly regulate
sfTSLP expression in vivo. The ultrasonic extract of Beas-2B
cells cross-linked with formaldehyde was immunoprecipitated with
the anti-AP-2α antibody. PCR was performed based on the relevant
chromatin DNA fragments, using primers flanking the AP-2α binding
site in the core promoter region. The reactions with anti-histone
H3 antibodies were used as positive controls, while those of
anti-IgG were used as negative controls. As shown in Fig. 4A, the anti-AP-2α antibody was more
abundant in the Beas-2B cells than in the AP-2α promoter
precipitated by the IgG antibody. The DNA fragments were further
quantified by RT-qPCR (Fig. 4B).
In conclusion, the present study suggested that AP-2α could
directly bind to the core promoter region of sfTSLP.
Discussion
It has been reported that sfTSLP/lfTSLP ratio
changes in certain diseases, such as gut disorders, atopic
dermatitis and skin keratosis exposed to inflammatory stimuli
(6,19). In viral respiratory infections, the
aberrant expression of long isoform thymic stromal lymphopoietin
(lfTSLP) triggers Th2 overreaction and pathological exacerbation,
eventually resulting in asthma (20,21),
while the role of short isoform TSLP (sfTSLP) in airway epithelial
cells attacked by respiratory viruses is still unclear. Previous
research has demonstrated that sfTSLP is almost uninduced by
proinflammatory cytokines, such as poly(I:C), lipopolysaccharide,
or macrophage-activating lipopeptide 2 (6) and appears to be downregulated in
inflammation (22). However, the
expression of sfTSLP mRNA increases in bronchial epithelial cell
lines challenged with human pneumovirus (23). To elucidate the underlying
mechanism, the present study explored the differential expression
of lfTSLP and sfTSLP in RSV infection. It has been confirmed that
lfTSLP is secreted by RSV-attached airway epithelial cells
(13,24), but the role of sfTSLP has been
rarely reported. The present study detected the expression profiles
of both isoforms in Beas-2B cells following RSV infection.
Polyclonal antibodies, with a previously verified feasibility, were
used to detect the protein level of lfTSLP (6,19,22).
Consistent with the findings of previous studies (20,21),
lfTSLP showed a significant increase at the mRNA and protein
levels. As the sfTSLP protein sequence overlaps the lfTSLP sequence
in the C-terminal region, it was not possible to distinguish the
expression of sfTSLP at the protein level, but a significant
increase was observed at the mRNA level.
The present study further investigated the potential
transcriptional pathways mediating RSV-induced TSLP expression in
Beas-2B cells. It has been reported that pro-inflammatory cytokines
can induce lfTSLP by a transcriptional mechanism regulated by
nuclear factor (NF)-κB and transcription factor AP-1 (25–27).
However, sfTSLP is not sensitive to proinflammatory cytokines
(6,28), meaning that the activation of NF-κB
and AP-1 is critical for inflammation-induced expression of lfTSLP,
but not sfTSLP.
AP-2α is a transcription factor that regulates the
proliferation and differentiation of mammalian cells (29). It has been reported that AP-2α can
upregulate Toll-like receptor 2 (TLR2) gene expression, and
expression of lfTSLP is induced through the TLR2-TLR6 pathway in
human monocyte cell lines (30,31).
It has also been reported that the reduced AP-2α protein binds to
TSLP promoter polymorphism reference SNP rs2289276 site to increase
a child's susceptibility to atopic asthma (13). However, no functional studies have
been carried out. In the present study, limited AP-2α expression
was observed in RSV-infected Beas-2B cells. It was first
hypothesized that the increase in sfTSLP expression was caused by a
decrease in AP-2α expression, since sfTSLP cannot be induced by
NF-κB and AP-1. Further experiments showed that the overexpression
of AP-2α reduced the promoter activity, mRNA levels of sfTSLP and
the mRNA and protein levels of lfTSLP, while inhibition of AP-2α
expression increased sfTSLP and lfTSLP expression. Transcriptional
activation on 5′ deletion of the human lfTSLP promoter has been
studied in airway epithelial cells (27). Harada et al (13) showed that AP-2α directly binds to
the promoter of lfTSLP and may act as a transcription-suppressing
factor. However, no studies, to the best of the authors' knowledge,
have investigated the association between AP-2α and sfTSLP
transcriptional promoter activity. In the present study, AP-2α was
identified as an important regulator in sfTSLP expression in
Beas-2B cells and the AP-2α binding site in the core promoter
region of sfTSLP was shown to be critical for sfTSLP modulation.
Notably, the mutation of the imperfect binding site increased the
luciferase activity of sfTSLP core promoter, but the mutation of
the main AP-2α site located at −10/-2nt significantly reduced the
luciferase activity (P<0.05). This meant that the activity of
reporter genes was weakened, instead of increased (as expected),
following the mutation of the main AP-2α site. Adjacent to the
transcription initiation site, AP-2α may compete to inhibit certain
positive or general transcription factors (32), including general transcription
factor IIB (33) and influence the
formation of pre-initiation complex. These observations indicate
that AP-2α is a transcription-suppressing factor of both lfTSLP and
sfTSLP.
SfTSLP is a homeostatic and anti-inflammatory
isoform expressed in steady-state. By contrast, lfTSLP is
pro-inflammatory and only expressed during inflammatory processes
(6,19). LfTSLP-induced inflammation can be
partially reversed by sfTSLP, especially in HDM-induced asthmatic
airway epithelial cells (34).
SfTSLP can condition both mature dendritic cells and
monocyte-derived dendritic cells and limit their inflammatory
potential on monocyte-derived dendritic cells (19). The reduction of interferon (IFN) λ
is also associated with sfTSLP. IFN λ is also anti-inflammatory in
that it enhances adaptive mucosal immunity by promoting the release
of TSLP (35). The increase of
sfTSLP in RSV infection could counter inflammation, especially
lfTSLP-induced inflammation. However, the interaction between
sfTSLP and lfTSLP in RSV infection should be confirmed by further
functional studies.
In the present study, higher TSLP production
appeared with the reduction of AP-2α in bronchial epithelial cells
undergoing viral respiratory infections. It has been demonstrated
that lfTSLP is increased in RSV infection (19–21).
To the best of the authors' knowledge, this is the first study to
investigate the expression of sfTSLP in RSV infection in bronchial
epithelial cells. Previous studies have mentioned the possible
association between lfTSLP and AP-2α: AP-2α binds to the lfTSLP
promoter and this binding is inhibited in inflammation to exert a
protective effect (13,27). However, this is a conjecture that
has not been verified by experiments. The present study showed that
AP-2α regulated lfTSLP expression at both mRNA and protein levels.
The present study also showed that AP-2α regulated sfTSLP
expression and that AP-2α directly bound to sfTSLP promoter. It
also identified the transcription of sfTSLP in bronchial epithelial
cells and the association between sfTSLP and AP-2α. Further studies
should be performed to uncover the molecular mechanisms by which
sfTSLP expression regulates RSV infection, cellular function and
disease pathophysiology.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This work was supported by grants from the National
Natural Science Foundation of China (grant no. 81970579 to GPZ),
Jiangsu Province Science and Education Enhancing Health Project
Innovation Team (Leading Talent) Program (grant no. CXTDA2017018 to
GPZ) and A Project Funded by the Priority Academic Program
Development of Jiangsu Higher Education Institutions.
Availability of data and materials
The analyzed datasets generated during the study are
available from the corresponding author on reasonable request.
Authors' contributions
JH, QC and GPZ made substantial contributions to the
conception and design of the study. JH carried out most of the
experiments and wrote the manuscript. QC provided suggestions for
the first draft. DDF and JHC participated in data acquisition,
analysis and interpretation. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Malinczak CA, Fonseca W, Rasky AJ,
Ptaschinski C, Morris S, Ziegler SF and Lukacs NW: Sex-associated
TSLP-induced immune alterations following early-life RSV infection
leads to enhanced allergic disease. Mucosal Immunol. 12:969–979.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lee HC, Headley MB, Loo YM, Berlin A, Gale
M Jr, Debley JS, Lukacs NW and Ziegler SF: Thymic stromal
lymphopoietin is induced by respiratory syncytial virus-infected
airway epithelial cells and promotes a type 2 response to
infection. J Allergy Clin Immunol. 130:1187–1196.e5. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lin SC, Cheng FY, Liu JJ and Ye YL:
Expression and regulation of thymic stromal lymphopoietin and
thymic stromal lymphopoietin receptor heterocomplex in the
innate-adaptive immunity of pediatric asthma. Int J Mol Sci.
19:12312018. View Article : Google Scholar
|
|
4
|
Martin Mena A, Langlois A, Speca S,
Schneider L, Desreumaux P, Dubuquoy L and Bertin B: The Expression
of the short isoform of thymic stromal lymphopoietin in the colon
is regulated by the nuclear receptor peroxisome proliferator
activated receptor-gamma and is impaired during ulcerative colitis.
Front Immunol. 8:10522017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wilson SR, Thé L, Batia LM, Beattie K,
Katibah GE, McClain SP, Pellegrino M, Estandian DM and Bautista DM:
The epithelial cell-derived atopic dermatitis cytokine TSLP
activates neurons to induce itch. Cell. 155:285–295. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bjerkan L, Schreurs O, Engen SA, Jahnsen
FL, Baekkevold ES, Blix IJ and Schenck K: The short form of TSLP is
constitutively translated in human keratinocytes and has
characteristics of an antimicrobial peptide. Mucosal Immunol.
8:49–56. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jartti T and Gern JE: Role of viral
infections in the development and exacerbation of asthma in
children. J Allergy Clin Immunol. 140:895–906. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rossi GA and Colin AA: Infantile
respiratory syncytial virus and human rhinovirus infections:
Respective role in inception and persistence of wheezing. Eur
Respir J. 45:774–789. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dumas O, Hasegawa K, Mansbach JM, Sullivan
AF, Piedra PA and Camargo CA Jr: Severe bronchiolitis profiles and
risk of recurrent wheeze by age 3 years. J Allergy Clin Immunol.
143:1371–1379.e7. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Coultas JA, Smyth R and Openshaw PJ:
Respiratory syncytial virus (RSV): A scourge from infancy to old
age. Thorax. 74:986–993. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Feldman AS, He Y, Moore ML, Hershenson MB
and Hartert TV: Toward primary prevention of asthma. Reviewing the
evidence for early-life respiratory viral infections as modifiable
risk factors to prevent childhood asthma. Am J Respir Crit Care
Med. 191:34–44. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Qiao J, Li A and Jin X: TSLP from
RSV-stimulated rat airway epithelial cells activates myeloid
dendritic cells. Immunol Cell Biol. 89:231–238. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Harada M, Hirota T, Jodo AI, Hitomi Y,
Sakashita M, Tsunoda T, Miyagawa T, Doi S, Kameda M, Fujita K, et
al: Thymic stromal lymphopoietin gene promoter polymorphisms are
associated with susceptibility to bronchial asthma. Am J Respir
Cell Mol Biol. 44:787–793. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang XH, Shu J, Jiang CM, Zhuang LL, Yang
WX, Zhang HW, Wang LL, Li L, Chen XQ, Jin R, et al: Mechanisms and
roles by which IRF-3 mediates the regulation of ORMDL3
transcription in respiratory syncytial virus infection. Int J
Biochem Cell Biol. 87:8–17. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Reed LJ and Muench H: A simple method of
estimating fifty percent endpoint. Am J Hyg. 27:493–497. 1938.
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−ΔΔC(T)) Method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
He J, Cao Q and Zhou GP: Identification
and characterization of human short isoform TSLP promoter. J
Nanjing Med Univ. 40:10–14. 2020.(In Chinese).
|
|
18
|
Fornes O, Castro-Mondragon JA, Khan A, van
der Lee R, Zhang X, Richmond PA, Modi BP, Correard S, Gheorghe M,
Baranašić D, et al: JASPAR 2020: Update of the open-access database
of transcription factor binding profiles. Nucleic Acids Res.
48:D87–D92. 2020.PubMed/NCBI
|
|
19
|
Fornasa G, Tsilingiri K, Caprioli F, Botti
F, Mapelli M, Meller S, Kislat A, Homey B, Di Sabatino A, Sonzogni
A, et al: Dichotomy of short and long thymic stromal lymphopoietin
isoforms in inflammatory disorders of the bowel and skin. J Allergy
Clin Immunol. 136:413–422. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vázquez Y, González L, Noguera L, González
PA, Riedel CA, Bertrand P and Bueno SM: Cytokines in the
respiratory airway as biomarkers of severity and prognosis for
respiratory syncytial virus infection: An update. Front Immunol.
10:1154. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Stier MT, Bloodworth MH, Toki S, Newcomb
DC, Goleniewska K, Boyd KL, Quitalig M, Hotard AL, Moore ML,
Hartert TV, et al: Respiratory syncytial virus infection activates
IL-13-producing group 2 innate lymphoid cells through thymic
stromal lymphopoietin. J Allergy Clin Immunol. 138:814–824.e11.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bjerkan L, Sonesson A and Schenck K:
Multiple functions of the new cytokine-based antimicrobial peptide
thymic stromal lymphopoietin (TSLP). Pharmaceuticals (Basel).
9:412016. View Article : Google Scholar
|
|
23
|
Li Y, Lund C, Nervik I, Loevenich S,
Døllner H, Anthonsen MW and Johnsen IB: Characterization of
signaling pathways regulating the expression of pro-inflammatory
long form thymic stromal lymphopoietin upon human metapneumovirus
infection. Sci Rep. 8:8832018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhao Y, Jamaluddin M, Zhang Y, Sun H,
Ivanciuc T, Garofalo RP and Brasier AR: Systematic analysis of
cell-type differences in the epithelial secretome reveals insights
into the pathogenesis of respiratory syncytial virus-induced lower
respiratory tract infections. J Immunol. 198:3345–3364. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Redhu NS, Saleh A, Halayko AJ, Ali AS and
Gounni AS: Essential role of NF-κB and AP-1 transcription factors
in TNF-α-induced TSLP expression in human airway smooth muscle
cells. Am J Physiol Lung Cell Mol Physiol. 300:L479–L485. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cultrone A, de Wouters T, Lakhdari O,
Kelly D, Mulder I, Logan E, Lapaque N, Doré J and Blottière HM: The
NF-κB binding site located in the proximal region of the TSLP
promoter is critical for TSLP modulation in human intestinal
epithelial cells. Eur J Immunol. 43:1053–1062. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee H-C and Ziegler SF: Inducible
expression of the proallergic cytokine thymic stromal lymphopoietin
in airway epithelial cells is controlled by NFkappaB. Proc Natl
Acad Sci USA. 104:914–919. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Harada M, Hirota T, Jodo AI, Doi S, Kameda
M, Fujita K, Miyatake A, Enomoto T, Noguchi E, Yoshihara S, et al:
Functional analysis of the thymic stromal lymphopoietin variants in
human bronchial epithelial cells. Am J Respir Cell Mol Biol.
40:368–374. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Eckert D, Buhl S, Weber S, Jäger R and
Schorle H: The AP-2 family of transcription factors. Genome Biol.
6:2462005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Vu AT, Baba T, Chen X, Le TA, Kinoshita H,
Xie Y, Kamijo S, Hiramatsu K, Ikeda S, Ogawa H, et al:
Staphylococcus aureus membrane and diacylated lipopeptide induce
thymic stromal lymphopoietin in keratinocytes through the Toll-like
receptor 2-Toll-like receptor 6 pathway. J Allergy Clin Immunol.
126:985–993, 993.e1-993.e3. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li M, Li X, Wang E and Luo E: Upregulation
of Toll-like receptor 2 gene expression by acetylation of AP-2
alpha in THP-1 cells, a human monocytic cell line. Int J Biochem
Cell Biol. 45:1594–1599. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Haberle V and Stark A: Eukaryotic core
promoters and the functional basis of transcription initiation. Nat
Rev Mol Cell Biol. 19:621–637. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lagrange T, Kapanidis AN, Tang H, Reinberg
D and Ebright RH: New core promoter element in RNA polymerase
II-dependent transcription: Sequence-specific DNA binding by
transcription factor IIB. Genes Dev. 12:34–44. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dong H, Hu Y, Liu L, Zou M, Huang C, Luo
L, Yu C, Wan X, Zhao H, Chen J, et al: Distinct roles of short and
long thymic stromal lymphopoietin isoforms in house dust
mite-induced asthmatic airway epithelial barrier disruption. Sci
Rep. 6:395592016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ye L, Schnepf D, Becker J, Ebert K,
Tanriver Y, Bernasconi V, Gad HH, Hartmann R, Lycke N and Staeheli
P: Interferon-λ enhances adaptive mucosal immunity by boosting
release of thymic stromal lymphopoietin. Nat Immunol. 20:593–601.
2019. View Article : Google Scholar : PubMed/NCBI
|