Introduction
Glaucoma is the second leading cause of irreversible
visual field loss and blindness in the world (1). The two most common types are primary
open-angle glaucoma and primary angle-closure glaucoma (1). Pathological elevated intraocular
pressure (IOP) is the major primary risk factor for glaucoma
(2). Human trabecular meshwork
(HTM) cells, at the irido-corneal angle, serve a critical role in
regulating IOP (3). The anomalous
accumulation of extracellular matrix (ECM) may induce dysfunction
in aqueous humor outflow through HTM cells, thereby upregulating
IOP, leading to the onset of glaucoma (4,5). In
addition, increases in IOP induce HTM deformation, while decreases
in IOP restore the anatomy (6).
Therefore, an improved understanding of the biological function of
HTM cells may improve the understanding the pathogenesis of
glaucoma.
MicroRNAs (miRNAs/miRs) are a class of
single-stranded, non-coding, ~22 nucleotide (nt)-long small RNAs
(7), which are sequence-specific
regulators of post-transcriptional gene expression (8). miRNAs are associated with the
occurrence and development of disease, and they have been reported
to exert a wide range of effects through regulating the biological
processes of their associated genes (9). miRNAs are also involved in regulating
the normal or pathophysiological cellular functions of HTM cell
contraction and ECM turnover (10). miR-200c is one of the members of
the miR-200 family, which is located at chromosome 12p13 (11). Previous data have revealed that
miR-200c is downregulated in glaucoma (12). Furthermore, miR-200c may regulate
trabecular contraction and modulate IOP (13). However, the role of miR-200c and
its associated molecular mechanisms are largely unknown. Oxidative
stress is a critical risk factor in glaucoma; antioxidant
glutathione levels are decreased in patients with glaucoma
(14), and it also has alterative
effect on HTM cells, leading eventually to increased IOP (15,16).
Therefore, it is necessary to examine the effects of miR-200c in
HTM cells under oxidative stress.
In the present study, to elucidate the role of
miR-200c-3p in primary HTM cells, the expression of miR-200c-3p was
detected in H2O2-treated HTM cells. Then the
target gene of miR-200c-3p, phosphatase and tensin homolog (PTEN),
was verified. Notably, overexpression of miR-200c-3p promoted cell
proliferation and inhibited cell apoptosis by targeting PTEN. The
results of the present study may suggest a potential target for HTM
cells.
Materials and methods
Cell culture and
H2O2 treatment
HTM cells were purchased from ScienCell Research
Laboratories, Inc. The cells were cultured in TM cell medium (TMCM)
containing 2% FBS (ScienCell Research Laboratories, Inc.), 1% TM
cell growth supplements and 1% penicillin/streptomycin (ScienCell
Research Laboratories, Inc.). The cells were maintained in a
humidified incubator at 37°C with 5% CO2.
HTM cells were seeded into 6-well plates at a
density of 2×105 cells/well. The cells were treated at
37°C with 300 µM H2O2 (Sigma-Aldrich; Merck
KGaA) in serum-free medium for 2 h. Then, the medium was removed
and replaced with complete TMCM, and cells were then incubated for
an additional 2 h. These cells served as an oxidative stress model,
and were named H2O2-HTM cells.
Target prediction and dual-luciferase
reporter assay
The potential targets of miR-200c-3p were predicted
using the bioinformatics analysis tool TargetScan (http://www.targetscan.org/vert_72) (17). A dual-luciferase reporter assay was
then performed to confirm the prediction. The wild-type (WT) or
mutant sequences of the PTEN 3′ untranslated region (UTR),
containing the predicted miR-200c-3p target site, were inserted
into the pmirGLO vector (Promega Corporation), and named
pmirGLO-WT-PTEN and pmirGLO-mutant-PTEN, respectively. A density of
2×105 cells/well H2O2-HTM cells
were seeded in 24-well plates prior to transfection. A total of 24
h later, cells were co-transfected with pmirGLO-WT-PTEN or
pmirGLO-mutant-PTEN and miR-200c-3p mimics or miR-NC mimics using
Lipofectamine® 2000 reagent (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol.
Firefly and Renilla luciferase activities were measured with
a Dual-Glo Luciferase Assay System (Promega Corporation) at 48 h
post-transfection. The results for luciferase activity were
normalized to Renilla luciferase activity.
Cell transfection
miR-200c-3p mimics (5′-UAACACUGUCUGGUAAUGAUGUU-3′)
and negative control mimics (miR-NC mimics:
5′-UAACACUGUCUGGUAAUGAUGUU-3′) were designed and synthesized by
Biomics Biotechnologies Co., Ltd. A total of 4×105
H2O2-HTM cells/well were seeded in 6-well
plates and transfected with 20 nM miR-200c-3p mimics and miR-NC
mimics in serum-free medium using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.). A total of 6 h later,
the medium was replaced by complete medium. The cells were
harvested for incubation at 37°C for 48 h.
Reverse transcription-quantitative PCR
(RT-qPCR)
To detect the mRNA expression levels of miR-200c-3p,
total RNA was extracted from the cells using a miRNeasy FFPE kit
(Qiagen GmbH), according to the manufacturer's protocol. Then, cDNA
was synthesized using the miScript II RT kit (Qiagen GmbH),
according to the manufacturer's protocol. To detect PTEN
expression, total RNA was extracted using TRIzol®
reagent (Invitrogen; Thermo Fisher Scientific, Inc.), according to
the manufacturer's protocol. The reverse transcription was
performed with a Multiscribe Reverse Transcriptase kit (Invitrogen;
Thermo Fisher Scientific, Inc.), according to the manufacturer's
protocol. Expression of miRNAs and mRNAs was quantified using GoTaq
qPCR Master mix (Promega Corporation), according to the
manufacturer's protocol, on an ABI PRISM 7500 Real-Time PCR System
(Applied Biosystems; Thermo Fisher Scientific, Inc.). The following
qPCR thermocycling conditions were used for miR-200c-3p: Initial
denaturation at 95°C for 2 min; and 40 cycles of denaturation at
95°C for 5 sec, annealing at 55°C for 30 sec and extension at 72°C
for 30 sec. The following qPCR thermocycling conditions were used
for PTEN: Initial denaturation at 95°C for 2 min; and 40 cycles of
denaturation at 95°C for 15 sec and annealing and extension at 60°C
for 1 min. The sequences of the specific primers were as follows:
miR-200c-3p forward, 5′-TCGTCTTACCCAGCAGTG-3′ and reverse,
5′-CGGCAGTATTAGAGACTCC-3′; U6 forward, 5′-CTCGCTTCGGCAGCACATA-3′
and reverse, 5′-AACGATTCACGAATTTGCGT-3′; PTEN forward,
5′-TTGAAGACCATAACCCACCACAG-3′ and reverse,
5′-CATTACACCAGTTCGTCCCTTTC-3′; and GAPDH forward,
5′-CTGGGCTACACTGAGCACC-3′ and reverse, 5′-AAGTGGTCGTTGAGGGCAATG-3′.
U6 was used as an internal control for miR-200c-3p and GAPDH was
used as an internal control for PTEN. Data were analyzed using the
2−ΔΔCq method (18).
Western blot analysis
Protein was extracted from transfected cells in RIPA
lysis buffer (Beyotime Institute of Biotechnology). The protein
concentration was detected by BCA Protein Assay kit (Beyotime
Institute of Biotechnology). Then, 30 µg protein/lane was separated
by 12% SDS-PAGE and transferred to a PVDF membrane (Beyotime
Institute of Biotechnology). Following blocking with 5% skim milk
dissolved in TBS + 0.05% Tween-20 (TBST) for 1 h at room
temperature, the membrane was incubated with the following primary
antibodies (Abcam) at 4°C overnight: Anti-PTEN (cat. no. ab31392;
1:1,000), anti-AKT (cat. no. ab179463, 1:10,000),
anti-phosphorylated (p)-AKT (cat. no. ab131443; 1:1,000),
anti-serine/threonine-protein kinase mTOR (mTOR; cat. no. ab2732;
1:2,000), anti-p-mTOR (cat. no. ab109268; 1:1,000), anti-cleaved
caspase-3 (cat. no. ab32042; 1:500), anti-Bax (cat. no. ab32503;
1:2,000) and anti-GAPDH (cat. no. ab9485; 1:2,500). Following
washing with TBST, the membrane was incubated with a horseradish
peroxidase-conjugated goat anti-rabbit IgG secondary antibody (cat.
no. ab205718; 1:2,000; Abcam) for 1 h at room temperature.
Immunoblotting signals were developed with an ECL Plus Western
Blotting Substrate kit (Thermo Fisher Scientific, Inc.). The
intensity of each band sample was analyzed with Image J v.1.49
software (National Institutes of Health).
Cell proliferation assay
Cell proliferation was measured using a Cell
Counting Kit-8 (CCK-8; Dojindo Molecular Technologies, Inc.),
following the manufacturer's protocol. Transfected cells were
seeded in 96-well plates and incubated at 37°C with 5%
CO2 for 0, 12, 24 and 48 h. Following this, 10 µl CCK-8
was added to each well and cells were incubated at 37°C for an
additional 2 h. The absorbance was measured at 450 nm using a
microplate reader (BioTek Instruments, Inc.).
Cell apoptosis assay
Cell apoptosis levels were measured using an Annexin
V-fluorescein isothiocyanate (FITC) Apoptosis Detection kit
(eBioscience; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. Briefly, a total of 5×105
transfected cells/well were seeded in 6-well plates. A volume of 5
µl Annexin V-FITC and 10 µl propidium iodide (PI) were added into
each well and the cells were incubated in the dark for 15 min at
room temperature. The apoptotic cells were detected using a
FACSCanto II flow cytometer (BD Biosciences). Data analysis was
performed with Cell Quest Pro software (v5.1; BD Biosciences).
Statistical analysis
All statistical analyses were performed using
GraphPad Prism 7 software (GraphPad Software, Inc.). Differences
between two groups were analyzed by Student's t-test, and
differences among multiple groups were analyzed by one-way analysis
of variance followed by Student-Newman-Keuls post hoc analysis. All
data are presented as the mean ± SEM of ≥3 independent experimental
repeats. P<0.05 was considered to indicate a statistically
significant difference.
Results
miR-200c-3p is downregulated in HTM
cells under oxidative stress
To investigate the role of miR-200c-3p, its
expression was first measured in HTM cells and
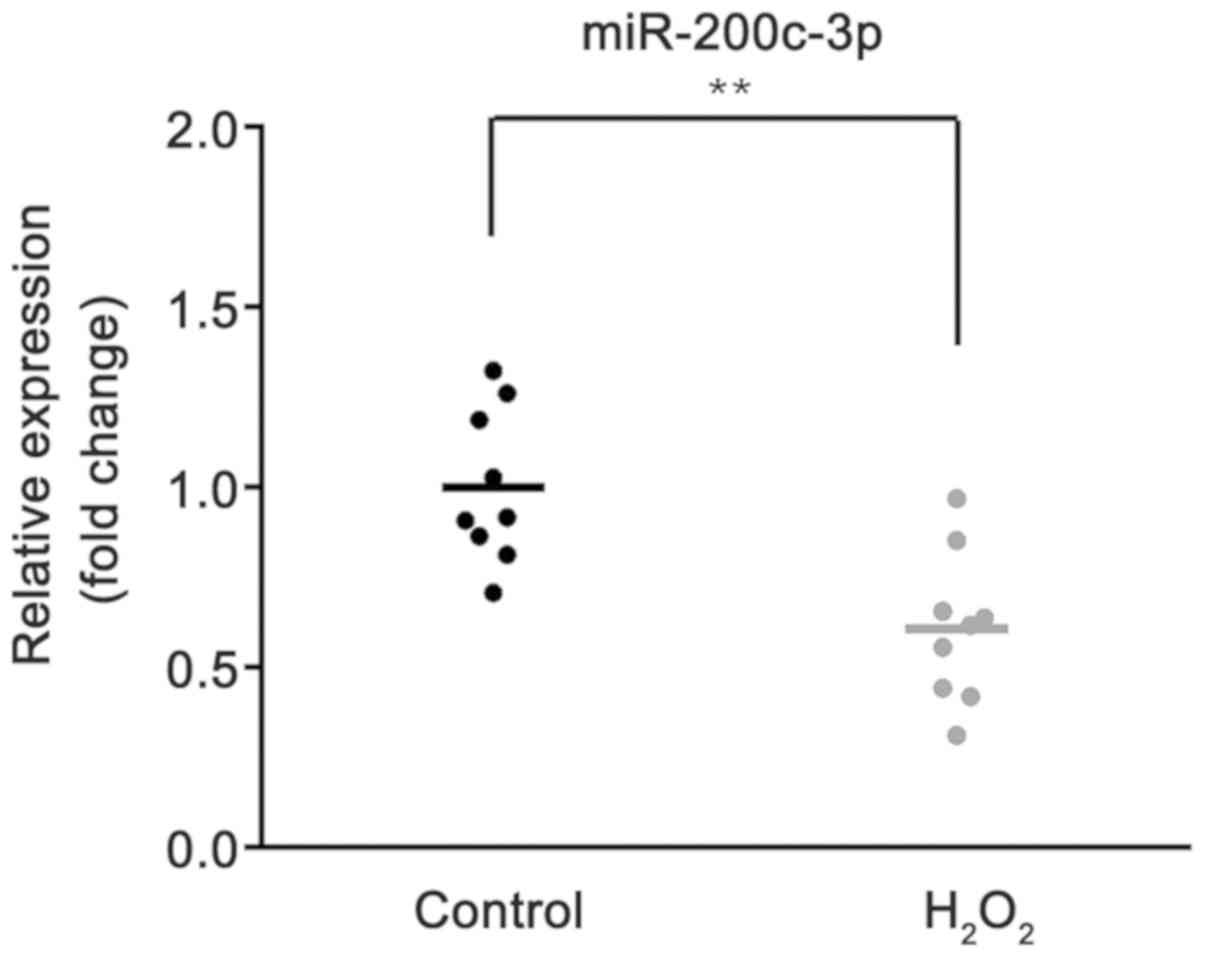
H2O2-HTM cells using RT-qPCR. As demonstrated
in Fig. 1, compared with control
group, the expression of miR-200c-3p was significantly decreased in
HTM cells treated with H2O2 (P<0.01).
PTEN is a target of miR-200c-3p
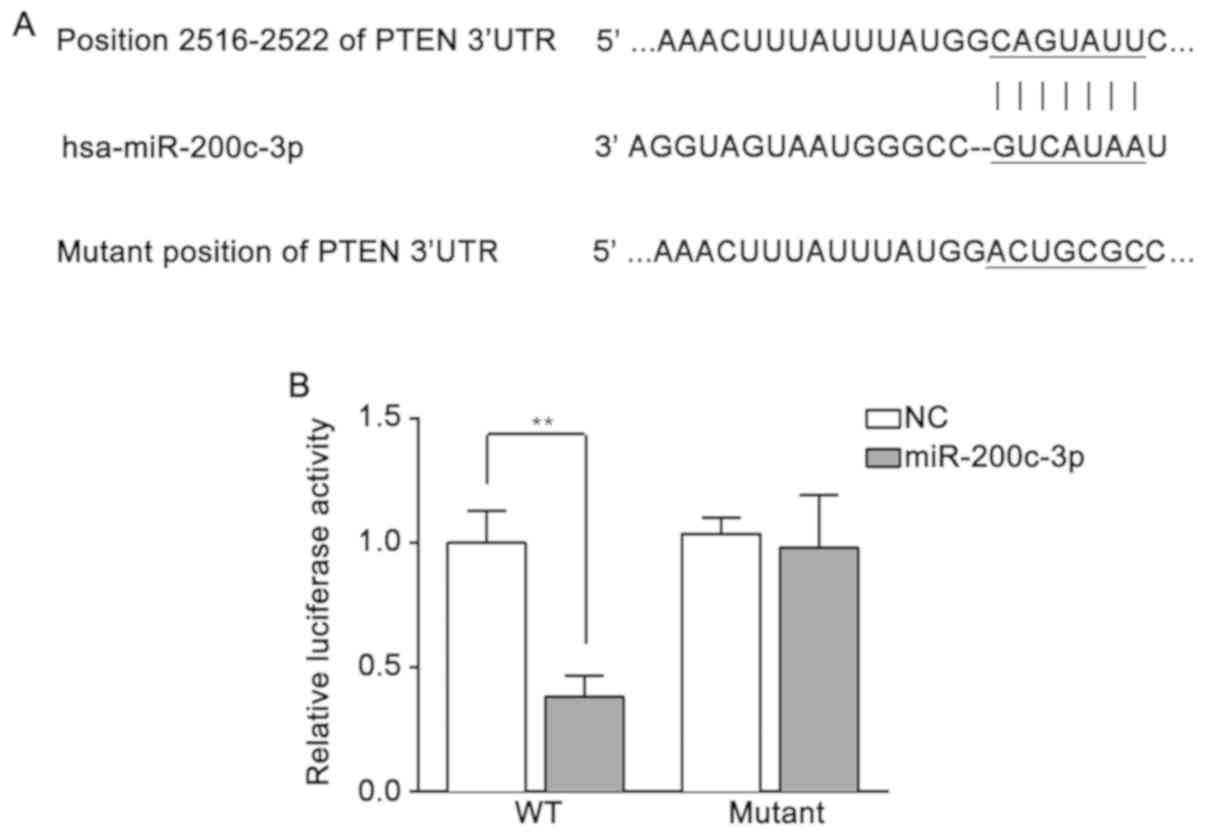
miR-200c-3p was predicted to be able to bind with
the 3′-untranslated region (UTR) of PTEN at position 2,516-2,522 nt
by TargetScan (Fig. 2A). To
confirm the prediction, H2O2-HTM cells were
co-transfected with pmirGLO-WT-PTEN or pmirGLO-mutant-PTEN and
miR-200c-3p mimics or miR-NC mimics. As demonstrated by the
detection of luciferase activity, compared with the miR-NC mimics,
the miR-200c-3p mimics evidently inhibited the luciferase activity
when co-transfected with pmirGLO-WT-PTEN (P<0.01). However,
there was no significant difference between miR-200c-3p and miR-NC
mimics when combined with pmirGLO-mutant-PTEN (Fig. 2B). These results suggested that
PTEN is a target of miR-200c-3p.
Expression of PTEN is upregulated in
HTM cells under oxidative stress
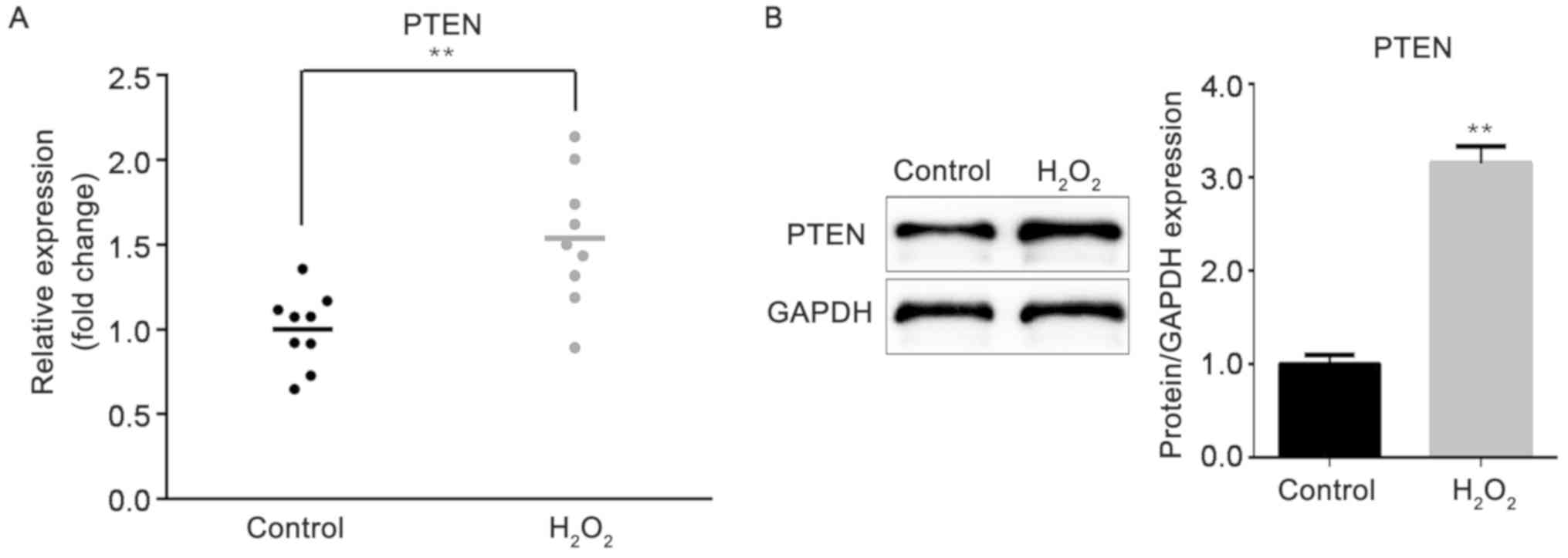
Following treatment of the HTM cells with
H2O2, the expression of the target gene PTEN
was measured by RT-qPCR. As indicated in Fig. 3A, PTEN expression was greatly
increased in H2O2-HTM cells, compared with
HTM cells in the control group (P<0.01). In addition, the
western blot analysis results demonstrated that the protein level
of PTEN was also increased under H2O2
treatment (P<0.01; Fig.
3B).
miR-200c-3p promotes cell
proliferation and inhibits cell apoptosis, while PTEN reverses the
effect of miR-200c-3p
To investigate the role of miR-200c-3p and PTEN in
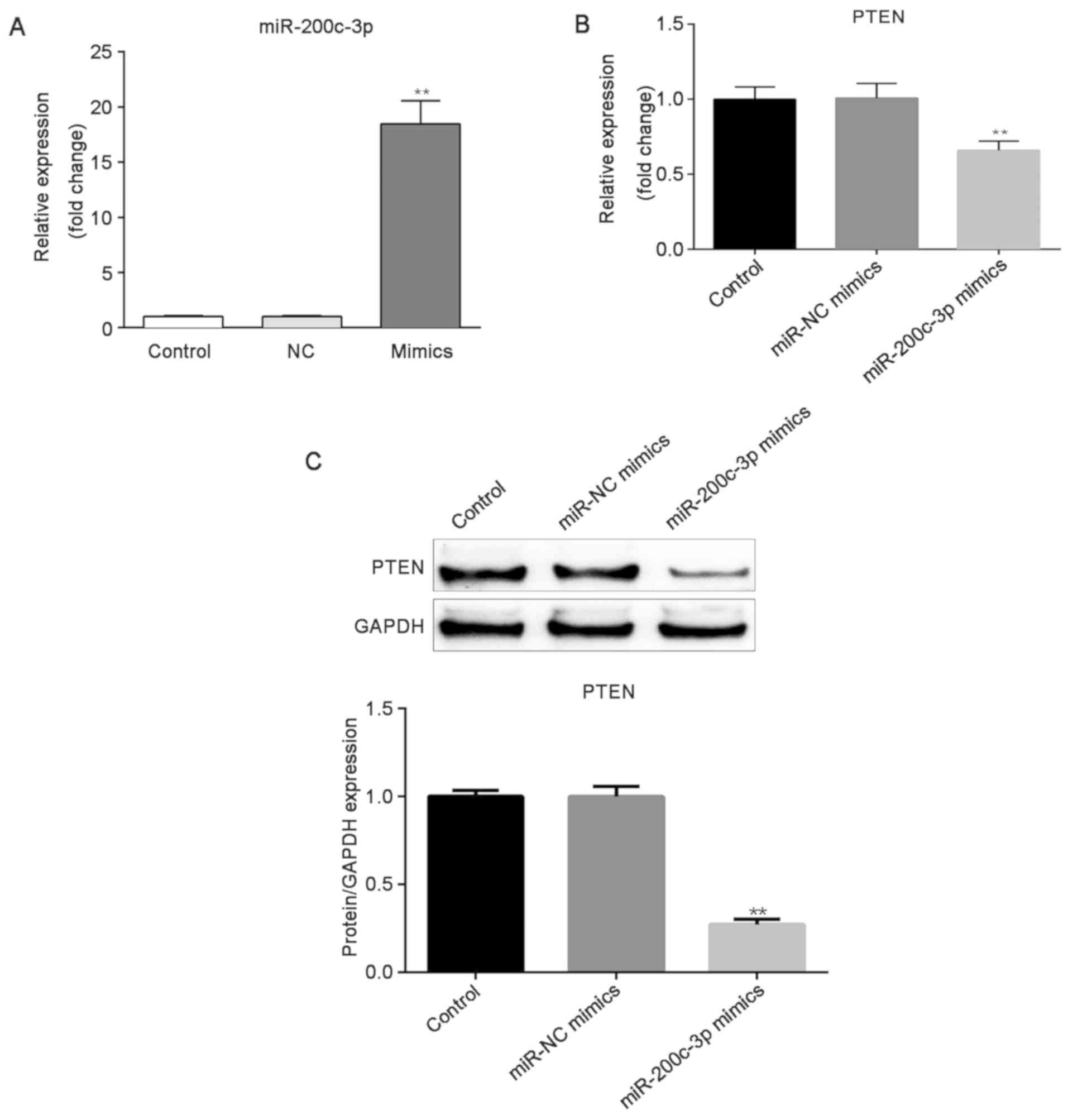
H2O2-HTM cells, the cells were transfected
with miR-200c-3p mimics, miR-NC mimics, and the transfection
efficiency was then evaluated by RT-qPCR. miR-200c-3p expression
was markedly increased in cells post transfection of miR-200c-3p
mimics, compared with the miR-NC mimics and control groups
(P<0.01; Fig. 4A). Then, it was
identified that the overexpression of miR-200c-3p induced the
downregulation of PTEN (P<0.01; Fig. 4B and C). These results indicated
that PTEN expression was negatively regulated by miR-200c-3p.
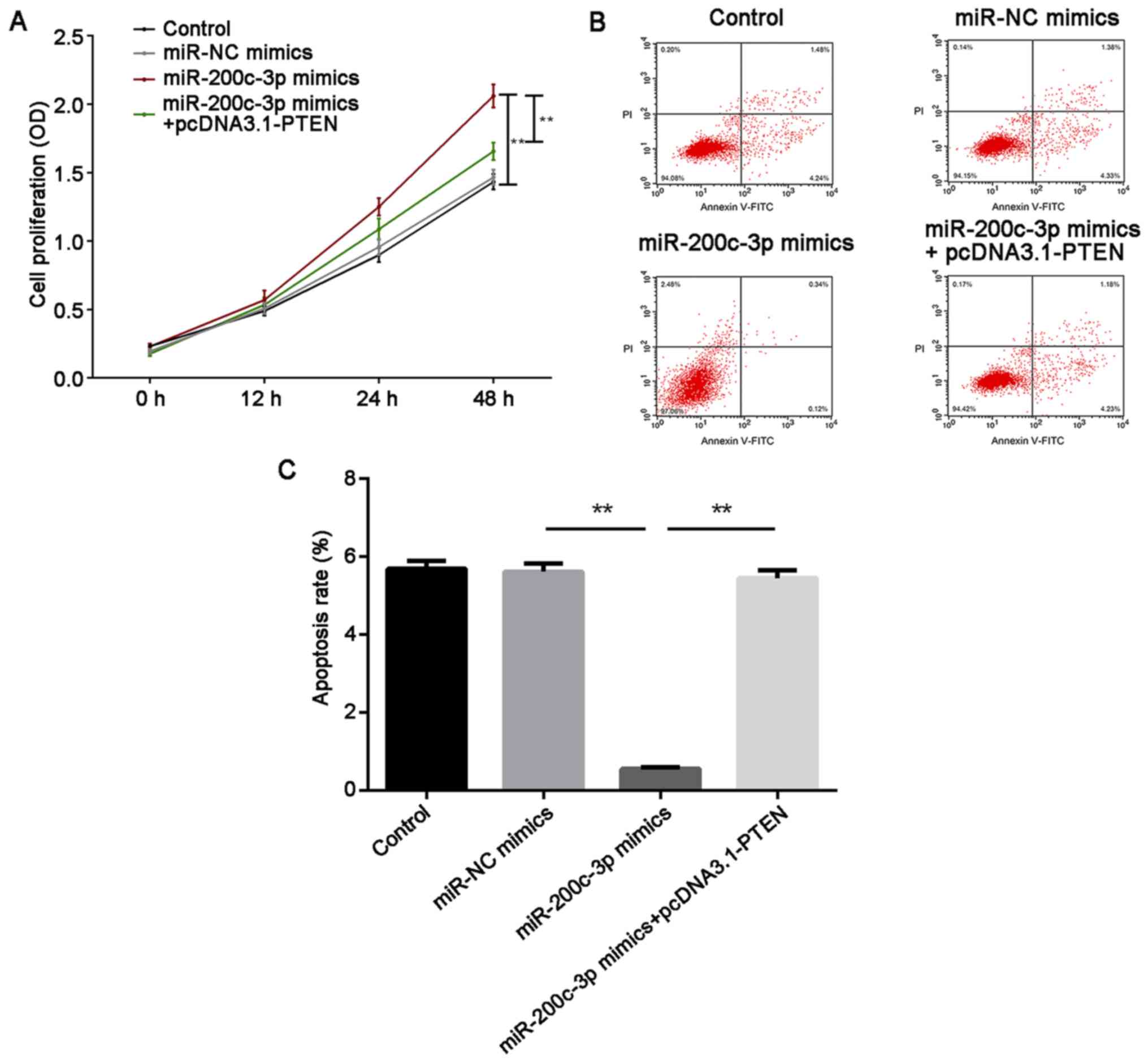
Furthermore, cell proliferation and cell apoptosis were measured in
HTM cells under oxidative stress by CCK-8 and flow cytometry
assays, respectively. For cell proliferation, miR-200c-3p enhanced
cell proliferation compared with the miR-NC mimics group
(P<0.01). Overexpression of PTEN attenuated cell proliferation
compared with the miR-200c-3p mimics group (P<0.01). However,
there was no significant difference between the miR-NC group and
the miR-200c-3p mimics + pcDNA3.1 PTEN group (Fig. 5A). By contrast, the overexpression
of miR-200c-3p reduced cell apoptosis, while PTEN abolished this
inhibition (P<0.01; Fig. 5B and
C). These results suggested that miR-200c-3p promoted cell
proliferation and suppressed cell apoptosis by targeting PTEN.
Overexpression of miR-200c-3p
suppresses the expression of cleaved caspase-3 and Bax, and
activates the PTEN/AKT/mTOR signaling pathway by targeting
PTEN
Finally, the expression of PTEN, cleaved caspase-3,
Bax, AKT, p-AKT, mTOR and p-mTOR at the protein level was measured
by western blot analysis. The results demonstrated that
overexpressed miR-200c-3p suppressed the expression of PTEN,
cleaved caspase-3 and Bax (P<0.01), while PTEN overexpression
reversed the suppression induced by miR-200c-3p mimics (P<0.01).
In addition, miR-200c-3p upregulated the protein expression of
p-AKT and p-mTOR (P<0.01), while the overexpression of PTEN
reversed the effect of miR-200c-3p (P<0.01). However,
miR-200c-3p did not have an effect on total AKT and mTOR expression
(Fig. 6A and B). These results
suggested that miR-200c-3p targeted PTEN to suppress the expression
of cleaved caspase-3 and Bax, and activated the PTEN/AKT/mTOR
signaling pathway.
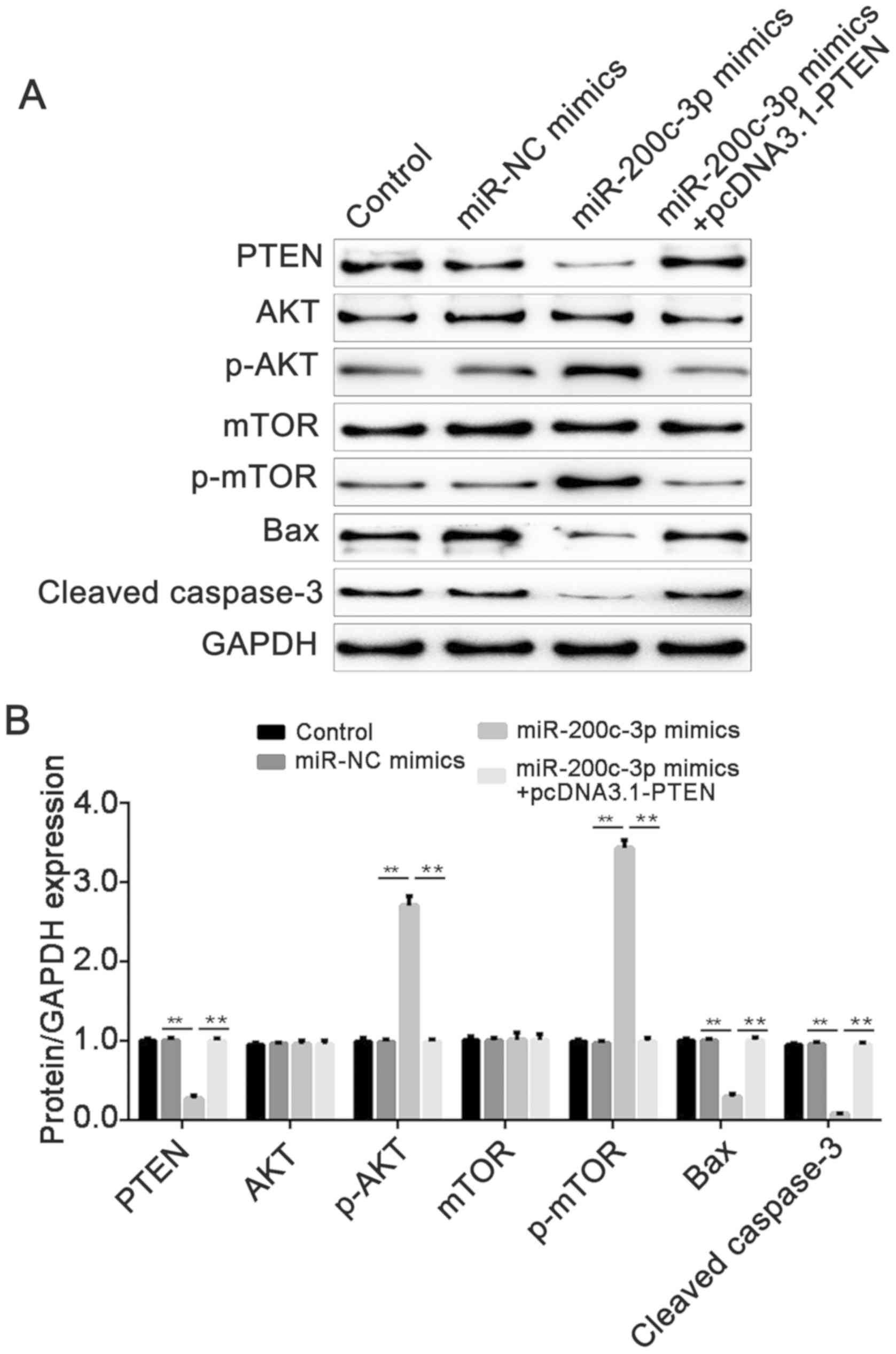 | Figure 6.miR-200c-3p decreases the expression
of PTEN, cleaved caspase-3, Bax, p-AKT and p-mTOR, while PTEN
abolishes the effect induced by miR-200c-3p mimics. (A) The protein
expression of PTEN, cleaved caspase-3, Bax, AKT, p-AKT, mTOR and
p-mTOR was measured by western blot analysis. GAPDH was used as an
internal control. (B) Densitometric analysis of the western blot
analysis grey values. Data are presented as means ± standard error
of the mean. **P<0.01. miR, microRNA; p-, phosphorylated; PTEN,
phosphatase and tensin homolog; mTOR, serine/threonine-protein
kinase mTOR; NC, negative control. |
Discussion
In the present study, miR-200c-3p was identified to
be downregulated in HTM cells under oxidative stress.
Overexpression of miR-200c-3p significantly promoted cell
proliferation, inhibited cell apoptosis, suppressed the expression
of cleaved caspase-3 and Bax, and activated the PTEN/AKT/mTOR
pathway, while overexpression of PTEN reversed the effects of
miR-200c-3p.
Greater resistance to aqueous humor outflow through
the HTM increases IOP and causes glaucoma (4). Oxidative stress is a risk factor in
glaucoma and one of the cellular factors that results in
alterations in the HTM (14,15).
In addition, oxidative stress treatment may lead to the changes in
miRNA expression; for example, H2O2 increased
miR-200c expression levels in human umbilical vein endothelial
cells (19). Additionally,
miR-181a levels in H2O2-treated HTM cells
were decreased (20). miR-200c is
a well-known tumor suppressor, which has been studied primarily in
the context of tumor development, proliferation, metastasis and
therapy resistance (21). However,
little is known about the role of miR-200c in HTM cells. A previous
study revealed that the expression levels of miR-200c were
upregulated in HTM cells (22) and
downregulated in glaucoma (12).
Furthermore, miR-200c inhibits the contraction of HTM cells and
leads to decrease in IOP (10,13).
In the present study, miR-200c-3p was highly expressed in HTM
cells, and oxidative stress induced a downregulation of the
expression. However, the underlying molecular mechanism of
miR-200c-3p in HTM cells is not clear.
The bioinformatics analysis results from the present
study indicated that PTEN is a potential target of miR-200c-3p, and
a dual-luciferase reporter assay verified the prediction. PTEN has
lipid and protein activities (23). A tumor suppresser, PTEN is also
associated with neurodegeneration, and deletion of PTEN has been
demonstrated to lead to axonal regeneration following optic nerve
injury (24). In addition, PTEN is
the target of miR-93-5p, which regulates the autophagy of retinal
ganglion cells in NMDA-induced glaucoma (25). However, to the best of our
knowledge, there is little known about PTEN in HTM cells. A
previous study revealed that oxidative stress may increase cellular
PTEN levels (26). Similarly, in
the present study, it was also identified that the expression of
PTEN was upregulated in HTM cells following treatment with
H2O2; its expression was the opposite of that
of miR-200c-3p.
Oxidative stress serves a critical role in promoting
HTM cell apoptosis, and it is reportedly triggered following the
alteration of trabecular meshwork tissue function and integrity,
which is caused by mitochondrial damage (27,28).
Several miRNAs have been suggested to regulate HTM cell viability
or apoptosis; for example, miR-181a enhanced HTM cell viability and
inhibited cell apoptosis induced by H2O2
(20). Upregulation of miR-1298
markedly suppressed chronic oxidative stress-induced apoptosis in
HTM cells (5). Additionally,
inhibition of miR-93 increased cell viability and suppresses cell
apoptosis in glaucoma HTM cells (29). miR-200c and PTEN are both factors
involved in regulating cell apoptosis (30,31).
The present study demonstrated that the level of PTEN was
negatively regulated by miR-200c-3p. In addition, miR-200c-3p
promoted cell proliferation and inhibited cell apoptosis in HTM
cells by targeting PTEN. These results suggested that miR-200c-3p
serves as an anti-apoptotic factor and PTEN serves a role as a
pro-apoptotic factor in H2O2-treated HTM
cells.
Apoptosis-associated modulator Bax (pro-apoptotic)
is expressed in both HTM cells and ex-vivo tissues (32). Bax is required for oxidative
stress-induced cell death (33).
In addition, cleaved caspase-3, an activated form of caspase 3,
exhibits negative responses to oxidative stress (34). The expression of PTEN has the same
variation trend as that of Bax and cleaved caspase-3 (35). In the PTEN/AKT/mTOR signaling axis,
the PI3K-AKT signaling pathway is one of the major pathways in HTM
cells responding to oxidative stress (34). PTEN is a key modulator involved in
the PI3K signaling pathway, which negative regulates phosphorylated
AKT (36). AKT phosphorylates and
activates mTOR, one of the downstream targets (37). In the present study, miR-200c-3p
suppressed Bax and cleaved caspase-3 levels, and increased the
phosphorylation of AKT and mTOR in H2O2-HTM cells, while PTEN
attenuated these effects. The aforementioned results indicated that
overexpression of miR-200c-3p suppressed Bax and cleaved caspase-3,
and activated the PTEN/AKT/mTOR signaling pathway via targeting
PTEN in HTM cells under oxidative stress.
In conclusion, overexpression of miR-200c-3p
enhanced and inhibited cell proliferation and apoptosis,
respectively, by suppressing cleaved caspase-3 and Bax expression
and activating the PTEN/AKT/mTOR signaling pathway by targeting
PTEN. The present study revealed the role of miR-200c-3p in
H2O2-treated HTM cells, and also assessed the
molecular mechanism underlying miR-200c-3p. These data may provide
an improved understanding of HTM cells and provide a therapeutic
target for glaucoma.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YS designed the study wrote the manuscript, whereas
YZ and FR performed the experiments and analyzed the data. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mantravadi AV and Vadhar N: Glaucoma. Prim
Care. 42:437–449. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nickells RW, Howell GR, Soto I and John
SW: Under pressure: Cellular and molecular responses during
glaucoma, a common neurodegeneration with axonopathy. Annu Rev
Neurosci. 35:153–179. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stamer WD and Clark AF: The many faces of
the trabecular meshwork cell. Exp Eye Res. 158:112–123. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Porter K, Hirt J, Stamer WD and Liton PB:
Autophagic dysregulation in glaucomatous trabecular meshwork cells.
Biochim Biophys Acta. 1852:379–385. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ruibin W, Zheng X, Chen J, Zhang X, Yang X
and Lin Y: Micro RNA-1298 opposes the effects of chronic oxidative
stress on human trabecular meshwork cells via targeting on EIF4E3.
Biomed Pharmacother. 100:349–357. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Johnstone MA: The aqueous outflow system
as a mechanical pump: Evidence from examination of tissue and
aqueous movement in human and non-human primates. J Glaucoma.
13:421–438. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pillai RS: MicroRNA function: Multiple
mechanisms for a tiny RNA? RNA. 11:1753–1761. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Guo R, Shen W, Su C, Jiang S and Wang J:
Relationship between the pathogenesis of glaucoma and miRNA.
Ophthalmic Res. 57:194–199. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gonzalez P, Li G, Qiu J, Wu J and Luna C:
Role of microRNAs in the trabecular meshwork. J Ocul Pharmacol
Ther. 30:128–137. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Feng X, Wang Z, Fillmore R and Xi Y:
MiR-200, a new star miRNA in human cancer. Cancer Lett.
344:166–173. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Romano GL, Platania CB, Forte S, Salomone
S, Drago F and Bucolo C: MicroRNA target prediction in glaucoma.
Prog Brain Res. 220:217–240. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Luna C, Li G, Huang J, Qiu J, Wu J, Yuan
F, Epstein DL and Gonzalez P: Regulation of trabecular meshwork
cell contraction and intraocular pressure by miR-200c. PLoS One.
7:e516882012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kimura A, Namekata K, Guo X, Noro T,
Harada C and Harada T: Targeting oxidative stress for treatment of
glaucoma and optic neuritis. Oxid Med Cell Longev.
2017:28172522017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao J, Wang S, Zhong W, Yang B, Sun L and
Zheng Y: Oxidative stress in the trabecular meshwork (Review). Int
J Mol Med. 38:995–1002. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Saccà SC, Gandolfi S, Bagnis A, Manni G,
Damonte G, Traverso CE and Izzotti A: The outflow pathway: A tissue
with morphological and functional unity. J Cell Physiol.
231:1876–1893. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Agarwal V, Bell GW, Nam JW and Bartel DP:
Predicting effective microRNA target sites in mammalian mRNAs.
Elife. 4:2015. View Article : Google Scholar
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta DeltaC(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Carlomosti F, D'Agostino M, Beji S,
Torcinaro A, Rizzi R, Zaccagnini G, Maimone B, Di Stefano V, De
Santa F, Cordisco S, et al: Oxidative stress-induced miR-200c
disrupts the regulatory loop among SIRT1, FOXO1, and eNOS. Antioxid
Redox Signal. 27:328–344. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang Y, Zhou H, Liu X, Han Y, Pan S and
Wang Y: MiR-181a inhibits human trabecular meshwork cell apoptosis
induced by H2O2 through the suppression of NF-κB and JNK pathways.
Adv Clin Exp Med. 27:577–582. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mutlu M, Raza U, Saatci Ö, Eyüpoğlu E,
Yurdusev E and Şahin Ö: miR-200c: A versatile watchdog in cancer
progression, EMT, and drug resistance. J Mol Med (Berl).
94:629–644. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li G, Luna C, Qiu J, Epstein DL and
Gonzalez P: Alterations in microRNA expression in stress-induced
cellular senescence. Mech Ageing Dev. 130:731–741. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Milella M, Falcone I, Conciatori F, Cesta
Incani U, Del Curatolo A, Inzerilli N, Nuzzo CM, Vaccaro V, Vari S,
Cognetti F and Ciuffreda L: PTEN: Multiple functions in human
malignant tumors. Front Oncol. 5:242015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Koriyama Y, Kamiya M, Arai K, Sugitani K,
Ogai K and Kato S: Nipradilol promotes axon regeneration through
S-nitrosylation of PTEN in retinal ganglion cells. Adv Exp Med
Biol. 801:751–757. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li R, Jin Y, Li Q, Sun X, Zhu H and Cui H:
MiR-93-5p targeting PTEN regulates the NMDA-induced autophagy of
retinal ganglion cells via AKT/mTOR pathway in glaucoma. Biomed
Pharmacother. 100:1–7. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Leslie NR, Bennett D, Lindsay YE, Stewart
H, Gray A and Downes CP: Redox regulation of PI 3-kinase signalling
via inactivation of PTEN. EMBO J. 22:5501–5510. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yu AL, Fuchshofer R, Kampik A and
Welge-Lüssen U: Effects of oxidative stress in trabecular meshwork
cells are reduced by prostaglandin analogues. Invest Ophthalmol Vis
Sci. 49:4872–4880. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Saccà SC, Pulliero A and Izzotti A: The
dysfunction of the trabecular meshwork during glaucoma course. J
Cell Physiol. 230:510–525. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang Y, Li F and Wang S: MicroRNA-93 is
overexpressed and induces apoptosis in glaucoma trabecular meshwork
cells. Mol Med Rep. 14:5746–5750. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yuan C, Xu M, Rong R, Mei Y, Cai W, Li L,
Xue Y, Zhu B, Sun K and Han L: miR-200c regulates endothelin-1
induced PASMCs abnormal proliferation and apoptosis. IUBMB Life.
69:877–886. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li MF, Guan H and Zhang DD: Effect of
overexpression of PTEN on apoptosis of liver cancer cells. Genet
Mol Res. 15:2016.
|
|
32
|
Baleriola J, García-Feijoo J,
Martínez-de-la-Casa JM, Fernández-Cruz A, de la Rosa EJ and
Fernández-Durango R: Apoptosis in the trabecular meshwork of
glaucomatous patients. Mol Vis. 14:1513–1516. 2008.PubMed/NCBI
|
|
33
|
Steckley D, Karajgikar M, Dale LB, Fuerth
B, Swan P, Drummond-Main C, Poulter MO, Ferguson SS, Strasser A and
Cregan SP: Puma is a dominant regulator of oxidative stress induced
Bax activation and neuronal apoptosis. J Neurosci. 27:12989–12999.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Awai-Kasaoka N, Inoue T, Kameda T,
Fujimoto T, Inoue-Mochita M and Tanihara H: Oxidative stress
response signaling pathways in trabecular meshwork cells and their
effects on cell viability. Mol Vis. 19:1332–1340. 2013.PubMed/NCBI
|
|
35
|
Han Z, Chen F, Ge X, Tan J, Lei P and
Zhang J: miR-21 alleviated apoptosis of cortical neurons through
promoting PTEN-Akt signaling pathway in vitro after experimental
traumatic brain injury. Brain Res. 1582:12–20. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chalhoub N and Baker SJ: PTEN and the
PI3-kinase pathway in cancer. Annu Rev Pathol. 4:127–150. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chen JS, Wang Q, Fu XH, Huang XH, Chen XL,
Cao LQ, Chen LZ, Tan HX, Li W, Bi J and Zhang LJ: Involvement of
PI3K/PTEN/AKT/mTOR pathway in invasion and metastasis in
hepatocellular carcinoma: Association with MMP-9. Hepatol Res.
39:177–186. 2009. View Article : Google Scholar : PubMed/NCBI
|