Introduction
Neonatal hypoxic-ischemic encephalopathy (HIE) is a
type of brain damage that occurs as a result of partial or complete
hypoxia of brain tissues, which decreases or suspends the cerebral
blood flow in neonates (1). HIE is
one of the main reasons for neonatal death, secondary mental
retardation, cerebral palsy and other disabilities (2). It is estimated that neonatal HIE
accounts for ~25% of global neonatal deaths annually (1). Brain damage due to HIE is the
predominant cause of mortality in neonates, and the proportion is
as high as 14.5% in pediatric patients with cerebral palsy caused
by HIE, resulting in a substantial burden on pediatrics, families
and society (3–6). Previous studies have demonstrated
that the cascade reaction of proinflammatory cytokines in brain
tissue is activated after hypoxia-ischemia condition, which in turn
activates inflammatory factors and further aggravates brain damage
(7–9). Therefore, secondary brain injury
plays an important role in the pathogenesis of HIE, and inhibition
of the inflammatory response may protect hypoxic-ischemic brain
tissues (1,10).
Toll-like receptors (TLRs) are crucial in the
induction and regulation of the inflammatory response (11). TLR4 activates downstream protein
kinases via MyD88-dependent and TIR-domain-containing
adapter-inducing interferon-β (TRIF)-dependent signaling pathways,
subsequently leading to the activation of NF-κB and interferon
regulatory factors (IRFs) (11).
This activation then induces and regulates the release of immune
inflammatory factors (12). NF-κB
is a transcriptional regulator commonly found in mammalian cells,
and specifically binds to sequence-tagged sites of promoters and
enhancers of various cellular immune-related genes (13). By regulating the expression levels
of various cytokines, NF-κB plays an essential role in immune and
inflammatory responses (13). The
release of various immune inflammatory factors depends on the
activation of the NF-κB signaling pathway induced by TLR4, thus
activating NF-κB acts as the central link for TLR4 in regulating
inflammatory responses (14).
The TLR4/NF-κB signaling pathway plays a vital role
in the activation of the inflammatory response and development of
brain damage caused by cerebral ischemia-hypoxia, carbon monoxide
poisoning and brain trauma (15–20).
A TLR4 antagonist alleviates various brain injuries, such as
ischemic stroke, subarachnoid hemorrhage and brain trauma (21–23).
However, the role of the TLR4/NF-κB signaling pathway in neonatal
HIE and its regulatory mechanisms are not fully understood. TAK-242
is a micromolecular TLR4-specific inhibitor that selectively binds
to the intracellular domain of TLR4 to inhibit the production of
inflammatory mediators (24). A
previous study has demonstrated that TAK-242 crosses the
blood-brain barrier, blocks the TLR4 signaling pathway, inhibits
phosphorylation of downstream proteins and downregulates the
expression level of inflammatory cytokines, thereby improving brain
neural function and reducing cerebral ischemia-reperfusion injury
in mice (25). However, the role
of TAK-242 in the inhibition of the TLR4/NF-κB signaling pathway
activation, the release of inflammatory cytokines and its effect as
a neuroprotectant after HIE in neonatal rats requires further
investigation.
In the present study, a neonatal HIE rat model was
established to investigate the role of the TLR4/NF-κB signaling
pathway in the infarct volume, degree of cerebral edema,
pathomorphological changes of brain tissue, neurobehavioral
function deficits and signaling pathway-related protein levels
after HIE. TAK-242 was intraperitoneally injected into rats 30 min
after hypoxia-ischemia to investigate whether TAK-242 alleviates
brain damage after HIE by inhibiting the TLR4/NF-κB signaling
pathway and reducing the release of inflammatory cytokines. The
present results may help to facilitate the development of a new
therapeutic method for HIE treatment in clinical practice.
Materials and methods
Laboratory animals
A total of 15 female Sprague-Dawley (SD) rats (age,
12 weeks; weight, 260–300 g) with a litter size of 10–12 neonatal
rats were purchased from the Laboratory Animal Center of Yangzhou
University [license no. SCXK (Su) 2017-0007]. Female rats were
allowed free access to food and water. Neonatal SD rats (age, 7
days; weight, 12–18 g) were freely fed by female rats at 24±2°C
with a relative humidity of 55±5% and an alternate 12 h light/dark
cycle.
All animal experimental procedures were approved by
the Laboratory Animal Ethics Committee of Yangzhou University, and
were conducted in strict accordance with the Regulation on the
Administration of Laboratory Animals issued by the Ministry of
Science and Technology of the People's Republic of China. All
efforts were made to reduce the suffering of the animals.
Animal grouping and animal models
A total of 114 SD rats (age, 7 days) were randomly
assigned into the sham (n=30), HIE + vehicle (n=42), HIE + 0.75
mg/kg TAK-242 (n=6), HIE + 1.5 mg/kg TAK-242 (n=6) or HIE + 3 mg/kg
TAK-242 (n=30) groups. The HIE model was established according to
the Rice-Vannucci method (26).
Anesthesia was induced by inhalation of 2–3% isoflurane (Merck Hoei
Ltd.) and maintained using 1–1.5% isoflurane using an animal
general anesthesia machine. After inhalation of anesthesia,
neonatal rats were fixed on an operating table at a constant
temperature in the supine position and disinfected with 75% alcohol
on the skin of middle neck. The skin was cut along the median line
~0.5 cm. The subcutaneous fat and superficial fascia were incised
layer by layer, the muscle and connective tissue were separated
blunt by bending forceps, and the left common carotid artery was
carefully separated. The 5-0 surgical suture was used to ligate
both ends and the blood vessel was disconnected in the middle.
Suturing was performed, and then the surgical incision was
disinfected with 75% alcohol. After surgery, the neonatal rats were
placed in an incubator at 37°C for recovery for 1 h, and then
placed in an anoxic box with a nitrogen-oxygen mixed gas (8%
O2 + 92% N2) delivered at a flow rate of 2l
/min and a controlled temperature at 36±1°C for 2.5 h. In the sham
group, only the left common carotid artery was separated, and no
ligation or hypoxic treatment were performed. Rats in HIE + TAK-242
group were intraperitoneally injected with 0.75, 1.5 and 3 mg/kg of
TAK-242 isopyknic drug solution, for 30 min after hypoxia-ischemia.
The HIE + vehicle group was injected with an equal volume (7.5
ml/kg) of vehicle (10% DMSO). TAK-242 (InvivoGen) solution was
prepared, and administered as previously described (25,27,28).
Behavioral experiments
Neurological deficit score
In total, six neonatal rats in each group were
randomly selected for neurological deficit score using the Longa
scale 48 h after hypoxia-ischemia (29) as follows: i) 0 points, no
significant neurological deficit, with normal mobility of limbs;
ii) 1 point, mild neurological deficit, with an inability to fully
extend the right forelimb when lifting the tails; iii) 2 points,
moderate neurological deficit, with turning rightwards while
walking; iv) 3 points, severe neurological deficit, with falling
down rightwards while walking; and v) 4 points, inability to walk
spontaneously and a loss of consciousness. This was assessed with
rats walking along a table.
Rotarod task and beam walking test
In total, six neonatal rats in each group were
randomly selected to undergo the rotarod task and beam walking test
at week 4 after hypoxia-ischemia, in order to test motor
integration and coordination abilities (19). The rats were trained for 3 days
before undergoing the formal experiment. For the rotarod task, rats
were placed on a rotating rod and the rotation speed was slowly
increased from 4 rpm to 40 rpm within 5 min. The time of
maintaining balance to falling down from the rotating rod was
recorded.
For the beam walking test, a balance beam with a
length of 100 cm and a width of 2.0 cm was placed at a height of
~50 cm from the ground. A black box was placed at one end of the
balance beam, and the time taken for the rats to cross the balance
beam to reach the black box was recorded.
Determination of infarct volume
Then, six neonatal rats in each group were randomly
selected for triphenyltetrazolium chloride (TTC) staining after 48
h of hypoxia-ischemia to determine the infarct volume. Neonatal
rats were deeply anesthetized with 3% isoflurane and sacrificed by
cervical dislocation. After the heartbeat, respiratory arrest and
reflex disappeared, the brains were dissected and coronally
sectioned to ~2 mm slices. The brain sections were placed in 2%
2,3,5-TTC solution (Thermo Fisher Scientific, Inc.), incubated for
30 min in dark place at 37°C and then immersed in 10% formaldehyde
solution for fixation at 4°C overnight. ImageJ software (version
1.8.0, National Institutes of Health) was used to trace and analyze
infarct volume (30).
Determination of brain water content
In total, six neonatal rats in each group were
randomly selected after 48 h of hypoxia-ischemia to calculate the
brain water content in order to assess the degree of cerebral
edema. Neonatal rats were deeply anesthetized, sacrificed by
cervical dislocation and the brains were removed after the
heartbeat, respiratory arrest and reflex disappeared. The water and
blood stains on the brain surface were removed using filter paper
to weigh the wet weight of the brain (31). The brain tissues were wrapped in a
tin foil paper and dried at 100°C in an oven for 12 h until a
constant weight was reached, and then were measured at room
temperature to weigh the dry weight of the brain. The brain water
content was calculated by dry-wet specific gravity method as
follows: Brain water content=(brain wet weight- brain dry
weight)/brain wet weight × 100% (31).
Hematoxylin and eosin (HE) staining, and
immunohistochemistry
Neonatal rats (n=6) were randomly selected from each
group to undergo heart perfusion with PBS, and were fixed with 4%
paraformaldehyde under deep anesthesia after 48 h of
hypoxia-ischemia. HE staining and immunohistochemical analysis of
5-µm brain tissue sections were performed as previously described
(17,19). Pathological changes were then
observed under a light microscope (magnification, ×400; Nikon
Corporation) after HE staining. Paraffin sections were dewaxed and
endogenous peroxidase activity was blocked with 3%
H2O2 solution at room temperature for 5 min,
and 5% goat serum (cat. no. C0265; Beyotime Biotech Co., Ltd.) was
used for mounting. The following primary antibodies were added:
Rabbit polyclonal anti-TLR4 (1:100; cat. no. 19811-1-AP;
ProteinTech Group, Inc.) and rabbit polyclonal anti-phosphorylated
(p)-NF-κB p65 (1:100; cat. no. AP0123; ABclonal Biotech Co., Ltd.),
which were then incubated overnight at 4°C and rewarmed at room
temperature. The anti-rabbit biotin-conjugated IgG secondary
antibody (1:100; cat. no. P0628; Beyotime Biotech Co., Ltd.) were
added, incubated at 37°C for 1 h and horseradish peroxidase was
added for labeling for 15 min at room temperature. Color
development was performed using 3,3′-diaminobenzidine, and
hematoxylin was used for counterstaining at room temperature.
Immunohistochemical images were then observed under a light
microscope (magnification, ×400; Nikon Corporation). Analysis was
performed using ImageJ software.
Western blotting
Neonatal rats (n=6) in each group were randomly
selected to undergo heart perfusion with PBS under deep anesthesia
at 24, 48 and 72 h after hypoxia-ischemia, and then the left
hippocampus was removed. The tissues were homogenized with RIPA
lysis buffer (cat. no. P0013K; Beyotime Biotech Co., Ltd.). The
samples were incubated on ice for 30 min, and the supernatants were
recovered by centrifugation at 13,400 × g at 4°C for 20 min.
Protein concentrations were determined by bicinchoninic acid
protein assay, and the samples were incubated with 5× Loading
buffer (cat. no. P0015; Beyotime Biotech Co., Ltd.) at 100°C for 5
min. Western blotting was performed as described previously
(19). In total, 50 µg of protein
was taken from each sample and separated by 10% SDS-PAGE, and then
transferred onto a PVDF membrane (EMD Millipore). Then, 5% skim
milk powder was used for blocking at room temperature for 2 h, and
the following primary antibodies, rabbit polyclonal anti-TLR4
(1:500; cat. no. 19811-1-AP; ProteinTech Group, Inc.), rabbit
polyclonal anti-MyD88 (1:1,000; cat. no. A0980; ABclonal Biotech
Co., Ltd.), rabbit polyclonal anti-TRIF (1:500; cat. no. ab13810;
Abcam), rabbit polyclonal anti-phospho-NF-κB p65 (1:1,000; cat. no.
AP0123; ABclonal Biotech Co., Ltd.) and rabbit polyclonal
anti-NF-kB (1:1,000; cat. no. A2547; ABclonal Biotech Co., Ltd.)
were added and incubated overnight at 4°C. The anti-rabbit
HRP-conjugated IgG secondary antibody (1:5,000; cat. no. AS014;
ABclonal Biotech Co., Ltd.) was incubated for 2 h at room
temperature, developed and visualized by Hypersensitive ECL
chemiluminescence Kit (cat. no. P0018AS; Beyotime Institute of
Biotechnology) in a dark room. Densitometry of protein bands was
performed using ImageJ software.
ELISA
The supernatant of the left hippocampus was
collected after homogenization and centrifugation as described
above. The levels of the inflammatory cytokines tumor necrosis
factor α (TNF-α) and interleukin (IL)-1β in each group were
determined using TNF-α and IL-1β ELISA kits (cat. nos. RK00029 and
RK00009; ABclonal Biotech Co., Ltd.) according to the
manufacturer's instructions. The absorbance was detected at 450 nm
and the content of each sample was calculated from the standard
curve.
Statistical analysis
Statistical analyses were performed using SPSS 19.0
software (SPSS, Inc.). Normally distributed data are presented as
the mean ± SD, and comparisons among groups was performed using a
one-way ANOVA followed by Tukey's test. Non-normally distributed
data are presented as the median (interquartile range). A
Mann-Whitney U test was used for the statistical analysis of
neurological deficit score, and the Dunn-Bonferroni test was used
for further comparison between the groups. All experiments were
repeated three times. P<0.05 was considered to indicate a
statistically significant difference.
Results
Activation of the
TLR4/MyD88/TRIF/NF-κB signaling pathway and release of inflammatory
cytokines during brain damage after HIE
The protein expression levels of TLR4, MyD88, TRIF,
p-NF-κB, TNF-α and IL-1β in the left hippocampus of neonatal rats
were significantly increased after HIE. These protein expression
levels reached a peak at 48 h, and started to decrease at 72 h
after HIE (Fig. 1).
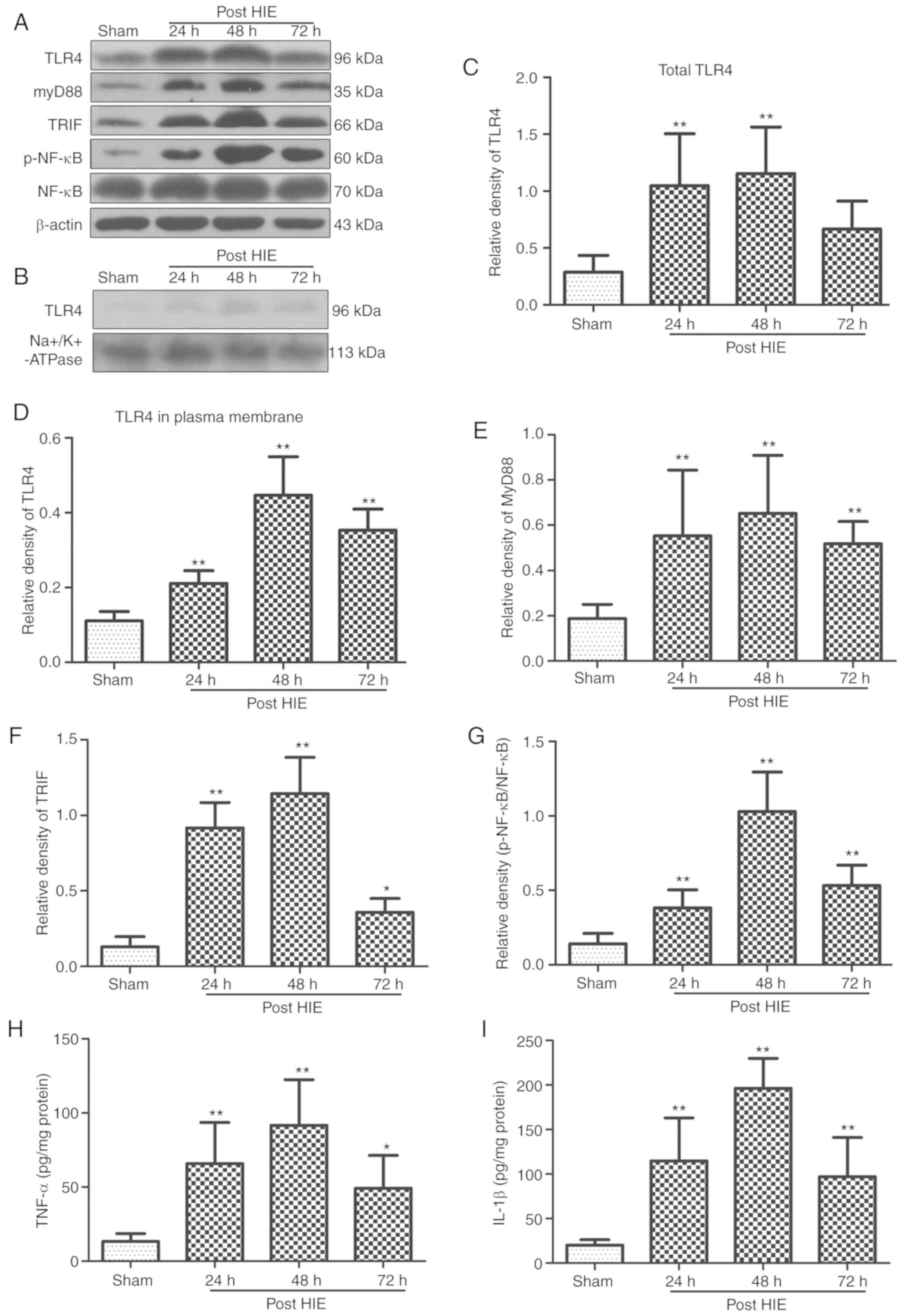 | Figure 1.Activation of the
TLR4/MyD88/TRIF/NF-κB signaling pathway and release of inflammatory
cytokines during brain damage after HIE. (A) Representative western
blotting bands of total TLR4, MyD88, TRIF, p-NF-κB, NF-κB and (B)
TLR4 in plasma membrane at different time points after HIE. Western
blotting and ELISA results indicated that the levels of (C) total
TLR4, (D) TLR4 in plasma membrane, (E) MyD88, (F) TRIF, (G)
p-NF-κB, (H) TNF-α and (I) IL-1β were significantly increased and
peaked at 48 h, but were gradually decreased at 72 h. n=6 per group
*P<0.05, **P<0.01 vs. sham group. P-, phosphorylated; TNF-α,
tumor necrosis factor α; IL, interleukin; TLR4, Toll like receptor
4; TRIF, TIR-domain-containing adapter-inducing interferon-β; HIE,
hypoxic-ischemic encephalopathy. |
TAK-242 alleviates neurological
deficits and improves neurobehavioral function after HIE
It was observed that the right forelimb of neonatal
rats in the HIE + vehicle group did not extend when tailing and the
rats turned rightwards while walking, four rats also fell down
towards the right side. Furthermore, rats in the HIE + vehicle
group could not walk spontaneously, and thus exhibited significant
signs of neurological deficit. All three doses of TAK-242
alleviated neurological deficit after HIE, and the high dose group
(3 mg/kg) was identified to be the most effective compared with the
vehicle group (Fig. 2A).
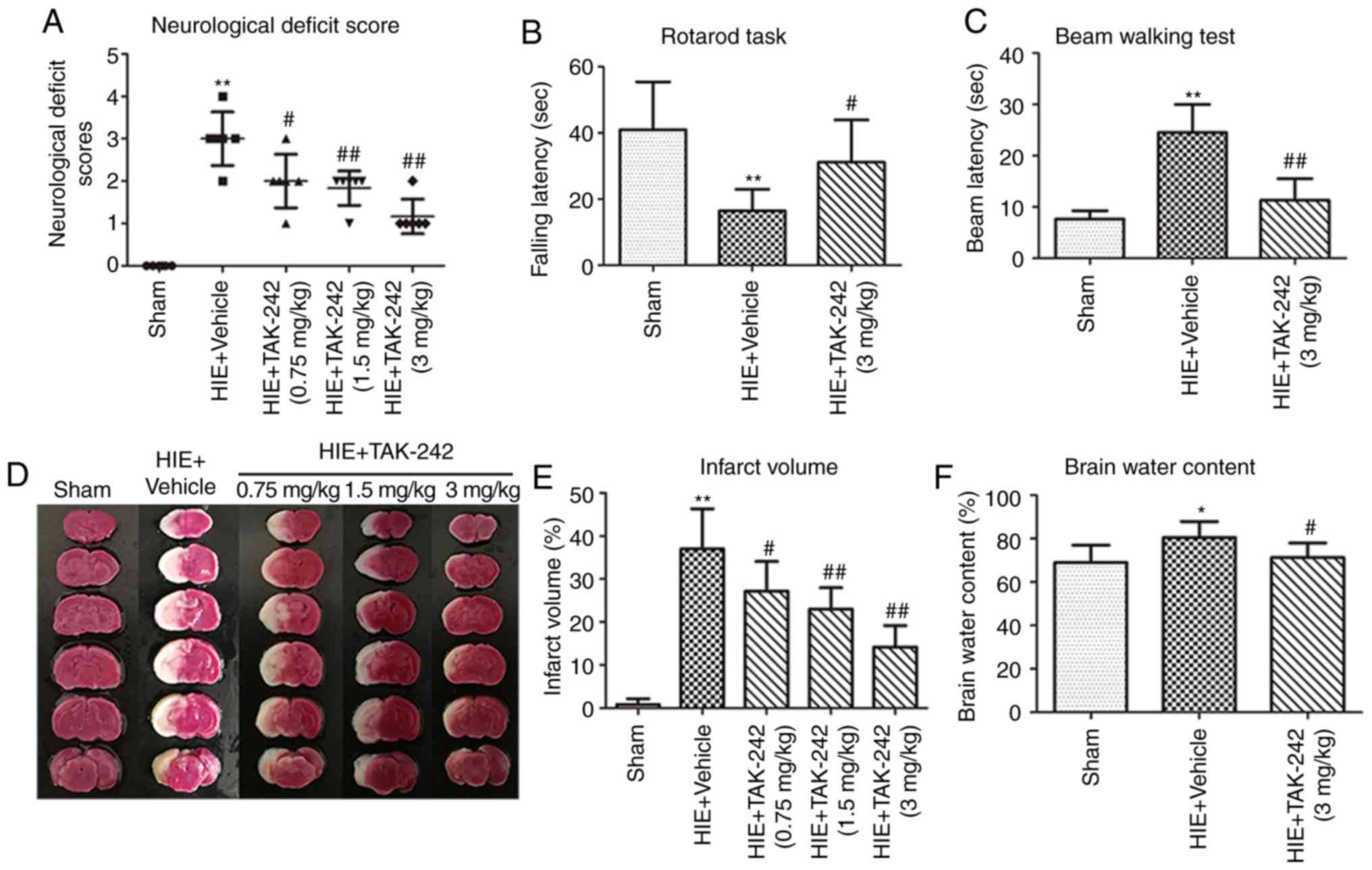 | Figure 2.TAK-242 alleviates neurological
deficits, improves neurobehavioral function, and reduces infarct
volume and cerebral edema content after HIE. Low, medium and high
doses (0.75, 1.5 and 3 mg/kg) of TAK-242 alleviated neurological
deficits in neonatal rats 48 h after HIE, with high dose group (3
mg/kg) appearing the most effective. (A) TAK-242 (3 mg/kg)
significantly improved the (B) rotarod task and (C) beam walking
test scores in neonatal rats 4 weeks after HIE. (D)
Triphenyltetrazolium chloride staining of representative brain
coronal sections in each group. (E) Low, medium and high doses of
TAK-242 significantly reduced the infarct volume of neonatal rats
after HIE, with high dose group demonstrating the most significant
decrease. (F) TAK-242 (3 mg/kg) significantly reduced the degree of
brain edema in neonatal rats after HIE. n=6 per group. *P<0.05,
**P<0.01 vs. sham group; #P<0.05,
##P<0.01 vs. HIE + vehicle group. HIE,
hypoxic-ischemic encephalopathy. |
The neurobehavioral function test results in the HIE
+ vehicle group were significantly lower compared with the sham
group. Moreover, compared with the HIE + vehicle group, the rotarod
running time exhibited a significant increase in the HIE + TAK-242
(3 mg/kg) group, while beam walking latency was significantly
reduced, thus demonstrating improved motor integration and
coordination abilities after TAK-242 treatment (Fig. 2B and C).
TAK-242 reduces infarct volume and the
content of cerebral edema 48 h after HIE
A large-area infarct was observed in the left
cerebral hemisphere of the neonatal rats in the HIE + vehicle
group, while the infarct volume of the neonatal rats in the three
TAK-242 groups was significantly reduced compared with the HIE +
vehicle group, with the high dose having the greatest significant
difference (Fig. 2D and E).
Furthermore, the present results suggested that the brain edema
content of neonatal rats in the TAK-242 group (3 mg/kg) was
significantly decreased compared with the HIE + vehicle group
(Fig. 2F).
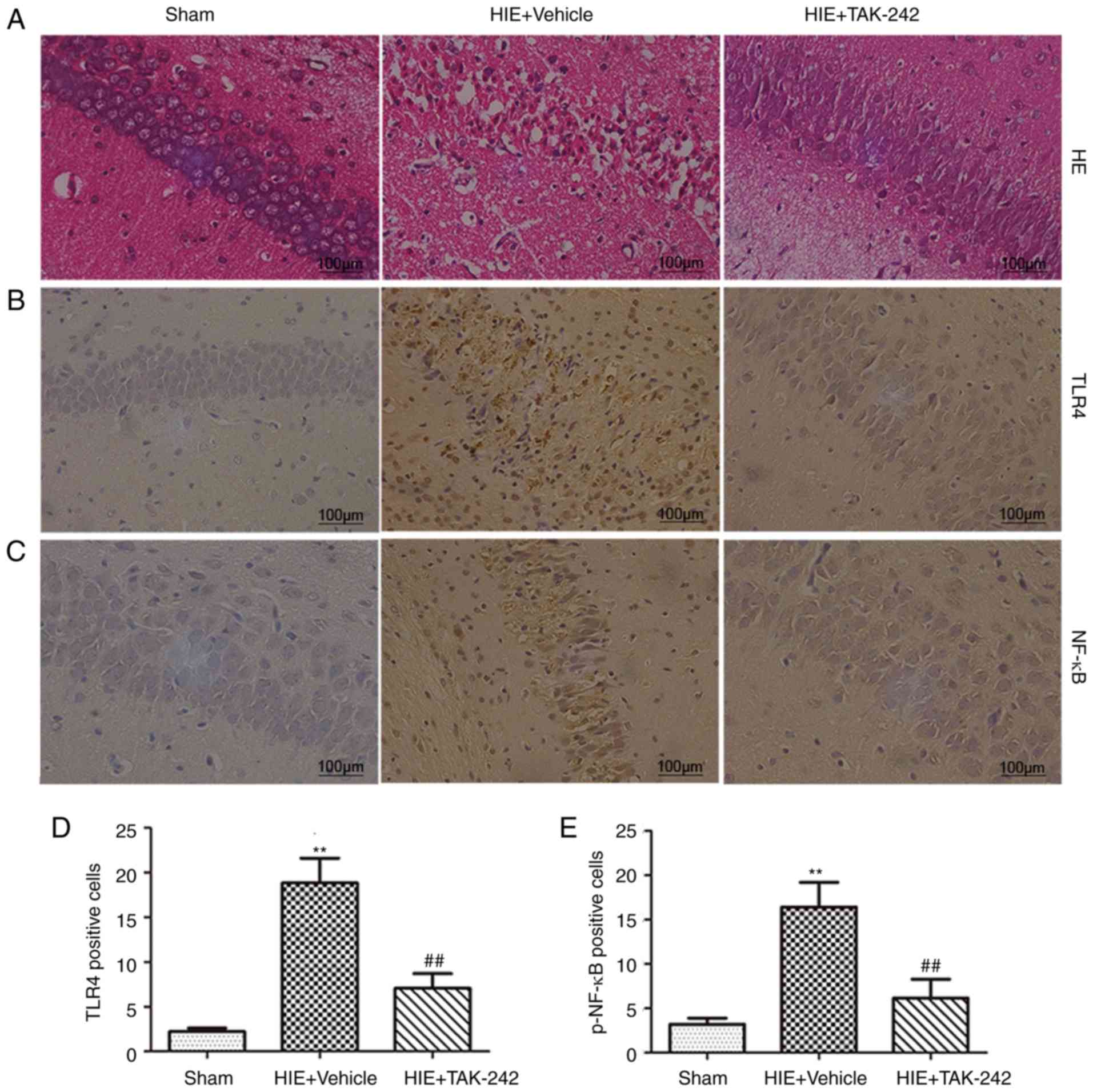
TAK-242 alleviates pathological damage
of brain tissue after HIE
It was demonstrated that the structure of left
hippocampus in the sham group was ordered, and the neuronal cells
were aligned and compact. Moreover, the cell morphology was normal.
In the HIE + vehicle group, the structure of the hippocampus was
disordered, and the neuronal cells were loosely arranged and
irregular. Furthermore, cellular edema, karyopyknosis, karyorrhexis
and necrosis of nerve cells were observed. Moreover, it was
demonstrated that in the HIE + TAK-242 group (3 mg/kg), hippocampal
damage was reduced compared with the HIE + vehicle group (Fig. 3A).
TAK-242 inhibits protein expression of
the TLR4/MyD88/ TRIF/NF-κB signaling pathway and the release of
downstream inflammatory cytokines in the hippocampus
To further investigate the neuroprotective mechanism
of TAK-242 in HIE, immunohistochemistry was performed to determine
the expression levels of TLR4 and p-NF-κB in the left hippocampus.
It was demonstrated that the expression levels of TLR4 and
p-NF-κB-positive cells in the hippocampus were significantly
increased after 48 h of HIE, but were significantly decreased after
treatment with 3 mg/kg TAK-242 (Fig.
3B-E). Furthermore, western blotting and ELISA results
suggested that the levels of TLR4, MyD88, TRIF, p-NF-κB, TNF-α and
IL-1β in the left hippocampus in the HIE + vehicle group peaked
after 48 h of HIE. Moreover, the present results suggested that
administration of 3 mg/kg TAK-242 significantly decreased the
expression levels of TLR4, MyD88, TRIF, p-NF-κB, TNF-α and IL-1β in
the hippocampus after 48 h of HIE, compared with the vehicle group
(Fig. 4).
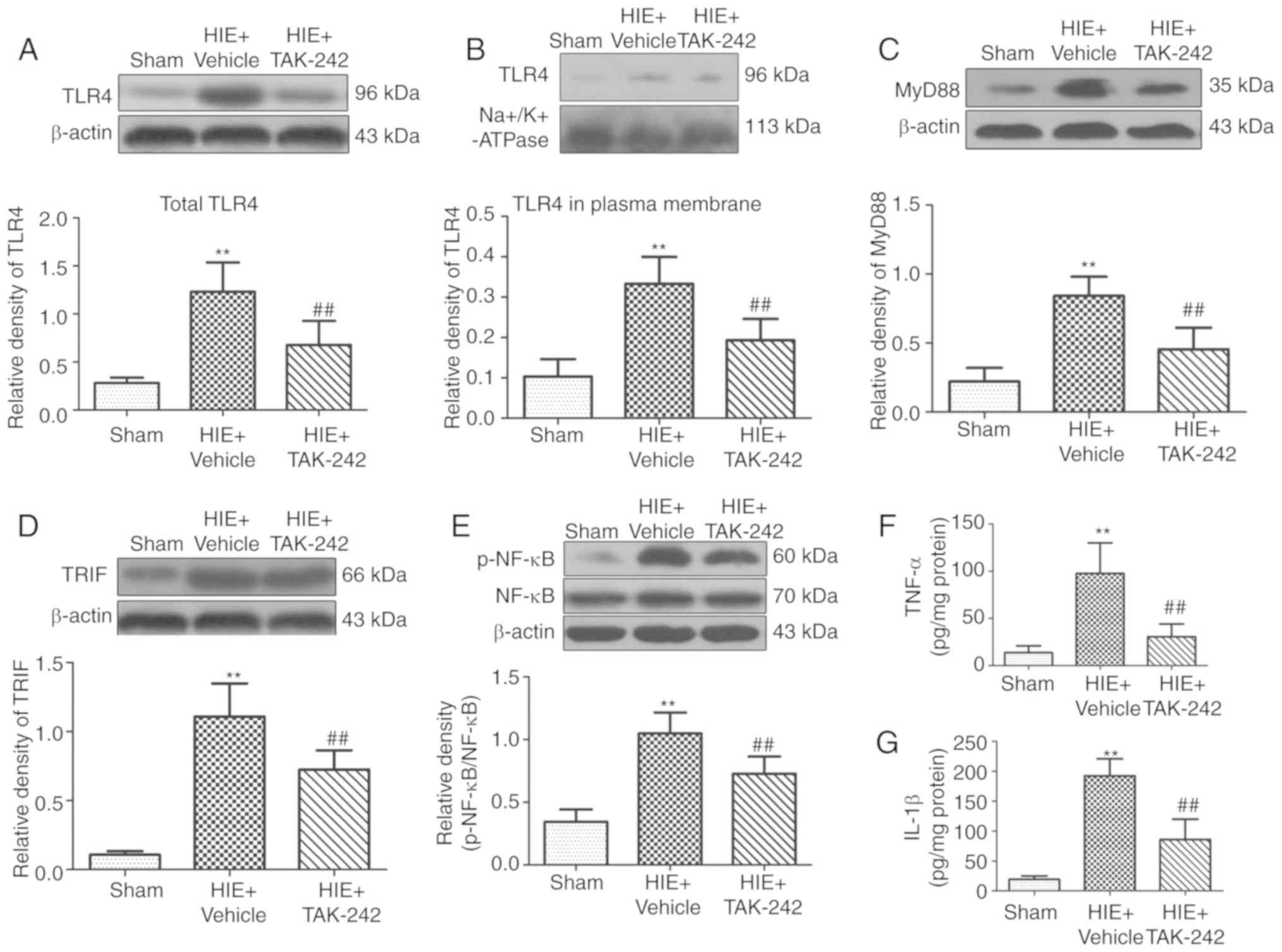 | Figure 4.TAK-242 inhibits protein expression
levels of the TLR4/MyD88/TRIF/NF-κB signaling pathway and the
release of downstream inflammatory cytokines in the hippocampus.
Western blotting results of TLR4, MyD88, TRIF and p-NF-κB in each
group demonstrated that 3 mg/kg TAK-242 significantly inhibited the
protein expression levels of total (A) TLR4, (B) TLR4 in plasma
membrane, (C) MyD88, (D) TRIF and (E) p-NF-κB in the hippocampus 48
h after HIE. ELISA results suggested that 3 mg/kg TAK-242
significantly decreased the levels of (F) TNF-α and (G) IL-1β in
the hippocampus 48 h after HIE. n=6 per group. **P<0.01 vs. sham
group; ##P<0.01 vs. HIE + vehicle group. HIE,
hypoxic-ischemic encephalopathy; TLR4, Toll like receptor 4; p-,
phosphorylated; TRIF, TIR-domain-containing adapter-inducing
interferon-β; IL, interleukin. |
Discussion
In the present study, results from the neonatal HIE
rat model suggested that TAK-242 treatment had significantly
reduced the infarct volume and cerebral edema content. Furthermore,
TAK-242 alleviated neuronal damage, improved neurobehavioral
function and decreased the protein expression levels of TLR4,
MyD88, TRIF, p-NF-κB, TNF-α and IL-1β in the hippocampus.
Therefore, TAK-242 may exert a neuroprotective effect after HIE by
inhibiting the TLR4/MyD88/TRIF/NF-κB signaling pathway and reducing
the release of inflammatory cytokines.
HIE is a hypoxic-ischemic brain damage caused by
perinatal asphyxia (1).
Inflammatory and immune responses serve an essential role in brain
ischemic injury (32). Therefore,
inhibition of secondary brain injury caused by inflammatory and
immune responses after hypoxia-ischemia may be a novel target for
treating HIE (1,10). TLR4 can regulate the process of
signal transduction in many immune and inflammatory diseases, and
is crucial in the inflammatory and immune responses associated with
the central nervous system (33).
Activation of the TLR4/NF-κB signaling pathway and
the release of inflammatory cytokines are present in the process of
brain damage caused by carbon monoxide poisoning, trauma and
cerebral hemorrhage, and inhibition of the TLR4/NF-κB signaling
pathway reduces the degree of brain damage (17,19,20,28).
Furthermore, activation of the TLR4/NF-κB signaling pathway is also
involved in cerebral ischemia-reperfusion injury in rats, and
inhibition of this signaling pathway can alleviate cerebral
ischemia-reperfusion injury (16,18).
In the present study, significant damage occurred to the neurons in
brain tissue, along with deficits in neurobehavioral function in
neonatal rats after HIE. In addition, large cerebral infarction
appeared in the left brain and cerebral edema content was also
significantly increased. The present study also demonstrated that
the expression levels of TLR4, p-NF-κB, TNF-α and IL-1β were
significantly increased in the left hippocampus, indicating that
the activation of TLR4/NF-κB signaling pathway and release of
inflammatory cytokines occurred during brain damage after HIE. The
present results were in line with results from Yao et al
(34). However, Yao et al
(34) did not elucidate the
specific molecular signaling mechanisms regarding the activation of
TLR4 and its downstream inflammatory cytokines after hypoxia.
TLR4 activates innate immune cells and inflammatory
response cells, and causes the release of the inflammatory
cytokines TNF-α, IL-1β, IL-6 and IL-8 via MyD88- and TRIF-dependent
signaling pathways, eventually leading to the activation of various
immuno-inflammatory responses (35). Previous studies have demonstrated
that the expression levels of TLR4 and its downstream signaling
molecules MyD88, TRIF and NF-κB are upregulated in the hippocampus
after traumatic brain injury in rats (20). Therefore, TLR4 may activate NF-κB
via MyD88- and TRIF-dependent signaling pathways, and promote the
release of inflammatory cytokines, such as TNF-α and IL-1β, further
aggravating the degree of brain damage; inhibition of the
TLR4/MyD88/TRIF/NF-κB signaling pathway plays a neuroprotective
role after brain trauma (20,36).
The activation of TLR4 and its downstream signaling molecules
MyD88, TRIF and NF-κB are observed in a mouse model with cerebral
hemorrhage, and inhibition of the TLR4/MyD88/TRIF/NF-κB signaling
pathway alleviates the degree of inflammatory brain damage and
impaired neurological function in mice (28). The present results suggested that
the levels of TLR4, p-NF-κB, MyD88, TRIF, TNF-α and IL-1β were
increased in the left hippocampus of neonatal rats after HIE,
indicating that TLR4 may activate NF-κB to promote the release of
downstream inflammatory cytokines via a signaling pathway mediated
by MyD88 and TRIF. Therefore, the activation of the
TLR4/MyD88/TRIF/NF-κB signaling pathway and release of inflammatory
cytokines after HIE may be important in the pathogenesis of HIE,
and inhibition of this signaling pathway may be a novel target for
HIE treatment.
TAK-242 is a specific TLR4 inhibitor that binds to
Cys747 in the intracellular domain of TLR4 and reduces TLR4
activity (37). Previous studies
have demonstrated that TAK-242 plays a neuroprotective role by
inhibiting the TLR4 signaling pathway, and reducing the activation
and release of inflammatory cytokines (16,20,38,39).
At present, no studies are available on the effect of TAK-242 on
HIE. Therefore, in the present study, intraperitoneal injection of
TAK-242 (25,27,28)
results in a significant reduction of neurobehavioral functional
deficits in neonatal HIE rats, and the pathological morphology of
brain tissue was improved. Moreover, the cerebral edema content and
infarct volume were significantly reduced, and the levels of TLR4,
MyD88, TRIF, p-NF-κB, TNF-α and IL-1β in hippocampus were also
significantly reduced. The present results suggested that TAK-242
may alleviate brain damage caused by hypoxia-ischemia by inhibiting
the TLR4/MyD88/TRIF/NF-κB signaling pathway and reducing the
release of inflammatory cytokines, thereby exerting a
neuroprotective effect after HIE.
In conclusion, to the best of our knowledge, the
present study was the first to use the neonatal HIE rat model to
identify that TAK-242 may serve a neuroprotective role in the brain
tissues after HIE. This neuroprotective effect is suggested to be
via an inhibition of the TLR4/MyD88/TRIF/NF-κB signaling pathway
and a reduction in the release of inflammatory cytokines.
Therefore, TAK-242 may be a promising medication for HIE. However,
due to the limited sample size and the fact that not all
pathological features of HIE were included in the present study,
further studies are required to investigate the neuroprotective
function of TAK-242 in HIE.
Acknowledgements
Not applicable.
Funding
This study was supported by the National Natural
Science Foundation of China (grant no. 81771625).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
LJ and XF conceived and designed the experiments.
LJ, ZX, HL, MW, FW, SL and JT performed the experiments and
analyzed the data. LJ and ZX wrote the manuscript. All authors read
and approved the manuscript and agree to be accountable for all
aspects of the research in ensuring that the accuracy or integrity
of any part of the work are appropriately investigated and
resolved.
Ethics approval and consent to
participate
All animal experimental procedures were approved by
the Laboratory Animal Ethics Committee of Yangzhou University
[approval no. SCXK (Su) 2017-0007], and were conducted in strict
accordance with the Regulation on the Administration of Laboratory
Animals issued by the Ministry of Science and Technology of the
People's Republic of China. All efforts were made to reduce the
suffering of animals.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
HIE
|
hypoxic-ischemic encephalopathy
|
|
TLRs
|
toll-like receptors
|
|
IRFs
|
interferon regulatory factors
|
|
OD
|
optical density
|
References
|
1
|
Qin X, Cheng J, Zhong Y, Mahgoub OK, Akter
F, Fan Y, Aldughaim M, Xie Q, Qin L, Gu L, et al: Mechanism and
treatment related to oxidative stress in neonatal hypoxic-ischemic
encephalopathy. Front Mol Neurosci. 12:882019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhao M, Zhu P, Fujino M, Zhuang J, Guo H,
Sheikh I, Zhao L and Li XK: Oxidative stress in hypoxic-ischemic
encephalopathy: Molecular mechanisms and therapeutic strategies.
Int J Mol Sci. 17:E20782016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu L, Oza S, Hogan D, Perin J, Rudan I,
Lawn JE, Cousens S, Mathers C and Black RE: Global, regional, and
national causes of child mortality in 2000-13, with projections to
inform post-2015 priorities: An updated systematic analysis.
Lancet. 385:430–440. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gale C, Statnikov Y, Jawad S, Uthaya SN
and Modi N; Brain Injuries expert working group, : Neonatal brain
injuries in England: Population-based incidence derived from
routinely recorded clinical data held in the national neonatal
research database. Arch Dis Child Fetal Neonatal Ed. 103:F301–F306.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Leigh S, Granby P, Turner M, Wieteska S,
Haycox A and Collins B: The incidence and implications of cerebral
palsy following potentially avoidable obstetric complications: A
preliminary burden of disease study. BJOG. 121:1720–1728. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Smith AL, Alexander M, Rosenkrantz TS,
Sadek ML and Fitch RH: Sex differences in behavioral outcome
following neonatal hypoxia ischemia: Insights from a clinical
meta-analysis and a rodent model of induced hypoxic ischemic brain
injury. Exp Neurol. 254:54–67. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Doycheva D, Shih G, Chen H, Applegate R,
Zhang JH and Tang J: Granulocyte-colony stimulating factor in
combination with stem cell factor confers greater neuroprotection
after hypoxic-ischemic brain damage in the neonatal rats than a
solitary treatment. Transl Stroke Res. 4:171–178. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shetty J: Neonatal seizures in
hypoxic-ischaemic encephalopathy-risks and benefits of
anticonvulsant therapy. Dev Med Child Neurol. 57 (Suppl 3):S40–S43.
2015. View Article : Google Scholar
|
|
9
|
Knox R, Brennan-Minnella AM, Lu F, Yang D,
Nakazawa T, Yamamoto T, Swanson RA, Ferriero DM and Jiang X: NR2B
phosphorylation at tyrosine 1472 contributes to brain injury in a
rodent model of neonatal hypoxia-ischemia. Stroke. 45:3040–3047.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jenkins DD, Rollins LG, Perkel JK, Wagner
CL, Katikaneni LP, Bass WT, Kaufman DA, Horgan MJ, Languani S,
Givelichian L, et al: Serum cytokines in a clinical trial of
hypothermia for neonatal hypoxic-ischemic encephalopathy. J Cereb
Blood Flow Metab. 32:1888–1896. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Leitner GR, Wenzel TJ, Marshall N, Gates
EJ and Klegeris A: Targeting toll-like recepter 4 to modulate
neuroinflammation in central nervous system disorders. Expert Opin
Ther Targets. 23:865–882. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tajalli-Nezhad S, Karimian M, Beyer C,
Atlasi MA and Azami Tameh A: The regulatory role of Toll-like
receptors after ischemic stroke: Neurosteroids as TLR modulators
with the focus on TLR2/4. Cell Mol Life Sci. 76:523–537. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mulero MC, Huxford T and Ghosh G: NF-kB,
IkB, and IKK: Integral components of immune system signaling. Adv
Exp Med Biol. 1172:207–226. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Brown J, Wang H, Hajishengallis GN and
Martin M: TLR-signaling networks: An integration of adaptor
molecules, kinases, and cross-talk. J Dent Res. 90:417–427. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu Y and Fassbender K: Deficiency of TLR4
ameliorates hypoperfusion-induced brain pathology. Theranostics.
8:6355–6356. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhao H, Chen Z, Xie LJ and Liu GF:
Suppression of TLR4/NF-kB signaling pathway improves cerebral
ischemia-reperfusion injury in rats. Mol Neurobiol. 55:4311–4319.
2018.PubMed/NCBI
|
|
17
|
Pang L, Zhang N, Dong N, Wang DW, Xu DH,
Zhang P and Meng XW: Erythropoietin protects rat brain injury from
carbon monoxide poisoning by inhibiting toll-like receptor
4/NF-kappa B-dependent inflammatory responses. Inflammation.
39:561–568. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang Y, Ge P, Yang L, Wu C, Zha H, Luo T
and Zhu Y: Protection of ischemic post conditioning against
transient focal ischemia-induced brain damage is associated with
inhibition of neuroinflammation via modulation of TLR2 and TLR4
pathways. J Neuroinflammation. 11:152014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Feng Y, Cui C, Liu X, Wu Q, Hu F, Zhang H,
Ma Z and Wang L: Protective role of apocynin via suppression of
neuronal autophagy and TLR4/NF-kB signaling pathway in a rat model
of traumatic brain injury. Neurochem Res. 42:3296–3309. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Feng Y, Gao J, Cui Y, Li M, Li R, Cui C
and Cui J: Neuroprotective effects of resatorvid against traumatic
brain injury in rat: Involvement of neuronal autophagy and TLR4
signaling pathway. Cell Mol Neurobiol. 37:155–168. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu T, Liu M, Zhang T, Liu W, Xu H, Mu F,
Ren D, Jia N, Li Z, Ding Y, et al: Z-Guggulsterone attenuates
astrocytes-mediated neuroinflammation after ischemia by inhibiting
toll-like receptor 4 pathway. J Neurochem. 147:803–815. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu FY, Cai J, Wang C, Ruan W, Guan GP,
Pan HZ, Li JR, Qian C, Chen JS, Wang L and Chen G: Fluoxetine
attenuates neuroinflammation in early brain injury after
subarachnoid hemorrhage: A possible role for the regulation of
TLR4/MyD88/NF-KB signaling pathway. J Neuroinflammation.
15:3472018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tang R, Lin YM, Liu HX and Wang ES:
Neuroprotective effect of docosahexaenoic acid in rat traumatic
brain injury model via regulation of TLR4/NF-Kappa B signaling
pathway. Int J Biochem Cell Biol. 99:64–71. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hussey SE, Liang H, Costford SR, Klip A,
DeFronzo RA, Sanchez-Avila A, Ely B and Musi N: TAK-242, a
small-molecule inhibitor of Toll-like receptor 4 signalling,
unveils similarities and differences in lipopolysaccharide- and
lipid-induced inflammation and insulin resistance in muscle cells.
Biosci Rep. 33:37–47. 2012.PubMed/NCBI
|
|
25
|
Hua F, Tang H, Wang J, Prunty MC, Hua X,
Sayeed I and Stein DG: TAK-242, an antagonist for Toll-like
receptor 4, protects against acute cerebral ischemia/reperfusion
injury in mice. J Cereb Blood Flow Metab. 35:536–542. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Vannucci RC, Connor JR, Mauger DT, Palmer
C, Smith MB, Towfighi J and Vannucci SJ: Rat model of perinatal
hypoxic-ischemic brain damage. J Neurosci Res. 55:158–163. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hwang JW, Jeon YT, Lim YJ and Park HP:
Sevoflurane postconditioning-induced anti-inflammation via
inhibition of the toll-like receptor-4/nuclear factor Kappa B
pathway contributes to neuroprotection against transient global
cerebral ischemia in rats. Int J Mol Sci. 18:E23472017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang YC, Wang PF, Fang H, Chen J, Xiong XY
and Yang QW: Toll-like receptor 4 antagonist attenuates
intracerebral hemorrhage-induced brain injury. Stroke.
44:2545–2552. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Longa EZ, Weinstein PR, Carlson S and
Cummins R: Reversible middle cerebral artery occlusion without
craniectomy in rats. Stroke. 20:84–91. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li Z, Wang J, Zhao C, Ren K, Xia Z, Yu H
and Jiang K: Acute blockage of notch signaling by DAPT induces
neuroprotection and neurogenesis in the neonatal rat brain after
stroke. Transl Stroke Res. 7:132–140. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cui C, Cui Y, Gao J, Sun L, Wang Y, Wang
K, Li R, Tian Y, Song S and Cui J: Neuroprotective effect of
ceftriaxone in a rat model of traumatic brain injury. Neurol Sci.
35:695–700. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ystgaard MB, Scheffler K, Suganthan R,
Bjoras M, Ranheim T, Sagen EL, Halvorsen B, Saugstad OD and
Yndestad A: Neuromodulatory effect of NLRP3 and ASC in neonatal
hypoxic ischemic encephalopathy. Neonatology. 115:355–362. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kong Y and Le Y: Toll-like receptors in
inflammation of the central nervous system. Int Immunopharmacol.
11:1407–1414. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yao L, Kan EM, Lu J, Hao A, Dheen ST, Kaur
C and Ling EA: Toll-like receptor 4 mediates microglial activation
and production of inflammatory mediators in neonatal rat brain
following hypoxia: Role of TLR4 in hypoxic microglia. J
Neuroinflammation. 10:232013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li M, Liu J, Bi Y, Chen J and Zhao L:
Potential medications or compounds acting on toll-like receptors in
cerebral ischemia. Curr Neuropharmacol. 16:160–175. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhu HT, Bian C, Yuan JC, Chu WH, Xiang X,
Chen F, Wang CS, Feng H and Lin JK: Curcumin attenuates acute
inflammatory injury by inhibiting the TLR4/MyD88/NF-KB signaling
pathway in experimental traumatic brain injury. J
Neuroinflammation. 11:592014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lei C, Wu B, Cao T, Liu M and Hao Z: Brain
recovery mediated by toll-like receptor 4 in rats after
intracerebral hemorrhage. Brain Res. 1632:1–8. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhao Y, Zhao Y, Zhang M, Zhao J, Ma X,
Huang T, Pang H, Li J and Song J: Inhibition of TLR4
signalling-induced inflammation attenuates secondary injury after
diffuse axonal injury in rats. Mediators Inflamm. 2016:47069152016.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Li XQ, Lv HW, Tan WF, Fang B, Wang H and
Ma H: Role of the TLR4 pathway in blood-spinal cord barrier
dysfunction during the bimodal stage after ischemia/reperfusion
injury in rats. J Neuroinflammation. 11:622014. View Article : Google Scholar : PubMed/NCBI
|