Introduction
Retinoblastoma (RB) is the most common intraocular
malignant tumor in pediatric patients <5 years old (1,2).
Furthermore, RB has an incidence rate of 1 in 15,000-20,000 live
births (3). Currently, the primary
approaches for the clinical treatment of RB include laser
photo-coagulation, chemotherapy and radiotherapy (4–6).
Despite substantial improvements in the treatment of RB, the
survival of patients remains poor (7). Thus, it is important to investigate
novel therapeutic targets to facilitate the development of
treatments for RB.
Previous studies have shown that microRNAs (miRNAs/
miRs) are potential therapeutic targets for tumors (8–11).
miRNAs are small endogenous non-coding RNAs, 18–22 nucleotides in
length, that can modulate mRNA translation via binding to the
3′-untranslated region (3′-UTR) of their target genes (12). A variety of miRNAs have been
reported to serve oncogenic or anti-tumor roles in RB cells by
regulating cell proliferation, apoptosis, migration, invasion and
the cell cycle (9–11). miR-106b promotes the proliferation,
migration and invasion of RB cells by inhibiting Zinc Finger and
BTB Domain Containing 4 (13). In
addition, miR-101-3p suppresses the proliferation of RB cells by
targeting Enhancer of zeste homolog 2 and Histone deacetylase 9
(14). Exogenous miR-34a inhibits
cell proliferation and increases the apoptotic activity of RB cells
(15). However, the specific
regulatory role of miR-34a on the chemosensitivity of RB cells is
not fully understood.
The anti-tumor potential of miR-34a is closely
associated with the regulation of various signaling pathways, such
as the Wnt/β-Catenin (16),
PI3K/AKT/survivin (17) and Notch
signaling pathways (18). The
Notch signaling pathway is a highly conserved pathway that is
involved in the regulation of several fundamental cellular
processes, such as cell proliferation, stem cell maintenance and
differentiation (19).
Furthermore, the Notch family of proteins, which consists of
Notch1, Notch2 and Notch3, serve a key regulatory role in RB
(20). Li et al (21) indicated that miR-433 suppresses
cell proliferation and metastasis in RB by inhibiting Notch1.
However, whether the regulatory effect of miR-34a on RB is related
with the Notch signaling pathway is unknown.
The present study investigated the regulatory
effects of miR-34a on the proliferation and chemosensitivity of RB
cells, as well as the related regulatory mechanism involving the
Notch signaling pathway. It was found that miR-34a may inhibit the
proliferation, and promote the apoptosis and chemosensitivity of RB
cells by downregulating Notch1. Therefore, the present study may
provide a novel theoretical basis for the treatment of RB.
Materials and methods
Cell culture
The immortalized human normal retinal vascular
endothelial cell line ACBRI-181, and the RB cell lines HXO-RB44 and
Y79 were obtained from the American Type Culture Collection. Cells
were grown in RPMI-1640 (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% FBS (BD Biosciences) and 1%
penicillin/streptomycin (Gibco; Thermo Fisher Scientific, Inc.).
All cells were maintained in a humidified 5% CO2
atmosphere at 37°C.
Dual-luciferase reporter gene
assay
TargetScan software (22) was used to predict the targeting
relationship between miR-34a and Notch1. The 3′-UTR containing the
miR-34a binding site of Notch1 was amplified and cloned into
Psi-CHECK2 reporter vector (Promega Corporation) to construct
wild-type (WT) Psi-CHECK2-WT-Notch1-3′-UTR (Notch1-WT) and mutant
(MUT) Psi-CHECK2-MUT-Notch1-3′-UTR (Notch1-MUT). For the luciferase
assay, miR-34a mimics or miR-34a negative control (NC) mimics (20
nmol/l) were co-transfected with reporter plasmids (20 nmol/l) into
HXO-RB44 and Y79 cells using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.). HXO-RB44 and Y79
cells were grouped as follows: i) MUT + mimics group, transfected
with Notch1-MUT and miR-34a mimics; ii) MUT + NC group, transfected
with Notch1-MUT and miR-34a NC mimics; iii) WT + mimics group,
transfected with Notch1-WT and miR-34a mimics; and iv) WT + NC
group, transfected with Notch1-WT and miR-34a NC mimics. Luciferase
activity was assessed using a dual-luciferase kit (Promega
Corporation) after 48 h of transfection, and was normalized to
Renilla luciferase activity.
Cell transfection and experimental
grouping
miR-34a mimics, Notch1 small interfering RNAs
(Notch1 siRNA-1 and −2) and their corresponding NC (miR-34a NC and
Notch1 siRNA NC) were supplied by Shanghai GenePharma Co., Ltd. The
sequences were: miR-34a mimics forward,
5′-UGGCAGUGUCUUAGCUGGUUGU-3′ and reverse,
5′-ACAACCAGCUAAGACACUGCCA-3′; miR-34a NC forward,
5′-UCACAACCUCCUAGAAAGAGUAGA-3′ and reverse,
5′-UCUACUCUUUCUAGGAGGUUGUGA-3′; Notch 1 siRNA-1 forward,
5′-CACCAGUUUGAAUGGUCAAdTdT-3′ and reverse,
5′-UUGACCAUUCAAACUGGUGTdTd-3′; Notch 1 siRNA-2 forward,
5′-UGGCGGGAAGUGUGAAGCGdTdT-3′ and reverse,
5′-CGCUUCACACUUCCCGCCATdTd-3′; Notch 1 siRNA NC forward,
5′-UUCUCCGAACGUGUCACGUTT-3′ and reverse,
5′-ACGUGACACGUUCGGAGAATt-3′. These substances (20 nmol/l) were
transfected into HXO-RB44 and Y79 cells using
Lipofectamine® 2000 reagent (Invitrogen; Thermo Fisher
Scientific, Inc.). HXO-RB44 and Y79 cells were randomly divided
into five groups: i) BLANK, cells without transfection; ii) miR-NC
+ si-NC, transfected with miR-34a NC and Notch1 siRNA NC; iii)
miR-34a mimics + si-NC, transfected with miR-34a mimics and Notch1
siRNA NC; iv) miR-NC + Notch1 siRNA, transfected with miR-34a NC
and Notch1 siRNA-1; and v) miR-34a mimics + Notch1 siRNA,
transfected with miR-34a mimics and Notch1 siRNA-1. All cells were
cultured for 48 h at 37°C with 5% CO2. Some HXO-RB44 and
Y79 cells (1×105 cells/well) were further treated with
40 µM Z-VAD (Selleckhchem) for 48 at 37°C with 5% CO2.
Cells were randomly divided into four groups: i) Z-VAD; ii) Z-VAD +
miR-34a mimics; iii) Z-VAD + Notch1 siRNA; and iv) Z-VAD + miR-34a
mimics + Notch1 siRNA.
Cell Counting Kit-8 (CCK-8) assay
The viability of HXO-RB44 and Y79 cells was detected
using a CCK-8 assay kit (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol. Cells were seeded
into 96-well plates (2×103 cells/well), and cultured for
24, 48, 72 or 96 h at 37°C with 5% CO2. CCK-8 solution
(10 µl) was then added to each well and the cells were incubated at
37°C for 2 h. The absorbance of each well was measured at 450 nm
using a microplate reader.
5-Bromo-2-deoxyuridine (BrdU)
assay
The proliferation of HXO-RB44 and Y79 cells was
measured using a BrdU kit (6813; Cell Signaling Technology, Inc.).
Cells were seeded into 96-well plates (1×104 cells/well)
and incubated overnight at 37°C. Subsequently, cells were labeled
with 10 µM BrdU solution for 3 h at 25°C, denatured with 100 µl
FixDenta solution for 30 min at 25°C and incubated with
peroxidase-conjugated anti-BrdU antibody (6813; 1:1,000; Cell
Signaling Technology, Inc.) for 1.5 h at 25°C. The absorbance of
each well was measured at 450 nm using a microplate reader.
Flow cytometry assay
The apoptotic rate of HXO-RB44 and Y79 cells was
examined using an Annexin V-FITC apoptosis detection kit (KGA108-1,
Nanjing KeyGen Biotech Co., Ltd.). Cells were washed three times
with PBS, suspended in 500 µl binding buffer, and incubated with 5
µl Annexin V-FITC and 5 µl PI for 15 min in the dark at room
temperature. Cell apoptosis was detected using a Cytomics FC500
flow cytometer (Beckman Coulter, Inc.), and the data were analyzed
by CytoDiff CXP software (version 2.0, https://www.beckmancoulter.com, Beckman Coulter, Inc.)
(23).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from ACBRI-181, HXO-RB44 and
Y79 cells using TRIzol® (Invitrogen; Thermo Fisher
Scientific, Inc.). A total of 500 ng RNA was then RT into cDNA with
a Revert Aid First Strand cDNA Synthesis kit at 42°C for 45 min
(Thermo Fisher Scientific, Inc.). RT-qPCR was performed on a
RT-qPCR instrument (Bio-Rad Laboratories, Inc.) with SYBR green
qPCR Master mix (Thermo Fisher Scientific, Inc.). The program
included 94°C for 3 min, 40 cycles of 94°C for 15 sec and 60°C for
30 sec. Primers used for RT-qPCR were as follows: miR-34a forward,
5′-GCCACTATGTAGCGGGTTTC-3′ and reverse, 5′-ACCTGCGCTAAGAACTGAGG-3′;
Notch1 forward, 5′-TCAACGCCGTAGATGACCT-3′ and reverse,
5′-TCTCCTCCCTGTTGTTCTGC-3′; Notch1 siRNA-1 forward,
5′-CAUGGUAGUCACUAACAUATT-3′ and reverse,
5′-UAUGUUAGUGACUACCAUGTT-3′; Notch1 siRNA-2 forward,
5′-GCACGCGGAUUAAUUUGCCA-3′ and reverse, 5′-UGCAAAUUAAUCCGCGUGC-3′;
si-NC forward, 5′-UUCUCCGAACGUGUCACGUTT-3′ and reverse,
5′-ACGUGACACGUUCGGAGAATT-3′; U6 forward, 5′-CTCGCTTCGGCAGCACA-3′
and reverse, 5′-ACGCTTCACGAATTTGCGT-3′; and GADPH forward,
5′-AGCCACATCGCTCAGACA-3′ and reverse, 5′-TGGACTCCACGACGTACT-3′.
Relative expression level was calculated by the 2−ΔΔCq
method (24).
Western blot analysis
Total proteins were extracted from HXO-RB44 and Y79
cells using RIPA lysis buffer (Beyotime Institute of
Biotechnology). Protein concentration was measured with a
bicinchoninic acid kit (Yeasen Biotechnology Co., Ltd.). Protein
samples (30 µg) were subjected to 10% SDS-PAGE and then transferred
to a nitrocellulose membrane. After being blocked with 5% skim milk
for 30 min at 37°C, the membrane was incubated with the following
primary antibodies: Notch1 (14-5785-81; 1:1,000; Chemicon
International; Thermo Fisher Scientific, Inc.), p16 (101169-T38;
1:1,000; Sino Biological, Inc.), proliferating cell nuclear antigen
(PCNA, 101118-T46; 1:1,000, Sino Biological, Inc.), Bcl-2
(ab185002; 1:1,000; Abcam), Bax (2774s; 1:1,000; Cell Signaling
Technology, Inc.), matrix metalloproteinase (MMP)-9 (3852s;
1:1,000; Cell Signaling Technology, Inc.), cleaved caspase-3
(9661s; 1:1,000; Cell Signaling Technology, Inc.) and GAPDH
(ab8245; 1:1,000; Abcam) at 4°C overnight. The membrane was
subsequently incubated with peroxidase-labeled secondary antibody
(anti-rabbit IgG; ab6721; 1:5,000; Abcam) at room temperature for 1
h. The protein blots were visualized using an enhanced
chemiluminescence kit (Invitrogen; Thermo Fisher Scientific, Inc.).
The density of western blot bands was analyzed using Quantity One
1-D Analysis software (version 4.6.9, Bio-Rad Laboratories,
Inc.).
Evaluation of drug sensitivity
HXO-RB44 and Y79 cells were seeded into 96-well
plates at a density of 4×103/ml. When the cells had
adhered, carboplatin (CBP; Sigma-Aldrich; Merck KGaA) was added
into each well at a concentration of 0, 3, 6, 9 or 12 µg/ml.
Subsequently, the cells were cultured for 48 h at 37°C. Then, CCK-8
reaction solution (10 µl) was added into each well and the cells
were incubated at 37°C for 2 h. The absorbance of each well was
measured at 450 nm using a microplate reader. The inhibition rate
(IR; %) was calculated according to the following formula: IR (%) =
(1-AMedicine/AControl)x100%. The CBP
concentration that caused the death of half of HXO-RB44 or Y79
cells was calculated, and considered the IC50.
Statistical analysis
All statistical analyses were performed using SPSS
version 22.0 (IBM Corp.). All experiments were repeated three
times. Data are presented as the mean ± standard deviation. A
one-way ANOVA followed by Tukey's post hoc test was used to analyze
the differences among multiple groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
miR-34a is downregulated and Notch1 is
upregulated in RB cells
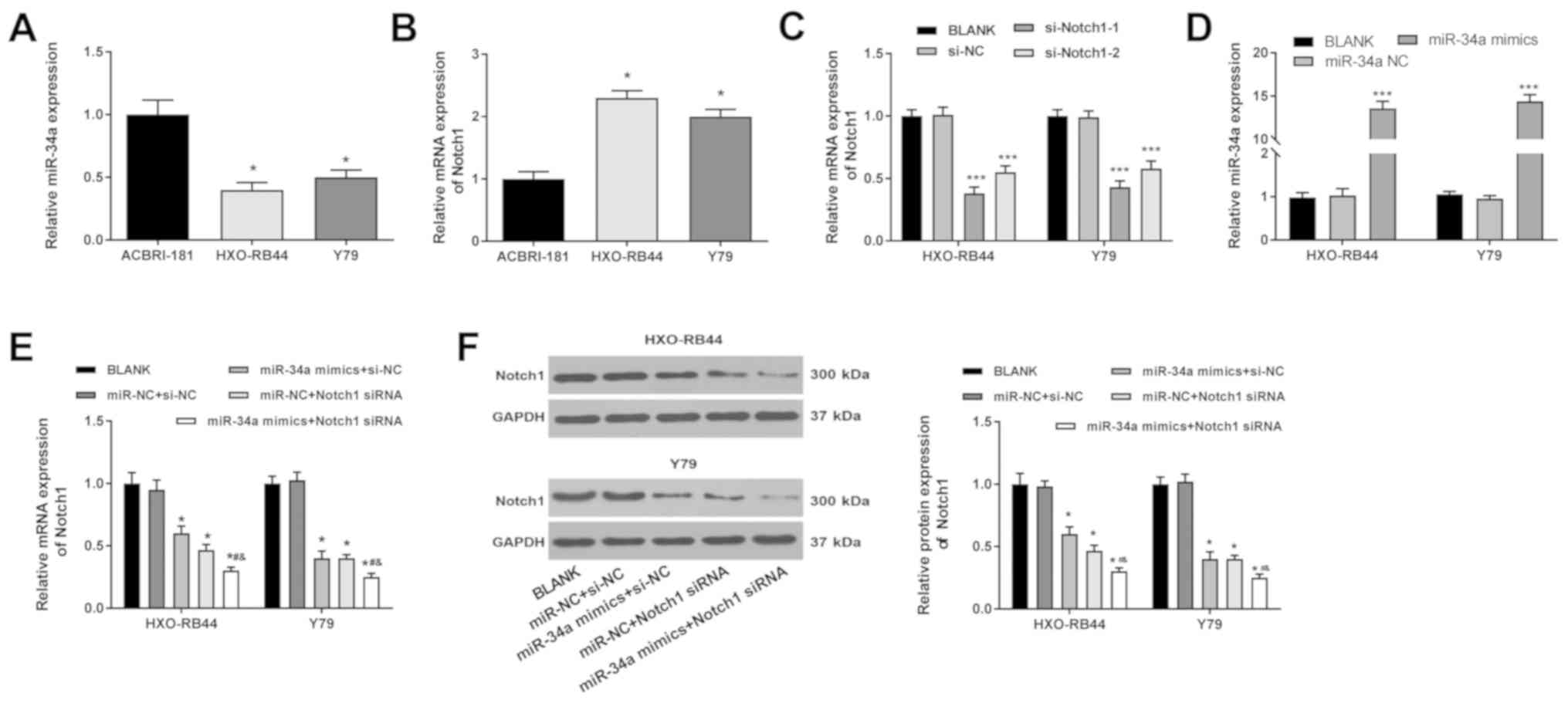
The expression levels of miR-34a and Notch1 in
ACBRI-181, HXO-RB44 and Y79 cells were detected using RT-qPCR. It
was found that the mRNA expression level of miR-34a was
significantly lower in HXO-RB44 and Y79 cells compared with
ACBRI-181 cells (P<0.05; Fig.
1A). However, the mRNA expression level of Notch1 in HXO-RB44
and Y79 cells was significantly higher compared with ACBRI-181
cells (P<0.05; Fig. 1B).
miR-34a downregulates Notch1 in RB
cells
Notch1 was silenced in HXO-RB44 and Y79 cells via
the transfection of si-Notch1-1 and si-Notch1-2. The RT-qPCR
results indicated that that the expression levels of Notch1 was
significantly decreased by si-Notch1-1 and si-Notch1-2 (P<0.001;
Fig. 1C). In addition, miR-34a was
overexpressed in HXO-RB44 and Y79 cells via the transfection of
miR-34a mimics. The RT-qPCR results showed that the mRNA expression
levels of miR-34a were significantly increased by miR-34a mimics in
HXO-RB44 and Y79 cells (P<0.001; Fig. 1D). Furthermore, the effect of
miR-34a mimics on the expression levels of Notch1 was analyzed
using si-Notch1-1. Compared with the BLANK group, the mRNA and
protein expression levels of Notch1 in HXO-RB44 and Y79 cells were
significantly decreased by miR-34a mimics and Notch1 siRNA
transfections (P<0.05). In addition, it was found that Notch1
was further downregulated in the miR-34a mimics + Notch1 siRNA
group compared with the miR-NC + Notch 1 siRNA group (P<0.05;
Fig. 1E and F).
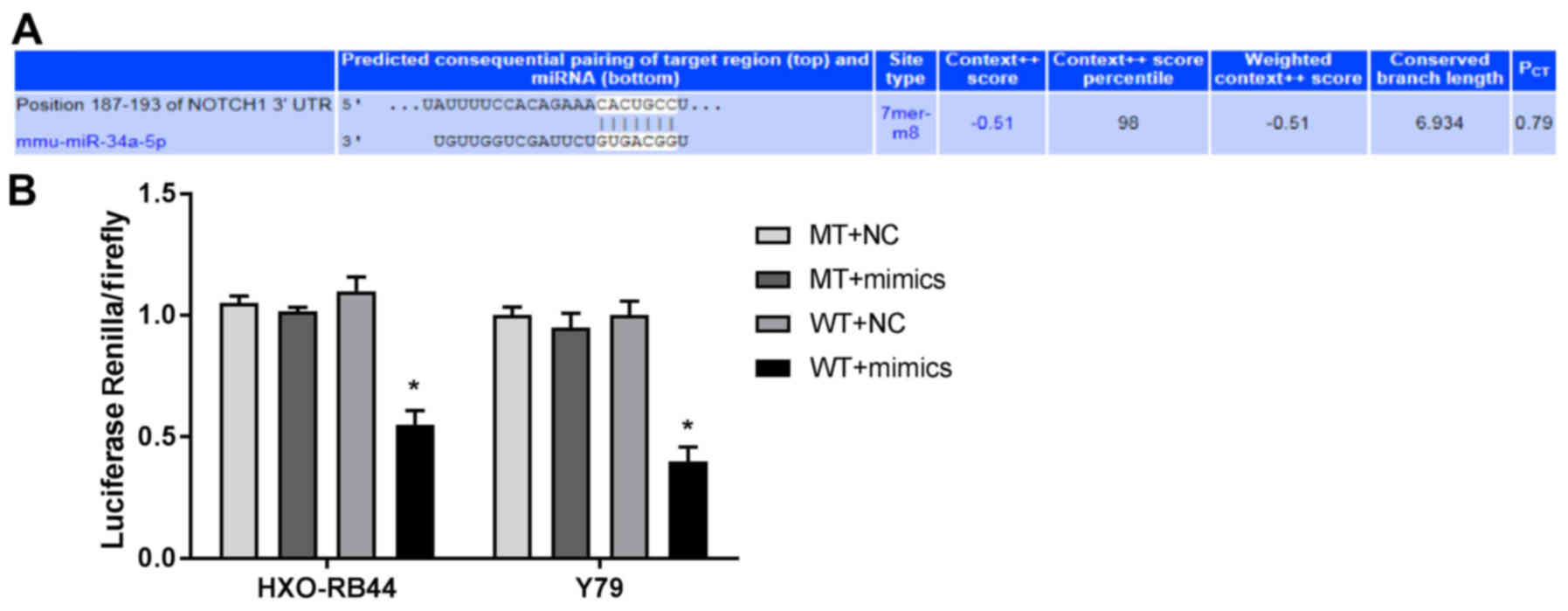
Notch1 is the target gene of
miR-34a
TargetScan analysis predicted that the binding site
of Notch1 to miR-34a was at the 3′-UTR region (Fig. 2A). Based on the result that the
Notch1 promoter contains the putative miR-34a binding site, the
target relationship between Notch1 and miR-34a was further analyzed
by dual-luciferase reporter gene assay. It was demonstrated that
the luciferase activity of the WT + mimics group was significantly
decreased compared with the WT + NC group (P<0.05; Fig. 2B).
miR-34a upregulation and Notch1
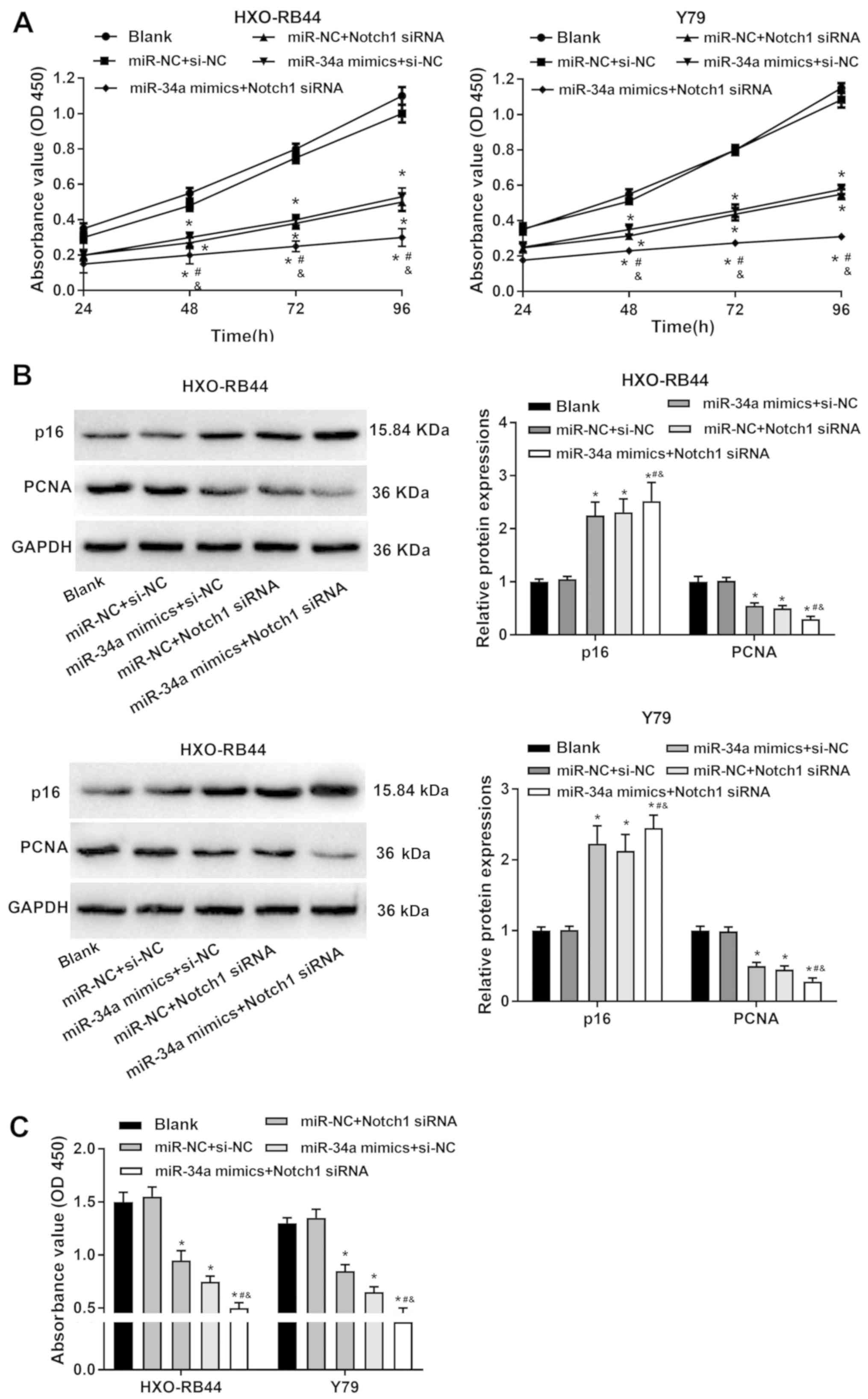
downregulation inhibit the proliferation of RB cells
The viability of HXO-RB44 and Y79 cells was detected
with a CCK-8 assay. The viability of HXO-RB44 and Y79 cells was
significantly decreased by the transfection of miR-34a mimics and
Notch1 siRNA compared with the BLANK group (P<0.05; Fig. 3A). Furthermore, the expression
levels of the proliferation-related proteins p16 and PCNA were
measured using western blotting. Compared with the BLANK group,
PCNA expression levels were significantly decreased, and p16
expression levels were significantly increased by the transfection
of miR-34a mimics and Notch1 siRNA in HXO-RB44 and Y79 cells
(P<0.05; Fig. 3B). The
proliferation of RB cells was further analyzed using a BrdU assay.
Compared with the BLANK group, the optical density (OD) 450 values
of HXO-RB44 and Y79 cells were significantly decreased by miR-34a
mimics and Notch1 siRNA (P<0.05; Fig. 3C). Furthermore, it was found that
the transfection of miR-34a mimics + Notch1 siRNA decreased cell
viability, upregulated p16 expression, downregulated PCNA
expression and decreased OD450 values of HXO-RB44 and Y79 cells,
compared with the transfection of miR-34a mimics and Notch1 siRNA
alone (P<0.05; Fig. 3A-C).
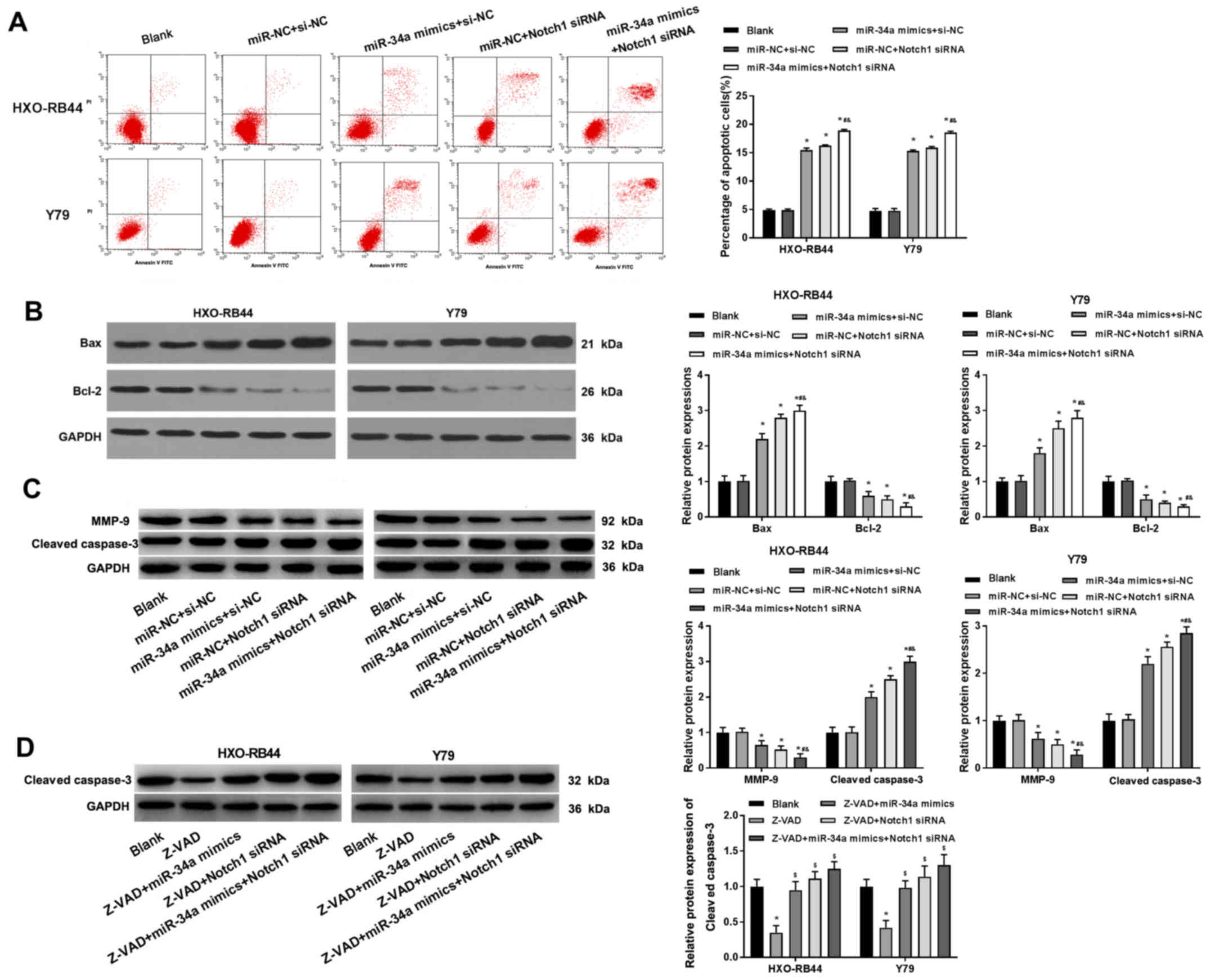
miR-34a upregulation and Notch1
downregulation promotes the apoptosis of RB cells
The apoptotic rate of HXO-RB44 and Y79 cells was
detected using Annexin/PI double staining. It was demonstrated that
the transfection of miR-34a mimics and Notch1 siRNA alone
significantly increased the percentage of apoptotic cells compared
with the BLANK group (P<0.05). Furthermore, the transfection of
miR-34a mimics + Notch1 siRNA further increased the percentage of
apoptotic cells (P<0.05; Fig.
4A). The expression levels of the apoptosis-related proteins
Bax, Bcl-2, MMP-9 and cleaved caspase-3 were measured by western
blotting. Compared with the BLANK group, the transfection of
miR-34a mimics and Notch1 siRNA alone significantly upregulated Bax
and cleaved caspase-3 expression, and downregulated Bcl-2 and MMP-9
expression (P<0.05). Furthermore, it was found that the changes
in expression of these apoptosis-related proteins were more
significant in cells transfected with miR-34a mimics + Notch1
siRNA, compared with those transfected with miR-34a mimics and
Notch1 siRNA alone (P<0.05; Fig. 4B
and C). In addition, the inhibiting effect of Z-VAD, a caspase
inhibitor, on cleaved caspase-3 expression level was significantly
reversed by the transfection of miR-34a mimics and Notch1 siRNA
alone, and particularly by the transfection of miR-34a mimics +
Notch1 siRNA (P<0.05; Fig.
4D).
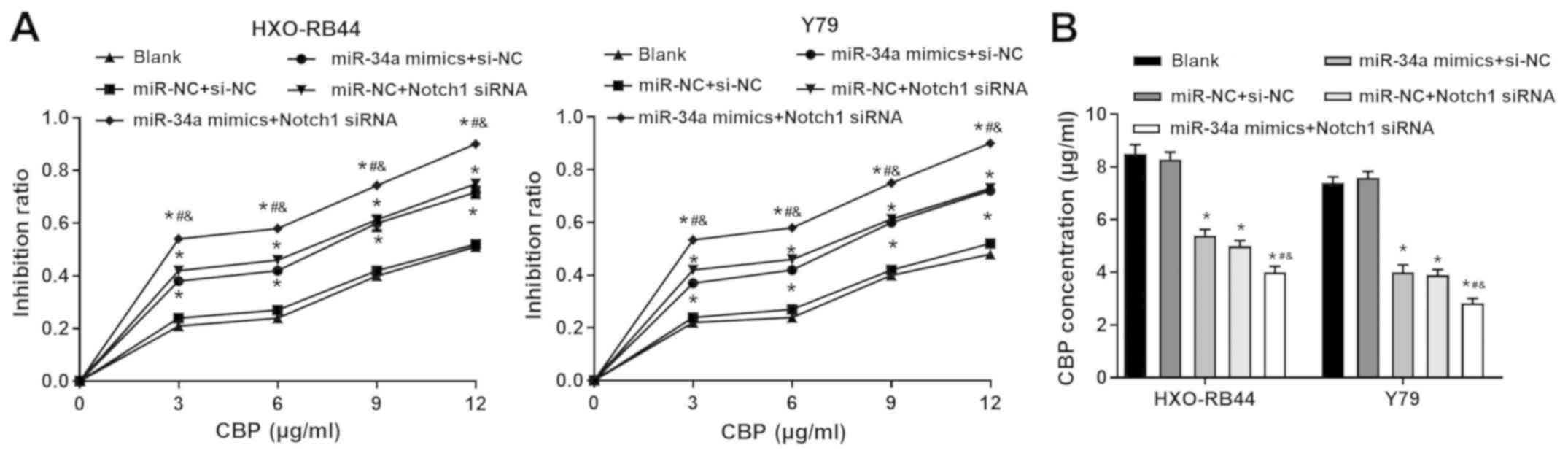
miR-34a upregulation and Notch1
downregulation increases the sensitivity of RB cells to CBP
The sensitivity of HXO-RB44 and Y79 cells to CBP was
evaluated by CKK-8 assay. Compared with the BLANK group, the
transfection of miR-34a mimics and Notch1 siRNA alone significantly
increased the IR of cells treated with different concentrations of
CBP. Furthermore, it was demonstrated that the transfection of
miR-34a mimics + Notch1 siRNA further increased the IR (P<0.05;
Fig. 5A). These results suggest
that the CBP IC50 was significantly lower in cells
transfected with miR-34a mimics and Notch1 siRNA alone compared
with the BLANK group. The CBP IC50 was lowest in cells
transfected with miR-34a mimics + Notch1 siRNA compared with the
different groups (P<0.05; Fig.
5B).
Discussion
miRNAs serve key regulatory roles in the occurrence
and development of RB (25,26).
It has been previously reported that miR-34a expression levels are
decreased in breast cancer, cervical cancer, pancreatic cancer,
cholangiocarcinoma and RB (15,27–29).
In relation to this, the results of the present study showed that
the expression levels of miR-34a were significantly lower in RB
cell lines compared with human normal retinal vascular endothelial
cells. Therefore, the present results suggested that miR-34a is
downregulated in RB, which is consistent with the aforementioned
previous studies. In addition, previous studies suggested that
miR-34a acts as a potential tumor suppressor by modulating cell
proliferation, apoptosis, migration and invasion (15). miR-34a suppresses colorectal cancer
metastasis by attenuating cell migration and invasion (30). In addition, miR-34a significantly
decreases the proliferation rate and increases the apoptotic rate
of gastric cancer cells (31).
Exogenous miR-34a also inhibits the proliferation and promotes the
apoptosis of RB cells (15). In
the present study, it was found that miR-34a inhibited the
proliferation and promoted the apoptosis of HXO-RB44 and Y79 cells.
Therefore, the present results are consistent with previous
studies, and demonstrated that miR-34a may be a potential
therapeutic target for RB.
The Notch signaling pathway has been identified as a
potential therapeutic target for a variety of cancer types,
including pancreatic cancer (32),
hepatocellular carcinoma (33) and
RB (20). In addition, the Notch
family of proteins serve vital roles in retinal development
(34). During early retinal
development in mammals, Notch1 and Notch3 are primarily present in
the central portion of the retina, and Notch2 is predominantly
expressed in the retinal periphery and pigment epithelial cells
(35,36). A previous study showed that Notch1
expression levels were significantly higher compared with Notch2
and Notch3 expression levels in Y79 cells (37). The present results suggested that
Notch1 was upregulated in RB cells compared with human normal
retinal vascular endothelial cells, consistent with the results
from a previous study, which identified that Notch1 is highly
expressed in human RB cells (20).
Furthermore, previous studies showed that Notch1 is a target of
miR-34a (38–40). miR-34a inhibits cell invasion via
the downregulation of Notch1 in cervical carcinoma and
choriocarcinoma (38). In
addition, miR-34a inhibits the proliferation, and induces the
apoptosis of glioblastoma cells by targeting Notch1 (39). miR-34a inhibits the proliferation
and invasion of endometrial cancer cells by downregulating Notch1
(40). In line with previous
studies, the present results indicated that Notch1 may be a target
gene of miR-34a, and that Notch1 may be downregulated by miR-34a
upregulation. In addition, downregulation of Notch1 inhibited the
proliferation and promoted apoptosis of HXO-RB44 and Y79 cells.
Thus, Notch1 may act as an oncogene in RB (37). In addition, miR-34a-induced
downregulation of Notch1 may contribute to the anti-tumor response.
Thus, miR-34a may inhibit the proliferation and promote the
apoptosis of RB cells by downregulating Notch1.
Pediatric patients with RB are primarily treated
with chemotherapy using drugs such as CBP, vincristine and
etoposide (ETO) (41). However,
drug resistance greatly limits the prognosis of patients with RB
(42,43). Previous studies have revealed that
miR-34a can, to a certain extent, increase the chemosensitivity of
RB cells (44,45). miR-34a downregulation elevates the
survival rate and viability of RB cells following carboplatin,
adriamycin and vincristine treatment (44). Furthermore, miR-34a restores ETO
and CBP chemosensitivity, and increases the apoptosis of RB cells
(45). The present results
suggested that miR-34a increased the IR and CBP IC50 of
HXO-RB44 and Y79 cells. This result is consistent with previous
studies, and indicates that miR-34a promotes the chemosensitivity
of RB cells to CBP treatment. In addition, miR-34a increases the
chemosensitivity of breast cancer stem cells to Paclitaxel by
downregulating Notch1 (46).
miR-34a sensitizes the chemosensitivity of breast cancer cells to
adriamycin by targeting Notch1 (47). As Notch1 is a target gene of
miR-34a, the present results suggested that miR-34a may improve the
chemosensitivity of RB cells by downregulating Notch1, and thus aid
in the treatment of RB.
In conclusion, it was found that miR-34a was
downregulated and Notch1 was upregulated in HXO-RB44 and Y79 cells.
In addition, miR-34a upregulation inhibited the proliferation,
promoted the apoptosis and enhanced the CBP chemosensitivity of RB
cells by downregulating Notch1. The present results provide a novel
regulatory mechanism of miR-34a in RB cells, and may facilitate the
treatment of RB as overexpression of miR-34a may be a potential
therapeutic strategy.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets generated and/or analyzed during the
current study are not publicly available due to research related to
future studies, but are available from the corresponding author on
reasonable request.
Authors' contributions
SZ was responsible for the conception and design of
the study, and development of the manuscript. FG and WY performed
the experiments, and were involved in the collection and
supervision of data. WY participated in drafting the manuscript or
revising it critically for important intellectual content. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Dimaras H, Dimba EA and Gallie BL:
Challenging the global retinoblastoma survival disparity through a
collaborative research effort. Br J Ophthalmol. 94:1415–1416. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mahoney MC, Burnett WS, Majerovics A and
Tanenbaum H: The epidemiology of ophthalmic malignancies in New
York State. Ophthalmology. 97:1143–1147. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ortiz MV and Dunkel IJ: Retinoblastoma. J
Child Neurol. 31:227–236. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shields CL and Shields JA: Retinoblastoma
management: Advances in enucleation, intravenous chemoreduction,
and intra-arterial chemotherapy. Curr Opin Ophthalmol. 21:203–212.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Assayag F, Nicolas A, Vacher S, Dehainault
C, Bieche I, Meseure D, Aerts I, Cassoux N, Houdayer C, Doz F, et
al: Combination of Carboplatin and Bevacizumab Is an Efficient
Therapeutic Approach in Retinoblastoma Patient-Derived Xenografts.
Invest Ophthalmol Vis Sci. 57:4916–4926. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shehata HH, Abou Ghalia AH, Elsayed EK,
Ahmed Said AM and Mahmoud SS: Clinical significance of high levels
of survivin and transforming growth factor beta-1 proteins in
aqueous humor and serum of retinoblastoma patients. J AAPOS. 20:444
e1–444 e9. 2016. View Article : Google Scholar
|
|
7
|
Kivelä T: The epidemiological challenge of
the most frequent eye cancer: Retinoblastoma, an issue of birth and
death. Br J Ophthalmol. 93:1129–1131. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
de Carvalho IN, de Freitas RM and Vargas
FR: Translating microRNAs into biomarkers: What is new for
pediatric cancer? Med Oncol. 33:492016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fabian MR, Sonenberg N and Filipowicz W:
Regulation of mRNA translation and stability by microRNAs. Annu Rev
Biochem. 79:351–379. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu Y, Zhou Y, Gong X and Zhang C:
MicroRNA-30a-5p inhibits the proliferation and invasion of gastric
cancer cells by targeting insulin-like growth factor 1 receptor.
Exp Ther Med. 14:173–180. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chandra S, Vimal D, Sharma D, Rai V, Gupta
SC and Chowdhuri DK: Role of miRNAs in development and disease:
Lessons learnt from small organisms. Life Sci. 185:8–14. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jin D and Lee H: Prioritizing
cancer-related microRNAs by integrating microRNA and mRNA datasets.
Sci Rep. 6:353502016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bu W, Wang Y and Min X: MicroRNA-106b
promotes the proliferation, migration and invasion of
retinoblastoma cells by inhibiting the expression of ZBTB4 protein.
Exp Ther Med. 16:4537–4545. 2018.PubMed/NCBI
|
|
14
|
Jin Q, He W, Chen L, Yang Y, Shi K and You
Z: MicroRNA-101-3p inhibits proliferation in retinoblastoma cells
by targeting EZH2 and HDAC9. Exp Ther Med. 16:1663–1670.
2018.PubMed/NCBI
|
|
15
|
Dalgard CL, Gonzalez M, deNiro JE and
O'Brien JM: Differential microRNA-34a expression and tumor
suppressor function in retinoblastoma cells. Invest Ophthalmol Vis
Sci. 50:4542–4551. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Si W, Li Y, Shao H, Hu R, Wang W, Zhang K
and Yang Q: MiR-34a Inhibits Breast Cancer Proliferation and
Progression by Targeting Wnt1 in Wnt/β-Catenin Signaling Pathway.
Am J Med Sci. 352:191–199. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cao W, Yang W, Fan R, Li H, Jiang J, Geng
M, Jin Y and Wu Y: miR-34a regulates cisplatin-induce gastric
cancer cell death by modulating PI3K/AKT/survivin pathway. Tumour
Biol. 35:1287–1295. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang XP, Zhou J, Han M, Chen CB, Zheng YT,
He XS and Yuan XP: MicroRNA-34a regulates liver regeneration and
the development of liver cancer in rats by targeting Notch
signaling pathway. Oncotarget. 8:13264–13276. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kidd S, Lockett TJ and Young MW: The Notch
locus of Drosophila melanogaster. Cell. 34:421–433. 1983.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xiao W, Chen X and He M: Inhibition of the
Jagged/Notch pathway inhibits retinoblastoma cell proliferation via
suppressing the PI3K/Akt, Src, p38MAPK and Wnt/β catenin signaling
pathways. Mol Med Rep. 10:453–458. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li X, Yang L, Shuai T, Piao T and Wang R:
MiR-433 inhibits retinoblastoma malignancy by suppressing Notch1
and PAX6 expression. Biomed Pharmacother. 82:247–255. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nam JW, Rissland OS, Koppstein D,
Abreu-Goodger C, Jan CH, Agarwal V, Yildirim MA, Rodriguez A and
Bartel DP: Global analyses of the effect of different cellular
contexts on microRNA targeting. Mol Cell. 53:1031–1043. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Faucher JL, Lacronique-Gazaille C, Frébet
E, Trimoreau F, Donnard M, Bordessoule D, Lacombe F and Feuillard
J: “6 markers/5 colors” extended white blood cell differential by
flow cytometry. Cytometry A. 71:934–944. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Castro-Magdonel BE, Orjuela M, Camacho J,
García-Chéquer AJ, Cabrera-Muñoz L, Sadowinski-Pine S,
Durán-Figueroa N, Orozco-Romero MJ, Velázquez-Wong AC,
Hernández-Ángeles A, et al: miRNome landscape analysis reveals a 30
miRNA core in retinoblastoma. BMC Cancer. 17:4582017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Montoya V, Fan H, Bryar PJ, Weinstein JL,
Mets MB, Feng G, Martin J, Martin A, Jiang H and Laurie NA: Novel
miRNA-31 and miRNA-200a-Mediated Regulation of Retinoblastoma
Proliferation. PLoS One. 10:e01383662015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lodygin D, Tarasov V, Epanchintsev A,
Berking C, Knyazeva T, Körner H, Knyazev P, Diebold J and Hermeking
H: Inactivation of miR-34a by aberrant CpG methylation in multiple
types of cancer. Cell Cycle. 7:2591–2600. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang X, Xie Y and Wang J: Overexpression
of MicroRNA-34a-5p Inhibits Proliferation and Promotes Apoptosis of
Human Cervical Cancer Cells by Downregulation of Bcl-2. Oncol Res.
26:977–985. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kwon H, Song K, Han C, Zhang J, Lu L, Chen
W and Wu T: Epigenetic Silencing of miRNA-34a in Human
Cholangiocarcinoma via EZH2 and DNA Methylation: Impact on
Regulation of Notch Pathway. Am J Pathol. 187:2288–2299. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang X, Ai F, Li X, Tian L, Wang X, Shen
S and Liu F: MicroRNA-34a suppresses colorectal cancer metastasis
by regulating Notch signaling. Oncol Lett. 14:2325–2333. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Deng X, Zheng H, Li D, Xue Y, Wang Q, Yan
S, Zhu Y and Deng M: MicroRNA-34a regulates proliferation and
apoptosis of gastric cancer cells by targeting silent information
regulator 1. Exp Ther Med. 15:3705–3714. 2018.PubMed/NCBI
|
|
32
|
Ponnurangam S, Dandawate PR, Dhar A,
Tawfik OW, Parab RR, Mishra PD, Ranadive P, Sharma R, Mahajan G,
Umar S, et al: Quinomycin A targets Notch signaling pathway in
pancreatic cancer stem cells. Oncotarget. 7:3217–3232. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ke X, Zhao Y, Lu X, Wang Z, Liu Y, Ren M,
Lu G, Zhang D, Sun Z, Xu Z, et al: TQ inhibits hepatocellular
carcinoma growth in vitro and in vivo via repression of Notch
signaling. Oncotarget. 6:32610–32621. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mitter D, Ullmann R, Muradyan A,
Klein-Hitpass L, Kanber D, Ounap K, Kaulisch M and Lohmann D:
Genotype-phenotype correlations in patients with retinoblastoma and
interstitial 13q deletions. Eur J Hum Genet. 19:947–958. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lindsell CE, Boulter J, diSibio G, Gossler
A and Weinmaster G: Expression patterns of Jagged, Delta1, Notch1,
Notch2, and Notch3 genes identify ligand-receptor pairs that may
function in neural development. Mol Cell Neurosci. 8:14–27. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bao ZZ and Cepko CL: The expression and
function of Notch pathway genes in the developing rat eye. J
Neurosci. 17:1425–1434. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Asnaghi L, Tripathy A, Yang Q, Kaur H,
Hanaford A, Yu W and Eberhart CG: Targeting Notch signaling as a
novel therapy for retinoblastoma. Oncotarget. 7:70028–70044. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Pang RT, Leung CO, Ye TM, Liu W, Chiu PC,
Lam KK, Lee KF and Yeung WS: MicroRNA-34a suppresses invasion
through downregulation of Notch1 and Jagged1 in cervical carcinoma
and choriocarcinoma cells. Carcinogenesis. 31:1037–1044. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Li WB, Ma MW, Dong LJ, Wang F, Chen LX and
Li XR: MicroRNA-34a targets notch1 and inhibits cell proliferation
in glioblastoma multiforme. Cancer Biol Ther. 12:477–483. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang Z, Wang W, Huang K, Wang Y, Li J and
Yang X: MicroRNA-34a inhibits cells proliferation and invasion by
downregulating Notch1 in endometrial cancer. Oncotarget.
8:111258–111270. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Jo DH, Lee K and Kim JH, Jun HO, Kim Y,
Cho YL, Yu YS, Min JK and Kim JH: L1 increases adhesion-mediated
proliferation and chemoresistance of retinoblastoma. Oncotarget.
8:15441–15452. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Rao R and Honavar SG: Retinoblastoma.
Indian J Pediatr. 84:937–944. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Saakyan SV, Tsygankov АY, Moiseeva NI,
Karamysheva АF, Zhil'tsova MG and Tadevosyan SS: Retinoblastoma
Cell Culturing and Evaluation of Their Drug Resistance. Bull Exp
Biol Med. 165:148–153. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yang G, Fu Y, Lu X, Wang M, Dong H and Li
Q: miR 34a regulates the chemosensitivity of retinoblastoma cells
via modulation of MAGE A/p53 signaling. Int J Oncol. 54:177–187.
2019.PubMed/NCBI
|
|
45
|
Liu K, Huang J, Xie M, Yu Y, Zhu S, Kang
R, Cao L, Tang D and Duan X: MIR34A regulates autophagy and
apoptosis by targeting HMGB1 in the retinoblastoma cell. Autophagy.
10:442–452. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kang L, Mao J, Tao Y, Song B, Ma W, Lu Y,
Zhao L, Li J, Yang B and Li L: MicroRNA-34a suppresses the breast
cancer stem cell-like characteristics by downregulating Notch1
pathway. Cancer Sci. 106:700–708. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Li XJ, Ji MH, Zhong SL, Zha QB, Xu JJ,
Zhao JH and Tang JH: MicroRNA-34a modulates chemosensitivity of
breast cancer cells to adriamycin by targeting Notch1. Arch Med
Res. 43:514–521. 2012. View Article : Google Scholar : PubMed/NCBI
|