Introduction
Myocardial infarction is defined worldwide as one of
the most common cardiovascular diseases with an estimated number of
cases of more than 600,000 in the US (1). A stress environment, such as
oxidative stress and hypoxia, is thought to be a significant
inducement for the irreversible damage to myocardial cells
(2). Therefore, maintaining the
normal function and prolonging the survival of myocardial cells are
of great importance for the heart (1,3).
Hypoxia induces the death of myocardial cells mainly by triggering
apoptosis (4–6). Strategies that can effectively
suppress cell apoptosis alleviate hypoxia-induced cell death.
Diosmetin (3′,5,7-trihydroxy-4′-methoxy flavone) is
the aglycone of the flavonoid glycoside diosmin, which is abundant
in Citrus species, olive leaves and spermine (7). Studies have demonstrated that
diosmetin exhibits various medicinal properties including
anticancer (8), anti-microbial
(9), anti-inflammatory (10) and anti-oxidant (11) activities. Diosmetin was found to
reduce secretion of TNF-α, thus decreasing the inflammatory level,
and the anti-oxidant effect by diosmetin was mainly ascribed to the
upregulation of superoxide dismutase (11). The anticancer effect of diosmetin
was reportedly related to its regulation of cell apoptosis
(8). Studies of hepatocellular
carcinoma and prostate cancer revealed that diosmetin functions as
a tumor inhibitor mainly by inducing apoptosis (8,12,13).
However, the effect of diosmetin on apoptosis is not consistent.
Zhang et al (14)
documented that diosmetin suppresses neuronal apoptosis. Yet, the
role of diosmetin in cardiomyocytes has not yet been
investigated.
Autophagy is characterized as a survival mechanism
that is responsible for removal of misfolded or wrongly assembled
proteins, clearance of damaged organelles and elimination of
intracellular pathogens (15). The
relationship between autophagy and apoptosis has previously been
discussed. Nikoletopoulou et al (16) summarized that autophagy and
apoptosis may antagonize or assist each other, which is possibly
attributed to the interplay among core factors involved in both
processes (17). Autophagy is a
process that can be accurately regulated by multiple signals
including the nuclear factor (NF)-κB pathway (10), the p53/Bcl-2 pathway (17) and BECN1/adenosine
5′-monophosphate-activated protein kinase (AMPK) (18,19),
which indicates an inconsistent role of autophagy in different
diseases or diseases with different stages (15).
The present study was designed with an aim to
explore the effects of diosmetin on hypoxia-injured myocardial
cells. Subsequently, the potential involvement of autophagy in the
diosmetin-mediated effects was focused on. Then the activity of
AMPK signaling in diosmetin-treated cells was assessed to elucidate
the intrinsic molecular mechanisms.
Materials and methods
Cell culture and treatment
The cardiomyocyte cell line H9c2 derived from the
rat was purchased from the American Type Culture Collection (ATCC,
Manassas, VA, USA). Cells were maintained in DMEM (HyClone
Laboratories Inc./GE Healthcare) as recommended. All medium used
were supplemented with 10% fetal bovine serum (FBS; HyClone
Laboratories Inc./GE Healthcare) and cells were routinely cultured
in a humidified incubator at 37°C under 5% CO2.
H9c2 cells at 70–80% confluence were maintained in
serum-free medium with low glucose for 12 h for cell starvation.
Subsequently, 5, 10 or 15 µg/ml diosmetin (Selleck Chemicals)
dissolved in DMSO, 5 mM 3-methyladenine (3-MA; Selleck Chemicals)
dissolved in PBS or 10 µM compound C (Selleck Chemicals) dissolved
in DMSO were added to the cell cultures immediately after serum
starvation. The cells were then cultured in normoxic conditions
(74% N2, 5% CO2 and 21% O2) for 1
h prior to being cultured in hypoxic conditions (94% N2,
5% CO2 and 1% O2) for 48 h, a duration which
was frequently used in several previous studies (20,21).
As the non-treated group (NT), cells were maintained in normoxic
conditions for the duration of the experiment.
Cell viability assay
For determination of cell viability, H9c2 cells
exposed to hypoxia or the non-treated group were plated into a flat
bottom 96-well plate at 4×103 cells per well in
triplicate with 100 µl medium. Ten microliters of Cell Counting
Kit-8 reagent (CCK-8; Dojindo) was added into each well. After
incubation for 2 h, optical density (OD) value at the wavelength of
450 nm was measured using a microplate reader (Bio-Rad
Laboratories, Inc.).
Cell apoptosis assay
Cell apoptosis was detected using the Annexin V-FITC
Apoptosis Detection Kit (Calbiochem/EMD/Merck KGaA) according to
the manufacturer's instructions.
Western blot analysis
Cell lysates were prepared using RIPA lysis buffer
(Cell Signaling Technology) supplemented with protease inhibitor
and phosphatase inhibitors for protein extraction. Concentration of
total protein was determined by the Pierce Coomassie (Bradford)
Protein Assay Kit (Thermo Fisher Scientific, Inc.). Then protein
samples (20 µg) were resolved on 8% SDS-PAGE and transferred to
polyvinylidene difluoride membranes. The rinsed membranes were
incubated with primary antibodies targeting cleaved caspase-3 (cat.
no. 9654), phospho-AMPKα (cat. no. 2531), AMPK (cat. no. 5832),
phospho-ULK1 (cat. no. ab229537), ULK1 (cat. no. 8054) or β-actin
(cat. no. 4970) at 4°C overnight. After washing, the membrane was
incubated with a secondary antibody conjugated with horseradish
peroxidase (cat. no. 7074). The signals were developed using Super
Signal West Dura Extended Duration chemiluminescence substrate
(Thermo Fisher Scientific, Inc.) and measured by ChemiDoc™ XRS+
System (Bio-Rad Laboratories, Inc.). All primary antibodies and
secondary antibodies were purchased from Cell Signaling Technology
except for phospho-ULK1 antibody which was purchased from Abcam.
All antibodies were used in a 1:1,000 dilution as recommended by
the supplier.
Autophagy detection
Cell autophagy was detected using a
CYTO-ID® Autophagy detection kit (Enzo Life Sciences)
following the manufacturer's instructions. Briefly, H9c2 cells were
harvested and washed with PBS. Cells were then stained with CYTO-ID
green fluorescence regents for 30 min at 37°C. After being washed
with assay buffer provided in the CYTO-ID® Autophagy
detection kit, cells were fixed with 4% paraformaldehyde in PBS for
20 min. Cells labeled with fluorescence were analyzed by flow
cytometry and photographed using a Leica DM6000B fluorescence
microscope (Leica Microsystems, Inc.). Higher fluorescence
intensity indicated a higher level of autophagy.
Autophagy LC3 HiBiT Reporter
Assay
An Autophagy LC3 HiBiT Reporter Assay System
(Promega) provides a quick, efficient and common method with which
to assess the effects of compounds on autophagy. The effects of
diosmetin on autophagy were confirmed using this assay. Briefly,
293T cells provided by this system were routinely cultured with
DMEM supplemented with 10% FBS and 500 µg/ml G418 (Selleck
Chemicals). For the assays, 293T cells were plated into an opaque,
white tissue-culture 96-well plate at 2×104 cells per
well in triplicate. After incubation for 24 h, the cells were
treated with diosmetin at different concentrations (5, 10 and 15
µg/ml); cells treated with an equal amount of solvent were as
control. After incubation at 37°C for another 48 h, the
luminescence signal was detected with an EnVision 2105 Multimode
Plate Reader (PerkinElmer) following the manufacturer's
instructions. The luminescence signal of treated cells was
normalized to the control. Three independent experiments were
performed.
Statistical analysis
Statistical analysis was performed using SPSS 19.0
(SPSS, Inc.). Data are presented as a mean ± standard deviation of
three parallel experiments. One-way ANOVA analysis was used for
comparison of more than two groups, followed by Newman-Keuls post
hoc test. A P-value <0.05 was considered to indicate a
statistically significant difference.
Results
Diosmetin alleviates hypoxia-induced
myocardial apoptosis
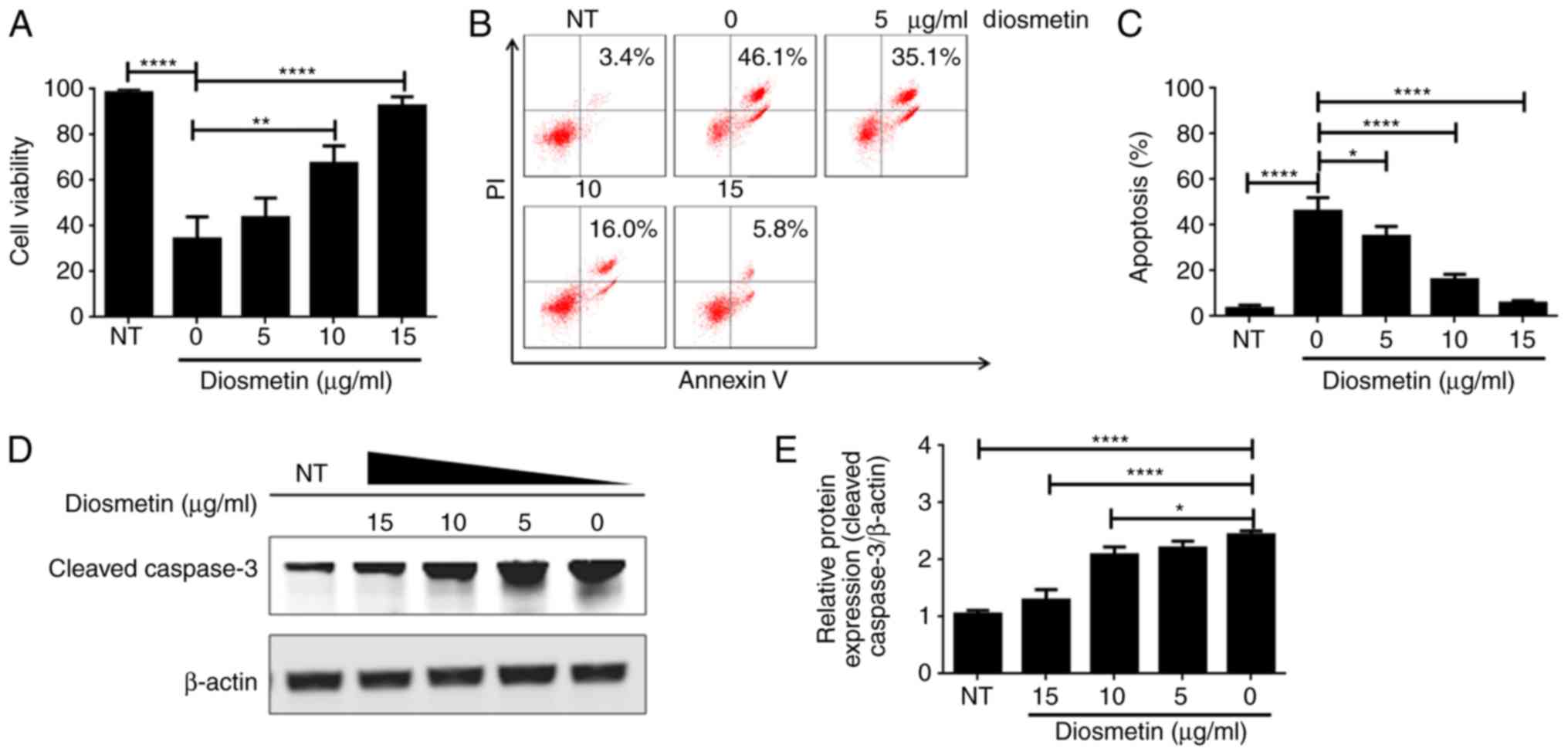
To ascertain the effects of diosmetin on
hypoxia-induced injury in cardiomyoblasts, H9c2 cells were treated
with gradient dilutions of diosmetin (5, 10, 15 µg/ml) before
exposure to hypoxia. Results of the CCK-8 assay indicated that cell
viability was significantly lower in the cells exposed to hypoxia
when compared to the cells not exposed to hypoxia (NT) (Fig. 1A). It was also observed that cell
viability was obviously higher in the cells treated with 10 and 15
µg/ml diosmetin than that in cells exposed only to hypoxia among
all hypoxia-treated groups (Fig.
1A). These data demonstrated that exposure to hypoxia decreased
cell survival, while diosmetin protected H9c2 cells from
hypoxia-induced decrease in cell survival in a dose-dependent
manner. Furthermore, cell apoptosis was detected by PI/Annexin V
staining assay. The results revealed that exposure to hypoxia
significantly induced H9c2 apoptosis, resulting in considerable
double-labeled population (46.1%, Fig.
1B). Notably, the double-labeled apoptotic population was 35.1,
16.0 and 5.8 in the cells pretreated with 5, 10 and 15 µg/ml
diosmetin, respectively (Fig. 1B and
C). Moreover, western blot analysis showed that hypoxia
upregulated expression of cleaved caspase-3, while the upregulated
cleaved caspase-3 was effectively suppressed when cells were
treated with diosmetin at increasing concentrations (Fig. 1D and E). Collectively, diosmetin
may protect H9c2 cells from hypoxia-induced cell death via
alleviating hypoxia-induced cell apoptosis in a dose-dependent
manner.
Cell autophagy induced by
diosmetin
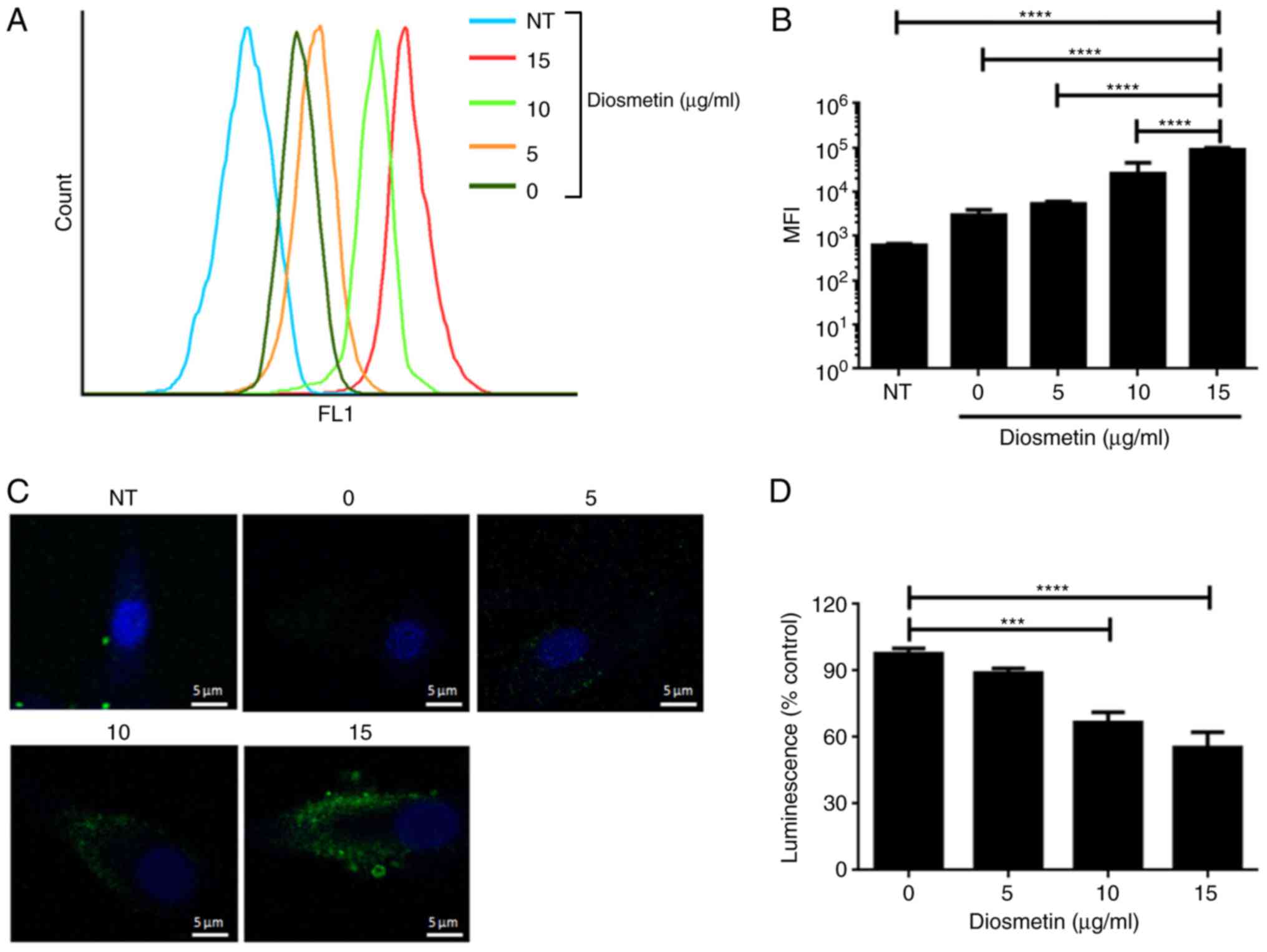
The effects of diosmetin on cell autophagy were also
investigated in the present study. CYTO-ID® Autophagy
detection kit was used to detect cell autophagy, providing a rapid,
specific and quantitative approach for monitoring autophagy. As
shown in Fig. 2A-C, exposure to
hypoxia induced slight cell autophagy which was subsequently
strengthened by additional treatment of diosmetin in a
dose-dependent manner. An Autophagy LC3 HiBiT Reporter Assay System
was used to investigate whether diosmetin functions as an autophagy
inducer or inhibitor. Currently, the direct effects of diosmetin on
cell autophagy were estimated using this reporter assay system. The
results indicated that luminescence intensity was comparably lower
in cells which were treated with 10 and 15 µg/ml diosmetin
(Fig. 2D), confirming the role of
diosmetin as an autophagy inducer in a dose-dependent manner.
Autophagy inhibition by
3-methyladenine neutralizes the protective effects of diosmetin on
hypoxia-injured cells
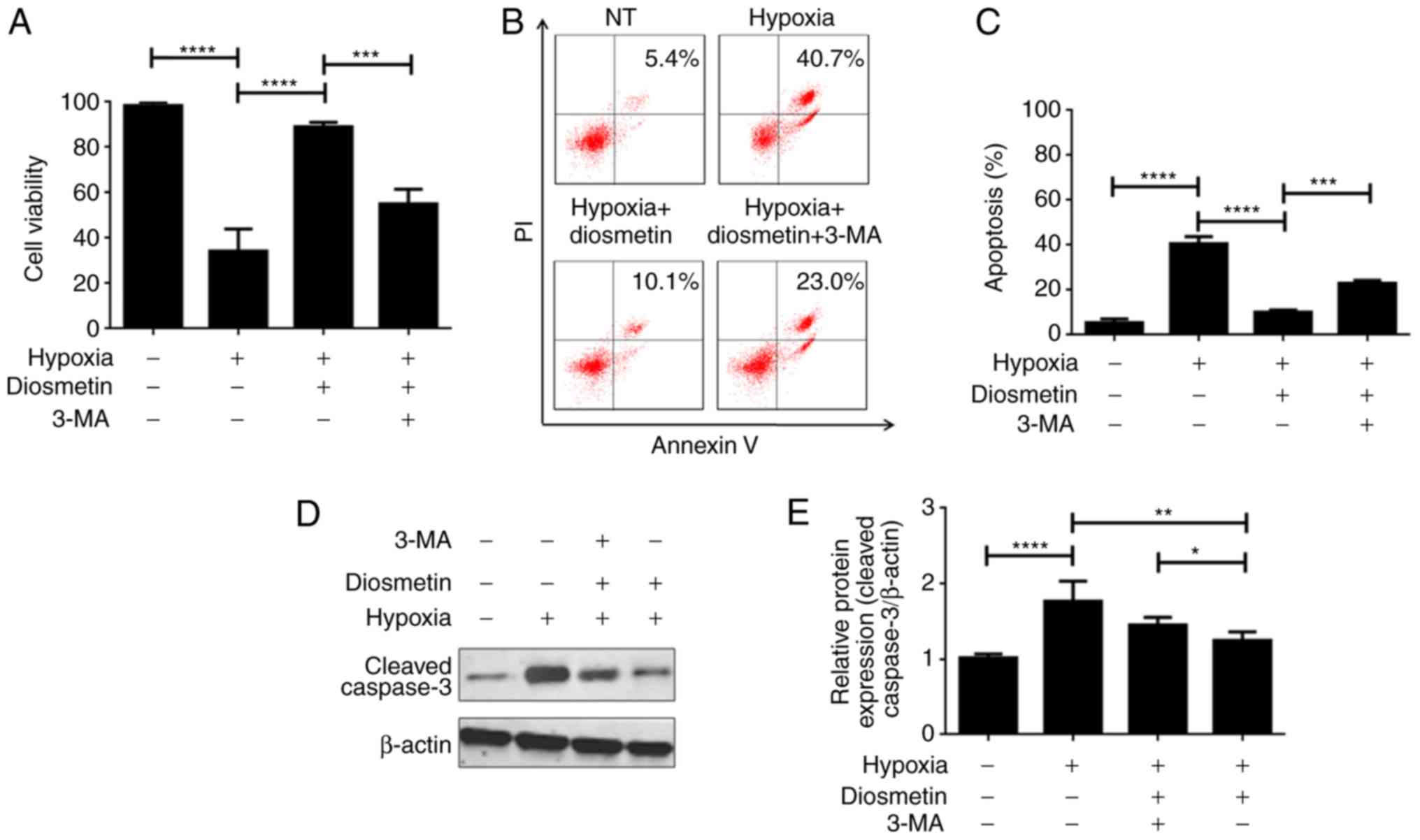
3-Methyladenine (3-MA) is a well-studied autophagy
inhibitor. 3-MA was used as a cell autophagy inhibitor to confirm
whether diosmetin-induced autophagy is essential in maintaining its
protective effect on cell survival. Results of the CCK-8 assay
illustrated that diosmetin treatment significantly enhanced the
cell viability of the hypoxia-injured cells, which was previously
evidenced. Subsequently, it was demonstrated that an additional
treatment of 3-MA observably decreased cell viability in the
hypoxia-injured cells treated with diosmetin (Fig. 3A). It can be concluded that the
protective effects of diosmetin on hypoxia-injured cells were
suppressed by autophagy inhibition; namely, autophagy is an
indispensable mechanism that mediates the protection of diosmetin
in regards to cell proliferation. apoptosis analysis also revealed
that 3-MA partially reversed apoptosis inhibition induced by
diosmetin (Fig. 3B and C). Cleaved
caspase-3 was also upregulated in cells treated with diosmetin and
3-MA as compared to cells only treated with diosmetin (Fig. 3D and E). The above findings imply
that autophagy is the main mechanism responsible for
diosmetin-induced proliferation protection.
Activation of AMPK by diosmetin
results in induction of autophagy and inhibition of apoptosis
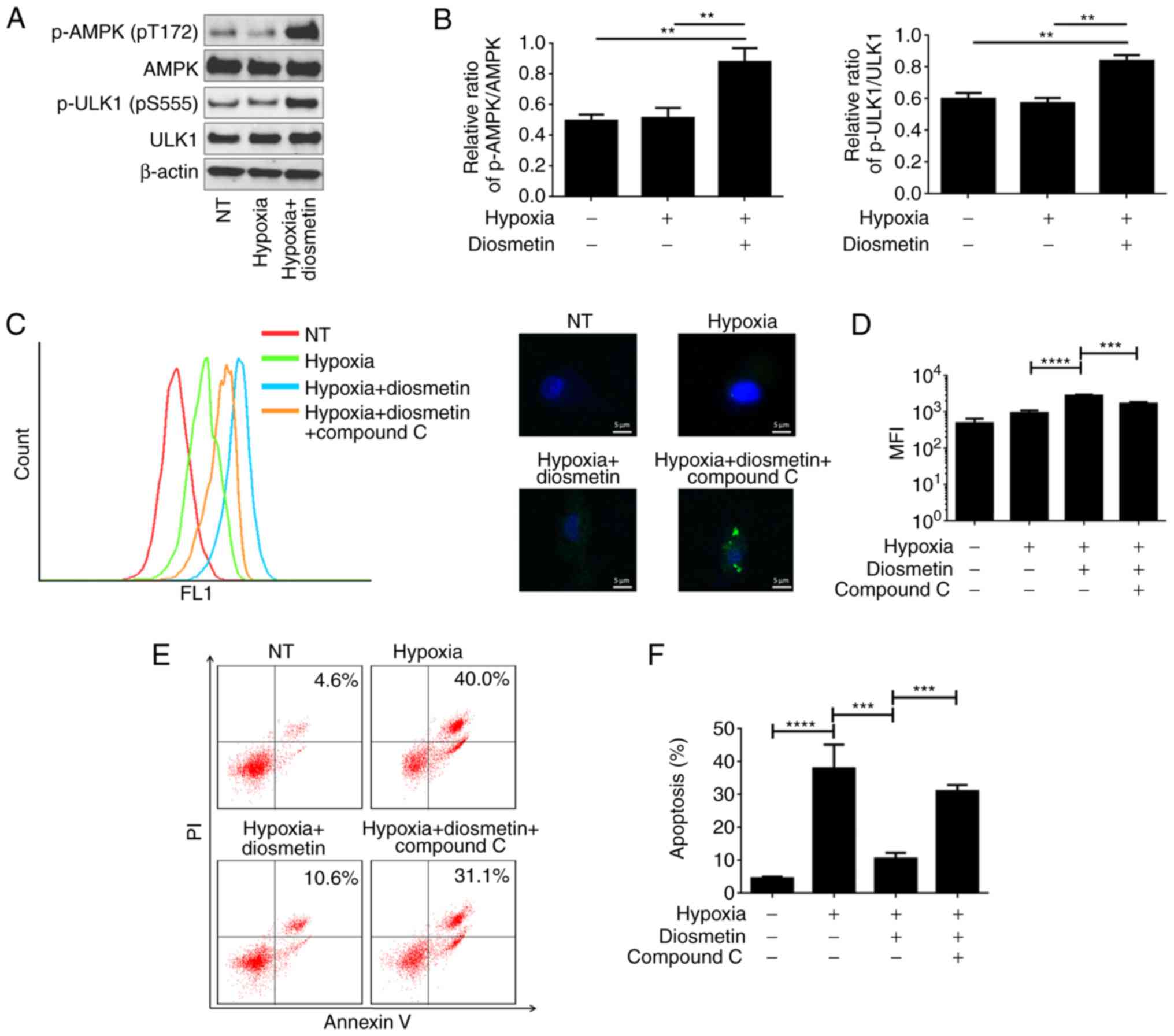
Activation of Unc-51 like autophagy activating
kinase 1 (ULK1), a kinase that is importantly involved in cell
autophagy, can be regulated by adenosine 5′-monophosphate-activated
protein kinase (AMPK) in a direct or even an indirect manner
(19). Therefore, we aimed to
ascertain whether the AMPK signaling pathway is involved in
diosmetin-induced autophagy and apoptosis inhibition.
Phosphorylated (p)-AMPK, AMPK, p-ULK1 and ULK1 were detected by
western blot analysis. The results indicated that the levels of
p-AMPK and p-ULK1 in hypoxia-injured cells were significantly
increased by diosmetin (Fig. 4A and
B). Compound C, an effective and widely used AMPK inhibitor,
was used together with diosmetin in cells before hypoxia treatment.
Cell autophagy was detected using CYTO-ID® Autophagy
detection kit in hypoxia-injured cells treated with diosmetin alone
or combined with compound C. It was found that additional
administration of compound C indeed significantly attenuated
autophagy as compared to treatment with diosmetin alone (Fig. 4C and D). PI/Annexin V staining
assay also showed that the apoptotic population in the
hypoxia-injured cells treated with diosmetin was increased from
10.6 to 31.1% when compound C was additionally applied (Fig. 4E and F). Based on the observed
results, it was proposed that diosmetin-induced autophagy and
apoptosis inhibition were partially mediated by activation of
AMPK.
Discussion
Myocardial infarction is globally recognized as one
of the most common cardiovascular diseases consistently threatening
human health (3). Despite the fact
that various advanced strategies have been developed, myocardial
infarction is still diagnosed with a continually increasing
incidence and high mortality rate (22,23).
Thus, effective treatment strategies are urgently needed. Recent
studies have established that myocardial infarction is mainly
caused by ischemia or hypoxia which may further result in
myocardial cell apoptosis (2,24).
Based on these mechanistic findings, studies have reported several
compounds as potential and promising candidates for myocardial
infarction treatment. Canstatin and atorvastatin were respectively
found to improve myocardial infarction via attenuation of cell
apoptosis (3,25). Diosmetin has recently received
extensive concerns for its anti-oxidative, anti-inflammatory and
anti-apoptotic effects in various diseases (8,10,11,26).
Hence, diosmetin was investigated as a potential candidate for
improvement of myocardial infarction caused by hypoxia.
The results of the present study revealed that
diosmetin efficiently protected and increased the cell viability of
H9c2 cells by inhibiting apoptosis indicated by decreased cleaved
caspase-3. It was further found that diosmetin promoted cell
autophagy, although hypoxia induced slight autophagy. Autophagy is
characterized as a conserved process involving the degradation of
cytoplasmic components (15).
Previous research has demonstrated a significant relationship
between cell autophagy and apoptosis. Zhang et al (27) reported that hypoxia-induced
apoptosis is negatively correlated with autophagy. Another
investigation demonstrated that attenuation of hypoxia-induced
cardiomyocyte apoptosis by overexpressed BAG3 was mainly ascribed
to induction of autophagy (21).
To ascertain whether induction of autophagy is indispensable for
diosmetin-associated protection from apoptosis, 3-MA, an autophagy
inhibitor, was used to suppress autophagy. Similar to previous
studies (21,27,28),
the present results revealed that inhibition of autophagy indeed
compromised the protective effects of diosmetin, indicating an
essential role of autophagy in the diosmetin-associated protection
from apoptosis. The role of autophagy, described as a double-edged
sword, has been recently proposed in several reviews which
explained that autophagy may also lead to cell death under certain
conditions (16,17). Thus, it can be seen that the
interplay between autophagy and apoptosis is more complex than
imaged and is of great significance for cell fate decision when
facing stress conditions. In the present study, a hypoxia duration
of 48 h was used to successfully induce cell death. However, the
determination of autophagy and apoptosis in cells treated with
hypoxia for different durations is of great significance for a full
understanding of autophagy and apoptosis under hypoxia.
ULK1, a protein kinase, is required for autophagy
and has been reported as a substrate of adenosine
monophosphate-activated protein kinase (AMPK) (29). AMPK is frequently known as a sensor
that can be activated in response to environmental stress like
hypoxia (19,30). A previous study described that AMPK
is positively involved in mitophagy regulation under chronic
hypoxia (31). In the present
study, it was found that AMPK was activated in cells receiving
diosmetin prior to exposure to hypoxia. Appropriately, we
hypothesized that diosmetin-induced autophagy is mediated by the
AMPK/ULK1 signaling pathway. Therefore, an AMPK inhibitor (compound
C) was used to evaluate the role of AMPK in diosmetin-induced
autophagy. As expected, compound C treatment suppressed
diosmetin-induced autophagy as well as the inhibitive effects on
apoptosis. In fact, research demonstrated that single exposure to
hypoxia was able to activate AMPK in H9c2 cells (31); however, this was not observed in
our study. The different observations might be attributed to the
slightly discrepant methods of hypoxia exposure in the two
articles. Even so, slight autophagy was currently observed in cells
exposed to hypoxia only. Presumably, autophagy as a protective
mechanism is synchronously induced when cells are exposed to
hypoxia. While ULK1 is not the only autophagy-associated protein
that can be regulated by the AMPK signaling pathway. Beclin1, which
plays a vital role in autophagy nucleation and elongation, was
found to be regulated by AMPK (18). Diosmetin has been demonstrated to
influence activation of the PI3K/Akt and NF-κB signaling pathways
(10,14,32).
The present study is the first to report that diosmetin is involved
in regulation of the AMPK signaling pathway.
The in vitro evaluation of diosmetin as a
promising drug candidate has been conducted in several diseases,
among which diosmetin may function as an inhibitor of inflammation,
an inducer or an inhibitor of apoptosis (11,14,26,32).
Existing research suggests that diosmetin may ameliorate the
severity of chronic asthma and pancreatitis by anti-inflammatory
action (10,33). Investigations of the effects of
diosmetin on several types of cancer suggest that diosmetin
inhibits the proliferation of prostate cancer and hepatoma cells
via induction of apoptosis and cell cycle arrest (8,12,13).
A study assessed the effects of diosmetin on kidney injury and
revealed that diosmetin was able to prevent ischemia-induced acute
kidney injury via restraining apoptosis (34). Meanwhile, our present study was the
first to investigate diosmetin in light of its protective role in
promoting cardiomyocyte survival under a hypoxic environment. To
date, diosmetin is a candidate agent with high potential in disease
treatment. However, systemic and detailed research regarding the
mechanisms related to its observed effects is limited. Apoptosis is
not the only mechanism that may lead to hypoxia-induced cell death
(16); other mechanisms will be
given advanced attention in future investigations by our group.
Mitochondrial membrane potential, oxygen consumption rate,
endoplasmic reticulum stress, ATP generation rate and glycolysis
(16) are suitable methods with
which to comprehensively ascertain the mechanisms mediating
diosmetin-associated protection of myocardial cell survival.
In conclusion, the present study revealed that
diosmetin may protect myocardial cells from hypoxia-mediated cell
death by inducing autophagy. Further investigation demonstrated
that AMPK is involved in the regulation of diosmetin-induced
autophagy. This study suggests diosmetin as a drug candidate for
myocardial infarction treatment, and the results regarding AMPK
contribute to the clinical application of diosmetin.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
NZ was involved in the conception and design of the
study. QS, YS and DH were responsible for the data acquisition,
analysis and interpretation. QS wrote the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Li R, Geng HH, Xiao J, Qin XT, Wang F,
Xing JH, Xia YF, Mao Y, Liang JW and Ji XP: miR-7a/b attenuates
post-myocardial infarction remodeling and protects H9c2
cardiomyoblast against hypoxia-induced apoptosis involving Sp1 and
PARP-1. Sci Rep. 6:290822016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Huang Y, Chen JB, Yang B, Shen H, Liang JJ
and Luo Q: RhoA/ROCK pathway regulates hypoxia-induced myocardial
cell apoptosis. Asian Pac J Trop Med. 7:884–888. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cheng WP, Lo HM, Wang BW, Chua SK, Lu MJ
and Shyu KG: Atorvastatin alleviates cardiomyocyte apoptosis by
suppressing TRB3 induced by acute myocardial infarction and
hypoxia. J Formos Med Assoc. 116:388–397. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu B, Che W, Xue J, Zheng C, Tang K,
Zhang J, Wen J and Xu Y: SIRT4 prevents hypoxia-induced apoptosis
in H9c2 cardiomyoblast cells. Cell Physiol Biochem. 32:655–662.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Luo S, Gu X, Ma F, Liu C, Shen Y, Ge R and
Zhu Y: ZYZ451 protects cardiomyocytes from hypoxia-induced
apoptosis via enhancing MnSOD and STAT3 interaction. Free Radic
Biol Med. 92:1–14. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ashok A, Kanwar JR, Krishnan UM and Kanwar
RK: SurR9C84A protects and recovers human cardiomyocytes from
hypoxia induced apoptosis. Exp Cell Res. 350:19–31. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Patel K, Gadewar M, Tahilyani V and Patel
DK: A review on pharmacological and analytical aspects of
diosmetin: A concise report. Chin J Integr Med. 19:792–800. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Oak C, Khalifa AO, Isali I, Bhaskaran N,
Walker E and Shukla S: Diosmetin suppresses human prostate cancer
cell proliferation through the induction of apoptosis and cell
cycle arrest. Int J Oncol. 53:835–843. 2018.PubMed/NCBI
|
|
9
|
Chan BC, Ip M, Gong H, Lui SL, See RH,
Jolivalt C, Fung KP, Leung PC, Reiner NE and Lau CB: Synergistic
effects of diosmetin with erythromycin against ABC transporter
over-expressed methicillin-resistant Staphylococcus aureus (MRSA)
RN4220/pUL5054 and inhibition of MRSA pyruvate kinase.
Phytomedicine. 20:611–614. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yu G, Wan R, Yin G, Xiong J, Hu Y, Xing M,
Cang X, Fan Y, Xiao W, Qiu L, et al: Diosmetin ameliorates the
severity of cerulein-induced acute pancreatitis in mice by
inhibiting the activation of the nuclear factor-κB. Int J Clin Exp
Pathol. 7:2133–2142. 2014.PubMed/NCBI
|
|
11
|
Yang Y, Gong XB, Huang LG, Wang ZX, Wan
RZ, Zhang P, Zhang QY, Chen Z and Zhang BS: Diosmetin exerts
anti-oxidative, anti-inflammatory and anti-apoptotic effects to
protect against endotoxin-induced acute hepatic failure in mice.
Oncotarget. 8:30723–30733. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu B, Shi Y, Peng W, Zhang Q, Liu J, Chen
N and Zhu R: Diosmetin induces apoptosis by upregulating p53 via
the TGF-β signal pathway in HepG2 hepatoma cells. Mol Med Rep.
14:159–164. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Qiao J, Liu J, Jia K, Li N, Liu B, Zhang Q
and Zhu R: Diosmetin triggers cell apoptosis by activation of the
p53/Bcl-2 pathway and inactivation of the Notch3/NF-κB pathway in
HepG2 cells. Oncol Lett. 12:5122–5128. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang Y, Jiang Y and Lu D: Diosmetin
suppresses neuronal apoptosis and inflammation by modulating the
phosphoinositide 3-kinase (PI3K)_AKT_nuclear factor-κB (NF-κB)
signaling pathway. Med Sci Monit. 25:82019.
|
|
15
|
Glick D, Barth S and Macleod KF:
Autophagy: Cellular and molecular mechanisms. J Pathol. 221:3–12.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nikoletopoulou V, Markaki M, Palikaras K
and Tavernarakis N: Crosstalk between apoptosis, necrosis and
autophagy. Biochim Biophys Acta. 1833:3448–3459. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fan YJ and Zong WX: The cellular decision
between Apoptosis and autophagy. Chin J Cancer. 32:121–129.
2013.PubMed/NCBI
|
|
18
|
Zhang D, Wang W, Sun X, Xu D, Wang C,
Zhang Q, Wang H, Luo W, Chen Y, Chen H and Liu Z: AMPK regulates
autophagy by phosphorylating BECN1 at threonine 388. Autophagy.
12:1447–1459. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mihaylova MM and Shaw RJ: The AMPK
signalling pathway coordinates cell growth, autophagy and
metabolism. Nat Cell Biol. 13:1016–1023. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yuan M, Zhang L, You F, Zhou J, Ma Y, Yang
F and Tao L: MiR-145-5p regulates hypoxia-induced inflammatory
response and apoptosis in cardiomyocytes by targeting CD40. Mol
Cell Biochem. 431:123–131. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang J, He Z, Xiao W, Na Q, Wu T, Su K
and Cui X: Overexpression of BAG3 attenuates hypoxia-induced
cardiomyocyte apoptosis by inducing autophagy. Cell Physiol
Biochem. 39:491–500. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jia Z, Lin L, Huang S, Zhu Z, Huang W and
Huang Z: Inhibition of autophagy by berberine enhances the survival
of H9C2 myocytes following hypoxia. Mol Med Rep. 16:1677–1684.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang S, Zhao Y, Xu M, Yu L, Zhao Y, Chen
J, Yuan Y, Zheng Q and Niu X: FoxO3a modulates hypoxia stress
induced oxidative stress and apoptosis in cardiac microvascular
endothelial cells. PLoS One. 8:e803422013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Su Y, Tian H, Wei L, Fu G and Sun T:
Integrin β3 inhibits hypoxia-induced apoptosis in cardiomyocytes.
Acta Biochim Biophys Sin (Shanghai). 50:658–665. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kanazawa H, Imoto K, Okada M and Yamawaki
H: Canstatin inhibits hypoxia-induced apoptosis through activation
of integrin/focal adhesion kinase/Akt signaling pathway in H9c2
cardiomyoblasts. PLoS One. 12:e01730512017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu Q, Ci X, Wen Z and Peng L: Diosmetin
alleviates lipopolysaccharide-induced acute lung injury through
activating the Nrf2 pathway and inhibiting the NLRP3 inflammasome.
Biomol Ther (Seoul). 26:157–166. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang Z, Li H, Chen S, Li Y, Cui Z and Ma
J: Knockdown of microRNA-122 protects H9c2 cardiomyocytes from
hypoxia-induced apoptosis and promotes autophagy. Med Sci Monit.
23:4284–4290. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sung HK, Chan YK, Han M, Jahng JWS, Song
E, Danielson E, Berger T, Mak TW and Sweeney G: Lipocalin-2 (NGAL)
attenuates autophagy to exacerbate cardiac apoptosis induced by
myocardial ischemia. J Cell Physiol. 232:2125–2134. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Egan DF, Shackelford DB, Mihaylova MM,
Gelino S, Kohnz RA, Mair W, Vasquez DS, Joshi A, Gwinn DM, Taylor
R, et al: Phosphorylation of ULK1 (hATG1) by AMP-activated protein
kinase connects energy sensing to mitophagy. Science. 331:456–461.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhou C, Gu J, Zhang G, Dong D, Yang Q,
Chen MB and Xu D: AMPK-autophagy inhibition sensitizes
icaritin-induced anticolorectal cancer cell activity. Oncotarget.
8:14736–14747. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang H, Liu B, Li T, Zhu Y, Luo G, Jiang
Y, Tang F, Jian Z and Xiao Y: AMPK activation serves a critical
role in mitochondria quality control via modulating mitophagy in
the heart under chronic hypoxia. Int J Mol Med. 41:69–76.
2018.PubMed/NCBI
|
|
32
|
Xu Z, Yan Y, Xiao L, Dai S, Zeng S, Qian
L, Wang L, Yang X, Xiao Y and Gong Z: Radiosensitizing effect of
diosmetin on radioresistant lung cancer cells via Akt signaling
pathway. PLoS One. 12:e01759772017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ge A, Liu Y, Zeng X, Kong H, Ma Y, Zhang
J, Bai F and Huang M: Effect of diosmetin on airway remodeling in a
murine model of chronic asthma. Acta Biochim Biophys Sin
(Shanghai). 47:604–611. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yang K, Li WF, Yu JF, Yi C and Huang WF:
Diosmetin protects against ischemia/reperfusion-induced acute
kidney injury in mice. J Surg Res. 214:69–78. 2017. View Article : Google Scholar : PubMed/NCBI
|