Introduction
Cerebrovascular disease is a common disease that
poses a significant threat to human health, particularly in
individuals >50 years old, and ischemic cerebrovascular disease
accounts for 60–80% of cases (1,2).
Ischemia-reperfusion injury is an important pathophysiological
process in the pathogenesis of ischemic cerebrovascular disease
(3,4). Under ischemic conditions, the brain
cannot provide the energy required for biosynthesis and positive
ions transformation of neurotransmitters, and for proper function
of enzymes and cell membrane structures, resulting in excessive
release and difficulty in the reuptake of excitatory
neurotransmitters, cytoskeleton cleavage and antioxidant damage,
resulting in neurological damage (5–7). The
energy changes caused by mitochondrial dysfunction can directly
induce neuronal apoptosis or necrosis. A large number of
pro-apoptotic molecules that are stored in the mitochondria and do
not exhibit their effects under physiological conditions; however,
these molecules result in apoptosis through a series of
physiological changes induced by a variety of apoptosis signals,
which also suggests mitochondrial apoptosis serves a key role in
cell death induced by hypoxic-ischemic brain damage (8–10).
Butorphanol is a synthetic selective opioid receptor
antagonist with substantial analgesic effects (11). The mechanisms underlying its
function is dependent on the dual pharmacological functions of
metabolite activated κ-opioid receptor and μ-receptor (12,13).
Butorphanol can be administered in a variety of ways, including
intravenous injection, muscular injection and nasal sprays.
Previous studies have demonstrated that butorphanol exhibits
potential for a wide range of clinical applications, including the
treatment of opioid-induced pruritus (14). Additionally, butorphanol can be
used as an adjuvant therapy in opioid-dependent patients (15). Huang et al (16) reported that butorphanol could
attenuate myocardial ischemia reperfusion injury through inhibiting
mitochondria-mediated apoptosis in mice. Zhang et al
(17) demonstrated that fentanyl
combined with butorphanol protected against myocardial
ischemia-reperfusion injury via activation of nuclear factor
erythroid 2-related factor 2-antioxidant response elements
signaling. In addition, in vivo experiments reported that
butorphanol exhibits a significant protective effect on myocardial
ischemia-reperfusion injury in rats, reducing ischemic myocardial
ischemia and infarct size, reducing the levels of pro-inflammatory
cytokines or increasing the production of anti-inflammatory
cytokines to reduce myocardial reperfusion-induced myocardial
injury, and it also reduced myocardial ischemic injury by
inhibiting mitochondrial-mediated apoptosis (18). However, the neuroprotective effects
of butorphanol on oxygen glucose deprivation/reoxygenation (OGD/R)
injury of nerve cells has not been determined previously, to the
best of our knowledge.
PC12 cells are derived from a pheochromocytoma of
the rat adrenal medulla. Use of this cell line has several
advantages, including high purity, rapid growth and reproduction.
It is a widely used cell line for studying the function,
differentiation, apoptosis and potential molecular mechanism of
nerve cells (19,20). In the present study, the effects of
butorphanol on hypoxic-induced ischemia-reperfusion injury of PC12
cells were investigated. In addition, the involvement of
mitochondrial apoptosis in the butorphanol-mediated effects was
assessed.
Materials and methods
Reagents and chemicals
Butorphanol Tartrate Injection was purchased from
Jiangsu Hengrui Medicine Co., Ltd. PC12 cells were obtained from
the American Type Culture Collection. DMEM, Earle's balanced salt
solution (EBSS), FBS, 0.25% trypsin and PBS were obtained from
Gibco (Thermo Fisher Scientific, Inc.). Cell Counting Kit-8 (CCK-8)
was obtained from Dojindo Molecular Technologies, Inc. Tumor
necrosis factor (TNF)-α (cat. no. PT512), interleukin (IL)-6 (cat.
no. PI326), IL-1β (cat. no. PI301) and monocyte chemotactic
protein-1 (MCP-1; cat. no. PC125) detection kits were provided by
Beyotime Institute of Biotechnology. Reactive oxygen species (ROS
cat. no. AAT-16053), lactate dehydrogenase (LDH; cat. no.
10008882-96), myeloperoxidase (MPO; cat. no. HK105-01) and ATP
(cat. no. 701004-4) detection kits were provided by AmyJet
Scientific, Inc. Annexin V-FITC reagents were purchased from BD
Biosciences. PVDF membranes were provided by EMD Millipore. DAPI
reagents (cat. no. C1002) and FITC-conjugated goat anti-rabbit IgG
antibodies (cat. no. A0562) were purchased from Beyotime
Biotechnology Co., Ltd. Primary antibodies against Bcl-2 (cat. no.
2875), Bax (cat. no. 14796), cleaved-poly ADP-ribose polymerase
(PARP) (cat. no. 9532), caspase-3 (cat. no. 14220), caspase-9
(1:1,000; cat. no. 9508), X-linked inhibitor of apoptosis protein
(XIAP; cat. no. 14334), and β-actin (cat. no. 3700) were all from
Cell Signaling Technology, Inc. The horseradish peroxidase
(HRP)-conjugated goat anti-rabbit secondary antibody (cat. no.
bs-0296G-HRP) was purchased from BIOSS. The FITC-conjugated goat
anti-rabbit IgG antibody (cat. no. sc-2359-FITC) was obtained from
Santa Cruz Biotechnology. All the other chemicals and reagents were
of analytical grade.
Cell culture
PC12 cells were cultured in a glass culture flask
filled with DMEM containing 10% FBS and placed in a CO2
incubator (95% O2 and 5% CO2) at 37°C. The
medium was changed every 48 h and cells were subcultured at 80–90%
confluency. Subsequently, cells were subcultivated. Cells in the
logarithmic growth phase were used for subsequent experiments.
OGD/R model
DMEM was replaced by glucose-free EBSS solution, and
2×105 cells/ml PC12 cells were cultured in the three-gas
incubator (94% N2, 5% CO2 and 1%
O2) at 37°C for 2 h. Subsequently, the medium was
replaced with DMEM and cells were incubated in the CO2
incubator (95% O2 and 5% CO2) at 37°C for 24
h. Control cells were routinely cultured under normoxic
conditions.
Experimental groups
To determine a suitable dose of butorphanol for
treating PC12 cells, cells were cultured with gradually increasing
concentrations (0–8 µM) of butorphanol at 37°C for 24 h. Cell
viability was analyzed using a CCK-8 assay according to the
manufacturer's instructions, and three doses of butorphanol (1, 2
and 4 µM) were selected for the subsequent experiment. PC12 cells
were divided into five groups in the study: i) Control group; ii)
OGD/R group; iii) OGD/R+1 µM butorphanol group; iv) OGD/R+2 µM
butorphanol group; and v) OGD/R+4 µM butorphanol group.
CCK-8 assay
Cell viability was detected using a CCK-8 assay
according to the manufacturer's protocol. PC12 cells were seeded
into 96-well plates at a density of 5×103 cells/well and
incubated at 37°C for 24 h. CCK-8 solution (20 µl) was added to
each well followed by continuous incubation at 37°C for 2 h.
Absorbance was measured at a wavelength of 450 nm and cell activity
was expressed as the ratio of the measured wavelength to the
control wavelength.
ELISA assay
The supernatant of the PC12 cells was collected and
centrifuged at 1,000 × g for 20 min at 4°C to remove any impurities
and cell fragments. The levels of TNF-α, IL-1β, IL-6 and MCP-1 were
detected according to the manufacturer's protocol. The standard
curve was drawn with the standard concentration as abscissa and the
absorbance at 450 nm wavelength as the ordinate values. The actual
concentrations of samples were obtained according to the absorbance
value.
Detection of oxidative stress
Oxidative stress was measured based on ROS, LDH and
MPO activity using the corresponding kits. When determining ROS
content, the time between loading the probe and measuring should be
shortened as much as possible, to reduce various possible errors.
LDH activity (mU/ml) was calculated as follows: (absorbance of
sample - absorbance of background blank control)/(absorbance of
standard - absorbance of standard blank) × concentration of
standard substance (mU/ml).
Flow cytometry analysis
PC12 cells were digested with trypsin and
resuspended to a density of 5×105−1×106
cells/ml. The digested cells were centrifuged at 1,000 × g for 10
min at 4°C, and the supernatant was discarded. After adding 1 ml
PBS, the above steps were repeated three times, and cells were
resuspended in 200 µl buffer solution. A total of 10 µM Annexin
V-FITC was added to the cells and incubated at room temperature
without light for 15 min, and then 300 µl buffer and 5 µl propidium
iodide were added. Apoptosis was detected using flow cytometry (FC
5000; BD Biosciences) within 1 h, and data were analyzed by using
FlowJo version 7.6.5 (Treestar).
Western blot analysis
Total proteins were extracted using lysis buffer
(Pierce; Thermo Fisher Scientific, Inc.). The protein concentration
was determined by bicinchoninic acid protein assay (Thermo Fisher
Scientific, Inc.) and then denatured by heating the mixture of the
protein and the loading buffer at 95°C for 5 min. A total of 40 µg
protein per lane was resolved by 12.5% SDS-PAGE and transferred to
a PVDF membrane. Membranes were blocked with 5% skimmed milk at
37°C for 2 h and incubated with primary antibodies against Bcl-2
(1:1,000), Bax (1:1,000), XIAP (1:1,000), cleaved-PARP (1:1,000),
caspase-3 (1:1,000), caspase-9 (1:1,000), cleaved-caspase-3 and −9
(1:1,000) overnight at 4°C. After washing the membrane with TBS
with 0,1% Tween-20, the HRP-conjugated goat anti-rabbit secondary
antibody (1:5,000) was added at room temperature for 1 h, and
signals were visualized using ECL reagent (Cytiva). The gel images
were scanned by the Typhoon 9400 Gel Imaging System (Cytiva) and
analyzed by Quantity One software (version 4.6.9; Bio-Rad
Laboratories, Inc.). β-actin was used as the internal reference,
and the relative expression levels of the target protein was
expressed as the ratio of the gray value of target protein to
β-actin.
Immunofluorescence
Cells were cultured on a 6-well plate at a density
of 2×104 cells/ml. When the cell density grew to 80–90%,
the cells were digested using a trypsin-EDTA (0.05% trypsin)
solution and washed with PBS three times. Cells were fixed with 4%
paraformaldehyde at 4°C for 30 min and permeabilized in 0.5% Triton
X-100 for 20 min on ice. Blocking was performed by incubation with
5% goat serum (Zymed; Thermo Fisher Scientific, Inc.) at room
temperature for 30 min. Cells were incubated with anti-XIAP
antibodies (1:200) at 4°C overnight and subsequently incubated with
FITC-conjugated goat anti-rabbit IgG antibodies (1:2,000) in the
dark at 4°C for 2 h. The cell nuclei were stained using DAPI and
incubated at room temperature for 10 min. The experimental results
were observed using an Olympus BX60 fluorescence microscope at a
magnification of ×400 (Olympus Corporation).
ATP activity analysis
Cells were seeded in 6-well plates at a density of
5×103 cells/200 µl. After the incubation, the cells and
the supernatants were collected. The supernatant was centrifuged at
12,000 × g for 5 min at 4°C after pyrolysis at 4°C. The
concentration of ATP was determined using an ATP detection kit
according to the manufacturer's protocol.
Statistical analysis
All experiments were repeated three times, and the
data are expressed as the mean ± standard deviation. The data were
analyzed using SPSS 22.0 (IBM Corp.). The statistical comparisons
among multiple groups were performed using ANOVA followed by a post
hoc Tukey's test. P<0.05 was considered to indicate a
statistically significant difference.
Results
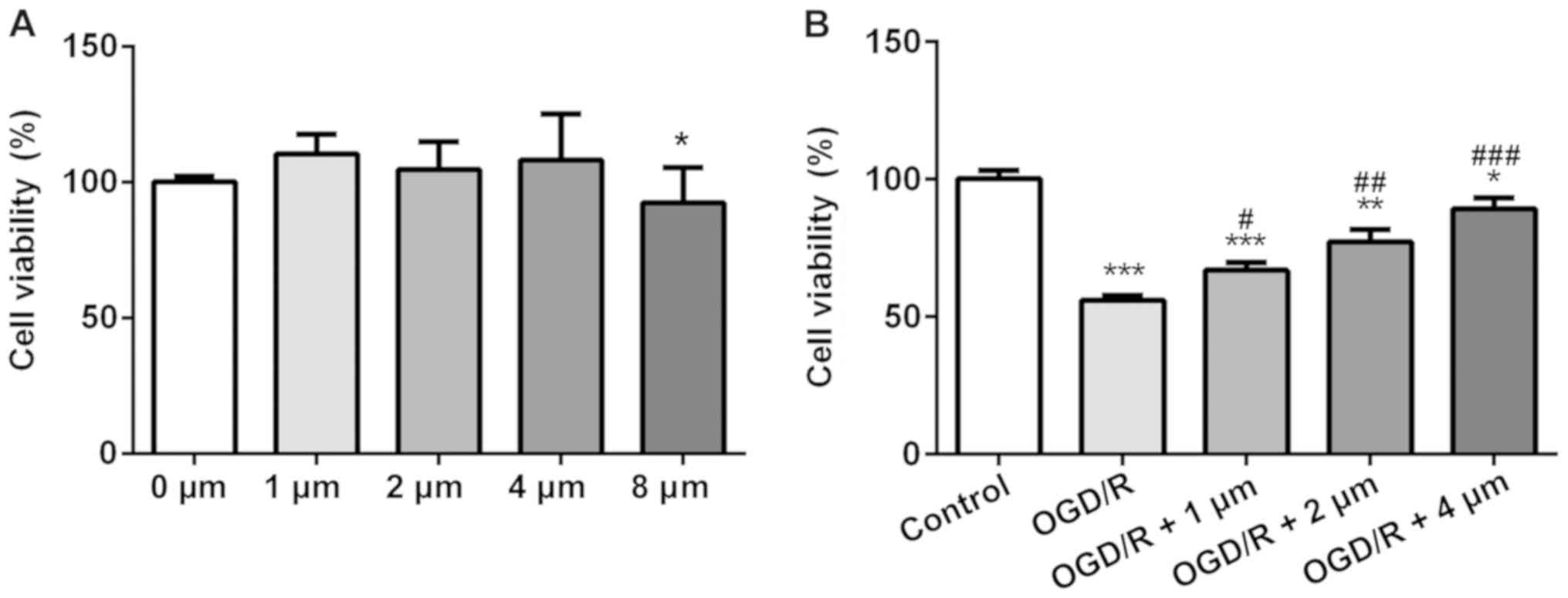
Butorphanol increases cell viability
following OGD/R injury
The effects of different concentrations of
butorphanol on the viability of PC12 cells was assessed using a
CCK-8 assay. Compared with the control group, butorphanol (1, 2 and
4 µM) had no effect on cell vitality (Fig. 1A), whereas in cells treated with 8
µM butorphanol, viability was decreased. Thus, the former three
groups were selected for subsequent study. To determine the
protective effects of butorphanol on neurons in OGD/R injury, cells
were treated with hypoxia and reoxygenation, and then subsequently
with butorphanol. As shown in Fig.
1B, cell viability in the OGD/R group decreased significantly
compared with the control group. Whereas, butorphanol increased
cell viability compared with the OGD/R group in a dose-dependent
manner. Cell viability was highest in cells treated with 4 µM.
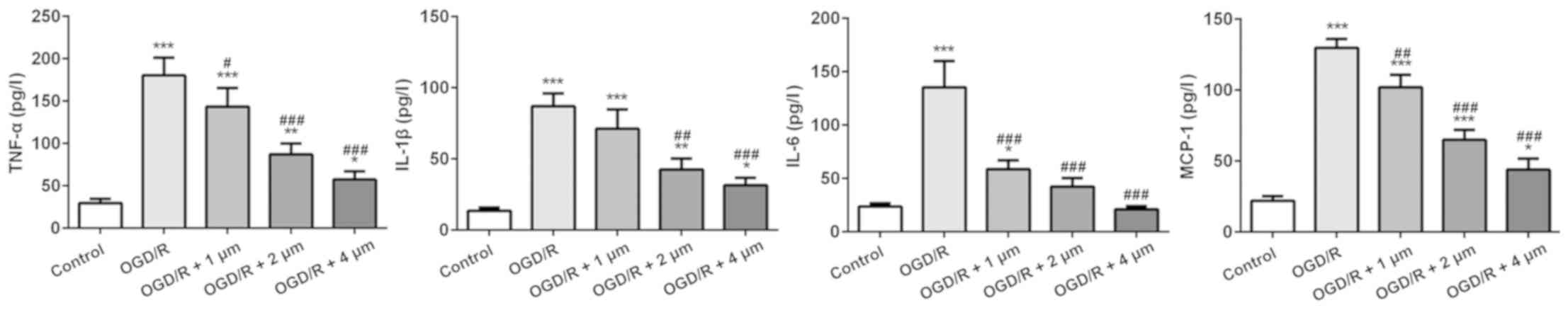
Effect of butorphanol on expression of
inflammatory factors following OGD/R injury
The inflammatory response following ischemic injury
is accompanied by the production of a large number of inflammatory
factors. Thus, ELISA kits were used to detect the expression of
several typical inflammatory factors (TNF-α, IL-1β, IL-6 and
MCP-1). As shown in Fig. 2,
compared with the control group, the expression of inflammatory
cytokines in the OGD/R-treated cells was significantly increased,
particularly MCP-1 expression. Butorphanol significantly reduced
the expression of inflammatory factors compared with the OGD/R
group, and the inhibitory effect was negatively associated with
concentration. In addition, there was no significant difference in
the expression of IL-6 between the 4 µM group and the blank control
group.
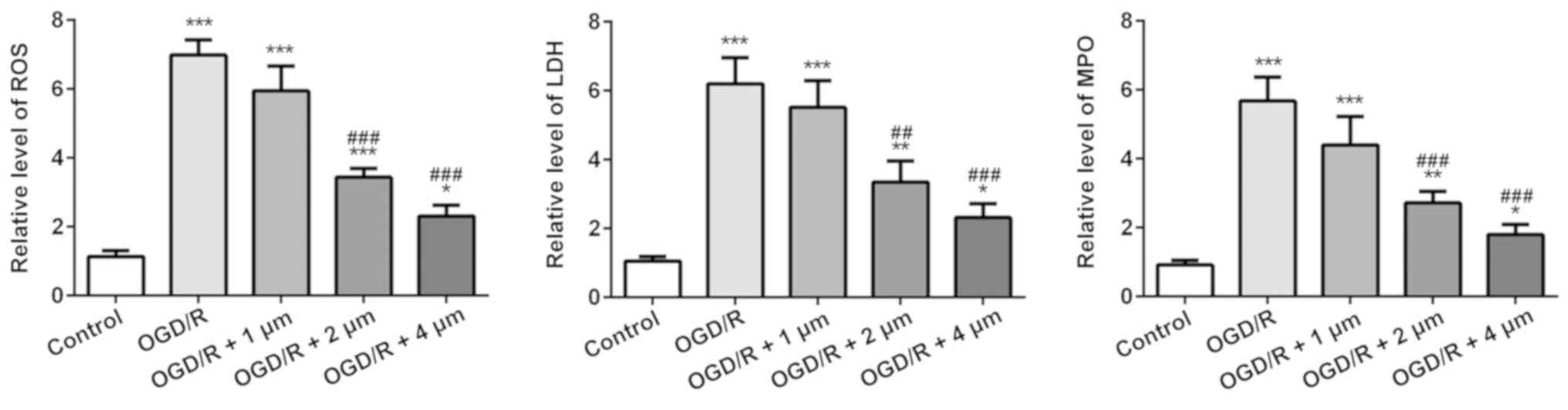
Effect of butorphanol on oxidative
stress in OGD/R injury
The effect of butorphanol on oxidative stress
induced by OGD/R in PC12 cells was assessed. As shown in Fig. 3, the OGD/R group significantly
increased the levels of ROS, the release of LDH and the MPO content
compared with the control group. Treatment with butorphanol
significantly decreased ROS, LDH and MPO levels, compared with the
OGD/R group, in a dose-dependent manner. Consistent with the
results of inflammatory factors, the inhibitory effect at 4 µM was
the most prominent.
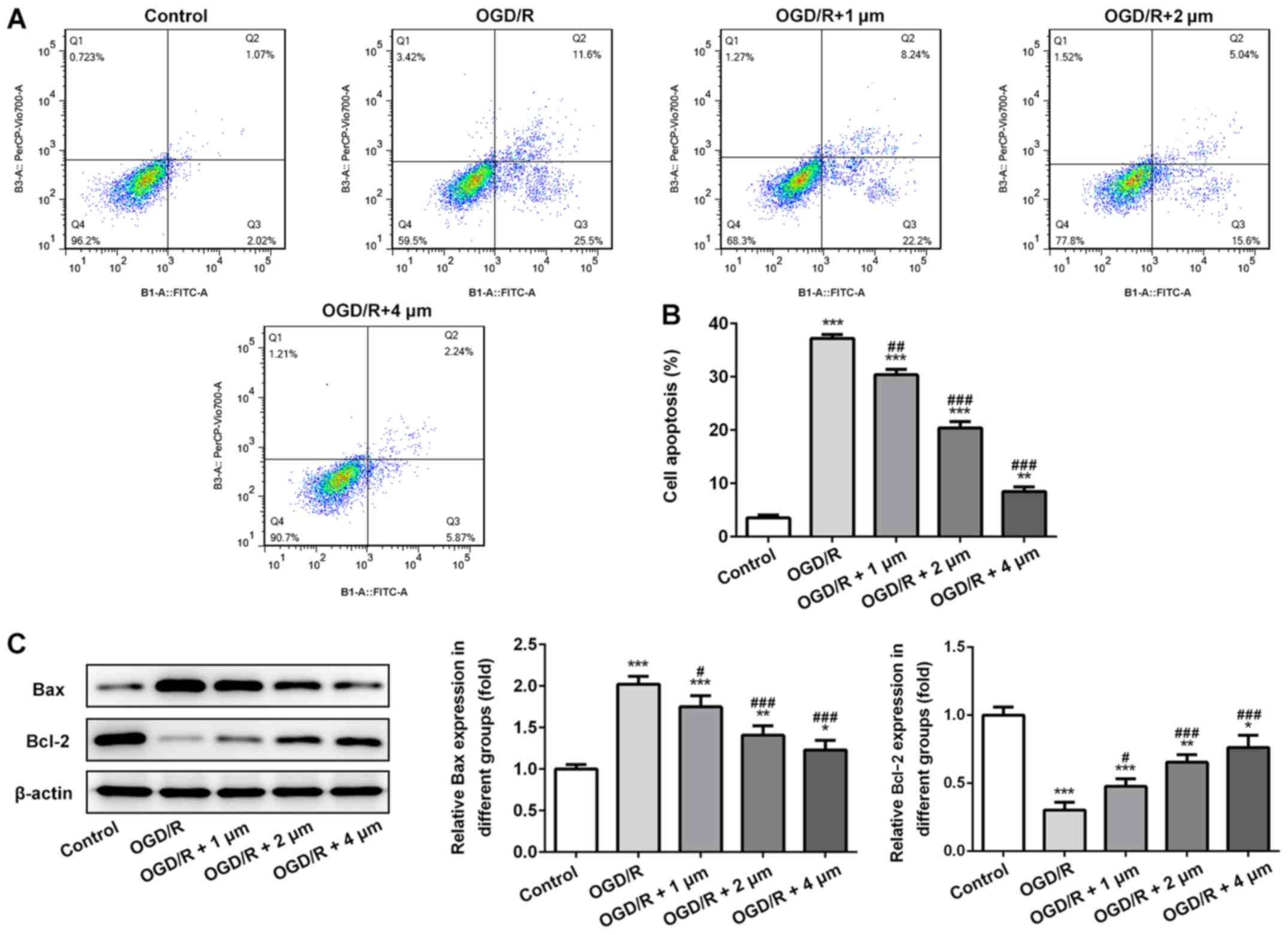
Effect of butorphanol on apoptosis of
PC12 cells following OGD/R injury
Subsequently, apoptosis of PC12 cells was assessed
by measuring the expression of the anti-apoptotic protein Bcl-2 and
the pro-apoptotic protein Bax by western blotting. As shown in
Fig. 4A and B, OGR/D significantly
increased the apoptotic rate, and this was significantly alleviated
by butorphanol. Similarly, as shown in Fig. 4C, compared with the control group,
OGD/R significantly decreased the expression of Bcl-2 and increased
the expression of Bax. Following treatment with butorphanol, there
was a negative association between the expression levels of Bax and
Bcl-2. The expression levels of Bax were decreased and the
expression levels of Bcl-2 increased in a dose-dependent manner.
Compared with the OGD/R group, there were significant differences
between expression levels of Bax and Bcl-2 after butorphanol
treatment.
Effects of butorphanol on expression
of mitochondrial-mediated apoptosis associated protein and ATP
activity
The expression of mitochondrial-mediated apoptosis
associated proteins and ATP activity in PC12 cells induced by OGD/R
were assessed. As shown in Fig. 5A and
B, the expression levels of XIAP and cleaved-PARP was
significantly decreased following OGD/R, whereas the expression of
cleaved-caspase-3 and −9 was significantly increased. Following
treatment with butorphanol, compared with the OGD/R group,
butorphanol significantly increased the expression of XIAP and
cleaved-PARP, and decreased the activation of caspase-3 and −9. In
addition, the activity of ATP in mitochondria was detected.
Compared with the control group, OGD/R resulted in a significant
reduction of ATP activity, and butorphanol increased activity in a
dose-dependent manner. The expression levels of XIAP was also
evaluated in PC12 cells treated with OGD/R by immunofluorescence.
As shown in Fig. 5C, compared with
the control group, OGD/R significantly reduced the expression
levels of XIAP. Butorphanol increased the expression of XIAP in a
dose-dependent manner.
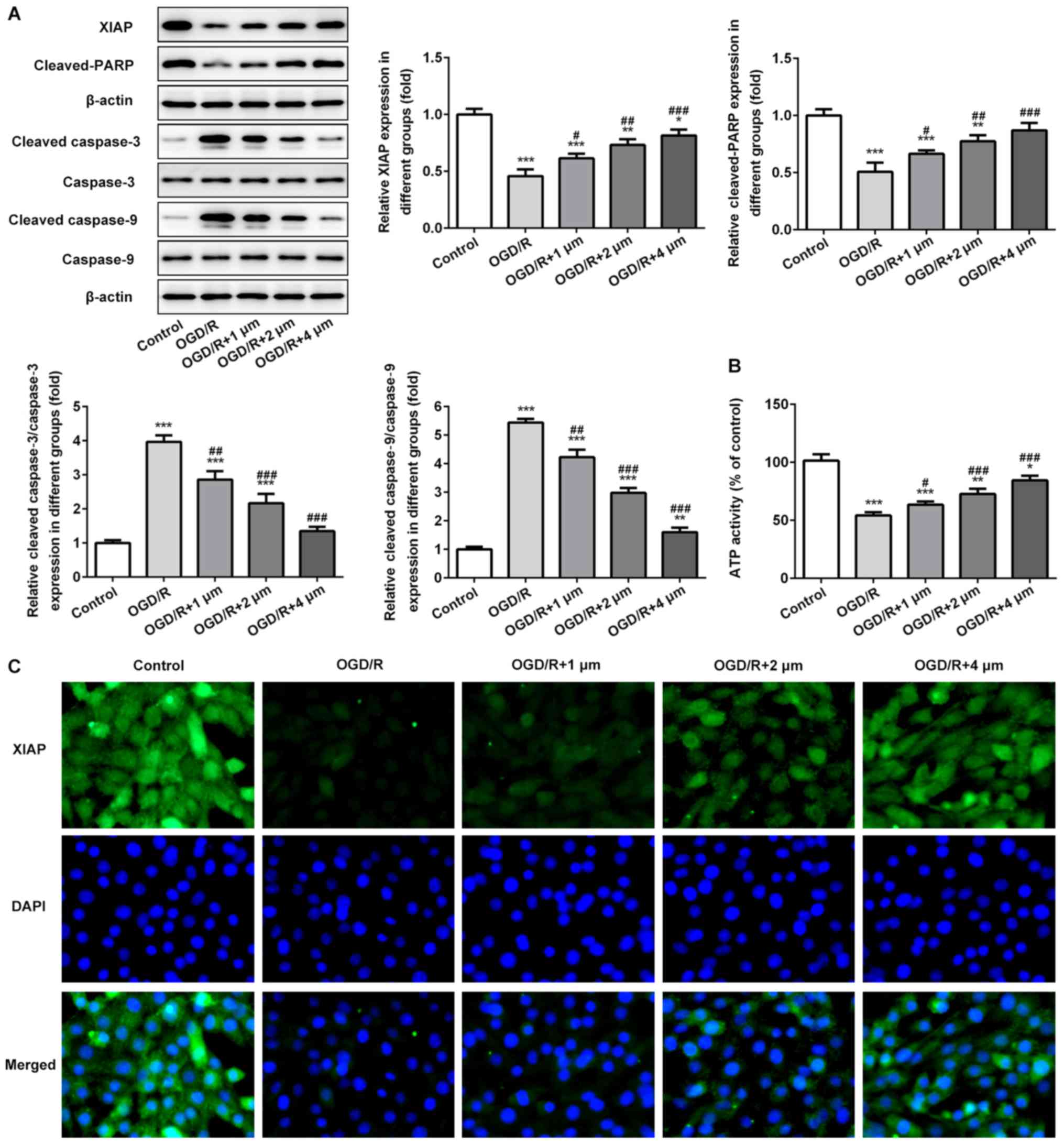 | Figure 5.Effects of butorphanol on expression
of mitochondrial-mediated apoptosis associated proteins and ATP
activity. (A) Relative protein levels of XIAP, cleaved-PARP,
caspase-3, caspase-9, cleaved caspase-3 and cleaved caspase-9 in
each group, β-actin was used as the control. (B) ATP activity of
PC12 cells was determined by a detection kit. (C) XIAP protein
expression was examined by an immunofluorescence assay. *P<0.05,
**P<0.01 and ***P<0.001 vs. control; #P<0.05,
##P<0.01 and ###P<0.001 vs. OGD/R.
OGD/R, oxygen glucose deprivation/reoxygenation; XIAP, X-linked
inhibitor of apoptosis protein; PARP, poly ADP-ribose
polymerase. |
Discussion
In the present study, the potential protective
effects of butorphanol on nerve injury induced by OGD/R were
assessed. To elucidate the effects of butorphanol on
ischemia-reperfusion injury of PC12 cells, cell viability,
expression of inflammatory factors, oxidative stress and apoptosis
were measured. Butorphanol increased viability and decreased the
intracellular levels of ROS, LDH and MPO in PC12 cells following
OGD/R. Butorphanol also ameliorated the downregulation of Bcl-2,
the upregulation of Bax and the activation of mitochondrial
apoptosis-related proteins. Additionally, butorphanol significantly
enhanced ATP activity in the mitochondria.
A previous study demonstrated that butorphanol
attenuated myocardial ischemia-reperfusion injury in mice by
inhibiting mitochondrial-mediated apoptosis (16). In the present study, it was shown
that butorphanol exhibited a protective effect on the activity of
PC12 neurons against OGD/R-induced damage, consistent with previous
studies.
There are a large number of inflammatory factors in
the ischemic area, and the presence of inflammatory cells alters
brain tissue from ischemia to inflammation by activating
inflammatory signaling pathways during cerebral
ischemia-reperfusion. Therefore, inflammation serves an important
role during cerebral ischemia-reperfusion injury (21–23).
In addition, butorphanol has been shown to exhibit
anti-inflammatory effects. A previous study showed that butorphanol
alleviated brain injury and neurological damage, and reduced the
expression of serum inflammatory factors (TNF-α, IL-6 and IL-1) by
regulating the NF-κB signaling pathway in septic rats (24). In the present study, the expression
of inflammatory cytokines in PC12 cells treated with OGD/R
increased significantly, whereas butorphanol decreased the
expression of inflammatory cytokines in a dose-dependent manner.
Therefore, butorphanol can reduce OGD/R-induced neuronal injury by
inhibiting the expression of inflammatory factors.
Oxidative stress serves a key role in neuronal
injury following cerebral ischemia (25). Wu et al (18) found that butorphanol
postconditioning significantly reduced MPO content in
cardiomyocytes following ischemia injury. In the present study, the
levels of ROS, LDH and MPO in the OGD/R group were all
significantly increased, and addition of butorphanol reduced their
levels. Therefore, it is hypothesized that butorphanol may protect
PC12 cells from OGD/R-induced injury by inhibiting oxidative
stress.
The causes and pathways underlying apoptosis are
complex and diverse, and numerous genes are involved in the
regulation of apoptosis, including lethal genes and survival genes.
Among them, members of the Bcl-2 family serve an important role in
gene regulation. They are primarily divided into anti-apoptotic
gene Bcl-2 and pro-apoptotic gene Bax, which regulates apoptosis by
activating a series of downstream genes (26,27).
In the present study, OGD/R significantly decreased the expression
of Bcl-2 and increased the expression of Bax. Following treatment
with butorphanol, the expression of Bcl-2 was significantly
increased and the expression of Bax was significantly decreased.
These data suggested that butorphanol attenuated nerve injury
induced by OGD/R, which may be associated with the inhibition of
apoptosis.
The process of ischemia-reperfusion injury
drastically alters the steady-state balance of mitochondria,
reduces the production of ATP and induces apoptosis and necrosis
(28,29). In the present study, it was shown
that following hypoxia, the expression levels of XIAP and
cleaved-PARP were decreased significantly, whereas the expression
levels of cleaved-caspase-3 and −9 were increased significantly.
Butorphanol significantly blocked the activation of caspase-3 and
−9, and increased the expression of XIAP and cleaved-PARP.
Additionally, compared with the OGD/R group, butorphanol also
significantly enhanced ATP activity following mitochondrial injury.
Based on the above results, it was demonstrated that butorphanol
reduced OGD/R-induced neuronal injury by inhibiting mitochondrial
apoptosis.
In summary, it was shown that butorphanol can be
used to protect neuronal PC12 cells from OGD/R-induced damage,
including inflammatory and apoptotic damage, whilst also reducing
mitochondrial damage. However, the specific underlying mechanisms
should be further studied. The present study expands the potential
application of butorphanol and highlights a novel method for the
treatment of nerve-related ischemia-reperfusion injury.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
ZY and LW designed the research. ZY, LW, YH and FW
performed the experiments. ZY analyzed the data and wrote the
paper. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Yitshak Sade M, Novack V, Ifergane G,
Horev A and Kloog I: Air pollution and ischemic stroke among young
adults. Stroke. 46:3348–3353. 2105. View Article : Google Scholar
|
|
2
|
Dong JY, Iso H, Kitamura A and Tamakoshi
A; Japan Collaborative Cohort Study Group, : Multivitamin use and
risk of stroke mortality: The Japan collaborative cohort study.
Stroke. 46:1167–1172. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kim JY, Kawabori M and Yenari MA: Innate
inflammatory responses in stroke: Mechanisms and potential
therapeutic targets. Curr Med Chem. 21:2076–2097. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nordestgaard LT, Tybjærg-Hansen A,
Nordestgaard BG and Frikke-Schmidt R: Loss-of-function mutation in
ABCA1 and risk of Alzheimer's disease and cerebrovascular disease.
Alzheimers Dement. 11:1430–1438. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dai Y, Wang Z, Quan M, Lv Y, Li Y, Xin HB
and Qian Y: Asiatic acid protests against myocardial
ischemia/reperfusion injury via modulation of glycometabolism in
rat cardiomyocyte. Drug Des Devel Ther. 12:3573–3582. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Aon MA, Cortassa S, Marbán E and O'Rourke
B: Synchronized whole cell oscillations in mitochondrial metabolism
triggered by a local release of reactive oxygen species in cardiac
myocytes. J Biol Chem. 278:44735–44744. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shah VK and Shalia KK: Reperfusing the
myocardium - a damocles Sword. Indian Heart J. 70:433–438. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Suhail AS, Suhel P and Heena T: Melatonin
and ischemic stroke: Mechanistic roles and action. Adv Pharmacol
Pharm Sci. 31:1–8. 2015.[J].
|
|
9
|
Waseem M, Tabassum H and Parvez S:
Melatonin modulates permeability transition pore and
5-hydroxydecanoate induced KATP channel inhibition in isolated
brain mitochondria. Mitochondrion. 31:1–8. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sun M, Izumi H, Shinoda Y and Fukunaga K:
Neuroprotective effects of protein tyrosine phosphatase 1B
inhibitor on cerebral ischemia/reperfusion in mice. Brain Res.
1694:1–12. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tsukamoto A, Iimuro M, Sato R, Yamazaki J
and Inomata T: Effect of midazolam and butorphanol premedication on
inhalant isoflurane anesthesia in mice. Exp Anim. 64:139–145. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gupta A, Kaur S, Attri JP and Saini N:
Comparative evaluation of ketamine - propofol, fentanyl - propofol
and butorphanol-propofol on haemodynamics and laryngeal mask airway
insertion conditions. J Anaesthesiol Clin Pharmacol. 27:74–78.
2011.PubMed/NCBI
|
|
13
|
Du BX, Song ZM, Wang K, Zhang H, Xu FY,
Zou Z and Shi XY: Butorphanol prevents morphine-induced pruritus
without increasing pain and other side effects: A systematic review
of randomized controlled trials. Can J Anaesth. 60:907–917. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee H, Naughton NN, Woods JH and Ko MC:
Effects of butorphanol on morphine-induced itch and analgesia in
primates. Anesthesiology. 107:478–485. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kuzumaki N, Suzuki A, Narita M, Hosoya T,
Nagasawa A, Imai S, Yamamizu K, Morita H, Nagase H, Okada Y, et al:
Effect of κ-opioid receptor agonist on the growth of non-small cell
lung cancer (NSCLC) cells. Br J Cancer. 106:1148–1152. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huang LH, Li J, Gu JP, Qu MX, Yu J and
Wang ZY: Butorphanol attenuates myocardial ischemia reperfusion
injury through inhibiting mitochondria-mediated apoptosis in mice.
Eur Rev Med Pharmacol Sci. 22:1819–1824. 2018.PubMed/NCBI
|
|
17
|
Zhang XT, Jun L, Yang JP, et al: Fentanyl
combined with butorphanol protects myocardial ischemia/reperfusion
injury via κ-opioid receptor-mediated Nrf2-ARE signaling. Int J
Clin Exp Med. 9:2500–2506. 2016.
|
|
18
|
Wu Y, Wan J, Zhen WZ, Chen LF, Zhan J, Ke
JJ, Zhang ZZ and Wang YL: The effect of butorphanol
postconditioning on myocardial ischaemia reperfusion injury in
rats. Interact Cardiovasc Thorac Surg. 18:308–312. 2014.[J].
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu X, Zhu X, Chen M, Ge Q, Shen Y and Pan
S: Resveratrol protects PC12 cells against OGD/ R-induced apoptosis
via the mitochondrial-mediated signaling pathway. Acta Biochim
Biophys Sin (Shanghai). 48:342–353. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wu Y, Shang Y, Sun SG, Liu RG and Yang WQ:
Protective effect of erythropoietin against
1-methyl-4-phenylpyridinium-induced neurodegenaration in PC12
cells. Neurosci Bull. 23:156–164. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Suzuki S, Tanaka K and Suzuki N:
Ambivalent aspects of interleukin-6 in cerebral ischemia:
Inflammatory versus neurotrophic aspects. J Cereb Blood Flow Metab.
29:464–479. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Maddahi A, Kruse LS, Chen QW and Edvinsson
L: The role of tumor necrosis factor-α and TNF-α receptors in
cerebral arteries following cerebral ischemia in rat. J
Neuroinflammation. 8:1072011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
He ZJ, Huang ZT, Chen XT and Zou ZJ:
Effects of matrix metalloproteinase 9 inhibition on the blood brain
barrier and inflammation in rats following cardiopulmonary
resuscitation. Chin Med J (Engl). 122:2346–2351. 2009.PubMed/NCBI
|
|
24
|
Meng J, Jiang SJ, Jiang D and Zhao Y:
Butorphanol attenuates inflammation via targeting NF-κB in septic
rats with brain injury. Eur Rev Med Pharmacol Sci. 23 (Suppl
3):161–170. 2019.PubMed/NCBI
|
|
25
|
Liu PK, Grossman RG, Hsu CY and Robertson
CS: Ischemic injury and faulty gene transcripts in the brain.
Trends Neurosci. 24:581–588. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tsukahara S, Yamamoto S, Tin-Tin-Win-Shwe,
Ahmed S, Kunugita N, Arashidani K and Fujimaki H: Inhalation of
low-level formaldehyde increases the Bcl-2/Bax expression ratio in
the hippocampus of immunologically sensitized mice.
Neuroimmunomodulation. 13:63–68. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Prakasa Babu P, Yoshida Y, Su M, Segura M,
Kawamura S and Yasui N: Immunohistochemical expression of Bcl-2,
Bax and cytochrome c following focal cerebral ischemia and effect
of hypothermia in rat. Neurosci Lett. 291:196–200. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fantinelli JC, González Arbeláez LF, Pérez
Núñez IA and Mosca SM: Protective effects of
N-(2-mercaptopropionyl)-glycine against ischemia-reperfusion injury
in hypertrophied hearts. Exp Mol Pathol. 94:277–284. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Penna C, Perrelli MG and Pagliaro P:
Mitochondrial pathways, permeability transition pore, and redox
signaling in cardioprotection: Therapeutic implications. Antioxid
Redox Signal. 18:556–599. 2013. View Article : Google Scholar : PubMed/NCBI
|