Introduction
Systemic sclerosis (SSc) is a chronic connective
tissue disease that causes widespread microvascular damage and
excessive collagen deposition in the skin and internal organs
(1). However, the etiology,
pathogenesis, and progression of this disease are not fully
understood.
Alpha2-antiplasmin (α2AP), is a 65–70 kDa protein
that inactivates plasmin and thereby inhibits fibrinolysis
(2,3). α2AP exists in various tissues, such
as the liver, kidney, intestine, spleen, lung, muscle, ovary,
testis, cerebral cortex, hippocampus, cerebellum, bone, skin, and
placenta of murine tissue (4).
Apart from the inhibition of plasmin, α2AP regulates various cell
functions, including proliferation, differentiation, and cytokine
production, and also associates with angiogenesis, tissue repair,
vascular remodeling, and fibrosis progression (5–13).
In patients with rheumatic diseases, including SSc, plasma levels
of the plasmin-α2AP complex are increased (14,15).
α2AP affects myofibroblast differentiation, extracellular matrix
(ECM) production, vascular dysfunction, and progression of SSc. In
addition, we have shown that α2AP levels are elevated in an SSc
mouse model and dermal fibroblasts (16,17).
Matrix metalloproteinase-3 (MMP-3) plays a pivotal
role in ECM turnover as it can degrade ECM components, including
proteoglycans, collagen III, IV, V, and IX, laminin, fibronectin,
gelatin, and elastin (18,19). MMP-3 is expressed by fibroblasts,
chondrocytes, osteoblasts, endothelial cells, smooth muscle cells
and macrophages (20). Jinnin
et al (21) observed
similar MMP-3 serum levels in SSc patients and healthy controls;
however, serum levels of anti-MMP-3 autoantibody and tissue
inhibitors of metalloproteinase-1 (TIMP-1) were higher in SSc
patients, suggesting that MMP-3 activity may be decreased in SSc
(20,22). Since it has been reported that
cleavage by MMP-3 inactivates α2AP (23), the suppression of MMP-3 activity in
SSc may promote the activation of α2AP.
In the present study, we investigated the
relationship between α2AP and MMP-3 to gain insights into the
pathogenesis of SSc.
Materials and methods
The experiments with human samples in this study
were approved by the Gifu University Graduate School of Medicine
Ethics Committee (Approved ID:29-152). We received written informed
consent from the patients and volunteers involved.
Cell culture
Human normal and SSc dermal fibroblasts were
obtained from seven patients with SSc and four healthy controls as
previously described (6).
Fibroblasts were seeded onto 60-mm diameter dishes and cultured in
2 ml Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal
calf serum (FCS) at 37°C in a humidified atmosphere with 5%
CO2/95% air. After 6 days, the medium was replaced with
serum-free DMEM. Human normal dermal fibroblasts were stimulated by
α2AP, MMP-3 or mixture of α2AP and MMP-3 for 24 h. In other
studies, human SSc dermal fibroblasts were stimulated by MMP-3 for
24 h.
Western blot analysis
Cells were washed twice with cold PBS, harvested,
and then sonicated in lysis buffer containing 10 mM Tris-HCl buffer
(pH 7.5), 1% SDS, 1% Triton X-100, and a protease inhibitor
cocktail (Roche Diagnostics GmbH). The skin samples from the
subjects were homogenized and sonicated in the lysis buffer. The
protein concentration in each lysate was measured using a BCA
protein assay kit (Pierce; Thermo Fisher Scientific). Proteins in
the supernatant were separated by electrophoresis on 10%
SDS-polyacrylamide gels and transferred to a PVDF membrane. We
detected each protein by incubation with the relevant primary
antibodies followed by horseradish peroxidase-conjugated antibodies
to IgG.
Enzyme-linked immunosorbent assay
(ELISA)
Blood samples were obtained from 10 SSc patients and
10 healthy volunteers, and were subsequently centrifuged for 10 min
at 1,600 × g. The supernatant was then collected and used for the
assay. The serum levels of α2AP and MMP-3 were determined using
ELISA kits, Human Serpin F2/α 2-Antiplasmin (R&D Systems, MN,
USA) and Human Total MMP-3 Immunoassay (R&D Systems),
respectively. The absorbance was measured at 450 nm using an iMark
Microplate Reader (Bio-Rad Laboratories, Inc.).
miRNA study
SSc dermal fibroblasts were transfected with miR-29a
(sequence: ACUGAUUUCUUUUGGUGUUCAG, Bioneer) or negative control
miRNA using Lipofectamine 2000 (Invitrogen; Thermo Fisher
Scientific) according to the manufacturer's instructions. Cells
were harvested 48 h after transfection for further analysis.
Statistical analysis
All data were expressed as mean ± SEM and analyzed
using Statmate III version 3.06 (ATMS Co., Ltd.). The statistical
analysis was conducted with unpaired t-test for two-group
comparisons, with one-way ANOVA Tukey's for multiple comparison.
P<0.05 was considered to indicate a statistically significant
difference.
Results
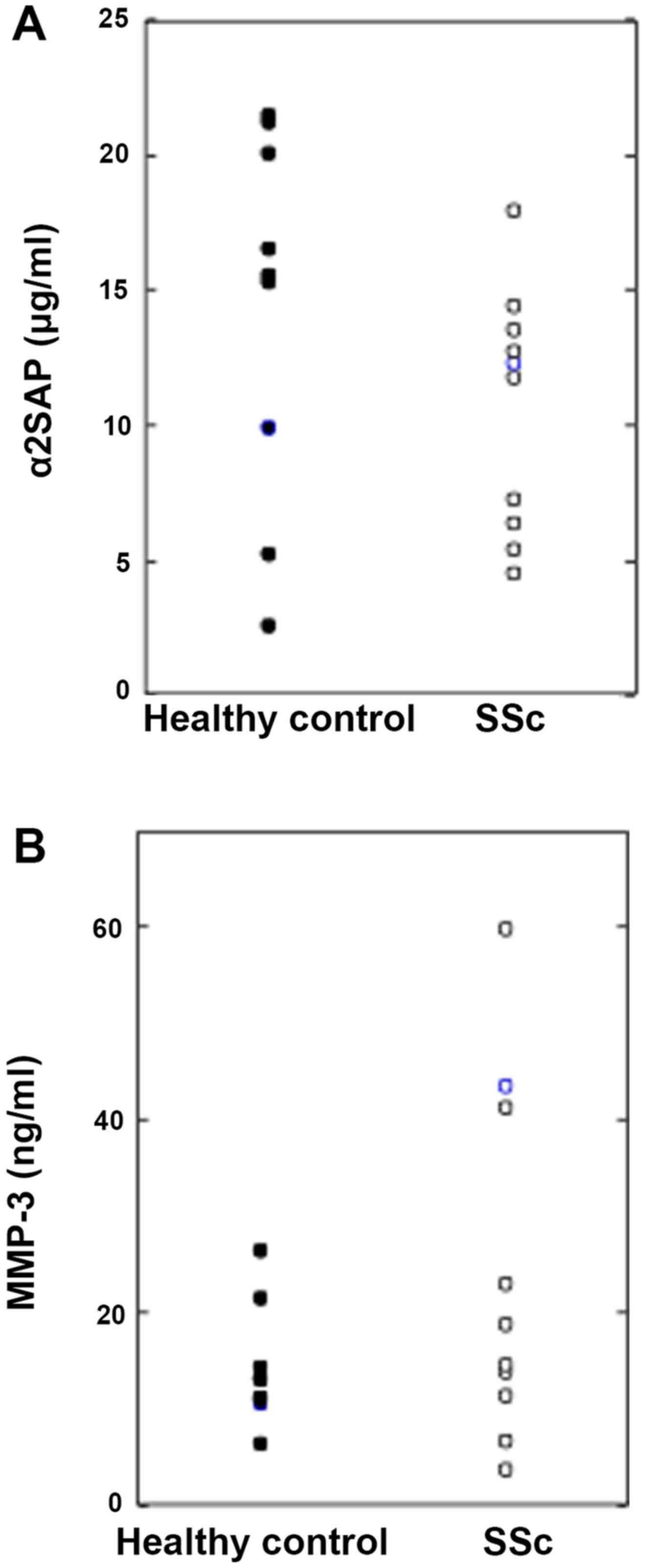
Serum levels of a2AP and MMP-3 are
similar in healthy controls and SSc patients
We examined the levels of α2AP and MMP-3 in the sera
from healthy controls and SSc patients by ELISA and found no
significant differences (healthy controls: n=10, SSc patients:
n=10) (Fig. 1).
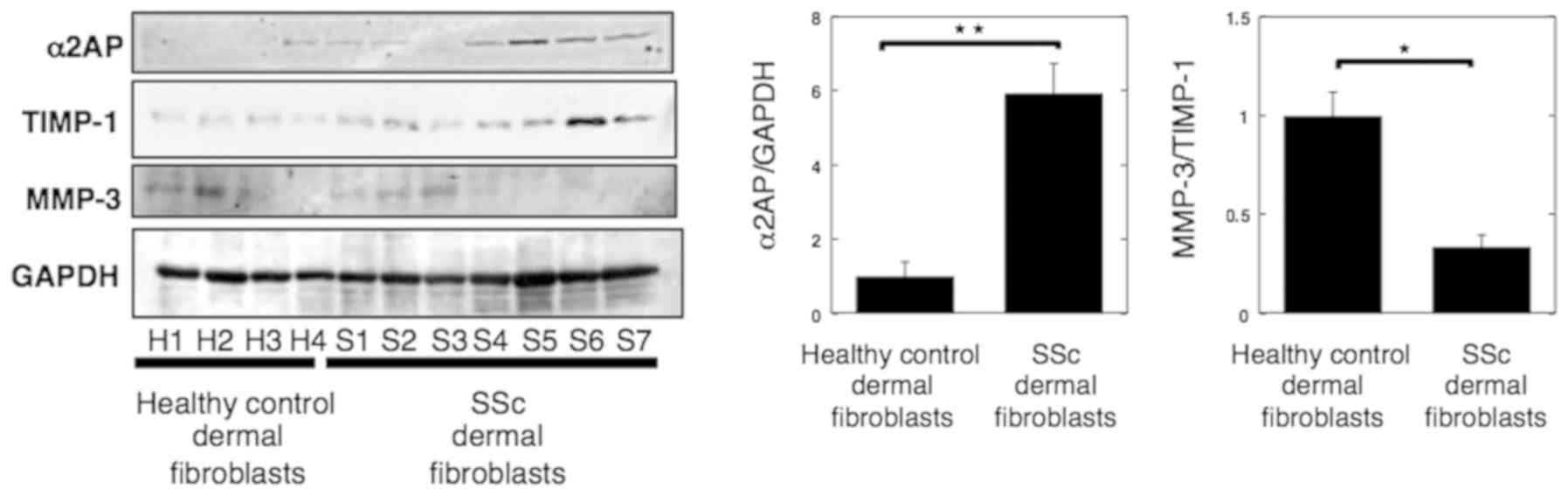
α2AP expression increased while
MMP-3/TIMP-1 ratio decreased in SSc dermal fibroblasts
Bonaventura et al (24) reported that the ratio of
MMP-3/TIMP-1 indicates the activity of MMP-3. Hence, we next
assessed α2AP expression and MMP-3/TIMP-1 ratio in normal human and
SSc dermal fibroblasts by western blotting (normal fibroblasts:
n=4, SSc fibroblasts: n=7). As shown in Fig. 2, we found that α2AP expression in
dermal fibroblasts was increased in SSc, whereas the MMP-3/TIMP-1
ratio was reduced.
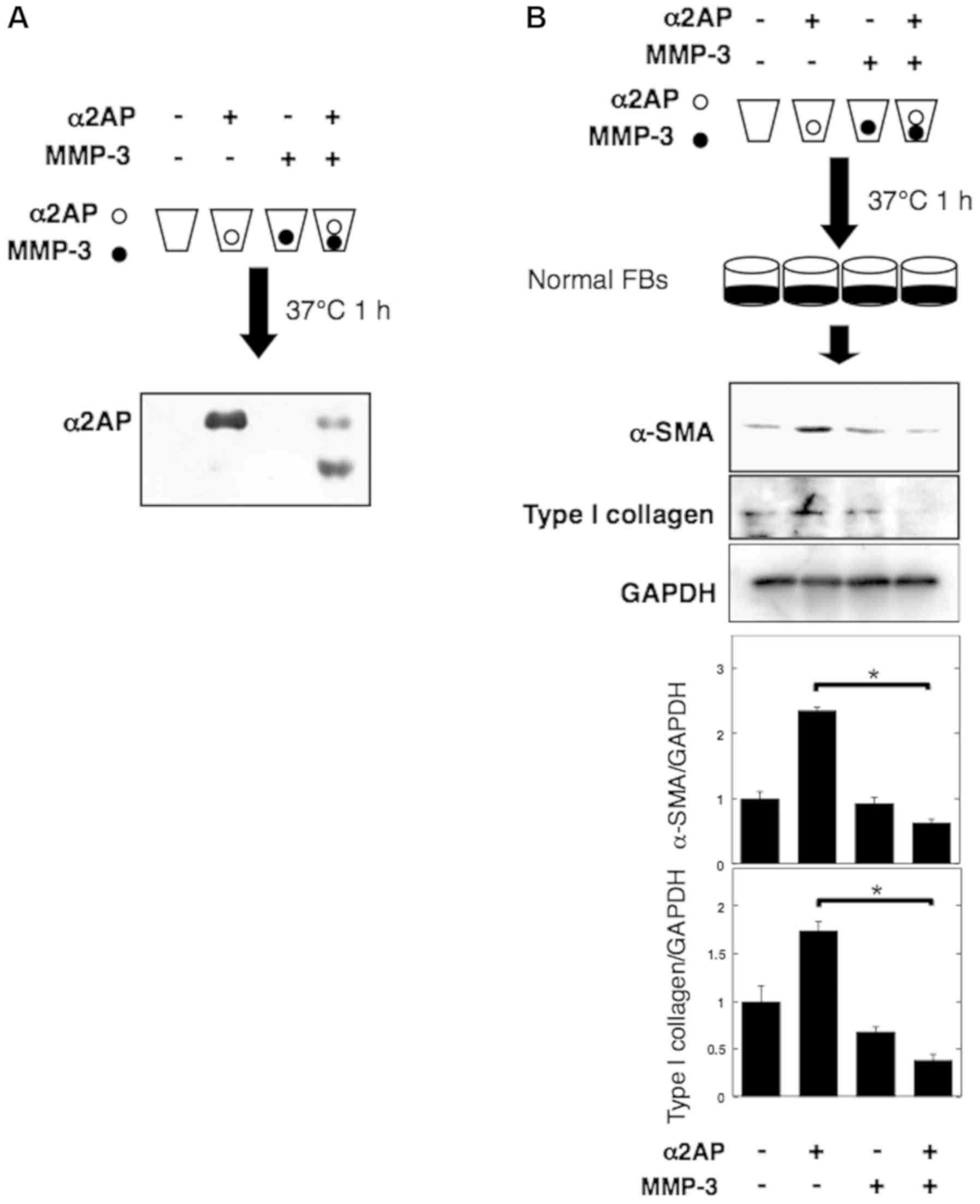
MMP-3-mediated degradation inhibits
pro-fibrotic effects of α2AP
Cleavage by MMP-3 inactivates α2AP (23). We thus incubated α2AP for 1 h at
37°C in the presence or absence of MMP-3 and showed that the enzyme
degraded α2AP (Fig. 3A). To assess
the effect of MMP-3-mediated degradation on α2AP, we performed the
same reactions and added the mixtures to normal human fibroblasts.
Pre-incubation of α2AP with MMP-3 led to attenuation of the
expression of α-smooth muscle actin (α-SMA) (a marker of the
myofibroblast phenotype) and type I collagen (Fig. 3B).
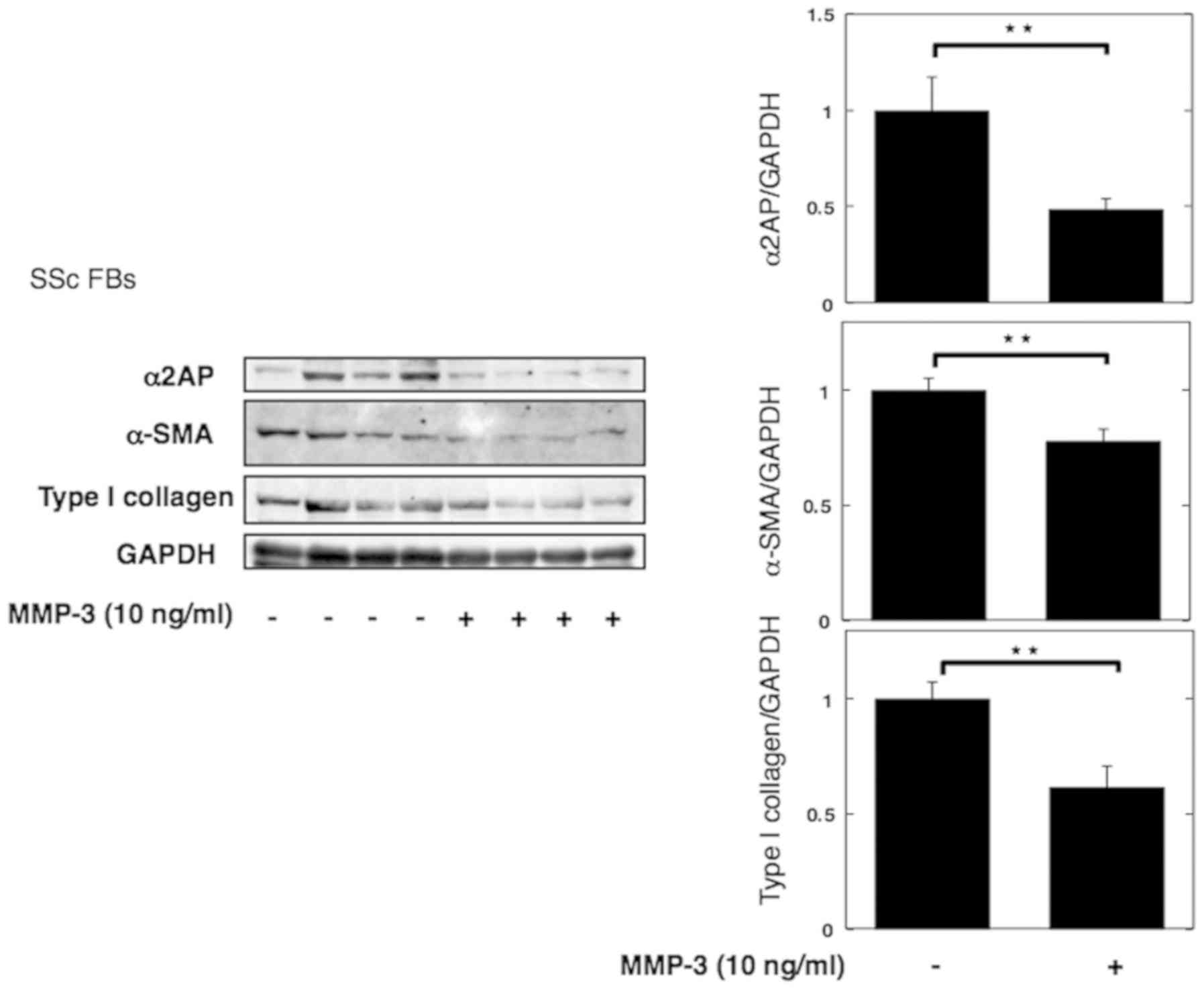
MMP-3 reverses pro-fibrotic phenotype
of SSc dermal fibroblasts
Next, we stimulated SSc dermal fibroblasts with
MMP-3 to investigate its anti-fibrotic effect. Consistent with the
previous results, stimulation with MMP-3 decreased the expression
of α2AP, αSMA, and type I collagen (Fig. 4).
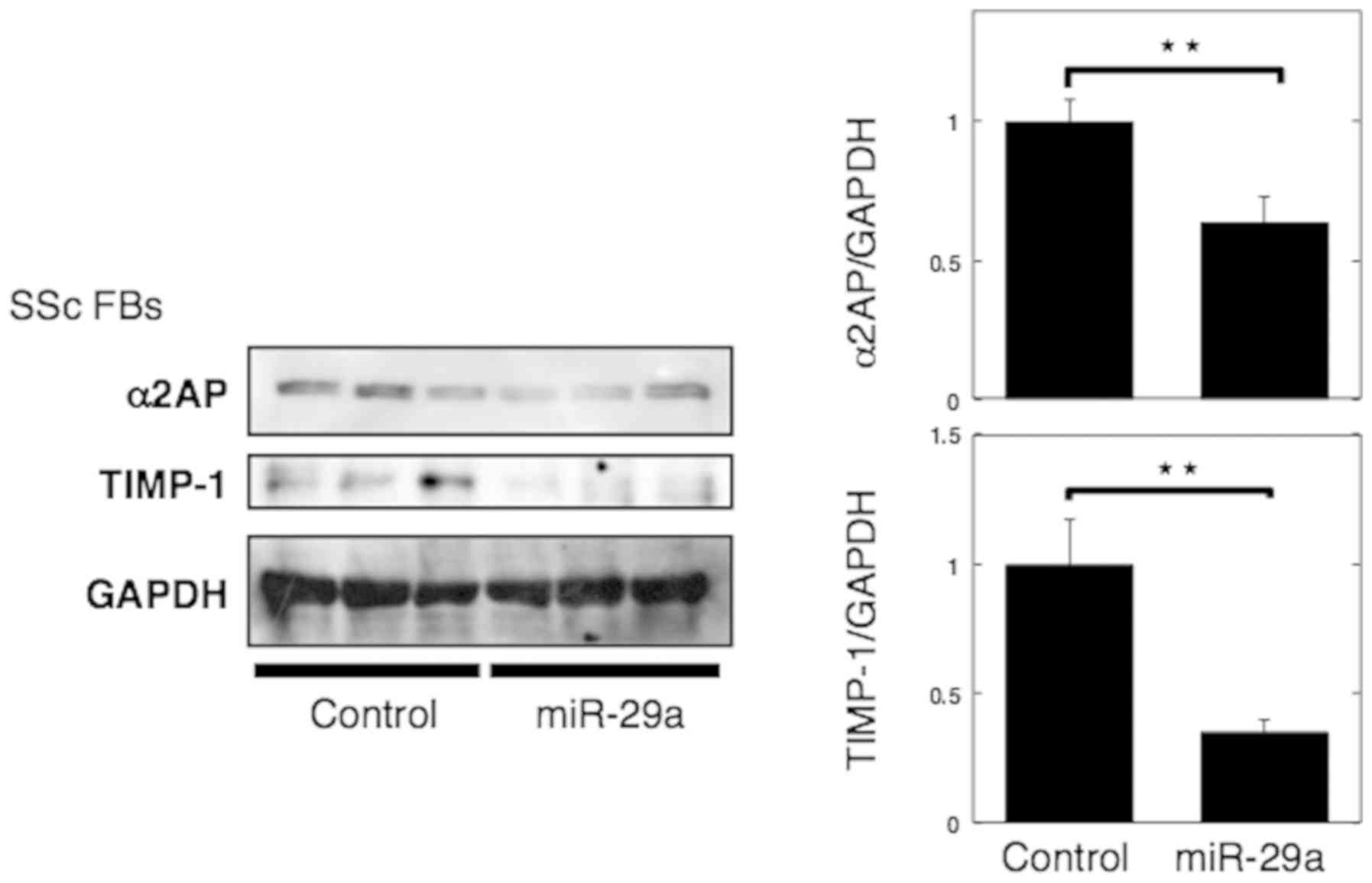
MicroRNA-29a attenuates α2AP
deposition in SSc dermal fibroblasts by inhibiting TIMP-1
Ciechomska et al (25) showed that miR-29a, decreased TIMP-1
expression and reversed the pro-fibrotic phenotype of SSc dermal
fibroblasts. In the present study, we confirmed that miR-29a
transfection in SSc fibroblasts caused a decrease in TIMP-1
expression, in line with the previous report (Fig. 5). We also showed that miR-29a
caused a decrease in α2AP expression in SSc fibroblasts (Fig. 5).
Discussion
SSc causes fibrosis of the skin and internal organs.
Previously, we showed that α2AP induces TGF-β production through
adipose triglyceride lipase, and is associated with myofibroblast
differentiation and ECM production (9). We also showed that the expression of
α2AP is elevated in SSc model mice and SSc fibroblasts, and that
its inactivation attenuates disease severity in SSc model mice and
SSc fibroblasts (16,17). These findings suggest that α2AP
contributes to the development of fibrosis in SSc. MMP-3, an
ECM-degrading enzyme, inactivates α2AP by cleaving its Pro19-Leu20
peptide bond (23). Here, we
focused on α2AP and MMP-3 to clarify their roles in the
pathogenesis of SSc.
In this study, we showed that serum levels of α2AP
and MMP-3 did not vary between healthy controls and SSc patients.
However, consistent with our previous findings, α2AP expression in
SSc dermal fibroblasts was increased. To determine whether MMP-3
contributes to the high expression of α2AP in SSc fibroblasts, we
measured the ratio of MMP-3/TIMP-1 as an indication of MMP-3
activity. Our results showed that this ratio was low in SSc dermal
fibroblasts. Taken together, these data suggest that the decrease
in MMP-3 activity might induce α2AP expression in SSc fibrotic
tissue. Moreover, skin-specific induction and development of
fibrosis may be due to increased α2AP expression and decreased
MMP-3 activity in tissue but not in serum.
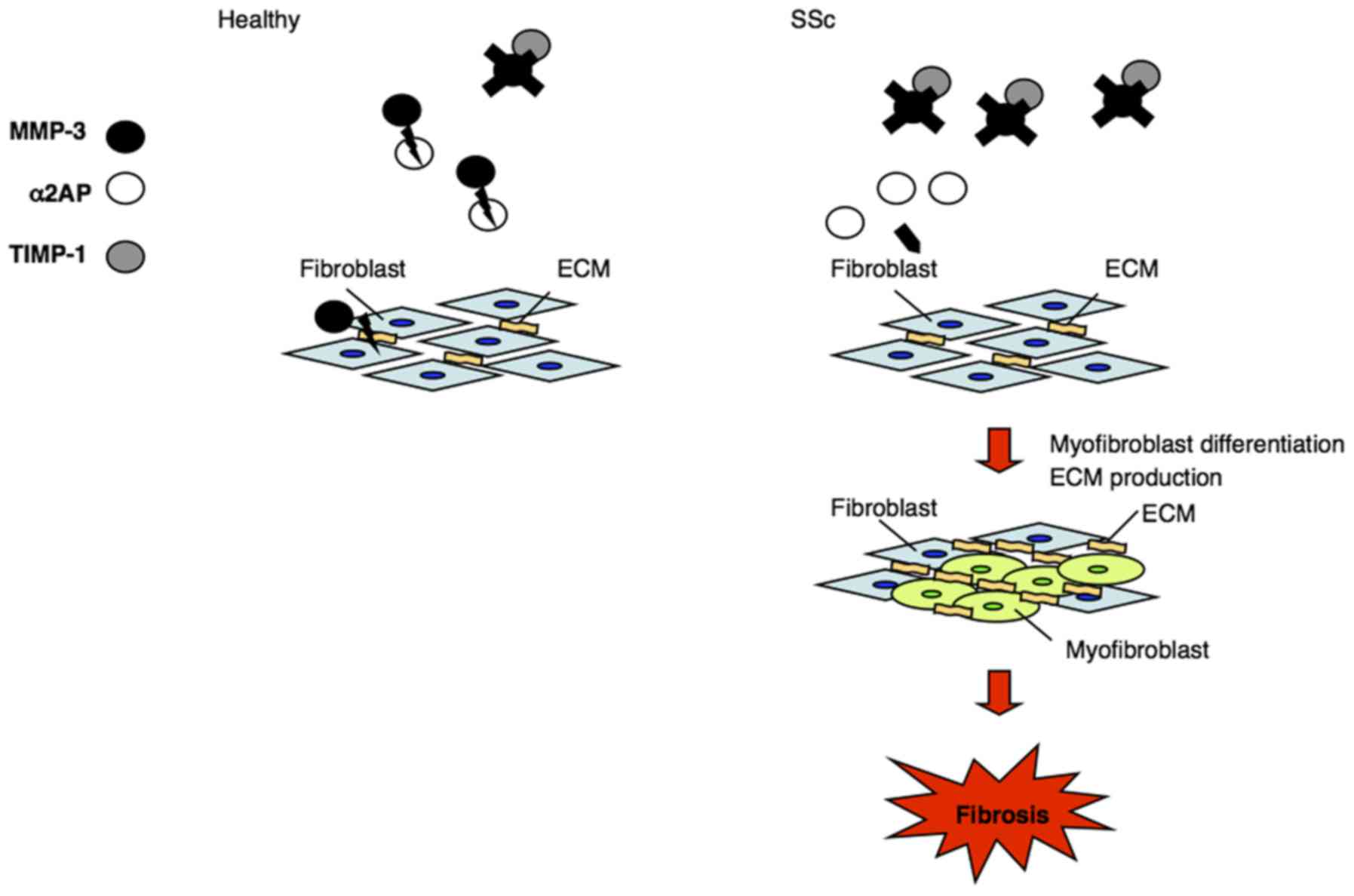
MMP-3 inactivates α2AP by proteolytic cleavage
(23). Here, we confirmed that
MMP-3 cleaved α2AP into two fragments and attenuated the
α2AP-induced pro-fibrotic effects, such as myofibroblast
differentiation and collagen production in normal fibroblasts. In
addition, treatment with MMP-3 suppressed the pro-fibrotic response
of SSc fibroblasts by reducing the expression of collagen and
myofibroblast markers. Moreover, MMP-3 is known to degrade ECM
components, such as collagen. The decrease in MMP-3 activity in SSc
dermal fibroblasts not only attenuates α2AP inactivation but also
promotes ECM deposition, and contributes to SSc progression
(Fig. 6).
miR-29a represses TIMP-1 expression, thereby
reversing the pro-fibrotic phenotype of SSc dermal fibroblasts
(25). In the present study, we
showed that miR-29a-transfected SSc fibroblasts exhibited a low
α2AP and TIMP-1 expression. Collectively, our data suggest that
TIMP-1-induced MMP-3 inhibition may lead to α2AP deposition in
SSc.
In conclusion, we found that the activity of MMP-3
was decreased and that α2AP expression was increased in SSc
fibroblasts. Moreover, treatment with MMP-3 or suppression with a
MMP-3 inhibitor, TIMP-1, reversed the pro-fibrotic phenotype of SSc
dermal fibroblasts. Our results suggest that decrease in MMP-3
activity in SSc fibroblasts causes α2AP and ECM deposition, and
contributes to the development of fibrosis. Thus, our findings
might contribute to a novel therapeutic approach for treatment of
SSc.
Acknowledgements
Not applicable.
Funding
The current study was partially supported by the
Takeda Science Foundation.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HN, YK, ES and MS designed the current study. HN, YK
and ES performed the experiments and analyzed data. HN, YK, ES and
MS wrote and edited the manuscript. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
The experiments utilizing human samples in the
current study were approved by the Gifu University Graduate School
of Medicine Ethics Committee (approval no. 29-152). Written
informed consent was obtained from the patients and volunteers
involved.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that there are no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
SSc
|
systemic sclerosis
|
|
α2AP
|
α2-antiplasmin
|
|
ECM
|
extracellular matrix
|
|
TIMP-1
|
tissue inhibitor of
metalloproteinase-1
|
References
|
1
|
Gilbane AJ, Denton CP and Holmes AM:
Scleroderma pathogenesis: A pivotal role for fibroblasts as
effector cells. Arthritis Res Ther. 15:30012013. View Article : Google Scholar
|
|
2
|
Collen D: Identification and some
properties of a new fast-reacting plasmin inhibitor in human
plasma. Eur J Biochem. 69:209–216. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kanno Y, Ishisaki A, Kawashita E, Kuretake
H, Ikeda K and Matsuo O: uPA attenuated LPS-induced inflammatory
osteoclastogenesis through the plasmin/PAR-1/Ca(2+)/CaMKK/AMPK
axis. Int J Biol Sci. 12:63–71. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Menoud PA, Sappino N, Boudal-Khoshbeen M,
Vassalli JD and Sappino AP: The kidney is a major site of
alpha(2)-antiplasmin production. J Clin Invest. 97:2478–2484. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kanno Y: The role of fibrinolytic
regulators in vascular dysfunction of systemic sclerosis. Int J Mol
Sci. 20:6192019. View Article : Google Scholar
|
|
6
|
Kanno Y, Hirade K, Ishisaki A, Nakajima S,
Suga H, Into T, Matsushita K, Okada K, Matsuo O and Matsuno H: Lack
of alpha2-antiplasmin improves cutaneous wound healing via
over-released vascular endothelial growth factor-induced
angiogenesis in wound lesions. J Thromb Haemost. 4:1602–1610. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kanno Y, Kuroki A, Okada K, Tomogane K,
Ueshima S, Matsuo O and Matsuno H: Alpha2-antiplasmin is involved
in the production of transforming growth factor beta1 and fibrosis.
J Thromb Haemost. 5:2266–2273. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kanno Y, Kawashita E, Minamida M, Kaneiwa
A, Okada K, Ueshima S, Matsuo O and Matsuno H: Alpha2-antiplasmin
is associated with the progression of fibrosis. Am J Pathol.
176:238–245. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kanno Y, Kawashita E, Kokado A, Okada K,
Ueshima S, Matsuo O and Matsuno H: Alpha2-antiplasmin regulates the
development of dermal fibrosis in mice by prostaglandin F(2α)
synthesis through adipose triglyceride lipase/calcium-independent
phospholipase A(2). Arthritis Rheum. 65:492–502. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kanno Y, Kawashita E, Kokado A, Kuretake
H, Ikeda K, Okada K, Seishima M, Ueshima S, Matsuo O and Matsuno H:
α2AP mediated myofibroblast formation and the development of renal
fibrosis in unilateral ureteral obstruction. Sci Rep. 4:59672014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kanno Y, Ishisaki A, Kuretake H, Maruyama
C, Matsuda A and Matsuo O: α2-antiplasmin modulates bone formation
by negatively regulating osteoblast differentiation and function.
Int J Mol Med. 40:854–858. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kawashita E, Kanno Y, Asayama H, Okada K,
Ueshima S, Matsuo O and Matsuno H: Involvement of α2-antiplasmin in
dendritic growth of hippocampal neurons. J Neurochem. 126:58–69.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hou Y, Okada K, Okamoto C, Ueshima S and
Matsuo O: Alpha2-antiplasmin is a critical regulator of angiotensin
II-mediated vascular remodeling. Arterioscler. Thromb Vasc Biol.
28:1257–1262. 2008. View Article : Google Scholar
|
|
14
|
Jinnin M, Ihn H, Yamane K, Asano Y, Yazawa
N and Tamaki K: Plasma plasmin-alpha2-plasmin inhibitor complex
levels are increased in systemic sclerosis patients with pulmonary
hypertension. Rheumatology (Oxford). 42:240–243. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kawakami M, Kawagoe M, Harigai M, Hara M,
Hirose T, Hirose W, Norioka K, Suzuki K, Kitani A and Nakamura H:
Elevated plasma levels of alpha 2-plasmin inhibitor-plasmin complex
in patients with rheumatic diseases. Possible role of fibrinolytic
mechanism in vasculitis. Arthritis Rheum. 32:1427–1433. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kanno Y, Shu E, Kanoh H and Seishima M:
The antifibrotic effect of α2AP neutralization in systemic
sclerosis dermal fibroblasts and mouse models of systemic
sclerosis. J Invest Dermatol. 136:762–769. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kanno Y, Shu E, Kanoh H, Matsuda A and
Seishima M: α2AP regulates vascular alteration by inhibiting VEGF
signaling in systemic sclerosis: The roles of α2AP in vascular
dysfunction in systemic sclerosis. Arthritis Res Ther. 19:222017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen Q, Jin M, Yang F, Zhu J, Xiao Q and
Zhang L: Matrix metalloproteinases: Inflammatory regulators of cell
behaviors in vascular formation and remodeling. Mediators Inflamm.
Jun 12–2013.(Epub ahead of print). View Article : Google Scholar
|
|
19
|
Van Hove I, Lemmons K, Van de Velde S,
Verslegers M and Moons L: Matrix metalloproteinase-3 in the central
nervous system: A look on the bright side. J Neurochem.
123:203–216. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nishijima C, Hayakawa I, Matsushita T,
Komura K, Hasegawa M, Takehara K and Sato S: Autoantibody against
matrix metalloproteinase-3 in patients with systemic sclerosis.
Clin Exp Immunol. 138:357–363. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jinnin M, Ihn H, Asano Y, Yamane K, Yazawa
N and Tamaki K: Serum matrix metalloproteinase-3 in systemic
sclerosis. Arch Dermatol Res. 296:25–29. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Young-Min SA, Beeton C, Laughton R,
Plumpton T, Bartram S, Murphy G, Black C and Cawston TE: Serum
TIMP-1, TIMP-2, and MMP-1 in patients with systemic sclerosis,
primary Raynaud's phenomenon, and in normal controls. Ann Rheum
Dis. 60:846–851. 2001.PubMed/NCBI
|
|
23
|
Lijnen HR, Van Hoef B and Collen D:
Inactivation of the serpin alpha(2)-antiplasmin by stromelysin-1.
Biochim Biophys Acta. 1547:206–213. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bonaventura P, Lamboux A, Albarède F and
Miossec P: Regulatory effects of zinc on cadmium-induced
cytotoxicity in chronic inflammation. PLoS One. 12:e01808792017.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ciechomska M, O'Reilly S, Suwara M,
Bogunia-Kubik K and van Laar JM: miR-29a reduces TIMP-1 production
by dermal fibroblasts via targeting TGF-β activated kinase 1
binding protein 1, implications for systemic sclerosis. PLoS One.
9:e1155962014. View Article : Google Scholar : PubMed/NCBI
|