Introduction
Rheumatoid arthritis (RA) is a chronic autoimmune
disease characterized by swelling, pain, stiffness and deformity of
peripheral joints, which affects 0.5–1% of the adult population
worldwide (1). Although a variety
of antirheumatic drugs, such as cytokine antagonists, and B cell
depletion and T cell co-stimulation blockers, display clinical
efficacy for the treatment of RA, the life expectancy in patients
with RA is 10–15 years shorter compared with the general population
(2). Therefore, developing novel
and more potent therapeutic agents for RA is important.
RA is an inflammatory condition that primarily
affects the small synovial joints of the hands and feet (3). The main pathological features of RA
include synovial hyperplasia and the secretion of proinflammatory
cytokines, such as tumor necrosis factor-α (TNF-α), interleukin
(IL)-1, IL-6 and IL-8, in the affected joints (4,5).
Activated fibroblast-like synoviocytes (FLSs) serve a central role
in RA pathogenesis by producing inflammatory cytokines, chemokines
and proteinases that degrade the extracellular matrix (4,6). A
number of proinflammatory cytokines, such as TNF-α, IL-1 and IL-6,
are involved in the pathogenesis of RA, serving as therapeutic
targets for the development of novel drugs against RA (7).
Toll-like receptors (TLRs), a family of type I
transmembrane glycoproteins, have been implicated in the
inflammatory and immune responses in RA. When exposed to an
immunogenic stimulus, cells within the joint display increased TLR
expression and increased expression of the corresponding ligands,
such as bacterial peptidoglycan and heat shock protein 60 (8), which triggers rapid expression of
proinflammatory cytokines and chemokines that orchestrate immune
responses, leading to inflammation and damage to the joints in
patients with RA (9). Previous
studies have highlighted the importance of TLR2 in RA via in
vitro systems and animal models (10,11).
Highly expressed TLR2 in RA synovial tissue-lining macrophages and
fibroblasts heterodimerizes with TLR1 or TLR6 upon ligand binding
and interacts with myeloid differentiation factor 88 (MYD88) to
initiate a signaling cascade that results in activation of key
transcription factors, including NF-κB (12). Previous studies have demonstrated
that TLR2 ligation in RA FLSs enhances proinflammatory cytokine
secretion (13), and TLR2 blockade
significantly inhibits cytokine secretion (14), indicating an indispensable role of
TLR2 signaling in RA development. Therefore, targeting TLR2 and the
downstream signaling pathways may serve as a potential therapeutic
approach in RA treatment.
Baicalin (7-glucuronic acid, 5,6-dihydroxyflavone)
is a flavonoid compound isolated from the root of Scutellaria
baicalensis (15), which
possesses multiple pharmacological effects, including
anti-inflammatory, antioxidative, antiapoptotic and immune
regulatory properties (16–18).
Previous studies have suggested that baicalin could ameliorate CIA
in animal models (19,20). In addition, baicalin can attenuate
periodontitis and lipopolysaccharide (LPS)-induced fever in rat
models via inhibition of the TLR2 or TLR4/MYD88/p38 mitogen
activated protein kinase (MAPK)-NF-κB signaling pathways (21,22).
However, whether TLR2 signaling is associated with the beneficial
role of baicalin in CIA is not completely understood.
In the present study, a CIA rat model that is widely
used to mimic the joint inflammation observed in human RA (23) was established to evaluate the
potential therapeutic effect of baicalin in CIA. Alterations to the
serum levels of proinflammatory cytokines and the expression of
TLR2, MYD88 and NF-κB p65 in response to baicalin treatment
were examined to investigate the mechanisms underlying the
therapeutic effects of baicalin in CIA. The present study indicated
that baicalin may serve as a promising therapeutic agent to target
the inflammatory response and TLR2/MYD88/NF-κB signaling in
RA.
Materials and methods
Animals and CIA modeling
A total of 100 male Sprague-Dawley (SD) rats (age, 8
weeks; weight, 180±20 g) were obtained from the Experimental Animal
Center of Ningxia Medical University (certificate no. SCXK
2015-0001). The rats were fed and housed in a standard
pathogen-free environment with a temperature of 22±2°C, 55±5%
humidity, 12-h light/dark cycles and free access to food and water.
All animal care and procedures described in the present study were
approved by the Ethics Committee of Ningxia Medical University
(approval no. 2015-125). All animal experiments were performed in
accordance with the Guidelines for the Care and Use of Animals
published by the P.R. China Ministry of Health (January 1998)
(24). The CIA model was
established in 84 rats as previously described (13,14).
The normal group consisted of the remaining 16 rats. Briefly, 0.1
ml bovine collagen II (Sigma-Aldrich; Merck KGaA) emulsified in
complete Freund's adjuvant (Sigma-Aldrich; Merck KGaA) was
administered twice at 7-day intervals by intra-dermal injection
into the back, base of tail and the footpad of each rat. At 14 days
after the initial immunization, each rat was administered with a
booster subcutaneous injection at the base of tail. After the
booster injection, the degree of joint redness and swelling were
evaluated using a 5-grade arthritis scoring method, as previously
described (15,16). The paw thickness (mm) of each rat
was measured with a Vernier caliper every 7 days during the
establishment of the CIA rat model (28 days). At 28 days after the
initial immunization, 80 rats with an arthritis score of ≥2 were
selected as CIA model rats.
Baicalin treatment
At 28 days after the initial immunization, the CIA
model rats were randomly divided into five groups (n=16
rats/group): i) Model; ii) Tripterygium Glycosides Tablet (TGT;
Yuanda Pharmaceutical Huangshi Feiyun Pharmaceutical Co., Ltd.; 10
mg/kg/day); iii) 15 mg/kg/day baicalin (purity 98%; Nanjing Chunqiu
Biological Engineering Co., Ltd.); (iv) 30 mg/kg/day baicalin; and
v) 60 mg/kg/day baicalin. The doses of baicalin used in the present
study were determined based on a previous study (1). TGT was dissolved in normal saline and
baicalin was dissolved in saline (pH 8.0). Rats were administered
once a day with TGT or baicalin by oral gavage. Rats in the normal
and model groups were administered once a day with an equivalent
volume of saline by oral gavage. After 40 days of treatment, the
rats were fasted for 8 h (with free access to water) and then
anaesthetized with 10% chloral hydrate (300 mg/kg) via
intraperitoneal injection. Blood (~5 ml) was obtained from the
retro-orbital plexus and maintained at room temperature for 2 h.
Serum was collected by centrifugation at 2,000 × g, 4°300*12C for
15 min and stored at −80°C until further use. Subsequently, the
rats were sacrificed by cervical dislocation. No signs of
peritonitis were observed prior to sacrifice. The synovium was
immediately isolated from the hind knee joint, washed with sterile
normal saline and stored at −80°C.
Cell culture and treatment
Normal human fibroblast-like synoviocytes (HFLS;
cat. no. 338586; BeNa Culture Collection) and RA human
fibroblast-like synoviocytes (HFLS-RA; cat. no. 337864; BeNa
Culture Collection) were maintained in synoviocyte growth medium
(Gibco; Thermo Fisher Scientific, Inc.) supplemented with 100 IU/ml
penicillin and 100 mg/ml streptomycin at 37°C in a humidified
atmosphere of 5% CO2. Passage 4–7 cells were used for
subsequent in vitro experiments. HFLS-RAs were serum-starved
for 12 h at 37°C prior to baicalin treatment (25, 50 or 100 µg/ml)
for 48 h at 37°C. Untreated HFLSs were used as controls.
Radiographic assessment
Following sacrifice, the rat hind paws were
subjected to X-ray scans to observe the radiologic alterations
using a MRAD-D50S RADREX–I system (Toshiba Medical Manufacturing
Co., Ltd.) with the following parameters: 40 kV, 100 mA and 0.02
millisecond. Images were read and scored by a blinded independent
radiologist as previously described (25): 0, no radiologic alterations; 1,
mild alterations with tissue swelling and edema; 2, moderate
alterations with joint erosion and disfiguration; 3, severe
alterations with bone erosion and osteophyte formation. The
radiologic score for each rat was expressed as the sum of the
results of both hind paws.
Hematoxylin and eosin (H&E)
staining
Rat synovium was fixed using 4% paraformaldehyde for
48 h at room temperature, dehydrated using a graded alcohol series,
cleared with xylene and embedded in paraffin at room temperature.
Tissues were cut into 5-µm thick paraffin sections using a tissue
microtome. The deparaffinized tissue sections were stained with
H&E for 10 min at room temperature. Synovial hyperplasia was
observed in three randomly selected fields of view using a CKX41SF
inverted microscope (Olympus Corporation; magnification, ×200).
ELISA
Serum levels of IL-1β, TNF-α and IL-6 were
determined using ELISA kits (Dakewe Biotech, Ltd.; cat. nos.
DKW12-3720, DKW12-3012 and DKW12-3060, respectively) according to
the manufacturer's instructions.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was isolated from rat synovial tissues or
in vitro cultured human cells using TRIzol®
(Invitrogen) according to the manufacturer's instructions. Total
RNA was reverse transcribed into cDNA at 42°C for 60 min, then 70°C
for 10 min using M-MLV reverse transcription kit (Promega
Corporation), according to the manufacturer's protocol. RT-qPCR was
performed using a 20 µl volume containing 1.5 µl cDNA, 0.5 µl
forward primer (10 pmol/µl), 0.5 µl reverse primer (10 pmol/µl), 10
µl SYBR-Green mix (Bio-Rad Laboratories, Inc.) and 7.5 µl deionized
distilled H2O. The sequences of the primers (Sangon
Biotech Co., Ltd.) used for qPCR are listed in Tables I and II. The following thermocycling
conditions were used for qPCR: Initial denaturation at 94°C for 15
min; followed by 40 cycles of denaturation at 94°C for 15 sec,
annealing at 60°C for 34 sec, and extension at 72°C for 15 sec; and
final extension at 72°C for 10 min. mRNA expression levels were
quantified using the 2−ΔΔCq method (26) and normalized to the internal
reference gene GAPDH.
 | Table I.Primer sequences used to detect gene
expression in rat synovial tissue by reverse
transcription-quantitative PCR. |
Table I.
Primer sequences used to detect gene
expression in rat synovial tissue by reverse
transcription-quantitative PCR.
| Gene | Sequence
(5′-3′) |
|---|
| TLR2 | F:
GACTCTGGAAGCAGGTGACA |
|
| R:
CAGTCAACCAGGACATGGAC |
| MYD88 | F:
TGGTGGTTGTTTCTGACGAT |
|
| R:
GATCAGTCGCTTCTGTTGGA |
| GAPDH | F:
CAACTCCCTCAAGATTGTCAGCAA |
|
| R:
GGCATGGACTGTGGTCATGA |
 | Table II.Primer sequences used to detect gene
expression in human fibroblast-like synoviocytes by reverse
transcription-quantitative PCR. |
Table II.
Primer sequences used to detect gene
expression in human fibroblast-like synoviocytes by reverse
transcription-quantitative PCR.
| Gene | Sequence
(5′-3′) |
|---|
| TLR2 | F:
AAGCACTGGCCAAAGTCTTG |
|
| R:
TGTCCTGTGACATTCCGACA |
| MYD88 | F:
CCTGCAGAGCAAGGAATGTG |
|
| R:
GTCGCAGACAGTGATGAACC |
| GAPDH | F:
GGGTGTGAACCATGAGAAGT |
|
| R:
GACTGTGGTCATGAGTCCT |
Western blotting
Total protein was extracted from rat synovial
tissues or in vitro cultured human cells in pre-cooled cell
lysis solution (Beijing Solarbio Science & Technology Co.,
Ltd.) containing protease and phosphatase inhibitors to inhibit
protein degradation (Beijing Solarbio Science & Technology Co.,
Ltd.). Total protein was quantified using a bicinchoninic acid
protein assay method. Proteins were separated by 10% SDS-PAGE and
transferred to PVDF membranes. The membranes were blocked with 5%
skim milk diluted by 2% PBS for 60 min at room temperature and then
incubated overnight at 4°C with the following primary antibodies:
Anti-TLR2 (Santa Cruz Biotechnology, Inc.; cat. no.sc-10739;
1:1,000), anti-MYD88 (Abcam; cat. no. ab2064; 1:2,000), anti-NF-κB
p65 (CST Biological Reagents Co., Ltd.; cat. no.4764; 1:3,000) and
anti-GAPDH (Abcam; cat. no. ab8245; 1:500). The membranes were
washed with TBS containing 0.1% Tween™ 20 and incubated with
horseradish peroxidase-conjugated secondary antibodies (OriGene
Technologies, Inc.; cat. no. ZB-2301; 1:1,000 for TLR2; 1:2,000 for
MYD88; 1:2,000 for NF-κB p65; 1:10,000 for GAPDH) at 37°C for 60
min. After washing with TBS three times, protein bands were
visualized using electrochemiluminescence reagent (Santa Cruz
Biotechnology, Inc.). Protein expression levels were quantified
using Image-Pro Plus software (version 6.0; Media Cybernetics,
Inc.) with GAPDH as the loading control.
Statistical analysis
Data are expressed as the mean ± standard deviation
of three experiments (n=6 rats in each group). Statistical analyses
were conducted using SPSS software (version 17.0; SPSS, Inc.).
Comparisons among multiple groups were analyzed using one-way ANOVA
followed by Tukey's post hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
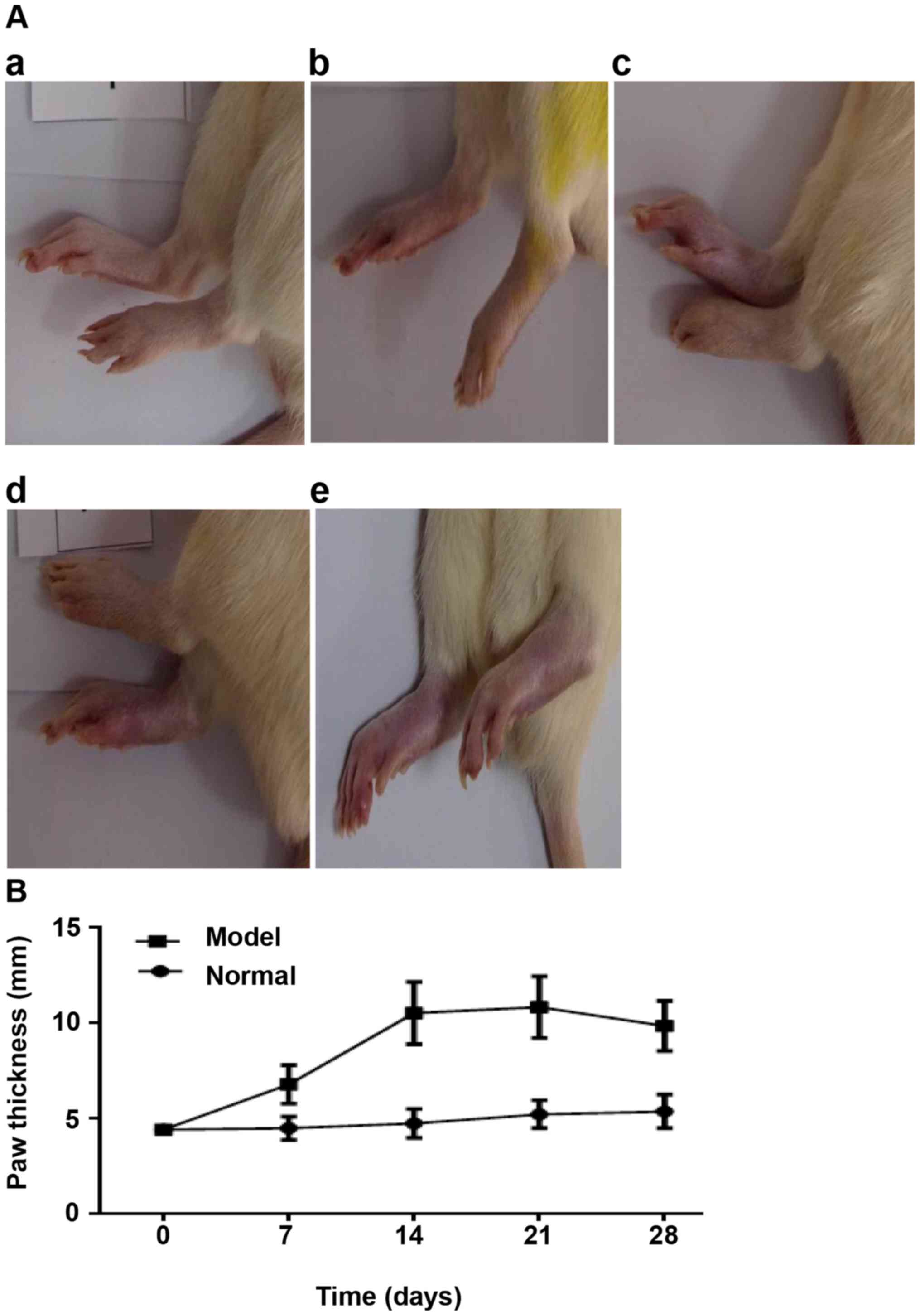
Baicalin treatment alleviates
collagen-induced joint injury in rats
To investigate the potential therapeutic effects of
baicalin on RA, a CIA rat model that is widely used to mimic the
joint inflammation observed in patients with RA was established
(23). The onset of arthritis in
the model group occurred at day 7 following primary collagen
immunization, as evidenced by a notable increase in hind paw
thickness from day 7 to day 28 (Fig.
1B) and clinical manifestations, such as functional impairment
and swollen red paws, compared with the normal group (Fig. 1A). No joint destruction was
observed in the normal group, whereas notable bone erosion was
observed in the model group (Fig.
2). Compared with the model group, the radiographic change in
the 15 mg/kg baicalin group was minimal. Moreover, moderate
alterations were observed in rats treated with TGT or 30 mg/kg
baicalin, whereas obvious alleviation was observed in the joints of
rats treated with 60 mg/kg baicalin compared with the model group
(Fig. 2). Consistently, the mean
radiologic scores of rats receiving TGT, 30 mg/kg baicalin and 60
mg/kg baicalin were significantly lower compared with the model
group (P<0.01; Fig. 2),
suggesting that both TGT and baicalin could alleviate
collagen-induced bone erosion and destruction of the joints. CIA
model rats receiving 60 mg/kg baicalin exhibited the most potent
protection, as indicated by the lowest radiologic score recorded
among the five different groups (Fig.
2).
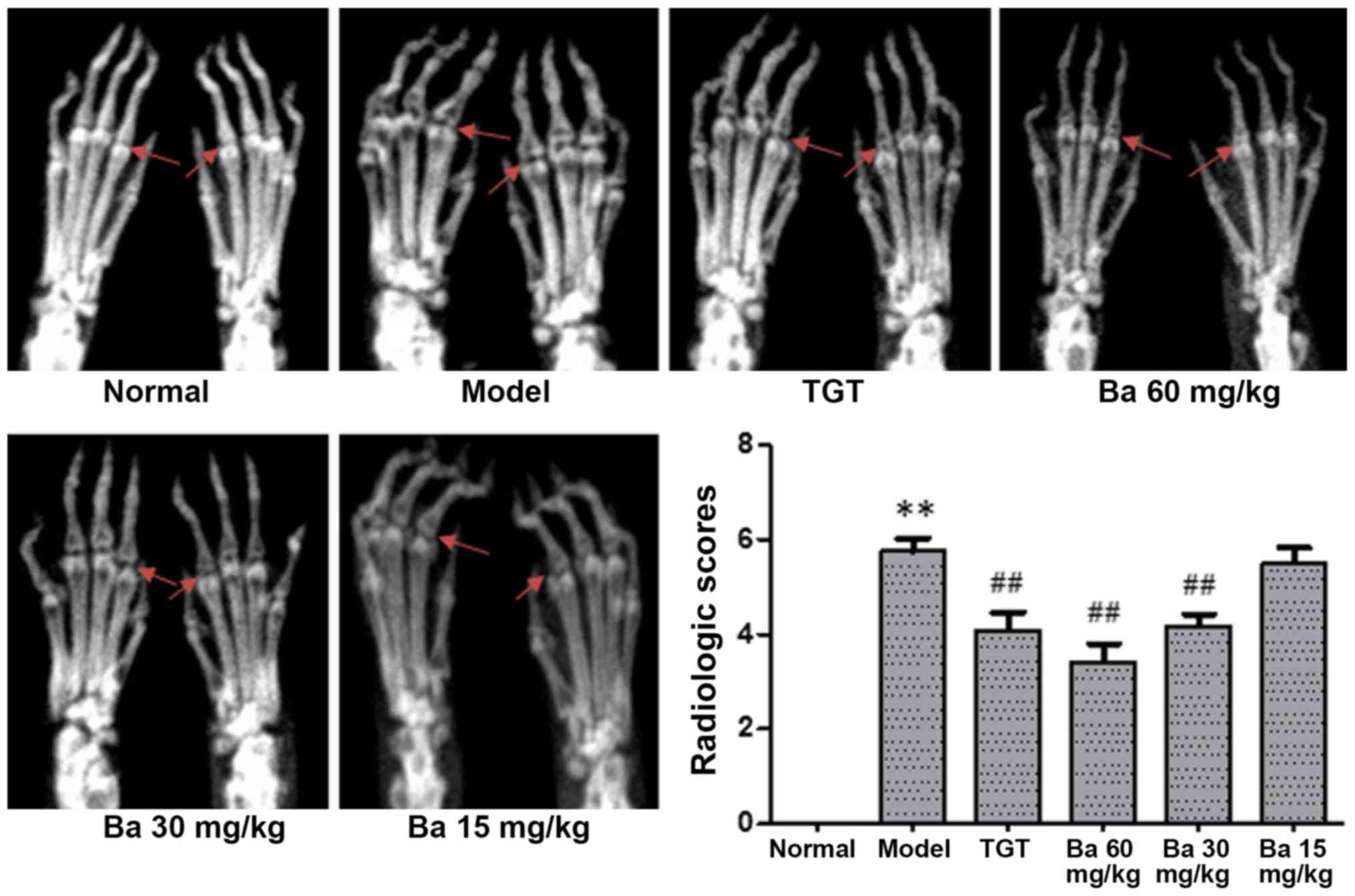 | Figure 2.Therapeutic effects of baicalin on
joint destruction in collagen-induced arthritis model rats. After
40 days of treatment, the rats were sacrificed. In each group, six
rats were randomly selected and their hind limbs were subjected to
radiographic assessment using the following scoring method: 0, no
radiologic alterations; 1, mild alterations with tissue swelling
and edema; 2, moderate alterations with joint erosion and
disfiguration; 3, severe alterations with bone erosion and
osteophyte formation. As indicate by the red arrows, no joint
destruction was observed in the normal group, whereas notable bone
erosion was observed in the model group. **P<0.01 vs. normal;
##P<0.01 vs. model. TGT, tripterygium glycosides
tablet; Ba, baicalin. |
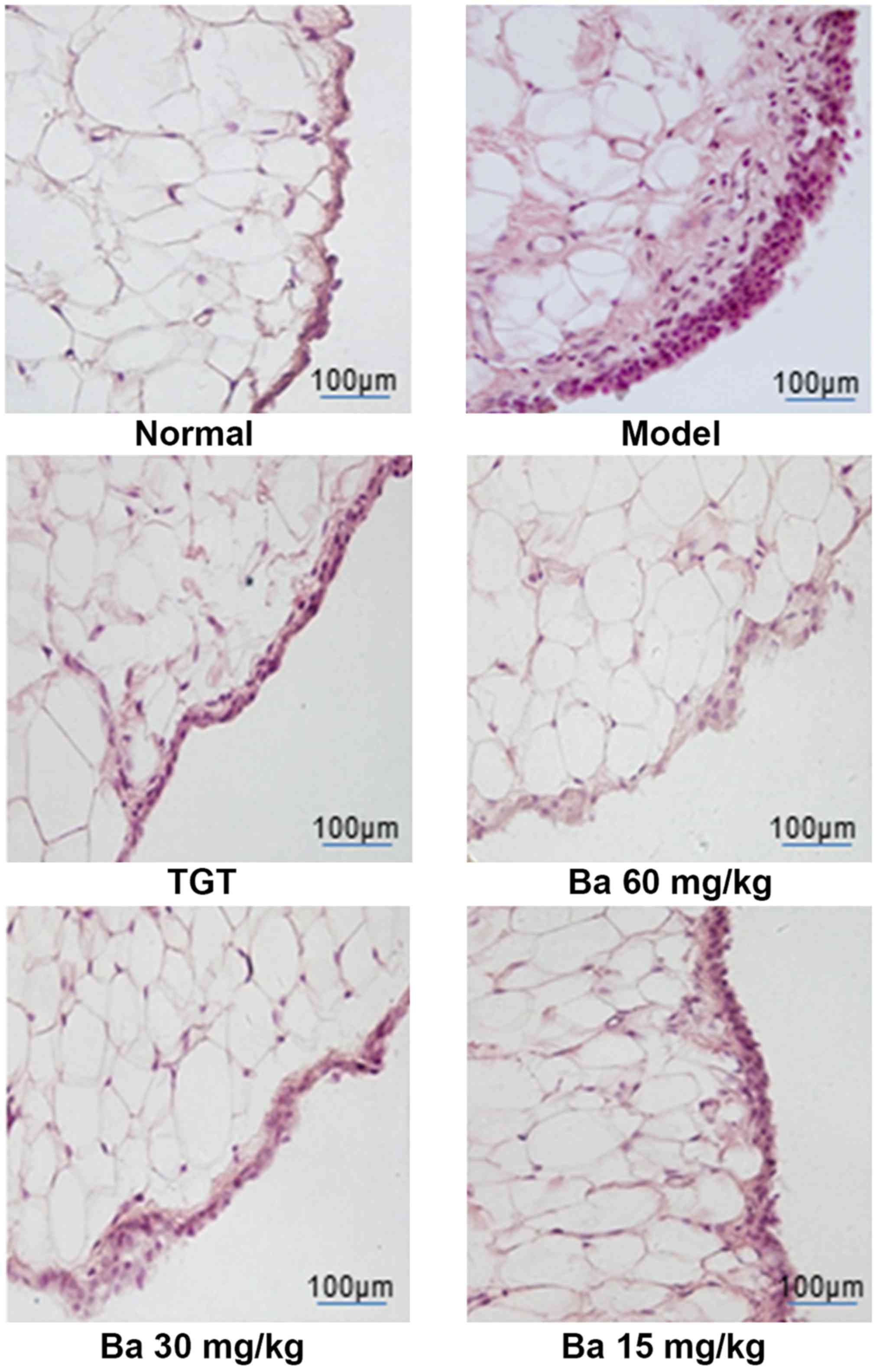
Subsequently, histologic analysis of synovial tissue
from the knee joint after 40 days of treatment was performed to
further evaluate the therapeutic effect of baicalin. H&E
staining indicated that the normal group exhibited a normal
synovium appearance, whereas the model group demonstrated notable
histological abnormalities, including synovial thickening,
inflammatory cell infiltration and excessive proliferation of
synovial fibroblasts. Compared with the model group,
baicalin-treated rats displayed a dose-dependent improvement to
histological changes, further indicating the beneficial effect of
baicalin on collagen-induced joint injury (Fig. 3).
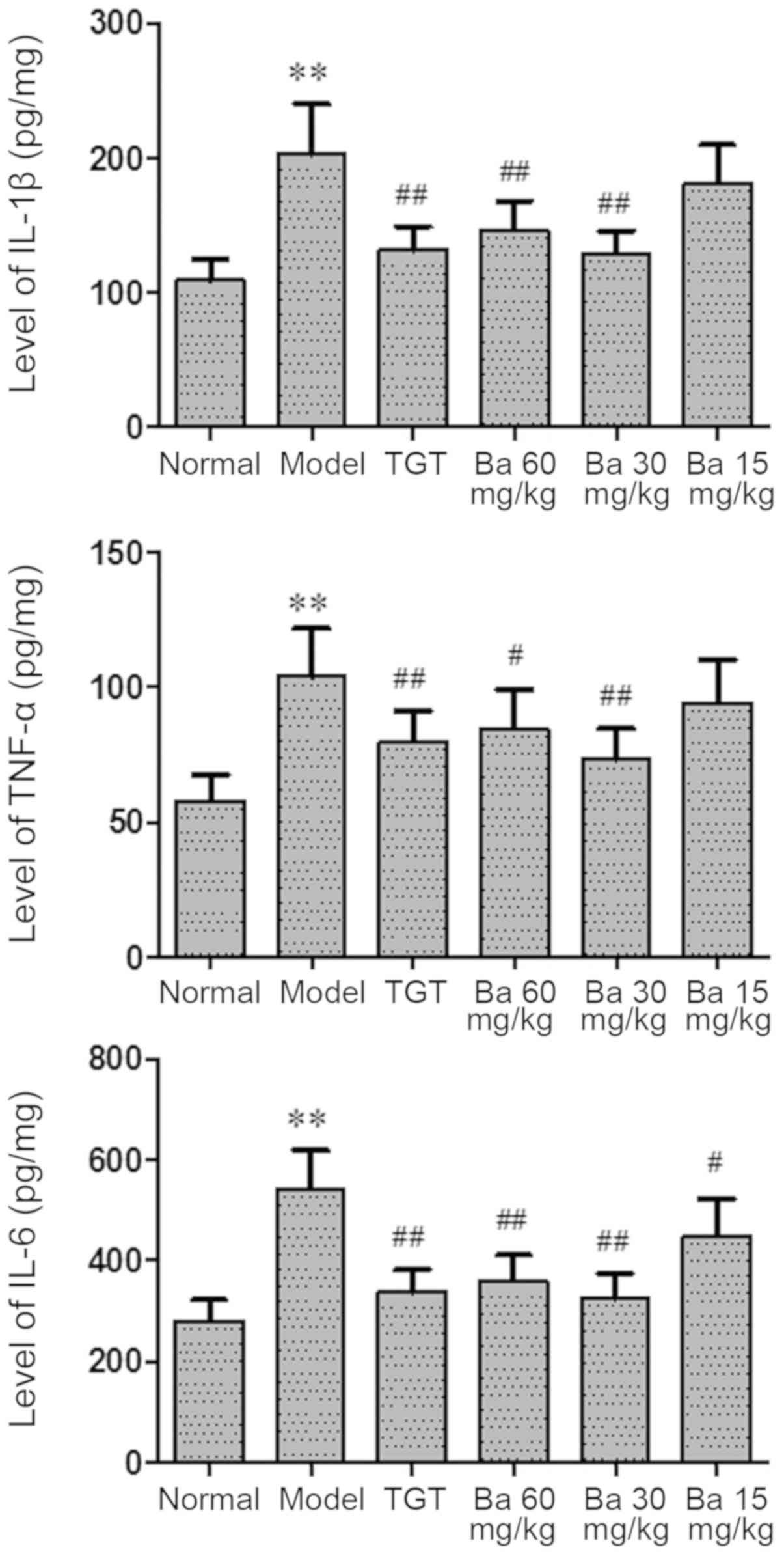
Baicalin inhibits the production of
IL-1β, TNF-α and IL-6
RA is a chronic inflammatory disease (6); therefore, whether baicalin could
modulate the inflammatory process in CIA model rats was
investigated. The serum levels of proinflammatory cytokines,
including IL-1β, TNF-α and IL-6, were significantly increased in
the model group compared with the normal group (P<0.01), whereas
the serum levels of proinflammatory cytokines were significantly
decreased in the TGT (P<0.01), 30 mg/kg baicalin (P<0.01) and
60 mg/kg baicalin (P<0.05 for TNF-α; P<0.01 for IL-1β and
IL-6) groups compared with the model group, suggesting an
anti-inflammatory role of baicalin in CIA model rats (Fig. 4).
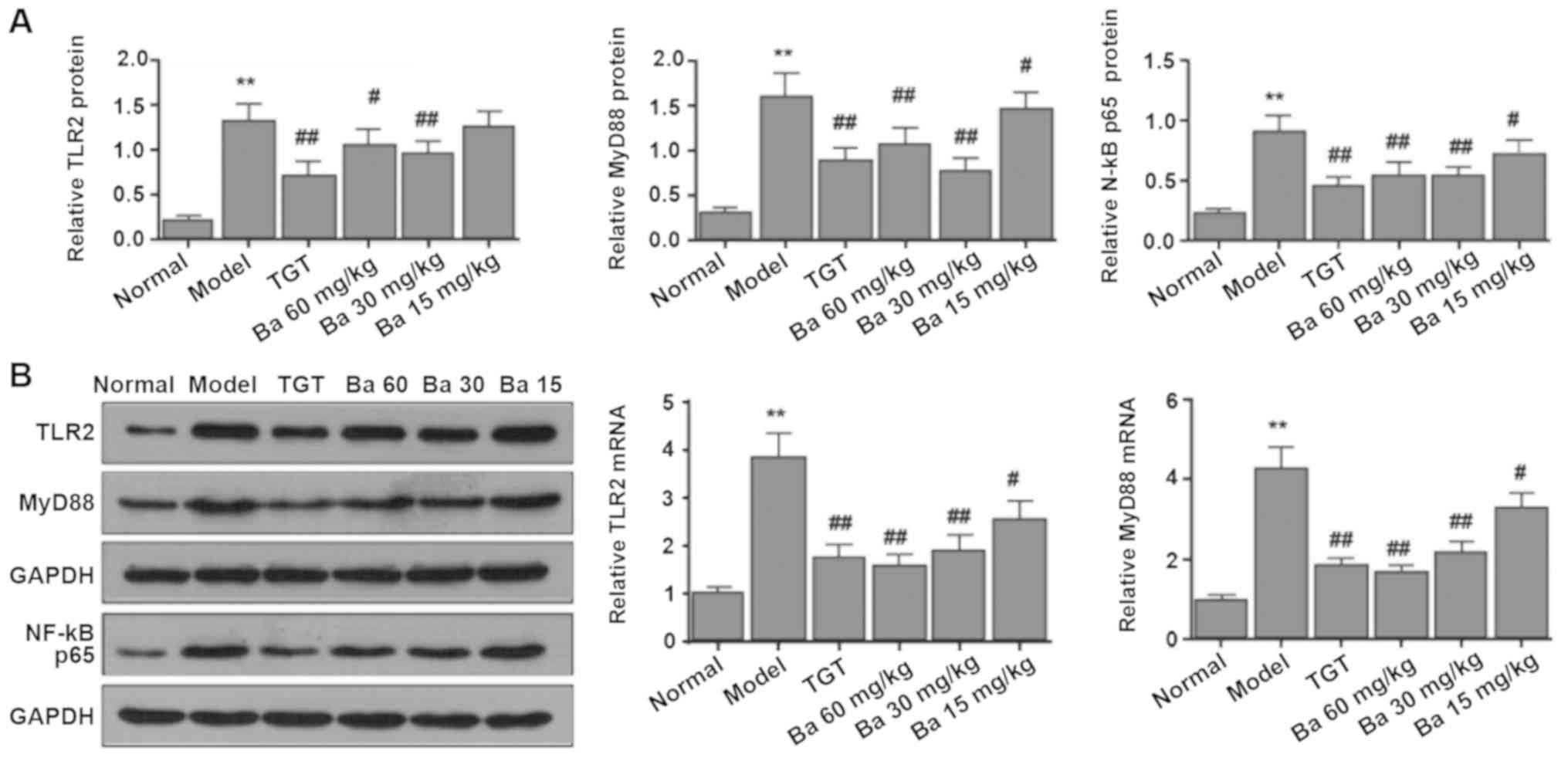
Baicalin suppresses the
TLR2/MYD88/NF-κB signaling pathway in the synovial tissue of
rats
To further investigate the mechanism underlying the
anti-inflammatory effect of baicalin in CIA model rats, the
expression levels of TLR2, MYD88 and NF-κB p65 in the synovial
tissue from the knee joint of rats were measured, as TLR signaling
is extensively involved in the inflammatory response in human RA
(27). Compared with the model
group, treatment with different doses of baicalin significantly
attenuated CIA-induced mRNA and protein expression of TLR2 and
MYD88; however, TLR2 protein expression levels were not
significantly decreased by 15 mg/kg baicalin compared with the
model group. Similar results were observed for the protein
expression levels of NF-κB (Fig.
5). The results suggested an involvement of the
TLR2/MYD88/NF-κB signaling pathway in the development of CIA in
rats.
Baicalin suppresses the
TLR2/MYD88/NF-κB signaling pathway in cultured HFLSs
Subsequently, the expression levels of TLR2, MyD88
and NF-κB p65 in cultured HFLSs were measured. Compared with
untreated HFLS-RA cells, the mRNA expression levels of TLR2 and
MYD88, as well as the protein expression levels of TLR2, MYD88 and
NF-κB p65 were significantly decreased in baicalin-treated HFLS-RA
cells. However, compared with untreated HFLA-RAs, the protein
expression level of NF-κB p65 was not significantly decreased by 25
mg/kg baicalin (Fig. 6). The
results were consistent with the results observed in CIA model
rats, further indicating the involvement of the TLR2/MYD88/NF-κB
signaling pathway in synovial inflammation.
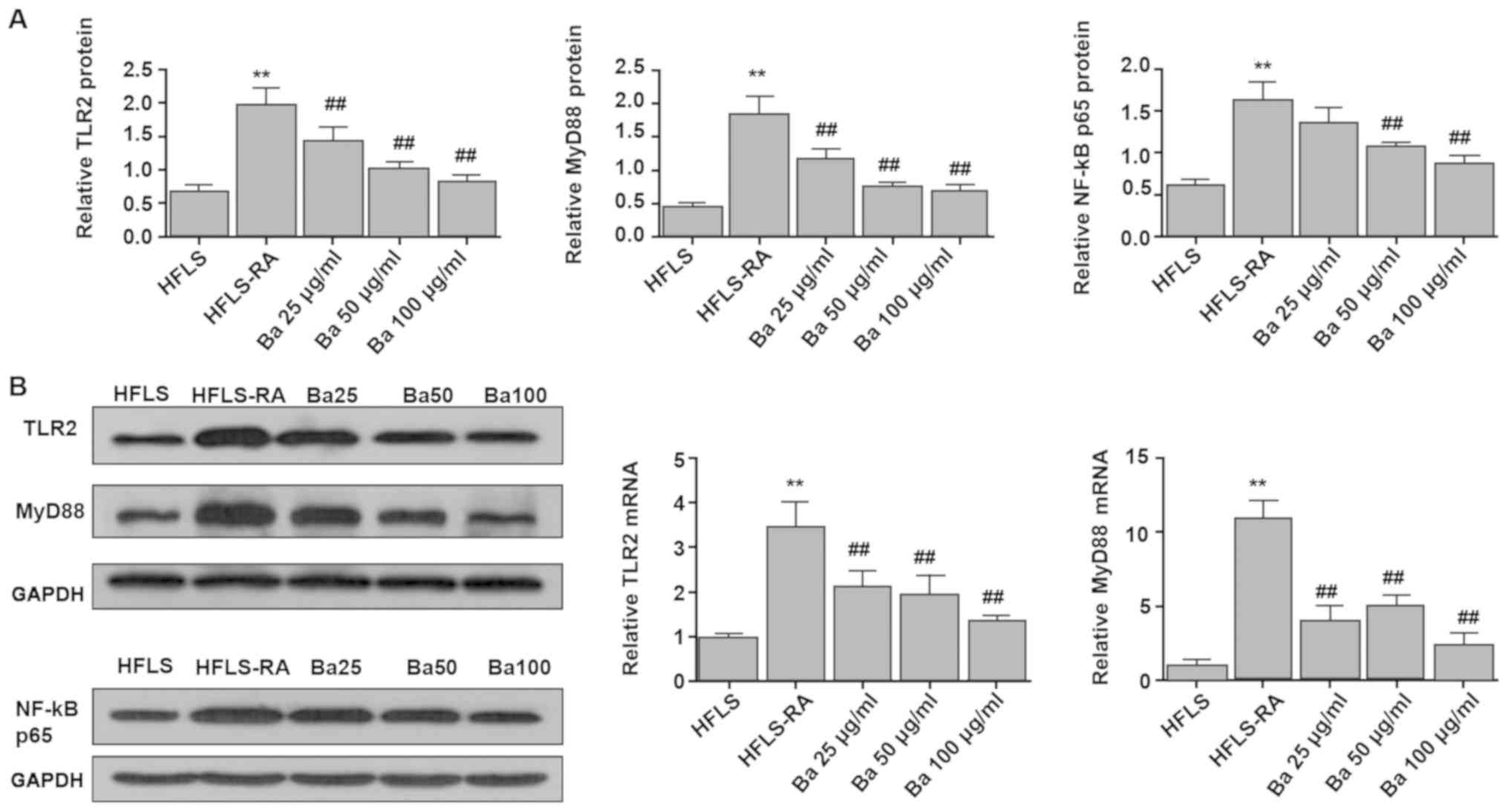 | Figure 6.TLR2, MYD88 and NF-κB p65 expression
in HFLSs and HFLS-RAs. The (A) protein expression levels of TLR2,
MYD88 and NF-κB p65, and (B) mRNA expressions of TLR2 and MYD88
were measured. Compared with untreated HFLS-RA cells, the mRNA
expression levels of TLR2 and MYD88, as well as the protein
expression levels of TLR2, MYD88 and NF-κB p65 were significantly
decreased in baicalin-treated HFLS-RA cells. **P<0.01 vs. HFLS;
##P<0.01 vs. HFLS-RA. TLR2, toll-like receptor 2;
MYD88, myeloid differentiation factor 88; HFLS, human
fibroblast-like synoviocyte; HFLS-RA, human fibroblast-like
synoviocyte-rheumatoid arthritis; TGT, tripterygium glycosides
tablet; Ba, baicalin. |
Discussion
The CIA model is the most widely used animal model
for RA studies due to the similar pathological and arthritic
presentations between CIA model animals and patients with RA
(23). In the present study, CIA
was induced in male SD rats, and a notable increase in hind paw
thickness was observed in the model group compared with the normal
group from day 7 post-primary collagen immunization. In the later
stages of CIA model induction, acute inflammation gradually
alleviated, leading to the redness and swelling of hind paws
gradually subsiding. As chronic inflammation continued, CIA model
rats displayed clinical manifestations, such as functional
impairment and notable histological abnormalities in the synovial
tissue, such as synovial thickening and inflammatory cell
infiltration. The results indicated that the CIA model was
successfully established in the present study. The results also
indicated that baicalin dose-dependently alleviated joint injury in
CIA model rats. Furthermore, to the best of our knowledge, the
present study demonstrated for the first time that baicalin could
suppress the TLR2/MYD88/NF-κB signaling pathway in the synovial
tissue of CIA model rats as well as in HFLS-RAs in vitro,
suggesting that blockade of TLR2/MYD88/NF-κB signaling may be
associated with the beneficial role of baicalin in CIA model
rats.
The pathogenic mechanism underlying RA is complex,
with genetic, environmental and immunological factors contributing
to RA incidence and development (28,29).
Increasing evidence indicates that various immune cells and
cytokines are involved in the pathogenesis of RA (30). The present study indicated that
there was a notable increase in the number of inflammatory cells
and a significant increase in serum levels of IL-1β, TNF-α and IL-6
in the synovial tissue were observed in CIA model rats compared
with normal rats, which was consistent with previous studies
(31,32). It has been reported that baicalin
exerts anti-inflammatory effects in various disorders. For example,
baicalin inhibits LPS-induced inflammation caused by endotoxic
shock (21). Baicalin
administration also inhibits inflammatory cell infiltration and
expression of proinflammatory cytokines in an animal model of
multiple sclerosis (33). Similar
results were observed in the present study, highlighting the
potential of baicalin as an anti-inflammatory therapeutic agent in
RA.
The TLR2/MYD88/NF-κB signaling pathway serves an
essential role in RA development by enhancing the secretion of
proinflammatory cytokines and matrix metallopeptidases (10–14).
The present study indicated that the mRNA and protein expression
levels of TLR2, MYD88 and NF-κB p65 in synovial tissue, as well as
the systemic levels of IL-1β, TNF-α and IL-6 were significantly
increased in CIA model rats compared with normal rats. Previous
studies demonstrated that baicalin inhibits the TLR2 or
TLR4/MYD88/p38 MAPK/NF-κB signaling pathways in periodontitis and
LPS-induced fever in rats (21,22).
In addition, suppression of synovial NF-κB p65 expression is
involved in baicalin-relieved joint inflammation in CIA model rats
(20). In the present study,
following treatment with different doses of baicalin, the
amelioration of synovial lesions was accompanied by significant
suppression of the TLR2/MYD88/NF-κB p65 signaling pathway,
suggesting that the beneficial effect of baicalin on CIA may be
mediated via inhibiting activation of the TLR2/MYD88/NF-κB
signaling pathway in the synovial tissue of CIA model rats.
Collectively, the results of the present study
suggested that baicalin exerted an anti-inflammatory effect on CIA
model rats, and may serve as a potential therapeutic agent in RA.
The inhibitory role of baicalin in collagen-induced inflammatory
responses may be mediated by suppression of the TLR2/MYD88/NF-κB
p65 signaling pathway.
The present study had two main limitations. Firstly,
a large number of rats were used and secondly, TGT treatment was
not used in the in vitro experiments because sterile TGT
solution was not available. The TGT tables used for the in
vitro experiments contain starch that can easily block the mesh
of the filter during the filtration and degerming process.
Acknowledgements
Not applicable.
Funding
The present study was supported by The National
Natural Science Foundation of China (grant no. 81560724) and West
China Top Class Discipline Project in Basic Medical Sciences,
Ningxia Medical University (grant no. NXYLXK2017B07).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
QW and RM designed the study. LB, YB, YY, WZ, LH, LW
and HD performed the experiments. LB, WZ and LH contributed
reagents or analytical tools. LB and YB analyzed the data. QW, LB
and YB drafted the manuscript. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Ningxia Medical University (approval no. 2015-125).
All animal experiments were performed in accordance with the
Guidelines for the Care and Use of Animals published by the P.R.
China Ministry of Health (January 25, 1998).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
RA
|
rheumatoid arthritis
|
|
CIA
|
collagen-induced arthritis
|
|
TNF-α
|
tumor necrosis factor-α
|
|
IL-1
|
interleukin-1
|
|
TLRs
|
toll-like receptors
|
|
MYD88
|
myeloid differentiation factor 88
|
|
LPS
|
lipopolysaccharide
|
|
SD
|
Sprague-Dawley
|
|
HFLS
|
human fibroblast-like synoviocytes
|
|
RT-qPCR
|
reverse transcription-quantitative
PCR
|
References
|
1
|
Mclnnes IB and Schett G: The pathogenesis
of rheumatoid arthritis. N Engl J Med. 365:2833–2219. 2011.
|
|
2
|
van den Hoek J, Boshuizen HC, Roorda LD,
Tijhuis GJ, Nurmohamed MT, van den Bos GA and Dekker J: Mortality
in patients with rheumatoid arthritis: A 15-year prospective cohort
study. Rheumatol Int. 37:487–493. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bartok B and Firestein GS: Fibroblast-like
synoviocytes: Key effector cells in rheumatoid arthritis. Immunol
Rev. 233:233–255. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Guo Q, Wang Y, Xu D, Nossent J, Pavlos NJ
and Xu J: Rheumatoid arthritis: Pathological mechanisms and modern
pharmacologic therapies. Bone Res. 6:152018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Branimir A and Miroslav M: Pathogenesis of
rheumatoid arthritis. Reumatizam. 61:19–23. 2014.(In Croatian).
PubMed/NCBI
|
|
6
|
Araki Y and Mimura T: The Mechanisms
underlying chronic inflammation in rheumatoid arthritis from the
perspective of the epigenetic landscape. J Immunol Res.
2016:62906822016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wilsdon TD and Hill CL: Managing the drug
treatment of rheumatoid arthritis. Aust Prescr. 40:51–58. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Akira S, Uematsu S and Takeuchi O:
Pathogen recognition and innate immunity. Cell. 124:783–801. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Huang QQ and Pope RM: The role of
toll-like receptors in rheumatoid arthritis. Curr Rheumatol Rep.
11:357–364. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jimenez-Dalmaroni MJ, Gerswhin ME and
Adamopoulos IE: The critical role of toll-like receptors-from
microbial recognition to autoimmunity: A comprehensive review.
Autoimmun Rev. 15:1–8. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Huang QQ and Pope RM: The role of
glycoprotein 96 in the persistent inflammation of rheumatoid
arthritis. Arch Biochem Biophys. 530:1–6. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sampaio NG, Kocan M, Schofield L, Pfleger
KDG and Eriksson EM: Investigation of interactions between TLR2,
MyD88 and TIRAP by bioluminescence resonance energy transfer is
hampered by artefacts of protein overexpression. PLoS One.
13:e02024082018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Iwahashi M, Yamamura M, Aita T, Okamoto A,
Ueno A, Ogawa N, Akashi S, Miyake K, Godowski PJ and Makino H:
Expression of Toll-like receptor 2 on CD16+ blood monocytes and
synovial tissue macrophages in rheumatoid arthritis. Arthritis
Rheum. 50:1457–1467. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
McGarry T, Veale DJ, Gao W, Orr C, Fearon
U and Connolly M: Toll-like receptor 2 (TLR2) induces migration and
invasive mechanisms in rheumatoid arthritis. Arthritis Res Ther.
17:1532015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li BQ, Fu T, Gong WH, Dunlop N, Kung H,
Yan Y, Kang J and Wang JM: The flavonoid baicalin exhibits
anti-inflammatory activity by binding to chemokines.
Immunopharmacology. 49:295–306. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hou J, Wang J, Zhang P, Li D, Zhang C,
Zhao H, Fu J, Wang B and Liu J: Baicalin attenuates proinflammatory
cytokine production in oxygen-glucose deprived challenged rat
microglial cells by inhibiting TLR4 signaling pathway. Int
Immunopharmacol. 14:749–757. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xu XF, Cai BL, Guan SM, Li Y, Wu JZ, Wang
Y and Liu B: Baicalin induces human mucoepidermoid carcinoma Mc3
cells apoptosis in vitro and in vivo. Invest New Drugs. 29:637–645.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yin F, Liu J, Ji X, Wang Y, Zidichouski J
and Zhang J: Baicalin prevents the production of hydrogen peroxide
and oxidative stress induced by Abeta aggregation in SH-SY5Y cells.
Neurosci Lett. 492:76–79. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sun F and Gu W: Baicalin attenuates
collagen-induced arthritis via inhibition of JAK2-STAT3 signaling
and regulation of Th17 cells in mice. J Cell Commun Signal.
13:65–73. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang HZ, Wang HH, Huang SS, Zhao H, Cao
YG, Wang GZ, Wang D, Wang ZG and Liu YH: Inhibitory effect of
baicalin on collagen-induced arthritis in rats through the nuclear
factor-κB pathway. J Pharmacol Exp Ther. 350:435–443. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ye L, Tao Y, Wang Y, Feng T and Li H: The
effects of baicalin on the TLR2/4 signaling pathway in the
peripheral blood mononuclear cells of a lipopolysaccharide-induced
rat fever model. Int Immunopharmacol. 25:106–111. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sun JY, Li DL, Dong Y, Zhu CH, Liu J, Li
JD, Zhou T, Gou JZ, Li A and Zang WJ: Baicalin inhibits toll-like
receptor 2/4 expression and downstream signaling in rat
experimental periodontitis. Int Immunopharmacol. 36:86–93. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu W, Sun Y, Cheng Z, Guo Y, Liu P and
Wen Y: Crocin exerts anti-inflammatory and anti-arthritic effects
on type II collagen-induced arthritis in rats. Pharm Biol.
56:209–216. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lin ZP, Lin HL, Yu XP, Zheng YJ and Cheng
SY: TLR4 mediates inflammation and hepatic fibrosis induced by
chronic intermittent hypoxia in rats. Mol Med Rep. 22:651–660.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cai X, Zhou H, Wong YF, Xie Y, Liu ZQ,
Jiang ZH, Bian ZX, Xu HX and Liu L: Suppression of the onset and
progression of collagen-induced arthritis in rats by QFGJS, a
preparation from an anti-arthritic Chinese herbal formula. J
Ethnopharmacol. 110:39–48. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Elshabrawy HA, Essani AE, Szekanecz Z, Fox
DA and Shahrara S: TLRs, future potential therapeutic targets for
RA. Autoimmun Rev. 16:103–113. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Haag S, Tuncel J, Thordardottir S, Mason
DE, Yau AC, Dobritzsch D, Backlund J, Peters EC and Holmdahl R:
Positional identification of RT1-B (HLA-DQ) as susceptibility locus
for autoimmune arthritis. J Immunol. 194:2539–2550. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jutley G, Raza K and Buckley CD: New
pathogenic insights into rheumatoid arthritis. Curr Opin Rheumatol.
27:249–255. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mc Ardle A, Flatley B, Pennington SR and
FitzGerald O: Early biomarkers of joint damage in rheumatoid and
psoriatic arthritis. Arthritis Res Ther. 17:1412015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mateen S, Shahzad S, Ahmad S, Naeem SS,
Khalid S, Akhtar K, Rizvi W and Moin S: Cinnamaldehyde and eugenol
attenuates collagen induced arthritis via reduction of free
radicals and pro-inflammatory cytokines. Phytomedicine. 53:70–78.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Alam J, Jantan I, Kumolosasi E, Nafiah MA
and Mesaik MA: Suppressive effects of the standardized extract of
phyllanthus amarus on type ii collagen-induced rheumatoid arthritis
in sprague dawley rats. Curr Pharm Biotechnol. 19:1156–1169. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang Y, Li X, Ciric B, Ma CG, Gran B,
Rostami A and Zhang GX: Therapeutic effect of baicalin on
experimental autoimmune encephalomyelitis is mediated by SOCS3
regulatory pathway. Sci Rep. 5:174072015. View Article : Google Scholar : PubMed/NCBI
|