Introduction
Acute lung injury (ALI)/acute respiratory distress
syndrome (ARDS) is a critical condition characterized by neutrophil
infiltration of the lungs, injury of alveolar epithelial cells and
capillary endothelium, and protein-rich edema (1). ARDS is a severe lung condition
developing from ALI, and epidemiological studies from Europe
published in the past 20 years reported an ARDS incidence ranging
from 5–8 cases/1,00,000 individuals (1). If not treated correctly or in a
timely manner, ALI may lead to acute respiratory failure, including
diffuse pulmonary interstitial and alveolar edema and severe
hypoxic respiratory, insufficiency characterized by the progressive
hypoxemia and respiratory distress (2,3).
Despite the development of promising new therapies to treat ALI
during the past decade, including venovenous extracorporeal
membrane oxygenation and lung-protective ventilation, the mortality
of ALI remains high (40–50%) (1).
Therefore, it is important to investigate novel therapeutic methods
for treating ALI.
Macrophages in lung tissue include alveolar
macrophages, pulmonary interstitial macrophages and bronchial
macrophages, and these are an important regulator of the local
inflammatory microenvironment and immune response during ALI
(4). When stimulated by external
pathogenic factors, macrophages may be converted into a
pro-inflammatory phenotype (M1) and release a large number of
inflammatory mediators, such as tumor necrosis factor (TNF)-α,
interleukin (IL)-1 and IL-6, recruiting various immune cells and
initiating an inflammatory cascade (5). However, with an increase in the level
of pro-inflammatory cytokines, some macrophages will polarize
toward an immunosuppressive phenotype (M2) and in turn inhibit the
inflammatory reaction (6).
Moreover, the disruption of the balance between M1 and M2
macrophages is considered to be one of the most important causes of
the uncontrolled inflammatory cascade in ALI (4,6).
Mesenchymal stromal cells (MSCs) are a type of
fibroblast-like cells that have the ability of self-renewal and
differentiation (7). MSCs have
been detected in various tissues in the adult human body, such as
the bone marrow, adipose tissue, umbilical cord blood and synovial
fluid, from which they may be easily isolated (8). Although MSCs have a prominent ability
to proliferate and differentiate, the fact that they die soon after
being transplanted into the injured area suggests that their
paracrine properties largely constitute the basis for their medical
application (9,10). Furthermore, the clinical use of
MSCs has been limited by the long waiting times for cell
preparation and the potential tumorigenicity safety concerns
(11). To solve these
aforementioned issues, some researchers have collected the
bioactive factors secreted by MSCs in conditioned media (CM) as an
alternative for MSCs and have reported positive results on
improving cardiac function, skin wound repair and bone regeneration
(11–13). In addition, the advantages of
secure storage, transportation and administration also indicate CM
may be a promising alternative for MSCs.
CM from MSCs is composed of a broad repertoire of
bioactive factors, including cytokines, growth factors, microRNAs,
proteasomes and exosomes. The wide range of anti-inflammatory
cytokines secreted by MSCs, including transforming growth factor
(TGF)-β, IL-1 receptor antagonist (IL-1Ra) and IL-10, suggests that
CM can regulate the inflammatory process via a variety of
mechanisms. For example, TGF-β can induce M1 macrophage
polarization towards the M2-like phenotype, inhibit the maturation
of B cells and decrease the secretion of TNF-α and immunoglobulin
E. Moreover, IL-1Ra can block IL-1α/IL-1β signaling pathways,
inhibit the antigen-presenting ability of dendritic cells and
increase the number of immunosuppressive T cells, while IL-10 is a
potent anti-inflammatory cytokine that can markedly inhibit the
production of interferon (IFN)-γ, IL-2 and TNF-α (14–17).
Factors of the host microenvironment serve a key
role in MSC-mediated immunomodulation (18). For instance, exposure of MSCs to
inflammatory signals such as TNF-α and IL-1, is the basis of the
in vivo MSC-mediated immunosuppression (17,19).
Given that the in vitro cell culture condition does not
include inflammatory signals, preconditioning MSCs is a
prerequisite for MSC-based therapy (20). MSCs derived from various tissues
have been reported to express functional Toll-like receptors
(TLRs), a type of single-pass transmembrane proteins (21,22).
TLR ligations can activate the immune defensive function of MSCs
and alter their secretome profiles. For example, TLR4 ligands can
activate the NF-κB pathway in MSCs and induce MSCs to release a
variety of molecules, including IL-6 and vascular endothelial
growth factor (23). Therefore,
priming MSCs with TLR ligations may be a promising method for
enhancing their therapeutic effects.
Flagellin is a subunit protein of the flagellum, a
whip-like appendage that enables bacterial motility, and it is a
commonly used TLR5 ligand (24).
The majority of previous studies have focused on preconditioning
MSCs with TLR3 and TLR4 ligations (22,23,25).
While Linard et al (21)
reported the superior anti-inflammatory effect of
flagellin-activated MSCs in an irradiation-induced proctitis model
compared with normal MSCs, whether MSCs preconditioned with
flagellin are effective in other inflammation-related diseases and
the mechanism underlying the beneficial effects of flagellin
preconditioning on the MSCs remain elusive.
The aim of the present study was to investigate the
effects of CM from normal adipose derived-MSCs (ADSCs) and CM from
flagellin-activated ADSCs (F-CM) in a mouse model of ALI induced by
intraperitoneal injection of lipopolysaccharide (LPS). Experiments
were performed both in vitro and in vivo. The effects
of CM and F-CM on the polarization and expression levels of
inflammation-related factors were first investigated in RAW264.7
cells. Furthermore, CM and F-CM were injected into mice with
LPS-induced lung injury to assess their anti-inflammatory activity
in vivo. The current study also aimed to determine whether
flagellin preconditioning can increase the beneficial effects of CM
from ADSCs against LPS-induced ALI.
Materials and methods
Culture of human ADSCs
Human-ADSCs were purchased from the Type Culture
Collection of the Chinese Academy of Sciences. The eight donors
(men, 4; women, 4) were aged from 21–42 years old (the sex and age
range of each sample were provided by the Type Culture Collection
of the Chinese Academy of Sciences). The cells were centrifuged at
500 × g for 20 min at room temperature, and the cell pellet was
diluted with D10 media which was composed of 89% DMEM-low glucose
(DMEM-LG; Hyclone; Cytiva), 10% FBS (Gibco; Thermo Fisher
Scientific, Inc.) and 1% penicillin-streptomycin (Invitrogen;
Thermo Fisher Scientific, Inc.). Then, cells were cultured in T-75
flasks (BD Biosciences) at 37°C in a humidified incubator
containing 5% CO2, and the culture media was changed
twice a week. ADSCs were passaged when they were confluent, and the
cells obtained at the end of the third passage were used for the
experiments. ADSCs from different donors were separately cultured.
ADSCs from all eight donors were used in all subsequent
experiments, including the animal study.
Tri-lineage differentiation
(osteocyte, chondrocyte and adipocyte) of the flagellin-activated
ADSCs
For osteogenic and adipogenic differentiation, ADSCs
were seeded on 6-well plates at the density of 1×105
cells/well. Osteogenic induction was performed using
differentiation media consisting of DMEM-LG supplemented with 10%
FBS, 50 mg/ml of ascorbic acid, 10 mM β-glycerophosphate and 100 nM
dexamethasone (all Sigma-Aldrich; Merck KGaA). The media was
changed every 3 days until day 21.
Adipogenic studies were performed by culturing the
cells in differentiation media containing 1 mM dexamethasone, 50 mM
3-isobutyl-1-methylxanthine and 10 mg/ml insulin (all
Sigma-Aldrich; Merck KGaA).
For chondrogenesis, 1×105 cells were
centrifuged at 500 × g in a polypropylene tube (15 ml; BD
Biosciences) for 10 min at 4°C. Aggregates were incubated in
chondrogenic media consisting of DMEM supplemented with 10% FBS, 1%
insulin-transferrin-selenium, 1 mM sodium pyruvate and 50 mM
L-proline (all Sigma-Aldrich; Merck KGaA).
After the differentiation processes were complete,
cells were fixed in 4% paraformaldehyde for 30 min at 37°C and
stained with Alizarin Red (10 min at 37°C), Oil Red O (10 min at
37°C) and Toluidine Blue (30 min at 37°C; all Beyotime Institute of
Biotechnology), respectively. Then, cells were observed with a
light microscope (Optiphot; Nikon Corporation) at a high-power
magnification of ×100.
Flow cytometry
After being incubated with D10 media containing
flagellin (100 ng/ml; cat. no. P7388; Beyotime Institute of
Biotechnology) at 37°C for 48 h, ADSCs were harvested and suspended
in PBS containing 4% FBS. Non-specific detection of the Fc
component of the CD antibodies were blocked with 5% BSA (Beyotime
Institute of Biotechnology) for 30 min at room temperature. Then,
cells were incubated with fluorescence-conjugated antibodies
directed against mouse anti-human CD31 (cat. no. 555446), CD45
(cat. no. 560178), CD73 (cat. no. 550257), CD90 (cat. no. 555593)
and CD105 (cat. no. 562380; all from BD Biosciences; 1:20) for 30
min in the dark at 4°C After being washed three times with PBS, the
stained cells were sorted using a FACSCalibur flow cytometer (BD
Biosciences). The result was analyzed using BD FACStation software
(version 6.1X; BD Biosciences).
Preparation of CM and F-CM
When reaching 80–90% confluency, the culture media
was changed to a mixture of D10 media and flagellin (100 ng/ml).
After 48 h incubation at 37°C, flagellin-activated ADSCs were
washed three times with warm PBS and re-supplemented with
serum-free DMEM. After 48 h, the CM was collected, filtered with a
0.2-µm filter (EMD Millipore) and concentrated 50-fold using
ultrafiltration units with a 3-kDa molecular weight cutoff (EMD
Millipore) (26). The concentrated
F-CM was stored at −80°C before being used for the following
experiments. The CM was prepared using the same method, except no
flagellin was added.
Effect of serum deprivation on the
viability of ADSCs
Normal ADSCs and ADSCs pretreated with flagellin
(100 ng/ml) for 48 h at 37°C were cultured in 96-well plates at the
density of 2×104 cells/well with serum-free culture
media. After 0, 24 and 48 h, the serum-free culture media was
removed and 200 µl fresh culture media containing 10% Cell Counting
Kit-8 reagent (CCK-8; Dojindo Molecular Technologies, Inc.) were
added to each well according to the manufacturer's instructions.
After 2 h incubation at 37°C, the absorbance was measured at 450
nm.
Culture and treatment of RAW264.7
cells and THP-1 cells
RAW264.7 cells were purchased from the Type Culture
Collection of the Chinese Academy of Sciences. Cells were cultured
in 6-well plates at the density of 1×105 cells/well, and
randomly divided into the control (20 µl/ml PBS), LPS (1 µg/ml LPS;
Beyotime Institute of Biotechnology), CM (1 µg/ml LPS + 20 µl/ml
CM) and F-CM (1 µg/ml LPS + 20 µl/ml F-CM) groups. The cells were
cultured at 37°C for 24 h and then tested.
THP-1 cells were purchased from the Type Culture
Collection of the Chinese Academy of Sciences. Cells were cultured
in RPMI-1640 medium (Hyclone; Cytiva) containing 10% FBS, 100 U/ml
penicillin and 100 µg/ml streptomycin (complete medium) and
maintained at 37°C in a humidified, 5% CO2 environment
with medium changed every 2 days. Cells were cultured in 6-well
plates at the density of 1×105 cells/well, and randomly
divided into the control (20 µl/ml PBS), LPS (1 µg/ml LPS), CM (1
µg/ml LPS + 20 µl/ml CM) and F-CM (1 µg/ml LPS + 20 µl/ml F-CM)
groups. The cells were cultured at 37°C for 24 h and then
tested.
Reverse transcription-quantitative PCR
(RT-qPCR)
To extract RNA, 0.5 ml TRIzol® reagent
(Beyotime Institute of Biotechnology) was added in each well of
cells after 24 h of culture or small pieces of the mouse lung
tissue. mRNA dissolved in the TRIzol® reagent was
isolated via centrifugation (12,000 × g/min) at 4°C for 15 min.
cDNA was synthesized using a RT kit (cat. no. 4368813; Thermo
Fisher Scientific, Inc.), Mouse-specific or human-specific primers
with SYBR Green Master Mix (cat. no. 309155; Thermo Fisher
Scientific, Inc.) used in the study are presented in Table I. The thermocycling conditions were
as follows: Initial denaturation at 95°C for 5 min, 40 cycles of
denaturation at 95°C for 30 sec, annealing at 58°C for 30 sec and
extension at 72°C for 45 sec. The relative mRNA expression was
calculated using the 2−ΔΔCq method (27). The GAPDH gene was used as the
internal control.
 | Table I.Primer sequences (5′→3′) for reverse
transcription-quantitative PCR. |
Table I.
Primer sequences (5′→3′) for reverse
transcription-quantitative PCR.
| Gene | Species | Forward primer | Reverse primer |
|---|
| TNF-α | mice |
CCCTCACACTCAGATCATCTTCT |
GCTACGACGTGGGCTACAG |
| IL-6 | mice | GCC TTC CCT ACT TCA
CAA | ACA ACTCTT TTC TCA
TTT CCA C |
| GAPDH | mice |
GGAGCGAGATCCCTCCAAAAT |
GGCTGTTGTCATACTTCTCATGG |
| CD86 | mice |
TGTTTCCGTGGAGACGCAAG |
TTGAGCCTTTGTAAATGGGCA |
| IL-10 | mice |
GCTCTTACTGACTGGCATGAG |
CGCAGCTCTAGGAGCATGTG |
| IL-1 | mice |
CTATGTCTTGCCCGTGGAG |
CATCATCCCACGAGTCACA |
| CD163 | mice |
ATGGGTGGACACAGAATGGTT |
CAGGAGCGTTAGTGACAGCAG |
| TNF-α | human |
CTCCCAGGTCCTCTTCAAGG |
TTGATGGCAGAGAGGAGGTT |
| IL-6 | human |
CGGGAACGAAAGAGAAGCTC |
ACAACAACAATCTGAGGTGC |
| GAPDH | human |
CCACRCCRCCACCTTTGAC |
ACCCTGTTGTTGCTGTAGCCA |
| CD86 | human |
TGCTCATCTATACACGGTTACC3 |
TGCATAACACCATCATACTCGA |
| IL-10 | human |
GCTGTCATCGATTTCTTCCC |
TCAAACTCACTCATGGCTTTGT |
| IL-1 | human |
AATCTGTACCTGTCCTGCGTGTT |
TGGGTAATTTTTGGGATCTACACTCT |
| CD163 | human |
CCAGTCCCAAACACTGTCCT |
CACTCTCTATGCAGGCCACA |
| PPARγ | human |
ACCAAAGTGCAATCAAAGTGG A | ATG AGG GAG TTG GAA
GGC TCT |
| ALP | human |
ACTGGGGCCTGAGATACCC |
TCGTGTTGCACTGGTTAAAGC |
| RUNX2 | human |
TCAACGATCTGAGATTTGTGGG |
GGGGAGGATTTGTGAAGACGG |
| SOX9 | human |
AGCGAACGCACATCAAGAC |
CTGTAGGCGATCTGTTGGGG |
| COL2A1 | human |
TGGACGATCAGGCGAAACC |
GCTGCGGATGCTCTCAATCT |
| Adiponectin | human |
GGCTTTCCGGGAATCCAAGG |
TGGGGATAGTAACGTAAGTCTCC |
ELISA
Commercial ELISA kits for human [C-C chemokine
ligand 5 (CCL5; cat. no. DRN00B), IFN-γ (cat. no. DIF50), IL-1β
(cat. no. MLB00C), IL-1Ra (cat. no. DRA00B), IL-4 (cat. no. D4050),
IL-6 (cat. no. D6050), IL-8 (cat. no. D8000C), IL-10 (cat. no.
D1000B) and TGF-β (cat. no. DB100B)] and mouse [TNF-α (cat. no.
MTA00B), IL-1 (cat. no. MLB00C), IL-6 (cat. no. DY406), IL-10 (cat.
no. M1000B) and monocyte chemoattractant protein (MCP)-1 (cat. no.
MJE00B)] cytokines (all from R&D Systems, Inc.) were used to
determine the concentrations of inflammatory-associated factors in
the bronchoalveolar lavage fluid (BALF) according to manufacturer's
protocol.
Western blotting
To detect the expression of NF-κB pathway-related
proteins, ADSCs treated with 100 ng/ml flagellin at 37°C for 0, 30
and 120 min were harvested to obtain the cytoplasmic and nuclear
proteins using a Keygen Kit (Nanjing Keygen Biotech Co., Ltd.)
according to manufacturer's protocol. Total protein was quantified
using the bicinchoninic acid Protein assay kit (Beyotime Institute
of Biotechnology). The same amount of protein (20 µg/lane) was
placed into a 10% SDS-PAGE and transferred onto 0.22 µm PVDF
membranes (EMD Millipore). Then, the transferred membranes were
blocked in 5% BSA (Beyotime Institute of Biotechnology) at room
temperature for 2 h and incubated with primary antibodies [p65,
cat. no. AF5881, 1:1,000; IκBα, cat. no. AF5204, 1:1,000;
phosphorylated (p)-IκBα, cat. no. AF5851, 1:1,000; β-actin, cat.
no. AF5006, 1:2,000; histone deacetylase 1 (HDAC1), cat. no. AF5180
1:2,000; all from Beyotime Institute of Biotechnology] overnight at
4°C. β-actin and histone deacetylase 1 served as the internal
controls. The membranes were washed with PBST containing 0.05%
Tween (Shanghai Aladdin Bio-Chem Technology Co., Ltd.) three times
followed by incubation with the corresponding horseradish
peroxidase-conjugated secondary antibody (Beyotime Institute of
Biotechnology) for 1 h at 37°C. Protein bands were visualized using
ECL reagents (Roche Diagnostics GmbH) and then scanned with the
Image Quant LAS4000 system (Cytiva). Protein expression levels were
semi-quantified using Gel-Pro Analyzer software (version 4.0; Media
Cybernetics, Inc.), with β-actin or HDAC1 as the loading
control.
Animal model
All animal experiments were approved by the Animal
Care and Use Committee of the Shanghai Children's Hospital. In
total, 32 male BALB/c mice (age, 6–8 weeks; weight, 20–25 g) were
purchased from Shanghai Lab Animal Research Center. Mice were
maintained on a standard diet and water ad libitum (12 h
light/dark cycle with humidity of 60±5% and temperature 22±3°C).
All mice were randomly divided into the control group (n=8), the
LPS group (n=8; ALI mice received PBS), the CM group (n=8; ALI mice
received CM) and the F-CM group (n=8; ALI mice received F-CM). PBS,
CM or F-CM (5 µl/g of body mass of the animals) were injected into
the tail vein of mice in the LPS, CM and F-CM groups once a day for
2 days before the experiment. ALI was induced via intraperitoneal
injection of LPS (20 µl/g) (28,29).
Then, 24 h after injection, mice were euthanized with 0.9% saline
containing sodium pentobarbital (50 mg/ml) via intraperitoneal
injection at dose of 250 mg/kg of body mass of the animals.
Cell counting and protein content in
BALF
Each mouse was euthanized with sodium pentobarbital
via intraperitoneal injection. Both lungs of each mouse were
lavaged three times via a tracheal cannula with 0.5 ml PBS at 4°C
to obtain BALF. BALF was centrifuged (300 × g; 10 min; 4°C) and the
protein content of the BALF supernatant was determined using the
Braford method (Beyotime Institute of Biotechnology), according to
the manufacturer's instructions. The cell pellet was washed with
red blood cell lysis solution (Beyotime Institute of Biotechnology)
at 37°C for 1 min, centrifuged at 300 × g at room temperature for
10 min and resuspended in 200 µl PBS. The total cell count was
determined using a hemocytometer and differential cell count was
measured using Wright-Giemsa staining (incubated with Wright-Giemsa
for 1 min at room temperature; Beyotime Institute of
Biotechnology). Macrophages and neutrophils were quantified by
counting 200 cells per slide at ×400 magnification using light
microscope.
Hematoxylin and eosin (H&E)
staining
Lung tissue was washed three times with PBS and
immersed in 4% paraformaldehyde (Beyotime Institute of
Biotechnology) overnight at room temperature. Then, lung tissue was
dehydrated with an increasing gradient concentration of ethanol
(50, 70, 80, 90 and 100%) and embedded in paraffin (Beyotime
Institute of Biotechnology). Tissue slices with thickness of 4 µm
were attached to the slides coated with polylysine (Beyotime
Institute of Biotechnology) and were incubated overnight at 60°C.
Then, the slices were immersed in hematoxylin purple solution
(Beyotime Institute of Biotechnology) at 37°C for 5 min, washed
three times with deionized water, washed in 1% hydrochloric acid
alcohol (Shanghai Aladdin Bio-Chem Technology Co., Ltd.) for 5 sec
and rinsed in deionized water for 10 min. Slices were then immersed
in eosin dyeing solution at 37°C (Beyotime Institute of
Biotechnology) for 30 sec, in 95% ethanol for 2 min, in 100%
ethanol for 2 min, in 100% xylene (Beyotime Institute of
Biotechnology) for 5 min, and in the mixture of xylene and ethanol
(1:1), respectively.
Lung injury was evaluated by two blinded observers
according to a previous method by Smith et al (30). The parameters, lung edema, alveolar
and interstitial inflammation, alveolar and interstitial
hemorrhage, atelectasis and hyaline membrane formation, were scored
as followed: i) No injury = score of 0; ii) injury in 25% of the
field = score of 1; iii) injury in 50% of the field = score of 2;
iv) injury in 75% of the field = score of 3; and v) injury
throughout the field = score of 4. The lung injury score was the
sum of these five criteria. In total, 12 random light microscopic
(magnification, ×400) fields from each slide were imaged and the
injury area was calculated using ImageJ 1.8 software (National
Institutes of Health).
Lung wet/dry weight ratio
After the mice were euthanized, their lungs were
excised, rinsed briefly in PBS, blotted dry and weighed to obtain
the wet weight. Then, the lungs were placed in an oven at 80°C for
48 h to obtain the dry weight. The ratio of wet lung weight to dry
lung weight was calculated.
Statistical analysis
Data are presented as the mean ± standard deviation.
Differences between or among groups were examined for statistical
significance using a unpaired Student's t-test, one-way ANOVA with
Tukey's post hoc or Kruskal-Wallis with Dunn's post hoc test using
GraphPad Prism 6 (GraphPad Software, Inc.). P<0.05 was
considered to indicate a statistically significant difference.
Experiments were repeated ≥8 times.
Results
Characterization of ADSCs with
flagellin preconditioning
Flagellin-activated ADSCs were positive for MSC
markers, including CD73 (Fig. 1C),
CD90 (Fig. 1D) and CD105 (Fig. 1E), but negative for the endothelial
cell marker CD31 (Fig. 1A) and the
hematopoietic cell marker CD45 (Fig.
1B). Moreover, flagellin-activated ADSCs maintained a
fibroblast-like morphology (Fig.
1F). The osteogenic (Fig. 1G),
adipogenic (Fig. 1H) and
chondrogenic (Fig. 1I)
differentiation of flagellin-activated ADSCs was identified using
Alizarin Red S, Oil Red O and Toluidine Blue staining,
respectively.
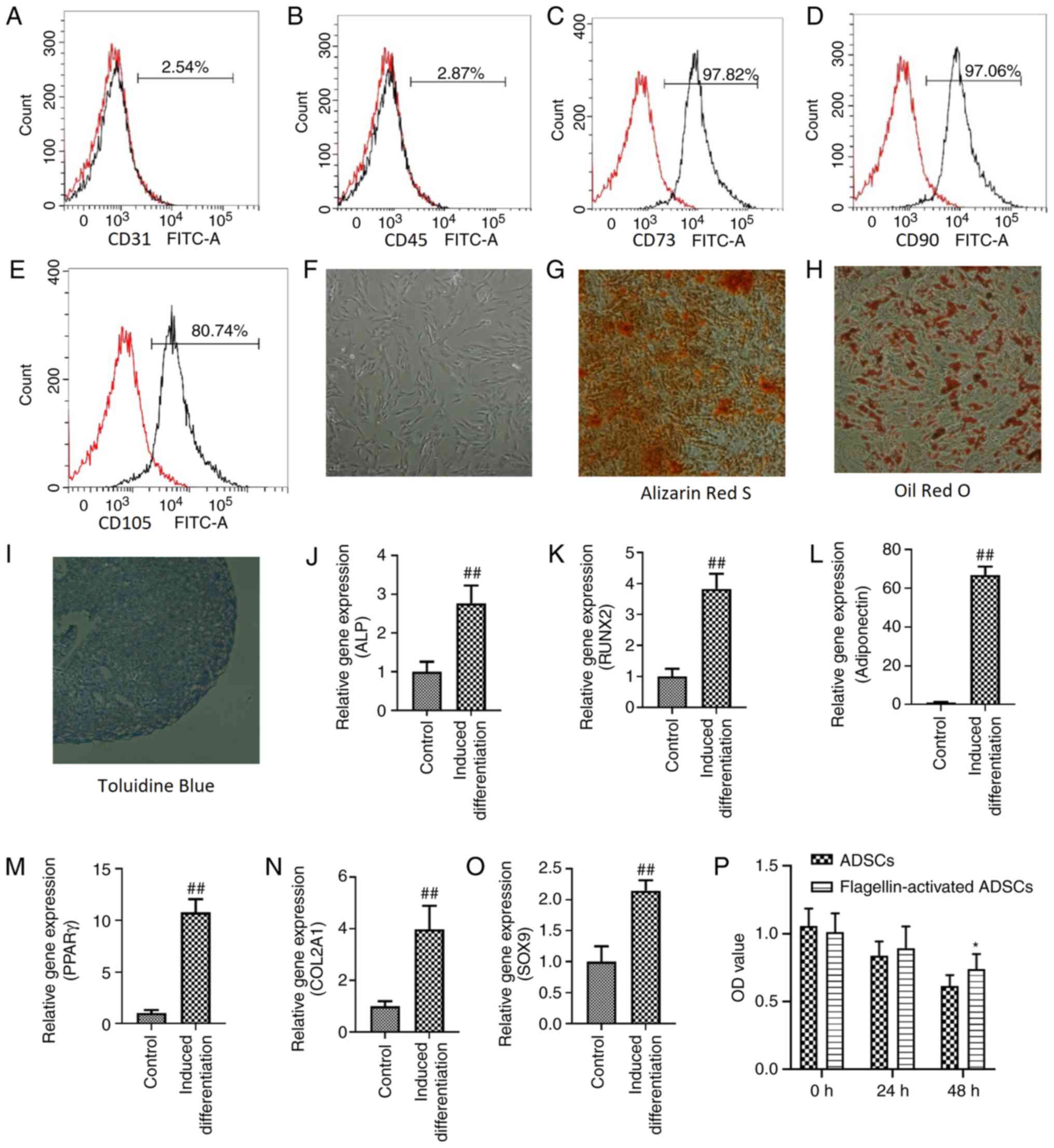 | Figure 1.Characterization of ADSCs with
flagellin preconditioning and effects of serum deprivation on the
viability of ADSCs. Flow cytometric analyses demonstrated that
ADSCs in passage 3 were negative for (A) CD31 and (B) CD45, but
positive for (C) CD73, (D) CD90 and (E) CD105. (F)
Flagellin-activated ADSCs maintained a fibroblast-like morphology.
Magnification, ×100. (G) Osteogenic, (H) adipogenic and (I)
chondrogenic differentiation of flagellin-activated ADSCs was
assessed using Alizarin Red S, Oil Red O and Toluidine Blue
staining, respectively. Magnification, ×100. Expression levels of
(J) ALP and (K) RUNX2 are used to investigate osteogenic
differentiation. Expression levels of (L) adiponectin and (M) PPARγ
are used to investigate adipogenic differentiation. Expression
levels of (N) COL2A1 and (O) SOX9 were used to investigate
chondrogenic differentiation. (P) Cell Counting Kit-8 was used to
detect the viability of ADSC and flagellin-activated ADSCs cultured
in serum-free media. Differences between groups were examined for
statistical significance using Student's t-test. *P<0.05 vs.
ADSCs group; ##P<0.01 vs. control group. RUNX2, RUNX
family transcription factor 2; PPARγ, peroxisome proliferator
activated receptor γ; ALP, alkaline phosphatase; COL2A1, collagen
type II α 1 chain; ADSC, adipose derived- mesenchymal stromal
cells; OD, optical density. |
The mRNA expression levels of the osteogenic genes
alkaline phosphatase (2.77±0.46) and RUNX family transcription
factor 2 (3.83±0.48), the adipogenic genes adiponectin (66.74±4.43)
and peroxisome proliferator activated receptor γ (10.77±1.29) and
the chondrogenic genes collagen type II α 1 chain (3.97±0.91) and
SOX9 (2.15±0.17), were significantly increased after
differentiation induction compared with the control (Fig. 1J-O).
Effect of serum deprivation on the
viability of ADSCs
Both the viability of ADSCs (0 h, 1.059; 24 h, 0.84;
and 48 h, 0.62) and flagellin-activated ADSCs (0 h, 1.013; 24 h,
0.89; and 48 h, 0.74) decreased over time (Fig. 1P). However, ADSCs retained 59%
viability and flagellin-activated ADSCs retained 73% viability. No
differences were found between ADSCs and flagellin-activated ADSCs
groups after 24 h incubation. At 48 h, the optical density value of
the flagellin-activated ADSCs group was significantly higher
compared with the ADSCs group.
Immunomodulatory effects of F-CM on
murine macrophages
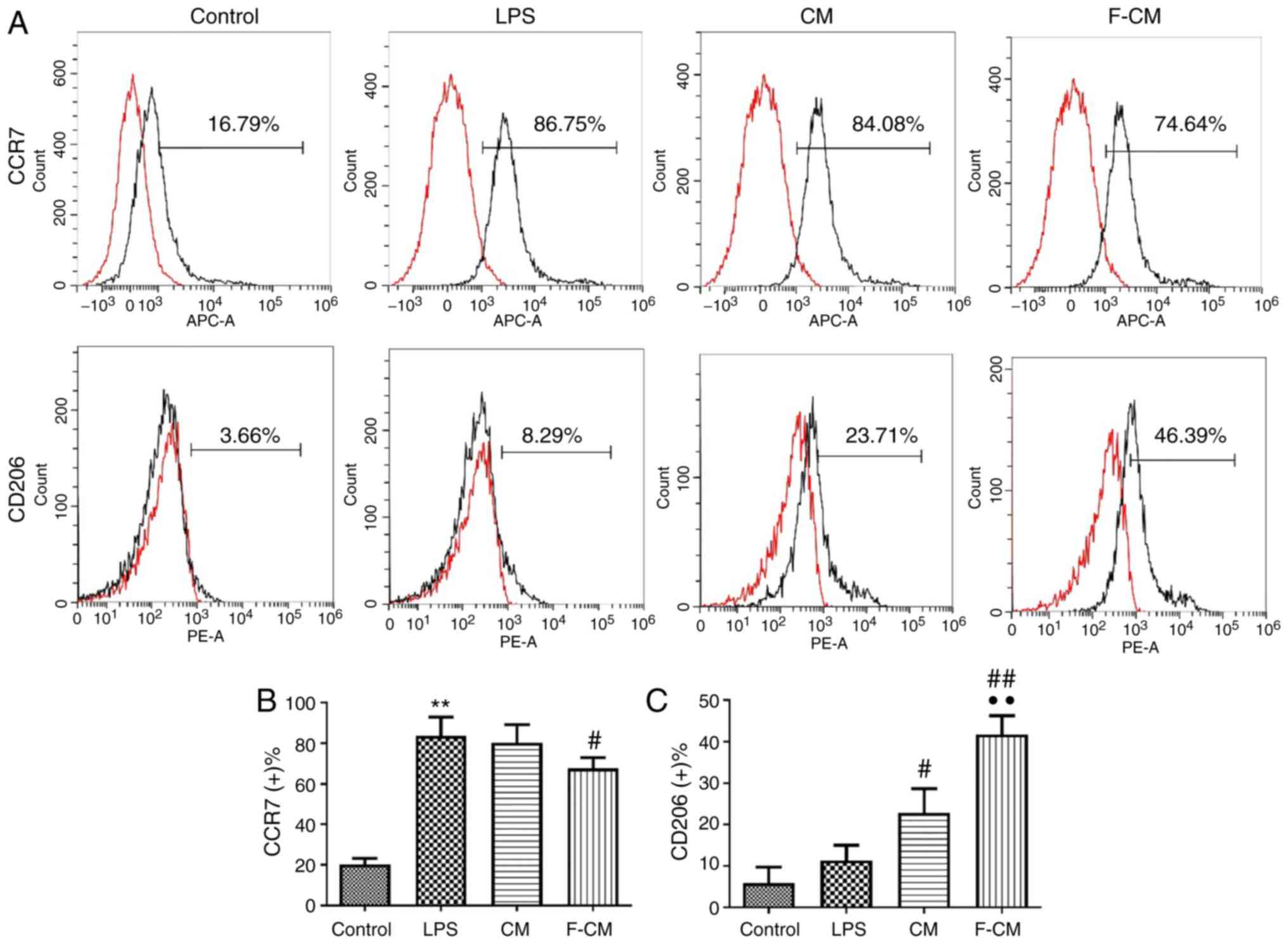
The immunomodulatory effect of F-CM was investigated
in vitro using RAW264.7 cells. The effects of F-CM on the
expression of the M1 marker C-C chemokine receptor (CCR)7 and the
M2 marker CD206 were evaluated with flow cytometry. After a 48-h
incubation, F-CM groups exhibited a significantly lower percentage
of CCR7-positive cells (67.66±5.32%; Fig, 2B), but a significantly higher
percentage of CD206-positive cells (41.75±4.48%; Fig. 2C) compared with the LPS group
(CCR7, 83.64±9.28%; CD206, 11.37±3.65%; Fig. 2). The CM group had a significantly
higher percentage of CD206-positive cells (22.79±5.87%) compared
with the LPS group, but the percentage of CCR7-positive cells of
the CM group (80.26±8.96%) was not significantly different compared
with the LPS group (Fig. 2).
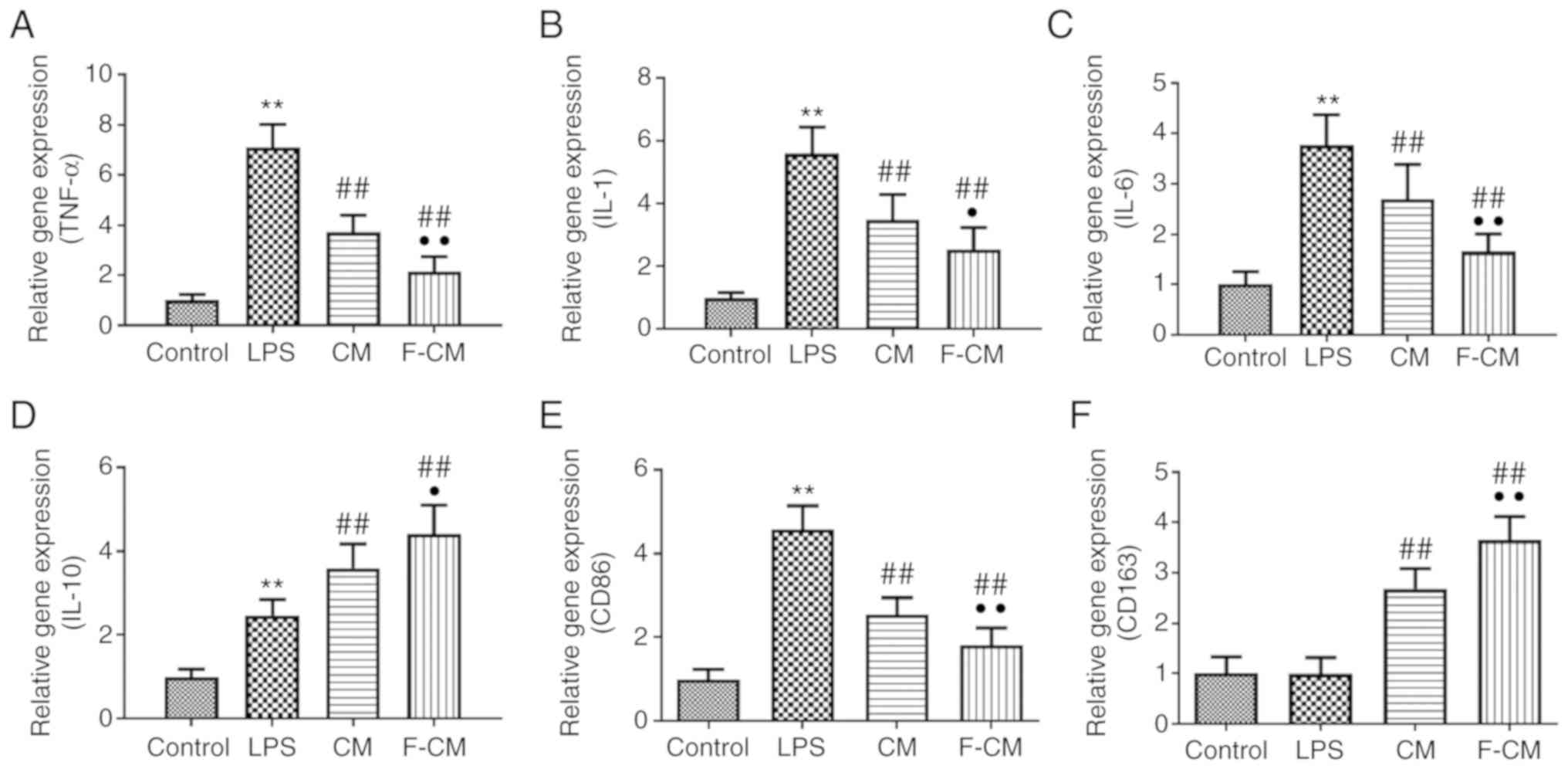
The effects of F-CM on the expression of
inflammation-related genes were investigated using PCR analysis.
Compared with PBS (the control group), LPS significantly increased
the expression levels of pro-inflammatory factors, including TNF-α
(7.11±0.91; Fig. 3A), IL-1
(5.60±0.83; Fig. 3B) and IL-6
(3.77±0.60; Fig. 3C), the
expression of the M1 macrophage marker CD86 (4.58±0.56; Fig. 3E) and even the anti-inflammatory
factor IL-10 (2.47±0.38; Fig. 3D),
but did not affect the expression of the M2 macrophage marker CD163
(0.99±0.32; Fig. 3F). Furthermore,
CM and F-CM significantly inhibited the expression levels of
pro-inflammatory TNF-α (CM, 3.73±0.67; F-CM, 2.14±0.61; Fig. 3A), IL-1 (CM, 3.48±0.80; F-CM,
3.22±0.95; Fig. 3B), IL-6 (CM,
2.70±0.68; F-CM, 1.65±0.35; Fig.
3C) and CD86 (CM, 2.55±0.38; F-CM, 1.81±0.42; Fig. 3E), but further enhanced the
expression levels of CD163 (CM, 2.69±0.40; F-CM, 3.65±0.46;
Fig. 3F) and the anti-inflammatory
factor IL-10 (CM, 3.60±0.58; F-CM, 4.42±0.68; Fig. 3D), compared with LPS.
Immunomodulatory effects of F-CM on
human macrophages
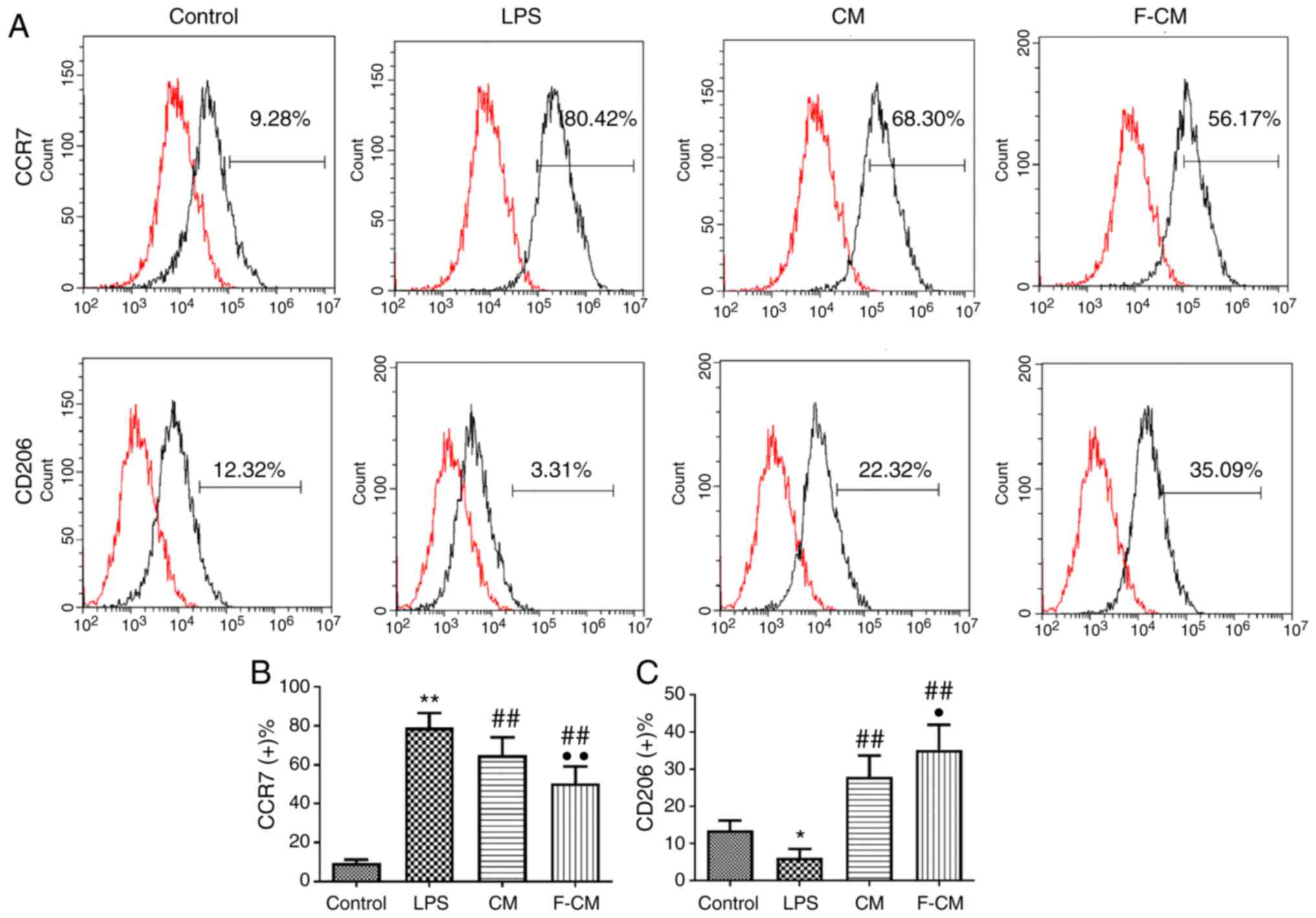
The immunomodulatory effect of F-CM was also
investigated in THP-1 cells (Fig.
4). Consistent with the aforementioned results, LPS
significantly increased the percentage of CCR7-positive cells
(79.23±7.40%), but CM and F-CM significantly decreased the
percentage of CCR7-positive cells (CM, 65.03±9.01%; F-CM,
50.56±8.57%; Fig. 4B). Moreover,
CM and F-CM groups also had a significantly higher ratio of
CD206-positive cells (CM, 27.98±5.75%; F-CM, 35.27±6.67%) compared
with the LPS group (6.19±2.40%; Fig.
4C).
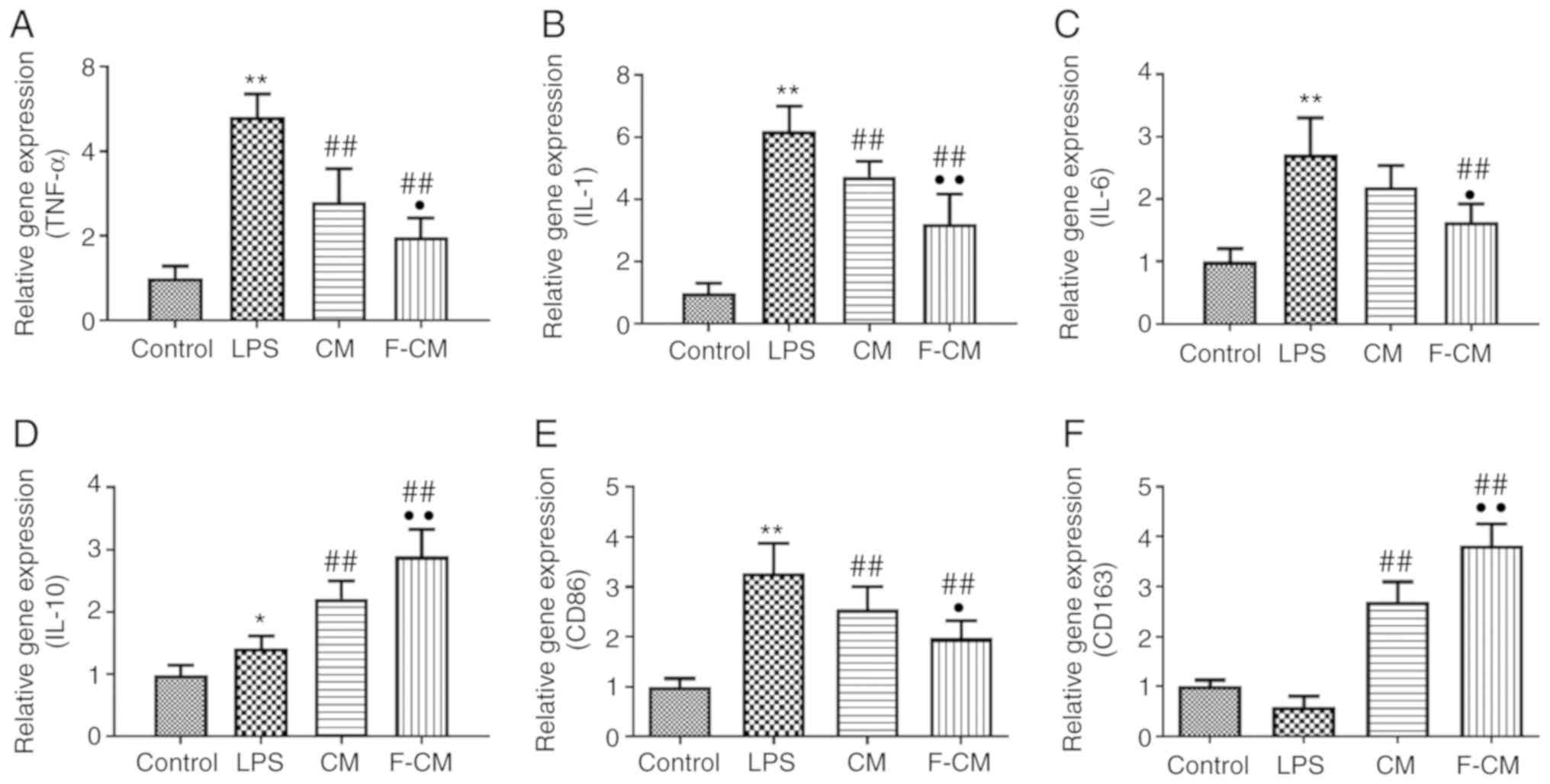
The effects of F-CM on the expression levels of
inflammation-related genes were examined using PCR analysis.
Compared with PBS (the control group), LPS significantly increased
the expression levels of TNF-α (4.81±0.54), IL-1 (6.20±0.79), IL-6
(2.71±0.59) and IL-10 (1.42±0.19), as well as the expression of the
M1 macrophage marker CD86 (3.28±0.59), but markedly decreased the
expression of the M2 macrophage marker CD163 (0.60±0.20) (Fig. 5A-F). In addition, CM and F-CM
significantly inhibited the expression levels of TNF-α (CM,
2.80±0.79; F-CM, 1.98±0.45), IL-1 (CM, 4.74±0.49; F-CM, 3.22±0.95),
IL-6 (CM, 2.19±0.34; F-CM, 1.64±0.29) and CD86 (CM, 2.56±0.46;
F-CM, 1.97±0.35), but enhanced the expression levels of CD163 (CM,
2.70±0.41; F-CM, 3.82±0.43; Fig.
5E) and the anti-inflammatory factor IL-10 (CM, 2.21±0.28;
F-CM, 2.89±0.43; Fig. 5D).
Changes of inflammatory cytokines in
CM from flagellin-activated ADSCs
The levels of CCL5, IFN-γ, IL-1β, IL-1Ra, IL-4,
IL-6, IL-8, IL-10 and TGF-β were assessed using ELISA (Fig. 6A). No significant differences in
the levels of CCL5 (CM, 36.75±10.87 pg/ml; F-CM, 35.25±10.50
pg/ml), IFN-γ (CM, 150.3±22.60 pg/ml; F-CM, 146.8±23.94 pg/ml),
IL-1β (CM, 58.75±6.85 pg/ml; F-CM, 45.50±13.48 pg/ml), IL-1Ra (CM,
45.50±13.67 pg/ml; F-CM, 59.25±18.48 pg/ml), IL-4 (CM, 19.25±12.61
pg/ml; F-CM, 19.75±10.24 pg/ml) or IL-10 (CM, 41.45±18.30 pg/ml;
F-CM, 34.38±15.64 pg/ml) were observed between the two types of CM.
However, flagellin preconditioning significantly increased the
levels of IL-6 (CM, 827.3±293 pg/ml; F-CM, 6,797±1,128 pg/ml), IL-8
(CM, 6,786±2,182 pg/ml; F-CM, 40,605±7,560 pg/ml) and TGF-β (CM,
130.3±31.19 pg/ml; F-CM, 436.8±105.5 pg/ml) compared with CM.
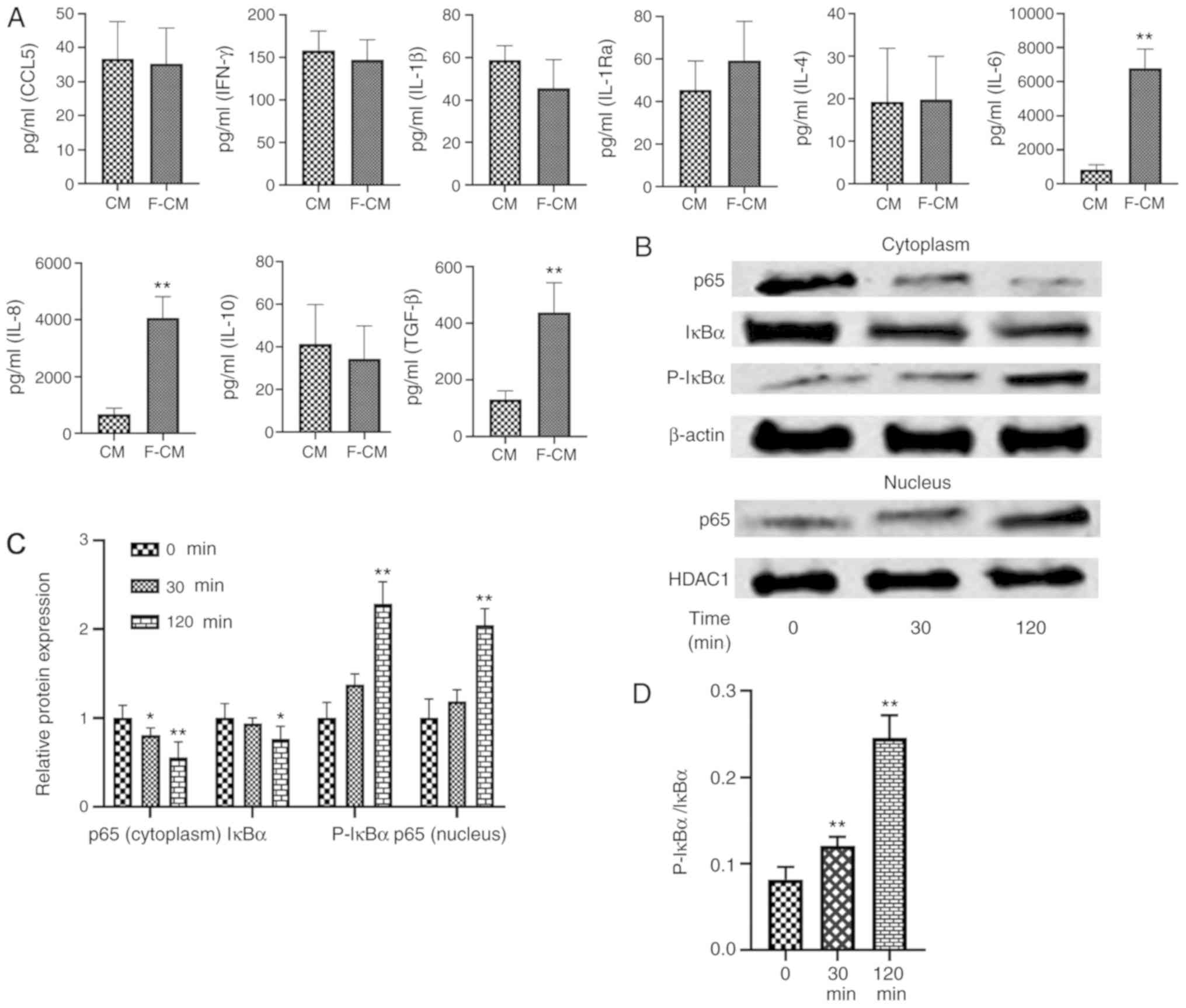 | Figure 6.Effects of flagellin preconditioning
on the production of inflammatory-related cytokines and NF-κB
signaling pathway in ADSCs. (A) Levels of inflammation-related
cytokines of MSCs and flagellin-activated MSCs. Differences between
groups were examined for statistical significance using Student's
t-test. **P<0.01 vs. CM group. (B) p65, IκBα and p-IκBα protein
expression levels in the cytoplasm and p65 protein expression in
nucleus were examined using western blotting. (C) Changes of
protein expressions (cytoplasm p65, cytoplasm lκBα, cytoplasm
p-lκBα and nucleus p65) and (D) p-IκBα/ lκBα ratio during
incubation. Differences among groups were examined for statistical
significance using ANOVA. The results are represented as a relative
ratio to the 0 min groups. *P<0.05 and **P<0.01 vs. 0 min
group. CM, conditioned medium; F-CM, flagellin-conditioned medium;
p-, phosphorylated; IκB, inhibitor of NF-κB; IL, interleukin; CCL,
C-C chemokine ligand; IFN, interferon; TGF, transforming growth
factor; HDAC1, histone deacetylase 1; IL-1Ra, IL-1 receptor
antagonist. |
Effects of flagellin on NF-κB
signaling pathway in ADSCs
Western blotting results indicated that the
expression level of p65 protein, a subunit of NF-κB, was
significantly decreased in the cytoplasm (30 min, 0.80±0.08; 120
min, 0.56±0.17), while its presence in the nucleus increased
significantly after ADSCs were treated with flagellin (30 min,
1.18±0.13; 120 min, 2.05±0.19; Fig. 6B
and C). Moreover, p-IκBα expression (30 min, 1.38±0.12; 120
min, 2.29±0.25) was increased, while total IκBα expression
decreased in the cytoplasm (30 min, 0.94±0.07; 120 min, 0.77±0.14;
Fig. 6B and C). The p-IκBα/IκBα
ratio was also significantly increased over time (0 min,
0.082±0.014; 30 min, 0.12±0.01; 120 min, 0.245±0.026; Fig. 6D). Thus, the results indicated that
NF-κB signaling was activated in ADSCs when treated with 100 ng/ml
flagellin.
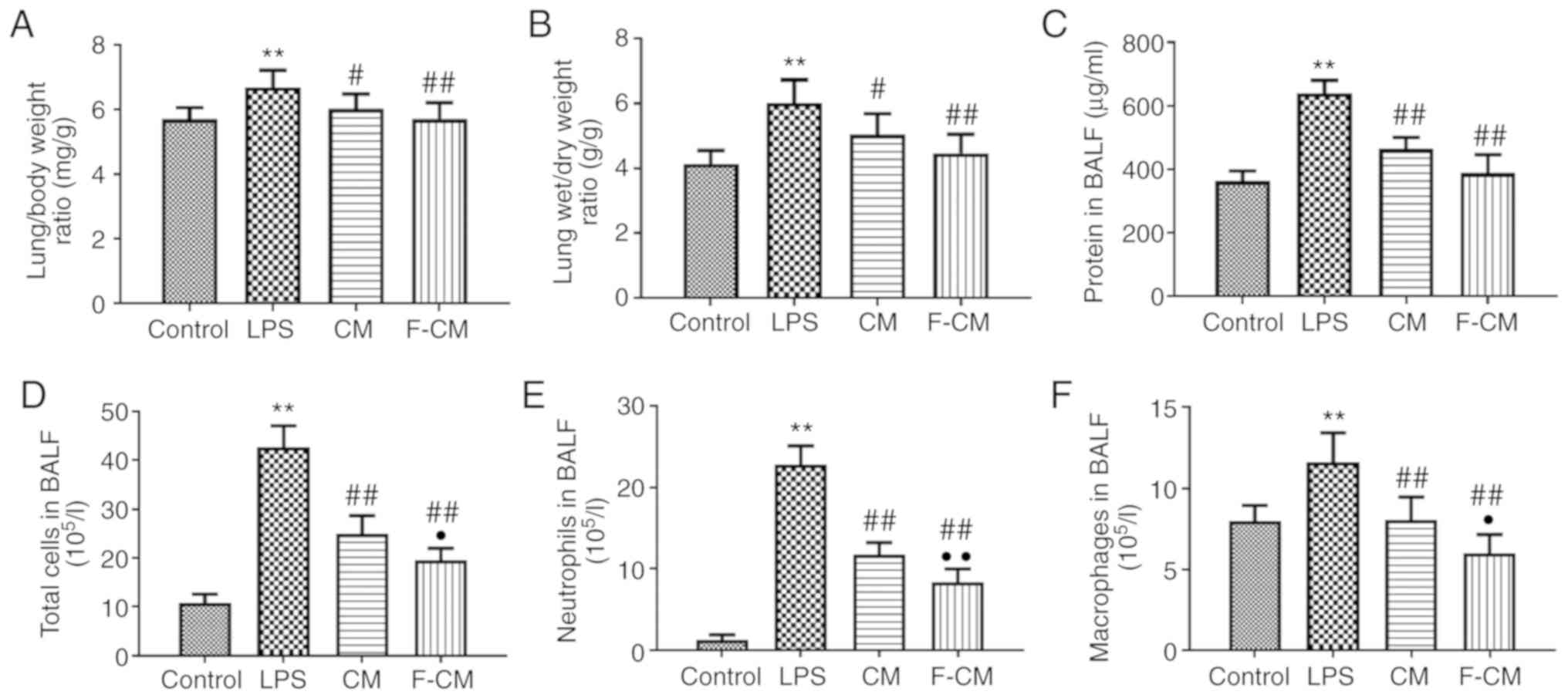
Effects of F-CM on lung exudation and
cell infiltration
The lung/body weight, lung wet/dry weight ratios and
the protein concentration in the BALF were detected to evaluate the
effects of F-CM on pulmonary edema and vascular permeability
(Fig. 7). The mice in the LPS
group exhibited significantly higher lung/body weight ratio
(6.69±0.52 mg/g; Fig. 7A), lung
wet/dry weight ratio (6.01±0.72 g/g; Fig. 7B) and protein concentrations
(639.1±40.23 µg/ml; Fig. 7C)
compared with the mice in the control group (5.70±0.36 mg/g;
4.13±0.42 g/g; and 362.1±32.36 µg/ml, respectively). Moreover, both
CM and F-CM significantly decreased the lung/body weight ratio (CM,
6.02±0.46 mg/g; F-CM, 5.71±0.51 mg/g), lung wet/dry weight ratio
(CM, 5.04±0.65 g/g; F-CM, 4.47±0.58 g/g) and protein concentrations
(CM, 463.8±36.48 µg/ml; F-CM, 388.5±57.51 µg/ml) compared with LPS,
but the effects of F-CM were more prominent.
The effect of F-CM on inflammatory cell recruitment
was investigated by counting the cell numbers in the BALF. LPS
significantly increased not only the total cell number (42.67±4.35
105/l; Fig. 7D), but
also the number of inflammatory cells, including macrophages
(11.63±1.78 105/l; Fig.
7F) and neutrophils (22.78±2.33 105/l; Fig. 7E) compared with the control group.
However, the increase in the numbers of total cells, macrophages
and neutrophils was significantly inhibited by CM (24.92±3.75
105/l; 8.04±1.43 105/l; and 11.77±1.43
105/l, respectively) and F-CM (19.56±2.38
105/l; 5.99±1.16 105/l; and 8.39±1.62
105/l, respectively). In addition, the BALF of mice
treated with F-CM contained the fewest macrophages and neutrophils
among mice undergoing a LPS challenge.
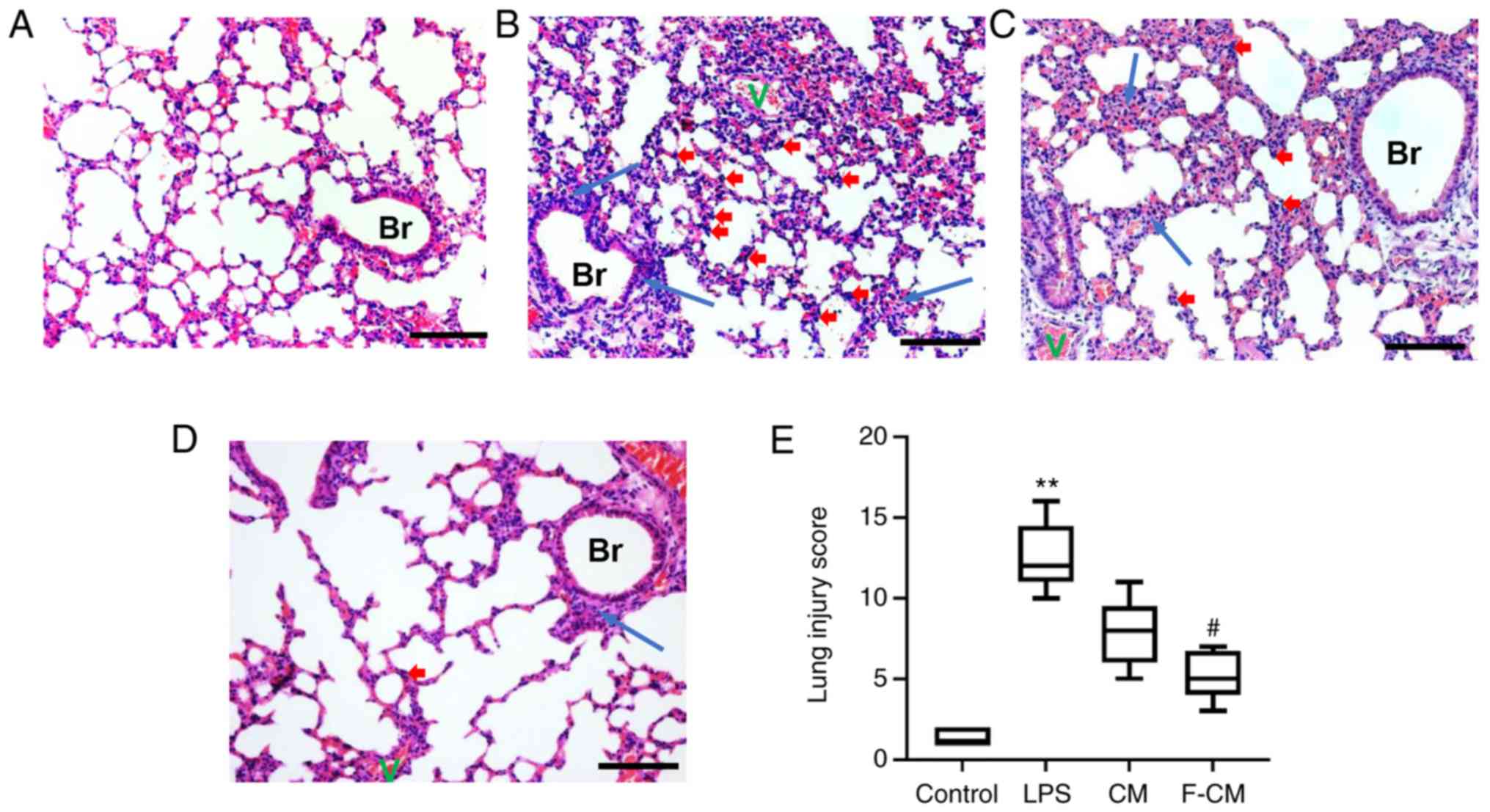
Effects of F-CM on reducing
inflammation caused by LPS in an animal model
H&E staining was performed to determine the
histological changes in the lung. In the control group, the
pulmonary architecture was intact and the alveoli were uniform in
size (Fig. 8A). Compared with the
control group, the LPS group exhibited marked inflammatory changes,
characterized by large areas of red serum, swelling of the alveolar
walls and numerous infiltrating inflammatory cells (Fig. 8B). However, the histological
changes caused by LPS administration were reversed by CM and F-CM
(Fig. 8C and D). Moreover, lung
injury score evaluation also identified the treatment effects of CM
and F-CM (Fig. 8E). The LPS group
(mean, 12.5) had significantly higher lung score compared with the
control group (mean, 1.38), but the lung injury score in F-CM group
(mean, 5.13) was significantly lower compared with the LPS group.
However, no significant differences were found between the CM
(mean, 7.75) and LPS groups.
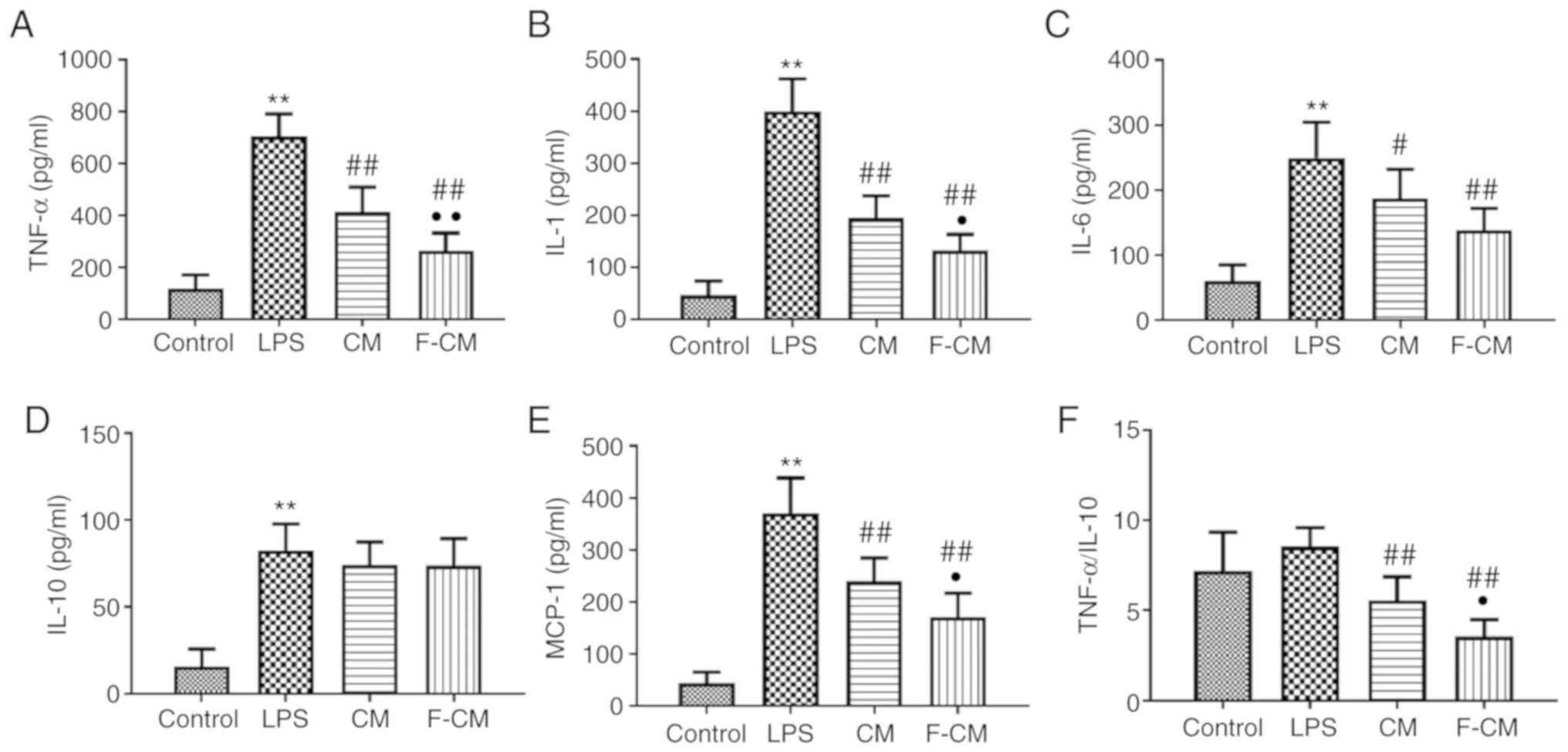
ELISA was performed to investigate the levels of
inflammatory mediators involved in ALI. The mice in the LPS group
had significantly higher levels of TNF-α (704.6±87.1 pg/ml), IL-1
(399.8±61.90 pg/ml), IL-6 (249.4±54.71 pg/ml), MCP-1 (370.8±67.46
pg/ml) and IL-10 (82.50±15.32 pg/ml) compared with the mice in the
control group (117.5±52.7; 42.75±26.68; 61.25±24.06; 44.25±21.02
pg/ml; and 15.75±10.18, respectively; Fig. 9A-E). Consistent with the results
from the in vitro study, CM and F-CM significantly reduced
the production of TNF-α (CM, 413.3±96.05 pg/ml; F-CM, 263.4±0.58
pg/ml), IL-1 (CM, 195.0±42.38 pg/ml; F-CM, 133.0±30.56 pg/ml), IL-6
(CM, 187.4±44.4 pg/ml; F-CM, 138.0±33.79 pg/ml) and MCP-1 (CM,
239.8±44.54 pg/ml; F-CM, 171.0±45.91 pg/ml) compared with PBS.
However, no differences in IL-10 (CM, 74.13±13.20 pg/ml; F-CM,
73.88±15.49 pg/ml) levels were observed among the LPS, CM and F-CM
groups. It was also demonstrated that the F-CM group had
significantly lower TNF-α, IL-1 and MCP-1 levels compared with the
CM group. In addition, the F-CM group (3.57±0.93) had a
significantly lower TNF-α/IL-10 ratio compared with the LPS group
(8.54±1.06) and the CM group (5.58±1.30; Fig. 9F). However, no significant
difference in TNF-α/IL-10 ratio was found between the Control
(7.21±2.14) and LPS group.
Discussion
The causes of ALI include a disruption in the
balance of pro- and anti-inflammatory factors, an imbalance between
oxidation and anti-oxidation, disorders of the coagulation system
and abnormal apoptosis (31). In
particular, LPS, which comprises part of the wall of Gram-negative
bacteria, can increase the expression of inflammatory factors,
promote inflammatory cells to enter lung tissue and increase
alveolar-capillary barrier permeability (28,32).
Thus, the focus of treating ALI is to inhibit the LPS-induced
inflammatory reaction (29).
Despite their effectiveness in regulating inflammation, the heavy
use of steroids is associated with severe adverse effects and may
increase the risk of infection (32). Moreover, other therapeutic methods,
such as mechanical ventilation, neuromuscular blocking agents and
antimicrobial therapy, are only moderately effective (7).
Previous clinical studies reported that the use of
MSCs is safe for patients with ARDS, with a superior
anti-inflammatory effect (7,33).
In addition, as the principal beneficial effects of MSCs are
mediated via paracrine mechanisms, CM from MSCs may be a promising
therapeutic approach to ALI (26).
However, the concentration of biofactors in CM is considered
insufficient for therapy; thus, CM must be concentrated for
treatment. Furthermore, external stimuli can alter the composition
of paracrine factors from MSCs, making them more useful in the
treatment of particular diseases. For instance, exposing MSCs to
pro-inflammatory cytokines for a short time, which mimics the acute
inflammatory microenvironment, significantly enhances their
anti-inflammatory effects (13,19).
Flagellin is a potent activator of a broad range of cell types
involved in innate and adaptive immunity. Following stimulation of
TLR5-expressing cells with flagellin, a signaling cascade is
triggered, which involves phosphorylation of IL-1
receptor-associated kinase 1, leading to activation of
mitogen-activated protein kinase kinases and inhibitor of NF-κB
kinases, ultimately activating inflammatory protein production via
NF-κB and p38 mitogen-activated protein kinase (24). However, most previous studies have
focused on the beneficial effects of ADSCs activated by cytokines
such as TNF-α, IL-1β and IFN-γ (17,19,34).
Thus, limited information is available regarding the
immunomodulatory properties of flagellin-exposed ADSCs.
In the present study, the MSC markers CD73, CD90 and
CD105 were highly expressed in flagellin-activated ADSCs, while the
expression levels of the endothelial cell marker CD31 and the
hematopoietic cell marker CD45 were low, suggesting that flagellin
preconditioning at 100 ng/ml for 2 days did not affect the
characteristics of ADSCs. Since FBS may induce severe immune
rejection, serum-free DMEM was used to collect paracrine factors
from MSCs. Although serum-free incubation decreased the viability
of ADSCs, flagellin-activated ADSCs retained 73% viability after 48
h. Previous studies have reported that LPS, a TLR5 ligand, could
protect MSCs from serum deprivation by stabilizing cell membrane
and activating ERK and PI3K/Akt signaling pathways (35,36).
Given the similarities between TLR4 and TLR5, flagellin may exert
its beneficial effects in similar ways (23). Therefore, ultrafiltration units
with a 3-kDa molecular weight cutoff were used to concentrate CM,
to retain the highest number of inflammatory regulators as
possible.
Alveolar macrophages include tissue-resident and
recruited macrophages, and account for 80% of permanent alveolar
cells (6). Alveolar macrophages
phagocytize foreign bodies, recognize antigens and secrete a
variety of inflammatory factors, forming the first line of the host
immune defense (6). Macrophages
exhibit high functional plasticity and may be induced into
different phenotypes when subjected to different biosignals
(37). In general, macrophages may
be divided into classically activated (M1) and alternatively
activated (M2) macrophages. M1 macrophages usually serve a
pro-inflammatory role and can secrete several pro-inflammatory
factors, such as TNF-α, IL-1, and IL-6, which contribute to the
clearance of pathogens (38). In
contrast, M2 macrophages secrete anti-inflammatory factors, such as
IL-10, and promote angiogenesis, wound healing and tissue
remodeling (39). The present
in vitro study demonstrated that CM from flagellin-activated
ADSCs significantly enhanced the expression of the
anti-inflammatory M2 macrophage marker CD163 and decreased the
expression of the inflammatory M1 macrophage marker CD86 compared
with CM from the regular ADSC model. These results indicated the
superior ability of F-CM to regulate macrophage characteristics and
immunosuppression.
To investigate the mechanism underlying the superior
anti-inflammatory effects of F-CM, the cytokines responsible for
the immunomodulatory properties of the CM were investigated. Since
the composition of CM is complex, flagellin preconditioning
significantly increased the levels of both pro-inflammatory (IL-6
and IL-8) and anti-inflammatory cytokines (TGF-β). Although IL-6 is
considered as a pro-inflammatory cytokine, it may exert both
pro-inflammatory and anti-inflammatory roles (37). Previous studies revealed that IL-6
can induce macrophages into the M2 phenotype in an animal model
(37,40). Furthermore, Liu et al
(15) and Chen et al
(41) reported that MSCs
reprogramed macrophages into the M2 phenotype mainly by secreting
TGF-β. Therefore, the increased IL-6 and TGF-β levels may partly
explain the beneficial effects of flagellin preconditioning. In the
current study, western blotting results indicated that NF-κB
signaling was activated in ADSCs after treatment with 100 ng/ml
flagellin for 30 min, which may partly explain the reason for the
altered cytokines levels.
An LPS-induced ALI mouse model was used in the
present study, as its reliability and reproducibility have been
previously confirmed (42). The
results of the examination of the lung exudate, H&E staining
and cell counting in the BALF identified that both CM and F-CM
significantly prevented LPS-induced lung injury in mice, and the
effects of F-CM treatment were superior. Furthermore, the levels of
inflammation-related factors in the BALF were detected using ELISA.
TNF-α is an inflammatory factor that increases rapidly in the early
stages of inflammation (43).
TNF-α can mobilize bone marrow leukocytes into the blood
circulation, aggregate monocytes in the lung tissue and promote the
release of inflammatory factors, such as IL-1, IL-6 and
colony-stimulating factor (44).
The role of IL-1 has numerous similarities with that of TNF-α in
ALI caused by inflammation (45).
For example, IL-1 can initiate inflammatory reactions together with
TNF-α, and enhance lung injury induced by TNF-α, but it does not
itself cause lung injury (46).
IL-6 can further promote macrophage aggregation, enhance
phagocytosis by macrophages and promote the expression of various
inflammatory factors (47). MCP-1
is another inflammatory chemokine that regulates the chemotactic
response of leukocytes to inflammatory stimuli (48). Moreover, IL-10 is a general
immunosuppressive cytokine, which has been shown to promote the
conversion of M1 macrophages into M2 macrophages, as well as
counteract the pro-inflammatory effects of TNF-α (47).
In the present study, LPS challenge not only
increased the levels of pro-inflammatory cytokines, but also those
of the anti-inflammatory cytokine IL-10 in mice, suggesting that
the anti-inflammation process was activated. F-CM significantly
decreased the production of TNF-α, IL-1, IL-6 and MCP-1; however,
there were no significant differences in IL-10 among the LPS, CM
and F-CM groups, possibly because the alleviated inflammation
reaction was associated with lowered production of
anti-inflammatory factors. Previous studies have reported that drug
interventions, such as Jaceosidin and Ginsenoside Rh2, greatly
decreased the levels of pro-inflammatory cytokines, but do not
alter the level of IL-10 (28,49,50).
Thus, the TNF-α/IL-10 ratio may be a more suitable indicator of the
anti-inflammatory effect. However, IL-10 can be produced by almost
all leukocytes, including macrophages, dendritic cells,
neutrophils, natural killer cells, B cells and T cells (43). Currently, the effects of CM on
cells other than macrophages remain unknown. In addition, the
reasons for the absence of an increase in IL-10 require further
investigation.
Despite the promising results, there were several
limitations to the present study. Due to the limited time and the
complexity of CM (including exosomes, cytokines and microRNAs),
only the changes in nine important cytokines in the CM following
flagellin preconditioning were examined. Although it was found that
three cytokines changed significantly after ADCSs were
preconditioned with flagellin, which one serves the key role in
regulating the polarization of macrophages and inhibiting
inflammation is yet to be elucidated. Furthermore, only one F-CM
administration mode was evaluated in the present study, and
additional experiments are required to determine the optimal dosage
and timing of administration.
In conclusion, the results of the present study
suggested that flagellin preconditioning significantly enhanced the
beneficial effects of CM from ADSCs against LPS-induced lung injury
in mice. These findings may indicate a promising novel approach to
the treatment of inflammation-induced ALI.
Acknowledgements
Not applicable.
Funding
This study was supported by the Shanghai
International Science and Technology Cooperation Fund (grant no.
18410721300).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XD and RL collaborated to design the study. RL was
responsible for experiments. YL analyzed the data and prepared the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable (MSCs were purchased from the Type
Culture Collection of the Chinese Academy of Sciences).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Villar J, Blanco J and Kacmarek RM:
Current incidence and outcome of the acute respiratory distress
syndrome. Curr Opin Crit Care. 22:2753–6. 2016. View Article : Google Scholar
|
|
2
|
Patel VJ, Biswas Roy S, Mehta HJ, Joo M
and Sadikot RT: Alternative and natural therapies for acute lung
injury and acute respiratory distress syndrome. Biomed Res Int.
2018:24768242018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Herrero R, Sanchez G and Lorente JA: New
insights into the mechanisms of pulmonary edema in acute lung
injury. Ann Transl Med. 6:322018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Laskin DL, Malaviya R and Laskin JD: Role
of macrophages in acute lung injury and chronic fibrosis induced by
pulmonary toxicants. Toxicol Sci. 168:287–301. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chazaud B: Inflammation and skeletal
muscle regeneration: Leave it to the macrophages! Trends Immunol.
41:481–492. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Huang X, Xiu H, Zhang S and Zhang G: The
role of macrophages in the pathogenesis of ALI/ARDS. Mediators
Inflamm. 2018:12649132018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wilson JG, Liu KD, Zhuo H, Caballero L,
McMillan M, Fang X, Cosgrove K, Vojnik R, Calfee CS, Lee JW, et al:
Mesenchymal stem (stromal) cells for treatment of ARDS: A phase 1
clinical trial. Lancet Respir Med. 3:24–32. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li Y and Zhang Z and Zhang Z: Porous
chitosan/nano-hydroxyapatite composite scaffolds incorporating
simvastatin-loaded PLGA microspheres for bone repair. Cells Tissues
Organs. 205:20–31. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Radwan SM, Ghoneim D, Salem M, Saeed M,
Saleh Y, Elhamy M, Wael K, Shokair O and Wahdan SA: Adipose
tissue-derived mesenchymal stem cells protect against
amiodarone-induced lung injury in rats. Appl Biochem Biotechnol.
191:1027–1041. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hamilton AM, Cheung WY, Gómez-Aristizábal
A, Sharma A, Nakamura S, Chaboureau A, Bhatt S, Rabani R, Kapoor M,
Foster PJ and Viswanathan S: Iron nanoparticle-labeled murine
mesenchymal stromal cells in an osteoarthritic model persists and
suggests anti-inflammatory mechanism of action. PLoS One.
14:e02141072019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Timmers L, Lim SK, Hoefer IE, Arslan F,
Lai RC, van Oorschot AA, Goumans MJ, Strijder C, Sze SK, Choo A, et
al: Human mesenchymal stem cell-conditioned medium improves cardiac
function following myocardial infarction. Stem Cell Res. 6:206–214.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zomer HD, Varela GKDS, Delben PB, Heck D,
Jeremias TDS and Trentin AG: In vitro comparative study of human
mesenchymal stromal cells from dermis and adipose tissue for
application in skin wound healing. J Tissue Eng Regen Med.
13:729–741. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lu Z, Chen Y, Dunstan C, Roohani-Esfahani
S and Zreiqat H: Priming adipose stem cells with tumor necrosis
factor-alpha preconditioning potentiates their exosome efficacy for
bone regeneration. Tissue engineering Part A. 23:1212–1220. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Park HH, Lee S, Yu Y, Yoo SM, Baek SY,
Jung N, Seo KW and Kang KS: TGF-β secreted by human umbilical cord
blood-derived mesenchymal stem cells ameliorates atopic dermatitis
by inhibiting secretion of TNF-α and IgE. Stem Cells. Apr
11–2020.doi: 10.1002/stem.3183. Online ahead of print. View Article : Google Scholar
|
|
15
|
Liu F, Qiu H, Xue M, Zhang S, Zhang X, Xu
J, Chen J, Yang Y and Xie J: MSC-secreted TGF-β regulates
lipopolysaccharide-stimulated macrophage M2-like polarization via
the Akt/FoxO1 pathway. Stem Cell Res Ther. 10:3452019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Harrell CR, Markovic BS, Fellabaum C,
Arsenijevic N, Djonov V and Volarevic V: The role of interleukin 1
receptor antagonist in mesenchymal stem cell-based tissue repair
and regeneration. Biofactors. 46:263–275. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Putra A, Ridwan FB, Putridewi AI, Kustiyah
AR, Wirastuti K, Sadyah NAC, Rosdiana I and Munir D: The role of
TNF-α induced MSCs on suppressive inflammation by increasing TGF-β
and IL-10. Open Access Maced J Med Sci. 6:1779–1783. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Saparov A, Ogay V, Nurgozhin T, Jumabay M
and Chen WC: Preconditioning of human mesenchymal stem cells to
enhance their regulation of the immune response. Stem Cells Int.
2016:39248582016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Redondo-Castro E, Cunningham C, Miller J,
Martuscelli L, Aoulad-Ali S, Rothwell NJ, Kielty CM, Allan SM and
Pinteaux E: Interleukin-1 primes human mesenchymal stem cells
towards an anti-inflammatory and pro-trophic phenotype in vitro.
Stem Cell Res Ther. 8:792017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ocansey DKW, Pei B, Yan Y, Qian H, Zhang
X, Xu W and Mao F: Improved therapeutics of modified mesenchymal
stem cells: An update. J Transl Med. 18:422020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Linard C, Strup-Perrot C, Lacave-Lapalun
JV and Benderitter M: Flagellin preconditioning enhances the
efficacy of mesenchymal stem cells in an irradiation-induced
proctitis model. J Leukoc Biol. 100:569–580. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jafari M, Asghari A, Delbandi AA, Jalessi
M, Jazayeri MH, Samarei R and Tajik N: Priming TLR3 and TLR4 in
human adipose- and olfactory mucosa-derived mesenchymal stromal
cells and comparison of their cytokine secretions. Cytotechnology.
72:57–68. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Evaristo-Mendonça F, Sardella-Silva G,
Kasai-Brunswick TH, Campos RMP, Domizi P, Santiago MF, de Melo Reis
RA, Mendez-Otero R, Ribeiro-Resende VT and Pimentel-Coelho PM:
Preconditioning of rat bone marrow-derived Mesenchymal stromal
cells with toll-like receptor agonists. Stem Cells Int.
2019:76929732019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
He L, Liang Y, Yu X, Peng W, He J, Fu L,
Lin H, Zhang Y and Lu D: Vibrio parahaemolyticus flagellin induces
cytokines expression via toll-like receptor 5 pathway in
orange-spotted grouper, Epinephelus coioides. Fish Shellfish
Immunol. 87:573–581. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li Y, Huang L, Cai Z, Deng W, Wang P, Su
H, Wu Y and Shen H: A study of the immunoregulatory function of
TLR3 and TLR4 on mesenchymal stem cells in ankylosing spondylitis.
Stem Cells Dev. 28:1398–1412. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Su VY, Lin CS, Hung SC and Yang KY:
Mesenchymal stem cell-conditioned medium induces neutrophil
apoptosis associated with inhibition of the NF-κB pathway in
endotoxin-induced acute lung injury. Int J Mol Sci. 20:22082019.
View Article : Google Scholar
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Huang XL, Wei XC, Guo LQ, Zhao L, Chen XH,
Cui YD, Yuan J, Chen DF and Zhang J: The therapeutic effects of
Jaceosidin on lipopolysaccharide-induced acute lung injury in mice.
J Pharmacol Sci. 140:228–235. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li L, Dong L, Zhang J, Gao F, Hui J and
Yan J: Mesenchymal stem cells with downregulated Hippo signaling
attenuate lung injury in mice with lipopolysaccharideinduced acute
respiratory distress syndrome. Int J Mol Med. 43:1241–1252.
2019.PubMed/NCBI
|
|
30
|
Smith KM, Mrozek JD, Simonton SC, Bing DR,
Meyers PA, Connett JE and Mammel MC: Prolonged partial liquid
ventilation using conventional and high-frequency ventilatory
techniques: Gas exchange and lung pathology in an animal model of
respiratory distress syndrome. Crit Care Med. 25:1888–1897. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Teixeira JP, Ambruso S, Griffin BR and
Faubel S: Pulmonary consequences of acute kidney injury. Semin
Nephrol. 39:3–16. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pedrazza L, Cunha AA, Luft C, Nunes NK,
Schimitz F, Gassen RB, Breda RV, Donadio MV, de Souza Wyse AT,
Pitrez PMC, et al: Mesenchymal stem cells improves survival in
LPS-induced acute lung injury acting through inhibition of NETs
formation. J Cell Physiol. 232:3552–3564. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Matthay MA, Calfee CS, Zhuo H, Thompson
BT, Wilson JG, Levitt JE, Rogers AJ, Gotts JE, Wiener-Kronish JP,
Bajwa EK, et al: Treatment with allogeneic mesenchymal stromal
cells for moderate to severe acute respiratory distress syndrome
(START study): A randomised phase 2a safety trial. Lancet Respir
Med. 7:154–162. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhu M, Chu Y, Shang Q, Zheng Z, Li Y, Cao
L, Chen Y, Cao J, Lee OK, Wang Y, et al: Mesenchymal stromal cells
pretreated with pro-inflammatory cytokines promote skin wound
healing through VEGFC-mediated angiogenesis. Stem Cells Transl Med.
Jun 13–2020.(Epub ahead of print). View Article : Google Scholar
|
|
35
|
Wang J, Li Z, Zhang Y, Liu X, Chen L and
Chen Y: CX43 change in LPS preconditioning against apoptosis of
mesenchymal stem cells induced by hypoxia and serum deprivation is
associated with ERK signaling pathway. Mol Cell Biochem.
380:267–275. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang ZJ, Zhang FM, Wang LS, Yao YW, Zhao Q
and Gao X: Lipopolysaccharides can protect mesenchymal stem cells
(MSCs) from oxidative stress-induced apoptosis and enhance
proliferation of MSCs via Toll-like receptor(TLR)-4 and PI3K/Akt.
Cell Biol Int. 33:665–674. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chen L, Wang S, Wang Y, Zhang W, Ma K, Hu
C, Zhu H, Liang S, Liu M and Xu N: IL-6 influences the polarization
of macrophages and the formation and growth of colorectal tumor.
Oncotarget. 9:17443–17454. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Rana AK, Li Y, Dang Q and Yang F:
Monocytes in rheumatoid arthritis: Circulating precursors of
macrophages and osteoclasts and, their heterogeneity and plasticity
role in RA pathogenesis. Int Immunopharmacol. 65:348–359. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Terai S, Ueda-Hayakawa I, Nguyen CTH, Ly
NTM, Yamazaki F, Kambe N, Son Y and Okamoto H: Palisaded
neutrophilic and granulomatous dermatitis associated with systemic
lupus erythematosus: Possible involvement of CD163(+) M2
macrophages in two cases, and a review of published works. Lupus.
27:2220–2227. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Braune J, Weyer U, Hobusch C, Mauer J,
Brüning JC, Bechmann I and Gericke M: IL-6 regulates M2
polarization and local proliferation of adipose tissue macrophages
in obesity. J Immunol. 198:2927–2934. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chen X, Yang B, Tian J, Hong H, Du Y, Li
K, Li X, Wang N, Yu X and Wei X: Dental follicle stem cells
ameliorate lipopolysaccharide-induced inflammation by secreting
TGF-β3 and TSP-1 to elicit macrophage M2 polarization. Cell Physiol
Biochem. 51:2290–2308. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kim YH, Kim YS, Kim BH, Lee KS, Park HS
and Lim CH: Remote ischemic preconditioning ameliorates indirect
acute lung injury by modulating phosphorylation of IκBα in mice. J
Int Med Res. 47:936–950. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kany S, Vollrath JT and Relja B: Cytokines
in inflammatory disease. Int J Mol Sci. 20:60082019. View Article : Google Scholar
|
|
44
|
Lewis TC, Metitiri EE, Mentz GB, Ren X,
Goldsmith AM, Eder BN, Wicklund KE, Walsh MP, Comstock AT, Ricci
JM, et al: Impact of community respiratory viral infections in
urban children with asthma. Ann Allergy Asthma Immunol.
122:175–183.e2. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Butt Y, Kurdowska A and Allen TC: Acute
lung injury: A clinical and molecular review. Arch Pathol Lab Med.
140:345–350. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Michaudel C, Couturier-Maillard A, Chenuet
P, Maillet I, Mura C, Couillin I, Gombault A, Quesniaux VF, Huaux F
and Ryffel B: Inflammasome, IL-1 and inflammation in ozone-induced
lung injury. Am J Clin Exp Immunol. 5:33–40. 2016.PubMed/NCBI
|
|
47
|
Garth J, Barnes JW and Krick S: Targeting
cytokines as evolving treatment strategies in chronic inflammatory
airway diseases. Int J Mol Sci. 19:34022018. View Article : Google Scholar
|
|
48
|
Betakova T, Kostrabova A, Lachova V and
Turianova L: Cytokines induced during influenza virus infection.
Curr Pharm Des. 23:2616–2622. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Hsieh YH, Deng JS, Chang YS and Huang GJ:
Ginsenoside Rh2 ameliorates lipopolysaccharide-induced acute lung
injury by regulating the TLR4/PI3K/Akt/mTOR, Raf-1/MEK/ERK, and
Keap1/Nrf2/HO-1 signaling pathways in mice. Nutrients.
10:12082018.
|
|
50
|
Li J, Li D, Liu X, Tang S and Wei F: Human
umbilical cord mesenchymal stem cells reduce systemic inflammation
and attenuate LPS-induced acute lung injury in rats. J Inflamm
(Lond). 9:332012. View Article : Google Scholar : PubMed/NCBI
|