Introduction
Osteoarthritis (OA), as a degenerative joint
inflammatory disease, influences the structure and function of
synovial joints, which is characterized by articular cartilage
degeneration, resulting in the narrowing of joint space,
subchondral sclerosis and osteophyte formation (1,2). OA
is one of the leading causes of global disability, and affects ~40%
of the elderly population (3).
Furthermore, OA causes a significant socioeconomic burden,
including direct cost of health care and indirect cost associated
with productivity loss (4). Thus,
it is important to investigate the mechanisms involved in OA
progression.
Cartilage degeneration as the pivotal cause in OA
has attracted increased attention (5). It is well-known that chondrocytes in
cartilage tissues exhibit cell hypertrophy and extracellular matrix
(ECM) degradation, followed by the vascular invasion to the
cartilage, thus exacerbating the injury of affected joints
(6). Chondrocytes also play an
important role in maintaining the balance of cartilage degeneration
and repair, and catabolic and abnormal differentiation of cytokines
and growth factors in chondrocytes can induce ECM degradation
(5,7). A previous study has reported that
transforming growth factor (TGF)-β1 signaling is a pivotal mediator
in cartilage injury and OA development (8). Moreover, TGF-β1 may induce ECM
mineralization, cell hypertrophy and angiogenesis, thus
accelerating cartilage degeneration (9). Smad2/3, as essential downstream
molecules of the TGF-β1 pathway, can induce tissue inhibitor of
matrix metalloproteinase-3 (TIMP3) expression in human chondrocytes
(10,11). Therefore, the TIMP3/TGF-β1 pathway
has the potential to participate in OA development; however, this
requires further investigation.
As mechanosensitive cells, chondrocytes in the
injured cartilage tissues are susceptible to mechanical loading,
and mechanical overloading can induce cartilage degeneration
(12). In addition, mechanical
overloading can lead to the activation of TGF-β1 signaling in
chondrocytes (13). Thus, it was
investigated whether mechanical loading can regulate chondrocyte
degeneration and angiogenesis via the TIMP3/TGF-β1 axis. In the
current study, primary human chondrocytes were isolated, and then
the effects of mechanical loading on chondrocyte metabolism were
examined. Furthermore, a TIMP3 overexpression lentivirus (LV-TIMP3)
was used to investigate the role of the TIMP3/TGF-β1 axis in
mechanical loading induced-chondrocyte degeneration and
angiogenesis.
Materials and methods
Ethical consideration
This study was approved by the Ethics Committee of
First Affiliated Hospital, Heilongjiang University of Chinese
Medicine (approval no. 2017HZYLL-021), and written patient consent
was obtained.
Isolation and culture of primary human
chondrocytes
Primary human chondrocytes were isolated from
healthy knee articular cartilage of a 70-year old male donor who
underwent lower extremity traumatic trauma surgery on 10 December
2018 at First Affiliated Hospital, Heilongjiang University of
Chinese Medicine (Harbin, China). First, knee articular cartilage
was processed into pieces, and then treated by enzymatic digestion
(25% trypsin and 0.2% type II collagenase) as previously described
(14). The isolated chondrocytes
were cultured in complete DMEM (Sigma-Aldrich; Merck KGaA)
containing 10% fetal calf serum (Gibco; Thermo Fisher Scientific,
Inc.) and 1% penicillin-streptomycin (Gibco; Thermo Fisher
Scientific, Inc.) under standard incubation conditions (5%
CO2 and 37°C).
Primary human chondrocytes were identified using 1%
toluidine blue (Sigma-Aldrich; Merck KGaA) staining for 2 h at room
temperature. In addition, the expression levels of chondrocyte
markers (collagen-I and collagen-II) were detected by
immunocytochemistry. In brief, the cells were subjected to 4%
paraformaldehyde for 15 min at room temperature for cell fixation
and goat serum for 30 min for cell blockage at room temperature.
Then, the cells were reacted with collagen-I (1: 50; cat. no.
ab34710; Abcam) or collagen-II antibody (1:50; cat. no. ab34712;
Abcam) overnight at 4°C, followed by incubation with goat
anti-rabbit horseradish peroxidase-conjugated secondary antibody
(1:500; cat. no. ab150077; Abcam) for 1 h at room temperature.
Following washing with PBS, cells were observed by light microscope
(MX 5200H; Meiji Techno Co., Ltd.) (magnification, ×100 and ×400).
Primary human chondrocytes at the third passage were used for the
following experiments.
Lentivirus infection
The LV-TIMP3 overexpression lentivirus (titer with
2×108 TU/ml) was purchased from Shanghai GeneChem Co.,
Ltd. Chondrocytes were infected with LV-TIMP3 (MOI=20). The stable
LV-TIMP3-infected cell lines (named as LV-TIMP3 chondrocytes) were
established using culture medium containing puromycin (10 µl/ml)
for 5 days before the expression level of LV-TIMP3 was assessed
using western blotting.
Mechanical loading to
chondrocytes
To evaluate the effect of mechanical overloading on
cartilage metabolism, chondrocytes were treated with TGF-β1 (5
ng/ml; Sigma-Aldrich; Merck KGaA) or LV-TIMP3 with or without
mechanical loading for 24 h at 37°C. Chondrocytes with mechanical
loading were subjected to a mechanical pressure unit (15) with 10 MPa compression for 72 h.
Chondrocytes without loading were set as the control group. After
72 h, the culture medium of chondrocytes in these groups was
collected for the Transwell experiments.
Cell treatment with lipopolysaccharide
(LPS) or LDN-193189
To establish the chondrocyte degeneration model,
chondrocytes were stimulated with 1 µg/ml LPS (Sigma-Aldrich; Merck
KGaA) for 24 h (LPS group) at 37°C. Then, in order to examine the
relationship of the TIMP3/TGF-β1 signaling pathway and cartilage
degeneration, chondrocytes treated with LDN-193189 (0.5 µM;
Sigma-Aldrich; Merck KGaA), an inhibitor of the TGF-β1 signaling
pathway, for 24 h (LDN-193189 group) at 37°C. The chondrocytes
without treatment were used as the control group.
Reverse transcription-quantitative
(RT-q)PCR
Chondrocytes after the various treatments were
collected and total RNA from these cells was obtained by
TRIzol® (Invitrogen; Thermo Fisher Scientific, Inc.).
Then cDNA was obtained by reverse transcription of RNA using a
PrimeScript™ RT reagent kit (Takara Biotechnology Co., Ltd.)
according to the manufacturers instructions. The RT-qPCR was
performed using SYBR Premix Ex Taq TM II (Takara Biotechnology Co.,
Ltd.). The PCR primers for collagen-I, proteoglycan, TIMP3, TGF-β1,
Smad2, Smad3 and GAPDH are presented in Table I. The PCR parameters were set as
follows: 95°C for 10 min, 40 cycles of 94°C for 30 sec, 58°C for 30
sec, and 72°C for 15 sec. GAPDH was used as the reference gene. The
relative expression levels of these genes were calculated using
comparative threshold (Ct) cycle method (2−ΔΔCt)
(16).
 | Table I.Primer sequences for PCR. |
Table I.
Primer sequences for PCR.
| Gene | Primer sequence |
|---|
| Collagen-I | F:
5′-GACATCCCTGAAGTCAGCTGC-3′ |
| Collagen-I | R:
5′-TCCCTTGGGTCCCTCGAC-3′ |
| Proteoglycan | F:
5′-ATGGAGTTCAGATTCTTCATC-3′ |
| Proteoglycan | R:
5′-TCAGTAGGCTTCACCGACCT-3′ |
| TIMP3 | F:
5′-CTCGAGCAAGGAGGAACTTGGGTG-3′ |
| TIMP3 | R:
5′-GCGGCCGCAATACAGAAGTGTCT-3′ |
| TGF-β1 | F:
5′-GACCGCAACAACGCAATCTA-3′ |
| TGF-β1 | R:
5′-AGGTGTTGAGCCCTTTCCA-3′ |
| Smad2 | F:
5′-GTTCCTGCCTTTGCTGAGAC-3′ |
| Smad2 | R:
5′-TCTCTTTGCCAGGAATGCTT-3′ |
| Smad3 | F:
5′-AGCACACAATAACTTGGACC-3′ |
| Smad3 | R:
5′-TAAGACACACTGGAACAGCGGATG-3′ |
| GAPDH | F:
5′-GCACCGTCAAGGCTGAGAAC-3′ |
| GAPDH | R:
5′-TGGTGAAGACGCCAGTGGA-3′ |
Western blotting
Chondrocytes after various treatments were
collected, and then lysed in RIPA lysis buffer (Gibco; Thermo
Fisher Scientific, Inc.) supplemented with PMSF (1 mM;
Sigma-Aldrich; Merck KGaA). Protein was extracted by centrifugation
at 12,000 × g for 20 min at 4°C and detected using a bicinchoninic
acid kit (Sangon Biotech Co., Ltd.). Protein samples (40 µg) were
separated via 10% SDS-PAGE, and transferred to PVDF membranes,
followed by the blocking with 5% non-fat milk for 1 h at room
temperature. Then, the membrane was incubated with anti-human
collagen-I (1:200; cat. no. sc-59772; Santa Cruz Biotechnology,
Inc.), proteoglycan (1:200; cat. no. MAB1582; Sigma-Aldrich; Merck
KGaA), TIMP3 (1:200; cat. no. sc-373839; Santa Cruz Biotechnology,
Inc.), TGF-β1 (1:200; cat. no. sc-130348; Santa Cruz Biotechnology,
Inc.), phosphorylated (p)-Smad2 (1:200; cat. no. sc-59772; Santa
Cruz Biotechnology, Inc.), Smad2 (1:200; cat. no. sc-393312; Santa
Cruz Biotechnology, Inc.), p-Smad3 (1:200; cat. no. ab-53100;
Abcam), Smad3 (1:200; cat. no. sc-101154; Santa Cruz Biotechnology,
Inc.) or β-actin (1:200; cat. no. sc-8432; Santa Cruz
Biotechnology, Inc.) antibodies overnight at 4°C. Membranes were
washed with PBS, followed by incubation with peroxidase-labeled
second antibody (1:5,000; cat. no. 115-035-003; Jackson
ImmunoResearch Laboratories, Inc.) for 2 h at room temperature.
Then, enhanced chemiluminescence (EMD Millipore) was used to detect
the protein expression levels. Band quantification was performed
using ImageJ software (version 1.54; National Institutes of
Health).
MTT assay
Chondrocytes (5×104/well) were cultured
in a 96-well plate. The next day, chondrocytes were treated with or
without mechanical loading as aforementioned and then the cells
were incubated for 72 h (saturation humidity, 37°C, 5%
CO2). MTT (10 µl; 5 mg/ml; Sigma-Aldrich; Merck KGaA)
was added into wells at 24, 48 or 72 h, and incubated for 4 h.
Then, 100 µl DMSO (Sigma-Aldrich; Merck KGaA) was added. The zero
hole (medium, MTT, DMSO) and blank hole were set up. The absorbance
values at 570 nm were measured by microplate reader (Molecular
Devices, LLC).
Flow cytometry analysis of cell
apoptosis
An Annexin V-FITC Apoptosis Detection kit (cat. no.
ab14085; Abcam) was used to evaluate the cell apoptosis.
Chondrocytes after various treatments were digested with 0.25%
trypsin. After washing with PBS, chondrocytes were resuspended with
1X Binding Buffer, followed by incubation with 5 propidium iodide
(PI) and 5 µl FITC-Annexin V for 15 min at 25°C in the dark, with a
mixture of 1X Binding Buffer. Cells were detected using a flow
cytometer (FACSCalibur; BD Biosciences) and analyzed using
CellQuest software (v. 3.0; Becton, Dickinson and Company). The
apoptotic rate was calculated as the percentage of early + late
apoptotic cells.
Transwell assay for migration of human
umbilical vein endothelial cells (HUVECs)
HUVECs were provided by Obio Technology (Shanghai)
Corp. and maintained in RPMI-1640 medium with 10% fetal bovine
serum (both Gibco; Thermo Fisher Scientific, Inc.) under 37°C and
5% CO2. The migration of HUVECs was analyzed using
Transwell assays (pore size, 0.4 µM; EMD Millipore). HUVECs
(5×105 cells/ml in 300 µl serum-free medium) were seeded
into the upper chamber. Then, 300 µl cell serum-free medium from
chondrocytes in the control, mechanical loading and LV-TIMP3 +
mechanical loading groups was placed in the lower chamber for 24 h.
Subsequently, the cells were stained with crystal violet for 10 min
at 37°C. Migration of HUVECs was observed using a light microscope
in five randomly selected fields (Olympus Corporation)
(magnification, ×200).
Co-culture of HUVECs and
chondrocytes
Co-culture of HUVECs with the chondrocytes was
constructed using Transwell inserts (EMD Millipore). HUVECs
(5×105 cells/ml in 300 µl medium) were seeded into the
upper chamber. Chondrocytes (control group) or LV-TIMP3
chondrocytes were cultured in the lower chamber and treated with 1
µg/ml LPS for 24 h at 37°C. Migration of HUVECs was observed under
a light microscope (Olympus Corporation) (magnification, ×200) and
HMVECs were collected for the following solid-phase binding
assay.
Solid-phase binding assay
The binding of vascular endothelial growth factor
(VEGF) to VEGF receptor (R)2 was detected by solid-phase binding
assays. Chondrocytes were collected, and then lysed in RIPA lysis
buffer supplemented with 1 mM PMSF. Recombinant VEGFR2 protein (50
ng/ml) in PBS was coated in a 96-well plate at 4°C overnight, and
then each well was washed with PBS. After blocking with 4% bull
serum albumin (Sigma-Aldrich; Merck KGaA) for 1 h at room
temperature, supernatant obtained from chondrocytes by
centrifugation at 12,000 × g for 15 min at 4°C was added into wells
at 4°C overnight. After washing with PBS, the bound proteins were
detected by biotinylated-anti-VEGF antibody (1:200; cat. no.
BAF293; R&D Systems China Co., Ltd.) at 37°C for 1 h, followed
by the incubation of avidin-horseradish peroxide at 37°C for 30
min. Then, plates were developed with 3,3,5,5-tetramethylbenzidine
(Sigma-Aldrich; Merck KGaA) for 10 min at room temperature and
measured at 450 nm.
ELISA
The culture supernatant from the aforementioned
treated cells (centrifugation at 2,500 × g for 5 min at room
temperature) was measured by commercial ELISA kits (Cusabio
Technology LLC) for the concentrations of proteoglycan (cat. no.
CSB-E14124h), interleukin (IL)-1β (cat. no. CSB-E08053h), IL-6
(cat. no. CSB-E04638h), IL-4 (cat. no. CSB-E04633h), IL-13 (cat.
no. CSB-E04601h) and TGF-β1 (cat. no. CSB-E04725h), according to
the manufacturers instructions. Supernatant samples (100 µl) were
incubated in a 96-well plate that was coated with antigen in each
well at 37°C for 1.5 h, followed by incubation with biotinylated
antibody (100 µl) at 37°C for 1 h. Then, avidin peroxidase (100 µl)
was added to each well and incubated at 37°C for 30 min, followed
by substrate solution (90 µl) in the dark at 37°C for 15 min and
stopping solution (50 µl). Absorbance at 450 nm was detected by a
microplate reader (Thermo Fisher Scientific, Inc.) to calculate the
level of each cytokine.
Statistical analysis
SPSS 12.0 statistical analysis software (SPSS, Inc.)
was used for statistical analysis. Data are presented as the mean ±
SD of three experimental repeats. Data were analyzed by one-way
ANOVA followed by post hoc Tukeys test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Mechanical loading influences
chondrocyte metabolism
To determine the effects of mechanical loading on
chondrocyte metabolism, primary human chondrocytes were isolated
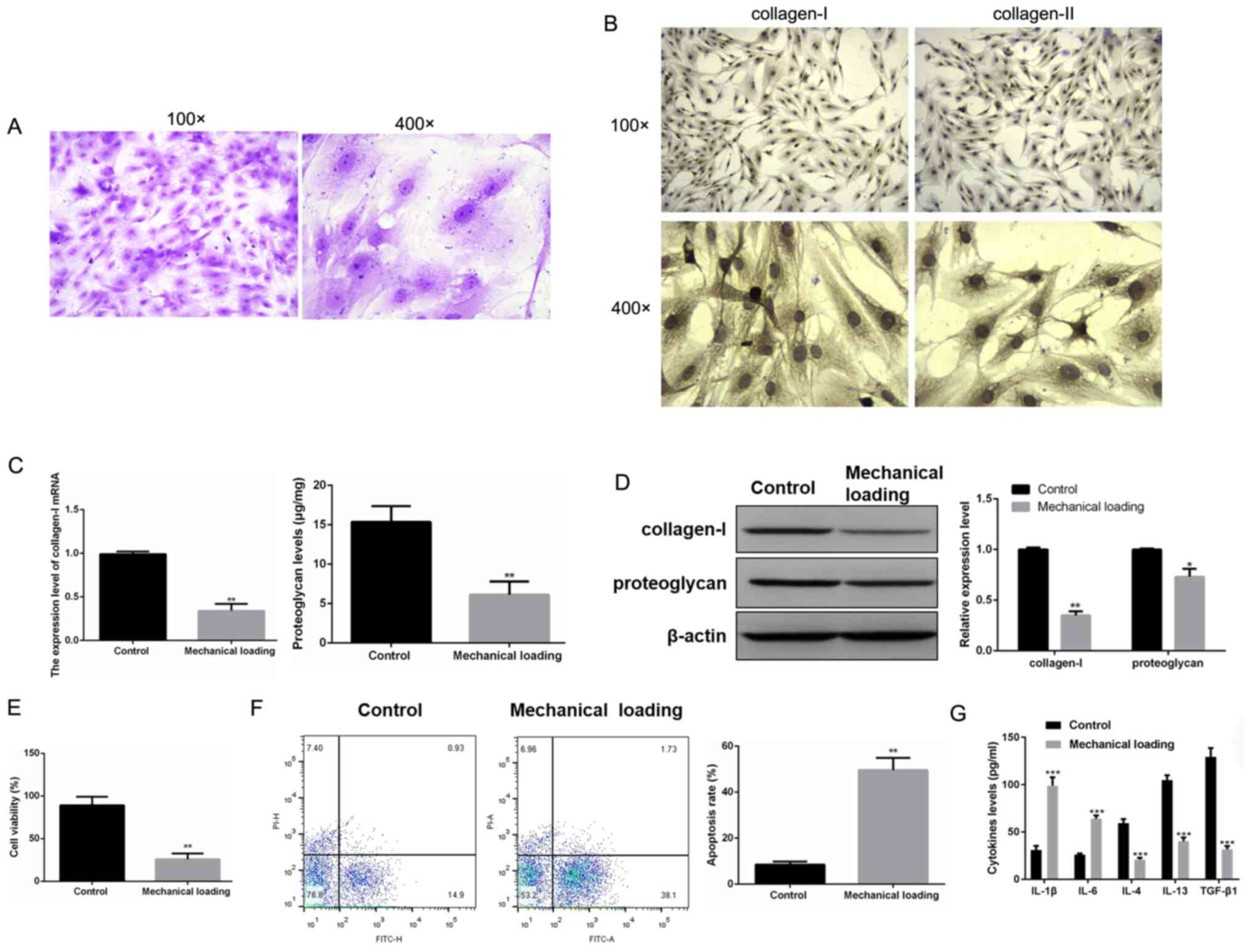
and subjected to mechanical loading. Toluidine blue staining
identified that primary human chondrocytes were stained as
blue-violet, which suggested the positive expression of
proteoglycan (Fig. 1A). Moreover,
immunocytochemistry results demonstrated the positive expression
levels of collagen-I and collagen-II (Fig. 1B). Thus, it was indicated that
primary human chondrocytes were successfully isolated.
The mRNA expression and protein levels of collagen-I
and proteoglycan were both significantly lower in chondrocytes
subjected to mechanical loading compared with normal chondrocytes
(P<0.05; Fig. 1C and D). MTT
assay results also suggested that mechanical loading inhibited
chondrocyte proliferation (P<0.01; Fig. 1E). Furthermore, flow cytometry
analysis also found that the cell apoptotic rate was significantly
increased in chondrocytes subjected to mechanical loading compared
with normal chondrocytes (P<0.01; Fig. 1F). Furthermore, ELISAs demonstrated
that the levels of IL-1β and IL-6 were significantly increased,
while the levels of IL-4, IL-13 and TGF-β1 were significantly
decreased in chondrocytes subjected to mechanical loading compared
with normal chondrocytes (P<0.001; Fig. 1G).
Relationship between the TIMP3/TGF-β1
axis, chondrocyte degeneration and angiogenesis
To investigate the effects of the TIMP3/TGF-β1
signaling pathway on chondrocyte degeneration, degeneration was
induced by LPS. It was found that LPS treatment significantly
inhibited the protein expression levels of collagen-I and
proteoglycan compared with in normal chondrocytes (P<0.05 and
P<0.01, respectively; Fig. 2A),
which indicated that chondrocyte degeneration was successfully
induced by LPS.
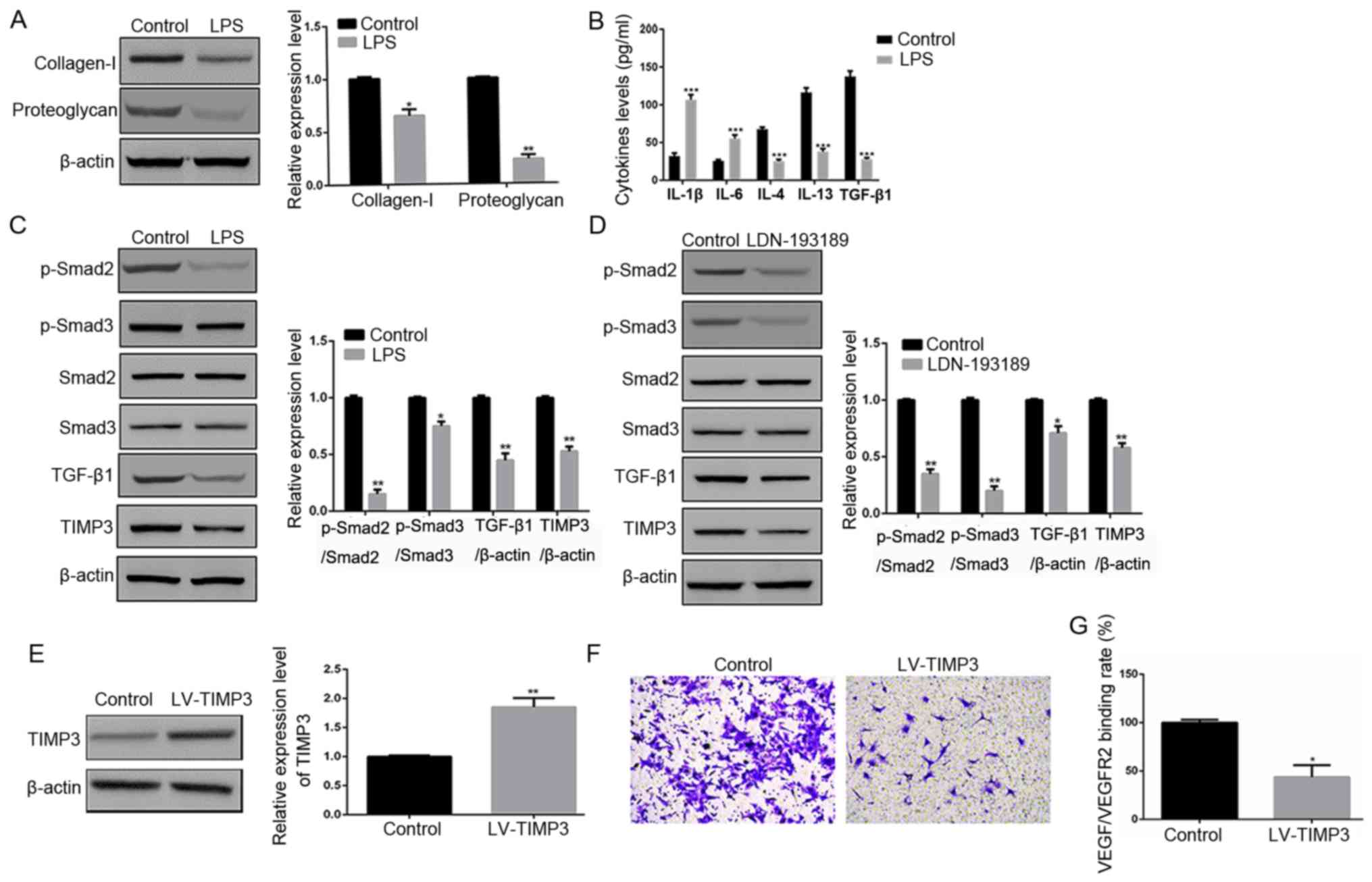 | Figure 2.Effects of the TIMP3/TGF-β1 axis on
chondrocyte degeneration and angiogenesis. (A) Protein expression
levels of collagen-I and proteoglycan in chondrocytes from the
control group and LPS group as determined via western blotting. (B)
Levels of inflammatory factors in chondrocytes from the control
group and LPS group as determined via ELISA. (C) Protein expression
levels of TIMP3, TGF-β1, p-Smad2 and p-Smad3 in chondrocytes from
the control group and LPS group assessed via western blotting. (D)
Protein expression levels of TIMP3, TGF-β1, p-Smad2 and p-Smad3 in
chondrocytes from the control group and LDN-193189 group determined
via western blotting. (E) Protein expression of TIMP3 in
chondrocytes from the control group and LV-TIMP3 group assessed via
western blotting. (F) Cell migration of HUVECs when co-cultured
with chondrocytes or LV-TIMP3 chondrocytes determined via Transwell
assays. (G) VEGF/VEGFR2 binding rate in HUVECs when co-cultured
with chondrocytes or LV-TIMP3 chondrocytes as determined via
solid-phase binding assays. *P<0.05, **P<0.01, ***P<0.001
vs. control group. LPS, lipopolysaccharide; p-, phosphorylated;
TGF-β1, transforming growth factor β1; TIMP3, tissue inhibitor of
matrix metalloproteinase-3; HUVECs, human umbilical vein
endothelial cells. |
In addition, the levels of IL-1β and IL-6 were
significantly increased, and the levels of IL-4, IL-13 and TGF-β1
were significantly decreased in chondrocytes treated with LPS
compared with normal chondrocytes (P<0.001; Fig. 2B). Western blotting results
identified that the protein expression levels of TIMP3, TGF-β1,
p-Smad2 and p-Smad3 were significantly decreased in chondrocytes
treated with LPS, compared with normal chondrocytes (P<0.05;
Fig. 2C).
It was also demonstrated that LDN-193189 (an
inhibitor of the TGF-β1 signaling pathway) significantly inhibited
the protein expression levels of TIMP3, TGF-β1, p-Smad2 and p-Smad3
(P<0.05; Fig. 2D). Furthermore,
LV-TIMP3 chondrocytes were constructed, and western blotting
results indicated that TIMP3 was overexpressed in LV-TIMP3
chondrocytes (P<0.01; Fig.
2E).
Co-culture of chondrocytes and HUVECs showed that
LV-TIMP3 chondrocytes suppressed cell migration of HUVECs compared
with normal chondrocytes (Fig.
2F). Moreover, the VEGF/VEGFR2 binding rate in HUVECs in the
LV-TIMP3 group was significantly lower compared with the control
group (P<0.05; Fig. 2G).
Mechanical loading influences
chondrocyte degeneration and angiogenesis via the TIMP3/TGF-β1
axis
It was found that, compared with control
chondrocytes, mechanical loading significantly inhibited the
protein expression levels of collagen-I, proteoglycan, TIMP3,
TGF-β1, p-Smad2 and p-Smad3, while TGF-β1 treatment or TIMP3
overexpression significantly increased their expression levels
compared with control chondrocytes. Furthermore, the combination of
mechanical loading and TGF-β1 treatment or TIMP3 overexpression
significantly reduced the protein expression levels of collagen-I,
proteoglycan, TIMP3, TGF-β1, p-Smad2 and p-Smad3 compared with
TGF-β1 treatment or TIMP3 overexpression alone, and significantly
increased their expression compared with chondrocytes subjected to
mechanical loading alone (P<0.05; Fig. 3A).
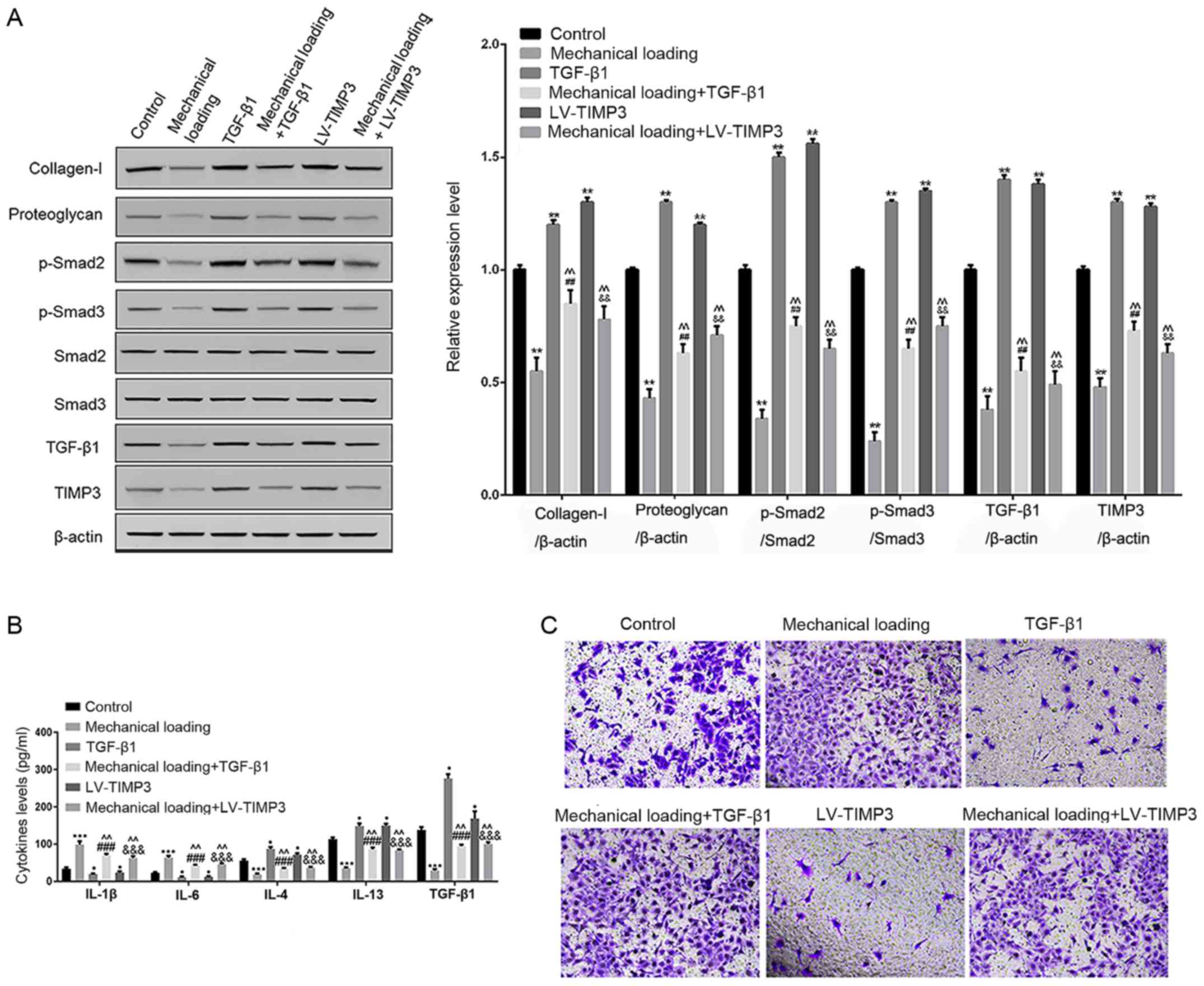 | Figure 3.Effects of mechanical loading on the
TIMP3/TGF-β1 axis and angiogenesis. (A) Protein expression levels
of collagen-I, proteoglycan, TIMP3, TGF-β1, p-Smad2 and p-Smad3 in
chondrocytes in the control group, mechanical loading group, TGF-β1
group, mechanical loading + TGF-β1 group, LV-TIMP3 group and
mechanical loading + LV-TIMP3 group assessed via western blotting.
(B) Levels of inflammatory factors in chondrocytes in the control
group, mechanical loading group, TGF-β1 group, mechanical loading +
TGF-β1 group, LV-TIMP3 group and mechanical loading + LV-TIMP3
group determined via ELISA. (C) Cell migration of HUVECs in the
control group, mechanical loading group, TGF-β1 group, mechanical
loading + TGF-β1 group, LV-TIMP3 group and mechanical loading +
LV-TIMP3 group determined using Transwell assays. *P<0.05,
**P<0.01, ***P<0.001 0.001 vs. control group.
##P<0.01, ###P<0.001 vs. TGF-β1 group.
&&P<0.01,
&&&P<0.001 vs. LV-TIMP3 group.
^^P<0.01 vs. mechanical loading group. p-, phosphorylated;
TGF-β1, transforming growth factor β1; TIMP3, tissue inhibitor of
matrix metalloproteinase-3; HUVECs, human umbilical vein
endothelial cells. |
Compared with control chondrocytes, TGF-β1 treatment
or TIMP3 overexpression alone significantly decreased the levels of
IL-1β and IL-6, but increased the levels of IL-4, IL-13 and TGF-β1;
however, the combination of mechanical loading and TGF-β1 treatment
or TIMP3 overexpression partly reversed their levels compared with
TGF-β1 treatment or TIMP3 overexpression alone (P<0.05; Fig. 3B).
Furthermore, cell migration of HUVECs was increased
in the mechanical loading group, while it was decreased in the
TGF-β1 and LV-TIMP3 groups, compared with the control group. It was
also indicated that the combination of mechanical loading and
TGF-β1 treatment or TIMP3 overexpression promoted cell migration of
HUVECs compared with cells subjected to TGF-β1 treatment or TIMP3
overexpression alone (Fig.
3C).
Discussion
In the present study, primary human chondrocytes
were successfully isolated. It was demonstrated that mechanical
loading significantly inhibited the expression levels of collagen-I
and proteoglycan in chondrocytes, as well as reducing cell
viability and promoting cell apoptosis, thus suggesting that
mechanical loading leads to chondrocyte degeneration. In addition,
the expression levels of TIMP3, TGF-β1, p-Smad2 and p-Smad3 were
significantly decreased in degenerated chondrocytes induced by LPS,
as well as in chondrocytes treated with LDN-193189. Furthermore,
TIMP3 overexpression suppressed cell migration and reduced the
VEGF/VEGFR2 binding rate in HUVECs. It was also identified that
mechanical loading significantly inhibited the expression levels of
collagen-I, proteoglycan, TIMP3, TGF-β1, p-Smad2 and p-Smad3 in
chondrocytes, increased the contents of IL-1β and IL-6, decreased
the levels of IL-4, IL-13 and TGF-β1, and promoted the migration of
HUVECs; TGF-β1 treatment or TIMP3 overexpression reversed these
results.
It is well-known that articular chondrocytes are
responsible for the maintenance of articular cartilage homeostasis,
and the dysregulation of this is related to the pathological
processes of cartilage degeneration in OA (17). TGF-β1 has been researched in
chondrocytes and OA for several years. A previous study reported
that TGF-β1 is an essential growth factor that maintains cartilage
integrity and ECM, and that the deactivation of TGF-β1 signaling
can induce cartilage degeneration (18). However, it has also been shown that
TGF-β1 is upregulated in subchondral bone of OA, and cartilage
degradation is attenuated by inhibiting the activity of TGF-β1,
indicating that TGF-β1 overexpression may be a trigger of OA
(19,20). Moreover, the present results
suggested that the expression levels of TGF-β1 and p-Smad2/3 were
reduced in degenerated chondrocytes induced by LPS.
Furthermore, it was demonstrated that TIMP3
expression was downregulated in degenerated chondrocytes. TIMPs are
endogenous inhibitors of matrix metalloproteinases (MMPs), and MMPs
are involved in ECM degradation in chondrocytes of OA (21). It has been previously revealed that
TGF-β-induced TIMP3 can inhibit MMP activity in cartilage (10,21).
In addition, Smad2/3, as classical intracellular mediators of the
TGF-β signaling pathway, can upregulate TIMP3 in human
chondrocytes; TIMP3 is considered as a target gene of Smad2/3 in
the TGF-β signaling pathway (10,22).
In line with these studies, the present results suggested that
TIMP3 expression was reduced when the TGF-β1/Smad2/3 pathway was
inhibited. Furthermore, it was identified that TIMP3 overexpression
suppressed cell migration and reduced the VEGF/VEGFR2 binding rate
in HUVECs. A previous study has also reported that the TGF-β
signaling pathway can promote angiogenesis in endothelial
progenitor cells by upregulating VEGF expression (23). Moreover, it has been shown that
TGF-β1 induces angiogenesis in cartilage of OA (9,19).
Collectively, it was speculated that the TIMP3/TGF-β1 axis may be
involved in chondrocyte degeneration and angiogenesis.
Mechanical stress is considered as a risk factor for
OA development (24). It has been
revealed that excessive loading in joints results in cartilage
degeneration (25,26). The present results indicated that
mechanical loading significantly inhibited the expression levels of
collagen-I and proteoglycan in chondrocytes. Collagen-I and
proteoglycan, as anabolic genes, are involved in ECM metabolism of
chondrocytes, and the abnormal expression of these genes may lead
to chondrocyte degeneration (27).
In addition, a previous study reported an increased cell apoptosis
rate in the chondrocytes of OA cartilage compared with healthy
cartilage, thus suggesting that chondrocyte apoptosis is closely
associated with OA progression (28). Moreover, it has been shown that
mechanical injury can induce chondrocyte apoptosis (29,30).
Consistent with these findings, the present results demonstrated
that mechanical loading reduced cell proliferation and promoted
cell apoptosis. Therefore, it was speculated that mechanical
loading led to chondrocyte degeneration. Furthermore, a previous
study revealed that mechanical overloading promotes the activation
of TGF-β1 signaling in chondrocytes, and upregulation of TGF-β1 is
responsible for mechanical stress-induced chondrocyte degradation
(13). In line with this, the
present results suggested that mechanical loading inhibited the
expression of the TIMP3/TGF-β1 pathway in chondrocytes, and also
increased cell migration of HUVECs. Moreover, TIMP3 overexpression
could reverse the role of mechanical loading in chondrocytes.
Collectively, it was indicated that the TIMP3/TGF-β1 axis may be
involved in mechanical loading-induced chondrocyte degeneration and
angiogenesis.
In conclusion, it was demonstrated that mechanical
loading led to chondrocytes degeneration. In addition, the
TIMP3/TGF-β1 axis may be a vital signaling pathway in mechanical
loading induced-chondrocytes degeneration and angiogenesis in OA
development.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The data used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors contributions
SHD designed the experiments. HTL provided the
patient sample and designed the experiments. DLZ and SHD carried
out the experiments. DLZ prepared the manuscript. All authors read
and approved the manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
First Affiliated Hospital, Heilongjiang University of Chinese
Medicine (approval no. 2017HZYLL-021). Written patient consent was
obtained from the donor.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mirandaduarte A: DNA methylation in
osteoarthritis: Current status and therapeutic implications. Open
Rheumatol J. 12:2637–49. 2018.
|
|
2
|
Vina ER and Kwoh CK: Epidemiology of
osteoarthritis: Literature update. Curr Opin Rheumatol. 30:160–167.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cross M, Smith E, Hoy D, Nolte S, Ackerman
I, Fransen M, Bridgett L, Williams S, Guillemin F, Hill CL, et al:
The global burden of hip and knee osteoarthritis: Estimates from
the global burden of disease 2010 study. Ann Rheum Dis.
73:1323–1320. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hunter DJ, Schofield D and Callander E:
The individual and socioeconomic impact of osteoarthritis. Nat Rev
Rheumatol. 10:437–441. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Varela-Eirin M, Loureiro J, Fonseca E,
Corrochano S, Caeiro JR, Collado M and Mayan MD: Cartilage
regeneration and ageing: Targeting cellular plasticity in
osteoarthritis. Ageing Res Rev. 42:56–71. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Man GS and Mologhianu G: Osteoarthritis
pathogenesis-a complex process that involves the entire joint. J
Med Life. 7:37–41. 2014.PubMed/NCBI
|
|
7
|
Kühn K, DLima DD, Hashimoto S and Lotz M:
Cell death in cartilage. Osteoarthritis Cartilage. 12:1–16. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhai G, Doré J and Rahman P: TGF-β signal
transduction pathways and osteoarthritis. Rheumatol Int.
35:1283–1292. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen JL, Chang Z, Chen Y, Zhu W, Wei L,
Huang J, Liu Q, Wang D, Li D, Xiong J, et al: TGFβ1 induces
hypertrophic change and expression of angiogenic factors in human
chondrocytes. Oncotarget. 8:91316–91327. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Qureshi HY, Ricci G and Zafarullah M: Smad
signaling pathway is a pivotal component of tissue inhibitor of
metalloproteinases-3 regulation by transforming growth factor beta
in human chondrocytes. Biochim Biophys Acta. 1783:1605–1612. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Leivonen SK, Lazaridis K, Decock J,
Chantry A, Edwards DR and Kähäri VM: TGF-β-elicited induction of
tissue inhibitor of metalloproteinases (TIMP)-3 expression in
fibroblasts involves complex interplay between Smad3, p38α, and
ERK1/2. PLoS One. 8:e574742013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kurz B, Lemke AK, Fay J, Pufe T,
Grodzinsky AJ and Schünke M: Pathomechanisms of cartilage
destruction by mechanical injury. Ann Anat. 187:473–485. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang RK, Li GW, Zeng C, Lin CX, Huang LS,
Huang GX, Zhao C, Feng SY and Fang H: Mechanical stress contributes
to osteoarthritis development through the activation of
transforming growth factor beta 1 (TGF-β1). Bone Joint Res.
7:587–594. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dvirginzberg M, Gagarina V, Lee EJ and
Hall DJ: Regulation of cartilage-specific gene expression in human
chondrocytes by SirT1 and nicotinamide phosphoribosyltransferase. J
Biol Chem. 283:36300–36310. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jun W, Yumin Z, Wei S, Tao M and Kunzheng
W: microRNA-590-5p targets transforming growth factor β1 to promote
chondrocyte apoptosis and autophagy in response to mechanical
pressure injury. J Cell Biochem. 116:9931–9940. 2018.
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Brandt KD, Dieppe P and Radin EL:
Etiopathogenesis of osteoarthritis. Med Clin North Am. 93:1–24.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Blaney Davidson EN, van der Kraan P and
van den Berg W: TGF-beta and osteoarthritis. Osteoarthritis
Cartilage. 15:597–604. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhen G, Wen C, Jia X, Li Y, Crane JL,
Mears SC, Askin FB, Frassica FJ, Chang W, Yao J, et al: Inhibition
of TGF-β signaling in mesenchymal stem cells of subchondral bone
attenuates osteoarthritis. Nat Med. 19:704–712. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Verdier MP, Seité S, Guntzer K, Pujol JP
and Boumediene K: Immunohistochemical analysis of transforming
growth factor beta isoforms and their receptors in human cartilage
from normal and osteoarthritic femoral heads. Rheumatol Int.
25:118–124. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yoshihara Y, Nakamura H, Obata K, Yamada
H, Hayakawa T, Fujikawa K and Okada Y: Matrix metalloproteinases
and tissue inhibitors of metalloproteinases in synovial fluids from
patients with rheumatoid arthritis or osteoarthritis. Ann Rheum
Dis. 59:455–461. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhu Y, Gu J, Zhu T, Jin C, Hu X and Wang
X: Crosstalk between Smad2/3 and specific isoforms of ERK in
TGF-β1-induced TIMP-3 expression in rat chondrocytes. J Cell Mol
Med. 21:1781–1790. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Harry LE and Paleolog EM: From the cradle
to the clinic: VEGF in developmental, physiological, and
pathological angiogenesis. Birth Defects Res C Embryo Today.
69:363–374. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Guilak F: Biomechanical factors in
osteoarthritis. Best Pract Res Clin Rheumatol. 25:815–823. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Andriacchi TP, Mündermann A, Smith RL,
Alexander EJ, Dyrby CO and Koo S: A framework for the in vivo
pathomechanics of osteoarthritis at the knee. Ann Biomed Eng.
32:447–457. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Felson DT, Joyce G, Jingbo N, Yuqing Z and
Hunter DJ: The effect of body weight on progression of knee
osteoarthritis is dependent on alignment. Arthritis Rheum.
50:3904–3909. 2014. View Article : Google Scholar
|
|
27
|
Liu Q, Hu X, Zhang X, Duan X, Yang P, Zhao
F and Ao Y: Effects of mechanical stress on chondrocyte phenotype
and chondrocyte extracellular matrix expression. Sci Rep.
6:372682016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Héraud F, Héraud A and Harmand MF:
Apoptosis in normal and osteoarthritic human articular cartilage.
Ann Rheum Dis. 59:959–965. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Colwell CW Jr, DLima DD, Hoenecke HR,
Fronek J, Pulido P, Morris BA, Chung C, Resnick D and Lotz M: In
vivo changes after mechanical injury. Clin Orthop Relat Res. 391
(Suppl):S116–S123. 2001. View Article : Google Scholar
|
|
30
|
DLima DD, Hashimoto S, Chen PC, Colwell CW
Jr and Lotz MK: Human chondrocyte apoptosis in response to
mechanical injury. Osteoarthritis Cartilage. 9:712–719. 2001.
View Article : Google Scholar : PubMed/NCBI
|