Introduction
Epilepsy is a complicated neurological condition
that reoccurs in patients (1).
Temporal lobe epilepsy (TLE), one of the most common types of
epileptic seizures, is a chronic neurological disorder that
originates from the temporal lobe of the brain (2). The etiology of TLE is complicated and
the prevalence of the disease is high, with an incidence rate of
61.4 in 100,000 individuals (95% CI 50.7-74.4) (3). Furthermore, ~1/3 of patients are not
effectively treated following the use of the common drugs, such as
carbamazepine and Phenytoin sodium (1,4,5). The
intractable nature of epilepsy renders both the treatment and
rehabilitation of the disorder difficult (6). Therefore, further studies are
required to increase the understanding of the pathogenesis of
epilepsy, and to discover effective therapeutic targets and
molecular therapies for the treatment of TLE.
MicroRNAs (miRNAs/miRs), which are found in
eukaryotes, area class of endogenous, highly conserved non-coding
small RNAs with regulatory functions (7). miRNAs identify target genes by base
pairing, which results in the degradation or the inhibition of
translation of the target gene (8,9).
Moreover, miRNAs have been reported to be involved in various
stages of biological growth and development, especially in cell
growth and tissue differentiation, which are closely related to
several types of disease, such as melanoma and chronic lymphocytic
leukemia (10–14). Previous studies have reported that
multiple miRNAs are differentially expressed in the central nervous
system, suggesting that miRNAs may be involved in the development
of neurological pathology, including TLE (15–20).
However, the specific regulatory mechanisms of these miRNAs require
further research. miR-15a is a conserved miRNA, which was
discovered to participate in cell progression in numerous types of
cancer, including thyroid cancer and prostate cancer (21,22),
in addition to epilepsy (23,24).
Furthermore, miR-15a has been suggested to serve as a biomarker, as
low expression levels of miR-15a were previously reported in
epilepsy (25,26). However, the function of miR-15a in
epilepsy is not fully understood.
Glial fibrillary acidic protein (GFAP) is a marker
of astrocyte activation and it is mainly distributed in astrocytes
of the central nervous system (27,28).
Moreover, GFAP has been discovered to be closely related to cell
progression and inflammation in numerous types of neurological
disease (29,30). For example, it was discovered that
GFAP was highly expressed in epilepsy, and increasing its
expression levels aggravated the neuroinflammatory response
(31,32).
To further examine whether miR-15a serves a role in
TLE, cell lines overexpressing miR-15a were constructed via cell
transfection. In addition, GFAP was predicted to be a target mRNA
of miR-15a using microT-CDS, followed by validation using
dual-luciferase reporter and RNA immunoprecipitation (RIP) assays.
Therefore, the present study hypothesized that miR-15a may be
associated with TLE by targeting GFAP.
Materials and methods
Patients studies
Temporal lobe cortical tissues (n=18) were removed
from drug-resistant patients with TLE. Control tissues from healthy
temporal neocortical tissues (n=18) were obtained during the
autopsy of patients who had no history of seizures or other
neurological diseases in The Second Hospital of Hebei Medical
University (Shijiazhuang, China) between March 2016 and August
2018. The age of the patients ranged from 25.7±12.4 years,
including ten females and 26 males. The inclusion criteria were as
follows: i) Patients who were diagnosed with TLE via pathological
examination; ii) patients who were diagnosed and treated for the
first time; and iii) patients who willing to join the study. The
exclusion criteria were: i) Patients with multiple diseases; and
ii) patients who received treatment within 90 days before
admission. All samples were stored at −80°C. The study was approved
by the Ethics Committee of The Second Hospital of Hebei Medical
University and all patients provided written, informed consent.
Construction of the epilepsy animal
model
To simulate the seizure process, the present study
constructed a pilocarpine-induced animal model with similar seizure
characteristics to human TLE to study the mechanisms of TLE
(33). All animal experiments were
approved by the Ethics Committee of The Second Hospital of Hebei
Medical University (Shijiazhuang, China). In total, 32 BALB/c
female Wistar rats (age, 8–10 weeks; weight, 200 g; Experimental
Animal Centre of the Academy of Military Medical Sciences, Beijing,
China) were randomly divided into four groups (8 rats in each
group): Normal group, epilepsy group, epilepsy + LV-miR-negative
control (NC; 5′-UUCUCCGAACGUGUCACGUUU-3′) group (hippocampi
transfected with miR-NC; final concentration 50 µM) and
epilepsy+LV-miR-15a group [hippocampi transfected with miR-15a
mimics (miR-15a: 5′-UAGCAGCACAUAAUGGUUU-3′), final concentration 50
µM]. Transfected LV-miR-NC or LV-miR-15a were subcutaneously
injected into the hippocampi of anesthetized rats. LV-miR-NC and
LV-miR-15a were obtained from Shanghai GenePharma Co., Ltd. Rats
were housed at room temperature of 22–25°C, with a relative
humidity of 50–60%, with a 12-h light/dark cycle. The food intake
of the rats was ~10 g per 100 g body weight, and the water intake
was 10–15 ml per 100 g body weight. Rats in the epilepsy, epilepsy
+ LV-miR-NC and epilepsy + LV-miR-15a groups were intraperitoneally
injected with lithium chloride (127 mg/kg; Sigma-Aldrich; Merck
KGaA). Then, 18 h later, pilocarpine (127 mg/kg; Sigma-Aldrich;
Merck KGaA) was repeatedly injected intraperitoneally every 30 min
until the rats had seizures with tonic-clonic (head and face
clonic, limb clonic) using an electroencephalogram and observation.
Rats in the normal group were intraperitoneally injected with an
equivalent volume of physiological saline. The epileptic seizure
was terminated after an epileptic state that lasted for 1 h. After
24 h, 10% chloral hydrate (300 mg/kg) was used to anesthetize the
rats at 5 ml/kg intraperitoneally and then the ratswere
euthanisedby cervial disclocation. The brain was removed and the
tissue was isolated from the rat hippocampus on iced saline and
stored at −80°C.
Cell culture and transfection
Rat hippocampus neurons were obtained from rat
hippocampus tissue. Briefly, Rat hippocampus tissue from the
removed brain was dissected and placed on a cell plate
(1×105 /ml) with neurobasal medium (neurobasal/B27;
Thermo Fisher Scientific, Inc.), containing 2% BC7 (Gibco; Thermo
Fisher Scientific, Inc.), and 10% FBS (Gibco; Thermo Fisher
Scientific, Inc.) cultured with 5% dihydrazide, at 37°C in a humid
environment. After plating, 5 µM cytosine arabinoside
(Sigma-Aldrich; Merck KGaA) was used to inhibit astrocyte
proliferation in hippocampal neurons at 37°C for 48 h. The medium
was replaced every 2 days. After a 2-week in vitro culture,
epilepsy induction was performed as followed. Briefly, the original
medium was replaced with a Mg2+-free medium
(Sigma-Aldrich; Merck KGaA) containing 2.5 mM KCl, 145 mM NaCl, 2
mM CaCl2, 10 mM HEPES, 10 mM glucose and 0.002 mM
glycine and the epileptic neuron cells were cultured for 6 days at
37°C. Subsequently, the neurons were cultured at 37°C in liquid
Mg2+-free medium for 3 h. Then, neuronal cells were
transferred to the conventional neurobasal/B27 medium for culture
at 37°C. Control (con) cells were cultured in neurobasal/B27 medium
under the same incubation conditions.
miR-15a and miR-NC, miR-15a inhibitors (anti-miR-15,
5′-AAACCAUUAUGUGCUGCUA-3′) and anti-miR-NC
(5′-CAGUACUUUUGUGUAGUACAA-3′), as well as pcDNA3.1 and pcDNA-GFAP
(GFAP; cat. no. KR712259.1) were obtained from Shanghai GenePharma
Co., Ltd. All 0.2 µg fragments and 0.5 µl oligos were transfected
into Mg2+-induced epilepsy cells (2×105
cells/well) using Lipofectamine® 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.), followed by incubation for 48
h.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from the hippocampal neural
tissue or cells using TRIzol® reagent (Invitrogen;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol. Total RNA [for GFAP, interleukin (IL)-1β, IL-6 and tumor
necrosis factor α (TNF-α)] was reverse transcribed into cDNA using
the High Capacity cDNA RT kit (Applied Biosystems; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol.
TaqMan®miR RT PCR assay reagents (Applied Biosystems;
Thermo Fisher Scientific, Inc.) was applied to synthesize cDNA
first strands, and SYBR Green PCR kit (Takara Bio, Inc.) was used
to determine the expression levels of miR-15a. The amplification
parameters were: Initial denaturation at 95°C for 10 min, followed
by 40 cycles of denaturation at 95°C for 30 sec, annealing at 60°C
for 30 sec and extension at 72°C for 1 min. GAPDH and U6 were used
as the internal reference genes for mRNA and miRNA, respectively.
Expression levels of all mRNAs and miRNA were calculated using the
2−ΔΔCq method (34).
The primer sequences used are as follows: miR-15a forward,
5′-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACCACAAAC-3′ and
reverse, 5′-GCGGCTAGCAGCACATAATGG-3′; U6 forward,
5′-GCTTCGGCAGCACATATACTAAAAT-3′ and reverse,
5′-CGCTTCACGAATTTGCGTGTCAT-3′; GFAP forward,
5′-TTGCACTGTGCACGTTC-3′ and reverse, 5′-TGGGGAAATGTGCCAG-3′; GAPDH
forward, 5′-GCACCGTCAAGGCTGAGAAC-3′ and reverse,
5′-TGGTGAAGACGCCAGTGGA-3′; TNF-α forward,
5′-TCAGCCGATTTGCCATTTCAT-3′ and reverse,
5′-ACACGCCAGTCGCTTCACAGA-3′; IL-1β forward,
5′-GTCCTTTCACTTGCCCTCAT-3′ and reverse,
5′-CAAACTGGTCACAGCTTTCGA-3′; and IL-6 forward,
5′-AAATGCCTCGTGCTGTCTGACC-3′ and reverse,
5′-GGTGGGTGTGCCGTCTTTCATC-3′.
TUNEL assay
Cell apoptosis was detected using TUNEL assay with a
Fluorescein FragELTMDNA fragmentation detection kit (Abcam)
according to the manufacturer's instructions. Briefly, cells fixed
in 4% paraformaldehyde (Beyotime Institute of Biotechnology) for 25
min at 4°C on the slides. After the sections (thickness, 5 µm) from
rat tissues were washed with H2O2 and PBS, 20
mg/l protease K solution was added at room temperature for 15 min.
Then, 40 µl stop buffer containing 20% FBS and 2 µl nucleoside was
added and the sections were incubated at 37°C for 1 h. At 10 min
post-addition of the stop buffer, peroxidase-conjugated
anti-digoxin antibody (1:1,000; cat. no. ab53510; Abcam) was added
and incubated at 37°C for 30 min. After washing with PBS, the
sections were re-stained with 0.1% hematoxylin for 3 min at room
temperature. In total, six non-overlapping fields of view were
selected randomly from each section, and apoptotic cells were
observed and counted using a fluorescent microscope (magnification,
×200).
Flow cytometric analysis of
apoptosis
An Annexin (An)-VFITC apoptosis detection kit (BD
Biosciences) was used to detect the levels of cell apoptosis. Cells
(1×105) were collected and digested with trypsin, washed
twice in PBS and centrifuged at 1,610 × g for 8 min at 37°C to
remove the supernatant. Subsequently, cells were resuspended in 100
µl binding buffer from the detection kit, and stained with 5 µl
Annexin V/FITC and 5 µl propidium iodide at room temperature for 15
min in the dark. Apoptotic cells were subsequently analyzed using a
FACSCalibur flow cytometer (BD Biosciences) and CELL Quest 3.0
software (BD Biosciences). In the scatter plot of cell apoptosis:
Lower left quadrant represented normal cells (An−
PI−); the lower right quadrant represented apoptotic
cells in early stage (An+ PI−); the upper
right quadrant represented apoptotic cells in advanced stage and
necrotic cells (An+ PI+); and the upper left
quadrant represented damaged cells in the process of collection
(An− PI+). The rate of apoptosis was
expressed as the percentage of the early apoptotic cells in the
total number of cells.
Dual-luciferase reporter assay
The underlying binding relationship between miR-15a
and GFAP was predicted using bioinformatics software microT-CDS
v5.0 (http://diana.imis.athena-innovation.
gr/Diana Tools/index.php?r=microT CDS/).
Then, a dual-luciferase reporter assay was used to
determine the relationship between miR-15a and GFAP. The GFAP 3′
untranslated region (UTR)-wild-type (WT) or GFAP 3′UTR-mutant (MUT)
were amplified and inserted into the pRL-TK plasmid (Promega
Corporation). Then, the vectors (0.1 µg) and miR-15a (40 nM) or
miR-NC (40 nM) were infected into hippocampal neurons using
Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's instructions. The relative
luciferase activity was measured at 48 h post-transfection using
the Dual-Luciferase Reporter assay system (Promega Corporation).
Renilla luciferase activities were used as the internal
control for the normalization of firefly luciferase activity.
RIP assay
The Magna RNA-binding protein immunoprecipitation
kit (EMD Millipore) was used for the RIP experiment according to
the manufacturer's protocol. Briefly, cells transfected with
miR-15a were collected by centrifugation (100 × g) at room
temperature for 2 min and resuspended in NP-40 lysis buffer
(Sigma-Aldrich; Merck KGaA; reagent to separate the nuclei)
containing 1 mM PMSF, 1 mM DTT, 1% protease inhibitor cocktail and
200 U/ml RNase inhibitor (Invitrogen; Thermo Fisher Scientific,
Inc.). Then, the supernatant was incubated 4°C with magnetic beads
labelled with human anti-Argonaute2 (Ago2, 1:1,000; cat. no.
ab32381; Abcam) antibody and IgG antibody (1:5,000; cat. no. PP64B,
EMD Millipore) as a positive control overnight, sonicated for 10
cycles in a Bioruptor Sonicator [Diagenode; High, 10 × (30
sec-ON/30 sec-OFF)] at 4°C and centrifuged (6,000 × g) at 4°C for
40 min. The beads were washed three times with buffer containing 20
mM HEPES (pH 7.9), 120 mM NaCl, 1 mM EDTA, 1 mM PMSF and 1 mM DTT
followed by centrifugation (10,000 × g; 40 min; 4°C). RNAs were
digested with proteinase K (0.5 mg/ml; Sigma-Aldrich; Merck KGaA)
for 15 min at 55°C, and then treated with TRIzol®
reagent (Invitrogen; Thermo Fisher Scientific, Inc.). The
expression levels of miR-15a and GFAP were analyzed using
RT-qPCR.
Western blot assay
Western blotting was used to detect the protein
expression levels of GFAP in hippocampal neurons. Cells were lysed
in RIPA lysis buffer (Beyotime Institute of Biotechnology). Protein
concentration and quality were detected with a bicinchoninic acid
protein assay kit (Sigma-Aldrich; Merck KGaA). Protein (50 µg)
samples were separated by 10% SDS-PAGE gels and then transferred
onto PVDF membranes (Bio-Rad Laboratories, Inc.). Subsequently, 5%
skim milk for 2 h at 37°C was used to block the membranes. Then,
the membranes were incubated with primary antibodies against GFAP
(1:1,000; cat. no. ab7260; Abcam) or β-actin (1:2,500; cat. no.
ab52614; Abcam) at 4°C overnight. After washing with TBS, the
membranes were incubated at 37°C with horseradish
peroxidase-conjugated goat anti-rabbit secondary antibody (1:5,000;
cat. no. SC-2301, Santa Cruz Biotechnology, Inc.) for 1 h. The band
of target protein was visualized using an ECL Plus western blotting
substrate (Thermo Fisher Scientific, Inc.) and analyzed with
Quantity One v4.6.2 software (Bio-Rad Laboratories, Inc.).
Statistical analysis
All statistical analyses and mapping were performed
using GraphPad Prism 7.0 (GraphPad Software, Inc.). Statistical
differences between two groups were determined using a paired and
unpaired Student's t-test, whereas an one-way ANOVA with Tukey's
test was used for ≥3 groups. Data are presented as the mean ± SD
from ≥3 independent experiments. P<0.05 was considered to
indicate a statistically significant difference.
Results
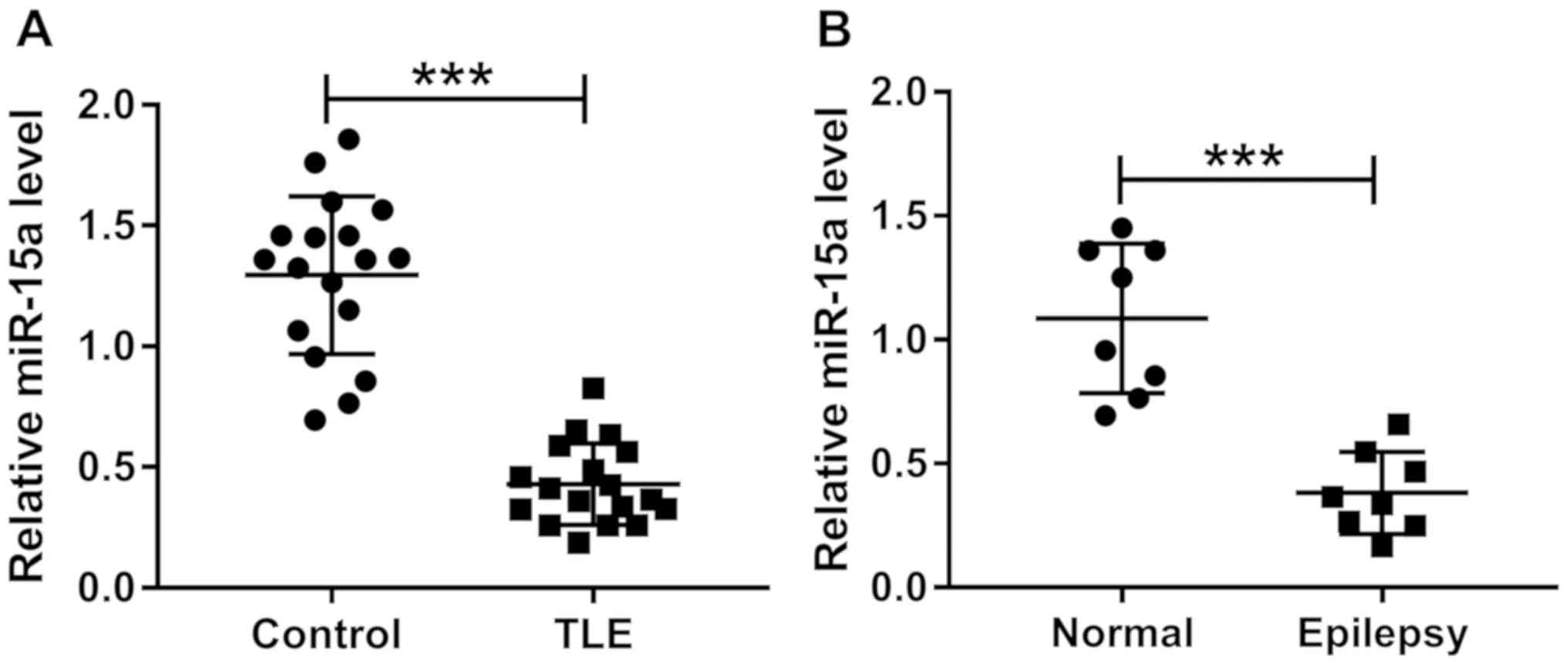
miR-15a expression levels are
downregulated in TLE and epilepsy tissues
In the present study, healthy tissues and TLE
tissues were obtained from 18 patients. In addition, healthy
tissues and epileptic model rat tissues were collected from eight
rats. RT-qPCR was performed to detect the expression levels of
miR-15a and it was revealed that compared with the control tissues,
the expression levels of miR-15a were significantly downregulated
in the TLE tissues (Fig. 1A).
Furthermore, miR-15a expression levels were significantly decreased
in the epilepsy group compared with the normal group (Fig. 1B).
Overexpression of miR-15a inhibits
cell apoptosis and inflammation in an in vivo epilepsy model
The transfection efficiency of LV-miR-15a in the
hippocampal neurons was determined (Fig. 2A). Moreover, to determine the
function of miR-15a, the present study transfected LV-miR-NC or
LV-miR-15a into the hippocampi to construct the epilepsy +
LV-miR-NC or epilepsy + LV-miR-15a groups, respectively. It was
demonstrated that miR-15a expression levels were downregulated in
the epilepsy group compared with the normal group, and miR-15a
expression was enhanced in epilepsy + LV-miR-15a group compared
with the epilepsy group (Fig. 2B).
In addition, miR-15a expression levels were significantly increased
in the epilepsy + LV-miR-15a group compared with the epilepsy +
LV-miR-NC group. The levels of cell apoptosis and expression levels
of inflammatory factors were subsequently analyzed in each group
using a TUNEL assay and RT-qPCR, respectively. The results
demonstrated that the expression levels of IL-1β, IL-6 and TNF-α,
which are important pro-inflammatory factors (35), were upregulated in the epilepsy
group vs. normal group, suggesting that there may be a strong
inflammatory response in the epilepsy model. Moreover, the
inflammatory factors were decreased in epilepsy + LV-miR-15a group
compared with epilepsy group, suggesting that LV-miR-15a could
inhibited inflammatory response in epilepsy model (Fig. 2D-F). Compared with the normal
group, the levels of cell apoptosis were also significantly
increased in the epilepsy group, and compared with the epilepsy
group, cell apoptosis index was reduced in epilepsy + LV-miR-15a
group (Fig. 2C). However, in the
epilepsy + LV-miR-15a group, the levels of cell apoptosis were
decreased, and the mRNA expression levels of IL-1β, IL-6 and TNF-α
were downregulated compared with the epilepsy + LV-miR-NC group
(Fig. 2C-F). Therefore, these
findings indicated that there were significant differences between
the epilepsy and epilepsy + LV-miR-15a groups, implying that the
upregulated expression levels of miR-15a may decrease the levels of
cell apoptosis and inflammation in epilepsy tissues.
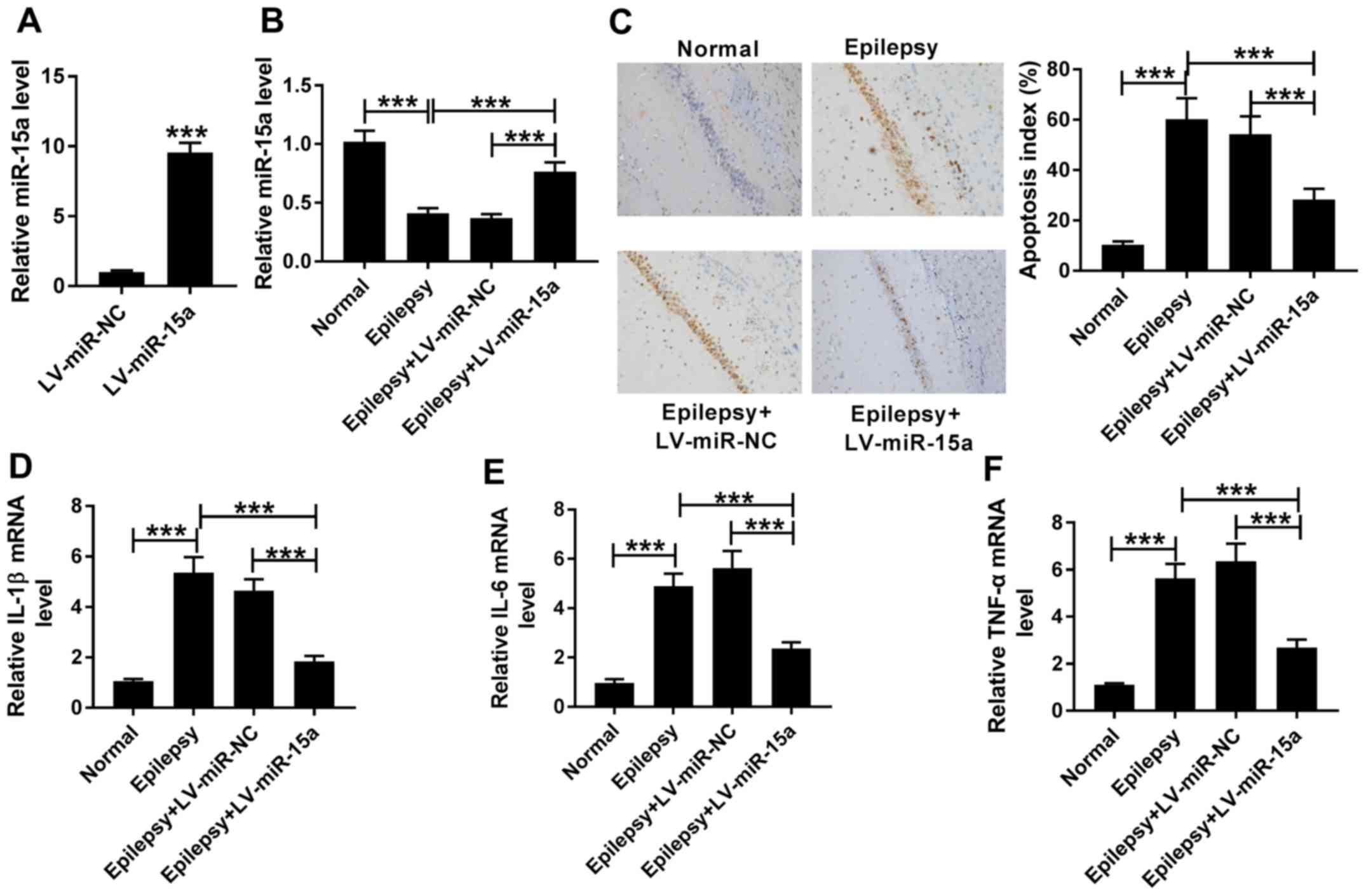 | Figure 2.Overexpression of miR-15a inhibits
cell apoptosis and inflammation in an in vivo epilepsy
model. (A) miR-15a expression levels were detected in the
hippocampal neurons transfected with the LV-miR-NC or LV-miR-15a
using RT-qPCR. (B) Expression levels of miR-15a were analyzed in
the normal, epilepsy, epilepsy + LV-miR-NC group and epilepsy +
LV-miR-15a groups using RT-qPCR. (C) Levels of cell apoptosis were
determined in the normal, epilepsy, epilepsy + LV-miR-NC and
epilepsy + LV-miR-15a groups using a TUNEL assay (magnification,
×200). Expression levels of (D) IL-1β, (E) IL-6 and (F) TNF-α were
analyzed in the normal, epilepsy, epilepsy + LV-miR-NC and epilepsy
+ LV-miR-15a groups using RT-qPCR. ***P<0.001. RT-qPCR, reverse
transcription-quantitative PCR; miR, microRNA; NC, negative
control; IL, interleukin; TNF-α, tumor necrosis factor α. |
Overexpression of miR-15a inhibits
cell apoptosis and inflammation in an in vitro epilepsy model
The overexpression transfection efficiency of
miR-15a in hippocampal neurons was determined (Fig. 3A). Subsequently, the epileptic
activity was induced in rat hippocampal neurons in vitro,
which were divided into four groups: Con, Mg2+-free,
Mg2+-free + miR-NC and Mg2+-free + miR-15a
groups. The apoptotic rate and mRNA expression levels of
inflammatory factors were analyzed for each group. Consistent with
the in vivo experiments, it was identified that the
expression levels of miR-15a were significantly downregulated in
the Mg2+-free-treated cells compared with the control
cells, while the expression levels of miR-15a were significantly
increased in Mg2+-free + miR-15a group compared with the
Mg2+-free + miR-NC group (Fig. 3B). Moreover, in the epileptic
cells, the apoptotic rate was significantly increased, and the
expression levels of IL-1β, IL-6 and TNF-α were significantly
upregulated in Mg2+-free group compared with the con
group (Fig. 3C-F). However,
increasing the expression levels of miR-15a significantly reduced
the high apoptotic rate and strong inflammatory response observed
in the epileptic cells (Fig.
3C-F). Collectively, these results suggested that miR-15a may
serve an important regulatory role in relieving the epileptic
symptoms.
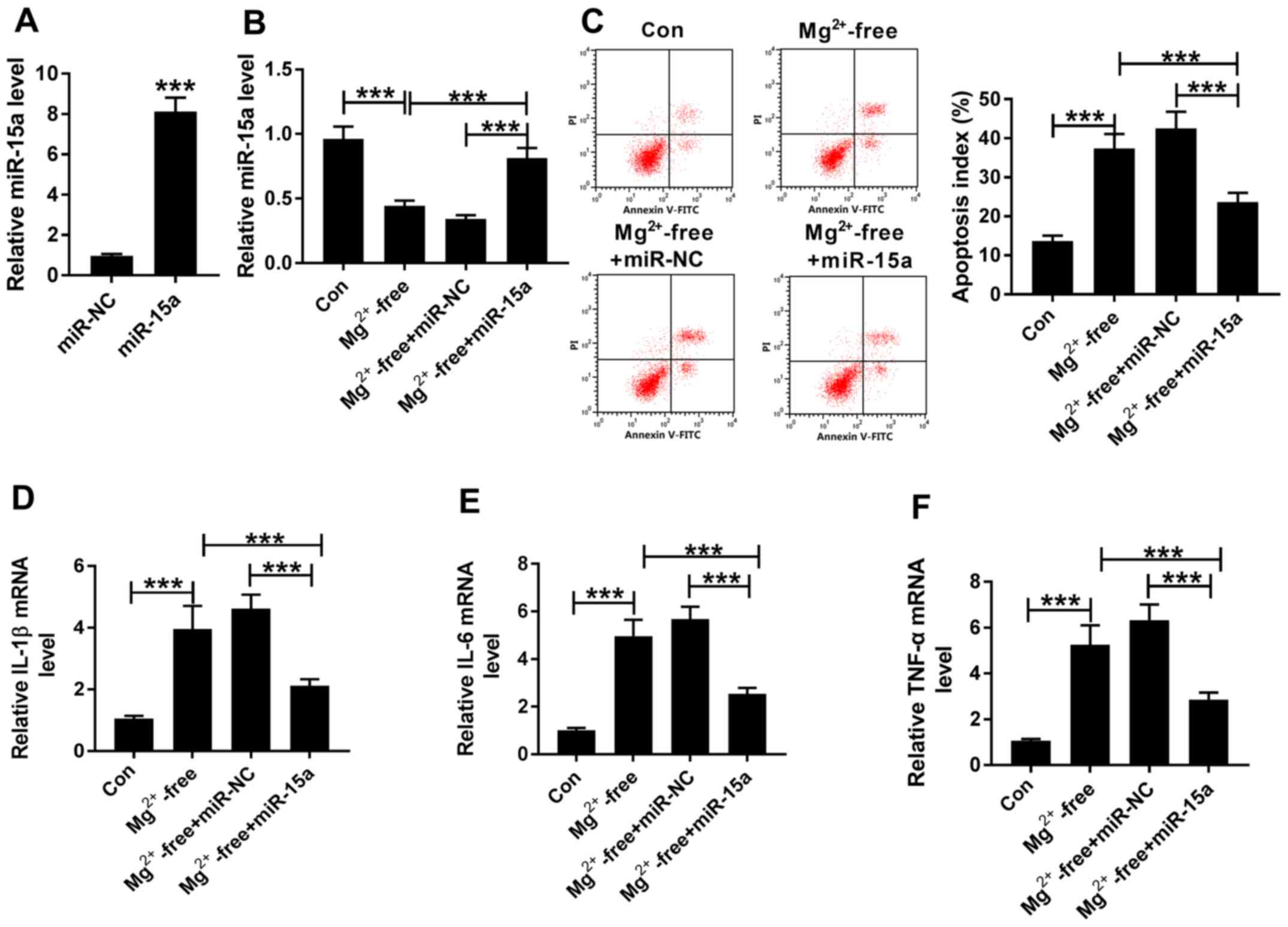 | Figure 3.Overexpression of miR-15a inhibits
cell apoptosis and inflammation in an in vitro epilepsy
model. (A) RT-qPCR was used to analyze the expression levels of
miR-15a in hippocampal neurons transfected with miR-NC or miR-15a.
(B) Expression levels of miR-15a wereanalyzed in the con,
Mg2+-free, Mg2+-free + miR-NC and
Mg2+-free+miR-15a groups via RT-qPCR. (C) Levels of cell
apoptosis were analyzed in the con, Mg2+-free,
Mg2+-free + miR-NC and Mg2+-free + miR-15a
groups using flow cytometry. Expression levels of (D) IL-1β, (E)
IL-6 and (F) TNF-α were determined in the con,
Mg2+-free, Mg2+-free + miR-NC and
Mg2+-free + miR-15a groups using RT-qPCR. ***P<0.001.
RT-qPCR, reverse transcription-quantitative PCR; miR, microRNA; NC,
negative control; IL, interleukin; TNF-α, tumor necrosis factor α;
con, control; PI, propidium iodide. |
miR-15a directly targets GFAP in
hippocampal neurons
Based on the prediction of the bioinformatics tool
DIANA TOOLS, it was discovered that the 3′untranslated region of
GFAP had a complementary binding site to miR-15a (Fig. 4A). Therefore, it was hypothesized
that GFAP may be a candidate target gene for miR-15a. The present
study constructed vectors for GFAP-WT and GFAP-MUT, which were
co-transfected alongside miR-NC and miR-15a into the hippocampal
neurons. The dual-luciferase reporter assay results identified that
miR-15a reduced the luciferase activity of GFAP-WT reporter, while
it had no notable effect on luciferase activity of GFAP-MUT
reporter (Fig. 4B). To further
validate these results, a RIP assay was performed to detect the
enrichment of GFAP in the cells. It was demonstrated that miR-15a
and GFAP were co-immunoprecipitated using the Ago2 group antibody
but not the IgG antibody (Fig.
4C). Subsequently, the transfection efficiency of miR-15a and
anti-miR-15a was determined (Fig.
4D). The western blotting analysis revealed that the increased
expression levels of miR-15a significantly inhibited the expression
levels of GFAP compared with the miR-NC group; however,
downregulating the expression levels of miR-15a significantly
induced the expression of GFAP compared with anti-miR-NC group
(Fig. 4E). Therefore, these
findings indicated that GFAP may be a target gene of miR-15a in
hippocampal neurons.
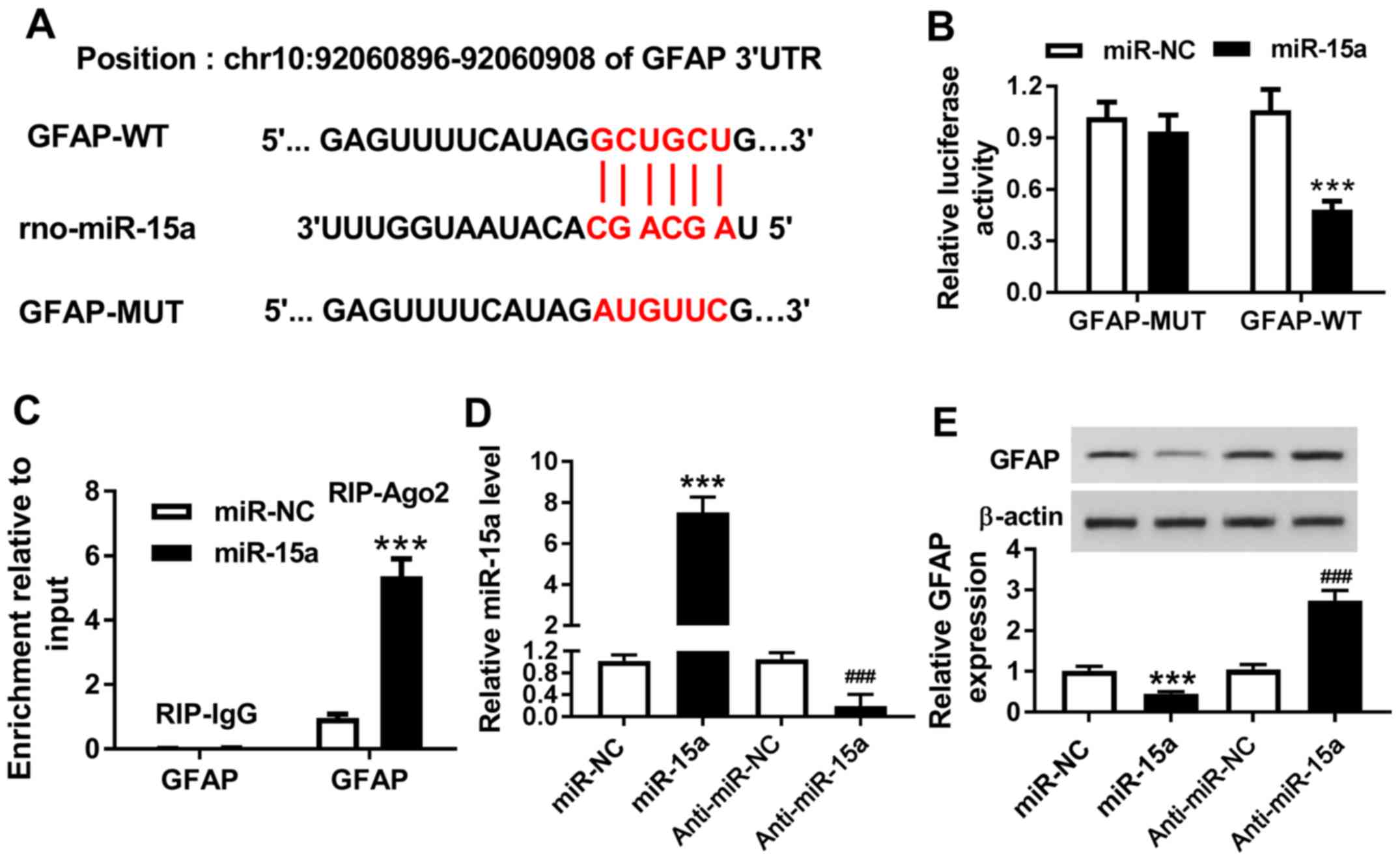 | Figure 4.miR-15a directly targets GFAP in
hippocampal neurons. (A) Schematic representation of the predicted
target site for miR-15a in the GFAP 3′UTR. (B) Dual-luciferase
reporter assay was performed to determine the target relationship
between miR-15a and GFAP. (C) RIP assay was performed to further
identify the relationship between miR-15a and GFAP in hippocampal
neurons. (D) Expression levels of miR-15a were analyzed in the
hippocampal neurons transfected with miR-NC, miR-15a, anti-miR-NC
and anti-miR-15a. (E) Protein expression levels of GFAP were
analyzed in miR-NC, miR-15a, anti-miR-NC and anti-miR-15a groups
using western blotting. ***P<0.001 vs. miR-NC;
###P<0.001 vs. anti-miR-NC. 3′UTR, 3′untranslated
region; miR, microRNA; NC, negative control; GFAP, glial fibrillary
acidic protein; WT, wild-type; MUT, mutant; Ago2, Argonaute2; RIP,
RNA immunoprecipitation. |
Upregulation of GFAP reverses the
effects of upregulated miR-15a expression-levels in an in vitro
epilepsy model
In order to determine whether miR-15a regulated
epilepsy by targeting GFAP, rescue experiments were performed. The
western blotting results demonstrated that the expression levels of
GFAP were significantly upregulated in the hippocampal neurons
transfected with GFAP compared with cells transfected with pcDNA3.1
(Fig. 5A). miR-NC or miR-15a were
transfected into Mg2+-free-induced hippocampal neurons,
with or without the co-transfection with pcDNA3.1 and GFAP. It was
subsequently demonstrated that the protein expression levels of
GFAP were significantly upregulated in the Mg2+-free
group compared with the con group (Fig. 5B). Moreover, the overexpression of
miR-15a significantly inhibited the expression levels of GFAP in
Mg2+-free + miR-15a group compared with the
Mg2+-free + miR-NC group, while upregulating the
expression levels of GFAP reversed this inhibitory effect. In
addition, the results identified that the apoptotic rate in the
Mg2+-free group was increased compared with the con
group (Fig. 5C). Furthermore, the
upregulation of GFAP reversed the miR-15a overexpression-mediated
decrease in the levels of cell apoptosis (Fig. 5C). It was also discovered that the
expression levels of IL-1β, IL-6 and TNF-α were upregulated in the
Mg2+-free group compared with the con group (Fig. 5D-F). Moreover, the overexpression
of GFAP also partially reversed the suppressive effects of miR-15a
on the expression levels of IL-1β, IL-6 and TNF-α in
Mg2+-free-induced hippocampal neurons (Fig. 5D-F). Thus, the present results
suggested that cell apoptosis and inflammation may be inhibited by
the upregulation of miR-15a, which may be impaired by the
overexpression of GFAP.
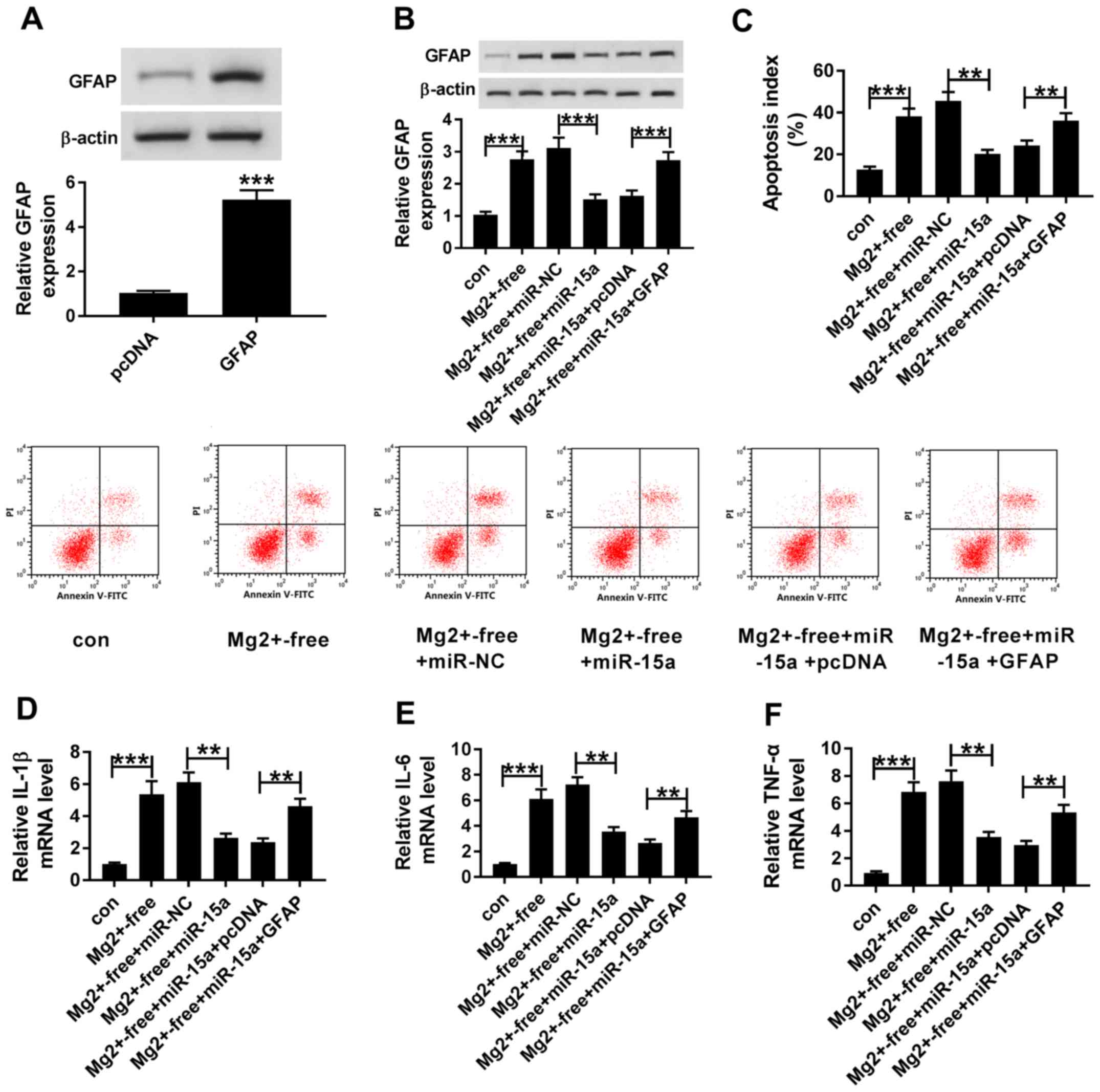 | Figure 5.Upregulation of GFAP reverses the
effects of upregulated miR-15a expression levels on cell apoptosis
and inflammation in an in vitro epilepsy model. (A) GFAP
protein expression levels were determined in hippocampal neurons
transfected with pcDNA and GFAP. (B) Protein expression levels of
GFAP were analyzed in the con, Mg2+-free,
Mg2+-free + miR-NC, Mg2+-free + miR-15a,
Mg2+-free + miR-15a + pcDNA and Mg2+-free +
miR-15a + GFAP groups using western blotting. (C) Levels of cell
apoptosis were examined in the con, Mg2+-free,
Mg2+-free + miR-NC, Mg2+-free+miR-15a,
Mg2+-free + miR-15a + pcDNA and Mg2+-free +
miR-15a + GFAP groups using flow cytometry. Expression levels of
(D) IL-1β, (E) IL-6 and (F) TNF-α were detected in the con,
Mg2+-free, Mg2+-free + miR-NC,
Mg2+-free + miR-15a, Mg2+-free + miR-15a +
pcDNA and Mg2+-free + miR-15a + GFAP groups using
reverse transcription-quantitative PCR. **P<0.01, ***P<0.001.
miR, microRNA; NC, negative control; IL, interleukin; TNF-α, tumor
necrosis factor α; GFAP, glial fibrillary acidic protein; con,
control; PI, propidium iodide. |
Discussion
The present study used a rat model of epilepsy and
epilepsy-induced hippocampal neurons to study the pathogenesis of
TLE. It was discovered that the expression levels of miR-15a were
downregulated in the epileptic tissue and TLE tissues. Furthermore,
increasing the expression levels of miR-15a effectively inhibited
GFAP expression, which in turn affected the levels of neuronal
apoptosis and inflammation. Therefore, the present results
indicated that the miR-15a/GFAP axis may be an important regulatory
mechanism and network in epilepsy, thus providing a novel target
site for the treatment of epilepsy.
miRNAs serve important regulatory roles in numerous
types of disease and they are indispensable regulators of cell
development and inflammation (36–39).
In cancer, miRNAs have been discovered to serve as either a tumor
suppressor or an oncogenic factor, where they were observed to have
roles in cell development, such as tumorigenesis and metastasis
(40–43). Moreover, numerous differentially
expressed miRNAs have been found in epileptic sequencing, including
miR-184, miR-124, miR-134, miR-132, miR-21 and miR-23a/b, where
they were identified to be involved in the occurrence and
development of epilepsy (20,25,44,45).
Previous studies have reported that miR-15a was involved in the
development of several types of human disease, cancer, the immune
response and angiogenesis (23,46–49).
Furthermore, Cai et al (24) revealed that the overexpression of
miR-15a induced cell apoptosis and the cell cycle in osteosarcoma.
However, research on the role of miR-15a in epilepsy is limited.
Thus, the present study investigated the function of miR-15a in
vitro and in vivo, and it was revealed that the
overexpression of miR-15a significantly inhibited the rate of
apoptosis and inflammation in hippocampal neurons.
miRNAs typically target multiple mRNAs, including
miR-15a (24,50). Previous studies have revealed that
cyclin D1, vascular endothelial growth factor A (VEGFA), forkhead
box protein O1 (FOXO1), brain-derived neurotrophic factor (BDNF)
and C-X-C motif chemokine 10 (CXCL10) were targets of miR-15a in
osteosarcoma, porcine pre-adipocytes, methyl CpG binding protein
2-deficient neurons, myasthenia gravis and multiple myeloma
(49,51–54).
Notably, these regulatory networks were discovered to regulate cell
progression and angiogenesis (24,47,49,51,53,54).
The results of the present study suggested that GFAP may be a
target gene for miR-15a. Previous studies have revealed that GFAP
encodes the GFAP protein, which was reported to be an important
regulator in the formation and development of astrocytes (55,56).
Furthermore, GFAP has been observed to affect the inflammatory
response in numerous types of disease. For example, Sun et
al (57) reported that GFAP
was related to the inflammatory response in the lumbo-sacral spinal
cord and medulla oblongata after chronic colonic inflammation in
rats. It has also been reported that GFAP was highly expressed in
epilepsy, and elevated GFAP expression levels subsequently
aggravated the neuroinflammatory response (27,55).
Consistent with these previous studies, the findings of the present
study indicated that the increased expression levels of GFAP may
inhibit the suppression of apoptosis and inflammation in
hippocampal neurons induced by increased expression levels of
miR-15a, thus suggesting the important role of the miR-15a/GFAP
axis in TLE.
In conclusion, the present study identified the
function of miR-15a in TLE and assessed the regulatory mechanism of
miR-15a. It was discovered that the upregulation of miR-15a
inhibited cell apoptosis and inflammation in TLE, which was
impaired by the overexpression of GFAP. Therefore, the results of
the present study may provide a novel therapeutic target for the
treatment of TLE.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WW and WL designed and conceptualized the study; WL
and XL analyzed and curated the data; YF and WL validated the data
and performed the experiments; and YF, WW and WL wrote the original
draft of the manuscript, and reviewed and analyzed the manuscript.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethical Review
Committee of The Second Hospital of Hebei Medical University
(Shijiazhuang, China). Written informed consent was obtained from
all enrolled patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kwan P and Brodie MJ: Early identification
of refractory epilepsy. N Engl J Med. 342:3504–319. 2000.
View Article : Google Scholar
|
|
2
|
Roggenhofer E, Santarnecchi E, Muller S,
Kherif F, Wiest R, Seeck M and Draganski B: Trajectories of brain
remodeling in temporal lobe epilepsy. J Neurol. 266:3150–3159.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fiest KM, Sauro KM, Wiebe S, Patten SB,
Kwon CS, Dykeman J, Pringsheim T, Lorenzetti DL and Jetté N:
Prevalence and incidence of epilepsy: A systematic review and
meta-analysis of international studies. Neurology. 88:296–303.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Engel J Jr; International League Against
Epilepsy (ILAE), : A proposed diagnostic scheme for people with
epileptic seizures and with epilepsy: Report of the ILAE task force
on classification and terminology. Epilepsia. 42:796–803. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lowenstein DH: Interview: The national
institute of neurological diseases and stroke/American epilepsy
society benchmarks and research priorities for epilepsy research.
Biomark Med. 5:531–535. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Beghi E: The epidemiology of epilepsy.
Neuroepidemiology. 54:185–191. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wahid F, Shehzad A, Khan T and Kim YY:
MicroRNAs: Synthesis, mechanism, function, and recent clinical
trials. Biochim Biophys Acta. 1803:1231–1243. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Witkos TM, Koscianska E and Krzyzosiak WJ:
Practical aspects of microRNA target prediction. Curr Mol Med.
11:93–109. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Saito T and Saetrom P: MicroRNAs-targeting
and target prediction. N Biotechnol. 27:243–249. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cheng AM, Byrom M, Shelton J and Ford LP:
Antisense inhibition of human miRNAs and indications for an
involvement of miRNA in cell growth and apoptosis. Nucleic Acids
Res. 33:1290–1297. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cloonan N, Wani S, Xu Q, Gu J, Lea K,
Heater S, Barbacioru C, Steptoe AL, Martin HC, Nourbakhsh E, et al:
MicroRNAs and their isomiRs function cooperatively to target common
biological pathways. Genome Biol. 12:R1262011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dar AA, Majid S, de Semir D, Nosrati M,
Bezrookove V and Kashani-Sabet M: miRNA-205 suppresses melanoma
cell proliferation and induces senescence via regulation of E2F1
protein. J Biol Chem. 286:16606–16614. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kovaleva V, Mora R, Park YJ, Plass C,
Chiramel AI, Bartenschlager R, Döhner H, Stilgenbauer S, Pscherer
A, Lichter P and Seiffert M: miRNA-130a targets ATG2B and DICER1 to
inhibit autophagy and trigger killing of chronic lymphocytic
leukemia cells. Cancer Res. 72:1763–1772. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mehta N and Cheng HY: Micro-managing the
circadian clock: The role of microRNAs in biological timekeeping. J
Mol Biol. 425:3609–3624. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bencurova P, Baloun J, Musilova K, Radova
L, Tichy B, Pail M, Zeman M, Brichtova E, Hermanova M, Pospisilova
S, et al: MicroRNA and mesial temporal lobe epilepsy with
hippocampal sclerosis: Whole miRNome profiling of human
hippocampus. Epilepsia. 58:1782–1793. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dogini DB, Avansini SH, Vieira AS and
Lopes-Cendes I: MicroRNA regulation and dysregulation in epilepsy.
Front Cell Neurosci. 7:1722013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu DZ, Tian Y, Ander BP, Xu H, Stamova
BS, Zhan X, Turner RJ, Jickling G and Sharp FR: Brain and blood
microRNA expression profiling of ischemic stroke, intracerebral
hemorrhage, and kainate seizures. J Cereb Blood Flow Metab.
30:92–101. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mckiernan RC, Jimenez-Mateos EM, Bray I,
Engel T, Brennan GP, Sano T, Michalak Z, Moran C, Delanty N,
Farrell M, et al: Reduced mature microRNA levels in association
with dicer loss in human temporal lobe epilepsy with hippocampal
sclerosis. PLoS One. 7:359212012. View Article : Google Scholar
|
|
19
|
Pitkanen A, Loscher W, Vezzani A, Becker
AJ, Simonato M, Lukasiuk K, Gröhn O, Bankstahl JP, Friedman A,
Aronica E, et al: Advances in the development of biomarkers for
epilepsy. Lancet Neurol. 15:843–856. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Song YJ, Tian XB, Zhang S, Zhang YX, Li X,
Li D, Cheng Y, Zhang JN, Kang CS and Zhao W: Temporal lobe epilepsy
induces differential expression of hippocampal miRNAs including
let-7e and miR-23a/b. Brain Res. 1387:134–140. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li D, Hao S and Zhang J: Long non-coding
RNA UCA1 exerts growth modulation by miR-15a in human thyroid
cancer TPC-1 cells. Artif Cells Nanomed Biotechnol. 47:1815–1822.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cui Y, Yang Y, Ren L, Yang J, Wang B, Xing
T, Chen H and Chen M: miR-15a-3p suppresses prostate cancer cell
proliferation and invasion by targeting SLC39A7 via downregulating
Wnt/β-catenin signaling pathway. Cancer Biother Radiopharm.
34:472–479. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Aqeilan RI, Calin GA and Croce CM: miR-15a
and miR-16-1 in cancer: Discovery, function and future
perspectives. Cell Death Differ. 17:215–220. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cai CK, Zhao GY, Tian LY, Liu L, Yan K, Ma
YL, Ji ZW, Li XX, Han K, Gao J, et al: miR-15a and miR-16-1
downregulate CCND1 and induce apoptosis and cell cycle arrest in
osteosarcoma. Oncol Rep. 28:1764–1770. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang J, Yu JT and Tan L, Tian Y, Ma J, Tan
CC, Wang HF, Liu Y, Tan MS, Jiang T and Tan L: Genome-wide
circulating microRNA expression profiling indicates biomarkers for
epilepsy. Sci Rep. 5:95222015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ma Y: The Challenge of microRNA as a
biomarker of epilepsy. Curr Neuropharmacol. 16:37–42.
2018.PubMed/NCBI
|
|
27
|
Jacque CM, Vinner C, Kujas M, Raoul M,
Racadot J and Baumann NA: Determination of glial fibrillary acidic
protein (GFAP) in human brain tumors. J Neurol Sci. 35:147–155.
1978. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Venkatesh K, Srikanth L, Vengamma B,
Chandrasekhar C, Sanjeevkumar A, Mouleshwara Prasad BC and Sarma
PV: In vitro differentiation of cultured human CD34+
cells into astrocytes. Neurol India. 61:383–388. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hol EM and Pekny M: Glial fibrillary
acidic protein (GFAP) and the astrocyte intermediate filament
system in diseases of the central nervous system. Curr Opin Cell
Biol. 32:121–130. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Storoni M, Verbeek MM, Illes Z, Marignier
R, Teunissen CE, Grabowska M, Confavreux C, Plant GT and Petzold A:
Serum GFAP levels in optic neuropathies. J Neurol Sci. 317:117–122.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhao T, Ding Y, Li M, Zhou C and Lin W:
Silencing lncRNA PVT1 inhibits activation of astrocytes and
increases BDNF expression in hippocampus tissues of rats with
epilepsy by downregulating the Wnt signaling pathway. J Cell
Physiol. Feb 25–2019.(Epub ahead of print).
|
|
32
|
Ahmadian SR, Ghasemi-Kasman M, Pouramir M
and Sadeghi F: Arbutin attenuates cognitive impairment and
inflammatory response in pentylenetetrazol-induced kindling model
of epilepsy. Neuropharmacology. 146:117–127. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Huang LG, Zou J and Lu QC: Silencing
rno-miR-155-5p in rat temporal lobe epilepsy model reduces
pathophysiological features and cell apoptosis by activating
Sestrin-3. Brain Res. 1689:109–122. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ng A, Tam WW, Zhang M, Ho CS, Husain SF,
McIntyre RS and Ho RC: IL-1β, IL-6, TNF-α and CRP in elderly
patients with depression or Alzheimer's disease: Systematic review
and meta-analysis. Sci Rep. 8:120502018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li Y and Kowdley KV: MicroRNAs in common
human diseases. Genomics Proteomics Bioinformatics. 10:246–253.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Iborra M, Bernuzzi F, Invernizzi P and
Danese S: MicroRNAs in autoimmunity and inflammatory bowel disease:
Crucial regulators in immune response. Autoimmun Rev. 11:305–314.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lei H, Tang J, Li H, Zhang H, Lu C, Chen
H, Li W, Xia Y and Tang W: MiR-195 affects cell migration and cell
proliferation by down-regulating DIEXF in Hirschsprung's disease.
BMC Gastroenterol. 14:1232014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Simpson LJ and Ansel KM: MicroRNA
regulation of lymphocyte tolerance and autoimmunity. J Clin Invest.
125:2242–2249. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang B, Pan X, Cobb GP and Anderson TA:
microRNAs as oncogenes and tumor suppressors. Dev Biol. 302:1–12.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hwang HW and Mendell JT: MicroRNAs in cell
proliferation, cell death, and tumorigenesis. Br J Cancer.
94:776–780. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Heinzelmann J, Henning B, Sanjmyatav J,
Posorski N, Steiner T, Wunderlich H, Gajda MR and Junker K:
Specific miRNA signatures are associated with metastasis and poor
prognosis in clear cell renal cell carcinoma. World J Urol.
29:367–373. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Fu X, Meng Z, Liang W, Tian Y, Wang X, Han
W, Lou G, Wang X, Lou F, Yen Y, et al: miR-26a enhances miRNA
biogenesis by targeting Lin28B and Zcchc11 to suppress tumor growth
and metastasis. Oncogene. 33:4296–4306. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Danis B, Van Rikxoort M, Kretschmann A,
Zhang J, Godard P, Andonovic L, Siegel F, Niehusmann P, Hanon E,
Delev D, et al: Differential expression of miR-184 in temporal lobe
epilepsy patients with and without hippocampal sclerosis-Influence
on microglial function. Sci Rep. 6:339432016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Peng J, Omran A, Ashhab MU, Kong H, Gan N,
He F and Yin F: Expression patterns of miR-124, miR-134, miR-132,
and miR-21 in an immature rat model and children with mesial
temporal lobe epilepsy. J Mol Neurosci. 50:291–297. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Li Y, Zhang B, Li W, Wang L, Yan Z, Li H,
Yao Y, Yao R, Xu K and Li Z: MiR-15a/16 regulates the growth of
myeloma cells, angiogenesis and antitumor immunity by inhibiting
Bcl-2, VEGF-A and IL-17 expression in multiple myeloma. Leuk Res.
49:73–79. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Chen H and Tian Y: MiR-15a-5p regulates
viability and matrix degradation of human osteoarthritis
chondrocytes via targeting VEGFA. Biosci Trends. 10:482–488. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kang W, Tong JH, Lung RW, Dong Y, Zhao J,
Liang Q, Zhang L, Pan Y, Yang W and Pang JC: Targeting of YAP1 by
microRNA-15a and microRNA-16-1 exerts tumor suppressor function in
gastric adenocarcinoma. Mol Cancer. 14:522015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Liu XF, Wang RQ, Hu B, Luo MC, Zeng QM,
Zhou H, Huang K, Dong XH, Luo YB, Luo ZH and Yang H: MiR-15a
contributes abnormal immune response in myasthenia gravis by
targeting CXCL10. Clin Immunol. 164:106–113. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Fan B, Chen LP, Yuan YH, Xiao HN, Lv XS
and Xia ZY: MiR-15a-3p suppresses the growth and metastasis of
ovarian cancer cell by targeting twist1. Eur Rev Med Pharmacol Sci.
23:1934–1946. 2019.PubMed/NCBI
|
|
51
|
Lines KE, Newey PJ, Yates CJ, Stevenson M,
Dyar R, Walls GV, Bowl MR and Thakker RV: MiR-15a/miR-16-1
expression inversely correlates with cyclin D1 levels in men1
pituitary NETs. J Endocrinol. 240:41–50. 2018. View Article : Google Scholar
|
|
52
|
Li YJ, Zhang BY, Li WJ, Wang LJ, Yan ZL,
Li H, Yao Y, Yao R, Xu K and Li Z: MiR-15a/16 regulates the growth
of myeloma cells angiogenesis and antitumor immunity by inhibiting
Bcl-2, VEGF-A and IL-17 expression in multiple myeloma. Leuk Res.
49:73–79. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Dong P, Mai Y, Zhang Z, Mi L, Wu G, Chu G,
Yang G and Sun S: MiR-15a/b promote adipogenesis in porcine
pre-adipocyte via repressing FoxO1. Acta Biochim Biophys Sin
(Shanghai). 46:565–571. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Gao Y, Su J, Guo W, Polich ED, Magyar DP,
Xing Y, Li H, Smrt RD, Chang Q and Zhao X: Inhibition of miR-15a
promotes BDNF expression and rescues dendritic maturation deficits
in MeCP2-Deficient neurons. Stem Cells. 33:1618–1629. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Isaacs A, Baker M, Wavrant-De Vrièze F and
Hutton M: Determination of the gene structure of human GFAP and
absence of coding region mutations associated with frontotemporal
dementia with parkinsonism linked to chromosome 17. Genomics.
51:152–154. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Reeves SA, Helman LJ, Allison A and Israel
MA: Molecular cloning and primary structure of human glial
fibrillary acidic protein. Proc Natl Acad Sci USA. 86:5178–5182.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Sun YN, Luo JY, Rao ZR, Lan L and Duan L:
GFAP and Fos immunoreactivity in lumbo-sacral spinal cord and
medulla oblongata after chronic colonic inflammation in rats. World
J Gastroenterol. 11:4827–4832. 2005. View Article : Google Scholar : PubMed/NCBI
|