Introduction
Acute myocardial infarction (AMI) is the leading
cause of mortality and disability worldwide (1). Epidemiological studies have
identified risk factors for AMI, including dyslipidemia, diabetes,
obesity, hypertension, smoking, sex and genetic variation (1,2).
Early prevention and the strict control of related risk factors
have become the standard therapies used to prevent AMI (3). AMI results from the abrupt occlusion
of coronary arteries, which leads to prolonged myocardial ischemia
and irreversible cardiomyocyte death (4). Currently, the most effective way to
save apoptotic cardiomyocytes is timely and successful
revascularization; however, re-establishing blood flow to the
ischemic area has been demonstrated to promote additional
myocardial damage, a process referred to as myocardial ischemia and
reperfusion injury (MIRI) (5).
Moreover, although strategies such as percutaneous coronary
intervention, intravenous thrombolysis and coronary artery bypass
grafting have markedly limited infarct expansion and decreased
in-hospital mortality, the incidence of subsequent chronic heart
failure has increased gradually (6). Thus, the identification of new drugs
that can attenuate MIRI through the aforementioned mechanisms is
urgently required.
MIRI involves a complex pathophysiological process,
in which inflammation and apoptosis have been recognized as the two
characteristics underlying the pathological mechanisms (7). Reperfusion was discovered to trigger
a strong inflammatory response, thus recruiting inflammatory cells
and releasing inflammatory factors, leading to serious
cardiomyocyte damage (8). In
addition, several apoptosis-related genes, including Bax and
cleaved caspase-3 were identified to be involved in MIRI (9). A previous study suggested that
cardiomyocyte apoptosis occurred during the early phase of ischemia
and that reperfusion could exacerbate the severity (10). Thus, it is hypothesized that the
regulation of the inflammatory response and the concurrent
inhibition of cardiomyocyte apoptosis, which are of great clinical
significance to the treatment of AMI, may mitigate MIRI.
Icariside II (ICAII) is a ubiquitous bioflavonoid
compound derived from Herba epimedii (11) and used in traditional Chinese
medicine. ICAII has demonstrated multiple biological benefits, such
as anti-oxidative, anti-inflammatory and anti-apoptotic properties
(12). To date, previous studies
have predominately focused on the antitumor efficacy of ICAII in
different types of cancer, such as lung carcinoma, prostate cancer,
melanoma and breast cancer (12),
although previous studies have demonstrated that ICAII also exerted
a positive effect in the pathological progression of cardiovascular
disease. For example, ICAII administration attenuated cardiac
remodeling and ameliorated diabetic cardiomyopathy, thereby
inhibiting myocardial hypertrophy and myocardial fibrosis (13,14).
However, to the best of our knowledge, whether ICAII can alleviate
MIRI remains unknown. Interestingly, it has been reported that
multiple flavonoid drugs exerted myocardial protective efficiency
in MIRI (10,15). Furthermore, a previous study
demonstrated that ICAII exerted an anti-inflammatory effect and
protected neurons from cerebral IR injury (16). Therefore, the aim of the present
study was to determine whether the pretreatment with ICAII
mitigated MIRI and to investigate the underlying mechanisms of
action.
Materials and methods
Construction of MIRI model rats
A total of 70 wild-type male Sprague-Dawley rats
(specific pathogen-free; weight, 200–250 g; age, 6–7 weeks) were
purchased from The Hubei Provincial Center for Disease Control and
Prevention. Rats were provided with free access to standard rat
chow and water until the time of the experiment, and were housed at
24°C and 55% humidity, with a 12 h-light/dark cycle. Animal health
and behaviour were monitored every two days for unexpected pain,
poor appetite, weight loss and weakness; no rat died unexpectedly
during the experiment (3 weeks). The maximum body weight observed
in the study was 241.6 g. After the intervention and reperfusion
procedures were completed, the rats were anesthetized with 60 mg/kg
pentobarbital sodium intraperitoneally, then sacrificed by air
embolization with 0.03% CO2. Euthanasia was confirmed by
loss of vital signs using an electrocardiogram (ECG). All
experimental procedures were approved by The Animal Care and Use
Committee of Hubei University (Wuhan, China) and performed in
accordance with the Institutional Guidelines and the Guide for the
Care and Use of Laboratory Animals (National Institutes of Health
publication no. 85–23, revised 1996 edition) (17).
The MIRI model rats were established by exerting
myocardial ischemia for 30 min and reperfusion for 24 h. Briefly,
animals were fasted 12 h before surgery, then anesthetized with 60
mg/kg pentobarbital sodium intraperitoneally and placed in the
supine position. A standard lead-II ECG was used to monitor
continuously throughout the operation. A median incision in the
neck was made to expose the trachea and the rats were artificially
ventilated with a small animal respirator at 70 strokes/min. A left
thoracotomy was performed and the left arterial descending (LAD)
coronary artery was located. A small curved needle with a 6-0 silk
suture was then passed through the myocardium beneath the LAD
artery and a ligation was performed to block the blood flow. MIRI
establishment was considered successful when the ST segment in the
lead II was elevated and the regional myocardial surface became
pale. Following 30 min of ischemia, the suture was untied to
reperfuse the myocardium for 24 h. After reperfusion, the animals
were sacrificed as described and the myocardial specimens were
harvested for further analysis.
Experimental design
ICAII (Shanghai Aladdin Biochemical Technology Co.,
Ltd.) was administered in varying concentrations to determine the
optimal drug dose for MIRI improvement. ICAII was dissolved in
normal saline containing 0.05% Tween-80 (Sigma-Aldrich; Merck KGaA)
and injected through the tail vein (300 µl) at the beginning of the
myocardial reperfusion period. Briefly, 30 rats were randomly
divided into five groups (6 rats/group): i) A sham-operated (SO)
group, in which thoracotomy surgery was performed without ligating
the LAD artery; ii) an IR group, in which rats were subjected to
LAD artery occlusion for 30 min, followed by reperfusion for 24 h;
iii) a 10 mg/kg ICAII and IR group (ICAII 10 + IR group); iv) a 20
mg/kg ICAII and IR group (ICAII 20 + IR group); and v) a 30 mg/kg
ICAII and IR group (ICAII 30 + IR group). Rats in the last three
groups were subjected to LAD artery ligation for 30 min and then
administered the indicated doses of ICAII, followed by a 24 h
myocardial reperfusion.
To investigate the specific underlying protective
mechanism of ICAII in MIRI, another experiment was performed, in
which rats were randomly divided into four groups (n=10 in each
group): i) An SO group; ii) an IR group; iii) an ICAII 20 + IR
group, in which the rat MIRI model was established and 20 mg/kg
ICAII was administered through the tail vein at the beginning of
reperfusion; and iv) an ICAII 20 + IR + LY294002 group, treated
like the ICAII 20 + IR group, with the addition of 0.3 mg/kg
LY294002 (Sigma-Aldrich; Merck KGaA), a specific inhibitor of the
PI3K/AKT signaling pathway. LY294002 was intraperitoneally injected
into the abdomen after the LAD artery was untied. An equal volume
of DMSO (0.3 mg/ml; Shanghai Aladdin Biotechnology Technology Co.,
Ltd.) was similarly administered to the rats in the three groups
which did not receive LY294002 treatment.
Histological examination
At the end of the experiment, the left ventricle
from each rat was harvested and immersed in a 4% paraformaldehyde
solution for 24 h at room temperature. After fixation, the
specimens were embedded in paraffin and the samples were cut into
5-µm thick sections on glass slides. Hematoxylin and eosin
(H&E) staining was then performed; sections were stained with
hematoxylin for 15 min at 25°C and eosin for 5 min at 25°C. The
morphology and structure of the myocardial tissue were observed
under a light microscope (magnification, ×200).
To quantify the severity of the myocardial injury,
five randomly selected fields from each group were chosen and
scored (damage score) according to the standards described in a
previous study (10): i) No
injury, 0; ii) interstitial edema and focal necrosis (mild injury),
1; iii) cardiomyocyte swelling and necrosis (moderate injury), 2;
iv) formation of necrotic contraction bands and inflammatory cell
enrichment (severe injury), 3; and v) expanded necrosis of
contraction bands, inflammatory cell infiltration and hemorrhage
(highly severe injury), 4.
Infarct size assessment
The size of the myocardial infarct was determined by
Evans blue and 2,3,5-triphenyltetrazolium chloride (TTC) staining,
as previously described (10)
(both from Sigma-Aldrich; Merck KGaA). Masson's trichrome staining
was not used in the present study as the experimental design did
not allow for the formation of collagen fibers in the myocardial
tissue (18). At the end of the
experiment (after 24 h reperfusion and prior to euthanasia), 5 rats
were anaesthetized with 60 mg/kg pentobarbital sodium
intraperitoneally. The LAD was re-ligated at the same location and
1 ml Evans blue solution (2%) was rapidly injected into the jugular
vein. The rats were subsequently euthanized as described above and
the heart was then removed, rinsed with an ice-cold saline solution
and incubated at −80°C for 8 min. The frozen heart was cut into
~2-mm longitudinal sections and incubated with 1% TTC solution at
37°C for 30 min, followed by fixation with 4% paraformaldehyde for
24 h at room temperature. The white infarct area and total left
ventricular area at the papillary muscle plane of the heart were
measured using a digital camera (Nikon Corporation) and Photoshop
CS6 software (Adobe Systems, Inc.). The relative myocardial infarct
size (%) was calculated using the following equation: (Infarct
area/the total left ventricular area) ×100.
Measurement of myocardial injury
markers
Myocardial enzymes are released when cardiomyocytes
are seriously damaged, with their levels representing the severity
of the myocardial injury (7).
Before the injection of Evans blue solution, 2 ml blood samples
were collected from the jugular vein and centrifuged at 1,006 × g
for 10 min at 4°C to obtain plasma. Subsequently, commercially
available biochemical kits (Nanjing Jiancheng Bioengineering
Institute) were used according to the manufacturers' instructions
to detect the levels of lactate dehydrogenase (LDH; cat. no.
A020-1-2) and creatine kinase-myocardial band (CK-MB; cat. no.
H197).
Examination of cardiac function
Ultrasonic cardiograms were performed to determine
the systolic function of the heart following IR injury using the
MyLab 30CV ultrasound system (Esaote SpA). Briefly, following the
24 h myocardium reperfusion, 5 rats were anesthetized by 1.5–2%
isoflurane inhalation (induction 2–3%), then placed in the supine
position. Ultrasound images were observed from the parasternal
short axis at the level of the mid-papillary muscle from ≥3
separate cardiac cycles. Left ventricular ejection fraction (LVEF)
was measured to determine cardiac function. Following the
ultrasound, the rats were anesthetized with 60 mg/kg sodium
pentobarbital, and blood was collected as described above, prior to
euthanasia by air embolization as previously described.
Assessment of myocardial
apoptosis
IR-induced apoptosis was evaluated using TUNEL
staining. Briefly, paraffin-embedded myocardial specimens were cut
into 5-µm-thick sections in the direction of the perpendicular
myocardial fibers. The samples were then fixed with 4%
paraformaldehyde for 24 h at 25°C. After deparaffinization with
dimethylbenzene at 60°C and rehydration with a descending alcohol
series, TUNEL staining was performed according to the
manufacturer's protocol using an In Situ Cell Death
Detection kit (cat. no. 11684817910; Roche Diagnostics). Briefly,
the sections were stained with TUNEL reagent for 1 h at 37°C and
cell nuclei were counterstained at 25°C for 10 sec with 3% ethyl
green, then mounted in 50–100 µl Permountä mounting medium.
Subsequently, ≥5 randomly selected high-power fields of view of
each slice were selected to count the number of apoptotic
cardiomyocytes using a fluorescent microscope (magnification,
×400). The nuclei of the apoptotic TUNEL-positive cells and normal
cells were stained brown and blue, respectively. The apoptotic
index (AI; %) was calculated using the following equation: Ratio of
TUNEL-positive cells to total cardiomyocytes.
Western blotting
Total protein was extracted from myocardial samples
using RIPA lysis buffer (Beyotime Institute of Biotechnology),
supplemented with 50 mM Tris, 150 mM NaCl, 1% Triton X-100, 1%
sodium deoxycholate, 0.1% SDS, 2 mM sodium pyrophosphate, 1 mM EDTA
and 100 mM PMSF. Total protein was quantified using a bicinchoninic
acid protein assay (Beyotime Institute of Biotechnology) and 36 µg
protein was separated via 10% SDS-PAGE. The separated proteins were
subsequently electrotransferred onto PVDF membranes and blocked
with 5% non-fat dried milk at room temperature for 2 h to prevent
nonspecific binding to the membranes. Then, the membranes were
incubated with the following primary antibodies overnight at 4°C
(all diluted 1:1,000): Anti-Bax (Abcam; cat. no. ab32503),
anti-Bcl-2 (Abcam; cat. no. ab196495), anti-cleaved caspase-3
(Abcam; cat. no. ab49822), anti-interleukin (IL)-6 (Abcam; cat. no.
ab9324), anti-tumor necrosis factor (TNF)-α (Abcam; cat. no.
ab6671), anti-PI3K (Cell Signaling Technology, Inc.; cat. no.
4249S), anti-phosphorylated (p)-PI3K (Cell Signaling Technology,
Inc. cat. no. 17366), anti-AKT (Abcam; cat. no. ab179463),
anti-p-AKT (Abcam; cat. no. ab182651) and anti-GAPDH (Abcam; cat.
no. ab181602). Following the primary antibody incubation, the
membranes were incubated with either a horseradish peroxidase
(HRP)-conjugated anti-rabbit IgG (Boster Biological Technology;
cat. no. BA1054; 1:10,000) or an HRP-conjugated anti-mouse IgG
(Abcam; cat. no. ab6789; 1:10,000) secondary antibody for 2 h at
25°C. The protein bands were visualized using an enhanced
chemiluminescence reagent (Thermo Fisher Scientific, Inc.) and
analyzed using ImageJ 1.52q software (National Institutes of
Health).
Statistical analysis
All statistical analyses were performed using SPSS
19.0 software (IBM Corp.) and data are presented as the mean ± SD
of 5 independent repeats. A one-way ANOVA followed by a Tukey's
post hoc test was used for multiple comparisons. P<0.05 was
considered to indicate a statistically significant difference.
Results
ICAII attenuates the morphological
damage and mitigates MIRI
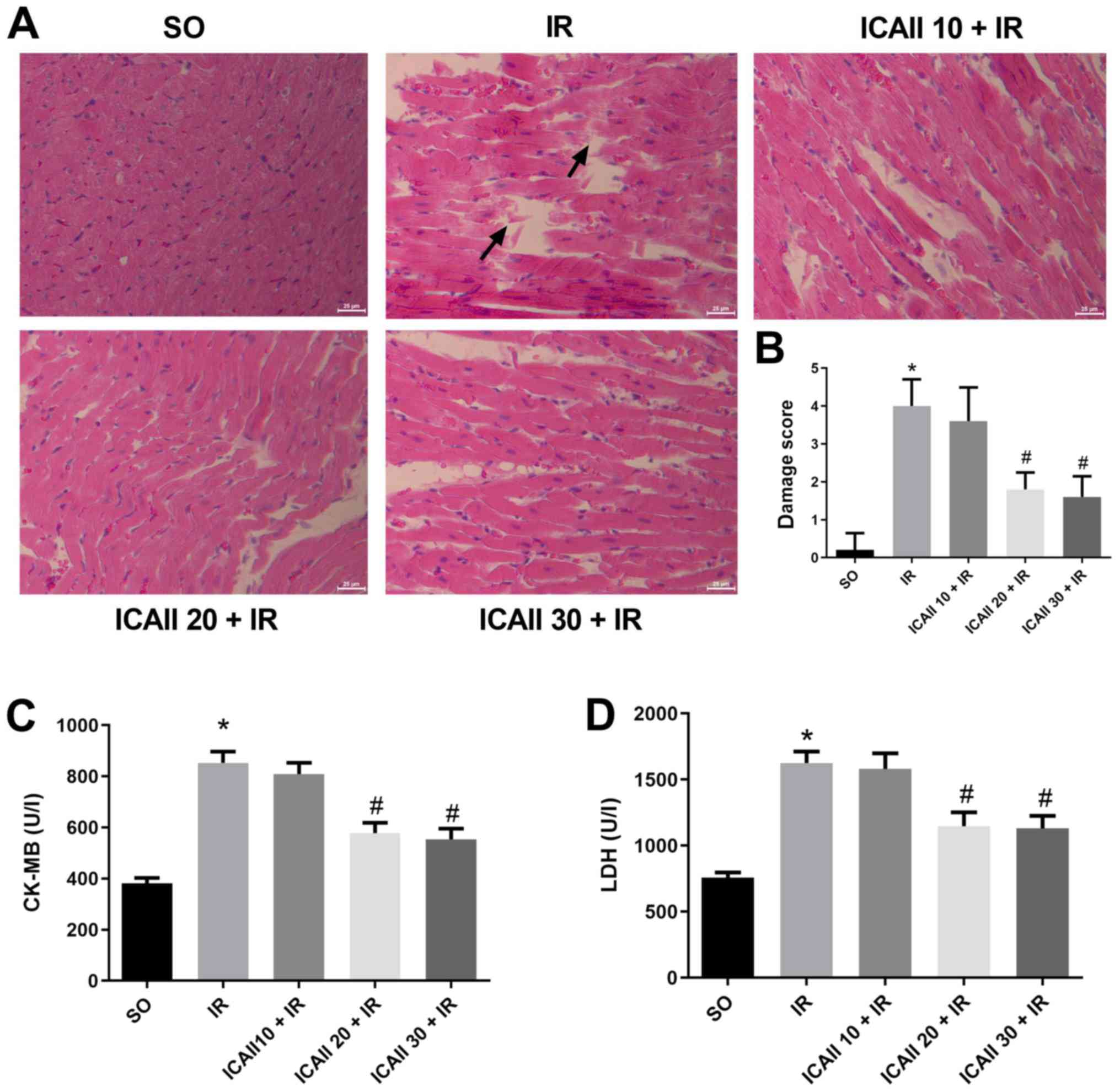
Myocardial fibres were intact and arranged regularly
in the SO group, and no necrosis was observed (Fig. 1A). However, compared with the SO
group, the myocardial fibres were disordered and partially ruptured
in the IR group, and interstitial edema and inflammatory cell
infiltration were observed. By contrast, myocardial damage was less
severe following ICAII pretreatment at all doses, and the damage
score was significantly decreased in the ICAII 20 + IR and ICAII 30
+ IR groups compared with the IR group. Notably, the cardiomyocyte
structure and morphology remained normal in the ICAII 20 + IR and
ICAII 30 + IR groups, with no significant differences identified in
the damage score between these two groups (Fig. 1B).
The levels of myocardial enzymes were also analyzed
in the different groups. Serum levels of LDH and CK-MB were
significantly increased in the IR group compared with the SO group
(Fig. 1C and D). However, only the
groups pretreated with 20 and 30 mg/kg ICAII exhibited
significantly decreased enzyme levels compared with the IR group.
No significant differences were identified in the levels of the
myocardial enzymes between the ICAII 20 + IR and ICAII 30 + IR
groups. As a result, 20 mg/kg was considered as the optimal dose to
alleviate myocardial damage.
ICAII pretreatment decreases infarct
size and improves cardiac function
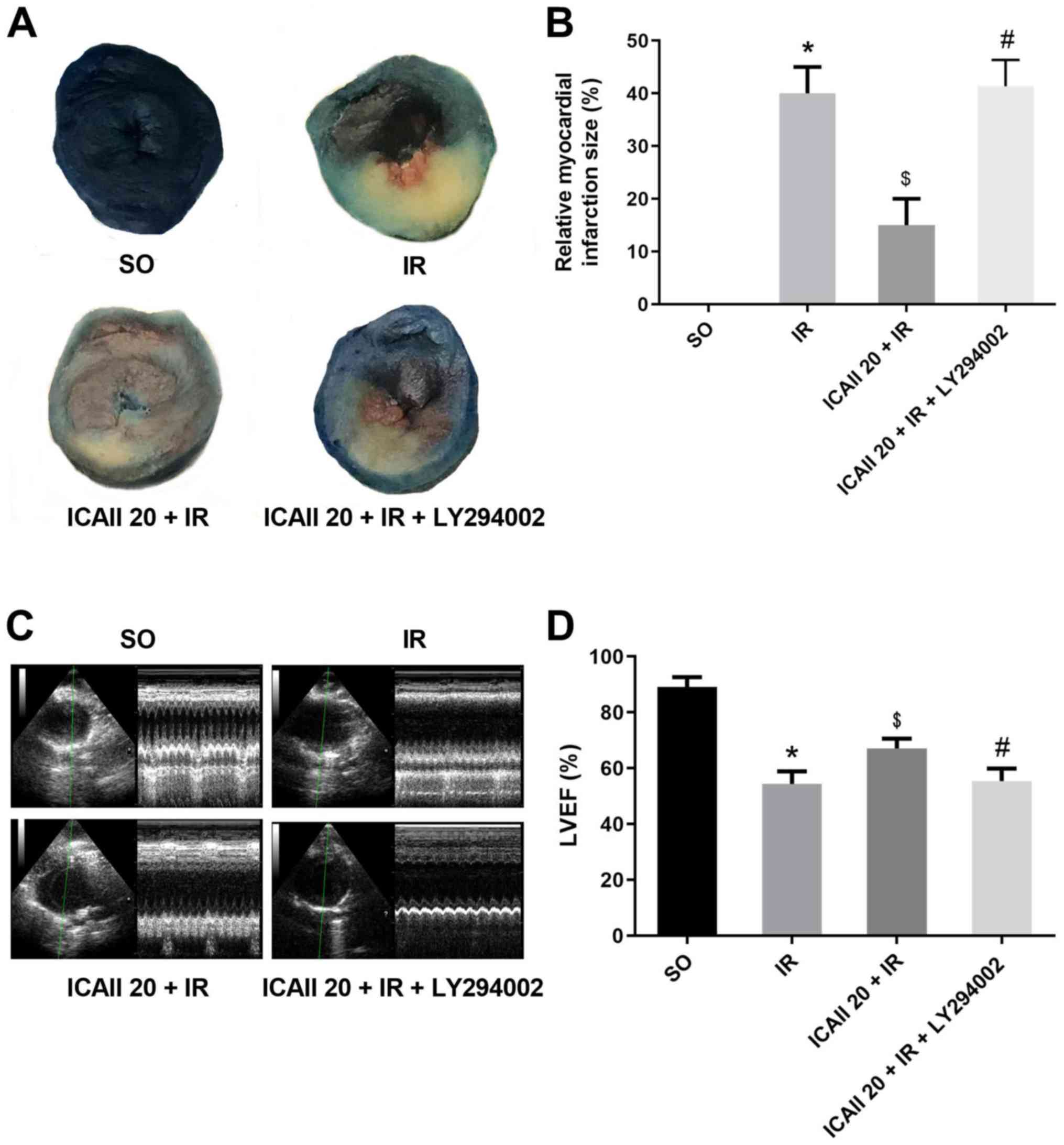
Sections of the papillary muscle plane of the heart
from each group were selected to compare the myocardial infarct
size; ICAII pretreatment notably decreased the IR-related infarct
size (Fig. 2A and B). Notably, the
cotreatment with ICAII and LY294002 significantly counteracted the
beneficial effect of ICAII pretreatment. Echocardiography data
revealed similar results (Fig. 2C and
D); IR injury resulted in the significant degeneration of
cardiac function compared with the SO group. However, compared with
the IR group, ICAII administration improved LVEF by 19.4%. Notably,
the cotreatment with ICAII and LY294002 abolished the improvement
in cardiac function observed with ICAII treatment alone.
ICAII pretreatment alleviates
IR-induced inflammatory responses
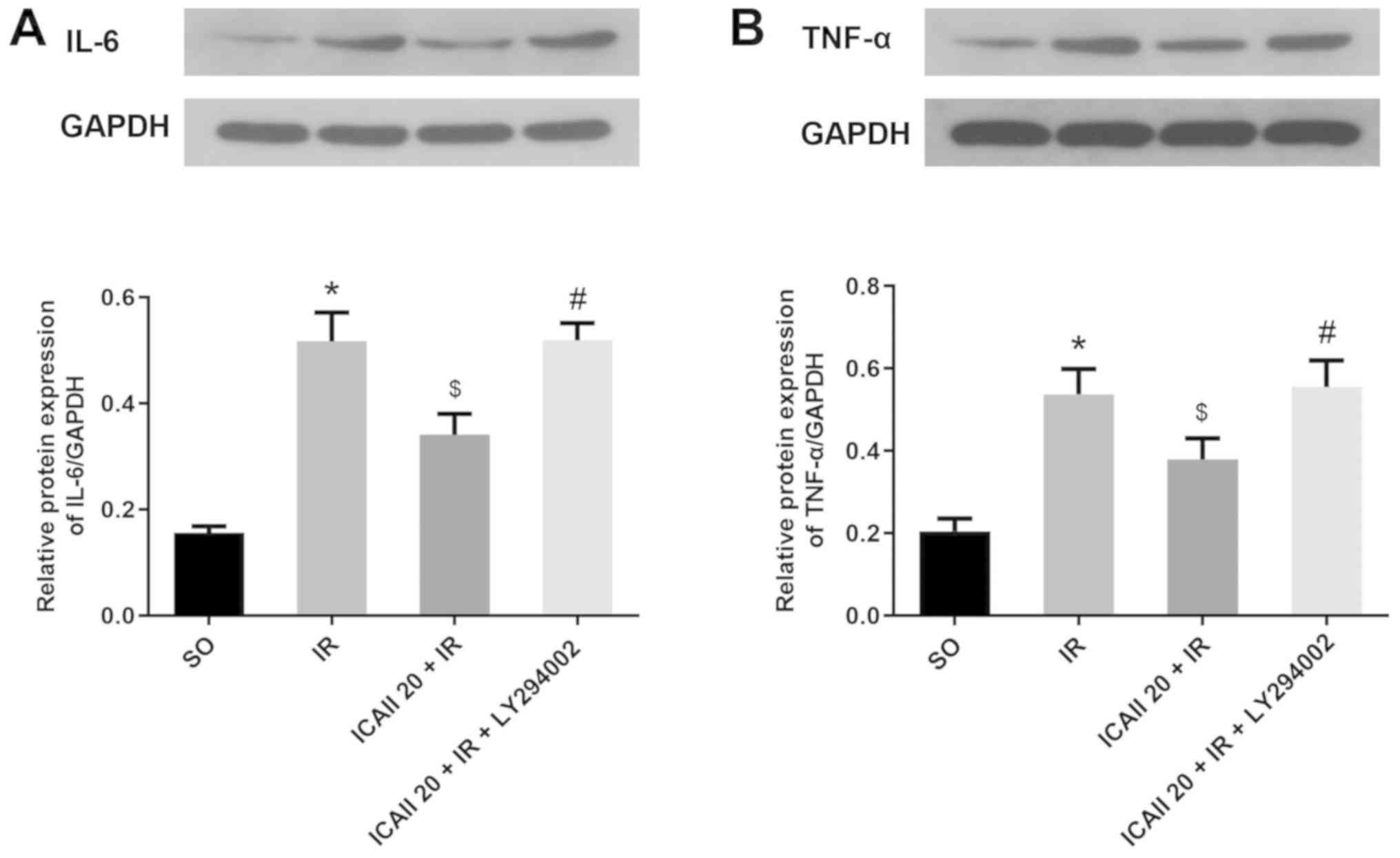
The expression levels of IL-6 and TNF-α in the
myocardial tissue were analyzed using western blotting. The
expression levels of IL-6 and TNF-α were significantly upregulated
following IR injury compared with the SO group (Fig. 3A and B). However, the pretreatment
with ICAII significantly downregulated the protein expression
levels of IL-6 and TNF-α in the ICAII 20 + IR group compared with
the IR group. By contrast, the anti-inflammatory effect of ICAII
was partially suppressed when LY294002 was also administered at the
same time.
ICAII pretreatment ameliorates
IR-induced myocardial apoptosis
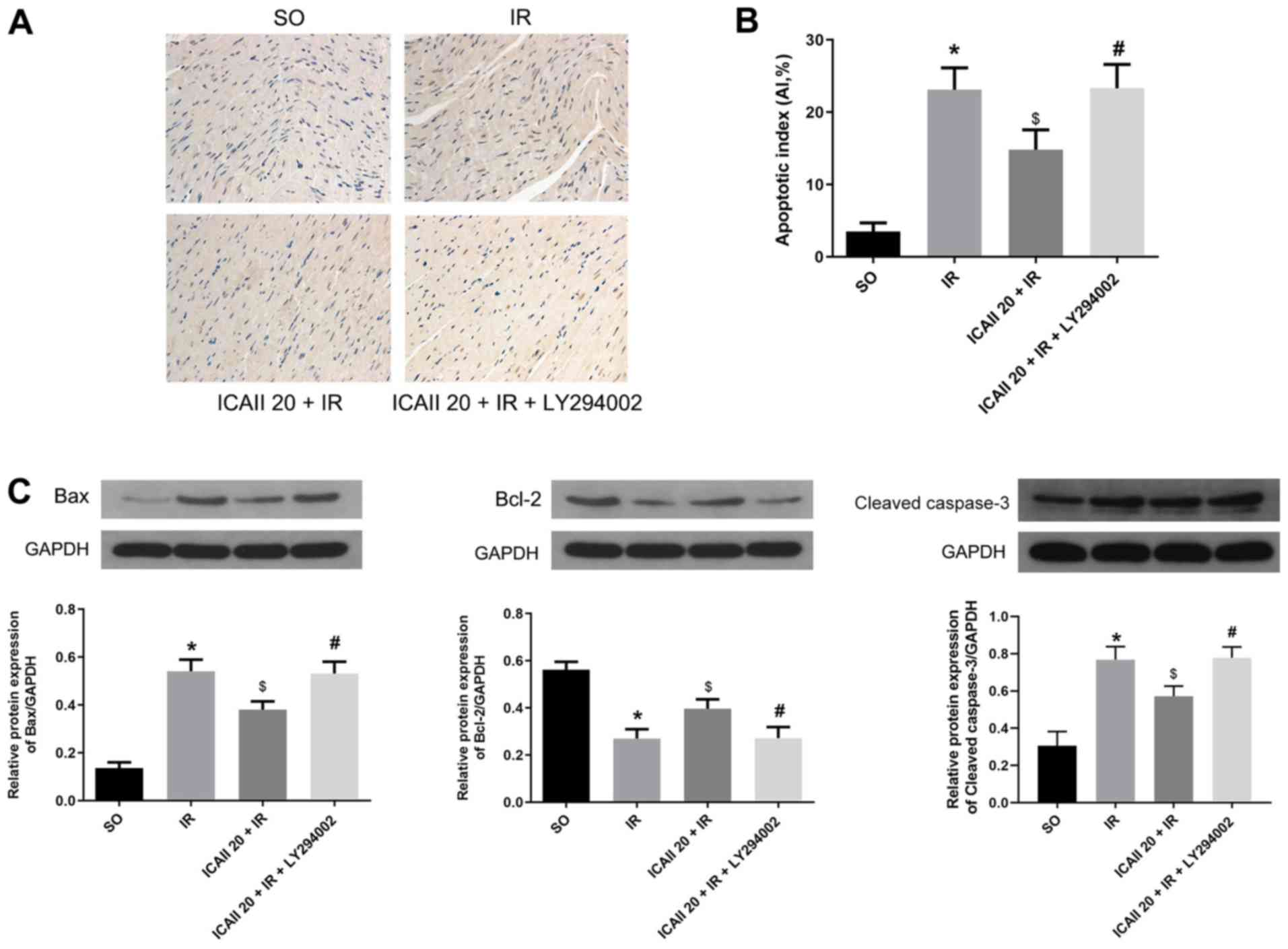
Few TUNEL-positive cells were identified in the SO
group, whereas the apoptotic rate of cardiomyocytes was
significantly increased following IR injury (Fig. 4A and B). ICAII pretreatment
significantly decreased the AI compared with the IR group. However,
LY294002 treatment significantly inhibited the effect of ICAII
treatment alone on apoptosis.
The expression levels of the proapoptotic protein
Bax and the anti-apoptotic protein Bcl-2, as well as cleaved
caspase-3, were also analyzed. IR injury significantly upregulated
the expression levels of Bax and cleaved caspase-3, whilst
downregulating the expression levels of Bcl-2 compared with the SO
group (Fig. 4C). However, ICAII
treatment significantly downregulated the expression levels of Bax
and cleaved caspase-3, while upregulating the expression levels of
Bcl-2, compared with the IR group. Notably, the cotreatment with
ICAII and LY294002 significantly reversed the trend observed
following ICAII treatment alone. These findings suggested that
ICAII may prevent IR-induced myocardial apoptosis, while LY294002
may partially inhibit this anti-apoptotic effect.
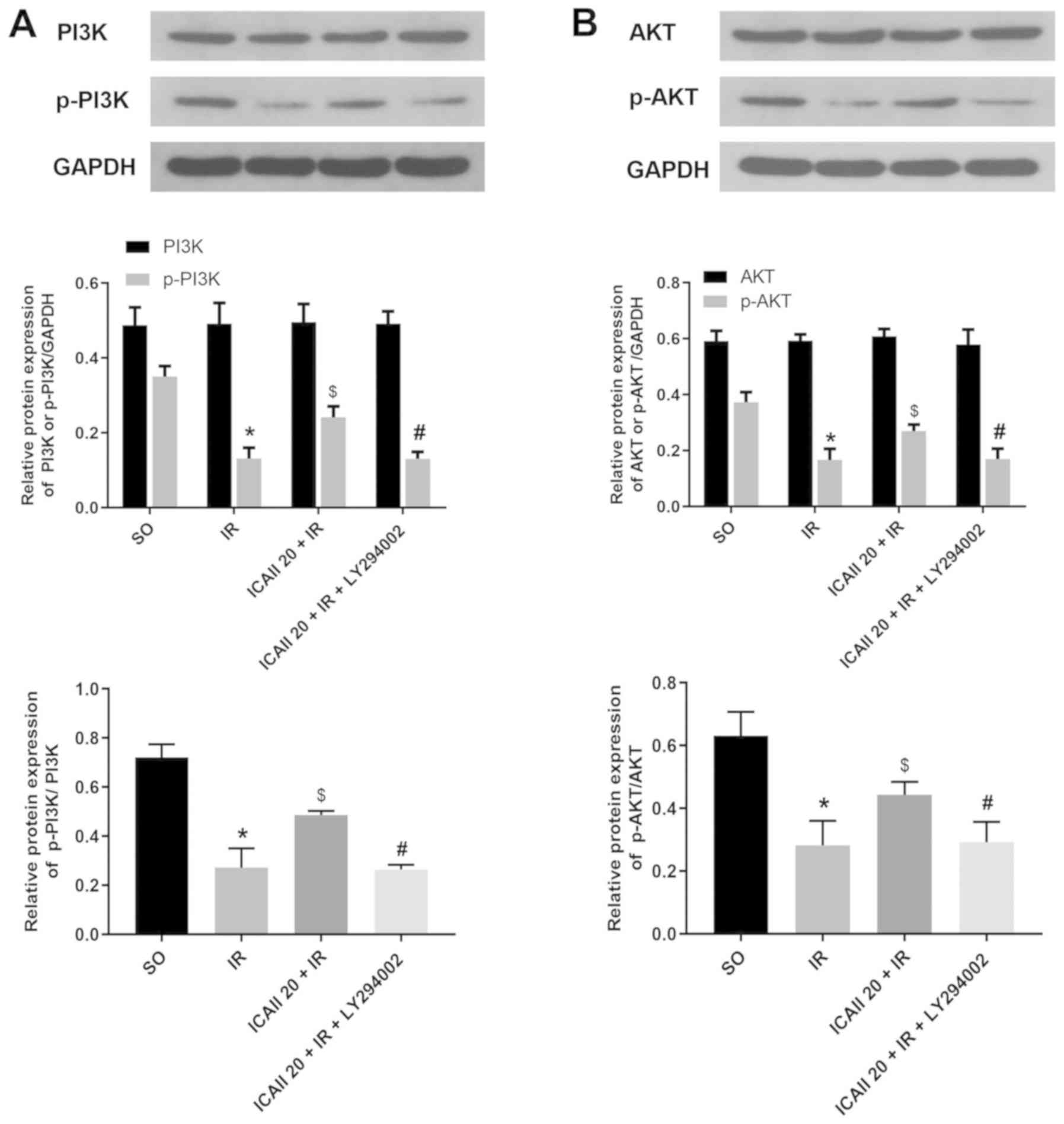
ICAII pretreatment activates the
PI3K/AKT signaling pathway
To further investigate the specific underlying
mechanisms of ICAII in alleviating IR injury, the expression levels
of molecules involved in the PI3K/AKT signaling pathway were
determined. IR injury significantly downregulated the expression
levels of p-PI3K and p-AKT compared with the SO group (Fig. 5A and B). However, the pretreatment
with ICAII at the onset of reperfusion significantly upregulated
the expression levels of p-PI3K and p-AKT compared with the IR
group. Conversely, LY294002 treatment significantly reversed the
upregulation of p-PI3K and p-AKT expression levels promoted by
ICAII pretreatment alone. In addition, no significant differences
were observed in the expression levels of PI3K and AKT between the
different groups. Notably, the ratio of p-PI3K/PI3K and p-AKT/AKT
exhibited a similar trend to the expression levels of the
phosphorylated proteins. Altogether, these observations indicated
that ICAII may activate the PI3K/AKT signaling pathway following IR
injury.
Discussion
Accumulating evidence has suggested that ICAII may
serve a beneficial impact in various types of disease, such as
Alzheimer's disease, osteoporosis, stroke, breast cancer and
leukemia, possibly due to its multiple bioactivities, including
antioxidative, anti-inflammatory and anti-apoptotic properties
(19). Recent studies have
reported that ICAII may also be beneficial for cardiovascular
disease; for example, in a rat model of spontaneous hypertension,
Wu et al (13) demonstrated
that the intra-gastric administration of ICAII (4, 8 or 16 mg/kg)
significantly decreased the blood pressure, promoted heart
functional recovery and improved ventricular remodeling.
Furthermore, mechanistically, ICAII inhibited myocardial apoptosis
and decreased the generation of reactive oxygen species (13). Fu et al (20) also identified that the pretreatment
with ICAII at the same dose improved myocardial fibrosis through
anti-inflammatory mechanisms in the same animal model (spontaneous
hypertension). In addition, other studies have indicated that ICAII
may serve a positive role during IR injury; for instance, in a rat
model of cerebral IR injury, Deng et al (16) demonstrated that 10 or 30 mg/kg
ICAII pretreatment protected neurons from damage and decreased
infarct size by regulating the crucial inflammatory factor NF-κB.
Yan et al (21) also
suggested that the oral administration of 20 mg/kg ICAII before IR
markedly inhibited IR-induced leukocyte adhesion and neuronal loss
in the hippocampal CA1 region. Thus, the aforementioned studies
indicated that ICAII may exhibit anti-inflammatory and
anti-apoptotic properties at doses of 4–30 mg/kg. However, to the
best of our knowledge, whether ICAII prevented MIRI remained
unclear.
In the present study, a dose-response experiment
demonstrated that ICAII attenuated MIRI at various doses, as
evidenced by the mitigation of morphological damage and a decline
in the release of LDH and CK-MB. Furthermore, no statistical
significances were reported in the damage scores and myocardial
enzyme levels between the middle and high-dose ICAII groups. Thus,
20 mg/kg ICAII was selected as the dose to elicit a sufficient
protective effect on the myocardium. Indeed, the pretreatment with
20 mg/kg ICAII markedly improved cardiac function, minimized
infarct size, reduced inflammatory reactions and decreased
cardiomyocyte apoptosis. Moreover, ICAII administration reversed
the downregulated expression levels of p-PI3K and p-AKT induced by
IR injury. Therefore, to the best of our knowledge, the present
study was the first to illustrate the protective effects of ICAII
against MIRI. The present findings indicated that ICAII may
represent a potential therapeutic strategy for MIRI, partly by
activating the PI3K/AKT signaling pathway.
It was previously reported that IR was associated
with an inflammatory cascade and neutrophil activation (8). The excessive release of cytokines was
discovered to be a crucial event in MIRI (22). In the present study, the expression
levels of classical inflammatory factors IL-6 and TNF-α were
evaluated. Consistent with previous studies, IR injury induced the
release of IL-6 and TNF-α from the myocardial tissue. However, the
administration of ICAII at the beginning of reperfusion
significantly downregulated IL-6 and TNF-α expression levels.
Apoptosis was reported to be the predominant form of cell death in
MIRI, leading to the loss of the myocardium and the deterioration
of cardiac function (23). The
imbalance between anti-apoptotic and proapoptotic protein
expression levels were identified as an important characteristic of
cell apoptosis (23). The present
study identified that IR injury upregulated the expression levels
of Bax and cleaved caspase-3, while downregulating the expression
levels of Bcl-2. Additionally, the number of TUNEL-positive cells
was significantly increased following IR injury. However, the
pretreatment with ICAII reduced the levels of cell apoptosis and
reversed the effects of IR on the expression levels of Bax and
Bcl-2 to a large extent. Notably, a previous study also identified
a feedback loop between inflammation and apoptosis in the
pathological progression of MIRI; apoptosis can be regulated by
cytokines such as TNF-α and IL-6 and reciprocally, the induced
apoptosis provokes a more severe inflammatory response (24). In the present study, ICAII reversed
the inflammation and apoptosis caused by IR injury. Nevertheless,
the mechanism through which ICAII exerted a myocardial protective
effect requires further investigations.
PI3K and its downstream target serine/threonine
kinase AKT belong to a conserved family of signal transduction
enzymes involved in multiple cellular biological processes
(25). The PI3K/AKT signaling
pathway is considered as an endogenous negative-feedback regulatory
mechanism that promotes cell survival in response to harmful
external stimuli (26). Numerous
previous studies have provided ample evidence suggesting that the
PI3K/AKT signaling pathway may serve a critical role in the
pathological progression of MIRI (27–29).
The activated form of AKT, p-AKT, regulates numerous
apoptosis-related mediators; for example, once activated, p-AKT
acts on intrinsic cell death pathways through Bcl-2 family members
(Bcl-2, Bcl-xl, Bax and Bad), as well as extrinsic cell death
pathways through caspase family members (cleaved caspase-3,
caspase-8 and caspase-9) (30). In
addition, PI3K/AKT signaling has also been identified to affect
several inflammatory-related regulators, such as high mobility
group box 1, TNF-α, NF-κB and IL-6 (10,31).
ICAII has been reported to affect the PI3K/AKT signaling pathway;
Luo et al (32) reported
that ICAII promoted the osteogenic differentiation of canine bone
marrow-derived stem cells by activating the PI3K/AKT signaling
pathway. In addition, ICAII exerted an antitumor effect in melanoma
and epidermoid carcinoma cells through the activation of the
PI3K/AKT signaling pathway (33,34).
In the present study, the pretreatment with ICAII not only
alleviated inflammation and inhibited the apoptosis induced by IR
injury, but it also upregulated the expression levels of p-PI3K and
p-AKT. To further verify the causal relationship between the
activation of the PI3K/AKT signaling pathway and ICAII, LY294002
(an inhibitor of the PI3K/AKT signaling pathway) was
co-administered at the onset of reperfusion. Interestingly, both
the cardioprotective effect of ICAII and the upregulated expression
levels of p-PI3K and p-AKT were significantly reversed in the
presence of LY294002.
In conclusion, the findings of the present study
suggested that the pretreatment with ICAII may alleviate MIRI by
activating the PI3K/AKT signaling pathway, thereby attenuating
inflammatory reactions and myocardial apoptosis. Nonetheless, the
present study had several limitations; for example, in addition to
anti-inflammatory and anti-apoptotic bioactivities, ICAII has been
discovered to possesses antioxidant and autophagy regulatory
properties (12,35). These pharmacological activities may
be involved in the underlying protective mechanism of ICAII in
MIRI; however, these cardioprotective effects of ICAII were not
further investigated in vitro in the present study, thus
further investigations are required to determine the involvement of
these functions of ICAII in MIRI. Moreover, in addition to the
PI3K/AKT signaling pathway, several other signaling pathways have
been reported to be involved in the pathological process of MIRI.
For instance, the activation of the STAT3 signaling pathway and the
mTOR signaling pathway alleviated myocardial IR injury through
suppressing cell apoptosis (36,37);
and the activation of the toll-like receptor 4/NF-κB and mitogen
activated protein kinase signaling pathways exacerbated IR injury
through facilitating inflammatory reactions and oxidative stress
(38,39). In addition, hypoxia-inducible
factor-1α/Bcl-2-interacting-protein 3-induced autophagy was
discovered to serve a protective role during MIRI (40), while glycogen synthase kinase-3β
signaling protected cardiomyocytes from hypoxia-reoxygenation
injury by preventing calcium overload (41). Therefore, further studies are
warranted to determine whether there are any other signaling
pathways involved in the protective effects of ICAII in MIRI.
Altogether, the findings of the present study indicated that ICAII
may be a promising therapeutic option for the treatment of
MIRI.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
BG, YZ and FA made substantial contributions to the
conception and design of the study; BG and XD acquired the data;
CC, QH, JS and DZ analyzed and interpreted the data; BG and QH were
involved in drafting the manuscript; and YZ and FA revised the
manuscript for critically important intellectual content. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
All experimental procedures were approved by The
Animal Care and Use Committee of Hubei University (approval no.
20190210; Wuhan, China) and were performed in accordance with the
Institutional Guidelines and Guide for the Care and Use of
Laboratory Animals (National Institutes for Health publication no.
85-23, revised 1996 edition).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CK-MB
|
creatine kinase-myocardial band
|
|
ICAII
|
icariside II
|
|
IL-6
|
interleukin-6
|
|
LAD
|
left anterior descending coronary
artery
|
|
LDH
|
lactate dehydrogenase
|
|
LVEF
|
left ventricular ejection fraction
|
|
MIRI
|
myocardial ischemia and reperfusion
injury
|
|
TNF-α
|
tumor necrosis factor-α
|
References
|
1
|
Lee SJ, Lee CK, Kang S, Park I, Kim YH,
Kim SK, Hong SP, Bae H, He Y, Kubota Y and Koh GY: Angiopoietin-2
exacerbates cardiac hypoxia and inflammation after myocardial
infarction. J Clin Invest. 120:3151–5033. 2018.
|
|
2
|
Corrrell CU, Robinson DG, Schooler NR,
Brunette MF, Mueser KT, Rosenheck RA, Marcy P, Addington J, Estroff
SE, Robinson J, et al: Cardiometabolic risk in patients with
first-episode schizophrenia spectrum disorders: Baseline results
from the RAISE-ETP study. JAMA Psychiatry. 71:1350–1363. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Maznyczka AM, Oldroyd KG, McCartney P,
McEntegart M and Berry C: The potential use of the index of
microcirculatory resistance to guide stratification of patients for
adjunctive therapy in acute myocardial infarction. JACC Cardiovasc
Interv. 12:951–966. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kimura T, Tajiri K, Sato A, Sakai S, Wang
Z, Yoshida T, Uede T, Hiroe M, Aonuma K, Ieda M and Imanaka-Yoshida
K: Tenascin-C accelerates adverse ventricular remodeling after
myocardial infarction by modulating macrophage polarization.
Cardiovasc Res. 115:614–624. 2018. View Article : Google Scholar
|
|
5
|
Bi X, Zhang G, Wang X, Nguyen C, May HI,
Li X, Al-Hashimi AA, Austin RC, Gillette TG, Fu G, et al:
Endoplasmic reticulum chaperone GRP78 protects heart from
Ischemia/Reperfusion injury through akt activation. Circ Res.
122:1545–1554. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shah M, Patil S, Patel B, Agarwal M,
Davila CD, Garg L, Agrawal S, Kapur NK and Jorde UP: Causes and
predictors of 30-day readmission in patients with acute myocardial
infarction and cardiogenic shock. Circ Heart Fail. 11:e0043102018.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhou H, Ma Q, Zhu P, Ren J, Reiter RJ and
Chen Y: Protective role of melatonin in cardiac
ischemia-reperfusion injury: From pathogenesis to targeted therapy.
J Pineal Res. 64:2018. View Article : Google Scholar
|
|
8
|
Yang Y, Lv J, Jiang S, Ma Z, Wang D, Hu W,
Deng C, Fan C, Di S, Sun Y and Yi W: The emerging role of Toll-like
receptor 4 in myocardial inflammation. Cell Death Dis. 7:e22342016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhao D, Feng P, Sun Y, Qin Z, Zhang Z, Tan
Y, Gao E, Lau WB, Ma X, Yang J, et al: Cardiac-derived CTRP9
protects against myocardial ischemia/reperfusion injury via
calreticulin-dependent inhibition of apoptosis. Cell Death Dis.
9:7232018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li X, Hu X, Wang J, Xu W, Yi C, Ma R and
Jiang H: Short-Term hesperidin pre-treatment attenuates rat
myocardial Ischemia/Reperfusion injury by inhibiting high mobility
group box 1 protein expression via the PI3K/Akt pathway. Cell
Physiol Biochem. 39:1850–1862. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li H, Xu Y, Guan R, Matheu M, Lei H, Tian
W, Gao Z, Lin G, Guo Y, Xin Z and Song W: Icariside II prevents
high-glucose-induced injury on human cavernous endothelial cells
through Akt-eNOS signaling pathway. Andrology. 3:408–416. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Deng Y, Long L, Wang K, Zhou J, Zeng L, He
L and Gong Q: Icariside II, a Broad-Spectrum anti-cancer agent,
reverses Beta-Amyloid-Induced cognitive impairment through reducing
inflammation and apoptosis in rats. Front Pharmacol. 8:392017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu Y, Qian Z, Fu S, Yue Y, Li Y, Sun R,
Huang B and Yang D: IcarisideII improves left ventricular
remodeling in spontaneously hypertensive rats by inhibiting the
ASK1-JNK/p38 signaling pathway. Eur J Pharmacol. 819:68–79. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang L, Peng C, Xia J, Zhang W, Tian L,
Tian Y, Yang X and Cao Y: Effects of icariside II ameliorates
diabetic cardiomyopathy in streptozotocin-induced diabetic rats by
activating Akt/NOS/NF-κB signaling. Mol Med Rep. 17:4099–4105.
2018.PubMed/NCBI
|
|
15
|
Rani N, Bharti S, Manchanda M, Nag TC, Ray
R, Chauhan SS, Kumari S and Arya DS: Regulation of heat shock
proteins 27 and 70, p-Akt/p-eNOS and MAPKs by Naringin Dampens
myocardial injury and dysfunction in vivo after
ischemia/reperfusion. PLoS One. 8:e825772013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Deng Y, Xiong D, Yin C, Liu B, Shi J and
Gong Q: Icariside II protects against cerebral ischemia-reperfusion
injury in rats via nuclear factor-κB inhibition and peroxisome
proliferator- activated receptor up-regulation. Neurochem Int.
96:56–61. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Guide for the Care and Use of Laboratory
Animals Institute of Laboratory Animal Research. Commission on Life
Sciences, National Research Council. 1401996.ISBN:0-309-58869-3,
6×9.
|
|
18
|
Valle RJ, Mauro AG, Devarakonda T,
Marchetti C, He J, Kim E, Filippone S, Das A, Toldo S, Abbate A and
Salloum FN: Reperfusion therapy with recombinant human relaxin-2
(Serelaxin) attenuates myocardial infarct size and NLRP3
inflammasome following ischemia/reperfusion injury via
eNOS-dependent mechanism. Cardiovasc Res. 113:609–619.
2017.PubMed/NCBI
|
|
19
|
Zheng Y, Deng Y, Gao JM, Lv C, Lang LH,
Shi JS, Yu CY and Gong QH: Icariside II inhibits
lipopolysaccharide-induced inflammation and amyloid production in
rat astrocytes by regulating IKK/IκB/NF-κB/BACE1 signaling pathway.
Acta Pharmacol Sin. 41:154–162. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fu S, Li YL, Wu YT, Yue Y, Qian ZQ and
Yang DL: Icariside II attenuates myocardial fibrosis by inhibiting
nuclear factor-κB and the TGF-β1/Smad2 signaling pathway in
spontaneously hypertensive rats. Biomed Pharmacother. 100:64–71.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yan BY, Pan CS, Mao XW, Yang L, Liu YY,
Yan L, Mu HN, Wang CS, Sun K, Liao FL, et al: Icariside II improves
cerebral microcirculatory disturbance and alleviates hippocampal
injury in gerbils after ischemia-reperfusion. Brain Res.
1573:63–73. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu JY, Shang J, Mu XD and Gao ZY:
Protective effect of down-regulated microRNA-27a mediating high
thoracic epidural block on myocardial ischemia-reperfusion injury
in mice through regulating ABCA1 and NF-κB signaling pathway.
Biomed Pharmacother. 112:1086062019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang J, Guo X, Yang J, Ding JW, Li S, Yang
R, Fan ZX and Yang CJ: RP105 protects against apoptosis in
ischemia/reperfusion-induced myocardial damage in rats by
suppressing TLR4-mediated signaling pathways. Cell Physiol Biochem.
36:2137–2148. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang BF, Jiang H, Chen J, Guo X, Li Y, Hu
Q and Yang S: Nobiletin ameliorates myocardial ischemia and
reperfusion injury by attenuating endoplasmic reticulum
stress-associated apoptosis through regulation of the PI3K/AKT
signal pathway. Int Immunopharmacol. 73:98–107. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yu JS and Cui W: Proliferation, survival
and metabolism: The role of PI3K/AKT/mTOR signaling in pluripotency
and cell fate determination. Development. 143:3050–3060. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Williams DL, Ozment-Skelton T and Li C:
Modulation of the phosphoinositide 3-kinase signaling pathway
alters host response to sepsis, inflammation, and
ischemia/reperfusion injury. Shock. 25:432–439. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hao YL, Fang HC, Zhao HL, Li XL, Luo Y, Wu
BQ, Fu MJ, Liu W, Liang JJ and Chen XH: The role of microRNA-1
targeting of MAPK3 in myocardial ischemia-reperfusion injury in
rats undergoing sevoflurane preconditioning via the PI3K/Akt
pathway. Am J Physiol Cell Physiol. 315:C380–C388. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu S, Ai Q, Feng K, Li Y and Liu X: The
cardioprotective effect of dihydromyricetin prevents
ischemia-reperfusion-induced apoptosis in vivo and in vitro via the
PI3K/Akt and HIF-1alpha signaling pathways. Apoptosis.
21:1366–1385. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li Q, Shen L, Wang Z, Jiang HP and Liu LX:
Tanshinone IIA protects against myocardial ischemia reperfusion
injury by activating the PI3K/Akt/mTOR signaling pathway. Biomed
Pharmacother. 84:106–114. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zeng C, Jiang W, Zheng R, He C, Li J and
Xing J: Cardioprotection of tilianin ameliorates myocardial
ischemia-reperfusion injury: Role of the apoptotic signaling
pathway. PLoS One. 13:e1938452018.
|
|
31
|
Pan T, Shi X, Chen H, Chen R, Wu D, Lin Z,
Zhang J and Pan J: Correction to: Geniposide suppresses
interleukin-1β-induced inflammation and apoptosis in rat
chondrocytes via the PI3K/Akt/NF-κB signaling pathway.
Inflammation. 42:404–405. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Luo G, Xu B and Huang Y: Icariside II
promotes the osteogenic differentiation of canine bone marrow
mesenchymal stem cells via the PI3K/AKT/mTOR/S6K1 signaling
pathways. Am J Transl Res. 9:2077–2087. 2017.PubMed/NCBI
|
|
33
|
Wu J, Xu J, Eksioglu EA, Chen X, Zhou J,
Fortenbery N, Wei S and Dong J: Icariside II induces apoptosis of
melanoma cells through the downregulation of survival pathways.
Nutr Cancer. 65:110–117. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wu J, Zuo F, Du J, Wong PF, Qin H and Xu
J: Icariside II induces apoptosis via inhibition of the EGFR
pathways in A431 human epidermoid carcinoma cells. Mol Med Rep.
8:597–602. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gao J, Long L, Xu F, Feng L, Liu Y, Shi J
and Gong Q: Icariside II, a phosphodiesterase 5 inhibitor,
attenuates cerebral ischaemia/reperfusion injury by inhibiting
glycogen synthase kinase-3β-mediated activation of autophagy. Br J
Pharmacol. 177:1434–1452. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cao X, Zhu N, Zhang Y, Chen Y, Zhang J, Li
J, Hao P, Gao C and Li L: Y-box protein 1 promotes
hypoxia/reoxygenation- or ischemia/reperfusion-induced
cardiomyocyte apoptosis via SHP-1-dependent STAT3 inactivation. J
Cell Physiol. Jan 22–2020.doi: 10.1002/jcp.29474 (Online ahead of
print). View Article : Google Scholar
|
|
37
|
Li CY, Yang P, Jiang YL, Lin Z, Pu YW, Xie
LQ, Sun L and Lu D: Ginsenoside Rb1 attenuates cardiomyocyte
apoptosis induced by myocardial ischemia reperfusion injury through
mTOR signal pathway. Biomed Pharmacother. 125:1099132020.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang L, Liu XH, Chen H, Chen ZY, Weng XD,
Qiu T and Liu L: Picroside II protects rat kidney against
ischemia/reperfusion-induced oxidative stress and inflammation by
the TLR4/NF-κB pathway. Exp Ther Med. 9:1253–1258. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liu K, Wang F, Wang S, Li WN and Ye Q:
Mangiferin attenuates myocardial ischemia-reperfusion injury via
MAPK/Nrf-2/HO-1/NF-κB in vitro and in vivo. Oxid Med Cell Longev.
2019:72854342019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang Y, Liu D, Hu H, Zhang P, Xie R and
Cui W: HIF-1α/BNIP3 signaling pathway-induced-autophagy plays
protective role during myocardial ischemia-reperfusion injury.
Biomed Pharmacother. 120:1094642019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
He X, Li S, Fang X and Liao Y: TDCPP
protects cardiomyocytes from hypoxia-reoxygenation injury induced
apoptosis through mitigating calcium overload and promotion GSK-3β
phosphorylation. Regul Toxicol Pharmacol. 92:39–45. 2018.
View Article : Google Scholar : PubMed/NCBI
|