Introduction
Postmenopausal osteoporosis (PMO), which results
from estrogen deficiency, is the most common type of osteoporosis
and is a systemic skeletal disease that is characterized by low
bone mass and micro-architectural deterioration of bone tissues
(1). Reduced ovarian production of
estrogen after menopause causes an initial phase of rapid bone loss
in women, with an annual bone loss rate of 3–5% for 5–10 years,
which mainly affects the trabecular bone (2). Estrogen replacement therapy is the
first line therapy in treating PMO (3); however, side effects such as
cardiovascular diseases, cerebrovascular diseases, and invasive
breast cancer offsets the beneficial effects on fracture and pain
(4). Other treatments, such as
bisphosphonates, denosumab and teriparatide, showed efficacy in
treating PMO, but extended treatment is challenging for a portion
of the population of patients with PMO due to gastrointestinal
disturbance or high price (5,6).
Acupuncture, which is one of the most popular complementary and
alternative therapies in Western countries, has been shown to be
effective in improving osteoporosis in several clinical trials
(7). Electroacupuncture (EA) is
widely used in clinics and is characterized by its precise and
reproducible electrical stimulation. EA has shown benefits for rats
with ovariectomy-induced osteoporosis by increasing bone mineral
density (BMD) (8). The possible
mechanisms by which acupuncture has improved BMD may be related to
the regulation of osteoblasts and osteoclasts. Runt-related
transcription factor 2 (RUNX2) is a key factor in the process of
differentiation of mesenchymal stem cells into osteoblasts
(9), while receptor activator of
nuclear factor-κB (RANK) ligand (RANKL) has a vital role in the
differentiation of macrophages into osteoclasts (10). EA can upregulate the expression of
RUNX2 and downregulate the expression of RANK and RANKL, these
EA-induced changes activated the formation of osteoblasts and
inhibited the formation of osteoclasts (11). Nevertheless, the intrinsic
mechanism by which EA regulates these two signaling pathways
remains unclear.
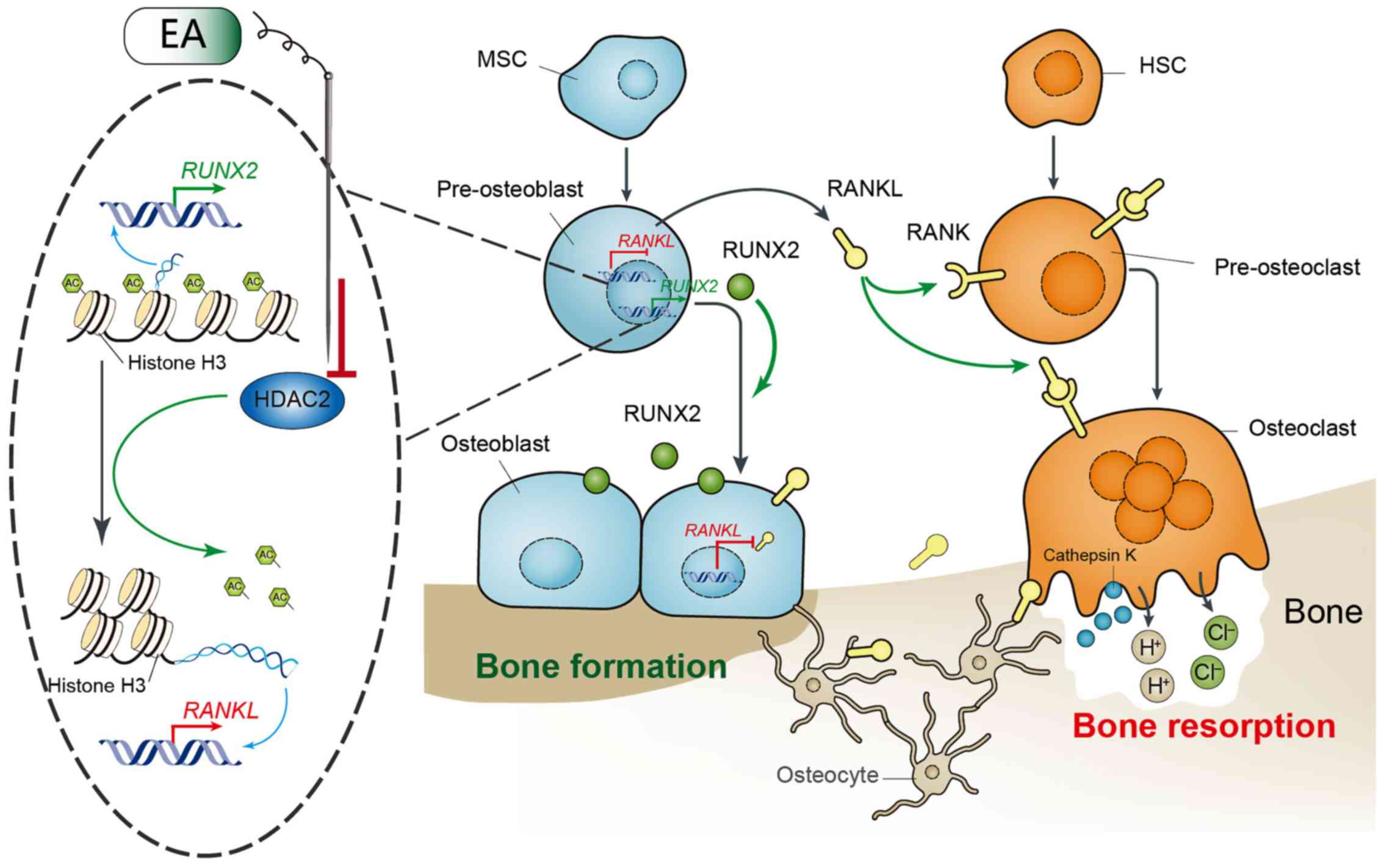
Epigenetics is a branch of genetics that addresses
the heritable changes in gene expression that does not involve
changes in the nucleotide sequence. It mainly includes a large
number of histone changes, such as methylation, acetylation and
phosphorylation, and these are known to alter gene expression
(12). Histones are basic proteins
that are present in the nucleus of all eukaryotes and bind to DNA.
The acetylation of histones, which is regulated by histone
deacetylases (HDACs), is an important mechanism that affects
chromatin conformation and regulates gene transcription. Histone
acetylation usually relaxes chromatin structure, and allows
transcription factors and RNA polymerase II to access DNA, which
promotes gene transcription (13).
In this way, it is associated with the promotion of gene
transcription, while histone deacetylation induces compaction of
the chromatin and is responsible for blocking gene expression
(14). HDAC2, which is widely
expressed in bone tissues, activates Akt and thus suppresses FoxO1
transcription, which results in RANKL-induced osteoclastogenesis
(15). In addition, HDAC2 has been
found to modulate the master transcription factor RUNX2, which is
essential in chondrocyte and osteoblast transformation (16). Acupuncture has been shown to
regulate the level of histone acetylation by inhibiting the
activity of HDAC2 (17). Based on
this finding, it was hypothesized that the mechanism by which EA
suppresses bone loss may be related to the acetylation modification
of histone H3 by HDAC2. In the current study four groups were
established: The sham operation group (SHAM), ovariectomy-induced
osteoporosis group (OVX), EA and 17β-estradiol (E2) treatment
groups, and the mechanism was investigated.
Materials and methods
Animals models and allocation
A total of 40 female Sprague-Dawley rats (age: 6
month; weight: 315–365 g) were acquired from Vital River
Laboratories Co., Ltd., (cat. no. 11400700298971). All rats were
placed in a controlled environment at 22±2°C and 50±10% relative
humidity with a 12-h light/dark cycle in the Experimental Animal
Center, Zhongnan Hospital of Wuhan University, with standard food
and water provided ad libitum for the duration of the study.
After acclimation to the facility for 1 week, all rats were given
random ID numbers and randomly allocated to four groups using SPSS
20.0 software. All the rats underwent either SHAM surgery with
intact ovaries (n=10) or bilateral ovariectomy (OVX, n=30). OVX
rats were randomly divided into an OVX control group (n=10), EA
treatment group (n=10) or E2 treatment group (n=10).
The OVX rats were anesthetized with isoflurane
(3-3.5% for anesthetic induction, 1.8-2.2% for maintenance of
anesthesia), after which both ovaries were removed. The SHAM group
underwent surgery where the fat tissue near the ovaries was
resected. Following surgery, rats received 9.75 mg/kg of gentamicin
by intramuscular injection each day for 3 days to prevent
infection.
Electroacupuncture treatment
Prior to treatment with EA, rats in the EA group
were restrained using an instrument made specifically for this
experiment to keep them calm (18). Acupoints of Shen Shu (BL23) and Pi
Shu (BL20) were subjected to EA by using 0.35×20 mm acupuncture
needles (Global; Suzhou Acupuncture Goods Co., Ltd.) according to
current standards (19), briefly:
i) BL20 is located at the depression lateral to the lower border of
the spinous process of the twelfth thoracic vertebra (~0.8 cm in
the adult rat, approximately one twentieth of the circumference of
the thorax). ii) BL23 is located at the depression lateral to the
lower border of the spinous process of the second lumbar vertebra
(~0.8 cm in the adult rat, approximately one twentieth of the
circumference of the thorax).
Bilateral BL20 and BL23 were linked with two
electrodes of a Han's Acupoint Nerve Stimulator (HANS LH202H,
Neuroscience Research Center, Peking University, Beijing, China).
The two acupoints were stimulated with successive waves of 2 Hz,
and the intensity varied from 0.5–1 mA, which produce local muscle
contractions. Rats in the EA group received EA treatment for 10 min
every other day for 8 weeks (20,21),
while other groups that did not receive EA were restrained at that
time.
E2 intragastric treatment
Rats in the E2 treatment group received E2
intragastric administration with 100 µg/kg E2 (20 µg/ml) at the
same time as the EA group underwent treatment for 8 weeks.
Hematoxylin and eosin (H&E) and
tartrate-resistant acid phosphatase (TRACP) staining
Caput femoris were randomly selected from each group
and fixed with a 10% ethylene diamine tetraacetic acid solution (pH
7.4) at 4°C for 7 weeks. H&E staining was performed on the
paraffin sections cut into 5 µm sections. Differences in morphology
of the H&E-stained sections of caput femoris were examined,
without any morphometric calculation, such as trabecular thickness
and trabecular number. TRACP staining was performed with a standard
immunohistochemical protocol. Sections were blocked in 0.1% goat
serum (cat. no. AR0009, Wuhan Boster Biological Technology, Ltd.)
for 30 min at room temperature, then incubated at 4°C overnight
with mouse anti-rat TRACP primary antibody (1:100; cat. no.
NBP2-45294; Novus Biologicals, LLC). Finally, a commercial
immunohistochemistry kit (cat. no. K5007; Dako; Agilent) including
horseradish peroxidase-conjugated anti-mouse secondary antibody and
DAB color development kit was used to visualize positive
expression, labeling osteoclasts as brown-labelled cells.
Micro-CT bone analysis
Three rats were randomly selected from each group.
After abdominal anesthesia with isoflurane and sacrifice by
cervical dislocation, the right femur was bluntly dissected to
avoid bleeding. All 12 right femurs were imaged on a micro-CT
system (SkyScan 1176; Bruker Biospin) according to the
manufacturer's protocol to scan the proximal femur with a spatial
resolution of 9 µm (X-ray source 74 kV, 338 µA; 12.59 µm filter
applied). The distance between each slide was 9 µm. After a short
offset of the first 191 slides from the reference slide, 2,284
continuous slides of CT scan images were included for the
trabecular region selection in the proximal femur. The trabecular
region of interest was selected in an unbiased analysis using
CT-Analyzer software (version 1.15, Bruker) with the suggested
order set. The trabecular analysis was performed to quantify BMD
and morphometric calculations. All radiographic studies were
conducted and quantified blindly with unbiased algorithms and
automatic calculations by an independent radiologist. The
trabecular metric parameters measured included cortical BMD,
trabecular BMD, bone volumetric fraction (BV/TV), trabecular
thickness (Tb Th), trabecular separation (Tb.Sp), and trabecular
number (Tb.N). Trabecular bone pattern factor (Tb.Pf), which is an
inverse measure of trabecular connectivity, and the structural
model index (SMI), which estimates the prevalence of rod-like or
plate-like trabecula, were also calculated to assess the trabecular
morphology.
Biomarkers of bone metabolism
After 8 weeks of intervention, five rats were
selected randomly from each group and anesthetized with isoflurane
as aforementioned. Serum was collected by cardiac puncture. The
level of serum estradiol (E2) was measured using a commercial
enzyme-linked immunosorbent assay (ELISA) kit (cat. no. E-EL-0065c;
Elabscience Biotechnology Co., Ltd.,). The levels of serum alkaline
phosphatase (ALP) and TRACP 5b were detected by a colorimetric
method using a commercial colorimetric assay kit (ALP: cat. no.
E-BC-K091, Elabscience Biotechnology Co., Ltd.; TRACP: cat. no.
A058, Nanjing Jiancheng Bioengineering Institute Co., Ltd.).
Western blotting
Rats were anesthetized and sacrificed by cervical
dislocation after 8 weeks of treatment. The femur was bluntly
dissected to avoid bleeding, frozen in liquid nitrogen, and stored
at −80°C. Caput femoris were cut off and ground into bone powder
and then homogenized in radioimmunoprecipitation assay buffer
(Beyotime Biotechnology Co., Ltd.). Protein supernatants were
quantified using the bicinchoninic acid (BCA) method. Denatured
proteins (30 µg) were separated on 12% sodium docecyl sulphate
polyacrylamide gels (Beyotime Biotechnology Co., Ltd.,).
Polyvinylidene fluoride (PVDF) membranes (Merck KGaA) were blocked
in phosphate-buffered saline (PBS) containing 0.1% v/v Tween-20
(TBST) and 5% w/v skimmed milk for 120 min at room temperature,
followed by overnight incubation at 4°C with primary antibodies
against HDAC2 (1:1,000; cat. no. 12922-3-ap; ProteinTech Group,
Inc.,), histone H3 (1:2,000, cat. no. 17168-1-ap; ProteinTech
Group, Inc.,), Ac-histone H3 (1:1,000, cat. no. Bs-3776r; Beijing
Biosynthesis Biotechnology Co., Ltd.,), RUNX2 (1:1,000, cat. no.
Bs-1134r; Beijing Biosynthesis Biotechnology Co., Ltd.,), and RANKL
(1:1,000, cat. no. Bs-0747r; Beijing Biosynthesis Biotechnology
Co., Ltd.,), Goat anti-rabbit and goat anti-mouse horseradish
peroxidase-conjugated IgG (1:5,000, cat. nos. BA1056 and BA1051;
Wuhan Boster Biological Technology Ltd.,) were used to detect
primary antibodies. Membranes were scanned and exposed to
photographic film. Optical density was semi-quantified by using
Glyco Bandscan software (Version 5.0; ProZyme). Protein expression
levels were calculated using the optical density (OD) ratio between
the target protein and β-actin loading control.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from rat caput femoris by
using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) and RNA concentrations were determined by the
absorbance ratio of 260/280 nm. Then, 1 µg of the extracted RNA was
reverse-transcribed into first-strand complementary DNA (cDNA)
using a ReverTra Ace® qPCR RT kit (cat. no. R101-01/02;
Vazyme Biotech Co., Ltd.). Gene expression of RUNX2 and RANKL were
quantified by using a SYBR-Green Real time PCR Master Mix Plus
(Q111-02; Vazyme Biotech Co., Ltd.) following the manufacturer's
protocols. Measurements were conducted in triplicate under standard
reaction conditions and normalized to β-actin. Primers were
obtained from TsingKe Biological Technology Co., Ltd. All
temperature cycling (two initial denaturation steps at 50°C for 2
min, then 95°C for 10 min, and followed by 40 cycles at 95°C 30 sec
and 60°C 30 sec for amplification) and gene amplification were
conducted in a QuantStudio™ 6 Real-Time PCR Detection System
(Thermo Fisher Scientific, Inc.). The primer sequences used are
presented in Table I.
 | Table I.Primer sequences |
Table I.
Primer sequences
| Primer name | Sequence | Size (bp) |
|---|
| Rat β-actin F |
5′-CACGATGGAGGGGCCGGACTCATC-3′ | 240 |
| Rat β-actin R |
5′-TAAAGACCTCTATGCCAACACAGT-3′ | 240 |
| Rat RUNX2 F |
5′-TCAGCGTCCTATCAGTTCCC-3′ | 174 |
| Rat RUNX2 R |
5′-ATTCAAAACGGTTGGGGAGC-3′ | 174 |
| Rat RANKL F |
5′-CATCGGGTTCCCATAAAGTC-3′ | 142 |
| Rat RANKL R |
5′-CTGAAGCAAATGTTGGCGTA-3′ | 142 |
Double-labeled immunofluorescence (IF)
staining
Double-labeled IF staining of Ac-histone H3/RUNX2,
histone H3/RANKL, RUNX2/ALP and RANKL/TRACP were performed in the 5
µm thick paraffin sections of the caput femoris. After antigen
retrieval, the sections were blocked in 0.1% goat serum [dissolved
in PBS with 0.02% Triton X-100 (Beyotime Biotechnology Co., Ltd.)
for 1 h at 37°C] and incubated at 4°C overnight with primary
antibodies against histone H3 (1:100; mouse; cat. no. bsm-33042 m;
BIOSS), Ac-histone-H3 (1:100, mouse, cat. no. bsm-33303 m), RUNX2
(1:100; Rabbit; cat. no. AF5186; Affinity Biosciences) and RANKL
(1:100; Rabbit; cat. no. ab62516; Abcam). Then sections were probed
with a Cy3-conjugated goat-anti-rabbit secondary antibody (1:50;
cat. no. BA1032; Wuhan Boster Biological Technology, Ltd.) or
Cy3-conjugated goat-anti-mouse secondary antibody (1:50; cat. no.
BA1031; Wuhan Boster Biological Technology, Ltd.) for 1 h at 37°C.
After blocking in goat serum a second time, the samples were
incubated at 4°C overnight with primary antibodies against RANKL
(1:100; Rabbit; cat. no. ab62516; Abcam), RUNX2 (1:100; Rabbit;
AF5186; Affinity Biosciences), ALP (1:100; cat. no. sc-36576;
mouse; Santa Cruz Biotechnology) and TRACP (1:100; cat. no.
NBP2-45294; mouse; Novus Biologicals, LLC) and then with
FITC-conjugated goat-anti-rabbit secondary antibody (1:50; cat. no.
BA1105; Wuhan Boster Biological Technology, Ltd.) or
FITC-conjugated goat-anti-mouse secondary antibody (1:50; cat. no.
BA1101; Wuhan Boster Biological Technology, Ltd.) for 1 h at 37°C.
Nuclei were stained with DAPI and images were acquired using an
Olympus BX53 digital fluorescence microscope (Olympus
Corporation).
Statistical analysis
Western blotting of the caput femoris in each group
were repeated three times and the OD of each target protein was
included in the statistical analysis. For IF, three sections were
randomly selected from each group for analysis. Optical density
analysis was performed on the immunofluorescence images using Image
Pro Plus software (version 6.0; Media Cybernetics, Inc.). Three
images at ×400 magnification were captured for each slice to
perform optical density analysis, and the mean density was
calculated according to integrated option density (IOD) and area in
each microscope view. The Pearson's correlation coefficient (PCC)
of the IOD of the two molecules in IF was calculated to confirm the
correlation. The difference in gene expression was calculated as
the gene expression in the treated group compared with that in the
control group, which were calculated using CFX Manager Software
version 3.0 (Bio-Rad Laboratories, Inc.). Analysis was performed by
blinded biostatisticians using SPSS software version 20.0 (SPSS
Statistics IBM Corp). The differences of trabecular parameters,
protein bands, optical density in IF and relative gene expression
among the four groups were analyzed by one-way ANOVA. To assess the
difference between two groups, post-hoc multiple comparisons with
the Tukey method were conducted if there were differences among all
four groups, the tests were two-sided. P<0.05 was considered to
indicate a statistically significant difference.
Results
EA improves the BMD and trabecular
morphology comparably to E2 in OVX rats
As shown in Fig.
1A, in the SHAM group, the caput femoris showed complete
structure in the trabecular and cortical bone, a considerable
number of trabeculae of normal thickness, and small separation
between trabeculae. However, in the OVX model group, the number of
abnormal trabeculae and amount of impaired cortical bone were
visibly increased and thickness was reduced, with frequent empty
bone lacunae. In the EA and E2 treatment groups, the caput femoris
showed better trabecular structure than in the OVX model group. The
E2 treatment group showed complete structure of cortical bone,
which was impaired in the EA treatment group. As shown in Fig. 1B, fewer TRACP-stained osteoclasts
were found in the SHAM group compared with the OVX group, while
there were less osteoclasts in the EA and E2 treatment groups than
in the OVX group.
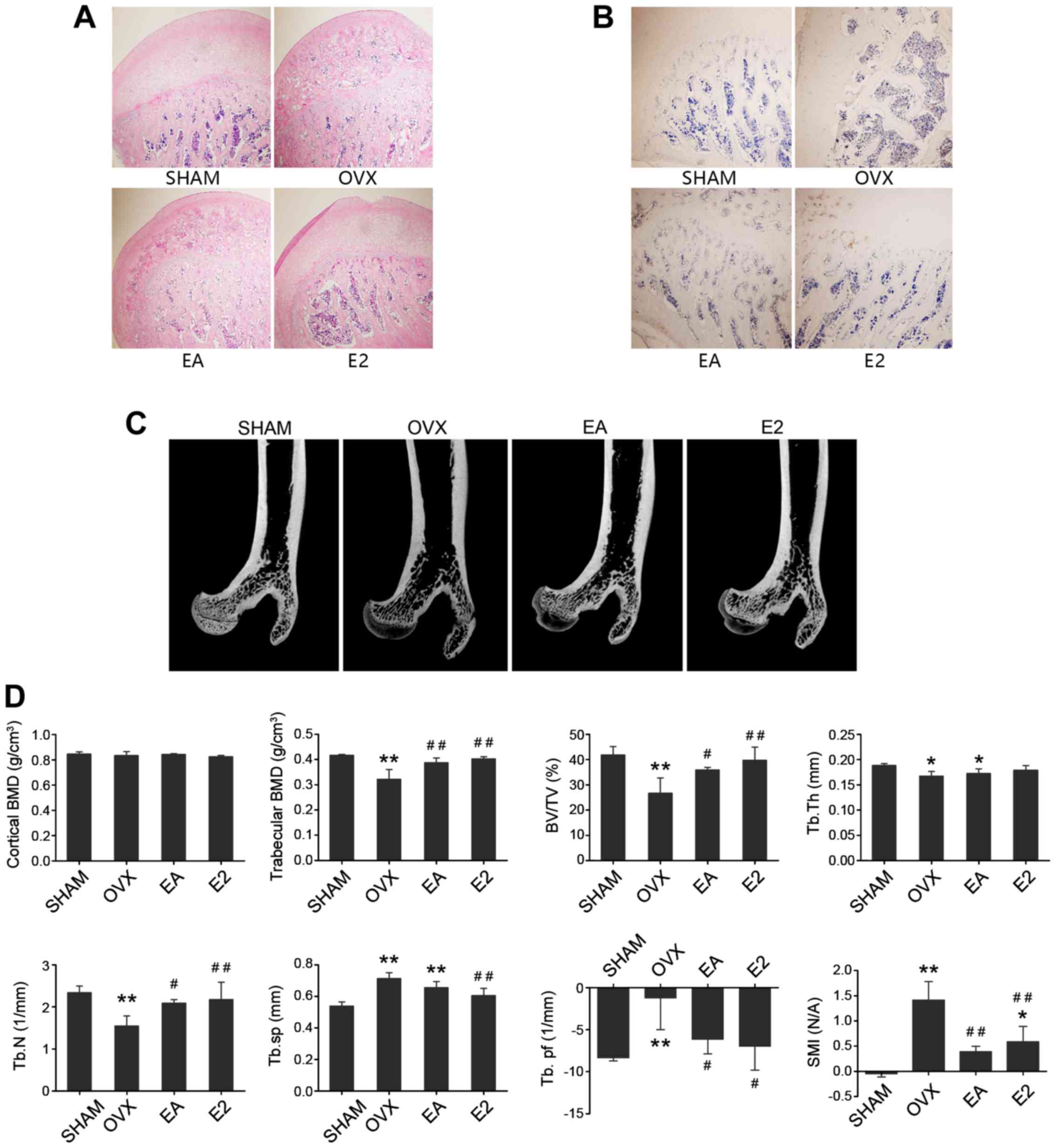 | Figure 1.Representative light micrographs of
H&E and TRACP-stained sections of caput femoris and comparison
of micro-CT and trabecular metric parameters of caput femoris. (A)
Representative images of H&E-stained sections of caput femoris
in SHAM group, OVX model group, EA and E2 treatment groups. (B)
Representative images of TRACP staining of caput femoris in SHAM
group, OVX model group, EA and E2 treatment groups. (C)
Representative images of micro-CT of caput femoris in SHAM group,
OVX model group, EA and E2 treatment groups. (D) Trabecular metric
parameters of caput femoris in SHAM group, OVX model group, EA and
E2 treatment groups. *P<0.05, **P<0.01, vs. SHAM;
#P<0.05, ##P<0.01, vs. OVX. SHAM, group
given sham surgery leaving the ovaries intact; OVX, bilateral
ovariectomy model group; EA, electroacupuncture treatment group;
E2, 17β-estradiol treatment control group; BMD, bone mineral
density; BV/TV, bone volumetric fraction; Tb.Th, trabecular
thickness; Tb.N, trabecular number; Tb.sp, trabecular separation;
Tb.pf, trabecular bone pattern factor; SMI, structural model
index. |
The caput femoris evaluated with micro-CT are shown
in Fig. 1C. After 8 weeks of
intervention, there was no notable loss of cortical bone from the
sagittal mid-section views of caput femoris after OVX. Compared
with the SHAM group, OVX rats showed a significant decrease in
trabecular bone BMD (P<0.01), while EA and E2 both spared this
trabecular bone mineral loss after OVX. In the OVX group, BV/TV,
Tb.Th and Tb.N were significantly lower, whereas Tb.Sp, Tb.Pf and
SMI were significantly higher compared with those in SHAM group.
Compared with the OVX model group, both EA and E2 treatment groups
increased the BV/TV and Tb.N significantly, but did not alter the
Tb.Th in caput femoris. The Tb.Pf and SMI were decreased after
treatment with EA or E2 (Fig. 1D).
All the results supported the hypothesis that EA mitigated the bone
loss from ovariectomy-induced osteoporosis in rats through the
regulation of the trabecular morphology, on par with E2
treatment.
EA improves biomarkers of bone
metabolism comparably with E2 in OVX rats
As shown in Fig.
2A, serum levels of TRACP 5b, which is indicative of the
activity of bone resorption, were increased after OVX (P<0.01).
After 8 weeks of treatment, TRACP 5b levels in the EA and E2
treatment groups were decreased, and treatment with E2 by
intragastric administration was shown to be significantly more
effective than treatment with EA. Levels of ALP and E2 were
decreased after OVX (P<0.01). After 8 weeks of treatment, serum
ALP and E2 in the EA and E2 treatment groups were increased, and no
significant differences were detected between the EA and E2
treatment groups (Fig. 2B and
C).
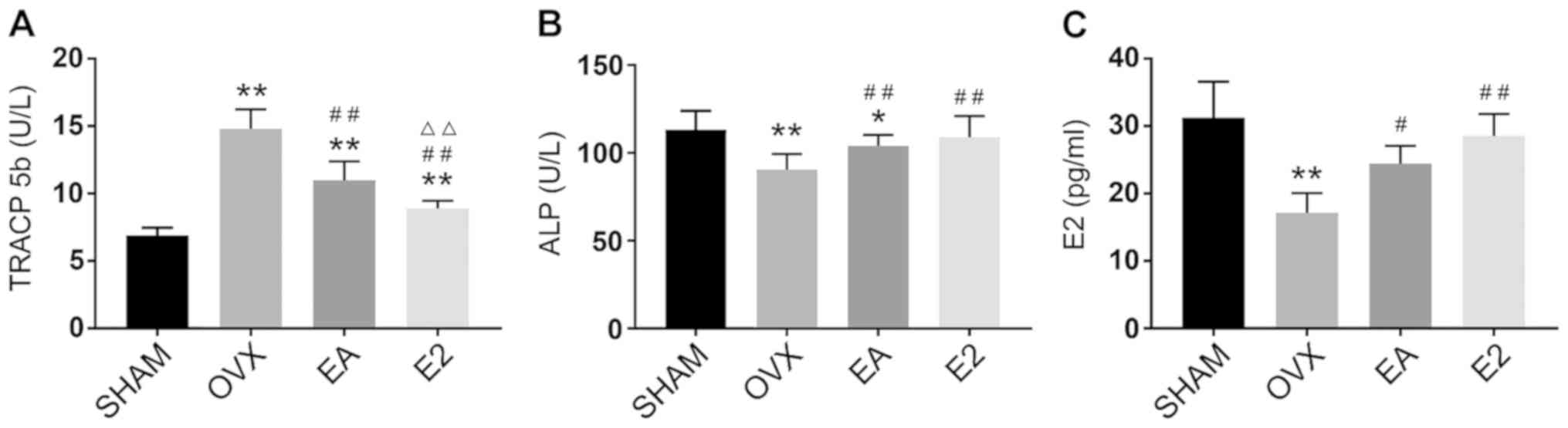 | Figure 2.Comparison of bone metabolism
biomarkers. (A) Serum TRACP levels in the SHAM, OVX model, EA and
E2 treatment groups (B) Serum ALP levels in the four groups. (C) E2
level of serum by colorimetric method in the four groups
*P<0.05, **P<0.01 vs. SHAM; #P<0.05,
##P<0.01 vs. OVX; ΔΔP<0.01 vs. EA.
SHAM, group given sham surgery leaving the ovaries intact; OVX,
bilateral ovariectomy model group; EA, electroacupuncture treatment
group; E2, 17β-estradiol treatment control group; TRACP,
tartrate-resistant acid phosphatase; ALP, alkaline phosphatase. |
EA affects the expression of RUNX2 and
RANKL by regulating the level of histone acetylation
As shown in Fig. 3,
following from OVX, HDAC2 protein expression levels in caput
femoris were upregulated, and this upregulation was suppressed
after treatment with EA (Fig. 3B).
Rats that received treatment with E2 intragastric administration
did not show changes in HDAC2 as compared with the OVX model group.
Protein expression of histone H3 showed a similar profile as HDAC2,
and was more highly expressed in the OVX model and E2 treatment
groups and less expressed in the SHAM group and EA treatment group
(Fig. 3C). By contrast, Ac-histone
H3 showed a higher expression in the SHAM group and lower
expression in the OVX model group. Ac-histone H3 levels were
restored following treatment with EA and were not significantly
different between the OVX model and E2 treatment groups (Fig. 3D), this indicated that EA can
mitigate OVX-suppressed histone H3 acetylation, while E2 has no
such effect. RUNX2 was highly expressed in the SHAM group compared
with the OVX model group. Compared with the OVX group, protein
expression of RUNX2 was upregulated by both the EA and E2 treatment
(Fig. 3E). RANKL was minimally
expressed in the SHAM group in comparison with the OVX group.
Compared with the OVX group, RANKL protein expression was
downregulated in both the EA and E2 groups, and RANKL protein
expression after E2 treatment was lower than that after EA
treatment (Fig. 3F).
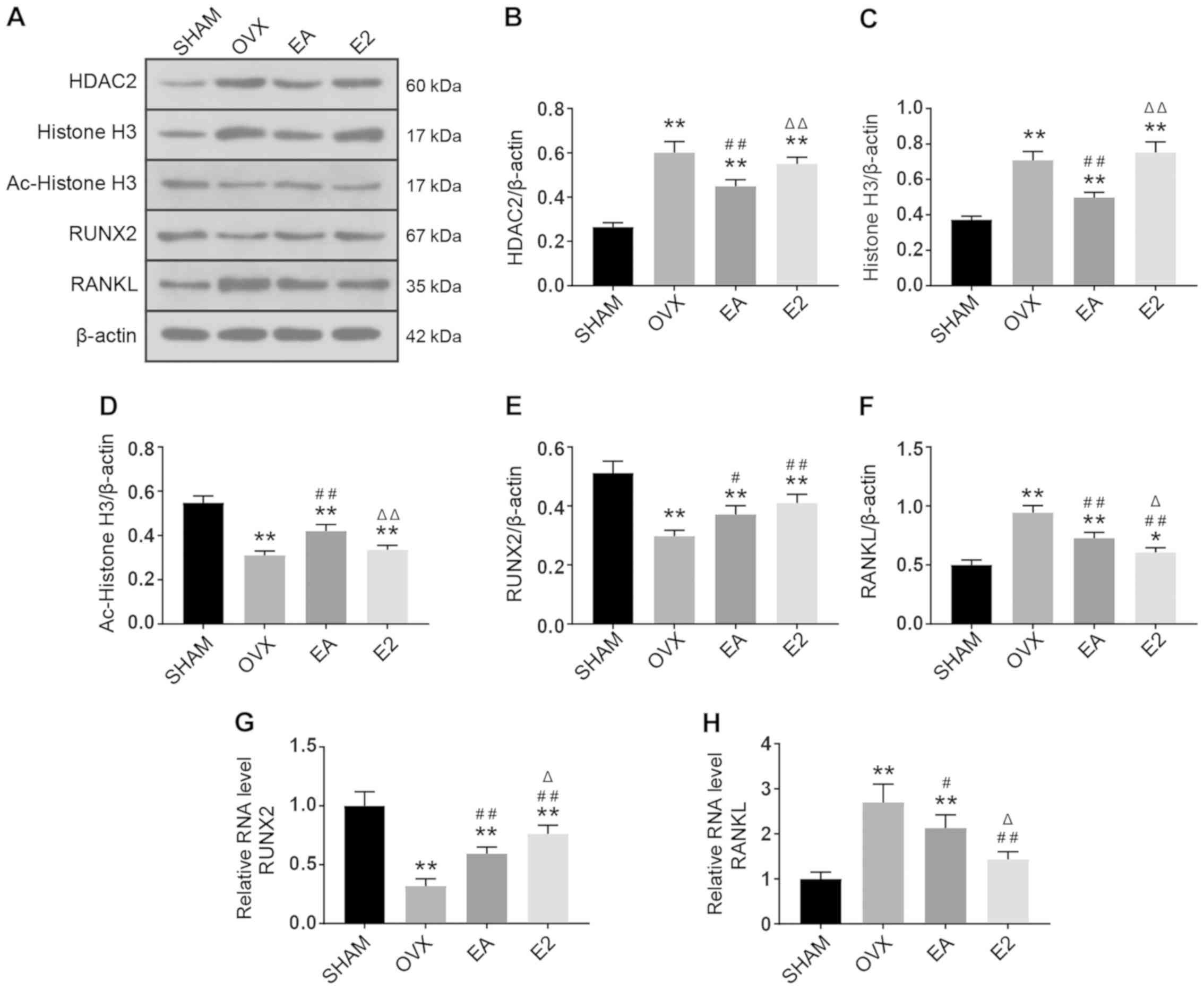 | Figure 3.Comparison of protein and gene
expression. (A) Representative western blot of HDAC2, histone H3,
Ac-histone H3, RUNX2, RANKL and β-actin proteins in the SHAM, OVX
model, EA and E2 treatment group; comparison of HDAC2 (B) histone
H3 (C) Ac-histone H3 (D) RUNX2 (E) RANKL (F) protein expression in
caput femoris of the four groups; comparison of (G) RUNX2 and (H)
RANKL gene expression in caput femoris of the four groups.
*P<0.05, **P<0.01 vs. SHAM; #P<0.05,
##P<0.01 vs. OVX; ΔP<0.05,
ΔΔP<0.01 vs. EA. SHAM, group given sham surgery
leaving the ovaries intact; OVX, bilateral ovariectomy model group;
EA, electroacupuncture treatment group; E2, 17β-estradiol treatment
control group; HDAC2, histone deacetylase 2; RUNX2, runt-related
transcription factor 2; RANKL, receptor activator of nuclear
factor-κB ligand. |
EA affects the gene expression of
RUNX2 and RANKL
As shown in Fig. 3G and
H, the RNA expression of RUNX2 was downregulated by OVX, and
was partially restored by treatment with either EA or E2. Compared
with E2, EA was less effective in promoting RUNX2 mRNA expression.
By contrast, RANKL mRNA was minimally expressed in the SHAM group
in comparison with the OVX group. After 8 weeks of treatment with
EA or E2, the mRNA expression of RANKL was suppressed in comparison
with the OVX group, with E2 being more effective.
EA-mediated acetylation of histone H3
regulates the differentiation of osteoblasts and osteoclasts
As shown in Fig. 4,
Ac-histone H3, RUNX2 and ALP, which represent the activity of
osteoblasts, were expressed throughout the caput femoris.
Ac-histone H3 showed far more expression in the SHAM group than in
the OVX group. After treatment with EA, the expression of
Ac-histone H3 was restored, and there was no significant difference
between the expressions of Ac-histone H3 in the E2 treatment group
vs. the OVX group. RUNX2 and ALP were highly expressed in the SHAM
group in comparison with the OVX group. After treatment with EA,
the expression of RUNX2 and ALP were partially restored in the EA
and E2 treatment groups. There were no significant differences
between the EA and E2 treatment groups in the expression of RUNX2
and ALP, while the expression levels of RUNX2 and ALP in both
groups were lower than those in the SHAM group. Correlation
analysis revealed that Histone/RANKL and RANKL/TRACP were positive
correlations, while the correlation of Histone/RANKL was weaker
than that of RANKL/TRACP. This may also be related to the inability
of E2 to regulate histone acetylation.
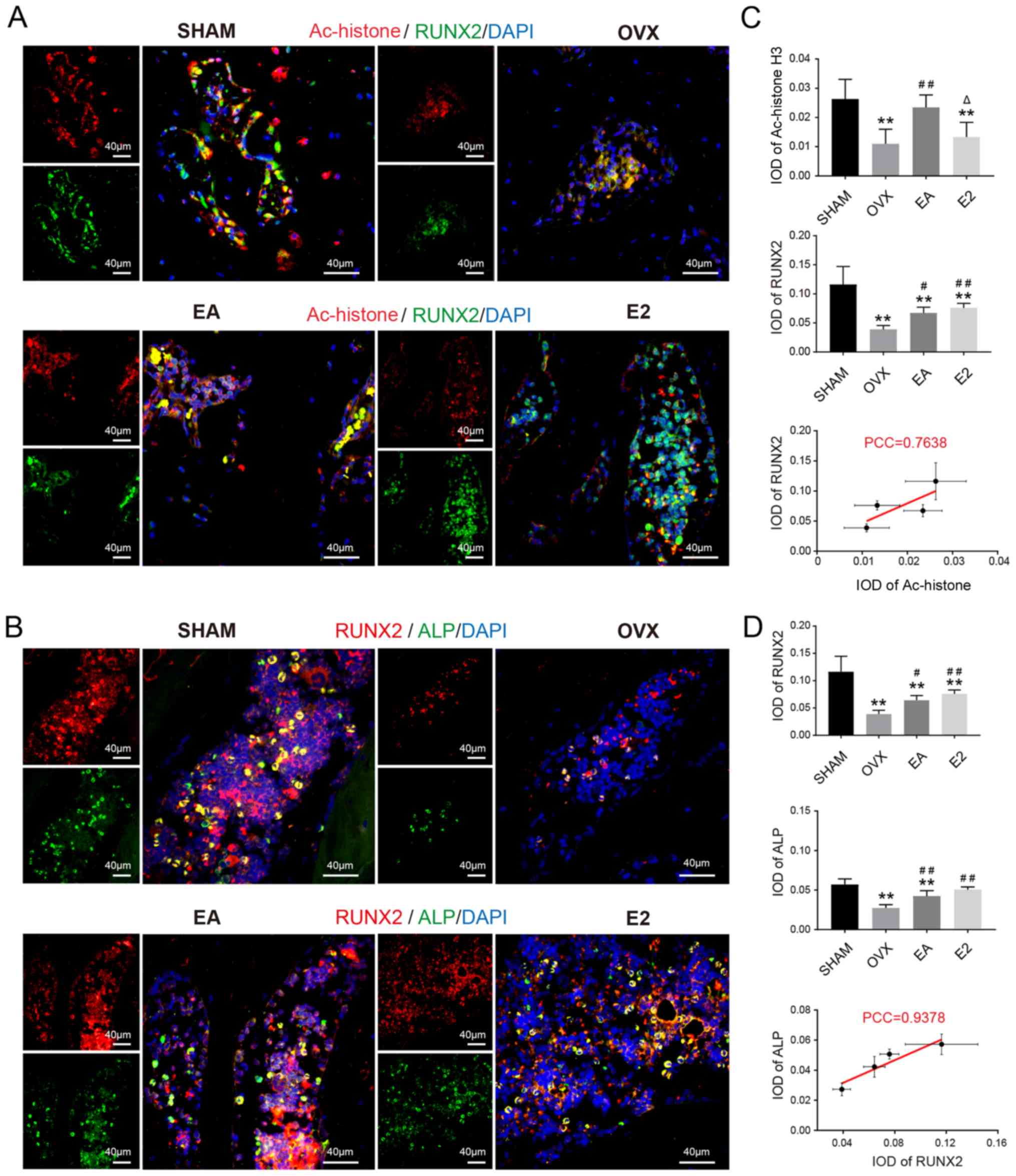 | Figure 4.Representative double-labeled
immunofluorescence images (A) Ac-histone H3 (red)/RUNX2 (green) and
nuclei (blue). (B) RUNX2 (red)/ALP (green) and nuclei (blue). IOD
from (C) Ac-histone H3/RUNX2 and (D) RUNX2/ALP. **P<0.01 vs.
SHAM; #P<0.05, ##P<0.01 vs. OVX;
ΔP<0.05 vs. EA. SHAM, group given sham surgery
leaving the ovaries intact; OVX, bilateral ovariectomy model group;
EA, electroacupuncture treatment group; E2, 17β-estradiol treatment
control group; PCC, Pearson correlation coefficient; IOD,
integrated option density; Ac, acetylated; RUNX2, runt-related
transcription factor 2; ALP, alkaline phosphatase. |
As shown in Fig. 5,
histone H3, RANKL and TRACP, which reflect the activity of
osteoclasts, were expressed throughout the caput femoris. Histone
H3 was minimally expressed in the SHAM group as compared with the
OVX group. After treatment with EA, histone H3 was downregulated
relative to that in the OVX group. After administration of E2,
histone H3 levels were similar to those in the OVX group. As
expected, based on the elevation of histone H3 after OVX, the
levels of RANKL and TRACP, which are derepressed by histone H3,
also increased after OVX. Treatment with EA or E2 mitigated these
increases in RANKL and TRACP, and treatment with E2 was more
effective than EA treatment. Correlation analysis revealed that
Ac-Histone/RUNX2, RUNX2/ALP had a positive correlation, and the
Histone/RUNX2 correlation was weaker than the RUNX2/ALP
correlation. This may be related to the inability of E2 to regulate
histone acetylation.
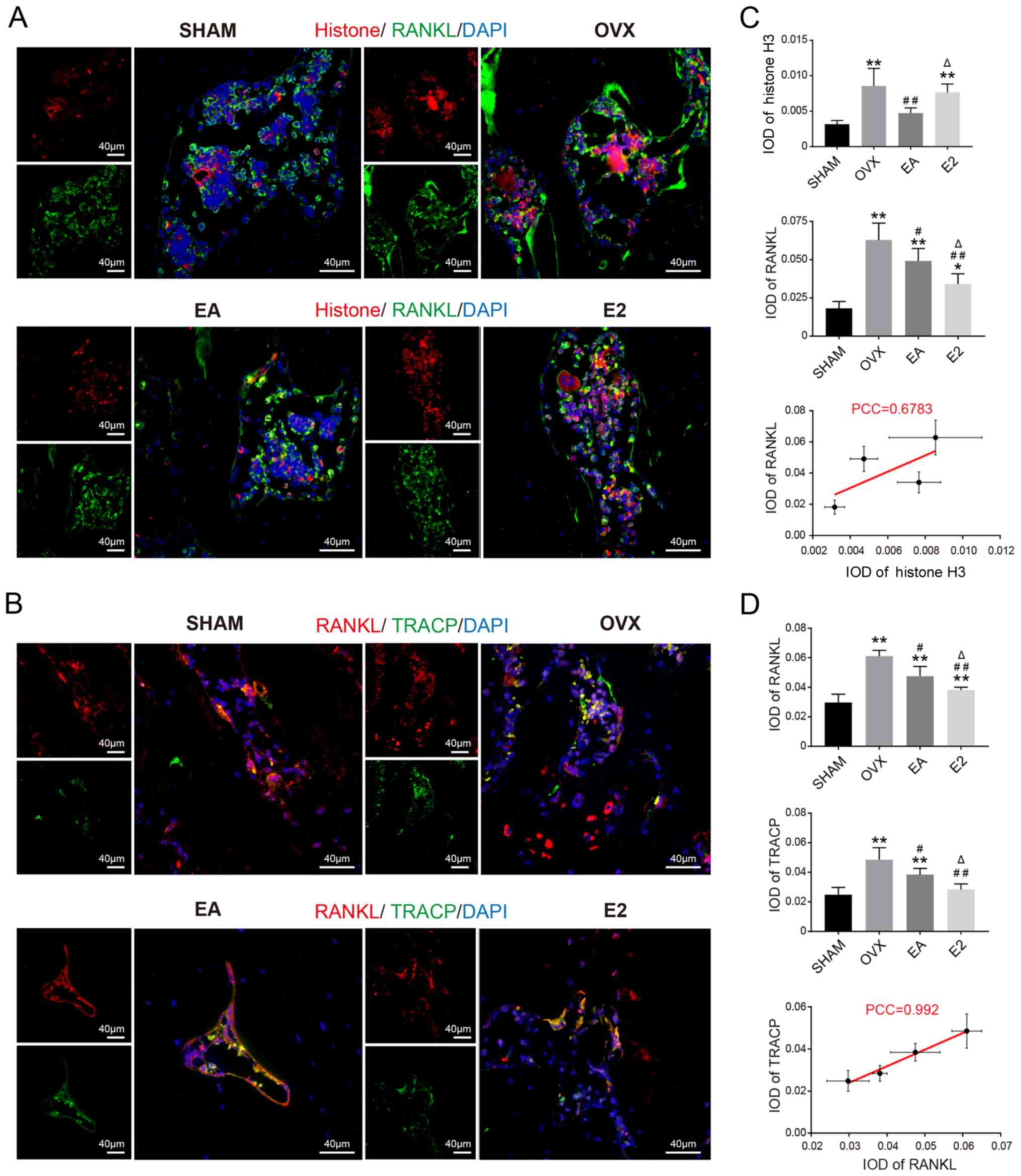 | Figure 5.Representative double-labeled
immunofluorescence images of (A) Histone H3 (red)/RANKL (green) and
nuclei (blue). (B) RANKL (red)/TRACP (green) and nuclei (blue). IOD
from (C) Histone H3/RANKL and (D) RANKL/TRACP. *P<0.05,
**P<0.01 vs. SHAM; #P<0.05, ##P<0.01
vs. OVX; ΔP<0.05 vs. EA. SHAM, group given sham
surgery leaving the ovaries intact; OVX, bilateral ovariectomy
model group; EA, electroacupuncture treatment group; E2,
17β-estradiol treatment control group; PCC, Pearson correlation
coefficient; IOD, integrated option density; RANKL, receptor
activator of nuclear factor-κB ligand; TRACP, tartrate-resistant
acid phosphatase. |
Discussion
Estrogen deficiency results in enhanced bone
resorption accompanied by impaired bone formation, which are the
main factors that affect BMD in patients with PMO. In the process
of bone cell differentiation, estrogen is involved in the formation
of osteoblasts and osteoclasts. RUNX2 is a transcription factor
that determines the osteoblast lineage from mesenchymal stem cells
via canonical Wnt signaling by blocking the inhibition of
chondrocyte differentiation (22).
Estrogen and selective estrogen receptor modulators induce RUNX2
mRNA expression and enhanced RUNX2 promoter activity and
play an important role in osteoblast differentiation (23,24).
RANKL, a type II transmembrane protein of the tumor necrosis factor
superfamily, is mainly produced by osteoblastic lineage cells
(mesenchymal stem cells, osteoblasts and osteocytes), and
transduces its signal by binding the receptor RANK on the membrane
of osteoclasts (25). This binding
activates nuclear factor of activated T-cells, cytoplasmic 1, the
master regulator of osteoclastogenesis, which induces the process
of osteoclast differentiation from monocyte/macrophage lineage
cells (10). Estrogen inhibits
osteoporosis by promoting osteoclastic apoptosis and suppressing
osteoclastogenesis (26). A
potential mechanism by which estrogen has these effects may be
related to the inhibitory effect of RANKL-induced osteoclastic
differentiation by increasing the expression of the transient
receptor potential cation channel subfamily V member 5 channel
(27). In addition, estrogen can
antagonize RUNX2-mediated osteoblast-driven osteoclastogenesis by
regulating RANKL membrane association (9). Based on these effects, hormone
replacement therapy (HRT) is still considered a first-line choice
for the prevention of bone loss and fracture in the early
postmenopausal period (28), and
HRT was included as a positive control in our study. However,
several side effects, including increased risk of endometrial
carcinoma, breast cancer and thromboembolism, limit the clinical
application of HRT (29).
According to the theory of epigenetics, the
regulation of downstream gene expression by regulating acetylation
of histones is characterized by effects on multiple gene targets
(13). Similarly, mechanisms of
acupuncture to prevent and treat diseases are related to the
regulation of various signaling pathways and molecules (30,31),
both these fields are characterized by pleotropic effects.
Recently, acupuncture has been shown to regulate the
hypothalamic-pituitary-ovarian axis (HPOA) in rats (32) and postmenopausal women (33), a finding which was widely applied
in treatment of endocrine diseases (34). EA, characterized by sustained
stimulation and the stability and controllability of its
parameters, has been applied extensively in research and in
clinical practice, including to HPOA dysfunction (35). When applied to improving
osteoporosis, acupuncture can prevent bone loss and improve bone
morphology by regulating osteoblast/osteoclast differentiation
(36), and this effect was also
observed in the present study. A possible mechanism may be through
the regulation of the RANKL/RANK and Wnt/β-catenin signaling
pathways, which are known to suppress osteoclastogenesis (11). In addition, acupuncture also
regulates downstream molecules including urine deoxypyridinoline,
serum bone specific alkaline phosphatase (21), cathepsin K (37), TRACP and osteoprotegerin (36) in rat models of osteoporosis. These
published studies have mainly explored the mechanism underlying
acupuncture with respect to one or more cytokines or signal
pathways related to osteoclastogenesis or osteoblastogenesis. The
results of these studies fully illustrate the multi-target effect
of acupuncture in improving osteoporosis. Nevertheless, whether
this multi-target effect is related to histone acetylation levels
has not been deeply studied. The underlying mechanisms of how
acupuncture regulates these signaling pathways and molecules may be
related to the modification of histones, which merits further study
(38).
In the present study, the E2 group served as the
positive treatment control, which was used to assess the efficacy
of EA and investigate the underlying mechanism. It was found that
both EA and E2 improved the trabecular BMD and bone morphology of
OVX-induced osteoporotic rats. However, neither EA nor E2 affected
the Tb.Th of caput femoris. In addition, EA and E2 showed
comparable improvements in the morphology of the trabecular bone.
These results demonstrated the effectiveness of hormone replacement
therapy and suggested that applying EA alone can achieve similar
effects. The study also found that the levels of HDAC2 in caputs
femoris were increased after OVX, which induced increased
expression of histone H3 and lower expression of Ac-histone H3.
Administration of EA mitigated these OVX-induced HDAC2 increases.
Enhancement of histone acetylation levels were correlated with the
expected changes in RUNX2 and RANKL, presumably via changes in the
three-dimensional structure of chromatin. Correspondingly, RUNX2
protein expression was upregulated and RANKL protein expression was
downregulated following EA treatment. In the results of IF,
ALP-labelled osteoblasts were decreased in caput femoris sections
after OVX. These decreases were mitigated by EA or E2 treatment,
and RUNX2 protein expression changes were similar to those of ALP
in the caput femoris tissues. As expected, the changes in
RANKL/TRACP labelling in caput femoris were generally opposite to
those of RUNX2/ALP. Consistent with the results of IF, the bone
metabolism biochemical markers including TRACP, ALP and E2 in serum
were also changed. These results suggested that the alterations in
the protein and gene expression of RUNX2 and RANKL affected the
differentiation of osteoblasts and osteoclasts, which regulated BMD
and trabecular morphology. By contrast, administration of E2 in OVX
rats did not change the protein expression of HDAC2 and did not
affect the level of histone acetylation. However, the protein and
gene expression of RUNX2 and RANKL were affected by E2, which
indicates that the underlying mechanisms by which EA and E2 treat
PMO were different.
In conclusion, the present study demonstrated that
EA suppressed bone loss and improved trabecular morphology in rats
with OVX-induced osteoporosis. Furthermore, a potential mechanism
was identified through EA-induced epigenetic changes which may be
linked to the observed effects. EA suppressed the activity of HDAC2
and increased the acetylation of histone H3 in caput femoris, which
increased the gene and protein expression of RUNX2 and decreased
those of RANKL. The alternations of expression of RUNX2 and RANKL
readjusted the differentiation of osteoblasts and osteoclasts
(Fig. 6). The mechanism of action
from treatment with EA was different from that of treatment with
E2, in that E2 did not modulate the activity of HDAC2. Despite
these findings, this study has limitations. Chromatin
immunoprecipitation (CHIP)-PCR was not used to detect the
relationship between histone H3 and the expression of the
RUNX2/RANKL genes, and the possible downstream genetics
affected by EA-induced histone H3 acetylation need further
investigation through CHIP-seq. Thus, the potential mechanisms
underlying how EA improves osteoporosis will be investigated in
further systematic studies that could provide additional evidence
for the application of acupuncture in preventing and treating
osteoporosis. Furthermore, based on the multi-target
characteristics of the effects of acupuncture and epigenetic
regulation, the results of this study show some possible directions
for further research on the mechanisms underlying acupuncture and
provide more evidence for application of acupuncture.
Acknowledgements
Not applicable.
Funding
This work was supported by Natural Science
Foundation of Hubei Province (grant no. 2017CFB787) and the
Fundamental Research Funds for the Central Universities (grant no.
2042018kf0097).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
JT and QS conceived the project. QS and RL designed
the experiments. QS, YS and YH performed the experiments. ZP
analyzed the data. QS and JT wrote the manuscript. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
All animal procedures were performed according to
the Declaration of Helsinki (European Union guidelines on use of
animals in scientific experiments) and Animal Research: Reporting
of In Vivo Experiments (ARRIVE) guidelines. The study was approved
by the Institutional Animal Care and Use Committee of Wuhan
University, Wuhan, Hubei, P.R. China (no. 2018122).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Eastell R, O'Neill TW, Hofbauer LC,
Langdahl B, Reid IR, Gold DT and Cummings SR: Postmenopausal
osteoporosis. Nat Rev Dis Primers. 2:34532016. View Article : Google Scholar
|
|
2
|
Manolagas SC, O'Brien CA and Almeida M:
The role of estrogen and androgen receptors in bone health and
disease. Nat Rev Endocrinol. 9:699–712. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Eastell R, Rosen CJ, Black DM, Cheung AM,
Murad MH and Shoback D: Pharmacological management of osteoporosis
in postmenopausal women: An endocrine society* clinical practice
guideline. J Clin Endocrinol Metab. 104:1595–1622. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Manson JE, Chlebowski RT, Stefanick ML,
Aragaki AK, Rossouw JE, Prentice RL, Anderson G, Howard BV, Thomson
CA, LaCroix AZ, et al: Menopausal hormone therapy and health
outcomes during the intervention and extended poststopping phases
of the women's health initiative randomized trials. JAMA.
310:1353–1368. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Axelsson KF, Wallander M, Johansson H,
Lundh D and Lorentzon M: Hip fracture risk and safety with
alendronate treatment in the oldest-old. J Intern Med. 282:546–559.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hiligsmann M, Williams SA, Fitzpatrick LA,
Silverman SS, Weiss R and Reginster JY: Cost-effectiveness of
sequential treatment with abaloparatide vs. teriparatide for United
States women at increased risk of fracture. Semin Arthritis Rheum.
49:184–196. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pan H, Jin R, Li M, Liu Z, Xie Q and Wang
P: The effectiveness of acupuncture for osteoporosis: A systematic
review and meta-analysis. Am J Chin Med. 46:489–513. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fan H, Ji F, Lin Y, Zhang M, Qin W, Zhou Q
and Wu Q: Electroacupuncture stimulation at CV4 prevents
ovariectomy-induced osteoporosis in rats via Wnt-β-catenin
signaling. Mol Med Rep. 13:2485–2491. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Martin A, Xiong J, Koromila T, Ji JS,
Chang S, Song YS, Miller JL, Han CY, Kostenuik P, Krum SA, et al:
Estrogens antagonize RUNX2-mediated osteoblast-driven
osteoclastogenesis through regulating RANKL membrane association.
Bone. 75:96–104. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ono T and Nakashima T: Recent advances in
osteoclast biology. Histochem Cell Biol. 149:325–341. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zheng X, Wu G, Nie Y and Lin Y:
Electroacupuncture at the governor vessel and bladder meridian
acupoints improves postmenopausal osteoporosis through
osteoprotegerin/RANKL/RANK and Wnt/β-catenin signaling pathways.
Exp Ther Med. 10:541–548. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shen Y, Wei W and Zhou DX: Histone
acetylation enzymes coordinate metabolism and gene expression.
Trends Plant Sci. 20:614–621. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gräff J and Tsai LH: Histone acetylation:
Molecular mnemonics on the chromatin. Nat Rev Neurosci. 14:97–111.
2013. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Marini F, Cianferotti L and Brandi ML:
Epigenetic mechanisms in bone biology and osteoporosis: Can they
drive therapeutic choices? Int J Mol Sci. 17:13292016. View Article : Google Scholar
|
|
15
|
Dou C, Li N, Ding N, Liu C, Yang X, Kang
F, Cao Z, Quan H, Hou T, Xu J and Dong S: HDAC2 regulates FoxO1
during RANKL-induced osteoclastogenesis. Am J Physiol Cell Physiol.
310:C780–C787. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Queirolo V, Galli D, Masselli E, Borzi RM,
Martini S, Vitale F, Gobbi G, Carubbi C and Mirandola P: PKCepsilon
is a regulator of hypertrophic differentiation of chondrocytes in
osteoarthritis. Osteoarthritis Cartilage. 24:1451–1460. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li J, Wu S, Tang H, Huang W, Wang L, Zhou
H, Zhou M, Wang H and Li J: Long-term effects of acupuncture
treatment on airway smooth muscle in a rat model of smoke-induced
chronic obstructive pulmonary disease. Acupunct Med. 34:107–113.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tu WZ, Cheng RD, Cheng B, Lu J, Cao F, Lin
HY, Jiang YX, Wang JZ, Chen H and Jiang SH: Analgesic effect of
electroacupuncture on chronic neuropathic pain mediated by P2X3
receptors in rat dorsal root ganglion neurons. Neurochem Int.
60:379–386. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yin CS, Jeong HS, Park HJ, Baik Y, Yoon
MH, Choi CB and Koh HG: A proposed transpositional acupoint system
in a mouse and rat model. Res Vet Sci. 84:159–165. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shu Q, Chen L, Wu S, Li J, Liu J, Xiao L,
Chen R and Liang F: Acupuncture targeting SIRT1 in the hypothalamic
arcuate nucleus can improve obesity in high-fat-diet-induced rats
with insulin resistance via an anorectic effect. Obes Facts.
13:40–57. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang HD, Chen Z, Inoue I, Fu SJ, Shi XL,
Tang L, Zhang FZ, Jiang Y and Jiang H: Effects of
electroacupuncture at GB points on markers of osteoporosis and
bodyweight in ovariectomised rats. Acupunct Med. 33:465–471. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Komori T: Runx2, an inducer of osteoblast
and chondrocyte differentiation. Histochem Cell Biol. 149:313–323.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Amzaleg Y, Ji J, Kittivanichkul D,
Törnqvist AE, Windahl S, Sabag E, Khalid AB, Sternberg H, West M,
Katzenellenbogen JA, et al: Estrogens and selective estrogen
receptor modulators differentially antagonize Runx2 in ST2
mesenchymal progenitor cells. J Steroid Biochem Mol Biol.
183:10–17. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang Y, Lu Y, Li Z, Zhou Y, Gu Y, Pang X,
Wu J, Gobin R and Yu J: Oestrogen receptor α regulates the
odonto/osteogenic differentiation of stem cells from apical papilla
via ERK and JNK MAPK pathways. Cell Prolif. 51:e124852018.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sobacchi C, Menale C and Villa A: The
RANKL-RANK Axis: A bone to thymus round trip. Front Immunol.
10:6292019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xiong Q, Tang P, Gao Y, Zhang L and Ge W:
Proteomic analysis of estrogen-mediated signal transduction in
osteoclasts formation. Biomed Res Int. 2015:5967892015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen F, Ouyang Y, Ye T, Ni B and Chen A:
Estrogen inhibits RANKL-induced osteoclastic differentiation by
increasing the expression of TRPV5 channel. J Cell Biochem.
115:651–658. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tella SH and Gallagher JC: Prevention and
treatment of postmenopausal osteoporosis. J Steroid Biochem Mol
Biol. 142:155–170. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gambacciani M and Levancini M: Hormone
replacement therapy and the prevention of postmenopausal
osteoporosis. Prz Menopauzalny. 13:213–220. 2014.PubMed/NCBI
|
|
30
|
Wang Y, Jiang H, Meng H, Li J, Yang X,
Zhao B, Sun Y and Bao T: Antidepressant mechanism research of
acupuncture: Insights from a genome-wide transcriptome analysis of
frontal cortex in rats with chronic restraint stress. Evid Based
Complement Alternat Med. 2017:16768082017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang A, Yan G, Sun H, Cheng W, Meng X,
Liu L, Xie N and Wang X: Deciphering the biological effects of
acupuncture treatment modulating multiple metabolism pathways. Sci
Rep. 6:199422016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fu H, Sun J, Tan Y, Zhou H, Xu W, Zhou J,
Chen D, Zhang C, Zhu X, Zhang Y, et al: Effects of acupuncture on
the levels of serum estradiol and pituitary estrogen receptor beta
in a rat model of induced super ovulation. Life Sci. 197:109–113.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen GZ, Xu YX, Zhang JW, Liu SH and Guo
ZY: Effect of acupoint catgut-embedding on the quality of life,
reproductive endocrine and bone metabolism of postmenopausal women.
Chin J Integr Med. 16:498–503. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Deng YZ, Li LB, Xu LG, Zhou D, Wei LJ and
Liu Y: Acupuncture in endocrine disorders: A critical appraisal. J
Biol Regul Homeost Agents. 30:1035–1040. 2016.PubMed/NCBI
|
|
35
|
Zhao H, Tian ZZ and Chen BY: An important
role of corticotropin-releasing hormone in electroacupuncture
normalizing the subnormal function of hypothalamus-pituitary-ovary
axis in ovariectomized rats. Neurosci Lett. 349:25–28. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zheng X, Nie Y, Sun C, Wu G, Cai Q, Huang
S and Lin Y: Long-term electroacupuncture stimulation prevents
osteoporosis in ovariectomised osteopaenic rats through multiple
signalling pathways. Acupunct Med. 36:176–182. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhou J, Li X, Liao Y, Feng W and Guo X:
Effects of electroacupuncture on bone mass and cathepsin K
expression in ovariectomised rats. Acupunct Med. 32:478–485. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mak JC: Acupuncture in osteoporosis: More
evidence is needed. Acupunct Med. 33:440–441. 2015. View Article : Google Scholar : PubMed/NCBI
|