Introduction
In-stent restenosis (ISR) after stent implantation
remains a serious clinical challenge, and ~26.4% of patients
experience ISR after implantation of stents (1). New-generation drug-eluting stents
have reduced the incidence of ISR to 10% (2–4);
however, local anti-proliferative therapy may interfere with
vascular healing, and incomplete neointimal coverage 3–6 months
after stent implantation has been identified to be associated with
late stent thrombosis (5).
Previous studies have demonstrated that early reendothelialization
can reduce vascular neointimal hyperplasia and restenosis,
indicating that endothelial regeneration is essential to prevent
unfavorable vascular events (6,7).
Endothelial progenitor cells (EPCs) can accelerate
reendothelialization and attenuate neointimal hyperplasia (8,9).
However, the concentration of circulating EPCs may be decreased in
patients with risk factors for heart disease, including elevated
low-density lipoprotein (LDL) cholesterol, diabetes mellitus and
hypertension (10–12). The aforementioned data indicate
that it is crucial for endothelial regeneration to mobilize more
circulating EPCs to enhance early reendothelialization.
AMD3100, also known as plerixafor, an antagonist of
C-X-C motif chemokine receptor (CXCR)4, has been proposed, instead
of granulocyte colony-stimulating factor, to mobilize
CD34+ hematopoietic stem or progenitor cells (HSCs)
derived from bone marrow (13,14).
The underlying mechanism of AMD3100 mobilization of progenitor
cells involves interfering with the stromal cell-derived factor 1
[SDF-1, also known as C-X-C motif chemokine ligand (CXCL)12]/CXCR4
signaling pathway, which is vital for the retention of EPCs in
niches, and then forcing the release of circulating EPCs (15). The process of EPC homing, including
mobilization, recruitment and adhesion, is regulated by key
angiogenic chemokines (CXCL1, CXCL7, CXCL12 and C-C motif chemokine
ligand 2) and their respective receptors (CXCR2, CXCR4 and C-C
motif chemokine receptor 2). Previous studies have reported that
the homing or recruitment of circulating EPCs to injury or ischemic
sites by SDF-1 is an important process for executing their
angiogenic and repair functions (16–18).
These results indicate that AMD3100 and SDF-1 may be useful for
endothelial regeneration. Therefore, the present study evaluated
the effects of AMD3100 and SDF-1 on endothelial repair in a rat
carotid artery injury model. Furthermore, the influence of AMD3100
and SDF-1 on the cellular function of EPCs and the expression
levels of CXCR4 and CXCR7 in EPCs after treatment with AMD3100 and
SDF-1 was assessed.
Materials and methods
Isolation, cultivation and
identification of EPCs
A total of 20 ml fresh human umbilical cord blood
was obtained from the Obstetrics Department of Shanghai Sixth
People's Hospital (Shanghai) and all participants (totally 20
patients; mean age: 24 years old) provided written informed
consent. EPCs were isolated from the human umbilical cord blood by
Ficoll gradient centrifugation (1,500 × g) for 10 min at room
temperature and cultured in endothelial basal medium (Lonza Group
Ltd.) containing growth factors (hydrocortisone, 0.2 ml; human
basic fibroblast growth factor-B, 2 ml; vascular endothelial growth
factor, 0.5 ml; Recombinant human R3 insulin-like growth factor-1,
0.5 ml; human epidermal growth factor, 0.5 ml; ascorbic acid, 0.5
ml; and gentamicin sulfate-amphotericin, 0.5 ml). Isolation,
cultivation and identification of EPCs were performed as described
previously (6). Fluorescent
staining was used to detect the uptake of Dil-ac-LDL (Molecular
Probes; Thermo Fisher Scientific, Inc.) and binding of FITC-UEA-l
(Sigma-Aldrich; Merck KGaA). Briefly, the cells were incubated with
Dil-ac-LDL (15 µg/ml) for 4 h, and then stained with FITC-UEA-l (10
µg/ml) for 1 h and with DAPI for 5 min at room temperature. The
cells were washed three times and analyzed under a fluorescence
microscope (Olympus Corporation). EPCs at passages 2–4 were used in
subsequent experiments.
Proliferation assay of EPCs
The proliferation assay of EPCs was performed to
construct a cell proliferation curve after treatment with AMD3100
and SDF-1. Briefly, EPCs were seeded into 96-well plates at a
density of 1×104 cells/well (Corning Life Sciences) and
cultured in 100 µl microvascular endothelial cell growth medium-2
(Lonza Group Ltd.) supplemented with 5% FBS (Gibco; Thermo Fisher
Scientific, Inc.) for 24 h at 37°C. Subsequently, the EPCs were
incubated with AMD3100 (MedChemExpress) at different concentrations
(10, 1, 0.1, 0.01 and 0.001 µM) or SDF-1α (Cedarlane) at various
concentrations (1,000, 100, 10, 1 and 0.1 nM) for 6, 12, 24 and 48
h at 37°C. Then, cells were incubated with Cell Counting Kit-8
solution (Dojindo Molecular Technologies, Inc.) for 2 h according
to the manufacturers' protocols. Saline was used instead of AMD3100
or SDF-1 in the control group. The absorbance was measured at 450
nm in each well using a Synergy Multi-Mode Microplate Reader
(BioTek Instruments, Inc.). The 50% effective concentration
(EC50) of AMD3100 and SDF-1 was calculated for
subsequent experiments based on the proliferative activity at
different concentrations following incubation for 24 h. The
EC50 value was calculated using GraphPad software
(GraphPad Prism 7.00; GraphPad Software, Inc.).
Adhesion assay
The adhesion ability of EPCs to fibronectin (FN;
Sigma-Aldrich; Merck KGaA) and human umbilical vein endothelial
cells (HUVECs; Yuchi (Shanghai) Biotechnology Co., Ltd.) was
assessed by plating EPCs into 24-well plates. Briefly, to
investigate the adhesion of EPCs to the extracellular matrix,
24-well plates were pretreated with FN (100 µg/ml) for 2 h at 37°C.
Then, EPCs (1×105 cells/well) were added into each well
and cultured in microvascular endothelial cell growth medium-2
(Lonza Group, Ltd.) supplemented with AMD3100 (34 nM; group A),
SDF-1 (212 nM; group S) or AMD3100 combined with SDF-1 (group AS)
for 1 h at 37°C. Unattached cells were washed away three times with
PBS. DAPI (10 µg/ml) was used to stain the adherent EPCs for 10 min
at 37°C. Adherent EPCs were counted in five randomly selected
fields under a fluorescence microscope (magnification, ×400;
Olympus Corporation).
To assess the adhesion of EPCs to HUVECs, HUVECs
(1×106 cells/well) were seeded into 24-well plates to
form a monolayer overnight. Unattached cells were washed away using
PBS. EPCs (1×106 cells/well) were cultured in
microvascular endothelial cell growth medium-2 (Lonza Group, Ltd.)
containing DiI stain (4 mg/ml) for 30 min at 37°C, according to the
manufacturer's protocol. DiI-labeled EPCs were digested and
harvested after washing with PBS three times. Subsequently,
DiI-labeled EPCs (1×105 cells/well) were seeded into
each well and cultured in medium supplemented with AMD3100 (34 nM),
SDF-1 (212 nM) or AMD3100 combined with SDF-1 at 37°C for 2 h.
Unattached cells were washed away three times with PBS. DAPI (10
µg/ml) was used to stain the HUVECs for 10 min at 37°C. Adherent
EPCs were counted in five randomly selected fields under a
fluorescence microscope (magnification, ×400; Olympus
Corporation).
Confocal immunofluorescence
microscopy
CXCR4 and CXCR7 are ligand receptors for SDF-1 and
have a role in regulating the biological activities of EPCs.
Therefore, the expression levels of CXCR4 and CXCR7 were assessed
in each group.
Confocal immunofluorescence microscopy was performed
to determine the expression levels of CXCR4 and CXCR7 in EPCs.
Briefly, EPCs (1×105 cells/well) were grown on glass
coverslips for 12 h, fixed with 4% paraformaldehyde at room
temperature for 30 min and incubated with 0.5% Triton X-100
(Sigma-Aldrich; Merck KGaA) for 5 min and 1% BSA (Sigma-Aldrich;
Merck KGaA) for 30 min at room temperature. Subsequently, EPCs were
incubated with anti-human CXCR4 (1:1,000; Abcam; ab197203) and
anti-human CXCR7 monoclonal antibodies (1:1,000; Abcam; ab72100)
overnight at 4°C, and then incubated with Alexa Fluor 488-(1:500;
Abcam; ab150077) and Alexa Fluor 647-conjugated secondary
antibodies (1:500; Abcam; ab150079) for 2 h at room temperature.
Cell nuclei were stained with DAPI for 10 min at room temperature.
Confocal immunofluorescence microscopy images were captured using a
Leica TCS SP8 confocal microscope (Leica Microsystems GmbH).
Western blot analysis
Western blotting was performed to investigate the
effects of AMD3100 and SDF-1 on the expression levels of CXCR4 and
CXCR7 in EPCs. Briefly, EPCs (1×106 cells/well) were
incubated with AMD3100 (34 nM), SDF-1 (212 nM) or AMD3100 combined
with SDF-1 for 2 h at room temperature prior to protein extraction.
Saline was used instead of AMD3100 or SDF-1 in the control group.
Total protein was extracted using a protein extraction kit (Beijing
Solarbio Science & Technology Co., Ltd.) and quantified using a
bicinchoninic acid protein assay kit (Beijing Solarbio Science
& Technology Co., Ltd.). Extracts (50 ng per lane) were
subjected to 10% SDS-PAGE (Nanjing KeyGen Biotech Co., Ltd.) and
then transferred onto PVDF membranes (Roche Diagnostics) before
blocking with 5% skimmed milk for 1 h at room temperature. The
membranes were incubated with diluted primary antibodies overnight
at 4°C. The following antibodies were used: Rabbit anti-CXCR7
antibody (1:250; Abcam; ab72100), rabbit anti-CXCR4 antibody
(1:150; Abcam; ab197203) and rabbit anti-GADPH antibody (1:3,000;
Cell Signaling Technology, Inc; 97166). Subsequently, the membranes
were incubated with secondary antibody (1:1,000; Beijing Boaosen
Biotechnology Co., Ltd.; bs-0295D) for 2 h at room temperature.
Protein bands were visualized using an Epson photo 1650 (Seiko
Epson Corporation). Immunodetection was performed using the
Supersignal West Femto Maximum Sensitivity Substrate (Thermo Fisher
Scientific Inc., and quantified using ImageJ software (v1.63,
National Institutes of Health).
Rat model of vascular injury and
treatment regimens
To assess the therapeutic effects of different
regimens (AMD3100 alone, SDF-1 alone or AMD3100 combined with
SDF-1) in promoting reendothelialization after vascular injury, 220
female Sprague-Dawley (SD) rat (age: 10 weeks; weight: 250–300 g;
Nanjing Better Biotechnology Co Ltd.) were used to create a model
of carotid artery injury as described previously (6). Briefly, vascular endothelium was
injured by guidewire, as used in percutaneous transluminal
angioplasty. The operation was performed under anesthesia with
pentobarbital sodium (30 mg/kg). Then the bifurcation of the left
carotid artery was exposed, and the common, internal and external
carotid arteries were separated to temporarily restrict blood flow.
The common carotid artery was denuded three times with a 0.38-mm
flexible angioplasty guidewire through the external carotid artery.
The total length of denudation was 5 mm from the bifurcation of
carotid arteries. The external carotid artery was permanently
ligated after removing the wire, and the temporary ligatures were
released to allow blood flow to be restored followed by skin
suture. Following injury of the common carotid artery, the SD rats
were randomly divided into four groups (n=55 per group): Control
group (treated with saline), group A (AMD3100 alone), group S
(SDF-1 alone) and group AS (AMD3100 combined with SDF-1). The
treatment was performed as follows: i) In group AS, AMD3100 (5
mg/kg) was injected via the tail vein immediately after the artery
injury model was established, followed by injection of SDF-1 (1
µg/kg) at 1, 6 and 12 h; ii) in group A, AMD3100 was injected into
the tail vein immediately after the artery injury model was
established; iii) in group S, an equivalent volume of saline was
used instead of AMD3100, and then SDF-1 was administered locally at
1, 6 and 12 h after administration of saline; iv) only an
equivalent volume of saline was used in the control group. All rats
were housed in a constant temperature room (25–27°C; humidity,
40–50%) with food and water ad libitum, under a 12-h light/dark
cycle.
Flow cytometric analysis of the number
of EPCs in the peripheral blood of each group
Flow cytometry was performed to detect the number of
EPCs in the peripheral blood of rats in each group after treatment.
Briefly, mononuclear cells (MNCs) were isolated from the peripheral
blood at baseline, and 1, 6, 12, 24 and 48 h after treatment. The
blood sample was collected from the jugular vein, and then diluted
in PBS at a ratio of 1:1. The MNCs were isolated from cell
suspension via gradient centrifugation (1,500 × g) for 10 min at
room temperature, and the number of EPCs in the peripheral blood
was determined by flow cytometry. EPCs were defined as
CD34+ kinase insert domain receptor (KDR)+
cells. Briefly, MNCs were first incubated with 0.5% BSA, and then
incubated with allophycocyanin (APC)-conjugated anti-mouse CD34
(Abcam; ab155377) and phycoerythrin-conjugated anti-mouse KDR
antibodies (Abcam; ab253080) or isotype antibody (10 µl/tube) for
10 min in the dark at 4°C. MNCs were washed once with PBS, followed
by flow cytometry using a NAVIOS flow cytometer (Beckman Coulter,
Inc.) according to the manufacturer's protocol. The results were
analyzed using FlowJo 7.6 software (FlowJo LLC).
Immunofluorescence analysis of the
number of EPCs recruited to the injury site
A denuded artery of each group was separated and
harvested from the rat one day after treatment to evaluate the
number of EPCs recruited to the injury site. Samples were fixed in
10% formalin for 24 h at room temperature and embedded in paraffin
using standard methods. For immunohistochemistry, paraffin-embedded
sections (4 µm) were heated at 60°C for 30 min, cleared with xylol
and anhydrous alcohol and rehydrated in descending alcohol series.
For antigen retrieval the sections were blocked with 10% goat serum
(Gibco; Thermo Fisher Scientific, Inc.) diluted 1:10 for 30 min at
room temperature. Subsequently, the injured artery was incubated
with rabbit anti-mouse CD34 (1:100; Abcam; ab81289) and murine
anti-mouse KDR (1:100; Abcam; ab9530) primary antibodies for
overnight at 4°C, and FITC-(1:50; Wuhan Boster Biological
Technology, Ltd.; BM2012) or APC-conjugated secondary antibodies
(1:50; Wuhan Boster Biological Technology, Ltd.; BA1011) for 1 h at
room temperature. Three fields were randomly selected for imaging
under an Olympus BX53 biological fluorescence microscope
(magnification, ×400; Olympus Corporation).
Histological assessment
Reendothelialization and neointimal hyperplasia were
assessed at 7 and 14 days after treatment as described in our
previous study (6). All operations
were performed under anesthesia with pentobarbital sodium via
intraperitoneal injection (30 mg/kg). A pathologist who was blinded
to the treatment regimen assessed all specimens. Analysis of the
digitalized images was performed using ImageJ 1.63 software
(National Institutes of Health).
Neointimal hyperplasia was evaluated using
hematoxylin and eosin (H&E) staining. Briefly, following
anesthesia with 30 mg/kg pentobarbital sodium, cardiac perfusion
was conducted by perfusing PBS via the bilateral jugular vein until
the effluent ran clear, followed by fixation with formaldehyde for
5 min. Subsequently, the carotid arteries were excised from the
rats, and the specimens were fixed in 10% formalin for 24 h at room
temperature. Subsequently, separated vessels (5 mm) were embedded
in paraffin and sectioned at 4 µm). Sections were stained with
H&E staining kit (Beijing Solarbio Science & Technology
Co., Ltd., G1120) for 2 h at room temperature according to the
manufacturer's protocol. Neointimal thickness was assessed in terms
of the intima/media area ratio, and was measured in H&E-stained
axial sections. A pathologist who was blinded to the treatment
regimen investigated all specimens. Analysis of the digitalized
images was performed using ImageJ 1.63 software (National
Institutes of Health).
Reendothelialization was assessed using Evans blue
staining. Briefly, 0.5 ml 0.5% Evans blue dye was injected
intravenously via the tail vein 30 min before the rats were
scarified. Subsequently, cardiac perfusion was used to perfuse PBS
via the bilateral jugular vein until the effluent ran clear,
followed by fixation with 4% formaldehyde for 5 min at room
temperature. The common carotid artery was harvested at 4 mm from
the bifurcation and opened longitudinally. The areas stained and
unstained in blue were measured in the entire injured area, and the
rate of reendothelialization (unstained area/total area) was used
to determine the difference in reendothelialization among all
groups. The analysis of digitalized images was performed using
ImageJ 1.63 software (National Institutes of Health).
Statistical analysis
Data are presented as the mean ± standard deviation.
One-way ANOVA with Tukey's post hoc test was used to determine
statistically significant differences among the same treatment
group at different time points in the proliferation assay, or among
subgroups. SPSS 20.0 software (IBM Corp.) was used to perform
statistical analysis. P<0.05 was considered to indicate a
statistically significant difference.
Results
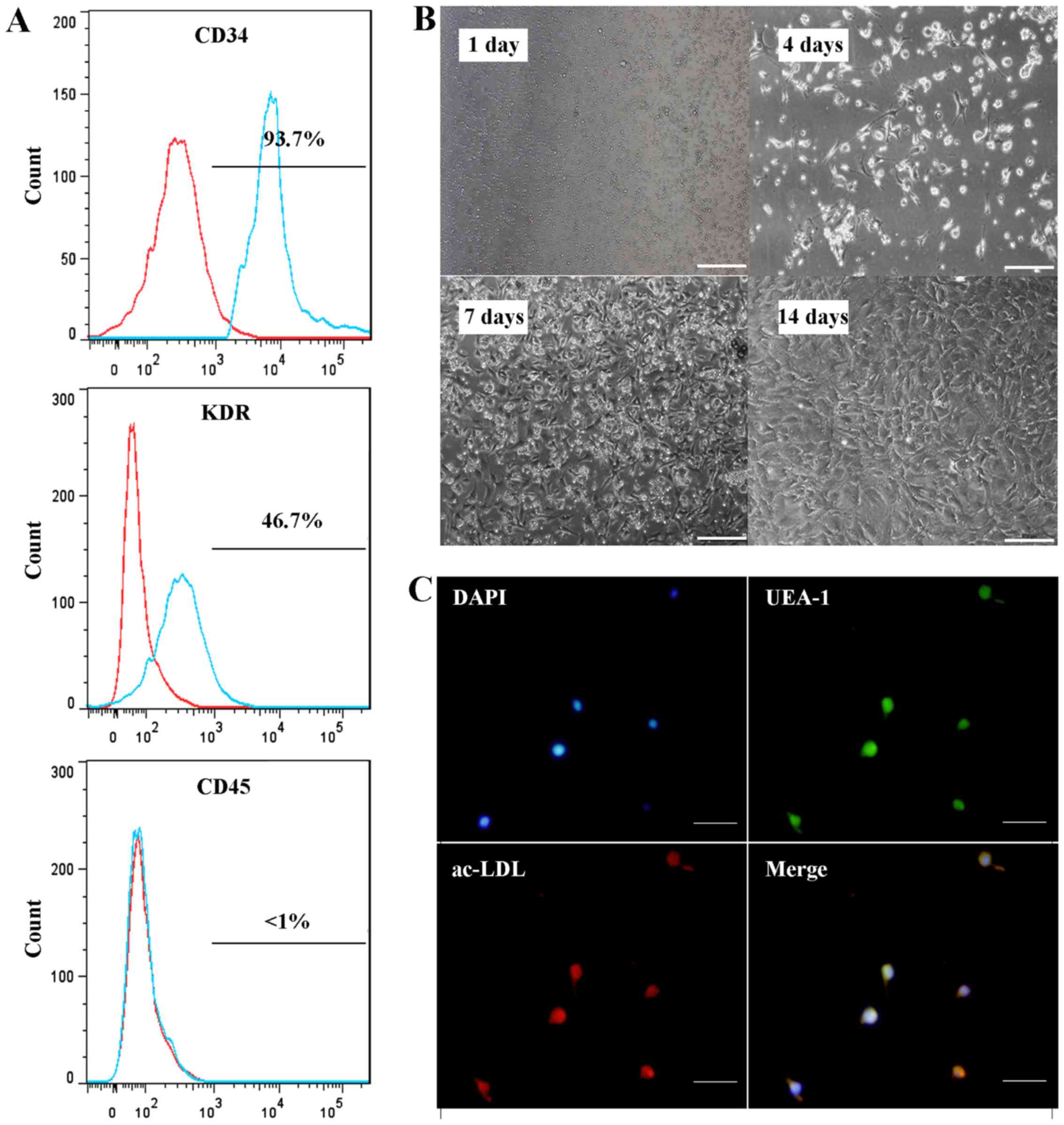
Characteristics of EPCs
EPCs appeared round or spindle-shaped at day 4, and
then typical cell clusters appeared after 7–10 days of culture.
After 21 days of culture, the cells formed colonies and appeared
pebble-shaped. Flow cytometry revealed that the EPCs in the present
study were positive for CD34 and KDR, while they were negative for
CD45. Furthermore, these cells could take up acetylated LDL and
bind to Ulex europaeus agglutinin I. These characteristics
identified the cells as EPCs (Fig.
1A-C).
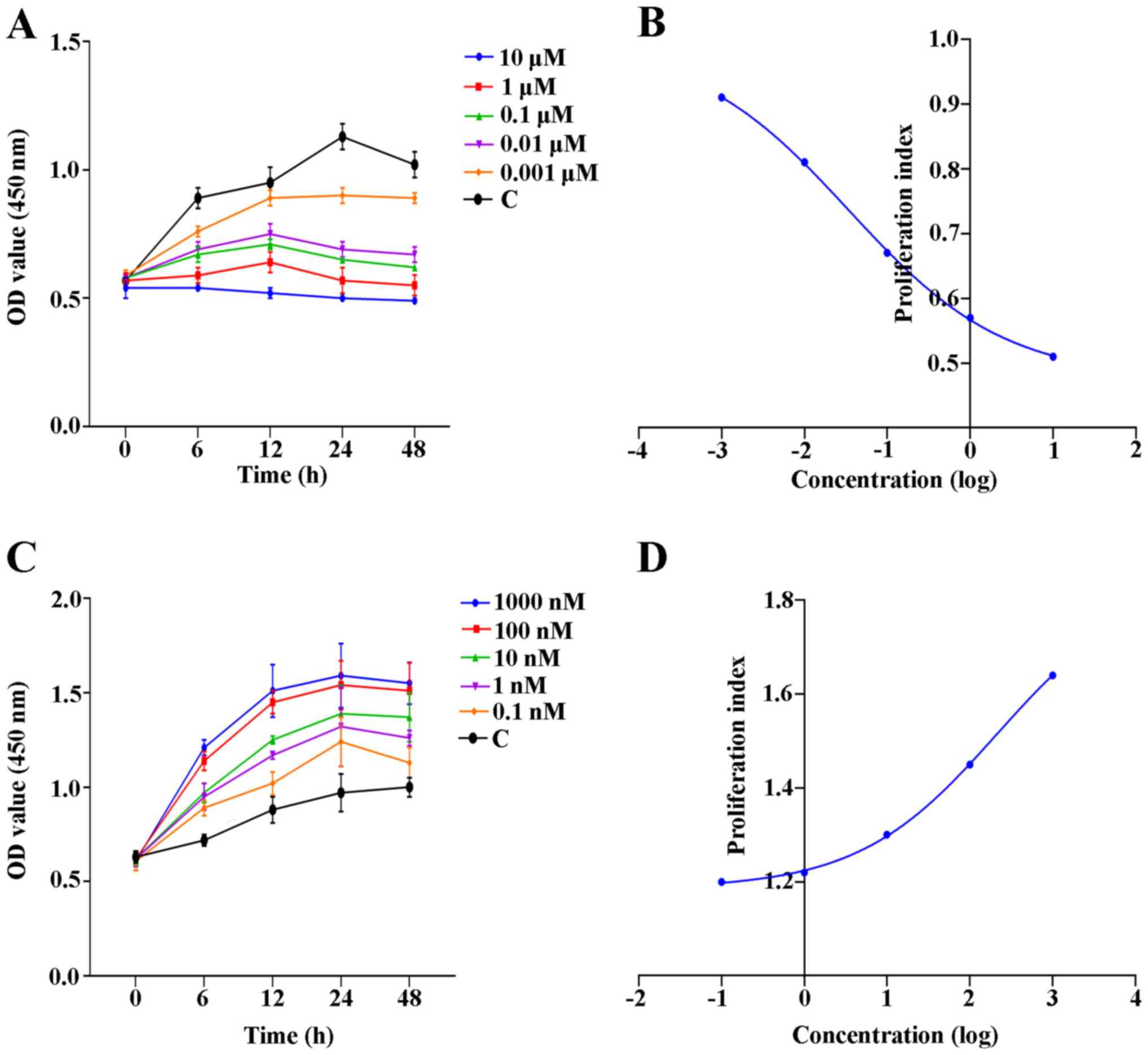
Proliferation of EPCs is attenuated by
AMD3100, whereas it is enhanced by SDF-1
Proliferation curves were plotted to reveal the
proliferative activity of EPCs after treatment with AMD3100 or
SDF-1 at various concentrations and different time points.
Furthermore, the EC50 of AMD3100 and SDF-1 was
calculated for subsequent experiments based on the proliferation
curves. The results revealed that AMD3100 reduced the proliferation
of EPCs effectively at various concentrations compared with the
control group (Fig. 2A). The
EC50 of AMD3100 was 34 nM (Fig. 2B). By contrast, SDF-1 could promote
proliferation of EPCs at various concentrations in a
concentration-dependent manner (Fig.
2C). The EC50 of SDF-1 was 212 nM (Fig. 2D).
AMD3100 stimulates adhesion of EPCs to
HUVECs rather than FN, while SDF-1 stimulates adhesion of EPCs to
HUVECs and FN
Adhesion assays demonstrated that fewer EPCs adhered
to FN after treatment with AMD3100 [60.3±20.1 (group A) vs.
80.7±16.7 (control group) cells/field, P=0.042; 60.3±20.1 (group A)
vs. 105.4±17.1 (group S) cells/field, P=0.007], whereas following
treatment with AMD3100, more EPCs adhered to HUVECs compared with
the control group [45.2±16.8 (group A) vs. 22.3±4.5 (control group)
cells/field, P=0.029]. Additionally, treatment with SDF-1
significantly enhanced the adhesion capacity of EPCs to both FN
[105.4±17.1 (group S) vs. 80.7±16.7 (control group) cells/field,
P=0.02] and HUVECs [52.7±12.6 (group S) vs. 22.3±4.5 (control
group) cells/field, P=0.031]. Furthermore, the adhesion capacities
of EPCs to HUVECs in groups S and A were not identified to be
significantly different [52.7±12.6 (group S) vs. 45.2±16.8 (group
A) cells/field, P=0.23]. The present study further revealed that
AMD3100 impaired the SDF-1-mediated adhesion capacity of EPCs to FN
[65.6±11.5 (group AS) vs. 105.4±17.1 (group S) cells/field,
P=0.015; 65.6±11.5 (group AS) vs. 80.7±16.7 (control group)
cells/field, P=0.047]. By contrast, even more EPCs adhered to
HUVECs in group AS compared with in the control group [62.7±17.4
(group AS) vs. 22.3±4.5 (control group) cells/field, P=0.029]. The
effect of AMD3100 combined with SDF-1 on the adhesion capacity of
EPCs to HUVECs was greater than that of AMD3100 [62.7±17.4 (group
AS) vs. 45.2±16.8 (group A) cells/field, P=0.031]. The detailed
results are shown in Fig. 3A.
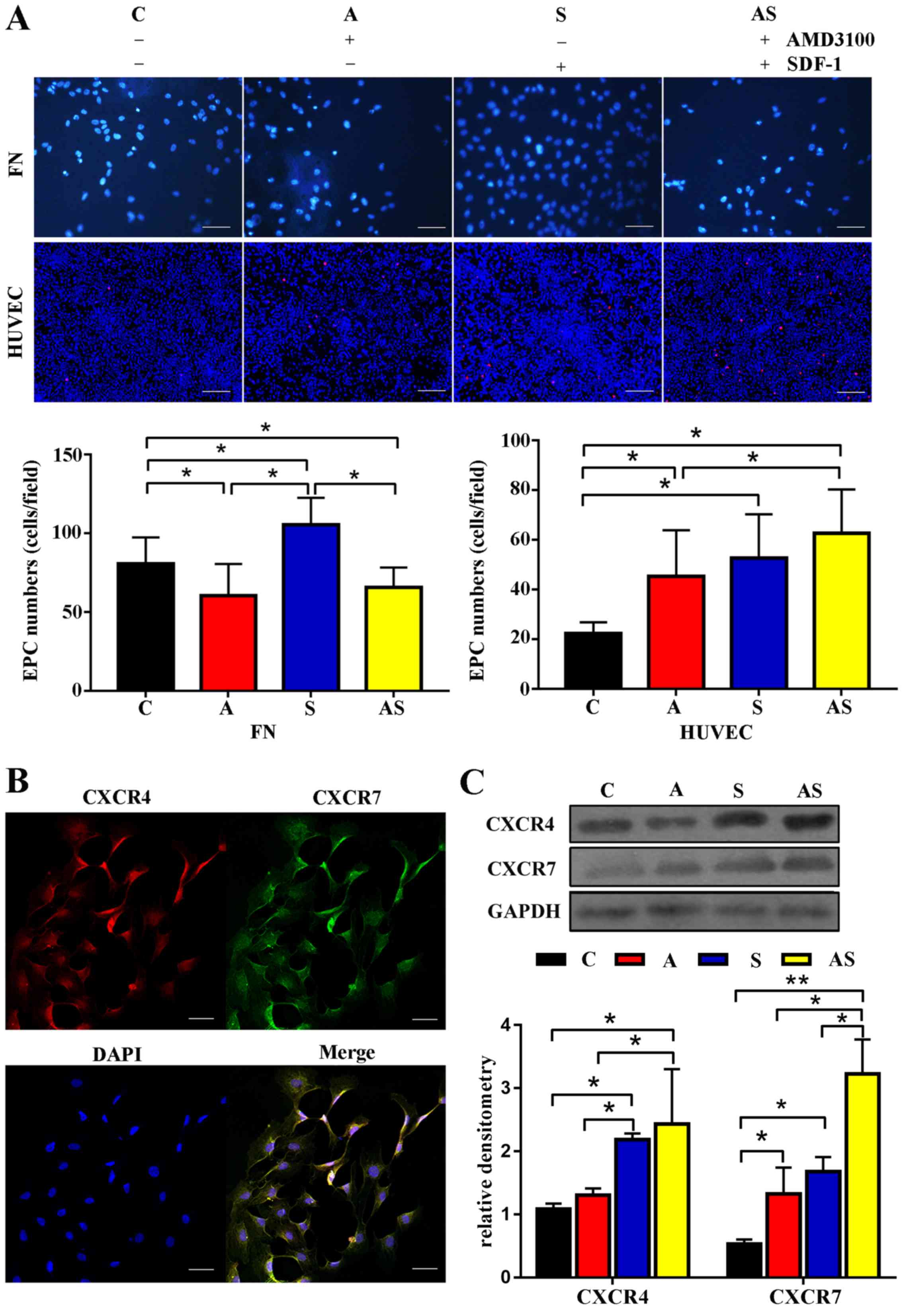 | Figure 3.Adhesion capacity, and the expression
levels of CXCR4 and CXCR7 are affected by AMD3100 and SDF-1
treatment. (A) Adhesion capacity to FN was impaired by AMD3100
treatment, whereas AMD3100 treatment stimulated the adhesion
capacity to HUVECs. A similar tendency was observed after treatment
with AMD3100 combined with SDF-1. Scale bar, 25 µm. (B) Confocal
immunofluorescence microscopy confirmed that both CXCR4 and CXCR7
were expressed in EPCs. Scale bar, 100 µm. (C) Western blotting
revealed that treatment with AMD3100 upregulated the expression
levels of CXCR7 but not CXCR4. However, SDF-1 or AMD3100 combined
with SDF-1 upregulated the expression levels of CXCR4 and CXCR7.
Furthermore, the effects of AMD3100 combined with SDF-1 on the
expression levels of CXCR7 were the greatest among the four groups.
n=5. *P<0.05; **P<0.001. C, control; A, AMD3100 alone; S,
SDF-1 alone; AS, AMD3100 combined with SDF-1; CXCR, C-X-C motif
chemokine receptor; EPCs, endothelial progenitor cells; FN,
fibronectin; HUVECs, human umbilical vein endothelial cells; SDF-1,
stromal cell-derived factor 1. |
AMD3100 only stimulates the expression
levels of CXCR7, while SDF-1 upregulates the expression levels of
CXCR4 and CXCR7 in EPCs
Both CXCR4 and CXCR7 are involved in regulating the
function of EPCs; therefore, the present study evaluated the
expression levels of CXCR4 and CXCR7 in EPCs before and after
treatment. Confocal immunofluorescence microscopy revealed that
both CXCR4 and CXCR7 were expressed by EPCs (Fig. 3B), and AMD3100 treatment
upregulated the expression levels of CXCR7 in EPCs. In addition,
the expression levels of CXCR4 and CXCR7 were significantly
increased after incubation with SDF-1 or AMD3100 combined with
SDF-1 for 24 h compared with the control group. Furthermore,
upregulation of CXCR7 was identified in group AS compared with in
groups A and S. The expression levels of CXCR4 in group S were
higher than those in group A. However, the expression levels of
CXCR7 were similar in groups S and A (Fig. 3C).
AMD3100 mobilizes circulating EPCs and
promotes the incorporation of EPCs into the injury site, while
SDF-1 does not
Different treatment regimens were used to reveal the
effects of AMD3100, SDF-1 or AMD3100 combined with SDF-1 in
vivo. The circulating EPCs were increased in group A compared
with in the control group at 1, 6, 12 and 24 h after treatment. The
number of circulating EPCs was significantly increased after 1 h
and reached the peak at 6 h after treatment with AMD3100. However,
treatment with SDF-1 did not mobilize circulating EPCs (Fig. 4A). An immunofluorescence assay
revealed that an increased number of EPCs were recruited to the
site of endothelial injury in group A compared with in the control
group [16.7±1.2 (group A) vs. 7.3±0.9 (control group) cells/field;
P=0.041]. However, treatment with SDF-1 did not recruit more EPCs
to the site of endothelial injury [8.7±1.1 (group S) vs. 7.3±0.9
(control group) cells/field, P=0.52] (Fig. 4B). Notably, pretreatment with
AMD3100, followed by local administration of SDF-1, recruited
significantly more EPCs to the site of vascular injury compared
with AMD3100 treatment alone [22.8±1.3 (group AS) vs. 16.7±1.2
(group A) cells/field, P=0.049].
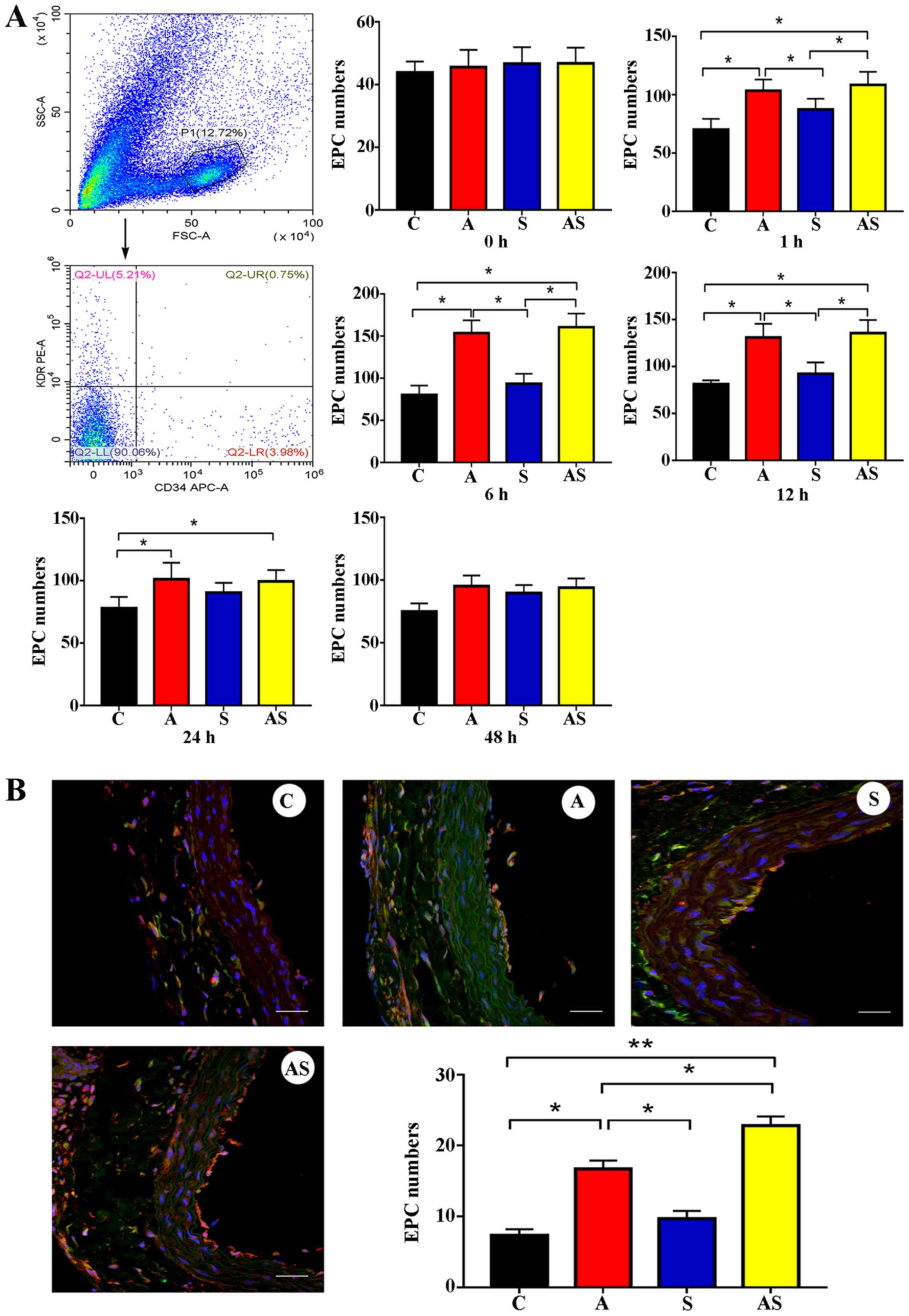 | Figure 4.Number of EPCs in circulation and at
the injury site. (A) AMD3100 treatment could effectively mobilize
circulating EPCs in a time-dependent manner. However, SDF-1
treatment did not increase the number of circulating EPCs at any
time point. Additionally, AMD3100 combined with SDF-1 (group AS)
increased the number of circulating EPCs. However, there was no
significant difference in circulating EPCs observed between group A
and group AS. (B) AMD3100 treatment recruited more EPCs to the
injury site. SDF-1 treatment did not increase the number of EPCs at
the injury site. However, pretreatment with AMD3100, followed by
local administration of SDF-1 recruited more EPCs to the injury
site. The effects of AMD3100 combined with SDF-1 (group AS) on
recruitment of EPCs were stronger than those of AMD3100 alone.
Scale bar, 50 µm. n=5. *P<0.05; **P<0.001. C, control; A,
AMD3100 alone; S, SDF-1 alone; AS, AMD3100 combined with SDF-1;
EPCs, endothelial progenitor cells; SDF-1, stromal cell-derived
factor 1. |
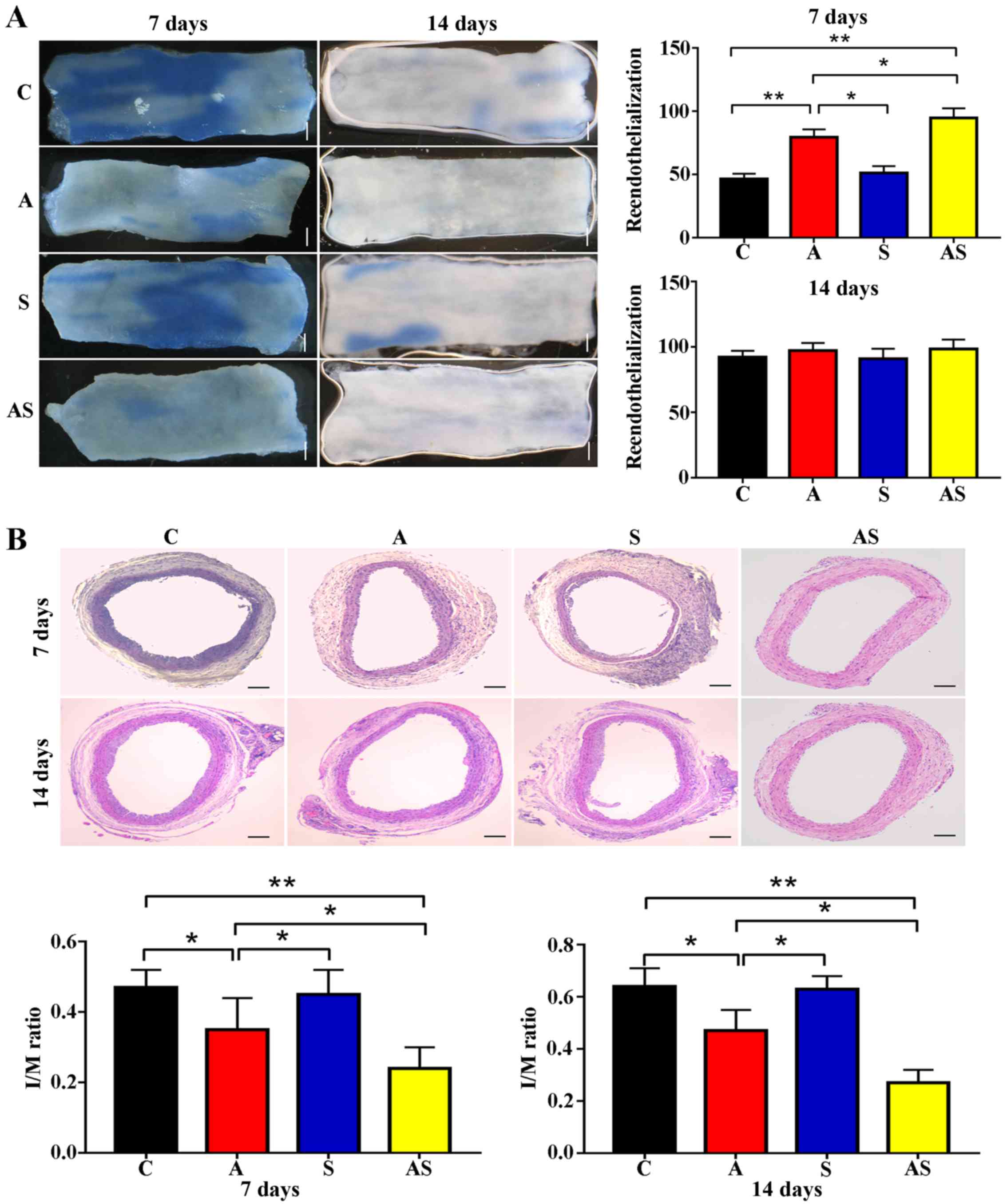
AMD3100 combined with SDF-1 promotes
reendothelialization and inhibits neointimal hyperplasia more
effectively than ADM3100 alone
Increased reendothelialization was identified in
groups A and AS at 7 days after treatment compared with in the
control group [80.4±6.1 (group A) vs. 46.3±4.2% (control group),
P<0.001; 92.7±7.6 (group AS) vs. 46.3±4.2% (control group),
P<0.001], and there was a significant difference in
reendothelialization between groups AS and A at day 7. By contrast,
no significant difference was observed in reendothelialization
after 14 days [97.2±5.9 (group A) vs. 92.3±4.7% (control group),
P=0.412; 98.5±7.2 (group AS) vs. 92.3±4.7% (control group),
P=0.43]. However, early reendothelialization inhibited neointimal
hyperplasia after 7 and 14 days of treatment in group A [7 days,
0.35±0.09 (group A) vs. 0.47±0.05 (control group), P=0.023; 14
days, 0.47±0.08 (group A) vs. 0.64±0.07 (control group), P=0.071]
and group AS [7 days, 0.24±0.06 (group AS) vs. 0.47±0.05 (control
group), P<0.001; 14 days, 0.27±0.05 (group AS) vs. 0.64±0.07
(control group), P<0.001]. The difference in the levels of
reendothelialization of group S compared with the control group was
not statistically significant at day 7 [51.2±5.4 (group S) vs.
46.3±4.2% (control group), P=0.22] and day 14 [91.1±7.6 (group S)
vs. 92.3±4.7% (control group), P=0.517], and neointimal hyperplasia
was not attenuated in this group [7 days, 0.45±0.07 (group S) vs.
0.47±0.05 (control group), P=0.049; 14 days, 0.63±0.04 (group S)
vs. 0.64±0.07 (control group), P=0.43] (Fig. 5).
Discussion
AMD3100 and SDF-1 are extensively used in
regenerative medicine, including hematological disease (14,19),
angiogenesis (17,20), intimal repairing (16), wound healing (21,22)
and brain repair after ischemic stroke (18,23).
Furthermore, previous studies have reported that AMD3100 and SDF-1
may be involved in mobilization and recruitment of EPCs (16,17,24,25).
These studies indicated that AMD3100 or SDF-1 may be used for
endothelial regeneration. The present study assessed the effects of
AMD3100 and SDF-1 on endothelial regeneration. Additionally, the
effects of AMD3100 and SDF-1 on EPCs were evaluated.
Intravenous or subcutaneous administration of
AMD3100 has been reported to effectively induce mobilization of
HSCs and EPCs (14,24). Furthermore, a single dose of
AMD3100 may mobilize EPCs into peripheral blood (25). Similar to previous studies
(13,25), the present study noted that a
single dose of AMD3100 was sufficient to mobilize EPCs into
circulation. Furthermore, the results of the present study
indicated that increased numbers of EPCs were involved in
reendothelialization following AMD3100 treatment. The underlying
mechanism by which AMD3100 treatment recruits more EPCs to
participate in endothelial repair is unclear. The in vivo
and in vitro results reported in the present study revealed
that endothelial cells (ECs) at the injury site may be crucial for
intimal repair after AMD3100 treatment, as it was observed that
more EPCs adhered to HUVECs compared with FN after AMD3100
treatment. Moreover, more EPCs were recruited into endothelial site
after intravenous injection of AMD3100. However, the detailed
mechanism should be considered in a further study. In addition, the
mismatch between the time circulating EPCs reached their highest
level after AMD3100 treatment and the median terminal half-life of
AMD3100 may contribute to EPCs ability to mediate intimal repair.
Stewart et al (26)
reported that the median terminal half-life of AMD3100 in
circulation was 4.6 h. However, in the present study, the number of
circulating EPCs reached its peak at 6 h and remained at high
levels for 24 h after AMD3100 treatment. The aforementioned
inhibitory effect of AMD3100 on EPCs was attenuated before
circulating EPCs decreased to baseline levels, as a result, more
circulating EPCs were recruited to the arterial injury site.
A previous study demonstrated that a decrease in the
level of SDF-1 at the injury site was associated with delayed
reendothelialization as fewer EPCs were recruited to the injury
site (27). By contrast, local
accumulation of fluorescence-labeled EPCs was observed in ischemic
muscle after local injection of SDF-1 in an athymic rat hind limb
ischemia model (28). Therefore,
it may be concluded that local accumulation of SDF-1 is essential
for recruitment of endothelial progenitors and in accelerating
repair of injury. Thus, the present study also assessed the effects
of SDF-1 on intimal repair via local injection. However, the
results of the present study indicated that local injection of
SDF-1 was ineffective in promoting reendothelialization. Hence, it
was revealed that insufficient EPCs in circulation were the main
contributing factor for delayed recovery of injury after SDF-1
treatment. Additionally, it was revealed that AMD3100 combined with
SDF-1 had superior therapeutic effects compared with AMD3100
alone.
Previous studies have demonstrated that SDF-1 may be
involved in regulating the mobilization, proliferation and adhesion
capacity of EPCs through binding to CXCR4 and CXCR7 (15,29,30).
In accordance with these studies, the present study revealed that
SDF-1 treatment stimulated the proliferation and adhesion capacity
of EPCs to FN and HUVECs. Furthermore, the present study
demonstrated that in EPCs the expression levels of CXCR4 and CXCR7
were upregulated after SDF-1 treatment. The results indicated that
SDF-1 exerted its positive regulatory effects on cellular function
not only via binding to and activating its receptor, but also by
upregulating the expression levels of CXCR4 and CXCR7. However, the
molecular mechanism by which SDF-1 can stimulate EPCs to upregulate
the expression levels of CXCR4 and CXCR7 needs to be investigated
further. The effects of AMD3100 on cellular function of EPCs were
also evaluated in the present study. A previous study demonstrated
that AMD3100 impaired the proliferation, migration and adhesion
capacity of EPCs via blocking the SDF-1/CXCR4 axis (31). The present results demonstrated
that AMD3100 could positively modulate adhesion of EPCs to HUVECs
via upregulation of the expression levels of CXCR7. It remains
elusive how AMD3100 can stimulate upregulation of the expression
levels of CXCR7 in EPCs. However, previous studies have
demonstrated that CXCR7 may be crucial in regulating cell adhesion
capacity, particularly the adhesion capacity to HUVECs (29,30,32).
Furthermore, Kalatskaya et al (33) revealed that AMD3100 may bind to
CXCR7 and positively modulate the effect of CXCL12 by inducing
β-arrestin recruitment to CXCR7. The aforementioned data indicated
that AMD3100 stimulated adhesion of EPCs to HUVECs via upregulation
of the expression levels of CXCR7 rather than CXCR4.
The present study demonstrated that the adhesive
activity of EPCs was pivotal for EPCs recruitment and EPC-mediated
endothelial repair. Furthermore, the in vitro results showed
that CXCR4 and CXCR7 molecules were associated with the adhesive
activity of EPCs after AMD3100 or SDF-1 treatment. Previous studies
identified that other molecules, including P-selectin (34) and E-selectin (35), and very late antigen-4 and its
ligand vascular cell adhesion molecule 1 (36) also contribute to the cellular
adhesion capacity of progenitor cells. All these results indicated
that upregulating these adhesive molecules may contribute to the
adhesive activity of EPCs and EPC-mediated intimal repair. However,
the present study was still inadequate and these underlying
mechanisms should be considered in the future.
There were several limitations of the present study.
Firstly, knockdown of CXCR4 and CXCR7 was not performed to identify
the detailed molecular mechanism by which SDF-1/CXCR4 and
SDF-1/CXCR7 are involved in regulating cellular function of EPCs
after treatment with AMD3100 or SDF-1. This should be investigated
in future studies. Secondly, the molecular mechanisms by which EPCs
exhibited upregulated expression levels of CXCR4 and CXCR7 after
treatment with AMD3100 or SDF-1 remain unclear. In addition, only
46.7% cells positive for KDR. The main reason underlying this
phenomenon may due to EPCs showing more characteristics of
progenitor cells but fewer characteristics of mature endothelial
cells.
In conclusion, AMD3100 positively regulated the cell
adhesion capacity of EPCs to HUVECs via elevation of the expression
levels of CXCR7 rather than CXCR4, whereas SDF-1 stimulated cell
proliferation and the adhesion capacity of EPCs to FN and HUVECs by
increasing the expression levels of CXCR4 and CXCR7. Treatment with
AMD3100 accelerated reendothelialization and inhibited neointimal
hyperplasia after endothelial injury, whereas SDF-1 treatment alone
failed to promote endothelial regeneration. AMD3100 combined with
SDF-1 outperformed AMD3100 alone, promoted early
reendothelialization and inhibited neointimal hyperplasia,
indicating that early reendothelialization attenuates neointimal
hypoplasia following endothelial injury.
Acknowledgements
Not applicable.
Funding
The present study was financially supported by the
National Natural Science Foundation of China (grant nos. 81271683
and 81671791) and the Shanghai Key Discipline of Medical Imaging
(grant no. 2017ZZ02005).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CJ, XM and RL performed the experiments. JZ designed
the experiments. HH conducted the statistical analysis of the data.
CJ, RL and XM drafted the manuscript. JG and JZ conceived the
study, participated in its design and coordination, and helped to
draft the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All procedures involving human beings and animals
were reviewed and approved by the Ethics Committee of Shanghai
Sixth People's Hospital (Shanghai, China). All clinical
investigations were conducted according to the principles of the
Declaration of Helsinki. All patients provided written informed
consent prior to the start of the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cassese S, Byrne RA, Tada T, Pinieck S,
Joner M, Ibrahim T, King LA, Fusaro M, Laugwitz KL and Kastrati A:
Incidence and predictors of restenosis after coronary stenting in
10 004 patients with surveillance angiography. Heart. 100:3201–159.
2014. View Article : Google Scholar
|
|
2
|
Alfonso F, Byrne RA, Rivero F and Kastrati
A: Current treatment of in-stent restenosis. J Am Coll Cardiol.
63:2659–2673. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jukema JW, Ahmed TA, Verschuren JJ and
Quax PH: Restenosis after PCI. Part 2: Prevention and therapy. Nat
Rev Cardiol. 9:79–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Byrne RA, Joner M and Kastrati A: Stent
thrombosis and restenosis: What have we learned and where are we
going? The Andreas Gruntzig Lecture ESC 2014. Eur Heart J.
36:3320–3331. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Finn AV, Joner M, Nakazawa G, Kolodgie F,
Newell J, John MC, Gold HK and Virmani R: Pathological correlates
of late drug-eluting stent thrombosis: Strut coverage as a marker
of endothelialization. Circulation. 115:2435–2441. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li X, Chen C, Wei L, Li Q, Niu X, Xu Y,
Wang Y and Zhao J: Exosomes derived from endothelial progenitor
cells attenuate vascular repair and accelerate reendothelialization
by enhancing endothelial function. Cytotherapy. 18:253–262. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kipshidze N, Ferguson JJ III, Keelan MH
Jr, Sahota H, Komorowski R, Shankar LR, Chawla PS, Haudenschild CC,
Nikolaychik V and Moses JW: Endoluminal reconstruction of the
arterial wall with endothelial cell/glue matrix reduces restenosis
in an atherosclerotic rabbit. J Am Coll Cardiol. 36:1396–1403.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Padfield GJ, Newby DE and Mills NL:
Understanding the role of endothelial progenitor cells in
percutaneous coronary intervention. J Am Coll Cardiol.
55:1553–1565. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Simard T, Jung RG, Motazedian P, Di Santo
P, Ramirez FD, Russo JJ, Labinaz A, Yousef A, Anantharam B,
Pourdjabbar A and Hibbert B: Progenitor cells for arterial repair:
Incremental advancements towards therapeutic reality. Stem Cells
Int. 2017:82704982017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Arcangeli A, Lastraioli E, Piccini B,
D'Amico M, Lenzi L, Pillozzi S, Calabrese M, Toni S and Arcangeli
A: Circulating endothelial progenitor cells in type 1 diabetic
patients: Relation with Patients' age and disease duration. Front
Endocrinol. 8:2782017. View Article : Google Scholar
|
|
11
|
Georgescu A, Alexandru N, Constantinescu
A, Titorencu I and Popov D: The promise of EPC-based therapies on
vascular dysfunction in diabetes. Eur J Oharmacol. 669:1–6. 2011.
View Article : Google Scholar
|
|
12
|
Gallagher KA, Liu ZJ, Xiao M, Chen H,
Goldstein LJ, Buerk DG, Nedeau A, Thom SR and Velazquez OC:
Diabetic impairments in NO-mediated endothelial progenitor cell
mobilization and homing are reversed by hyperoxia and SDF-1 alpha.
J Clin Invest. 117:1249–1259. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pantin J, Purev E, Tian X, Cook L,
Donohue-Jerussi T, Cho E, Reger R, Hsieh M, Khuu H, Calandra G, et
al: Effect of high-dose plerixafor on CD34(+) cell mobilization in
healthy stem cell donors: Results of a randomized crossover trial.
Haematologica. 102:600–609. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schroeder MA, Rettig MP, Lopez S, Christ
S, Fiala M, Eades W, Mir FA, Shao J, McFarland K, Trinkaus K, et
al: Mobilization of allogeneic peripheral blood stem cell donors
with intravenous plerixafor mobilizes a unique graft. Blood.
129:2680–2692. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Petit I, Jin D and Rafii S: The
SDF-1-CXCR4 signaling pathway: A molecular hub modulating
neo-angiogenesis. Trends Immunol. 28:299–307. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yin Y, Zhao X, Fang Y, Yu S, Zhao J, Song
M and Huang L: SDF-1alpha involved in mobilization and recruitment
of endothelial progenitor cells after arterial injury in mice.
Cardiovasc Pathol. 19:218–227. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Anderson EM, Kwee BJ, Lewin SA, Raimondo
T, Mehta M and Mooney DJ: Local delivery of VEGF and SDF enhances
endothelial progenitor cell recruitment and resultant recovery from
ischemia. Tissue Eng Part A. 21:1217–1227. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li Y, Chang S, Li W, Tang G, Ma Y, Liu Y,
Yuan F, Zhang Z, Yang GY and Wang Y: cxcl12-engineered endothelial
progenitor cells enhance neurogenesis and angiogenesis after
ischemic brain injury in mice. Stem Cell Res Ther. 9:1392018.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hoggatt J, Singh P, Tate TA, Chou BK,
Datari SR, Fukuda S, Liu L, Kharchenko PV, Schajnovitz A, Baryawno
N, et al: Rapid mobilization reveals a highly engraftable
hematopoietic stem cell. Cell. 172:191–204.e10. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tan Y, Li Y, Xiao J, Shao H, Ding C,
Arteel GE, Webster KA, Yan J, Yu H and Cai L: A novel CXCR4
antagonist derived from human SDF-1beta enhances angiogenesis in
ischaemic mice. Cardiovasc Res. 82:513–521. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vågesjö E, Öhnstedt E, Mortier A, Lofton
H, Huss F, Proost P, Roos S and Phillipson M: Accelerated wound
healing in mice by on-site production and delivery of CXCL12 by
transformed lactic acid bacteria. Proc Natl Acad Sci USA.
115:1895–1900. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nishimura Y, Ii M, Qin G, Hamada H, Asai
J, Takenaka H, Sekiguchi H, Renault MA, Jujo K, Katoh N, et al:
CXCR4 antagonist AMD3100 accelerates impaired wound healing in
diabetic mice. J Invest Dermatol. 132:711–720. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zamproni LN, Mundim MV, Porcionatto MA and
des Rieux A: Injection of SDF-1 loaded nanoparticles following
traumatic brain injury stimulates neural stem cell recruitment. Int
J Pharm. 519:323–331. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fu WL, Xiang Z, Huang FG, Cen SQ, Zhong G,
Duan X, Liu M and Leung F: Combination of granulocyte
colony-stimulating factor and CXCR4 antagonist AMD3100 for
effective harvest of endothelial progenitor cells from peripheral
blood and in vitro formation of primitive endothelial networks.
Cell Tissue Banking. 17:161–169. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shepherd RM, Capoccia BJ, Devine SM,
Dipersio J, Trinkaus KM, Ingram D and Link DC: Angiogenic cells can
be rapidly mobilized and efficiently harvested from the blood
following treatment with AMD3100. Blood. 108:3662–3667. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Stewart DA, Smith C, MacFarland R and
Calandra G: Pharmacokinetics and pharmacodynamics of plerixafor in
patients with non-Hodgkin lymphoma and multiple myeloma. Biol Blood
Marrow Transplant. 15:39–46. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Noels H, Zhou B, Tilstam PV, Theelen W, Li
X, Pawig L, Schmitz C, Akhtar S, Simsekyilmaz S, Shagdarsuren E, et
al: Deficiency of endothelial CXCR4 reduces reendothelialization
and enhances neointimal hyperplasia after vascular injury in
atherosclerosis-prone mice. Arterioscler Thromb Vasc Biol.
34:1209–1220. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yamaguchi J, Kusano KF, Masuo O, Kawamoto
A, Silver M, Murasawa S, Bosch-Marce M, Masuda H, Losordo DW, Isner
JM and Asahara T: Stromal cell-derived factor-1 effects on ex vivo
expanded endothelial progenitor cell recruitment for ischemic
neovascularization. Circulation. 107:1322–1328. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dai X, Tan Y, Cai S, Xiong X, Wang L, Ye
Q, Yan X, Ma K and Cai L: The role of CXCR7 on the adhesion,
proliferation and angiogenesis of endothelial progenitor cells. J
Cell Mol Med. 15:1299–1309. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dai X, Yan X, Zeng J, Chen J, Wang Y, Chen
J, Li Y, Barati MT, Wintergerst KA, Pan K, et al: Elevating CXCR7
improves angiogenic function of EPCs via Akt/GSK-3beta/Fyn-mediated
Nrf2 activation in diabetic limb ischemia. Circ Res. 120:e7–e23.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yin Y, Huang L, Zhao X, Fang Y, Yu S, Zhao
J and Cui B: AMD3100 mobilizes endothelial progenitor cells in
mice, but inhibits its biological functions by blocking an
autocrine/paracrine regulatory loop of stromal cell derived
factor-1 in vitro. J Cardiovasc Pharmacol. 50:61–67. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tarnowski M, Liu R, Wysoczynski M,
Ratajczak J, Kucia M and Ratajczak MZ: CXCR7: A new SDF-1-binding
receptor in contrast to normal CD34(+) progenitors is functional
and is expressed at higher level in human malignant hematopoietic
cells. Eur J Haematol. 85:472–483. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kalatskaya I, Berchiche YA, Gravel S,
Limberg BJ, Rosenbaum JS and Heveker N: AMD3100 is a CXCR7 ligand
with allosteric agonist properties. Mol Pharmacol. 75:1240–1247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Narasipura SD, Wojciechowski JC, Charles
N, Liesveld JL and King MR: P-Selectin coated microtube for
enrichment of CD34+ hematopoietic stem and progenitor cells from
human bone marrow. Clin Chem. 54:77–85. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sun J, Li Y, Graziani GM, Filion L and
Allan DS: E-selectin mediated adhesion and migration of endothelial
colony forming cells is enhanced by SDF-1alpha/CXCR4. PLoS One.
8:e608902013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Vanderslice P, Biediger RJ, Woodside DG,
Brown WS, Khounlo S, Warier ND, Gundlach CW IV, Caivano AR,
Bornmann WG, Maxwell DS, et al: Small molecule agonist of very late
antigen-4 (VLA-4) integrin induces progenitor cell adhesion. J Biol
Chem. 288:19414–19428. 2013. View Article : Google Scholar : PubMed/NCBI
|