Introduction
Among women, ~2.5% of all malignant tumors are
ovarian cancer (OC) (1), but OC
accounts for 5% of cancer-associated mortality in female patients;
this is due to the low survival rates, which are largely caused by
the late-stage diagnosis of OC (2). At present, interval debulking surgery
followed by platinum-based chemotherapy is the standard treatment
for OC, but there remains a lack of methods for screening and early
diagnosis of this disease (3).
Thus, OC is usually diagnosed at a terminal stage, and it is
important to study the mechanisms of OC carcinogenesis for improved
diagnosis and treatment.
In the past decade, studies have uncovered the role
of lncRNAs in carcinogenesis, suggesting that these genes may be
used as promising biomarkers in cancer (4–6).
Among them, lncRNA ANRIL is associated with an unfavorable
prognosis and aggravates invasion in malignant OC (5), whereas lncRNA homeobox (HOX)
A11-antisense suppresses the oncogenic phenotype of epithelial OC
(6). Genes of the HOX family act
as transcription factors that contribute to embryo and cancer
development (7). HOXA distal
transcript antisense RNA (HOTTIP) is an lncRNA that locates near
chromosome 7p15.2 and transcribes from the 5′ end of the HOXA gene
(8). A previous study has revealed
that HOTTIP regulates the stem cell characteristics of pancreatic
cancer by regulating HOXA9 (9).
Upregulation of HOTTIP promotes the invasion of esophageal squamous
carcinoma cells by inducing epithelial-to-mesenchymal transition
(EMT) (10). A previous study has
also revealed that HOTTIP is an indicator of OC progression and
enhances cell proliferation and invasion (11), but the underlying mechanism remains
to be elucidated. However, downregulation of HOTTIP regulates the
cell cycle and insulin secretion of islet β cells by inhibiting the
mitogen-activated protein kinase kinase (MEK)/ERK signaling pathway
(12).
The present study hypothesized that HOTTIP may
promote ovarian carcinogenesis by activating the MEK/ERK
pathway.
Materials and methods
Cell culture
Human OC cell lines including OVCAR3, SKOV3 and
human ovarian epithelial cells (HOSEpiC) were purchased from the
American Type Culture Collection. The cells were cultured in
Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher
Scientific, Inc.) and supplemented with 10% fetal bovine serum
(FBS; Thermo Fisher Scientific, Inc.). The cells were cultured in a
humidified atmosphere at 37°C with 5% CO2.
Plasmids
HOTTIP short hairpin (sh)RNA (sh-HOTTIP) or negative
controls (sh-NC) were obtained from Shanghai GenePharma Co., Ltd. A
pcDNA3.1 vector (Invitrogen; Thermo Fisher Scientific, Inc.) was
used for the construction of the overexpression plasmid
pcDNA3.1-HOTTIP. The HOTTIP sequence (RefSeq, NR_037843.3) was
purchased from Sangon Biotech Co., Ltd. and subcloned into the
pcDNA3.1 vector; an empty pcDNA 3.1 vector was used as the
control.
Cell transfection
OVCAR3 and SKOV3 cells were seeded in 6-well plates
at the density of 1×106 cells/well, 2.5 µg sh-HOTTIP or
sh-NC was transfected into the cells for shRNA experiments and
cells without transfection were used as control. 3.0 µg
pcDNA3.1-HOTTIP or empty pcDNA3.1 (vector) was transfected into the
cells for overexpression experiments and cells transfected vector
were used as control. All the plasmids were transfected using
Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.)
with Opti-MEM medium (Invitrogen; Thermo Fisher Scientific, Inc.)
at 37°C for 48 h. The medium was replaced by DMEM containing 2% FBS
6 h post transfection at 37°C according to the manufacturer's
instructions. Cells were collected 48 h post-transfection, and
HOTTIP expression levels were determined by reverse
transcription-quantitative (RT-q PCR).
RT-qPCR
RNA extraction with TRIzol® (Invitrogen;
Thermo Fisher Scientific, Inc.) from OVCAR3 or SKOV3 cells was
carried out according to the manufacturer's protocol. The RNA was
reverse-transcribed to cDNA at 42°C for 15 min with a cDNA
synthesis kit (cat. no. AE341-02; TransGen Biotech Co., Ltd.)
according to the manufacturer's instructions. QPCR was performed
using a Roche Light-Cycler (Roche Diagnostics) whit a
SYBR® Green Reaction mix (Qiagen GmbH) under the
following thermocycling conditions: Denaturation at 95°C for 15 sec
and annealing at 60°C for 30 sec, extension at 72°C for 30 sec for
a total of 40 cycles. The primers used were as follows: HOTTIP
forward, 5′-AGCTCTTTTCCCCGACAGTG-3′ and reverse,
5′-CCTTCACCAAGCTCCCTCTG-3′; and β-actin forward,
5′-ATTGCCGACAGGATGCAGAA-3′ and reverse. 5′-GCTGATCCACATCTGCTGGA-3′.
Experiments were performed in triplicate, the expression levels of
HOTTIP were calculated and normalized to β-actin using the
2−ΔΔCq method (13).
Colony formation assay
OVCAR3 or SKOV3 cells (200 cells/well) were seeded
in 6-well plates and cultured in complete DMEM for 14 days at 37°C,
culture medium changed regularly. Following washing with PBS, the
adherent colonies were fixed in cold 20% methanol at 4°C for 10 min
and stained with 1% crystal violet (Sigma-Aldrich; Merck KGaA)
dissolved in methanol for 15 min at room temperature. In some
instances, 10 µM U0126 (MedChemExpress) was added 12 h after the
transfection to block MEK/ERK pathway selectively. The colonies
were rinsed and counted under an inverted light microscope
(magnification, ×40).
Flow cytometry analysis of
apoptosis
An Annexin V-FITC Detection kit (Beyotime Institute
of Biotechnology) was used to detect apoptosis. OVCAR3 or SKOV3
cells grown in 6-cm dishes at the density of 1×106
cells/well were harvested by trypsinization without EDTA and
incubated with FITC-labelled Annexin V and propidium iodide (PI)
according to the manufacturer's instructions. FlowJo version 10
software (FlowJo LLC) was used for analysis. The experiments were
performed three times.
Western blotting
OVCAR3 or SKOV3 cells were harvested and lysed with
ice cold RIPA buffer (Sigma-Aldrich; Merck KGaA) supplemented with
a protease inhibitor cocktail (Roche Diagnostics), which was used
to extract the total protein. The lysates were centrifuged at
10,000 × g for 10 min at 4°C to remove debris and the protein
concentration was determined by BCA Kit (Takara Bio, Inc.). Cell
lysate containing 20 µg total protein samples was subjected to 10%
SDS-PAGE and transferred to PVDF membranes (EMD Millipore).
Following blocking with a BSA (BioFroxx)-TBST solution (1×TBS, 1%
Tween-20, 5% w/v BSA) for 1 h at room temperature, the membranes
were incubated with primary antibodies (dilution 1:1,000) overnight
at 4°C. The following antibodies were used: Anti-E-Cadherin (cat.
no. 14472S), cleaved caspase-3 (cat. no. 9661S), total caspase-3
(cat. no. 9662S), phosphorylated (p-)p44/42 mitogen-activated
protein kinase (MAPK; Erk1/2; Thr202/Tyr204; cat. no. 4370S) and
p44/42 MAPK (Erk1/2; cat. no. 4695S), p-mitogen-activated protein
kinase kinase (MEK)1/2 (Ser217/221; cat. no. 9154S), MEK1/2 (cat.
no. 9122S), Bcl-2 (cat. no. 4223S) and Bax (cat. no. 14796S) from
Cell Signaling Technologies, Inc; Ki67 (SP6; cat. no. GTX20833)
from GeneTex, Inc.; proliferating cell nuclear antigen (PCNA; cat.
no. 10205-2-AP), vimentin (cat. no. 10366-1-AP) and N-cadherin
(cat. no. 22018-1-AP) from ProteinTech Group, Inc.; and SNAIL
(Sn9H2, cat. no. ab229701) and β-actin (cat. no. ab179467) from
Abcam. Primary antibody incubation was followed by incubation with
a horseradish-peroxidase-conjugated secondary antibody (dilution
1:10,000; cat. no. ab205718, Abcam) at room temperature for 1 h,
and the protein blots on the membrane were imaged by
chemiluminescence kit (Pierce; Thermo Fisher Scientific, Inc). Data
were semi-quantified using ImageJ software v1.41 (National
institutes of Health).
Cell invasion assay
Cell invasion was determined using Transwell
invasion chambers with 8 µm pores (BD Diagnostics) pre-coated with
Matrigel overnight at 4°C according to the manufacturer's protocol.
Briefly, 1×105 OVCAR3 or SKOV3 cells were transfected,
resuspended in 200 µl DMEM without FBS and placed into the upper
chamber of the insert with or without 10 µM U0126. Complete DMEM
medium plus 10% FBS was used as a chemoattractant in the lower
chamber. The cells were incubated at 37°C for 1 day; subsequently,
the cells on the back surface of the membrane were fixed with 20%
methanol for 30 min at 4°C and stained with 0.1% crystal violet for
20 min at room temperature. The number of cells on the surface of
the membrane was determined in five random fields of view using an
IX71 inverted microscope (Olympus Corporation), and images were
captured at ×200 magnification. The experiments were performed
three times.
Cell migration assay
OVCAR3 or SKOV3 cells (2×106) with or
without transfection and 10 µM U0126 were plated into a 6-well
plate and cultured in complete DMEM at 37°C until the cells were
grown to confluence. Following a pre-incubation with 10 µg/ml
mitomycin C (Sigma-Aldrich; Merck KGaA) for 2 h at 37°C, wounds
were created in OC cell monolayers with a sterile 200 µl pipette
tip, and the medium was aspirated to remove the detached cells.
Serum-free medium was added to the 6-well plate, and images of the
wound were captured at 0 and 24 h. This experiment was performed
three times. The wound area was analyzed in five random fields of
view with an IX71 inverted microscope (Olympus Corporation) using
AlphaImager 2200 software v3.2 (ProteinSimple).
Statistical analysis
The data are presented as the mean ± standard
deviation of three independent experiments. GraphPad Prism 5.0
(GraphPad Software, Inc.) was used for analysis. Statistical
analyses were performed using one-way ANOVA with Tukey's post-hoc
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Knockdown of HOTTIP inhibits the
MEK/ERK pathway in OC cells
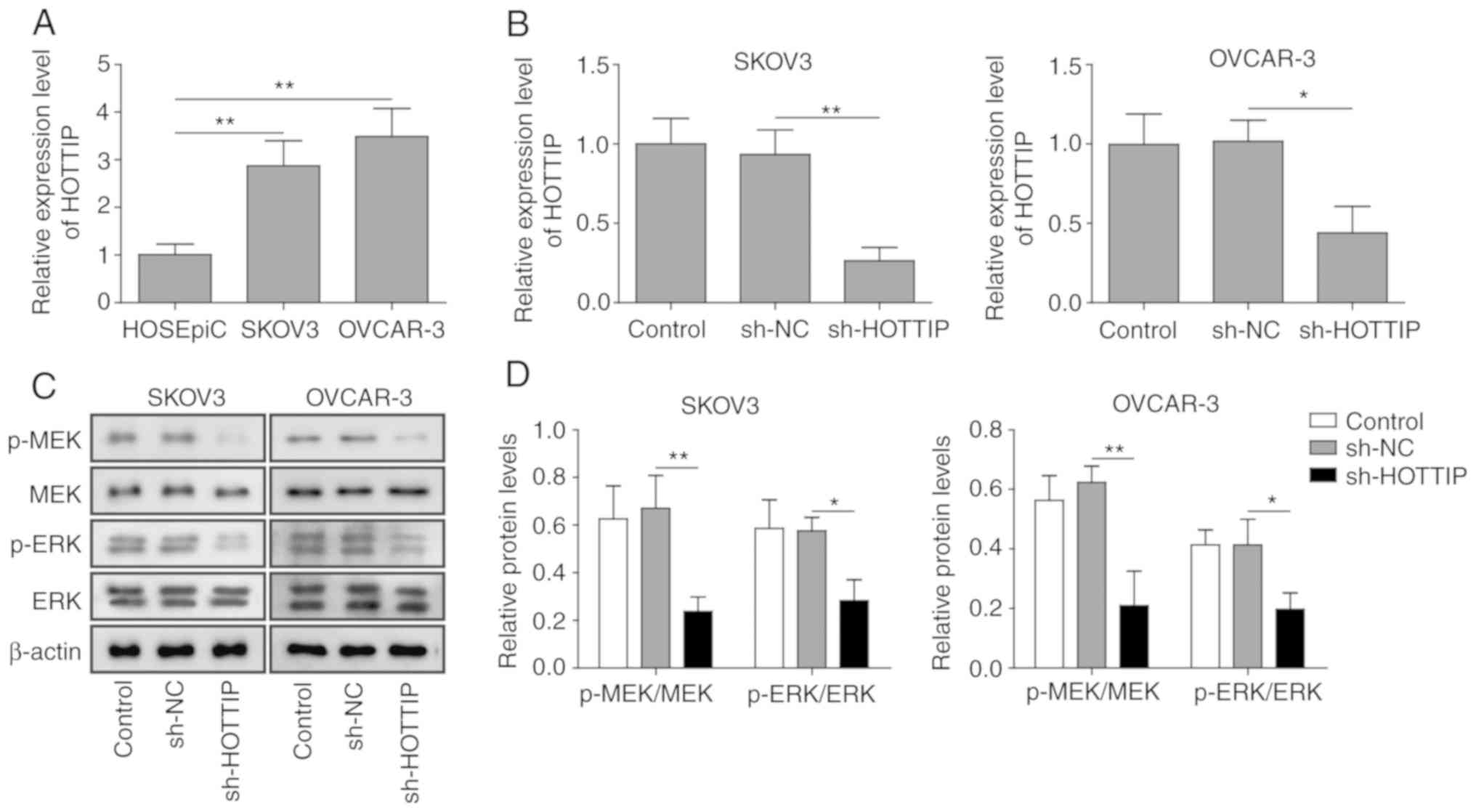
To explore the role of HOTTIP in OC progression,
HOTTIP expression levels in human ovarian epithelial and OC cells
were determined by RT-qPCR. SKOV3 and OVCAR-3 cells expressed
higher levels of HOTTIP compared with HOSEpiC ovarian epithelial
cells (Fig. 1A). To investigate
the function of HOTTIP in OC cells, HOTTIP was knocked down by
shRNA transfection. The expression of HOTTIP was significantly
downregulated following transfection with sh-HOTTIP in SKOV3 and
OVCAR-3 cells (Fig. 1B). In
addition, HOTTIP knockdown reduced the levels of MEK and ERK
phosphorylation compared with those in the cells transfected with
sh-NC (Fig. 1C and D), which
suggested that HOTTIP was involved in the MEK/ERK signaling
transduction in human OC cells.
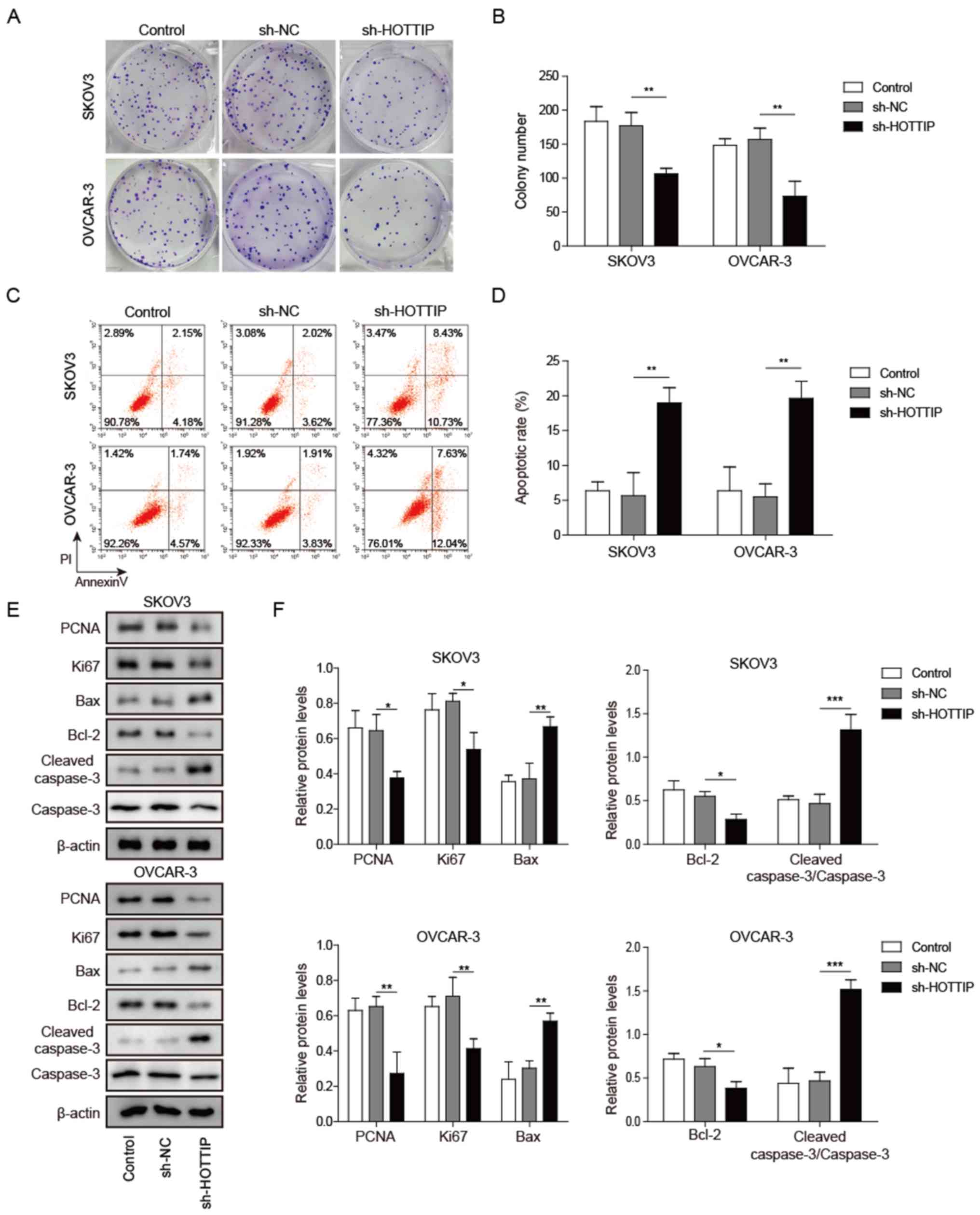
HOTTIP knockdown suppresses OC cell
proliferation and promotes apoptosis
The MEK/ERK pathway serves an important role in
regulating cell differentiation, proliferation, survival, migration
and malignant transformation (14). In the present study, HOTTIP
knockdown significantly reduced the proliferation of SKOV3 and
OVCAR-3 cells (Fig. 2A and B).
Furthermore, HOTTIP silencing promoted OC cell apoptosis as
detected by Annexin V/PI staining (Fig. 2C and D). To further investigate the
molecular mechanisms by which HOTTIP knockdown affected OC cell
proliferation and apoptosis, protein markers of cell proliferation
(PCNA and ki67) and apoptosis (cleaved caspase-3, Bax and Bcl-2)
were analyzed by western blotting. The results demonstrated that
knockdown of HOTTIP significantly reduced PCNA, ki67 and Bcl-2
protein levels, and elevated those of cleaved-caspase 3 and Bax in
SKOV3 and OVCAR-3 cells compared with cells transfected with sh-NC
(Fig. 2E and F). These results
indicated that HOTTIP knockdown suppressed OC cell proliferation
and promoted apoptosis.
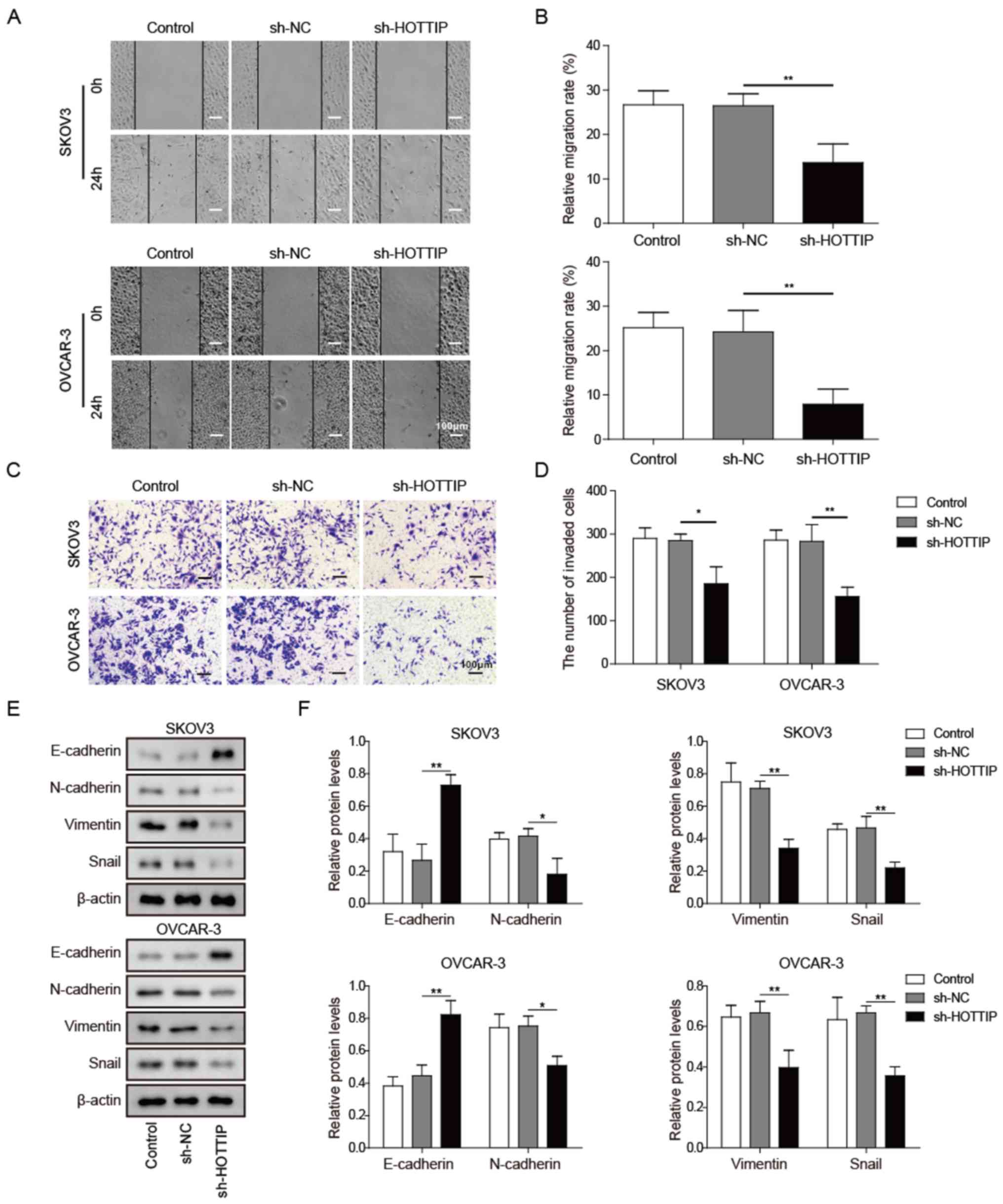
HOTTIP knockdown inhibits OC cell
migration, invasion and EMT
Metastasis is a major cause of poor outcomes in
patients with cancer; thus, the effects of HOTTIP on
metastasis-associated cell behaviors were assessed in vitro
using wound healing and Transwell assays. Wound healing assay
results demonstrated that knockdown of HOTTIP reduced the migration
of OC cells compared with that in cells transfected with sh-NC
(Fig. 3A and B). Cancer cell
invasion was also reduced by HOTTIP knockdown compared with that in
the control shRNA group as detected by the Transwell assay
(Fig. 3C and D). These results
demonstrated that HOTTIP may exert an oncogenic role in OC by
facilitating cell migration and invasion.
EMT is associated with tumor cell invasion and
metastasis (15). Epithelial
tumors become more aggressive during EMT, which is associated with
the upregulation of mesenchymal protein markers, including vimentin
and N-cadherin, and the downregulation of epithelial markers, such
as E-cadherin (16). In the
present study, HOTTIP knockdown notably elevated E-cadherin and
reduced N-cadherin, vimentin and Snail expression in SKOV3 and
OVCAR-3 cells compared with that in cells transfected with sh-NC
(Fig. 3E and F). These results
suggested that HOTTIP may promote the migration and invasion of OC
cells via EMT.
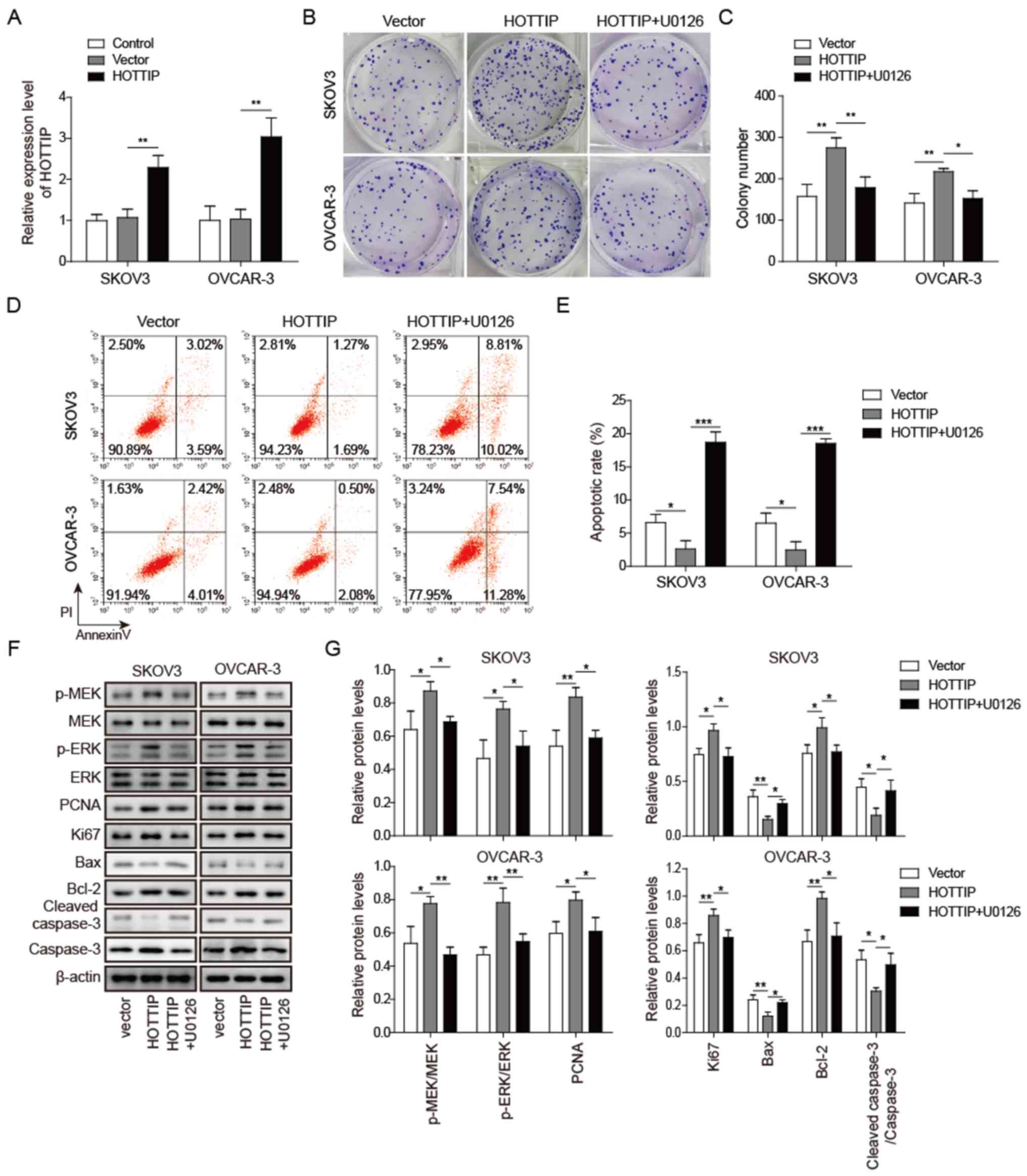
MEK/ERK pathway is involved in the
HOTTIP-induced proliferation of OC cells
HOTTIP serves crucial roles in OC cell proliferation
and apoptosis (11), and HOTTIP
knockdown significantly affected MEK and ERK phosphorylation in OC
cells. To further confirm that HOTTIP regulated OC cell
proliferation and apoptosis through the MEK/ERK pathway, SKOV3 and
OVCAR-3 cells were transfected with pcDNA3.1-HOTTIP and treated
with the MEK1/2-specific inhibitor U0126 simultaneously. The mRNA
expression level of HOTTIP was increased following transfection
with pcDNA3.1-HOTTIP in SKOV3 and OVCAR-3 cells compared with that
in cells transfected with an empty vector (Fig. 4A). HOTTIP overexpression promoted
OC cell colony formation, which was reversed by U0126 (Fig. 4B and C). In addition, the apoptotic
rate of OC cells was decreased when HOTTIP was overexpressed and
elevated in the presence of U0126 compared with the HOTTIP group,
as demonstrated by Annexin V/PI staining (Fig. 4D and E). Additionally, when HOTTIP
was overexpressed, the phosphorylation levels of MEK and ERK were
increased, the protein levels of PCNA, ki67 and Bcl-2 were
upregulated, and the levels of cleaved caspase-3 and Bax were
downregulated in SKOV3 and OVCAR-3 cells compared with those in the
cells transfected with an empty vector (Fig. 4F and G). U0126 inhibited the
HOTTIP-induced MEK and ERK phosphorylation and PCNA, ki67 and Bcl-2
expression, and decreased the levels of cleaved caspase-3 and Bax
in HOTTIP-overexpressing OC cells. These results suggested that the
MEK/ERK pathway was associated with HOTTIP-induced OC cell
proliferation and apoptosis.
MEK/ERK pathway participates in the
HOTTIP-induced invasion and migration of OC cells
As HOTTIP serves important roles in OC migration and
EMT (10–11), the present study further
investigated whether the MEK/ERK signaling pathway was involved in
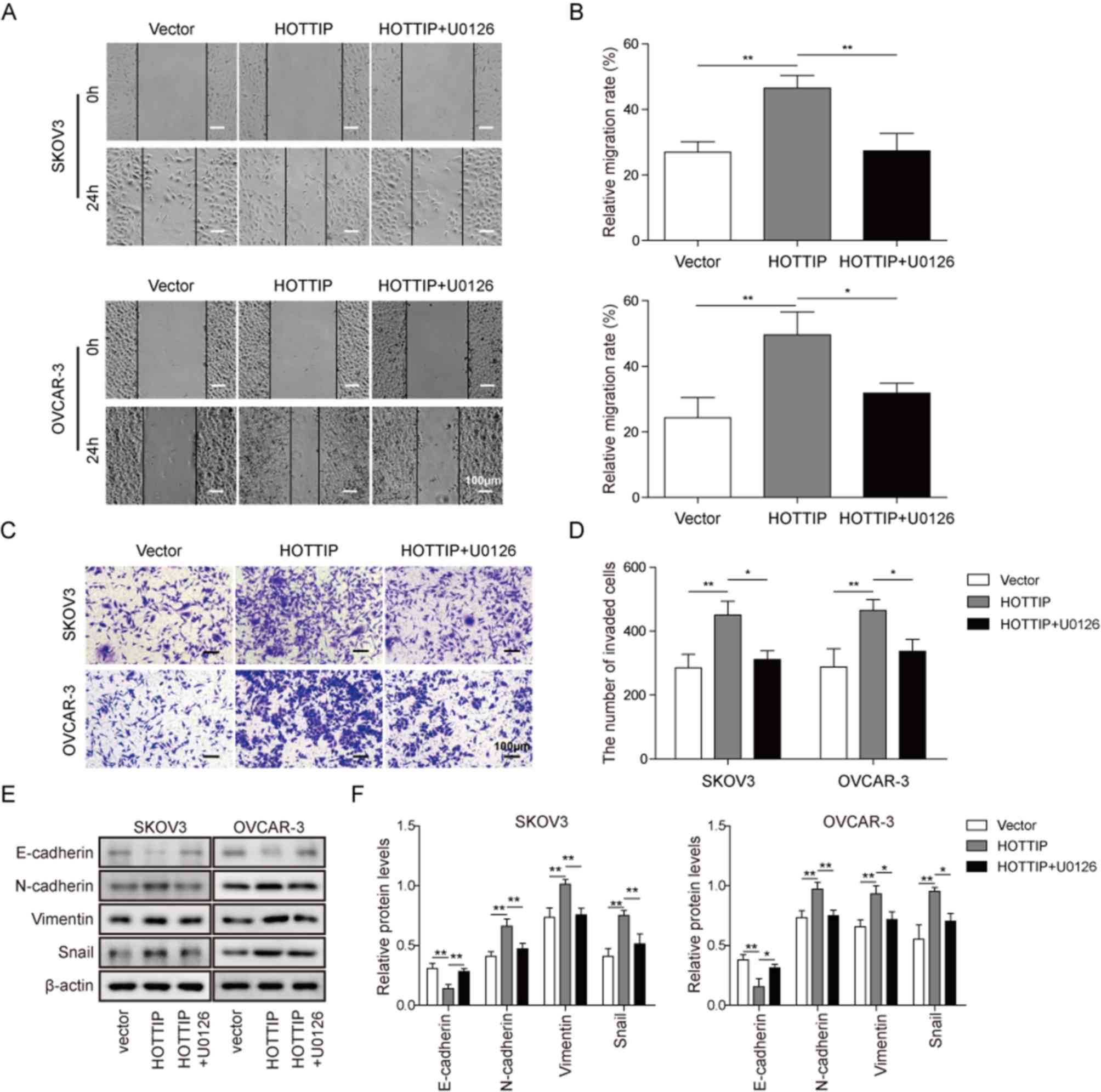
the HOTTIP-mediated OC cell migration and EMT. Overexpression of
HOTTIP promoted the migration and invasion of OC cells compared
with those of cells transfected with the empty vector, which was
demonstrated by the wound healing (Fig. 5A and B) and Transwell (Fig. 5C and D) assays. As presented in
Fig. 5A-D, the MEK1/2 inhibitor
U0126 significantly inhibited the HOTTIP-induced OC cell migration
and invasion, which suggested that the MEK/ERK pathway served a
role in HOTTIP-regulated OC cell migration. HOTTIP overexpression
downregulated the expression of the epithelial marker E-cadherin
and upregulated the expression of the mesenchymal markers
N-cadherin, vimentin and Snail in SKOV3 and OVCAR-3 cells compared
with those in vector-transfected cells; however, HOTTIP-induced OC
cell EMT was significantly reversed by U0126 (Fig. 5E and F). These results suggested
that HOTTIP promoted OC cell EMT via a mechanism involving the
activation of the MEK/ERK signaling pathway.
Discussion
OC is a serious threat to female health. Previous
studies have demonstrated that a number of lncRNAs are involved in
the development of OC (17–19),
including lncRNA HOTTIP, but the underlying mechanism remains to be
elucidated. The results of the present study revealed that HOTTIP
may be important for OC proliferation and invasion, and that the
cancer-promoting features of HOTTIP could be reversed by MEK1/2
inhibitor; this suggested that HOTTIP may promote the proliferation
and invasion of OC cells by activating the MEK/ERK pathway.
The function of lncRNA HOTTIP has been investigated
in multiple types of cancer, including esophageal squamous cell
(10) and thyroid (20) carcinoma, colorectal (21) and nasopharyngeal (22) cancer cells. The majority of studies
conclude that HOTTIP serves oncogenic roles (10,20–22).
The AKT survival pathway is upregulated by HOTTIP in various types
of cancer cells, including mammary (23), endometrial (24) and renal (25) cancer cells. In addition to cancer
cells, HOTTIP has been identified to regulate endothelial cell
proliferation and migration (26).
A recent study has suggested that HOTTIP is an indicator of OC
prognosis and facilitates OC cell proliferation and invasion
(11), but the underlying
mechanism remains to be elucidated. The present study demonstrated
that HOTTIP knockdown inhibited, whereas HOTTIP overexpression
promoted MEK and ERK phosphorylation in OC cells compared with that
in the respective negative control groups. The MEK/ERK pathway
regulates a number of important cellular functions including
proliferation, differentiation, survival and migration (27). Previous studies have demonstrated
that over-activation of MEK/ERK signaling is involved in the
oncogenesis of human cancers, which makes it an appealing target
for anticancer therapeutics (28–31).
The MEK/ERK pathway serves important roles in regulating gastric
cancer (29), retinoblastoma
(30) and pancreatic cancer
(31) oncogenesis. A recent study
has also revealed that the MEK/ERK cascade may be a promising
target to inhibit human OC cells via inducing apoptosis and cell
cycle arrest (32). The present
study identified that HOTTIP was involved in the regulation of OC
cell proliferation, apoptosis and invasion. The OC-promoting
properties of HOTTIP were reversed by a specific inhibitor of
MEK1/2, which suggested that HOTTIP promoted the proliferation and
invasion of OC cells by activating the MEK/ERK signaling
pathway.
Although a number of studies have revealed the
important role of lncRNAs in regulating different physiological and
pathological processes, the underlying mechanisms are mostly
uncharacterized (33). The sponge
or decoy function of lncRNA on micro (mi)RNAs has been revealed
(34). The results of the present
study suggested that lncRNA HOTTIP promoted OC via the MEK/ERK
pathway; however, no evidence was observed to identify whether
HOTTIP directly phosphorylated MEK or ERK. A recent study has
demonstrated that miRNA-145 inhibits the MEK/ERK pathway by
directly regulating ERK1/2 expression (35). miRNA-30a also suppresses MEK/ERK
signaling by targeting K-Ras mRNA (36). HOTTIP may regulate the activation
of the MEK/ERK pathway indirectly by interacting with other mRNA or
miRNAs; the specific regulatory mechanism of lncRNA HOTTIP on
MEK/ERK signaling transduction in OC needs to be further
investigated.
Tumor cells may be transformed into cancer stem
cell-like cells through EMT and lead to radiotherapy and
chemotherapy resistance, angiogenesis and distant metastasis, which
lead to the poor prognosis of tumors (37). EMT is accompanied by upregulation
of mesenchymal markers, such as N-cadherin and vimentin, and
downregulation of epithelial markers, including E-cadherin, and is
expected to be an important target in cancer treatment (16). The results of the present study
identified that the levels of the mesenchymal protein markers were
downregulated by HOTTIP knockdown and upregulated by HOTTIP
overexpression, whereas those of the epithelial protein marker
E-cadherin were upregulated by HOTTIP knockdown and downregulated
by HOTTIP overexpression. These results suggested that HOTTIP may
promote the migration and invasion of OC cells via EMT. In
addition, the HOTTIP-promoted changes in EMT marker expression in
OC cells were reversed by U0126, which suggested that HOTTIP
promoted OC cell EMT via a mechanism involving the activation of
the MEK/ERK signaling pathway.
In conclusion, HOTTIP promoted the proliferation,
invasion and EMT of OC cells by activating the MEK/ERK pathway.
These results indicated that HOTTIP and the MEK/ERK pathway axis
may contribute to OC tumorigenesis and metastasis, and that they
may be potential targets for the diagnosis and treatment of OC.
Acknowledgements
Not applicable.
Funding
This work was supported by Shaoguan Science and
Technology Planning Project in 2019 (grant no. 2019sn011).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
JL and ZL conceived and designed the present study.
JL and HH provided administrative support. YL and ZL provided study
materials. YL performed literature research and analyzed literature
suitability. FL and LZ performed the experiments. JL, ZL and HH
performed data analysis and interpretation. All authors wrote the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Trabert B, DeSantis CE, Miller
KD, Miller KD, Samimi G, Runowicz CD, Gaudet MM, Jemal A and Siegel
RL: Ovarian cancer statistics, 2018. Ca Cancer J Clin. 68:284–296.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cortez AJ, Tudrej P, Kujawa KA and
Lisowska KM: Advances in ovarian cancer therapy. Cancer Chemother
Pharmacol. 81:17–38. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ma DD, Yuan LL and Lin L: LncRNA HOTAIR
contributes to the tumorigenesis of nasopharyngeal carcinoma via
up-regulating FASN. Eur Rev Med Pharmacol Sci. 21:5143–5152.
2017.PubMed/NCBI
|
|
4
|
Nie GH, Li Z, Duan HF, Luo L, Hu HY, Yang
WQ, Nie LP, Zhu RF, Chen XF and Zhang W: lncRNA C22orf32-1
contributes to the tumorigenesis of nasopharyngeal carcinoma. Oncol
Lett. 13:4487–4492. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Qiu JJ, Lin YY, Ding JX, Feng WW, Jin HY
and Hua KQ: Long non-coding RNA ANRIL predicts poor prognosis and
promotes invasion/metastasis in serous ovarian cancer. Int J Oncol.
46:2497–2505. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Richards EJ, Permuth-Wey J, Li Y, Chen YA,
Coppola D, Reid BM, Lin HY, Teer JK, Berchuck A, Birrer MJ, et al:
A functional variant in HOXA11-AS, a novel long non-coding RNA,
inhibits the oncogenic phenotype of epithelial ovarian cancer.
Oncotarget. 6:34745–34757. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bhatlekar S, Fields JZ and Boman BM: HOX
genes and their role in the development of human cancers. J Mol Med
(Berl). 92:811–823. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang KC, Yang YW, Liu B, Sanyal A,
Corces-Zimmerman R, Chen Y, Lajoie BR, Protacio A, Flynn RA, Gupta
RA, et al: A long noncoding RNA maintains active chromatin to
coordinate homeotic gene expression. Nature. 472:120–124. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fu ZQ, Chen CH, Zhou QB, Wang YX, Zhao Y,
Zhao X, Li W, Zheng S, Ye H, Wang L, et al: LncRNA HOTTIP modulates
cancer stem cell properties in human pancreatic cancer by
regulating HOXA9. Cancer Lett. 410:68–81. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen X, Han H, Li Y, Zhang Q, Mo K and
Chen S: Upregulation of long noncoding RNA HOTTIP promotes
metastasis of esophageal squamous cell carcinoma via induction of
EMT. Oncotarget. 7:84480–84485. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zou T, Wang PL, Gao Y and Liang WT: Long
noncoding RNA HOTTIP is a significant indicator of ovarian cancer
prognosis and enhances cell proliferation and invasion. Cancer
Biomark. 25:133–139. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xu X, Tian J and Li QY: Downregulation of
HOTTIP regulates insulin secretion and cell cycle in islet β cells
via inhibiting MEK/ERK pathway. Eur Rev Med Pharmacol Sci.
22:4962–4968. 2018.PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
McCubrey JA, Steelman LS, Chappell WH,
Abrams SL, Wong EW, Chang F, Lehmann B, Terrian DM, Milella M,
Tafuri A, et al: Roles of the Raf/MEK/ERK pathway in cell growth,
malignant transformation and drug resistance. Biochim Biophys Acta.
1773:1263–1284. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang S, Yan Y, Cheng Z, Hu Y and Liu T:
Sotetsuflavone suppresses invasion and metastasis in non-small-cell
lung cancer A549 cells by reversing EMT via the TNF-α/NF-κB and
PI3K/AKT signaling pathway. Cell Death Discov. 4:262018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kang YB and Massagué J:
Epithelial-mesenchymal transitions: Twist in development and
metastasis. Cell. 118:277–279. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nikpayam E, Tasharrofi B, Sarrafzadeh S
and Ghafouri-Fard S: The role of long non-coding RNAs in ovarian
cancer. Iran Biomed J. 21:3–15. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fu Y, Biglia N, Wang Z, Shen Y, Risch HA,
Lu L, Canuto EM, Jia W, Katsaros D and Yu H: Long non-coding RNAs,
ASAP1-IT1, FAM215A, and LINC00472, in epithelial ovarian cancer.
Gynecol Oncol. 143:642–649. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Vafaee F, Colvin EK, Mok SC, Howell VM and
Samimi G: Functional prediction of long non-coding RNAs in ovarian
cancer-associated fibroblasts indicate a potential role in
metastasis. Sci Rep. 7:103742017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yuan Q, Liu Y, Fan Y, Liu Z, Wang X, Jia
M, Geng Z, Zhang J and Lu X: LncRNA HOTTIP promotes papillary
thyroid carcinoma cell proliferation, invasion and migration by
regulating miR-637. Int J Biochem Cell Biol. 98:1–9. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu T, Yu T, Hu H and He K: Knockdown of
the long non-coding RNA HOTTIP inhibits colorectal cancer cell
proliferation and migration and induces apoptosis by targeting
SGK1. Biomed Pharmacother. 98:286–296. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shen M, Li M and Liu J: Long noncoding RNA
HOTTIP promotes nasopharyngeal cancer cell proliferation,
migration, and invasion by inhibiting miR-4301. Med Sci Monit.
25:778–785. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gao W, Wu XL, Li DZ and Liu HD: HOTTIP
participates in mammary cancer by promoting cell proliferation via
PI3K/AKT pathway. Eur Rev Med Pharmacol Sci. 22:4181–4187.
2018.PubMed/NCBI
|
|
24
|
Guan Q, Zhang Q, Zhang C, Liu Q and Ren
QL: HOTTIP regulates progression of endometrial cancer via
activating PI3K/AKT pathway. Eur Rev Med Pharmacol Sci.
22:3727–3733. 2018.PubMed/NCBI
|
|
25
|
Su Y, Lu J, Chen X, Liang C, Luo P, Qin C
and Zhang J: Long non-coding RNA HOTTIP affects renal cell
carcinoma progression by regulating autophagy via the
PI3K/Akt/Atg13 signaling pathway. J Cancer Res Clin Oncol.
145:573–588. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liao B, Chen R, Lin F, Mai A, Chen J, Li
H, Xu Z and Dong S: Long noncoding RNA HOTTIP promotes endothelial
cell proliferation and migration via activation of the
Wnt/β-catenin pathway. J Cell Biochem. 119:2797–2805. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Guo L, Bai Y, Ji S and Ma H: MicroRNA-98
suppresses cell growth and invasion of retinoblastoma via targeting
the IGF1R/k-Ras/Raf/MEK/ERK signaling pathway. Int J Oncol.
54:807–820. 2019.PubMed/NCBI
|
|
28
|
Yu ZT, Ye SQ, Hu GY, Lv M, Tu Z, Zhou K
and Li QB: The RAF-MEK-ERK pathway: Targeting ERK to overcome
obstacles of effective cancer therapy. Future Med Chem. 7:269–289.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang HZ, Jiang HJ, Zhang HX, Liu JC, Hu X
and Chen L: Ribophorin II potentiates P-glycoprotein- and
ABCG2-mediated multidrug resistance via activating ERK pathway in
gastric cancer. Int J Biol Macromol. 128:574–582. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yu FF, Pang GL and Zhao GQ: ANRIL acts as
onco-lncRNA by regulation of microRNA-24/c-Myc, MEK/ERK and
Wnt/β-catenin pathway in retinoblastoma. Int J Biol Macromol.
128:583–592. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang J, Guo XJ, Xie CC and Jiang JX: KIF15
promotes pancreatic cancer proliferation via the MEK-ERK signalling
pathway. Br J Cancer. 117:245–255. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hua F, Li CH, Chen XG and Liu XP: Daidzein
exerts anticancer activity towards SKOV3 human ovarian cancer cells
by inducing apoptosis and cell cycle arrest, and inhibiting the
Raf/MEK/ERK cascade. Int J Mol Med. 41:3485–3492. 2018.PubMed/NCBI
|
|
33
|
Durruthy-Durruthy J, Sebastiano V,
Wossidlo M, Cepeda D, Cui J, Grow EJ, Davila J, Mall M, Wong WH,
Wysocka J, et al: The primate-specific noncoding RNA HPAT5
regulates pluripotency during human preimplantation development and
nuclear reprogramming. Nat Genet. 48:44–52. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Paraskevopoulou MD and Hatzigeorgiou AG:
Analyzing MiRNA-LncRNA interactions. Methods Mol Biol.
1402:271–286. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jafarnejad SM, Chapat C, Matta-Camacho E,
Gelbart IA, Hesketh GG, Arguello M, Garzia A, Kim SH, Attig J,
Shapiro M, et al: Translational control of ERK signaling through
miRNA/4EHP-directed silencing. Elife. 7:e350342018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhou K, Luo X, Wang Y, Cao D and Sun G:
MicroRNA-30a suppresses tumor progression by blocking
Ras/Raf/MEK/ERK signaling pathway in hepatocellular carcinoma.
Biomed Pharmacother. 93:1025–1032. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lamouille S, Xu J and Derynck R: Molecular
mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell
Biol. 15:178–196. 2014. View
Article : Google Scholar : PubMed/NCBI
|