Introduction
Liver cancer is a malignancy with a poor prognosis
worldwide that seriously threatens human health (1). China has a high prevalence of liver
cancer with an incidence of 466.1 per 100,000 population in 2015
(2). Liver cancer is asymptomatic
in its early stages and is usually diagnosed at an advanced stage
of the disease, which means successful surgery impossible.
Currently, the application of chemotherapy and molecule-targeted
drugs are the main approaches for the treatment of advanced liver
cancer (3). However, the side
effects of chemotherapy and resistance of target drugs limit the
therapeutic effects for patients. Therefore, researchers have
attempted to identify natural drugs with high efficiency and low
toxicity for the treatment and prevention of liver cancer (4,5).
Green tea (GT) is one of the most popular beverages
worldwide, particularly in China (6). It has been reported that GT has
beneficial effects on a number of human diseases including
diabetes, cardiovascular diseases, inflammatory diseases and cancer
(7–10). The main bioactive compounds in GT
are catechins, which comprise a mixture of catechin, epicatechin,
epigallocatechin, epicatechin gallate and epigallocatechin gallate
(EGCG) (11). Of the five
components, EGCG is the most abundant and bioactive catechin.
In the 1980s, our previous study investigated the
chemical prevention of liver cancer in China and identified that GT
can significantly inhibit hepatocarcinogenesis in rats induced by
aflatoxin B1 (AFB1) and diethylnitrosamine (DEN) (12,13).
Subsequently, GT was provided to high-risk groups in the high-risk
areas of liver cancer in the Chinese region of Guangxi and the
results demonstrated that GT can reduce the incidence of liver
cancer (14). EGCG exhibits
versatile antitumor functions, including inhibition of
proliferation, adhesion, migration, invasion and metastasis of
tumor cells, as well as reduction of angiogenesis, induction of
apoptosis and enhancement of anti-tumor immunity and cell cycle
arrest (15–19). It has been reported that EGCG
induces S phase arrest and inhibits cell proliferation in
hepatocellular carcinoma (HCC) (20). Moreover, EGCG may act via the
regulation of cell cycle regulatory proteins, including cyclin D1
and the cyclin B1/CDK1 complex, as shown in cell-based studies
(21,22). It has also been revealed that cell
division cycle 25A (CDC25A) can activate cell CDKs, including
cyclin B1/CDK1, to regulate the cell cycle (23). However, to the best of our
knowledge, no previous studies have investigated the antitumor and
preventive effects of EGCG on HCC via the inhibition of CDC25A.
Therefore, the present study evaluated CDC25A as the target of EGCG
in liver cancer. EGCG was compared with GT extract (GTE) under an
EGCG equivalence condition. The present findings provided a novel
mechanistic basis for EGCG application in HCC prevention and
treatment.
Materials and methods
Cell culture
The human hepatoma cell lines HepG2 and Huh7 were
obtained from Shanghai GeneChem Co., Ltd. The cell lines used have
been authenticated using short tandem repeat profiling. The cells
were maintained in DMEM (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.)
and 1% penicillin/streptomycin (Beyotime Institute of
Biotechnology) in a fully humidified incubator at 37°C with 5%
CO2.
Cell viability
HepG2 and Huh7 cells were seeded into a 96-well
plate at a density of 1×104 cells per well in 100 µl
culture medium. The cells were incubated for 24 h at 37°C, treated
with various concentrations (25, 50, 75, 100, 125 and 150 µg/ml) of
EGCG (purity >95%; Sigma-Aldrich; Merck KGaA) and cultured for
24, 48 or 72 h at 37°C with 5% CO2. Subsequently, 10
µl/well Cell Counting Kit (CCK)-8 solution (Dojindo Molecular
Technologies, Inc.) was added to each well, according to the
manufacturer's instructions. The plate was incubated at 37°C in a
5% CO2 atmosphere for 1 h and the absorbance per well
was recorded at 450 nm on a microplate reader. The resulting data
were analyzed from three independent experiments and then
normalized to the absorbance of the wells containing culture medium
alone. The cell viability was calculated using the following
formula: Cell viability (%)=[optical density
(OD)sample-ODblank)/(ODcontrol-ODblank)
×100. Inhibitory rate (%)=1-Cell viability (%). The half maximal
inhibitory concentration (IC50) values were determined
using GraphPad Prism 8.0 software (GraphPad Software, Inc.).
Cell cycle assay
HepG2 cells were treated with 127.09 µg/ml EGCG for
48 h at 37°C. Single-cell suspensions from the EGCG and control
groups (1×106 cells/ml in ice-cold PBS; Beijing Solarbio
Science & Technology Co., Ltd.) were prepared, washed with
ice-cold PBS and fixed with 70% ethanol at 4°C overnight.
Subsequently, the cells were stained with propidium iodide (70 µM;
Sigma-Aldrich; Merck KGaA) solution containing RNase A (10 µg/ml;
Fermentas; Thermo Fisher Scientific, Inc.) for 30 min at room
temperature. The cell cycle distribution was analyzed using a FACS
Calibur flow cytometer (BD Biosciences) and the percentage of each
population was measured using ModFIT software (version 2.0; BD
Biosciences).
Lentiviral particle generation and
infection
Lentivirus with CDC25A overexpression and control
lentivirus were provided by FitGene Biotechnology Co., Ltd. The
CDC25A coding sequence was amplified using PrimeSTAR Max polymerase
premix (Takara Biotechnology, Co., Ltd.) and verified by
sequencing. The primer sequences used for the amplification of
CDC25A were: Forward,
5′-CTACCGGACTCAGATCTCGAGGCCACCATGGAACTGGGCCCGGAG-3′ and reverse,
5′-GTAGTCAGATCCCATGGATCCGAGCTTCTTCAGACGACTG-3′. The following
thermocycling conditions were used: Initial denaturation at 96°C
for 4 min; followed by 30 cycles at 96°C for 20 sec, 60°C for 30
sec and 72°C for 1 min; followed by 72°C for 3 min. The CDC25A
coding sequence was inserted into the pLVX-Puro vector (FitGene
Biotechnology Co., Ltd.), and named Lenti-CDC25A. The empty
lentiviral vector (Lenti-vector) was generated as a negative
control. HepG2 cells seeded in 6-well plates at a density of
1×105 cells per well were cultured in serum-free DMEM.
The cells, which were 50–70% confluent, were mixed with the medium
containing lentiviruses and polybrene (10 µg/ml; Beijing Solarbio
Science & Technology Co., Ltd.) at a multiplicity of infection
of 10. After incubation for 24 h at 37°C, the supernatants were
replaced by the medium containing 10% FBS and cultured for a
further 48 h at 37°C for subsequent experimentation. The CDC25A
expression efficiency was measured using reverse
transcription-quantitative (RT-q)PCR and western blotting.
Animals and their treatment
Animal experiments were ethically approved and
supervised by the Ethics Committee of Ethics Committee of Guangxi
Medical University Cancer Hospital (approval no. LW2018022). A
total of 65 male SD rats [age, 4 weeks; weight, 100–120 g; Guangxi
Medical University (Nanning, China)] were housed individually in
CombiCages under controlled light (12-h light/dark cycle) and
temperature (22±3°C and 40–70% relative humidity) conditions. Food
and water were available ad libitum. The rats were randomly
divided into six groups and the treatment methods are presented in
Table I.
 | Table I.Distribution and treatment of
rats. |
Table I.
Distribution and treatment of
rats.
| Group | Number | Treatment
methods |
|---|
| Normal | 10 | Intraperitoneal
injection of physiological saline once a week from the 1st to 3rd
week and from the 11 to 13th week; intragastric administration of
physiological saline every day |
| HCC | 15 | Intraperitoneal
injection of DEN once a week from the 1st to 3rd week and from the
11 to 13th week; intragastric administration of physiological
saline every day |
| EGCG treatment | 10 | Intraperitoneal
injection of DEN once a week from the 1st to 3rd week and from the
11 to 13th week; intragastric administration of EGCG every day |
| GTE treatment | 10 | Intraperitoneal
injection of DEN once a week from the 1st to 3rd week and from the
11 to 13th week; intragastric administration of GTE every day |
| EGCG control | 10 | Intraperitoneal
injection of physiological saline once a week from the 1st to 3rd
week and from the 11 to 13th week; intragastric administration of
EGCG every day |
| GTE control | 10 | Intraperitoneal
injection of physiological saline once a week from the 1st to 3rd
week and from the 11 to 13th week; intragastric administration of
GTE every day |
The animal models of HCC were induced via DEN (20
mg/ml; Sigma-Aldrich; Merck KGaA) intraperitoneally injected (100
mg/kg body weight) once a week from the 1st-3rd week and from the
11–13th week. Moreover, 10 mg/ml EGCG (98%; Yibeijia Teatechnology)
dissolved in physiological saline and 20 mg/ml GTE (containing 49%
EGCG; Yibeijia Teatechnology) dissolved in physiological saline
were prepared to be used for intragastric administration (EGCG, 25
mg/kg and GTE, 50 mg/kg body weight every day of the experiment). A
curved, stainless steel 16-gauge gavage needle was used to deliver
the compound to the stomach and the needle was wiped between
animals. Rats that received an intraperitoneal injection of
physiological saline served as the control group. Vein blood
collection and liver biopsies were conducted during the 10–20th
weeks. Following routine disinfection at a site below the
left-sided groin of the rats, ~2 ml blood was drawn via the femoral
vein puncture and serum was separated following centrifugation at
2,000 × g for 10 min at room temperature. The concentrations of
alanine aminotransferase (ALT; cat. no. OSR6107; Beckman Coulter,
Inc.), aspartate transaminase (AST; cat. no. OSR6209; Beckman
Coulter, Inc.), alkaline phosphatase (ALP; cat. no. OSR6104;
Beckman Coulter, Inc.) and γ glutamyl transpeptidase (GGT; cat. no.
OSR6120; Beckman Coulter, Inc.) were determined using their
respective kits on a biochemical autoanalyzer (AU5800; Beckman
Coulter, Inc.).
Liver biopsies were performed as follows: Anesthesia
was induced by intraperitoneal injection of sodium pentobarbital
(40 mg/kg body weight). Following the preparation of the operation
site by removing fur, the anesthetized rat was disinfected with
iodine tincture and 75% alcohol, followed by a cut in the abdominal
wall along the middle of the abdomen near the xiphoid process. The
whole liver was exposed and ~1.0×0.6×0.6 cm in size was excised.
The incision was immediately pressed with sterile gauze for
hemostasis. Subsequently, 0.2 ml gentamicin sulfate (40,000 U/ml)
was injected into the abdominal cavity of rats and the abdominal
incision was sutured layer by layer. The harvested liver tissue was
divided into two pieces; one was immediately frozen with liquid
nitrogen (−196°C), the other was fixed with 4% paraformaldehyde for
48 h at room temperature. Animal health and behavior were monitored
daily.
When the animals exhibited a loss of appetite,
lethargy and clinical symptoms of severe loss of organ function or
when the maximum percentage weight loss was observed to be >10%,
the animals were sacrificed. The method used was intraperitoneal
injection of sodium pentobarbital (200 mg/kg). During the
experiment, a total of 25 mice died. The criteria for verifying
animal death were no breathing, no heartbeat and no corneal reflex.
All remaining rats were sacrificed at the 30th week, the liver
tissues were obtained and the tumor volume measured. For rats
presenting with multiple tumors, the largest tumor was selected for
statistical analysis of tumor volume. The tumor volume formula used
was as follows: Volume (mm3)=width2
(mm2) × length (mm)/2.
RT-qPCR
Total RNA was extracted from 2×105 cells
or 50 mg tissues using TRIzol® reagent (Invitrogen;
Thermo Fisher Scientific, Inc.) and first strand cDNA was
synthesized via reverse transcription from 1 µg RNA using a
PrimeScript RT Reagent kit with gDNA Eraser (Takara Biotechnology
Co., Ltd.), according to the manufacturer's protocol. Reverse
transcription was performed for 15 min at 37°C followed by 85°C for
5 sec. qPCR was performed using a standard protocol from the
SYBR® Premix Ex Taq kit (Takara Biotechnology Co., Ltd.)
on the CFX96 Real-Time PCR Detection system (Bio-Rad Laboratories,
Inc.), according to the manufacturer's protocol. The thermocycling
conditions were: Initial denaturation at 95°C for 30 sec, followed
by 40 cycles at 95°C for 5 sec and 60°C for 1 min. Primer sequences
used in the present study are provided in Table II. The relative mRNA expression
levels compared with the GAPDH control were analyzed with the
2−ΔΔCq method (24).
The experiments were replicated three times.
 | Table II.Primers set used for the detection of
human and rat CDC25A, p21waf1/Cip1 and GAPDH. |
Table II.
Primers set used for the detection of
human and rat CDC25A, p21waf1/Cip1 and GAPDH.
| Name | Sequence 5′→3′ | Product length
(base pairs) |
|---|
| Human CDC25A | Forward:
TTCCTCTTTTTACACCCCAGTCA | 173 |
|
| Reverse:
TCGGTTGTCAAGGTTTGTAGTTC |
|
| Human
p21waf1/Cip1 | Forward:
TGTCCGTCAGAACCCATGC | 139 |
|
| Reverse:
AAAGTCGAAGTTCCATCGCTC |
|
| Human GAPDH | Forward:
TGACTTCAACAGCGACACCCA | 121 |
|
| Reverse:
CACCCTGTTGCTGTAGCCAAA |
|
| Rat CDC25A | Forward:
CCAAAGGAACCATTGAGAAC | 138 |
|
| Reverse:
CAGATGCCATAATTTCTGGAG |
|
| Rat
p21waf1/Cip1 | Forward:
TGTTCCACACAGGAGCAAAG | 175 |
|
| Reverse:
AACACGCTCCCAGACGTAGT |
|
| Rat GAPDH | Forward:
AAGAAGGTGGTGAAGCAGGC | 200 |
|
| Reverse:
ACCACCCTGTTGCTGTAGCC |
|
Western blotting
HepG2 cells were treated with different
concentrations of EGCG (0, 75, 100 and 125 µg/ml) for 48 h at 37°C.
Subsequently, the cells were washed twice with ice-cold PBS. The
liver tissues obtained during the 10, 20 and 30th weeks of the
experiment were washed 2–3 times with cold PBS followed by
homogenization and incubation on ice for 30 min. The prepared cells
and tissues were lysed with RIPA lysis buffer (Sigma-Aldrich; Merck
KGaA) containing 1% phenylmethylsulfonyl fluoride (1 mM;
Sigma-Aldrich; Merck KGaA) and centrifuged (4°C; 10,000 × g; 10
min). The concentration of total protein was measured using the
Bradford assay. Each well was loaded with 50 µg protein. The
samples were fractionated on a 10% SDS-PAGE, stacked at 80 V for 35
min and separated at 120 V for 1 h. Following electrophoresis, the
proteins were transferred to PVDF membranes (EMD Millipore). The
membranes were blocked for 1 h at room temperature with 5% skimmed
milk and incubated overnight at 4°C with different primary
antibodies: Anti-CDC25A rabbit polyclonal antibody (1:1,000; cat.
no. GB11283; Wuhan Servicebio Technology Co., Ltd.),
anti-p21waf1/Cip1 rabbit polyclonal antibody (1:1,000; cat. no.
GB11153; Wuhan Servicebio Technology Co., Ltd.) and anti-GAPDH
mouse polyclonal antibody (1:50,000; cat. no. GB11002; Wuhan
Servicebio Technology Co., Ltd.). Following the primary antibody
incubation, the membranes were incubated for 1 h at 37°C with
anti-rabbit or anti-mouse HRP-conjugated polyclonal secondary
antibodies (1:5,000; cat. nos. A0208 and A0216; Beyotime Institute
of Biotechnology). The primary antibodies and secondary antibodies
were diluted in TBS-0.05% Tween 20 (TBST)-non-fat milk. The bands
were visualized by enhanced chemiluminescence detection reagents
(Applygen Technologies Inc.). The PVDF membranes were scanned with
a ChemiDoc MP imaging system (BioRad Laboratories, Inc.) and the
densitometry was performed using Image Lab software (version 3.0;
Bio-Rad Laboratories, Inc.).
Histopathological assessments
The livers of the rats were carefully removed and
fixed in 4% paraformaldehyde for 48 h at room temperature and
embedded in paraffin, then sliced into 4-µm thick sections. The
sections were dewaxed in xylene, rehydrated using a descending
series of ethanol, and stained with hematoxylin and eosin (H&E)
using a staining kit (Beyotime Institute of Biotechnology).
Briefly, the sections were stained with 30 mg/ml hematoxylin for 15
min at room temperature, washed three times with water for 1 min
for each time, and then dipped in 1% hydrochloric acid/alcohol for
10 sec. After, the sections were washed with tap water for 3 min
and stained in 1% eosin for 1 min at room temperature. The sections
were dehydrated in an ascending series of alcohol, transferred to
xylene for 5 min at room temperature, and then sealed with neutral
gum and covered with a glass coverslip. The sections were
visualized using a light microscope (magnification, ×100 or ×200;
Olympus Corporation).
Statistical analysis
Statistical analyses were performed using SPSS v19.0
statistical software (IBM Corp.). A one-way ANOVA followed by
Dunnett's test was performed for group comparisons. An unpaired
two-tailed Student's t-test was used to evaluate differences in
cell cycle distribution between EGCG group and the control group.
The cell experiment was conducted independently ≥3 times, and the
data are presented as the mean ± SD. The survival of the rats was
estimated using the Kaplan-Meier method. A log-rank test was
performed for the survival analysis after Kaplan-Meier test, and a
Bonferroni test was used for correction of the obtained values from
the log rank tests. P<0.05 was considered to indicate a
statistically significant difference.
Results
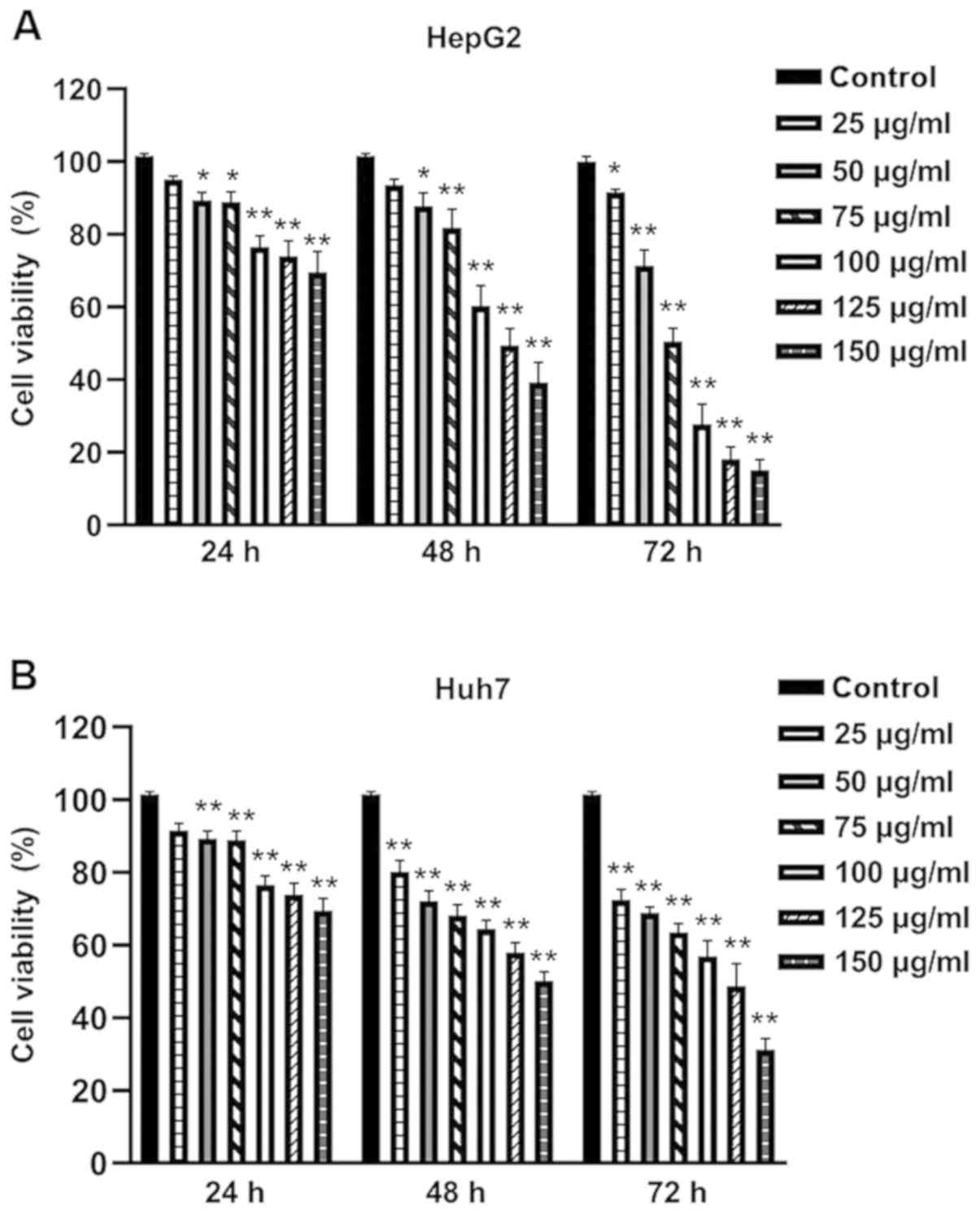
EGCG inhibits cell viability in human
hepatoma cell lines
The inhibitory effects of EGCG on HepG2 and Huh7
cells were evaluated using CCK-8 assay. Exposure of HepG2 and Huh7
cells to 25 µg/ml EGCG led to a notable inhibition of cell
viability, while 50, 75, 100, 125 and 150 µg/ml EGCG significantly
enhanced this inhibitory effect (Fig.
1). In addition, a time-effect analysis indicated that 25 µg/ml
EGCG markedly decreased the viability of HepG2 and Huh7 cells at 24
h, which became significant at 48 and 72 h. The decrease was
significant at all time points in HepG2 and Huh7 cells treated with
50–150 µg/ml EGCG. These results suggested that EGCG can inhibit
the proliferation of human hepatoma cell lines in a dose- and
time-dependent manner.
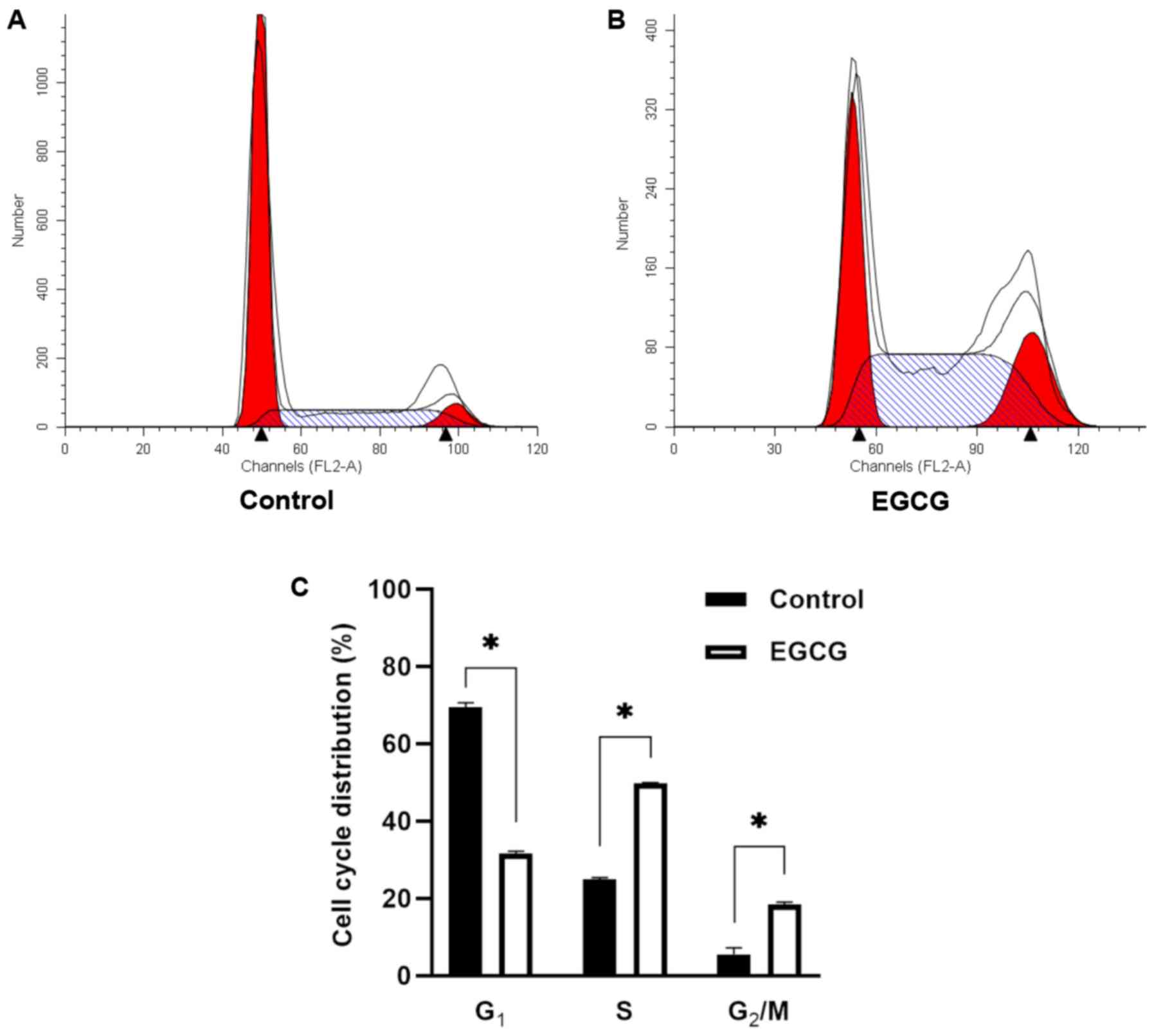
EGCG causes cell cycle arrest in HepG2
cells
According to the cell viability (%) presented in
Fig. 1, the inhibitory rate was
calculated (data not shown). The IC50 value of EGCG at
48 h for HepG2 cells was 127.09 µg/ml. Thus, HepG2 cells were
treated with 127.09 µg/ml EGCG for 48 h. Flow cytometry was used to
detect the cell cycle. The EGCG group demonstrated statistically
significant differences from the control group (Fig. 2). The proportion of cells in the S
phase and G2/M phases were significantly increased
compared with the control group, while the proportion of
G1 phase cells was reduced significantly. Therefore,
EGCG could arrest the cell cycle in the S phase and G2/M
phases of HepG2 cells.
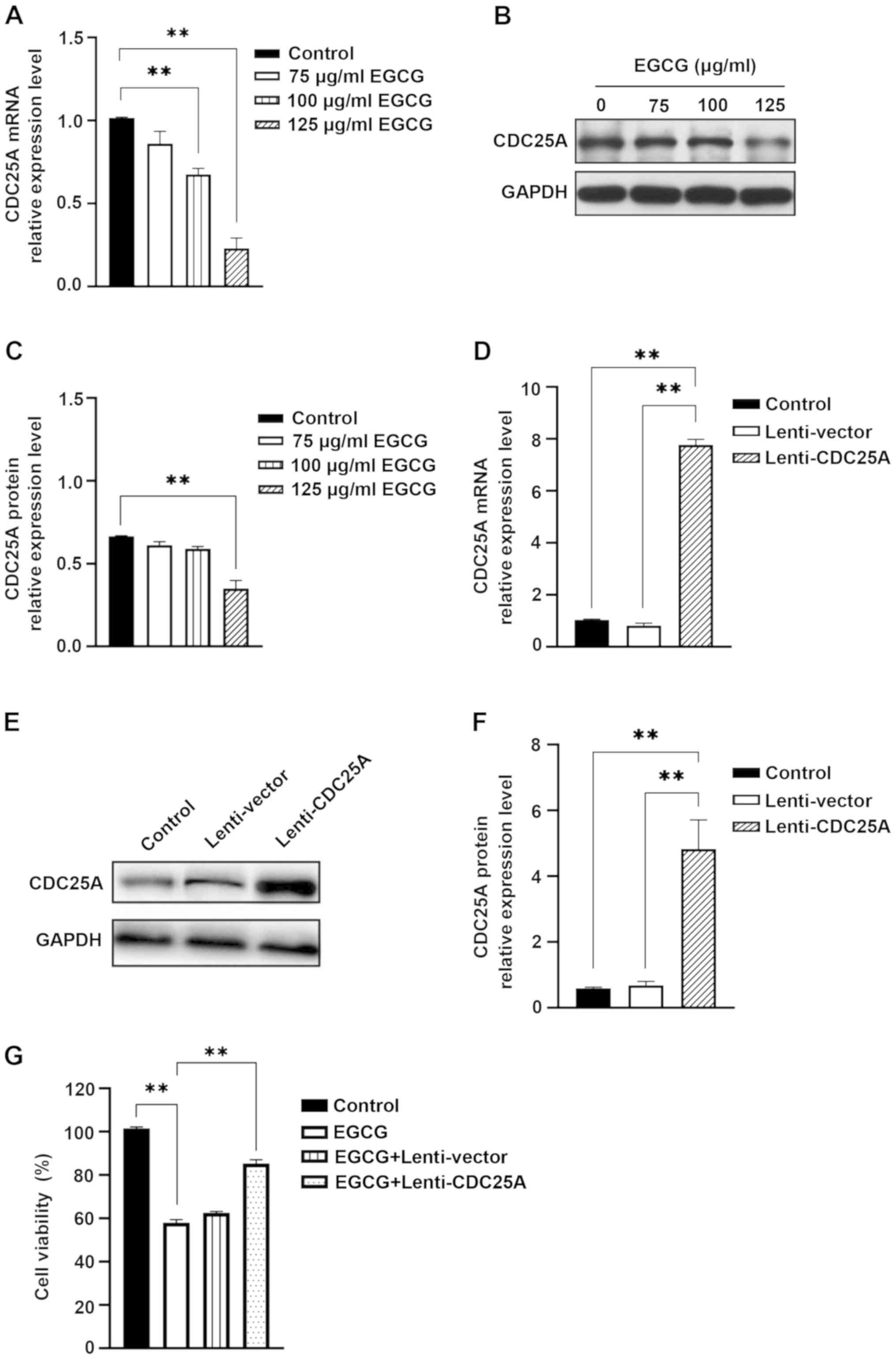
EGCG induces CDC25A downregulation in
HepG2 cells
The expression of CDC25A in HepG2 cells treated with
0, 75, 100 or 125 µg/ml EGCG for 48 h was examined using RT-qPCR
and western blotting. In HepG2 cells treated with 100 and 125 µg/ml
EGCG, the expression of CDC25A mRNA was significantly lower
compared with untreated HepG2 cells (Fig. 3A). It was also identified that
CDC25A protein expression decreased significantly in HepG2 cells
following treatment with 125 µg/ml EGCG for 48 h (Fig. 3B and C).
To further demonstrate that CDC25A was the target of
EGCG in inhibiting the proliferation of hepatoma cells, the effect
of CDC25A overexpression on EGCG-induced inhibition in HepG2 cells
was investigated. HepG2 cells overexpressing CDC25A were
constructed via transfection with Lenti-CDC25A (Fig. 3D-F). The viability changes in HepG2
cells treated with EGCG were reversed by transfection with
Lenti-CDC25A, which did not occur in HepG2 cells transfected with
Lenti-vector and untreated HepG2 cells (Fig. 3G). These findings indicated that
CDC25A overexpression blocked the EGCG-induced inhibition of HepG2
cell proliferation.
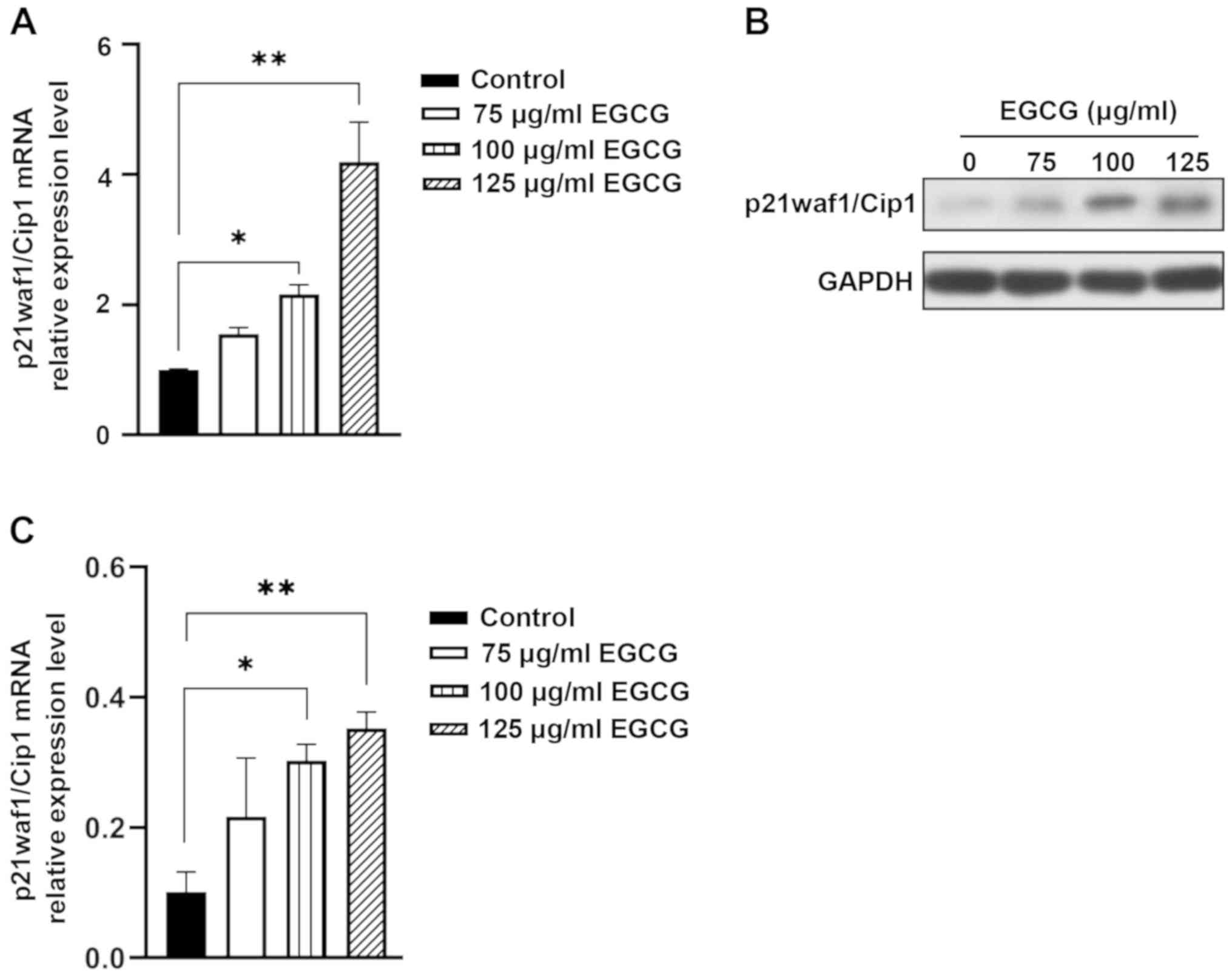
EGCG induces p21waf1/Cip1 upregulation
in HepG2 cells
As a negative transcription factor, p21waf1/Cip1 can
directly regulate the transcription activity of the CDC25A promoter
(25). However, whether EGCG
affected the expression of p21waf1/Cip1 in HCC required further
investigation. The present study identified that the expression of
p21waf1/Cip1 mRNA, which was reduced in HCC, increased with the
decrease of CDC25A expression in HepG2 cells following treatment
with 100 or 125 µg/ml EGCG for 48 h (Fig. 4A). p21waf1/Cip1 protein expression
was significantly upregulated in HepG2 cells treated with 100 and
125 µg/ml EGCG (Fig. 4B and C).
These results suggested that EGCG can simultaneously upregulate
p21waf1/Cip1 and downregulate CDC25A in vitro, which may be
one of the mechanisms for EGCG-induced inhibition of hepatoma cell
proliferation.
Chemopreventive effects of EGCG
against HCC in vivo
Maximum percentage weight loss observed in rat from
start to endpoint was 4.5%. During the experiment, liver biopsies
were performed for all surviving rats during the 10–20th weeks, to
observe the occurrence of liver cancer in rats. During the 10th
week, two rats in the HCC group were identified to have liver
cirrhotic nodules. During the 20th week, two rats in the EGCG
treatment group, three rats in the GTE treatment group and five
rats in the HCC group developed HCC. From the 15th week onward, rat
mortality in the HCC group occurred and fourteen rats died. In the
EGCG treatment group, five rats died during the 24, 26, 28 and 29th
weeks. From the 22nd-30th weeks, six rats in the GTE treatment
group died. The succumbed rats were diagnosed with liver cancer
using histopathologic analysis. At the end of the experiment, it
was observed that only one rat in the HCC group, five rats in the
EGCG treatment group and four rats in the GTE treatment group
remained alive. The remaining animals were sacrificed following
different drug treatments for 30 weeks and liver tissues were
obtained.
Histopathological analysis from the living rat in
the HCC group, in addition to three rats in the EGCG treatment
group and three rats in the GTE treatment group that developed HCC,
exhibited marked cellular infiltration, massive breakdown of
hepatic tissues, plate-like and diffuse cancer cells, as well as
more deeply stained nuclei and cytoplasm (Fig. 5A). A total of two rats in the EGCG
treatment group and one rat in the GTE treatment group developed
liver cirrhosis. The liver sections stained with H&E revealed
the formation of differently-sized liver cirrhosis pseudolobules,
deformation of the hepatocyte nuclei and atypical hyperplasia of
the hepatic cells (Fig. 5B).
However, all rats in the control groups, including the normal, EGCG
and GTE controls which were treated with EGCG or GTE only without
DEN, had no liver abnormalities (Fig.
5C). Fifteen rats in the HCC group, eight rats in the EGCG
treatment group and nine rats in the GTE treatment group developed
HCC. The incidence of HCC in the EGCG (80.0%, 8/10) and GTE groups
(90%, 9/10) was lower compared with the HCC group (100%, 15/15),
but the difference was not significant.
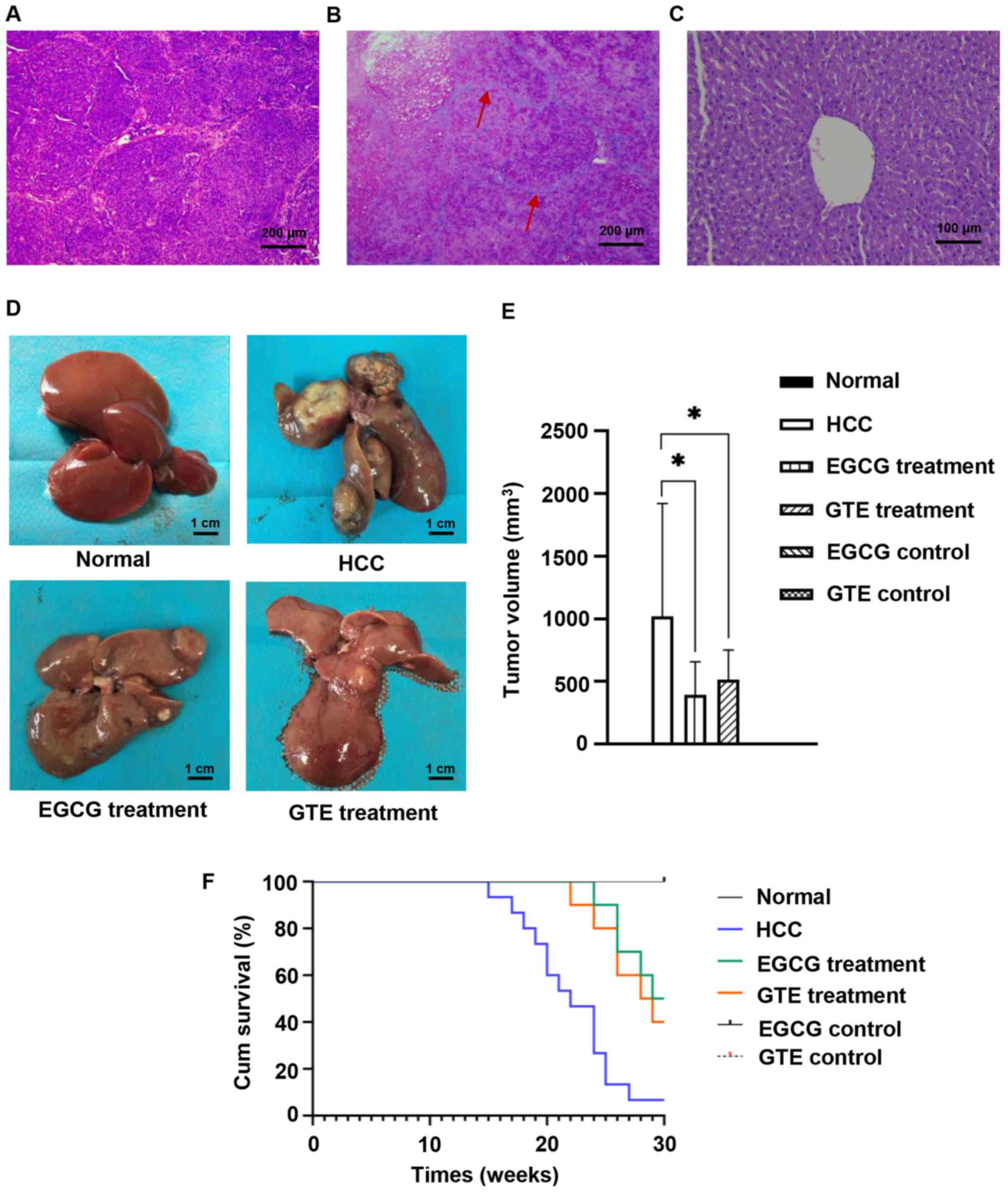 | Figure 5.EGCG induces the chemopreventive
effect against HCC in vivo. The surviving rats were
sacrificed and liver tissues were obtained at the end of the
experiment (the 30th week). Histopathological examination of liver
sections stained with hematoxylin and eosin. (A) HCC
(magnification, ×100), (B) liver cirrhosis (magnification, ×100)
and (C) healthy liver (magnification, ×200). The red arrows
indicate the formation of pseudo lobules in different sizes of
liver cirrhosis. (D) Representative images of the livers in the
normal group, HCC group, EGCG treatment group and GTE treatment
group. Scale bar, 1 cm. (E) Tumor volume in all groups was
calculated. All rats in control groups, including the normal, EGCG
and GTE controls, had no tumor in the liver, so the tumor volume
was zero. (F) Kaplan-Meier survival curves of rats. All rats in the
normal, EGCG and GTE control group survived at the end of the
experiment, so the survival curves overlaps. *P<0.05 vs. the
indicated group. EGCG, epigallocatechin gallate; HCC,
hepatocellular carcinoma; GTE, green tea extract. |
From the rats that died during the experiment and
those sacrificed at the end of the experiment, the livers were
harvested to observe the volume of the tumor (Fig. 5D). The width and length of each
tumor were measured and the tumor volume was calculated. Maximum
tumor diameter, volume and weight (as percentage of total body
weight) was 20 mm, 1,960 mm3 and 0.77% of total body
weight, respectively. It was found that, the tumor volume in the
EGCG treatment group and GTE treatment group was significantly
smaller compared with that in the HCC group (Fig. 5E).
Overall survival rates were also calculated
(Fig. 5F). The survival rate of
rats was 6.67% (1/15) in the HCC group. Notably, treatment with
EGCG for 30 weeks improved the rat survival to 50% (5/10) and
treatment with GTE improved the rat survival to 40% (4/10). These
data supported the hypothesis that EGCG and GTE significantly
inhibited tumor growth and prolonged the survival rates of rats
with HCC.
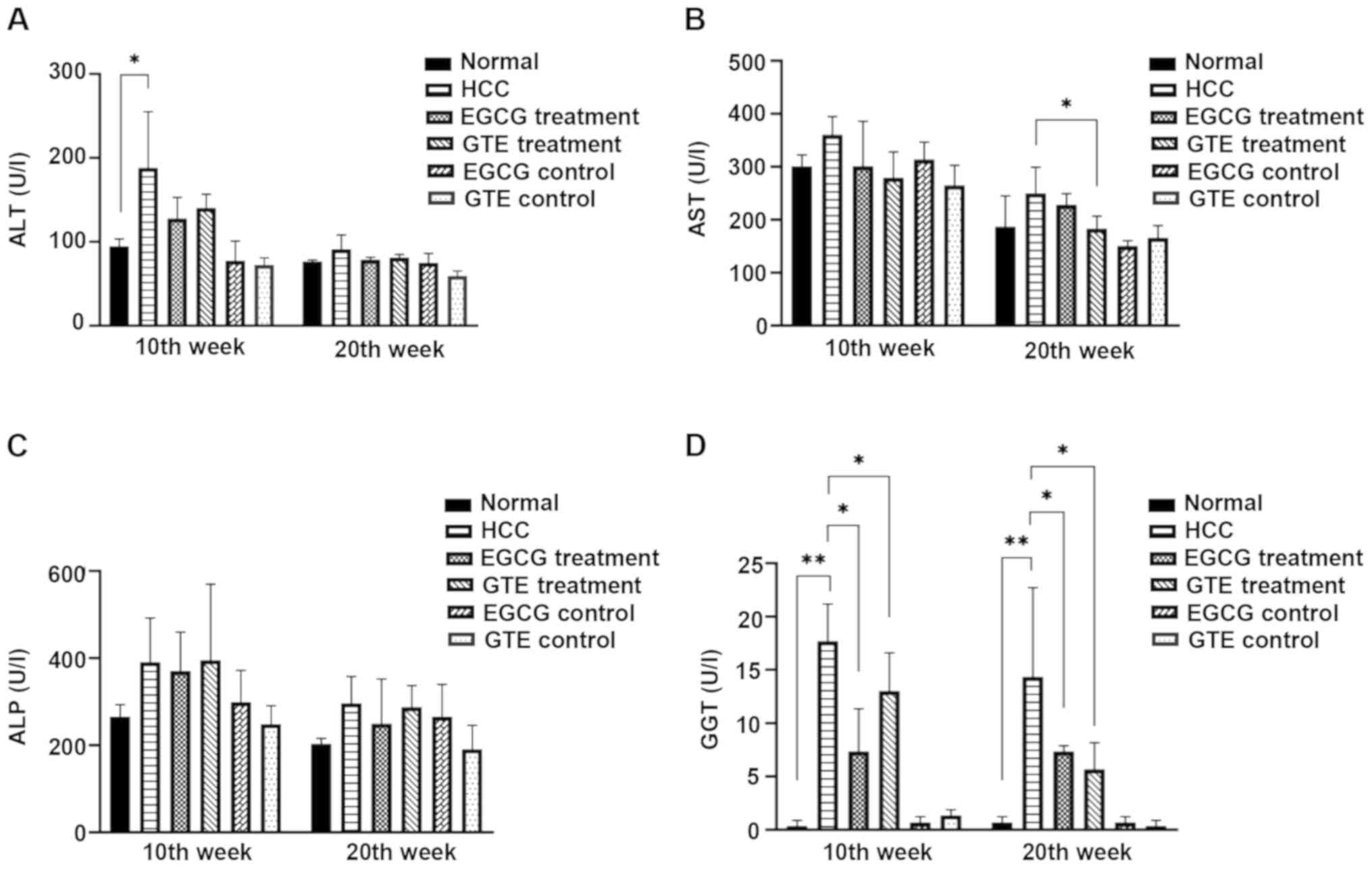
EGCG significantly reduces the serum
GGT level in rats with DEN-induced HCC
ALT, AST, ALP and GGT are the blood indexes
associated with the liver (26).
During the 10–20th weeks of the experiment, the levels of ALT, AST,
ALP and GGT in serum were detected. The levels of ALP, ALT and AST
exhibited no significant changes, with the exception of a transient
increase in ALT in the HCC group compared with the normal group
during the 10th week and a transient decrease in AST in the GTE
treatment group compared with the HCC group during the 20th week
(Fig. 6A-C). The results
demonstrated that serum GGT in HCC rats was significantly increased
compared with the normal group at 10 and 20 weeks; however, the
serum GGT level in rats treated with EGCG or GTE was significantly
lower compared with the HCC group at 10 and 20 weeks (Fig. 6D). Thus, treatment with EGCG and
GTE resulted in significant reductions in serum GGT.
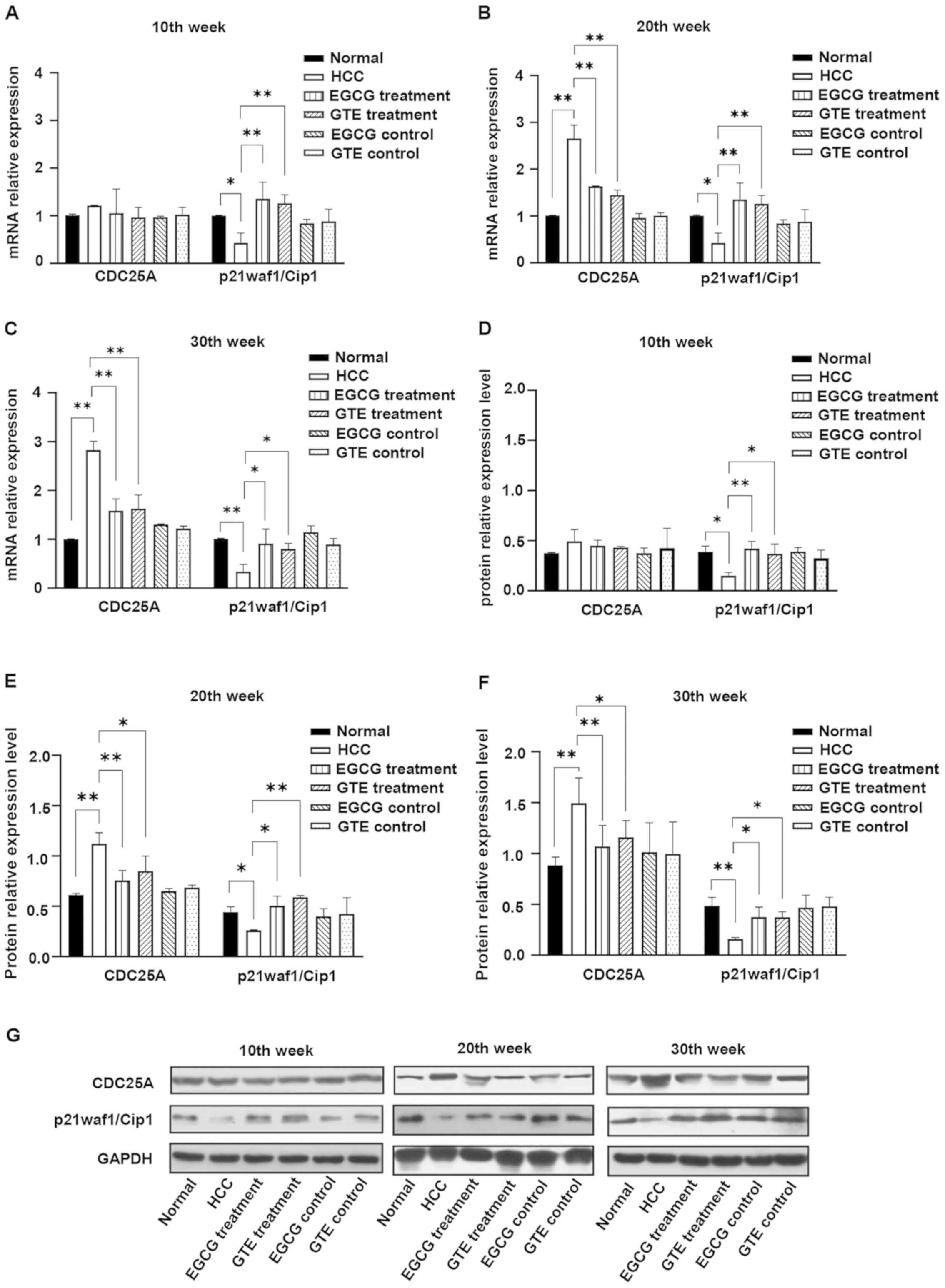
EGCG reverses HCC-induced elevation of
CDC25A and reduction of p21waf1/Cip1 in vivo
The dynamic changes in CDC25A and p21waf1/Cip1 were
observed by detecting the expression levels of CDC25A and
p21waf1/Cip1 mRNA and protein in liver tissues obtained during the
10, 20 and 30th weeks of the experiment. During the 10th week,
there was no obvious change in the expression of CDC25A mRNA
(Fig. 7A) and protein (Fig. 7D and G) in the liver tissues of
rats in the HCC group compared with the normal group. However, the
expression levels of CDC25A mRNA and protein in the HCC group
significantly increased from the 20th to the 30th week compared
with the normal group. Treatment with EGCG or GTE significantly
reduced the expression of CDC25A mRNA and protein in DEN-induced
HCC rats, which began at the 20th week (Fig. 7B,C and E-G).
It was identified that the expression levels of
p21waf1/Cip1 mRNA and protein in the HCC group were lower compared
with those of the normal group from the 10th week to the end of the
experiment. Furthermore, treatment with EGCG or GTE reversed the
expression of p21waf1/Cip1 in DEN-induced HCC rats, which started
at the 10th week (Fig. 7A and D).
It was demonstrated that EGCG and GTE did not affect the expression
levels of CDC25A and p21waf1/Cip1 in normal rats. Therefore, the
data supported the hypothesis that treatment with EGCG or GTE
reversed HCC-induced reduction of p21waf1/Cip1 and elevation of
CDC25A, but did not affect normal rats.
Discussion
EGCG, the most active biological constituent derived
from GT, has notable chemopreventive and antitumor effects
(27). The aim of the present
study was to simulate the process of drinking tea in daily life via
oral administration of EGCG instead of intraperitoneal injection,
as conducted in a previous study (28). In addition, the experiment lasted
for 30 weeks and is thus of benefit to the long-term understanding
of cancer prevention. The animal model selected could be used for
multiple liver biopsies during the experiment, which was
established early in our laboratory and continuously optimized over
the course of the current study (29). In the experiments, liver tissues
were collected three times for the dynamic detection of cell
cycle-related molecules.
Several previous studies have reported that EGCG can
exert chemopreventive effects on different carcinomas, including
skin tumors (30), lung cancer
(31), gastrointestinal cancer
(32) and prostate cancer
(33). In the present study, the
tumor incidence in rats treated with EGCG demonstrated a downward
trend compared with the HCC group, but no statistically significant
difference was observed; a possible explanation for this is that
the dose of oral EGCG used in the present study was not high
enough. Oral administration of EGCG has been reported to be first
absorbed in the intestines and the bioavailability is poor due to
oxidation, metabolism and efflux (34,35).
In order to observe the effect of EGCG and GTE on the survival time
of rats with HCC, the duration of this experiment was 30 weeks,
while numerous previous studies were only 20 weeks (36–38);
this may explain the high number of deaths. In the current study,
it was identified that EGCG can reduce tumor volume and inhibit
tumor growth. In addition, EGCG improved the survival of rats from
6.67% in the HCC group to 50.0% in the EGCG treatment group. The
results also suggested that 25 mg/day EGCG could prolong the
survival rates of HCC rats. In vitro, EGCG exhibited
dose-dependent reduction in the viability of HepG2 and Huh7 human
hepatoma cells. Furthermore, EGCG could also arrest the cell cycle
in the S phase and G2/M phases of HepG2 cells. Thus,
EGCG had chemopreventive effects on HCC to a certain degree.
It has been shown that the serum GGT levels are
significantly increased in patients with HCC (39). The elevation of the GGT level in
HCC may be associated with GGT gene activation by the
hypomethylation status of the CCGG sites of GGT genes (40), in addition to the release of GGT
from the liver cells into circulation due to DEN-induced liver
damage (41). GGT is also involved
in biotransformation, nucleic acid metabolism and tumorigenesis
(42). Moreover, EGCG inhibits the
GGT increase dose-dependently in HepG2 cells exposed to a lethal
dose of ethanol (43). In the
present study, treatment with EGCG reduced the elevated GGT in HCC
without affecting the control group, which aided the delay of
tumorigenesis.
Several mechanisms have been proposed to explain the
chemopreventive effects of EGCG. The generation of reactive oxygen
species (44), inhibition of
multiple signaling pathways and key enzymes are hypothesized to
suppress the processes of carcinogenesis (45–47).
However, EGCG can influence the different molecular mechanisms,
causing unique anti-cancer actions in various tumor cells (6). The present study hypothesized that
the mechanism underlying the preventive effects of EGCG may be
associated with the key genes involved in the pathogenesis and
progression of HCC. In our previous study, identification and
validation of CDC25A, candidate gene screening by gene set
enrichment analysis and a meta-analysis based on the cross-species
comparison strategy were performed in HCC. Subsequently, high
expression of CDC25A was also identified in HCC samples from
AFB1-induced rat and tree shrew HCC models, in addition to human
samples. Thus, CDC25A may serve an important role in the
development of HCC (48).
CDC25A, a member of the CDC25 phosphatase family,
can dephosphorylate and activate CDKs via tyrosine/threonine
phosphatase activity (49). CDC25A
is regarded as an important regulator of the cell cycle due to its
extensive role in cell cycle transformation and mitosis (50). A number of studies have confirmed
that CDC25A is an oncogene: Abnormal CDC25A can promote
G1/S and G2/M transition by dephosphorylating
CDK1 and CDK2 at T14/Y15, as well as CDK4 at Y17 (51–53),
leading to uncontrolled cell proliferation and malignant
transformation. In addition to affecting the cell cycle, CDC25A can
promote high expression levels of glycolysis- related genes Glucose
transporter 1, pyruvate kinase M1/2 (PKM2) and lactate
dehydrogenase A via the dephosphorylation of PKM2, the key enzyme
of glycolysis and participate in the metabolic regulation of tumor
cells (54). Upregulation of
CDC25A has frequently been identified in various tumors, and
appears to be closely associated with malignancy and poor prognosis
in patients with cancer (55–57).
In the process of DEN-induced HCC, the present study
identified that the expression levels of CDC25A mRNA and protein in
the liver tissue of rats in the HCC group were gradually increased
compared with the normal group. This is consistent with the
findings of our previous study, that CDC25A serves an important
role in the development of HCC (48). Thus, it was suggested that
treatment of HCC rats with EGCG restored CDC25A expression. In
addition, the present study demonstrated that EGCG significantly
reduced the expression of CDC25A in HepG2 cells. It was also found
that overexpression of CDC25A could reverse EGCG-induced inhibition
of HepG2 cell proliferation. Therefore, the present study provided
a possible mechanism for the inhibition of liver cancer development
via the repression of CDC25A in vitro and in
vivo.
p21waf1/Cip1 serves an important role in controlling
cell cycle progression (58). For
instance, p21waf1/Cip1 contributes to the inhibition of
retinoblastoma protein phosphorylation that is necessary for cell
cycle progression by binding CDKs in the G1 phase
(59). p21waf1/Cip1 functions as a
tumor suppressor and is usually deregulated in tumors due to the
function loss of transcriptional activators, including p53 and
smad3 (60). The downregulation of
p21waf1/Cip1 was also observed in the present study. p21waf1/Cip1
reduces the transcriptional activity of the CDC25A promoter as a
negative transcriptional repressor (25). The results of the present study
demonstrated that EGCG significantly upregulated the expression of
p21waf1/Cip1 between the 20–30th week, along with a decreased
expression of CDC25A in vivo, which is in line with a
previous study that reported that EGCG enhances the expression of
p21waf1/Cip1 in colorectal cancer cells (61). However, during the 10th week of the
present study, the rats treated with DEN remained in a precancerous
state. Although the expression of p21waf1/Cip1 decreased
significantly, it was not sufficient to affect the transcriptional
activity of CDC25A; CDC25A mRNA and protein appeared to increase,
but the change was not significant. Furthermore, reduced
p21waf1/Cip1 was observed in HepG2 cells. However, whether the
molecular mechanism of EGCG in decreasing CDC25A expression in
liver cancer cells is associated with the inhibition of CDC25A
promoter activity through elevated p21waf1/Cip1 remains to be
investigated. It was hypothesized that treatment with EGCG caused
p21waf1/Cip1 upregulation and CDC25A trans-inhibition, resulting in
decreased CDC25A mRNA and protein expression levels. Thus,
EGCG-induced p21waf1/Cip1 upregulation and CDC25A downregulation
may led to tumor growth inhibition.
Current research concentrates on EGCG, rather than
GT, in the mechanisms of prevention and treatment of tumors with
GT, as the advantages of EGCG (clear chemical structure and high
purity) are conducive to scientific research. In the present study,
GTE and EGCG were selected as research subjects. GT has application
advantages in safety, convenience, compliance and economy in daily
use. There are various chemical substances in GT that possess
complex biological antagonistic and synergistic properties
(6). A previous study reported
that GTE at an EGCG-equivalent concentration exhibits a stronger
inhibition compared with EGCG alone in human squamous cell
carcinoma lines (62). However,
another study demonstrated that EGCG and GTE exhibit some activity
as immune checkpoint inhibitors in lung cancer development
(63). The results of the present
study suggested that there was no significant difference in the
preventive effects of GTE and EGCG on liver cancer cells under an
EGCG equivalence condition. As with EGCG, treatment with GTE also
improved survival via p21waf1/Cip1 upregulation and CDC25A
downregulation.
To the best of our knowledge, the primary findings
of the current study are the first to have shown that EGCG
possesses chemopreventive properties, which can be partly explained
by the reduction in CDC25A, as a key liver cancer gene reported in
our previous study (48).
Collectively, the results from the present study demonstrated that
EGCG and GTE effectively reduced tumor volume, improved the
survival rate of rats with HCC and inhibited hepatoma cell
proliferation via the promotion of p21waf1/Cip1 and inhibition of
CDC25A expression. Further efforts to identify the mechanisms
underlying EGCG-induced inhibition in tumor cell viability via the
p21waf1/Cip1/CDC25A signal axis may facilitate the efficient
treatment of liver cancer. Given its poor stability and low
bioavailability, EGCG can be used in combination with other
antitumor drugs and have an auxiliary or synergistic effect on HCC
prevention and treatment.
Acknowledgements
The authors thank Professor Y Li and C Yang (Guangxi
Medical University Cancer Hospital, Nanning, China) for crucial
advice and assistance with animal experiments.
Funding
The present study was supported by the Guangxi
Natural Science Foundation Program under Grant (grant no.
2017GXNSFBA198003) and The Basic Ability Enhancement Program for
Young and Middle-aged Teachers of Guangxi under Grant (grant no.
2017KY0102).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YPT and KL designed the experiments and performed
the animal experiment. JC designed the experiments and analyzed the
data. ZC performed PCR and western blotting assays. HA, YL, YP, NC
and AL performed the animal experiment. HT performed the
histological examination. YPT wrote the manuscript. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
Animal experiments were conducted in accordance with
the guidelines for The Care and Use of Laboratory Animals issued by
the Ethics Committee of Guangxi Medical University Cancer Hospital
(approval no. LW2018022).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Yang JD, Hainaut P, Gores GJ, Amadou A,
Plymoth A and Roberts LR: A global view of hepatocellular
carcinoma: Trends, risk, prevention and management. Nat Rev
Gastroenterol Hepatol. 16:589–604. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Personeni N, Pressiani T, Bozzarelli S and
Rimassa L: Targeted agents for second-line treatment of advanced
hepatocellular carcinoma. World J Gastrointest Oncol. 11:788–803.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ilamathi M, Santhosh S and
Sivaramakrishnan V: Artesunate as an anti-cancer agent targets
stat-3 and favorably suppresses hepatocellular carcinoma. Curr Top
Med Chem. 16:2453–2463. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Qian YY, Liu ZS, Yan HJ, Yuan YF, Levenson
AS and Li K: Pterostilbene inhibits MTA1/HDAC1 complex leading to
PTEN acetylation in hepatocellular carcinoma. Biomed Pharmacother.
101:852–859. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gan RY, Li HB, Sui ZQ and Corke H:
Absorption, metabolism, anti-cancer effect and molecular targets of
epigallocatechin gallate (EGCG): An updated review. Crit Rev Food
Sci Nutr. 58:924–941. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu X, Xu W, Cai H, Gao YT, Li H, Ji BT,
Shu X, Wang T, Gerszten RE, Zheng W, et al: Green tea consumption
and risk of type 2 diabetes in Chinese adults: The Shanghai Women's
health study and the Shanghai Men's health study. Int J Epidemiol.
47:1887–1896. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Quan J, Jia Z, Lv T, Zhang L, Liu L, Pan
B, Zhu J, Gelb IJ, Huang X and Tian J: Green tea extract catechin
improves cardiac function in pediatric cardiomyopathy patients with
diastolic dysfunction. J Biomed Sci. 26:322019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu D, Wang J, Pae M and Meydani SN: Green
tea EGCG, T cells and T cell-mediated autoimmune diseases. Mol
Aspects Med. 33:107–118. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu J, Liu S, Zhou H, Hanson T, Yang L,
Chen Z and Zhou M: Association of green tea consumption with
mortality from all-cause, cardiovascular disease and cancer in a
Chinese cohort of 165,000 adult men. Eur J Epidemiol. 31:853–865.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nakagawa T and Yokozawa T: Direct
scavenging of nitric oxide and superoxide by green tea. Food Chem
Toxicol. 40:1745–1750. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yan RQ, Qin GZ, Chen ZY, Li Y and Qin LL:
The inhibition of green tea on the hepatocarcinogenesis induced by
aflatoxin b-1 in rats. Cancer. 2:83–87. 1987.(In Chinese).
|
|
13
|
Li Y, Qin GZ, Qin LL, Duan XX and Yan RQ:
A series of animal experiments on the prevention of liver cancer by
green tea. Cancer Res Clin. 4:22–24. 1997.(In Chinese).
|
|
14
|
Zhang ZQ, Liu QF, Huang TR, Wu YD, Zhong
SC and Yu TC: Experimental epidemiological study on the prevention
of liver cancer by green tea. Guangxi Prev Med. 1:5–7. 1995.(In
Chinese).
|
|
15
|
Shankar S, Ganapathy S, Hingorani SR and
Srivastava RK: EGCG inhibits growth, invasion, angiogenesis and
metastasis of pancreatic cancer. Front Biosci. 13:440–452. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hazgui S, Bonnomet A, Nawrocki-Raby B,
Milliot M, Terryn C, Cutrona J, Polette M, Birembaut P and Zahm JM:
Epigallocatechin-3-gallate (EGCG) inhibits the migratory behavior
of tumor bronchial epithelial cells. Respir Res. 9:332008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cerezo-Guisado MI, Zur R, Lorenzo MJ,
Risco A, Martín-Serrano MA, Alvarez-Barrientos A, Cuenda A and
Centeno F: Implication of Akt, ERK1/2 and alternative p38MAPK
signalling pathways in human colon cancer cell apoptosis induced by
green tea EGCG. Food Chem Toxicol. 84:125–132. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Youn HS, Lee JY, Saitoh SI, Miyake K, Kang
KW, Choi YJ and Hwang DH: Suppression of MyD88- and TRIF-dependent
signaling pathways of Toll-like receptor by
(−)-epigallocatechin-3-gallate, a polyphenol component of green
tea. Biochem Pharmacol. 72:850–859. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang CH, Tsai SJ, Wang YJ, Pan MH, Kao JY
and Way TD: EGCG inhibits protein synthesis, lipogenesis and cell
cycle progression through activation of AMPK in p53 positive and
negative human hepatoma cells. Mol Nutr Food Res. 53:1156–1165.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shen X, Zhang Y, Feng Y, Zhang L, Li J,
Xie YA and Luo X: Epigallocatechin-3-gallate inhibits cell growth,
induces apoptosis and causes S phase arrest in hepatocellular
carcinoma by suppressing the AKT pathway. Int J Oncol. 44:791–796.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Masuda M, Suzui M and Weinstein IB:
Effects of epigallocatechin-3-gallate on growth, epidermal growth
factor receptor signaling pathways, gene expression and
chemosensitivity in human head and neck squamous cell carcinoma
cell lines. Clin Cancer Res. 7:4220–4229. 2001.PubMed/NCBI
|
|
22
|
Lim YC and Cha YY:
Epigallocatechin-3-gallate induces growth inhibition and apoptosis
of human anaplastic thyroid carcinoma cells through suppression of
EGFR/ERK pathway and cyclin B1/CDK1 complex. J Surg Oncol.
104:776–780. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Esteban V, Vázquez-Novelle MD, Calvo E,
Bueno A and Sacristán MP: Human Cdc14A reverses CDK1
phosphorylation of Cdc25A on serines 115 and 320. Cell Cycle.
5:2894–2898. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vigneron A, Cherier J, Barré B, Gamelin E
and Coqueret O: The cell cycle inhibitor p21waf1 binds to the myc
and cdc25A promoters upon DNA damage and induces transcriptional
repression. J Biol Chem. 281:34742–34750. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Renner EL: Liver function tests.
Baillieres Clin Gastroenterol. 9:661–677. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Almatroodi SA, Almatroudi A, Khan AA,
Alhumaydhi FA, Alsahli MA and Rahmani AH: Potential therapeutic
targets of epigallocatechin gallate (EGCG), the most abundant
catechin in green tea, and its role in the therapy of various types
of cancer. Molecules. 25:31462020. View Article : Google Scholar
|
|
28
|
Darweish MM, Abbas A, Ebrahim MA and
Al-Gayyar MM: Chemopreventive and hepatoprotective effects of
Epigallocatechin-gallate against hepatocellular carcinoma: Role of
heparan sulfate proteoglycans pathway. J Pharm Pharmacol.
66:1032–1045. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liang HJ, Wei W, Kang XN, Guo K, Cao J, Su
JJ, Yang C, Ou C, Li Y and Liu YK: Differentially expressed
proteins in the precancerous stage of rat hepatocarcinogenesis
induced by diethylnitrosamine. Zhonghua Gan Zang Bing Za Zhi.
17:669–674. 2009.(In Chinese). PubMed/NCBI
|
|
30
|
Yoshizawa S, Horiuchi T, Fujiki H, Yoshida
T, Okuda T and Sugimura T: Antitumor promoting activity of
(−)-epigallocatechin gallate, the main constituent of ‘Tannin’ in
green tea. Phytother Res. 1:44–47. 1987. View Article : Google Scholar
|
|
31
|
Wang ZY, Hong JY, Huang MT, Reuhl KR,
Conney AH and Yang CS: Inhibition of N-nitrosodiethylamine- and
4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced
tumorigenesis in A/J mice by green tea and black tea. Cancer Res.
52:1943–1947. 1992.PubMed/NCBI
|
|
32
|
Xu Q, Yang CH, Liu Q, Jin XF, Xu XT, Tong
JL, Xiao SD and Ran ZH: Chemopreventive effect of
epigallocatechin-3-gallate (EGCG) and folic acid on the
N-methyl-N′-nitro-N-nitrosoguanidine (MNNG)-induced
gastrointestinal cancer in rat model. J Dig Dis. 12:181–187. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gupta S, Hastak K, Ahmad N, Lewin JS and
Mukhtar H: Inhibition of prostate carcinogenesis in TRAMP mice by
oral infusion of green tea polyphenols. Proc Natl Acad Sci USA.
98:10350–10355. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen L, Lee MJ, Li H and Yang CS:
Absorption, distribution, elimination of tea polyphenols in rats.
Drug Metab Dispos. 25:1045–1050. 1997.PubMed/NCBI
|
|
35
|
Kale A, Gawande S, Kotwal S, Netke S,
Roomi W, Ivanov V, Niedzwiecki A and Rath M: Studies on the effects
of oral administration of nutrient mixture, quercetin and red
onions on the bioavailability of epigallocatechin gallate from
green tea extract. Phytother Res. 24 (Suppl 1):S48–S55. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ding YF, Wu ZH, Wei YJ, Shu and Peng YR:
Hepatic inflammation-fibrosis-cancer axis in the rat hepatocellular
carcinoma induced by diethylnitrosamine. J Cancer Res Clin Oncol.
143:821–834. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cha JH, Bae SH, Kim HL, Park NR, Choi ES,
Jung ES, Choi JY and Yoon SK: Branched-chain amino acids ameliorate
fibrosis and suppress tumor growth in a rat model of hepatocellular
carcinoma with liver cirrhosis. PLoS One. 8:e778992013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Xu M, Zhao Q, Shao D, Liu H, Qi J and Qin
C: Chenodeoxycholic acid derivative HS-1200 inhibits
hepatocarcinogenesis and improves liver function in
diethylnitrosamine-exposed rats by downregulating MTH1. Biomed Res
Int. 2017:14659122017.PubMed/NCBI
|
|
39
|
Xu XS, Wan Y, Song SD, Chen W, Miao RC,
Zhou YY, Zhang LQ, Qu K, Liu SN, Zhang YL, et al: Model based on
γ-glutamyltransferase and alkaline phosphatase for hepatocellular
carcinoma prognosis. World J Gastroenterol. 20:10944–10952. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yao D, Jiang D, Huang Z, Lu J, Tao Q, Yu Z
and Meng X: Abnormal expression of hepatoma specific gamma-glutamyl
transferase and alteration of gamma-glutamyl transferase gene
methylation status in patients with hepatocellular carcinoma.
Cancer. 88:761–769. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Shaarawy SM, Tohamy AA, Elgendy SM,
Elmageed ZY, Bahnasy A, Mohamed MS, Kandil E and Matrougui K:
Protective effects of garlic and silymarin on NDEA-induced rats
hepatotoxicity. Int J Biol Sci. 5:549–557. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ma H, Zhang L, Tang B, Wang Y, Chen R,
Zhang B, Chen Y, Ge N, Wang Y, Gan Y, et al:
γ-Glutamyltranspeptidase is a prognostic marker of survival and
recurrence in radiofrequency-ablation treatment of hepatocellular
carcinoma. Ann Surg Oncol. 21:3084–3089. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lee SI, Kim HJ and Boo YC: Effect of green
tea and (−)-epigallocatechin gallate on ethanol-induced toxicity in
HepG2 cells. Phytother Res. 22:669–674. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yang GY, Liao J, Kim K, Yurkow EJ and Yang
CS: Inhibition of growth and induction of apoptosis in human cancer
cell lines by tea polyphenols. Carcinogenesis. 19:611–616. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Liang YC, Lin-shiau SY, Chen CF and Lin
JK: Suppression of extracellular signals and cell proliferation
through EGF receptor binding by (−)-epigallocatechin gallate in
human A431 epidermoid carcinoma cells. J Cell Biochem. 67:55–65.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Gupta S, Hastak K, Afaq F, Ahmad N and
Mukhtar H: Essential role of caspases in
epigallocatechin-3-gallate-mediated inhibition of nuclear factor
kappa B and induction of apoptosis. Oncogene. 23:2507–2522. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Berger SJ, Gupta S, Belfi CA, Gosky DM and
Mukhtar H: Green tea constituent (−-)-epigallocatechin-3-gallate
inhibits topoisomerase I activity in human colon carcinoma cells.
Biochem Biophys Res Commun. 288:101–105. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lu X, Sun W, Tang Y, Zhu L, Li Y, Ou C,
Yang C, Su J, Luo C, Hu Y and Cao J: Identification of key genes in
hepatocellular carcinoma and validation of the candidate gene,
cdc25a, using gene set enrichment analysis, meta-analysis and
cross-species comparison. Mol Med Rep. 13:1172–1178. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Terada Y, Tatsuka M, Jinno S and Okayama
H: Requirement for tyrosine phosphorylation of Cdk4 in G1 arrest
induced by ultraviolet irradiation. Nature. 376:358–362. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Shen T and Huang S: The role of Cdc25A in
the regulation of cell proliferation and apoptosis. Anticancer
Agents Med Chem. 12:631–639. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Bartek J and Lukas J: Mammalian G1- and
S-phase checkpoints in response to DNA damage. Curr Opin Cell Biol.
13:738–747. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Lindqvist A, Rodríguez-Bravo V and Medema
RH: The decision to enter mitosis: Feedback and redundancy in the
mitotic entry network. J Cell Biol. 185:193–202. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Lavarone A and Massagué J: Repression of
the CDK activator Cdc25A and cell-cycle arrest by cytokine TGF-beta
in cells lacking the CDK inhibitor p15. Nature. 387:417–422. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Liang J, Cao R, Zhang Y, Xia Y, Zheng Y,
Li X, Wang L, Yang W and Lu Z: PKM2 dephosphorylation by Cdc25A
promotes the Warburg effect and tumorigenesis. Nat Commun.
7:124312016. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Yamashita Y, Kasugai I, Sato M, Tanuma N,
Sato I, Nomura M, Yamashita K, Sonoda Y, Kumabe T, Tominaga T, et
al: CDC25A mRNA levels significantly correlate with Ki-67
expression in human glioma samples. J Neurooncol. 100:43–49. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Singh L, Pushker N, Sen S, Singh MK,
Bakhshi S, Chawla B and Kashyap S: Expression of CDC25A and CDC25B
phosphatase proteins in human retinoblastoma and its correlation
with clinicopathological parameters. Br J Ophthalmol. 99:457–463.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Brunetto E, Ferrara AM, Rampoldi F,
Talarico A, Cin ED, Grassini G, Spagnuolo L, Sassi I, Ferro A,
Cuorvo LV, et al: CDC25A protein stability represents a previously
unrecognized target of HER2 signaling in human breast cancer:
Implication for a potential clinical relevance in trastuzumab
treatment. Neoplasia. 15:579–590. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Weiss RH: p21Waf1/Cip1 as a therapeutic
target in breast and other cancers. Cancer Cell. 4:425–429. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Harper JW, Adami GR, Wei N, Keyomarsi K
and Elledge SJ: The p21 Cdk-interacting protein Cip1 is a potent
inhibitor of G1 cyclin-dependent kinases. Cell. 75:805–816. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Abbas T and Dutta A: p21 in cancer:
Intricate networks and multiple activities. Nat Rev Cancer.
9:400–414. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Zhang X, Min KW, Wimalasena J and Baek SJ:
Cyclin D1 degradation and p21 induction contribute to growth
inhibition of colorectal cancer cells induced by
epigallocatechin-3-gallate. J Cancer Res Clin Oncol. 138:2051–2060.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Liu X, Zhang DY, Zhang W, Zhao X, Yuan C
and Ye F: The effect of green tea extract and EGCG on the signaling
network in squamous cell carcinoma. Nutr Cancer. 63:466–475. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Rawangkan A, Wongsirisin P, Namiki K, Iida
K, Kobayashi Y, Shimizu Y, Fujiki H and Suganuma M: Green tea
catechin is an alternative immune checkpoint inhibitor that
inhibits PD-L1 expression and lung tumor growth. Molecules.
23:20712018. View Article : Google Scholar
|