Introduction
Osteosarcoma is one of the most common malignant
tumors, with a high incidence and metastasis rate, primarily
occurring in children and adolescents (1). In Portugal, the rate for African
Americans is 5.1–0.3% per 1 million children aged 0–14 years
(2). For several decades, the
treatment of osteosarcoma has primarily included surgery,
radiotherapy and chemotherapy, but the results of these treatment
are not satisfactory (3). The
challenge for anticancer research is to identify a novel
alternative approach that effectively targets osteosarcoma cells by
inducing apoptosis, combined with low toxicity (4).
Previous evidence has revealed that calycosin, a
bioactive phytoestrogen isoflavone that is extracted from
Trifolium pratense (red clover), possesses anti-allergic
(5), anti-inflammatory (6), antifibrotic (7), anti-myocardial injury (8) and antitumor (9–11)
properties. Calycosin may exert a potential antimetastatic effect
on several types of cancer, including breast cancer, colorectal
cancer, ovarian cancer and nasopharyngeal carcinoma (9,10).
It also promotes apoptosis and autophagy in adenocarcinoma cells
via sirtuin 1 (SIRT1)/AMP-activated protein kinase (AMPK)-induced
inhibition of the Akt/mammalian target of rapamycin (mTOR) pathway
(11). It inhibits the
phosphatidylinositol 3-kinase (PI3K)/Akt/mTOR pathway, which in
turn leads to apoptosis of osteosarcoma cells (12). Mitogen-activated protein kinases
(MAPKs) are one of the most important regulators of apoptotic cell
death (13) and the MAPK pathway
has been reported to be involved in the proliferation and apoptosis
of osteosarcoma cells (14).
Calycosin-induced apoptosis occurs in breast cancer cells through
regulation of the p38-MAPK and PI3K/Akt pathways (15). Therefore, a potential functional
mechanism through which the anti-osteosarcoma effects may be
mediated is inhibition of the MAPK pathway leading to cell death.
The aim of the present study was to investigate whether calycosin
exerted antitumor effects on osteosarcoma cells by inducing
apoptosis via the MAPK signaling pathway.
Materials and methods
Calycosin
Calycosin (purity 98%; Tianjin JAHE Science and
Technology Co., Ltd.) was diluted into a 250 µg/ml stock solution
with DMSO (Sigma-Aldrich; Merck KGaA). BIRB 796 was purchased from
R&D Systems, Inc.
Cell culture
The human osteosarcoma cell line 143B and the human
osteoblast cell line hFOB1.19 (Shanghai Institute of Biochemistry
and Cell Biology) were incubated in DMEM (Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 10% FBS (Gibco; Thermo Fisher
Scientific, Inc.), and 100 U/ml penicillin and 100 µg/ml
streptomycin in a humidified incubator containing 5% CO2
at 37°C. The medium was changed every 48 h.
Cells were cultured separately for 24 h, and were
then treated with various concentrations of calycosin (0–160 µg/ml)
at 37°C for 48 h. In addition, the p38 inhibitor BIRB 796 (Beyotime
Institute of Biotechnology) was applied to further explore the
expression of key proteins in osteosarcoma 143B cells following
treatment with calycosin. Specifically, the cells were incubated
with 1 µM BIRB 796 for 2 h at 37°C, and then treated with calycosin
(20 µg/ml) for 48 h.
MTT assay
Cell viability was determined by an MTT assay
(Sigma-Aldrich; Merck KGaA). Cells were harvested using 0.25%
trypsin and seeded into 96-well plates at a density of
3×104 cells/well at 37°C for 24 h. Then, the purple
formazan was dissolved with 100 µl DMSO, and cells were allowed to
shake for 10 min on a mini shaker, and analyzed in a
multi-well-plate reader at 570 nm on a microplate reader (Thermo
Fisher Scientific, Inc.). The proliferation rate (%) was calculated
as follows: Optical density (OD) treatment group/OD control
×100%.
Annexin V/PI staining assay
Early + late cell apoptosis was detected using flow
cytometry. Cells were collected and washed twice with PBS.
FITC-conjugated Annexin V (FITC-V) and PI double staining was used
to identify cells (BD Biosciences) according to the manufacturer's
instructions. Data were obtained and analyzed using a FACSCanto™
flow cytometer (Beckman Coulter, Inc.) with CellQuest software
(version 5.1; BD Biosciences).
JC-1 staining assay
Mitochondrial membrane potential (MMP) monitoring
was performed using the JC-1 MMP detection kit (Beyotime Institute
of Biotechnology), following the manufacturer's protocol. Cells
were collected, incubated with JC-1 at 37°C for 20 min, and then
washed twice with PBS. JC-1 green and red fluorescence were
recorded by flow using a FACSCanto™ flow cytometer (Beckman
Coulter, Inc.) as aforementioned at 527 and 590 nm, respectively.
Mitochondrial depolarization was manifested by a decrease in
red/green fluorescence intensity ratio.
Western blot analysis
Protein expression was detected by western blotting.
Cells were harvested, following which proteins were extracted from
the cells using a cocktail of cell lysis buffer supplemented with
protease inhibitor (Beyotime Institute of Biotechnology) in the
ratio of 100–200 µl of lysate to each well of the 6-well plate. The
lysates were centrifuged at 4°C at 700 × g for 10 min and
collected. Then, the total protein was measured with a
bicinchoninic protein assay kit (Tiangen Biotech Co., Ltd.). The
samples (20 µg) were separated via SDS-PAGE on a 12% gel and then
transferred to PVDF membranes (EMD Millipore). The membranes were
blocked with 5% non-fat dried milk in TBS with 0.2% Tween-20 (TBST)
buffer for 1 h at room temperature and then incubated with the
corresponding primary antibodies overnight at 4°C. The following
primary antibodies were used: Cleaved-caspase-3 (cat. no. 9661;
1:1,000), cleaved-caspase-9 (cat. no. 9509; 1:1,000),
cleaved-poly(ADP-ribose) polymerase (PARP; cat. no. 9548; 1:1,000),
B-cell lymphoma-2 (Bcl-2; cat. no. 15071; 1:1,000),
Bcl-2-associated X protein (Bax; cat. no. 5023; 1:1,000),
phosphorylated extracellular signal-regulated kinase (p-ERK; cat.
no. 4370; 1:2,000), ERK (cat. no. 4696; 1:2,000), p-p38 (cat. no.
4511; 1:1,000), p38 (cat. no. 8690; 1:1,000), c-Jun N-terminal
kinase (JNK; cat. no. 9252; 1:1,000), p-JNK (cat. no. 4668;
1:1,000), cytochrome c (cat. no. 11940; 1:1,000), Cytochrome
c oxidase IV (COX IV; cat. no. 4850; 1:1,000) and β-actin
(cat. no. 3700; 1:1,000). After three washes with TBST, the
membranes were subsequently incubated with anti-mouse IgG,
HRP-conjugated antibody (cat. no. 7076; 1:5,000) or anti-rabbit
IgG, HRP-conjugated antibody (cat. no. 7074; 1:5,000) for 1 h at
room temperature. The protein signal was detected via
electrochemiluminescence with an ECL-Plus kit (Beyotime Institute
of Biotechnology). All antibodies were purchased from Cell
Signaling Technology, Inc. All reagents were purchased from
Amersham (Cytiva). Cell Mitochondria Isolation kit (Beyotime
Institute of Biotechnology) was used to extract mitochondrial
proteins in order to analyze cytochrome c.
Statistical analysis
The present results were obtained from ≥3
independent experiments. Quantitative data are expressed as the
mean ± SD relative to the control value. Statistical analysis for
multiple comparisons were performed using one-way ANOVA followed by
Tukey's post hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
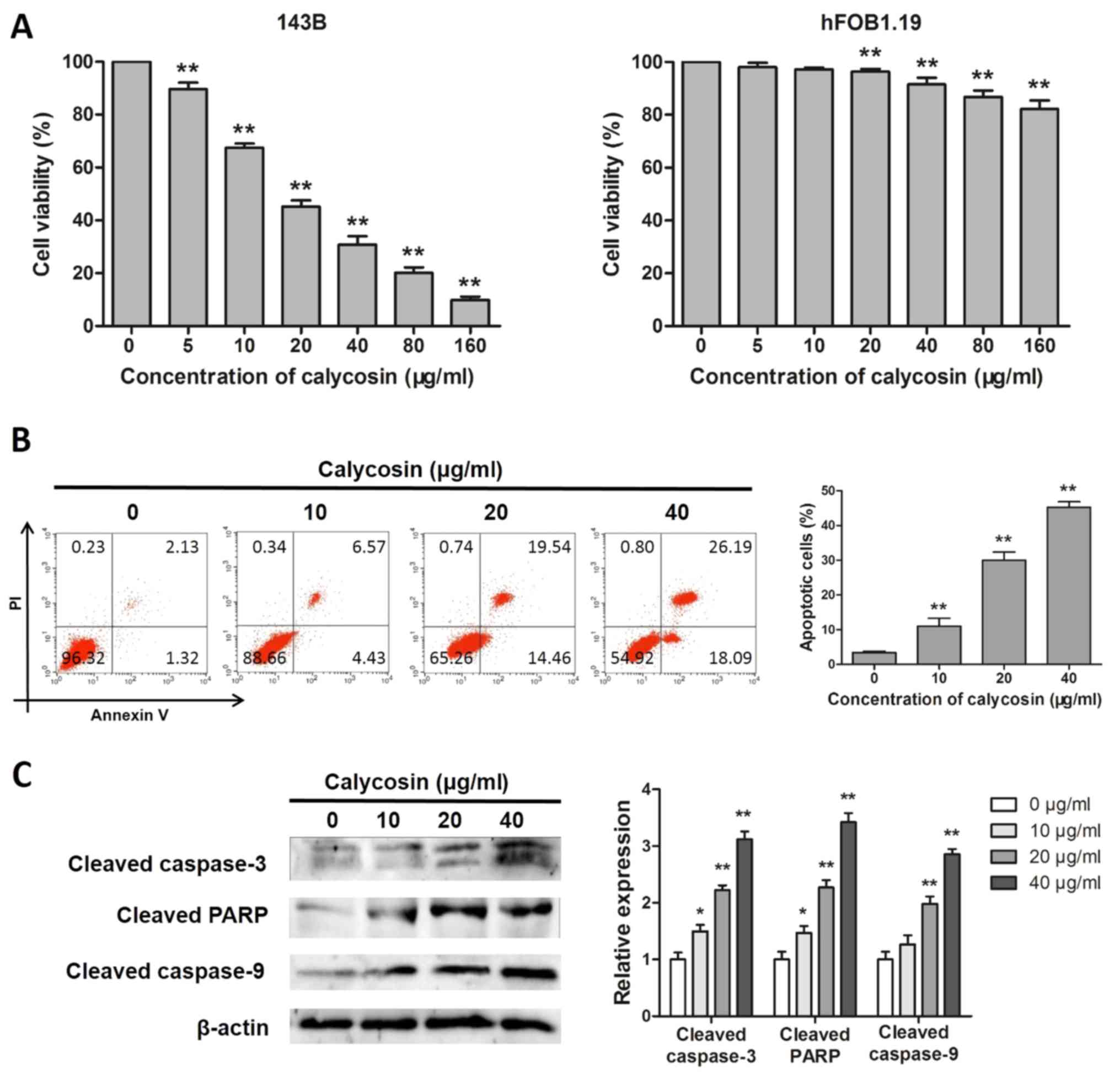
Calycosin suppresses cell viability
and promotes apoptosis of human osteosarcoma 143B cells
Human osteosarcoma 143B cells were exposed to
calycosin at different concentrations for 48 h to check whether
calycosin can inhibit the viability of osteosarcoma cells. As shown
in Fig. 1A, following calycosin
treatment for 48 h, the activity of osteosarcoma 143B cells was
inhibited in a dose-dependent manner (IC50, 21.22±1.54
µg/ml). In addition, although the survival rate of hFOB1.19 cells
decreased in a dose-dependent manner, the effect of calycosin on
normal osteoblasts was negligible (Fig. 1A).
Induction of apoptosis is a key mechanism through
which anticancer compounds exert their effects. Therefore, it was
investigated whether the cytotoxicity of calycosin was associated
with the induction of apoptosis in 143B cells. As shown in Fig. 1B, the flow cytometry assay results
demonstrated that, after calycosin treatment for 48 h, the
percentage of apoptotic cells increased significantly in a
concentration-dependent manner. As shown in Fig. 1C, western blotting revealed that
calycosin significantly increased the protein expression levels of
cleaved caspase-3, cleaved caspase-9, cleaved PARP, suggesting that
calycosin induced apoptosis in 143B cells.
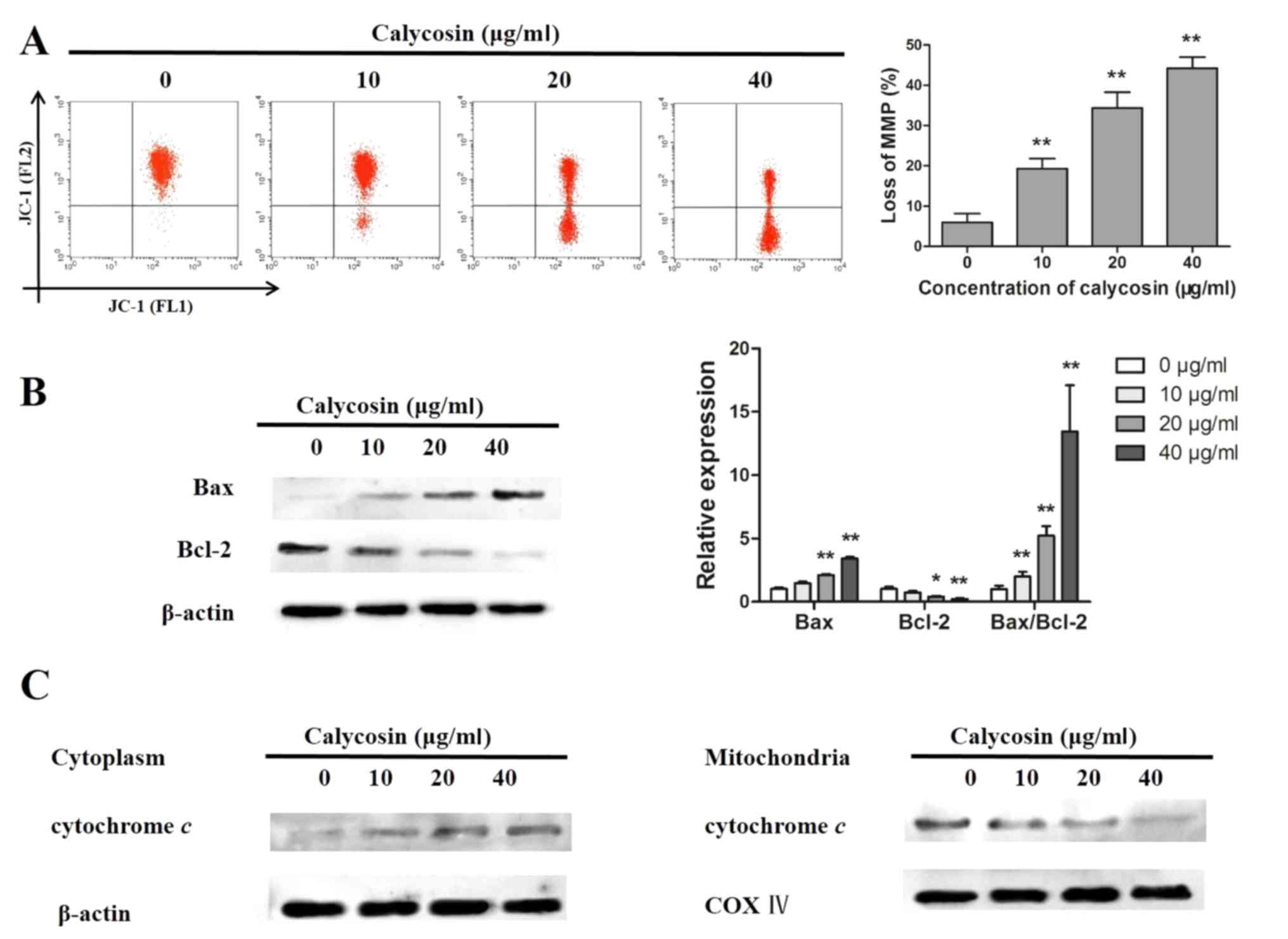
Calycosin regulates MMP in human
osteosarcoma 143B cells
The mitochondrial pathways are involved in the
initiation and development of the apoptotic process (16). Therefore, the alterations in MMP
and the expression of related proteins were investigated. Following
exposure to calycosin, loss of MMP increased significantly in a
dose-dependent manner in 143B cells, indicating that calycosin
significantly increased the proportion of depolarized cells
(Fig. 2A). In addition, calycosin
increased the expression of Bax and the Bax/Bcl-2 ratio, whereas it
decreased the expression of Bcl-2 (Fig. 2B). Furthermore, the levels of
cytochrome c were markedly reduced in the mitochondria and
increased in the cytoplasm following calycosin treatment, verifying
that calycosin-induced cell apoptosis was accompanied by
mitochondrial dysfunction (Fig.
2C).
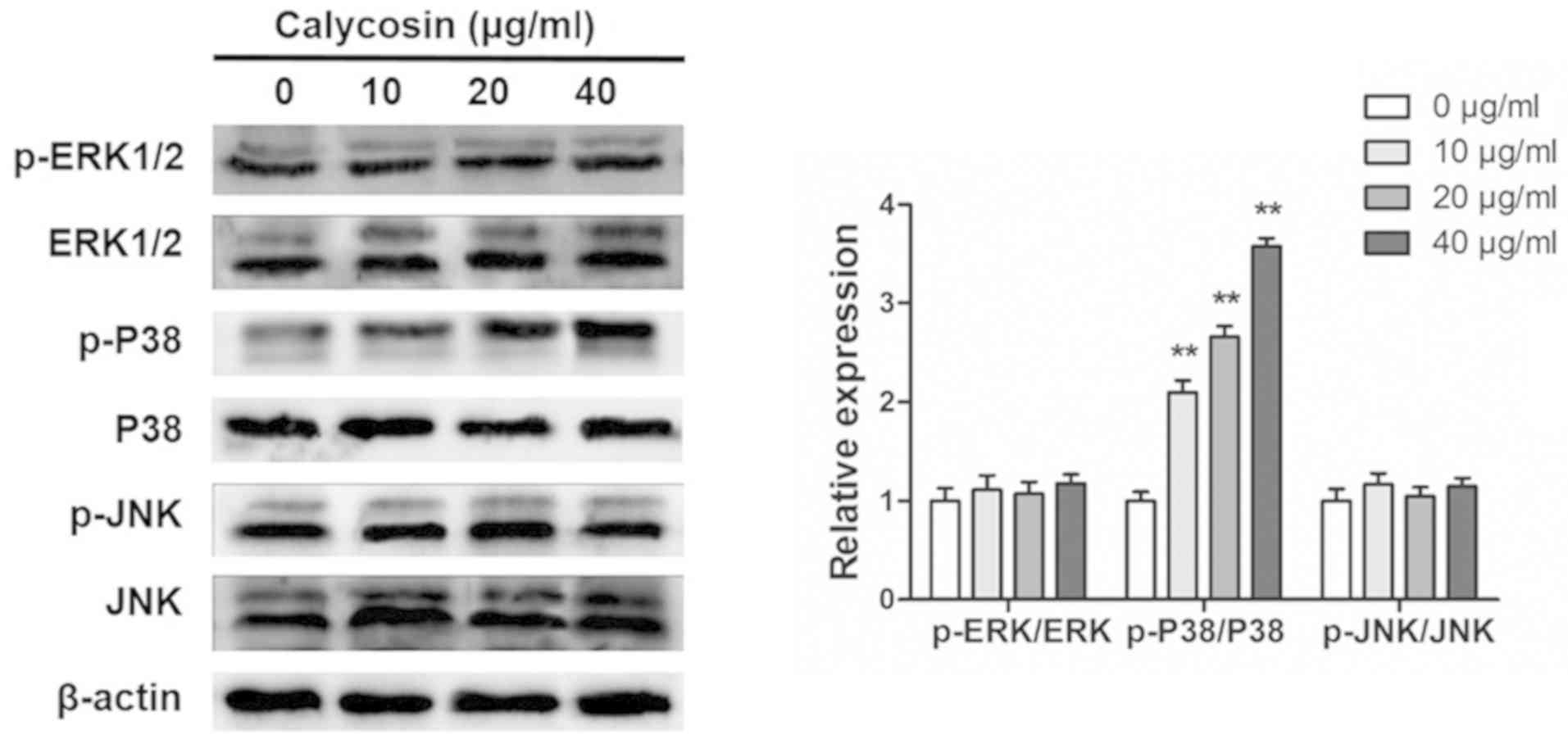
Calycosin activates p38-MAPK in human
osteosarcoma 143B cells
The MAPK signaling pathway regulates the apoptosis
pathways (17). Western blotting
was used to determine whether the MAPK signaling pathway was
involved in calycosin-induced apoptosis of osteosarcoma cells. It
was found that even at high concentrations, calycosin (40 g/ml) had
no effects on the phosphorylation of ERK1/2 and JNK (Fig. 3). By contrast, calycosin treatment
significantly increased p-p38-MAPK in a dose-dependent manner
(Fig. 3).
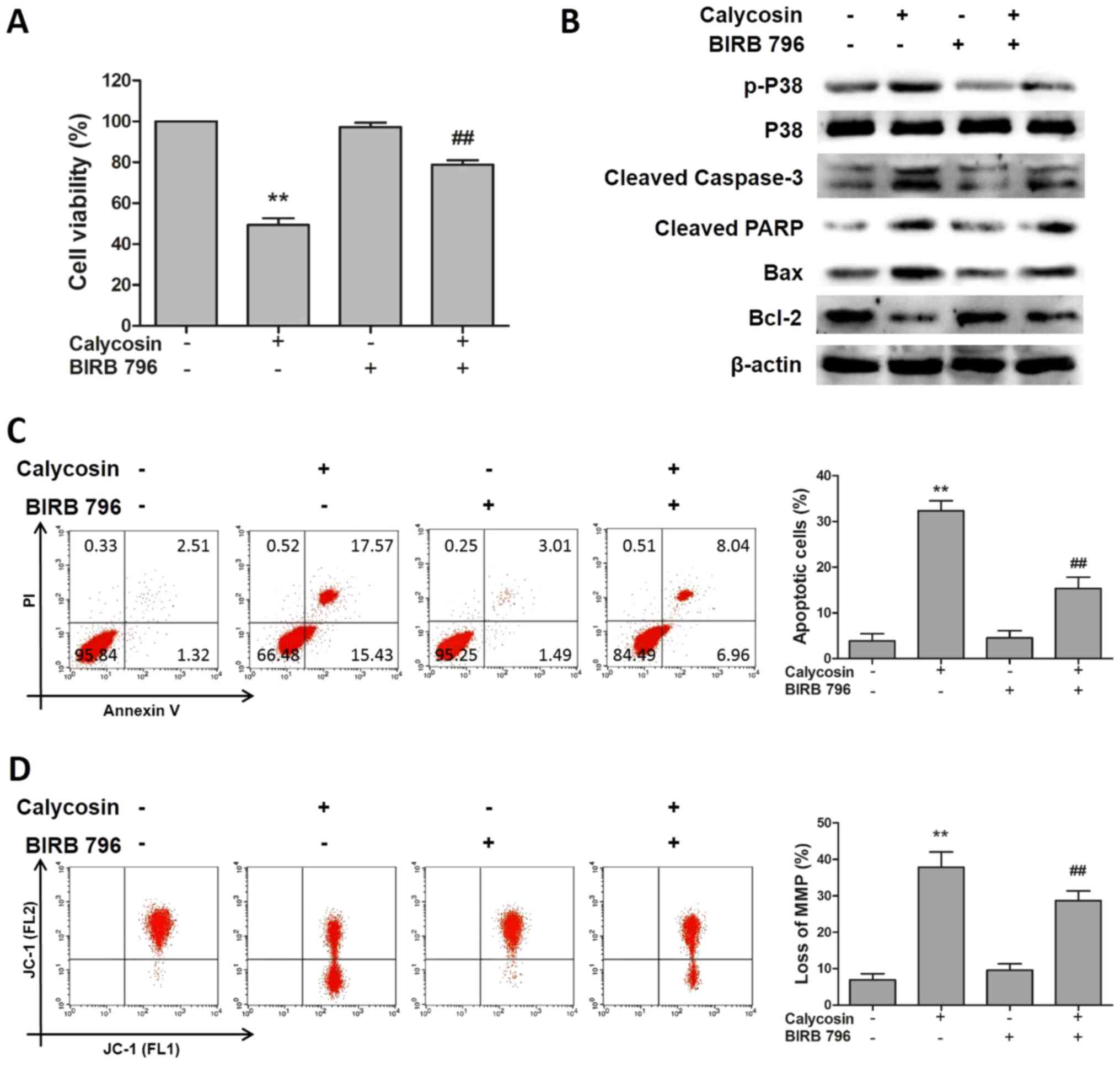
Calycosin inhibits the growth of human
osteosarcoma 143B cells via the p38-MAPK-mediated apoptotic
pathway
To investigate the role of the p38 signaling pathway
in calycosin-induced apoptosis, 143B cells were pretreated with the
p38 inhibitor BIRB 796. The results of flow cytometry analysis
demonstrated that BIRB 796 significantly ameliorated the
calycosin-induced apoptosis of osteosarcoma cells (Fig. 4A). The results of the western blot
analysis demonstrated that BIRB 796 treatment reversed the
upregulated expression levels of p-p38 and proapoptotic proteins,
including cleaved caspase-3 and cleaved PARP, and the downregulated
level of the antiapoptotic protein Bcl-2, caused by calycosin
treatment, whereas the total p38 protein levels remained constant
during all treatments (Fig. 4B).
The results of the flow cytometry analysis indicated that
inhibition of p38 by BIRB 796 abolished the effects of calycosin
treatment on the proportion of apoptotic and depolarized 143B cells
(Fig. 4C and D). In summary, these
results suggested that calycosin inhibited the growth of
osteosarcoma cells via the intrinsic apoptosis pathway mediated by
p38 signaling.
Discussion
Recent studies have extensively reported the
anticancer and antimetastatic activities of calycosin in various
types of cancer, including breast cancer, colorectal cancer,
ovarian cancer and nasopharyngeal carcinoma (10,11,15).
It was also reported that calycosin exerted an inhibitory effect on
the growth of osteosarcoma (9).
Sun et al (12) observed
that calycosin induced apoptosis of estrogen receptor+
MG-63 osteosarcoma cells via the PI3K/Akt/mTOR pathway. Qiu et
al (18) demonstrated that
calycosin was effective against osteosarcoma growth in vitro
and in vivo via suppression of the miR-223-IκBα pathway in
neoplastic cells. However, little is known about the mechanism
underlying the role of calycosin in osteosarcoma. In the present
study, it was examined whether calycosin inhibited osteosarcoma
growth by activating the p38-MAPK pathway in osteosarcoma 143B
cells.
Disruption of the apoptotic process plays an
important role in the pathogenesis of tumors, and the cytotoxic
effects of several antitumor drugs are often mediated by induction
of apoptosis (19). Apoptosis is
triggered by two major signaling pathways: The extrinsic pathway
(also referred to as the death receptor pathway) and the intrinsic
pathway (also referred to as the mitochondrial pathway) (20).
In the present study, calycosin at a concentration
of 0–160 µg/ml was used for 24, 48 and 72 h to detect the
inhibitory effect on osteosarcoma 143B cells. It was found that
calycosin reduced the viability of osteosarcoma 143B cells in a
concentration-dependent manner. Therefore, in the present study, a
concentration of 20 µg/ml, which was close to the IC50
concentration, were chosen to study the mechanism of action of
calycosin on 143B cells. However, smaller concentrations, such as 5
and 10 µg/ml, also have good effects on 143B cells, so the specific
mechanism of action using these concentrations still needs to be
explored.
Previous reports have demonstrated that calycosin
can inhibit cell growth and induce apoptosis of ovarian cancer
cells by upregulating the expression of caspases and Bcl-2 family
proteins (11,12,15,18,21).
Mitochondrial dependence signaling occurs through
cleavage of caspase-9, which then activates downstream caspase-3,
leading to cleavage of key cell substrates, including PARP, thereby
inducing apoptosis (22). It was
previously reported that calycosin exerts an anti-osteosarcoma
effect, and its mechanism was associated with the activation of
apoptosis (9). The present study
also demonstrated that treatment with calycosin led to the cleavage
of caspase-3, caspase-9 and PARP in osteosarcoma 143B cells.
Moreover, members of the Bcl-2 family play a key role in regulating
mitochondrial membrane permeability (23).
The balance between proapoptotic Bax and
antiapoptotic Bcl-2 is crucial for the determination of cell
survival. The increased Bax/Bcl-2 ratio and the transfer of Bax
from cytoplasm to the outer mitochondrial membrane renders cells
more susceptible to apoptosis (24). It was previously reported that the
release of cytochrome c is primarily regulated by Bcl-2/Bax
(25). The results of the present
study demonstrated that calycosin led to an increase in the
Bax/Bcl-2 ratio. Moreover, it was also observed that calycosin
treatment resulted in a marked increase in the level of cytochrome
c in the cytoplasm and a dose-dependent decrease in the
mitochondria, indicating that calycosin treatment caused cytochrome
c release from the mitochondria to the cytoplasm, further
confirming the hypothesis that calycosin-induced apoptosis is
mediated via the mitochondrial pathway.
In mammalian cells, the MAPK family is composed of
ERK1/2, JNK, and p38 (26). In
general, upregulation and activation of ERK1/2 and JNK promote
tumor development, whereas p38 is a tumor suppressor. The role of
p38-MAPK in cell proliferation and apoptosis has been extensively
investigated (27). Accumulating
evidence has suggested that the role of p38-MAPK is controversial
in several tumor cells, and the variation may be associated with
the applied stimuli and duration, as well as the specific
characteristics and types of cells (28). The evidence revealed that NK007
induces G1/S cell cycle arrest by promoting p38-MAPK
phosphorylation and suppressing the expression of hexokinase2,
which is associated with acidification and oxygen consumption in
ovarian cells (29). In
vivo and in vitro studies demonstrated that serotonin
yellow A induces apoptosis of HepG2 cells by significantly
inhibiting the phosphorylation of the p38-MAPK pathway in
hepatocellular carcinoma cells (30). Other findings have demonstrated
that calycosin can inhibit growth and induce apoptosis in breast
cancer cells via estrogen receptor β-dependent regulation of the
insulin-like growth factor 1 receptor, p38 MAPK and PI3K/Akt
pathways in breast cancer cells (15). In vitro and in vivo
research demonstrated that licochalcone A regulated the
mitochondrial-mediated internal apoptotic pathway via p38-MAPK, and
exerted antitumor effects on human osteosarcoma cells (28). Similarly, the present study
suggested that calycosin increased p-p38-MAPK expression, whereas a
specific p38 inhibitor, BIRB 796, significantly reversed the
effects of calycosin treatment on the viability, caspase
expression, MMP and apoptotic rate of osteosarcoma 143B cells.
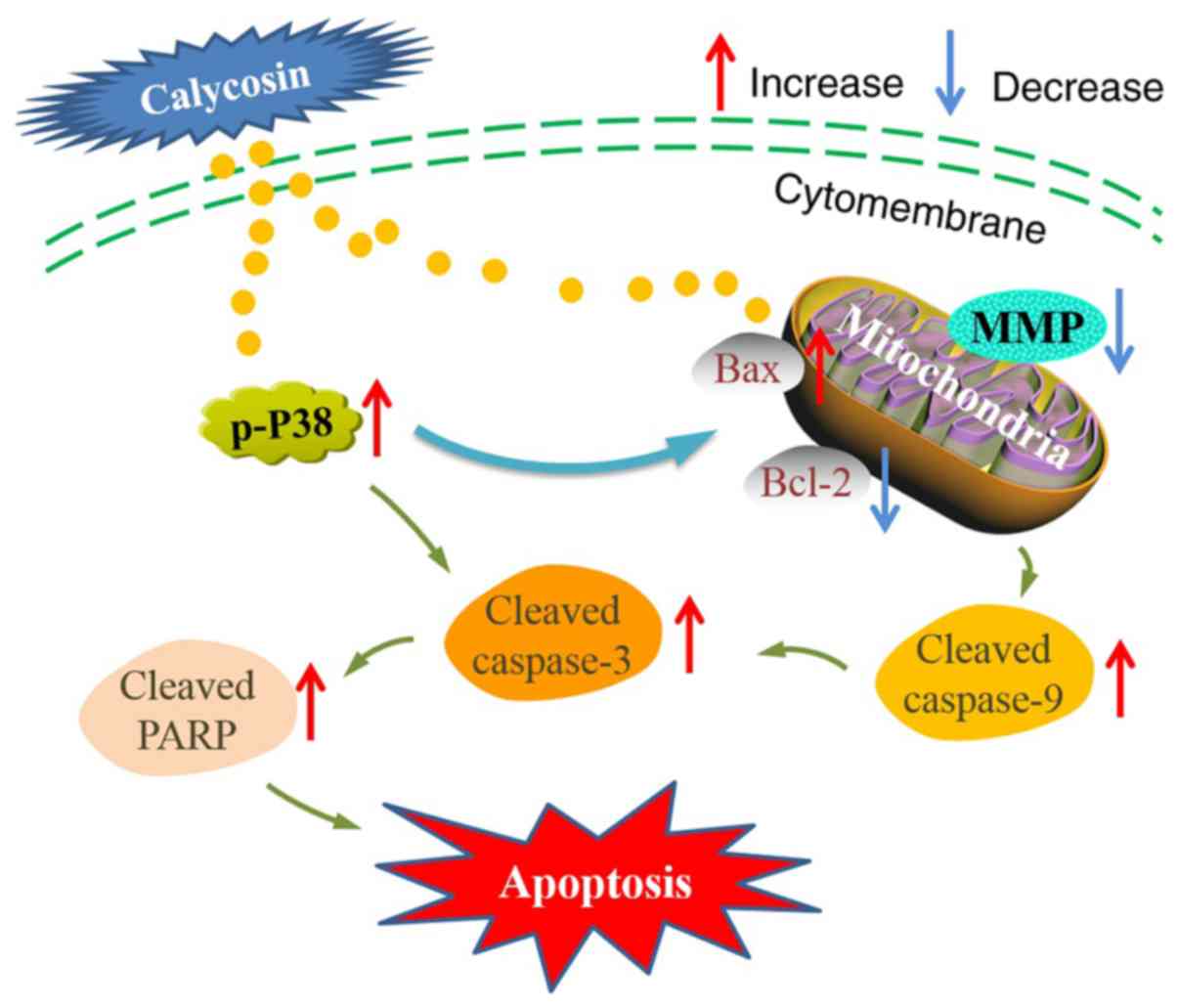
These results indicated that calycosin-induced apoptosis may be
caused by the activation of the p38-MAPK pathway-mediated
mitochondrial apoptotic pathway in human osteosarcoma cells
(Fig. 5). However, additional
studies are required to examine the molecular mechanisms through
which calycosin regulates the mitochondrial-related apoptotic
pathways that control the interaction between p38-MAPK and
apoptosis induction.
In conclusion, calycosin significantly induced
mitochondrial apoptosis in human osteosarcoma cells in
vitro, as indicated by the increased level of cleaved caspase-3
and −9 and PARP protein expression, and Bax/Bcl-2 ratio. Notably,
activation of caspases mediated by p38-MAPK or increased expression
of mitochondrial-related proteins may promote mitochondrial
apoptosis in human osteosarcoma cells. These results provided
insights into the potential value of calycosin in the clinical
treatment of osteosarcoma.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WT performed the majority of the experiments and
drafted the manuscript. ZWW helped perform the experiments. BMY
analyzed the data and drafted the manuscript. YGB conceived and
designed the study, supervised the experiments and edited the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
MMP
|
mitochondrial membrane potential
|
|
mTOR
|
mammalian target of rapamycin
|
|
PI3K
|
phosphatidylinositol 3-kinase
|
|
MAPK
|
mitogen-activated protein kinase
|
References
|
1
|
Mirabello L, Troisi RJ and Savage SA:
Osteosarcoma incidence and survival rates from 1973 to 2004: Data
from the surveillance, epidemiology, and end results program.
Cancer. 115:1531–1543. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Eyre R, Feltbower RG, Mubwandarikwa E,
Eden TO and McNally RJ: Epidemiology of bone tumours in children
and young adults. Pediatr Blood Cancer. 53:941–952. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Harrison DJ, Geller DS, Gill JD, Lewis VO
and Gorlick R: Current and future therapeutic approaches for
osteosarcoma. Expert Rev Anticancer Ther. 18:39–50. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Baudino TA: Targeted cancer therapy: The
next generation of cancer treatment. Curr Drug Discov Technol.
12:3–20. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tao Y, Wang Y, Wang X, Wang C, Bao K, Ji
L, Jiang G and Hong M: Calycosin suppresses epithelial derived
initiative key factors and maintains epithelial barrier in allergic
inflammation via TLR4 mediated NF-κB pathway. Cell Physiol Biochem.
44:1106–1119. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dong L, Yin L, Chen R, Zhang Y, Hua S,
Quan H and Fu X: Anti-inflammatory effect of Calycosin glycoside on
lipopolysaccharide-induced inflammatory responses in RAW 264.7
cells. Gene. 675:94–101. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Duan X, Meng Q, Wang C, Liu Z, Liu Q, Sun
H, Sun P, Yang X, Huo X, Peng J and Liu K: Calycosin attenuates
triglyceride accumulation and hepatic fibrosis in murine model of
non-alcoholic steatohepatitis via activating farnesoid X receptor.
Phytomedicine. 25:83–92. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu Y, Che G, Di Z, Sun W, Tian J and Ren
M: Calycosin-7-O-β-D-glucoside attenuates myocardial
ischemia-reperfusion injury by activating JAK2/STAT3 signaling
pathway via the regulation of IL-10 secretion in mice. Mol Cell
Biochem. 463:175–187. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Qiu R, Li X, Qin K, Chen X, Wang R, Dai Y,
Deng L and Ye Y: Antimetastatic effects of calycosin on
osteosarcoma and the underlying mechanism. Biofactors. 45:975–982.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu G, Niu M, Qin J, Wang Y and Tian J:
Inactivation of Rab27B-dependent signaling pathway by calycosin
inhibits migration and invasion of ER-negative breast cancer cells.
Gene. 709:48–55. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
El-Kott AF, Al-Kahtani MA and Shati AA:
Calycosin induces apoptosis in adenocarcinoma HT29 cells by
inducing cytotoxic autophagy mediated by SIRT1/AMPK-induced
inhibition of Akt/mTOR. Clin Exp Pharmacol Physiol. 46:944–954.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sun H, Yin M, Qian W and Yin H: Calycosin,
a phytoestrogen isoflavone, induces apoptosis of estrogen
receptor-positive MG-63 osteosarcoma cells via the
phosphatidylinositol 3-kinase (PI3K)/AKT/mammalian target of
rapamycin (mTOR) pathway. Med Sci Monit. 24:6178–6186. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang W and Liu HT: MAPK signal pathways
in the regulation of cell proliferation in mammalian cells. Cell
Res. 12:9–18. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ning L, Wan S, Jie Z, Xie Z, Li X, Pan X,
Wan X, Chen W, Huang H, Wang J, et al: Lycorine induces apoptosis
and G1 phase arrest through ROS/p38 MAPK signaling pathway in human
osteosarcoma cells in vitro and in vivo. Spine (Phila Pa 1976).
45:E126–E139. 2019. View Article : Google Scholar
|
|
15
|
Chen J, Hou R, Zhang X, Ye Y, Wang Y and
Tian J: Calycosin suppresses breast cancer cell growth via
ERβ-dependent regulation of IGF-1R, p38 MAPK and PI3K/Akt pathways.
PLoS One. 9:e912452014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang B, Wang D, Guo F and Xuan C:
Mitochondrial membrane potential and reactive oxygen species in
cancer stem cells. Fam Cancer. 14:19–23. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wada T and Penninger JM: Mitogen-activated
protein kinases in apoptosis regulation. Oncogene. 23:2838–2849.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Qiu R, Ma G, Li X, Shi Q, Li X, Zhou X,
Tang Y, Xie Z, Liao S, Qin Y, et al: Clinical case report of
patients with osteosarcoma and anticancer benefit of calycosin
against human osteosarcoma cells. J Cell Biochem. 120:10697–10706.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fleischer A, Ghadiri A, Dessauge F,
Duhamel M, Rebollo MP, Alvarez-Franco F and Rebollo A: Modulating
apoptosis as a target for effective therapy. Mol Immunol.
43:1065–1079. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Creagh EM: Caspase crosstalk: Integration
of apoptotic and innate immune signalling pathways. Trends Immunol.
35:631–640. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhou Y, Liu QH, Liu CL and Lin L:
Calycosin induces apoptosis in human ovarian cancer SKOV3 cells by
activating caspases and Bcl-2 family proteins. Tumour Biol.
36:5333–1339. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Palmer CS, Osellame LD, Stojanovski D and
Ryan MT: The regulation of mitochondrial morphology: Intricate
mechanisms and dynamic machinery. Cell Signal. 23:1534–1545. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xiong S, Mu T, Wang G and Jiang X:
Mitochondria-mediated apoptosis in mammals. Protein Cell.
5:737–749. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Siu WP, Pun PB, Latchoumycandane C and
Boelsterli UA: Bax-mediated mitochondrial outer membrane
permeabilization (MOMP), distinct from the mitochondrial
permeability transition, is a key mechanism in diclofenac-induced
hepatocyte injury: Multiple protective roles of cyclosporin A.
Toxicol Appl Pharmacol. 227:451–461. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jergens A, Young J, Moore D, Wang C,
Hostetter J, Augustine L, Allenspach K, Schmitz S and Mosher C:
Bcl-2/Caspase 3 mucosal imbalance favors T cell resistance to
apoptosis in dogs with inflammatory bowel disease. Vet Immunol
Immunopathol. 158:167–74. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xu T, Wang NS, Fu LL, Ye CY, Yu SQ and Mei
CL: Celecoxib inhibits growth of human autosomal dominant
polycystic kidney cyst-lining epithelial cells through the
VEGF/Raf/MAPK/ERK signaling pathway. Mol Biol Rep. 39:7743–7753.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cuenda A and Rousseau S: p38 MAP-kinases
pathway regulation, function and role in human diseases. Biochim
Biophys Acta. 1773:1358–1375. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lin RC, Yang SF, Chiou HL, Hsieh SC, Wen
SH, Lu KH and Hsieh YH: Licochalcone a-induced apoptosis through
the activation of p38MAPK pathway mediated mitochondrial pathways
of apoptosis in human osteosarcoma cells in vitro and in vivo.
Cells. 8:14412019. View Article : Google Scholar
|
|
29
|
Li Z, Tang X, Luo Y, Chen B, Zhou C, Wu X,
Tang Z, Qi X, Cao G, Hao J, et al: NK007 helps in mitigating
paclitaxel resistance through p38MAPK activation and HK2
degradation in ovarian cancer. J Cell Physiol. Feb 20–2019.(Epub
ahead of print). doi: 10.1002/jcp.28278.
|
|
30
|
Zhang J, Li J, Song H, Xiong Y, Liu D and
Bai X: Hydroxysafflor yellow A suppresses angiogenesis of
hepatocellular carcinoma through inhibition of p38 MAPK
phosphorylation. Biomed Pharmacother. 109:806–814. 2019. View Article : Google Scholar : PubMed/NCBI
|