Introduction
According to National Health and Nutrition
Examination Survey data between 2011 and 2014, the prevalence of
coronary heart disease (CHD) in the USA was 6.3% in adults ≥20
years of age (1). Unstable angina
(UA) is a severe and common complication of CHD. On the basis of
administrative claims data for US Medicare beneficiaries,
hospitalization for UA decreased from 1.5% in 1997 to 0.6% in 2009
(1). A previous study in Denmark
has demonstrated that with the introduction of the high-sensitivity
troponin test, the accuracy of UA diagnosis has been improved
(2). Overall, ~10% of all internal
medicine emergency patients present to the emergency department
with chest pain, but only 10% of these patients have an acute
myocardial infarction as the underlying cause (3). UA is more severe compared with
fatigue stable angina, but less severe compared with acute
myocardial infarction (4). The
main symptom of UA is severe chest pain, which can occur during
rest (4). Coronary atherosclerotic
plaque rupture, platelet aggregation and thrombosis are the main
causes of coronary blood flow occlusion, which induces UA (5). Due to the unique pathophysiological
mechanism UA, patients may develop acute myocardial ischemia or
acute myocardial infarction if UA is not detected and treated in
time (6). Therefore, the timely
diagnosis of UA is essential. The diagnosis of UA is primarily
based on electrocardiograms and the clinical symptoms of patients
(7); however, the subjective
nature of clinical symptoms and transient changes of
electrocardiograms are limitations. Although coronary angiography
is a reliable and accurate diagnostic method for UA, it is an
invasive procedure that may be unacceptable for certain patients.
Therefore, there is an urgent requirement for a non-invasive
technique to diagnose UA quickly and accurately.
As an emerging method of systematic biological
technology, metabolomics has provided application prospects in a
range of subject areas, such as disease pathophysiological process
research and molecular diagnosis of cancer (8,9).
Elucidating differences between healthy and diseased states is of
important clinical use (10).
Metabolomics techniques aim to quantitatively measure the systemic
effects of endogenous small molecule metabolites produced by
exogenous substances in the body (11,12).
Additionally, it elucidates pathological processes and the dynamic
metabolic responses of substances in the body (13). The use of metabolomics to identify
potential disease biomarkers has allowed for early diagnoses and
the determination of associated biological mechanisms (14,15).
In a recent study, the phospholipid profile in serum of patients
with Alzheimer's disease was studied by multi-platform metabonomics
to find a new diagnostic method (16). In this study, sera from 19 patients
with Alzheimer's disease and 17 healthy volunteers were collected
and significant changes were detected in the levels of several
phosphatidylcholine, phosphatidylethanolamine, plasma
alkenylcholine, plasma alkenylethanolamine and different
lysophospholipids, which provided a global insight into the
possible factors that triggered membrane rupture, a possible
underlying mechanism of Alzheimer's disease. Although the human
urine metabolome is a subset of the human serum metabolome,
compounds that are below the limit of detection in the blood are
detectable in urine (17,18). Urine is therefore becoming a
surrogate to blood for monitoring disease biomarkers. Samples are
easy to collect, repeatedly available and can be obtained in large
quantities from patients of all ages. Urine also contains low cell
and protein content, and rich chemical compositions, which provides
valuable bioinformation on metabolism (19,20).
In recent years, metabolomics has made progress in cardiovascular
research. Wang et al (21)
used ultra-high-performance liquid chromatography-quadrupole
time-of-flight mass spectrometry (UPLC-Q-TOF/MS) to conduct urine
metabolomics on patients with acute coronary syndrome. The results
revealed that nine biomarkers, such as isobutyryl-1-carnitine, were
significantly upregulated, and that the expression of 11
biomarkers, including l-lactic acid, were downregulated; in
addition, fatty acid metabolism, fatty acid β-oxidation metabolism,
amino acid metabolism and the tricarboxylic acid (TCA) cycle were
demonstrated to serve important roles in acute coronary syndrome.
Li et al (22) also
performed UPLC-Q-TOF/MS for metabolomics, and the results revealed
the interacting mechanism between Danhong injection and low-dose
aspirin, providing a reference for cardiovascular disease research,
which suggested that the emerging alterations caused by interaction
between Danhong injection and low-dose aspirin might influence the
co-administration of other drugs or the treatment of relevant
diseases. Urine metabolomics has therefore become a powerful
non-invasive technique for diagnosing and monitoring various human
diseases (18,23).
The current study aimed to use a UPLC-Q-TOF/MS-based
untargeted metabolomics platform to screen urine biomarkers in
patients with UA by principal component analysis (PCA) and partial
least squares discriminant analysis (PLS-DA). Receiver operating
characteristic (ROC) curves were used to determine whether these
biomarkers exhibited diagnostic significance for UA, and changes in
metabolic pathways during UA were examined using the metabolomics
pathway analysis (MetPA) web-based tool. The present findings
provide new insights into UA pathogenesis and its clinical
diagnosis.
Materials and methods
Clinical trial registration and ethics
statement
A total of 28 patients with UA and 28 healthy
controls (HCs) were recruited from Tianjin Chest Hospital (Tianjin,
China) and the General Hospital of Tianjin Medical University
(Tianjin, China). All participants enrolled in the current study
provided written informed consent. The present study conformed to
the Declaration of Helsinki and was approved by the Ethics
Committee of Tianjin University of Traditional Chinese Medicine
(approval no. TJUTCM-EC20180003).
Inclusion criteria
According to the Guidelines for the Diagnosis and
Treatment of Unstable Angina and Non-ST-Segment Elevation
Myocardial Infarction issued by the Chinese Medical Association
Cardiovascular Branch and the Editorial Board of the Chinese
Journal of Cardiovascular Diseases in 2007 (24), all patients were enrolled with the
following subtypes: i) Resting angina (the episode occurs at rest
with a duration >20 min; ii) initial angina (new angina
occurring within 1 month); iii) worsening exertional angina
pectoris (a history of angina pectoris with pain worsening in the
last month); and iv) variant angina pectoris (transient ST-segment
elevation with self-remission). The age-matched HCs adhered to
strict standards for inclusion: i) No abnormalities detected during
physical examination, including blood pressure, height, weight,
gait and appearance; ii) no abnormal medical history, family
history or female reproductive history; and iii) no abnormalities
in any of the following examinations: Cardiopulmonary, physical,
electrocardiogram, blood (routine, blood glucose, blood lipid,
liver function and renal function) and special examination for
female gynecology.
Exclusion criteria for patients
All patients were aged between 45–65 years and met
the diagnostic criteria for UA. Patients were excluded if they: i)
Had diseases that may cause chest pain, such as other heart
diseases, psychoneuroses, menopause, hyperthyroidism, cervical
spondylotic myelopathy, vertebral arteriopathy cervical
spondylosis, gastro-esophageal reflux disease or hiatal hernia; ii)
used >3 of the four types of drugs: β-receptor blockers, calcium
channel antagonists, energy metabolism drugs and nitrate drugs;
iii) exhibited hypertension following antihypertensive drug
treatment [systolic blood pressure (SBP) ≥160 mmHg, diastolic blood
pressure (DBP) ≥100 mmHg], severe cardiopulmonary insufficiency or
severe arrhythmia (rapid atrial fibrillation, atrial flutter and
paroxysmal ventricular tachycardia); iv) presented active liver
disease or unexplained elevated serum transaminase and alanine
aminotransferase levels, with aspartate aminotransferase twice the
upper limit of the normal reference value; v) had renal
dysfunction; vi) exhibited severe primary disease such as
hematopoietic system tumors or malignant tumors; vii) were
pregnant, lactating or of childbearing age and preparing for
pregnancy; viii) were mentally ill or exhibited impaired cognition;
ix) had severe metabolic diseases such as diabetic or gouty
nephropathy; and x) participated in other clinical trials within
the last 3 months.
Processing and preparation of
sample
Fasting morning blood was collected in the Tianjin
Chest Hospital and the General Hospital of Tianjin Medical
University and stored at 4°C. Fasting morning urine was collected
from the subjects and centrifuged at 4°C and 1,370 × g for 15 min
within 2 h. The supernatant was removed and centrifuged at 1,000 ×
g for 8 min at 4°C. Subsequently, sodium azide was added to the
sample at a volume ratio of 1:100 for preservation at −80°C for the
metabonomic analysis. Each group of samples was completely thawed
at room temperature, and 300 µl of each sample was centrifuged at
4°C and 11,180 × g for 10 min. Subsequently, 150 µl of the
supernatant was added and to 150 µl distilled water and vortexed
for 1 min. Following centrifugation at 18,890 × g for 15 min, 200
µl of the supernatant was analyzed in the vial for metabolomic
analysis. In addition, 100 µl of each urine sample was pipetted
into a centrifuge tube to prepare quality control (QC) samples to
validate the experimental precision, repeatability and
stability.
UPLC-Q-TOF/MS
Data were acquired using a UPLC-Q-TOF/MS system
(Waters Corporation). UPLC analysis was performed in a Waters
Acquity UPLC system (Waters Corporation). MS was performed on a
Waters Micro mass Q/TOF micro Synapt High Definition Mass
Spectrometer. Detailed parameters of the instrument are presented
in the Supplementary Materials and Methods (Data S1).
Statistical analysis
Raw UPLC-Q-TOF/MS data were subjected to peak
discovery, peak alignment and peak filtering through the MarkerLynx
applications manager data processing system (version 4.1; Waters
Corporation) for the identification of potential discriminant
variables. Subsequently, processed data (Excel format) were
imported into SIMCA-P 12.0 statistical software (Umetrics;
Sartorius AG), and unsupervised PCA was performed using SIMCA-P
software. If PCA could not completely separate data, a supervised
orthogonal PLS-DA was performed.
M/z values of the candidate markers were searched in
the human metabolome database (HMDB; http://www.hmdb.ca/) and were further determined based
on the secondary fragments obtained from the mass spectrum. The
‘Statistical Analysis’ (MetaboAnalyst 4.0; http://www.metaboanalyst.ca/) was used to analyze the
correlation between the biomarkers and Pearson correlation analysis
was used, and a correlation coefficient (Pearson's r) >0.3 was
considered to indicate a correlation if P<0.05. The ‘Pathway
Analysis’ online tool (MetaboAnalyst 4.0) was used to identify the
metabolic pathways associated with potential biomarkers.
ROC curves (IBM SPSS Statistics 21.0, IBM Corp.)
were used to determine whether these biomarkers exhibited
diagnostic significance for UA. The ROC curve had sensitivity
(true-positive rate) as the ordinate and 1-specificity
(false-positive rate) as the abscissa, which allows for the visual
observation of the association between sensitivity and specificity.
The area under the curve (AUC), reflects the diagnostic value of
the biomarker. The closer the AUC value is to 1, the more effective
the diagnostic effect.
Data between the UA and HC groups were compared by
independent sample Student's t-test using SPSS 21.0. Data are
presented as the mean ± SD for Gaussian variables or as the median
and IQR when normality was not tenable. P<0.05 was considered to
indicate a statistically significant difference.
Results
Demographic information
No significant differences were observed between
patients with UA and the HCs in age or height (P>0.05). SBP and
DBP levels were higher in the UA group compared with those in the
HCs (P<0.05). The detailed demographic analysis results are
presented in Table I. The
biochemical indicators of the subjects in the HC group are listed
in supplementary materials (Table
SI), all values of blood glucose, blood lipid and renal
function were within the normal range. The medications administered
to patients with UA following blood collection are presented in
Table SII, including antiplatelet
drugs, statins drugs, beta blocker drugs, nitrates drugs, energy
metabolism drugs, and so on.
 | Table I.Demographic information of patients
with UA and HCs. |
Table I.
Demographic information of patients
with UA and HCs.
| Parameter | UA (n=28) | HC (n=28) | P-value |
|---|
| Age, years | 51.86±3.83 | 50.18±5.29 | 0.179 |
| Sex,
male/female | 19/9 | 15/13 | 0.412 |
| Height, cm | 169.43±7.14 | 165.93±7.72 | 0.083 |
| SBP, mmHg | 132.32±11.85 | 122.68±9.53 | 0.001 |
| DBP, mmHg | 81.96±11.01 | 77.14±5.77 | 0.045 |
Metabolomics analysis
The relative standard deviation (RSD) of peak areas
and retention times of 20 randomly selected chromatographic peaks
were <15%, indicating that metabolomics requirements were met.
The QC results are presented in Table
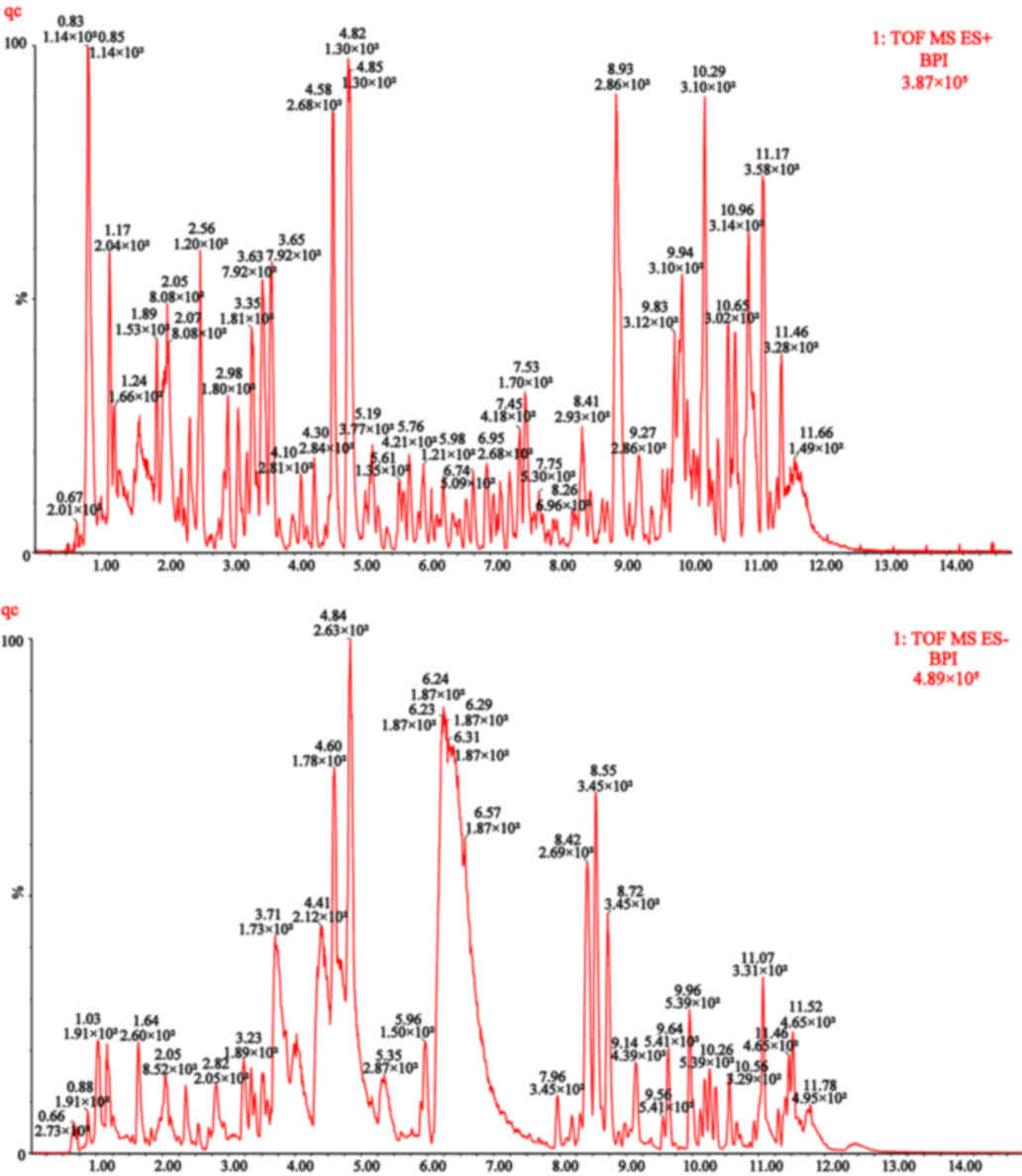
II. Based on UPLC-Q-TOF/MS analysis technology, the base peak
intensity (BPI) chromatograms of urine metabolomics were obtained
from 56 patients with UA and HCs, as well as QC samples. The
positive and negative ion maps of the QC samples are presented in
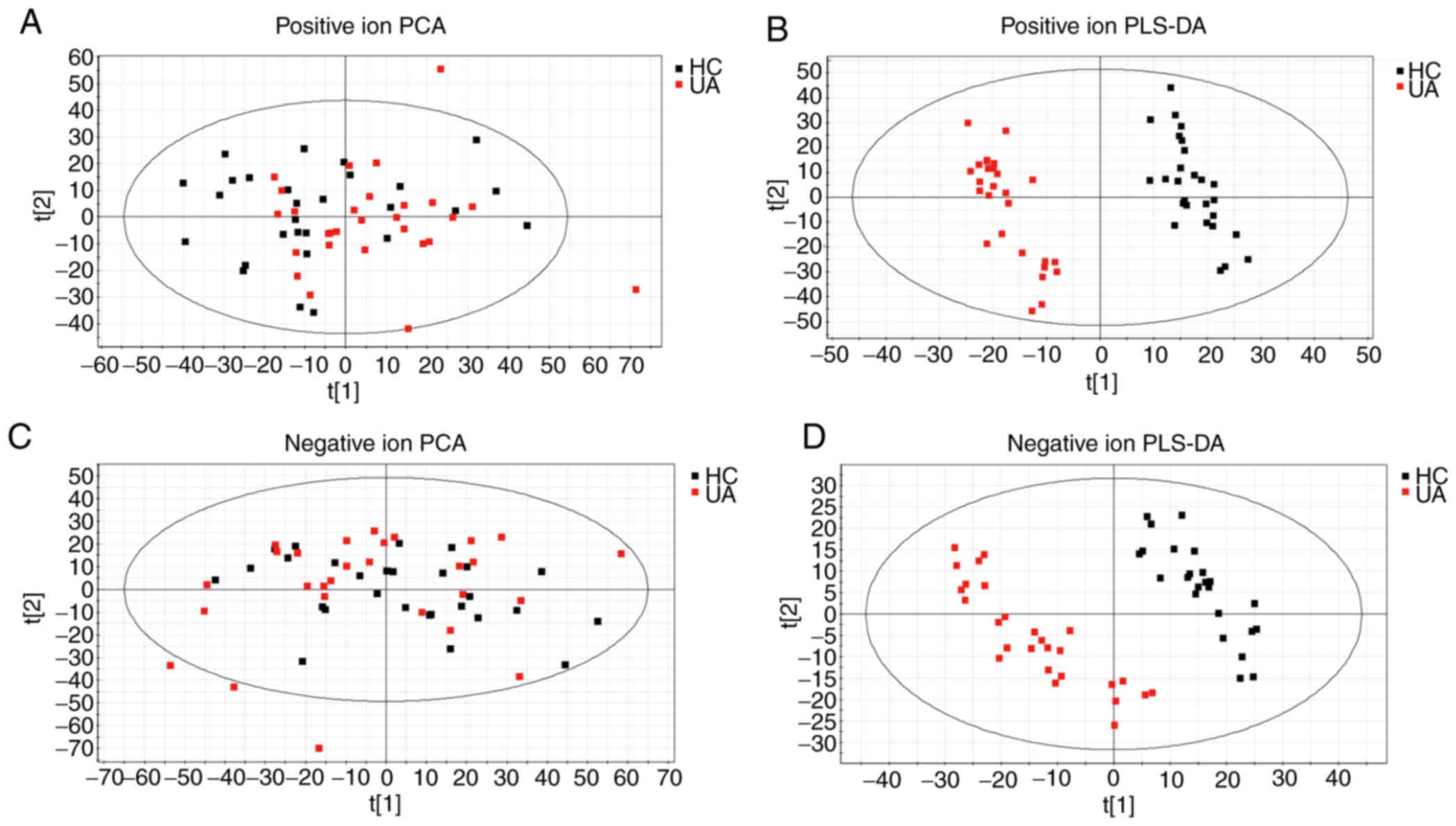
Fig. 1, and the PCA score map is
presented in Fig. 2A and C. It can
be observed from the PLS-DA score plots in Fig. 2B and D that the samples from
different groups were sorted into different quadrants, with no
overlap, which demonstrated that a wellfitted PLS-DA model was
established. The two groups of samples exhibited acceptable
classification and aggregation, indicating that the metabolic
patterns of the UA and HC groups were different.
 | Table II.Quality control experiment
results. |
Table II.
Quality control experiment
results.
| Experiment | Peak area, % | Retention time,
% |
|---|
| Instrument
precision | 0.10 | 0.89 |
| Method
repeatability | 0.10 | 0.98 |
| Sample
stability | 1.88 | 1.47 |
Identification of potential
biomarkers
According to the PLS-DA model and the t-test
results, substances with significant differences between the UA and
HC groups were screened. Subsequently, differentially expressed
small molecules were identified in patients with UA based on the
m/z values obtained from the HMDB. The urine biomarkers of 16
patients with UA were analyzed by comparing the secondary
characteristic fragments of compounds such as D-glucuronic acid,
creatinine, succinic acid and N-acetylneuraminic acid; the details
are presented in Table III.
Compared with the HC groups, the levels of biomarkers such as
glutathione, succinic acid semialdehyde, dihydrotestosterone and
cortisol in the UA group decreased significantly, whereas the
levels of succinic acid, N-acetylneuraminic acid, arachidonic acid,
glutaric acid and estradiol increased significantly. The metabolic
pathways can be further analyzed through the change trend of the
biomarkers, providing insight into the biological relevance of
these markers for UA.
 | Table III.Metabolites associated with unstable
angina based on ultra-high-performance liquid
chromatography-quadrupole time-of-flight mass spectrometry. |
Table III.
Metabolites associated with unstable
angina based on ultra-high-performance liquid
chromatography-quadrupole time-of-flight mass spectrometry.
| Marker no. | RT, min | Observed, m/z | Calculated,
m/z | Compound | Molecular
formula | Parent ion | Change |
|---|
| 1 | 1.8841 | 330.0704 | 330.0736 | Glutathione |
C10H17O6N3S | M+Na | ↓b |
| 2 | 0.8751 | 117.0177 | 117.0188 | Succinic acid |
C4H6O4 | M-H | ↑b |
| 3 | 1.1444 | 103.0393 | 103.0395 | Succinic acid
semialdehyde |
C4H6O3 | M+H | ↓b |
| 4 | 4.2378 | 112.0505 | 112.0511 | Creatinine |
C4H7N3O | M-H | ↑a |
| 5 | 7.3885 | 175.0239 | 175.0243 |
D-Glucurono-6,3-lactone |
C6H8O6 | M-H | ↓a |
| 6 | 4.2493 | 193.0348 | 193.0348 | D-Glucuronic
acid |
C6H10O7 | M-H | ↑a |
| 7 | 0.8662 | 308.0953 | 308.0982 | N-Acetylneuraminic
acid |
C11H19NO9 | M-H | ↑b |
| 8 | 5.7765 | 171.0642 | 171.0657 | 2-Octenedioic
acid |
C8H12O4 | M-H | ↑a |
| 9 | 11.2255 | 327.2304 | 327.2300 | Arachidonic
acid |
C20H32O2 | M+Na | ↑b |
| 10 | 0.8245 | 131.0345 | 131.0344 | Glutaric acid |
C5H8O4 | M-H | ↑b |
| 11 | 10.6419 | 201.1108 | 201.1127 | Sebacic acid |
C10H18O4 | M-H | ↑a |
| 12 | 11.3095 | 273.1864 | 273.1855 | Estradiol |
C18H24O2 | M+H | ↑b |
| 13 | 2.8365 | 225.0855 | 225.0875 |
Porphobilinogen |
C10H14N2O4 | M-H | ↓b |
| 14 | 11.5345 | 313.2173 | 313.2143 |
Dihydrotestosterone |
C19H30O2 | M+Na | ↓b |
| 15 | 9.7406 | 363.2197 | 363.2171 | Cortisol |
C21H30O5 | M+H | ↓b |
| 16 | 3.3935 | 134.0480 | 134.0467 | Adenine |
C5H5N5 | M-H | ↑a |
Analysis of potential biomarker
metabolic pathways
Small molecule metabolites were screened in patients
with UA to further elucidate the underlying metabolic pathways of
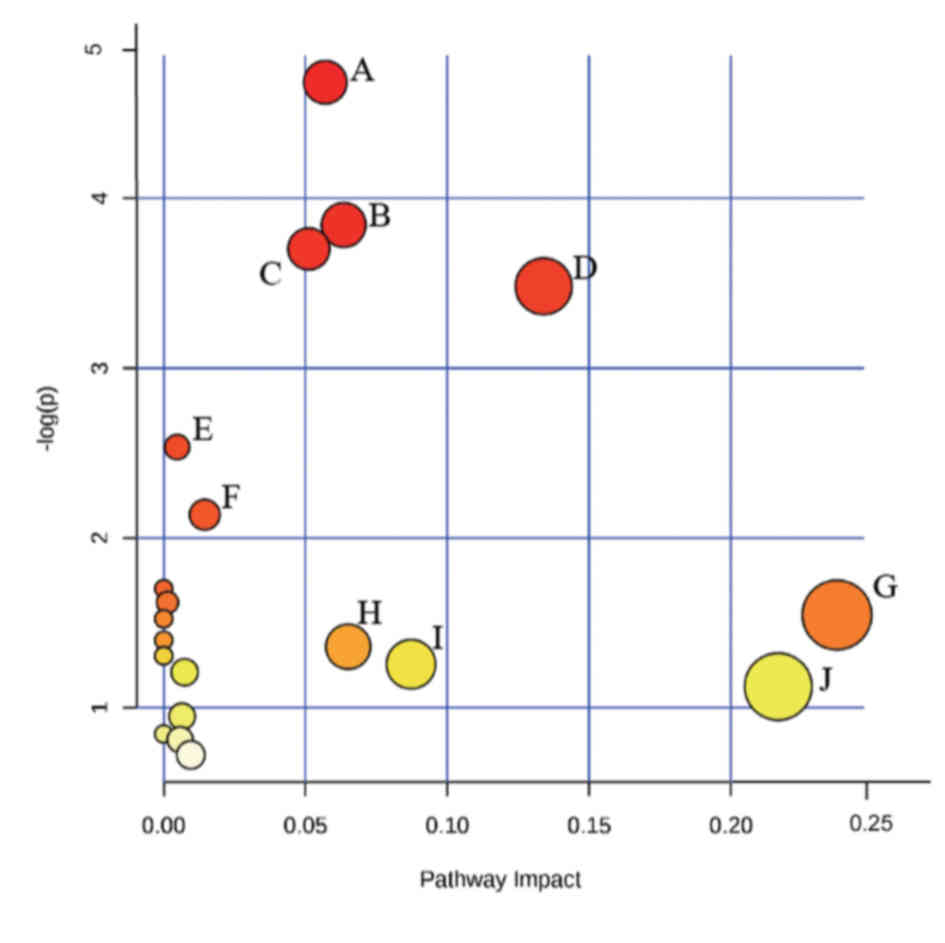
the disease. The association and metabolic pathways between the
small molecule metabolites were clarified using MetPA (Fig. 3). Metabolic pathways with a greater
contribution to the effects of UA relative to the HC groups were
considered to be involved in UA pathogenesis. The following
pathways were identified: ‘Alanine, aspartate and glutamate
metabolism’; ‘steroid hormone biosynthesis’; ‘butanoate
metabolism’, ‘ascorbate and aldarate metabolism’; ‘tyrosine
metabolism’; ‘the citrate cycle’; ‘glutathione metabolism’; ‘lysine
degradation’, ‘pentose and glucuronate interconversions’; and
‘arachidonic acid metabolism’. These may be the main pathways that
lead to the metabolic disorders of UA pectoris.
ROC curve analysis of potential
biomarkers
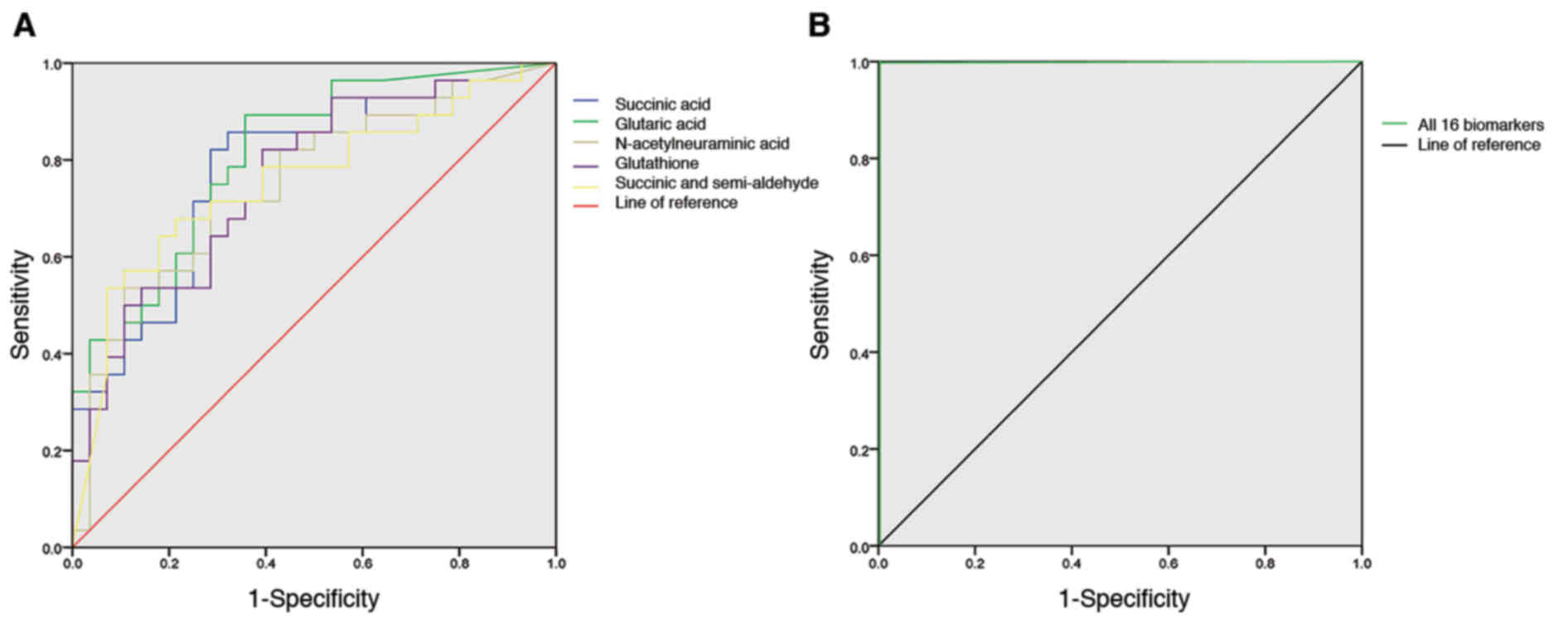
After 16 potential UA biomarkers were identified,
ROC curves were constructed and used to determine whether these
biomarkers exhibited diagnostic significance for UA. The different
colored ROC curves in Fig. 4A
represent an individual diagnostic biomarker. The AUC for each
diagnostic biomarker was >0.6 (Table IV). Overall, the 16 potential
biomarkers demonstrated diagnostic value for UA in Fig. 4B. The AUC for all diagnostic
biomarkers was 1, indicating a good accuracy for UA diagnosis.
 | Table IV.AUC of metabolites associated with
unstable angina. |
Table IV.
AUC of metabolites associated with
unstable angina.
|
|
|
| Asymptotic 95%
CI |
|---|
|
|
|
|
|
|---|
| Compound | AUC | Standard error | Lower bound | Upper bound |
|---|
| Sebacic acid | 0.714 | 0.068 | 0.581 | 0.848 |
| Succinic acid | 0.778 | 0.063 | 0.655 | 0.901 |
| Creatinine | 0.670 | 0.072 | 0.528 | 0.812 |
| Glutaric acid | 0.810 | 0.057 | 0.699 | 0.921 |
| Adenine | 0.656 | 0.073 | 0.512 | 0.800 |
|
D-Glucurono-6,3-lactone | 0.618 | 0.076 | 0.468 | 0.768 |
| D-Glucuronic
acid | 0.697 | 0.071 | 0.557 | 0.837 |
| N-Acetylneuraminic
acid | 0.753 | 0.066 | 0.624 | 0.881 |
| 2-Octenedioic
acid | 0.682 | 0.071 | 0.542 | 0.822 |
| Estradiol | 0.686 | 0.072 | 0.545 | 0.827 |
|
Porphobilinogen | 0.716 | 0.071 | 0.576 | 0.855 |
|
Dihydrotestosterone | 0.693 | 0.073 | 0.550 | 0.835 |
| Glutathione | 0.760 | 0.064 | 0.635 | 0.885 |
| Succinic acid
semialdehyde | 0.755 | 0.066 | 0.625 | 0.885 |
| Arachidonic
acid | 0.675 | 0.075 | 0.529 | 0.822 |
| Cortisol | 0.724 | 0.067 | 0.593 | 0.856 |
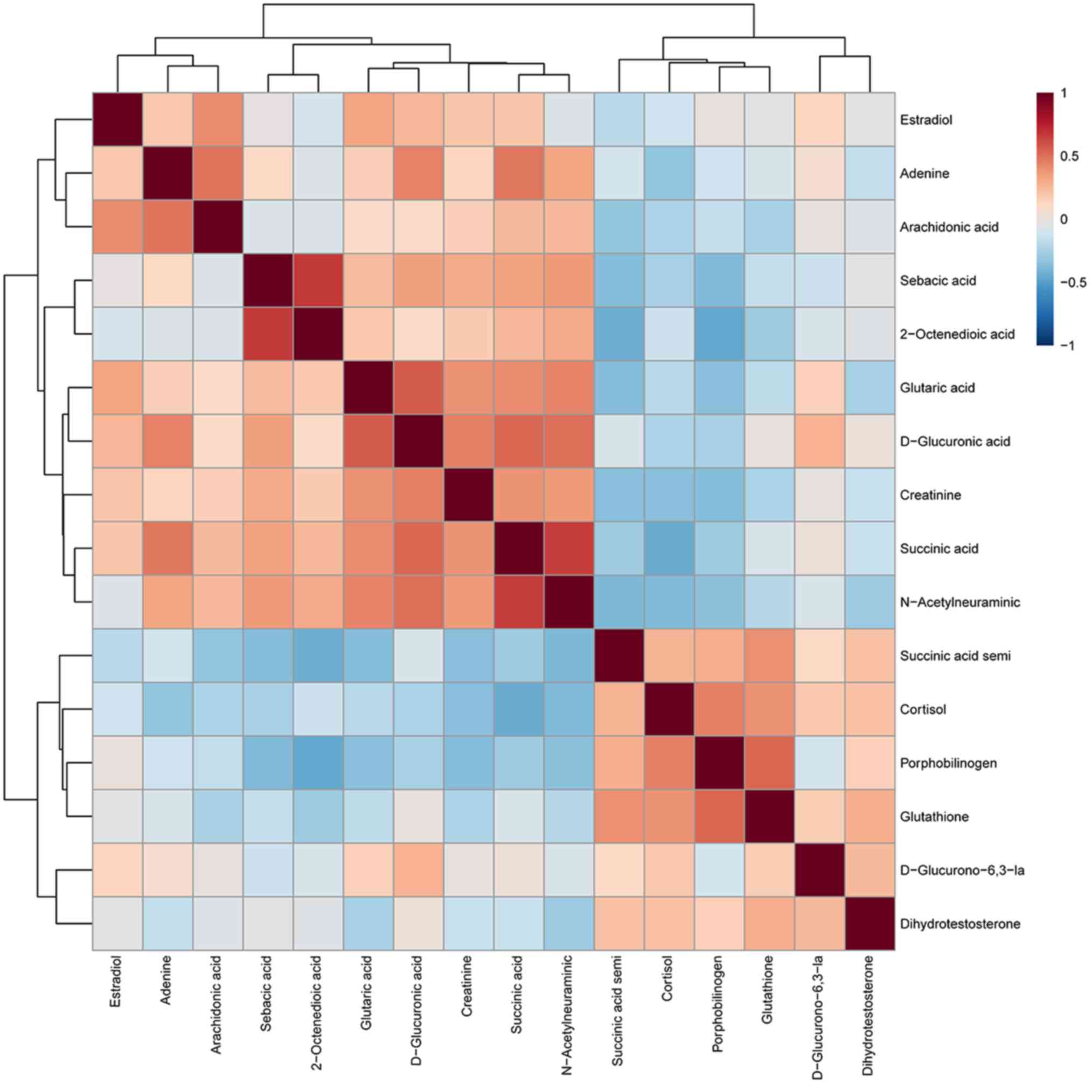
Correlation analysis of potential
biomarkers
The correlation between the 16 UA biomarkers was
subsequently determined (Fig. 5).
The results revealed that D-glucuronic acid was strongly correlated
with creatinine, succinic acid and N-acetylneuraminic acid,
(Pearson's r, 0.449, 0.527 and 0.502, respectively; P<0.001),
suggesting that UA may be caused by a metabolic disorder. The
correlation coefficient between adenine and arachidonic acid was
0.488 (P<0.001), indicating a potential role for purine and
fatty acid metabolism in UA. In addition, the correlation
coefficient between 2-octenedioic acid and sebacic acid was 0.669
(P<0.001), suggesting a potential association between fatty acid
metabolism and UA. Other biomarkers were also closely correlated,
such as porphobilinogen, cortisol and glutathione, which may
explain the pathogenesis of UA.
Discussion
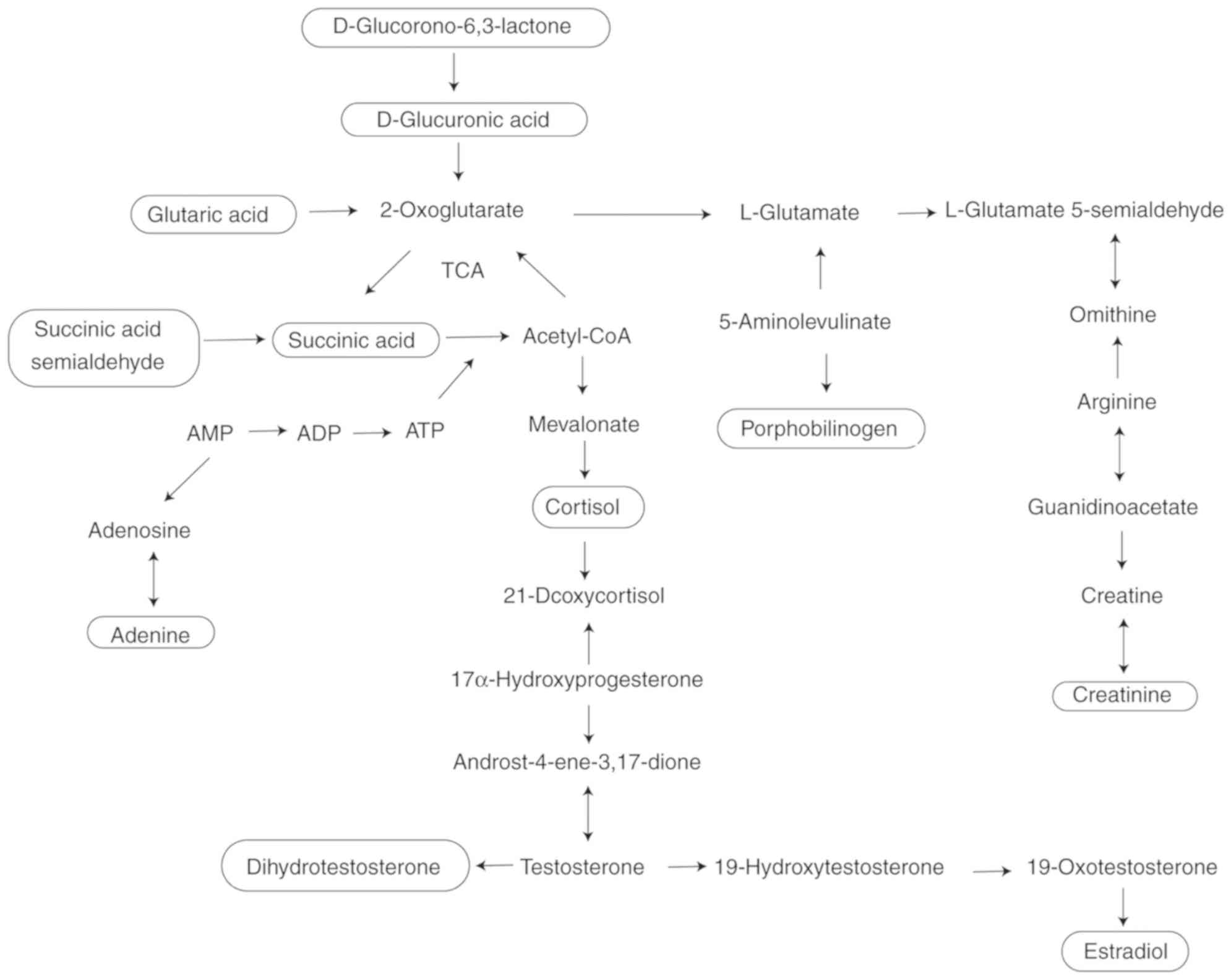
The current study identified 16 UA biomarkers via
urinary metabolomics analysis. The associations among these
biomarkers identified metabolic pathways that may be involved in
UA. Amino acid and energy metabolism, fatty acid metabolism, purine
metabolism and steroid hormone biosynthesis metabolic pathways may
be the main underlying cause of UA. The biomarkers and
associated-metabolic pathways are presented in Fig. 6.
Amino acid metabolism, as a precursor of energy
metabolism, serves a crucial role in increasing the formation of
ATP (25). Glucuronolactone is a
stable form of glucuronic acid; since glucuronic acid is usually
absent in free fatty acids, an increase of glucuronic acid and a
decrease of glucuronolactone indicates that amino acid metabolism
may be disturbed in patients with UA (26). Creatinine arises from creatine and
phosphocreatine, which is a type of nitrogenous organic acid
naturally produced in the human body (27–29).
In normal biological systems, the majority of creatine is converted
to phosphocreatine by reversible enzymes, increasing creatine
phosphate levels and maintaining and improving ATP levels during
tissue oxygen depletion (30,31).
In the absence of enzymes, creatine is converted to creatinine
(32,33). A previous study analyzed the plasma
creatinine level of 10,489 individuals and found that with the
increase of age and creatinine level, the cumulative incidence of
myocardial infarction and ischemic heart disease increased and the
survival rate decreased (logarithmic rank trend <0.001),
suggesting that creatinine is related to heart disease (34).
Glutathione is an endogenous antioxidant that
protects cells from reactive oxygen species, which makes it
essential for maintaining an appropriate immune response (35). A previous study has demonstrated
that glutathione peroxidase deficiency overstimulates the
proinflammatory pathway, leading to endothelial dysfunction and the
development of cardiovascular disease (36). Additionally, the low glutathione
peroxidase is associated with UA severity (37,38).
Succinic acid hemialdehyde can be oxidized by succinic acid
dehydrogenase to succinic acid, which is an intermediate in the TCA
cycle (39). A previous study has
demonstrated that the accumulation of succinic acid in tissue is a
universal metabolic feature of ischemia (39). The secretion of succinic acid
induces inflammation and neovascularization, which may contribute
to the pathogenesis of cardiovascular disease (40). N-acetylneuraminic acid is an
N-acetylated derivative of neuraminic acid, which is regulated by
neuraminidase-1 (41). Zhang et
al (41) used functional
metabolomics to explore its role as a signaling molecule in
triggering myocardial damage, and identified a key role for
N-acetylneuraminic acid in acute myocardial infarction, and
targeting neuraminidase-1 may represent an unrecognized therapeutic
intervention for CAD coronary artery disease.
Fatty acids are the main source of energy for the
heart, accounting for 40–80% of the heart's energy source (21). A previous study has demonstrated
that elevated free fatty acids levels are simply a biomarker of
poor overall health, associated with an increased risk of
cardiovascular (42). Glutaric,
octenedioic and sebacic acids are dicarboxylic acids with 5, 8 and
10 carbon atoms, respectively; they are converted into acetyl-CoA
through oxidation, which is subsequently further oxidized into the
TCA cycle, providing necessary energy for the body (43,44).
Compared with the healthy controls, these biomarkers are
significantly increased in the urine of patients with UA,
indicating that peroxisome dysfunction and β-oxidation processes
are reduced, causing excessive accumulation of dicarboxylic acid,
it causes swelling of mitochondria and inhibits respiration of
mitochondria (45,46). It has also been reported that high
levels of glutaric acid indicates that the body may be more
susceptible to hematological toxicity (47). Arachidonic acid is a
polyunsaturated ω-6 fatty acid that occurs in the phospholipids of
cell membranes; it is a precursor molecule during the synthesis of
thromboxane A2 (48). Arachidonic
acid generates thromboxane A2 through the thromboxane pathway to
cause platelet aggregation, which triggers various cardiovascular
diseases (49). For instance,
previous study has shown that platelet aggregation induced by
thromboxane A2 produced by arachidonic acid metabolism and
thrombosis mediated by vasoconstriction sometimes cause myocardial
infarction (50). Other studies
have also shown that thromboxane A2, isoprostaglandin and
prostacyclin can promote the formation and development of
atherosclerosis by controlling platelet activation and
leukocyte-endothelial cell interaction (51,52).
Platelet activation serves a role in blood clotting and thrombosis,
and high levels of arachidonic acid are therefore associated with
cardiovascular disease (53).
Platelet activation serves an important role in the
pathology of UA (54). Under
normal circumstances, uric acid is in a state of metabolic
equilibrium. Elevated levels of adenine can cause an imbalance of
uric acid metabolism, further promoting platelet aggregation
(55). Highuric acid levels are
associated with the mortality of patients with cardiovascular
disease (56,57). The antioxidant function of uric
acid was previously considered to result in resistance to aging and
oxidative stress; however, a recent clinical study has demonstrated
that hyperuricemia may be one of the risk factors for
cardiovascular disease (58).
Endogenous androgens represent a cardiovascular risk
factor in humans (59). Previous
studies have demonstrated that low testosterone levels can lead to
an increased risk of cardiovascular disease (60–62).
Dihydrotestosterone is an important hemostatic steroid with
inhibitory effects on platelets (63). Dihydrotestosterone cannot be
converted to estrogen by aromatase, but a previous study has
indicated that testosterone can be converted to estradiol and act
through estrogen receptors (64).
In the current study, a significant decrease in dihydrotestosterone
and estradiol levels were observed in patients with UA, indicating
that the body had entered extreme androgen deficiency, which may
lead to cardiovascular disease. In addition, high levels of
cortisol may lead to increased cardiovascular disease risk through
its associated effects on glucose, blood pressure and lipid
metabolic functions (65).
Porphobilinogen can generate heme via porphobilinogen deaminase; a
previous study has demonstrated that angioedema may be accompanied
with the temporary destruction of the blood-brain barrier, which
may be induced by intermediates in the heme biosynthetic pathway
(66).
Metabolomics systematically reveals the molecular
mechanism of metabolic pathways, determining the pathophysiological
processes of disease development and searching for novel disease
biomarkers. Based on the established non-targeted metabolomics
analysis platform, the current study assessed 16 biomarkers that
were associated with UA by analyzing the levels of metabolites in
patient urine. The results revealed strong correlations between
2-octenedioic acid and sebacic acid, amino acid metabolism, fatty
acid metabolism, purine metabolism and biosynthesis and steroid
hormone metabolism, indicating that these metabolic pathways were
affected in patients with UA pectoris. These pathways may also be
associated with UA pathogenesis. The results of the present study
also demonstrated the potential of biomarkers to assist in the
early diagnosis of UA, thus providing patients with personalized
clinical solutions that enable effective treatment of UA within a
feasible time frame, preventing progression to acute myocardial
ischemia and myocardial infarction. However, the biological
mechanisms of abnormal metabolic biomarkers in metabolic pathways
requires further study to provide more information for the clinical
diagnosis and treatment of UA.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Key
Basic Research and Development Program (973 Program; grant no.
2014CB542902).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YBL and CY researched the literature and conceived
the current study. SG and SF developed the protocol and obtained
the ethics approval. XC, YJL and ZL recruited the participants and
performed the statistical analysis. HZ and TZ analyzed the data.
YCL and YL performed the experiments and wrote the first draft of
the manuscript. All authors reviewed and edited the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The trial was registered with the Chinese Clinical
Trial Registration Center (registration no. ChiCTR-ROC-17013957) at
the Tianjin University of Traditional Chinese Medicine. The current
study conforms to the Declaration of Helsinki and was approved by
the Ethics Committee of Tianjin University of Traditional Chinese
Medicine (approval no. TJUTCM-EC20180003).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Benjamin EJ, Virani SS, Callaway CW,
Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling
FN, Deo R, et al: Heart disease and stroke statistics-2018 update:
A report from the American heart association. Circulation.
137:e67–e492. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
D'Souza M, Sarkisian L, Saaby L, Poulsen
TS, Gerke O, Larsen TB, Diederichsen AC, Jangaard N, Diederichsen
SZ, Hosbond S, et al: Diagnosis of unstable angina pectoris has
declined markedly with the advent of more sensitive troponin
assays. Am J Med. 128:852–860. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Möckel M, Searle J, Hamm C, Slagman A,
Blankenberg S, Huber K, Katus H, Liebetrau C, Müller C, Muller R,
et al: Early discharge using single cardiac troponin and copeptin
testing in patients with suspected acute coronary syndrome (ACS): A
randomized, controlled clinical process study. Eur Heart J.
36:369–376. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Amandeep G and Roman Z: Unstable Angina.
Treasure Island (FL): StatPearls Publishing; 2020, PubMed/NCBI
|
|
5
|
Yeghiazarians Y, Braunstein JB, Askari A
and Stone PH: Unstable angina pectoris. New Engl J Med.
342:101–114. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sun M, Gao X, Zhang D, Ke C, Hou Y, Fan L,
Zhang R, Liu H, Li K and Yu B: Identification of biomarkers for
unstable angina by plasma metabolomic profiling. Mol Biosyst.
9:3059–3067. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Anderson JL, Horne BD, Stevens SM, Grove
AS, Barton S, Nicholas ZP, Kahn SF, May HT, Samuelson KM,
Muhlestein JB, et al: Randomized trial of genotype-guided versus
standard warfarin dosing in patients initiating oral
anticoagulation. Circulation. 116:2563–2570. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
David SW: Metabolomics for investigating
physiological and pathophysiological processes. Physiol Rev.
99:1819–1875. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cheung PK, Ma MH, Tse HF, Yeung KF, Tsang
HF, Chu MKM, Kan CM, Cho WCS, Ng LBW, Chan LWC and Wong SCC: The
applications of metabolomics in the molecular diagnostics of
cancer. Expert Rev Mol Diagn. 19:785–793. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wheelock CE, Wheelock AM, Kawashima S,
Diez D, Kanehisa M, van Erk M, Kleemann R, Haeggström JZ and Goto
S: Systems biology approaches and pathway tools for investigating
cardiovascular disease. Mol Biosyst. 5:588–602. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Patti GJ, Yanes O and Siuzdak G:
Innovation: Metabolomics: The apogee of the omics trilogy. Nat Rev
Mol Cell Biol. 13:263–269. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Long NP, Nghi TD, Kang YP, Anh NH, Kim HM,
Park SK and Kwon SW: Toward a standardized srategy of clinical
metabolomics for the advancement of precision medicine.
Metabolites. 10:512020. View Article : Google Scholar
|
|
13
|
Nicholson JK and Lindon JC: Systems
biology: Metabonomics. Nature. 455:1054–1056. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Strimbu K and Tavel JA: What are
biomarkers? Curr Opin HIV AIDS. 5:463–466. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bujak R, Struck-Lewicka W, Markuszewski MJ
and Kaliszan R: Metabolomics for laboratory diagnostics. J Pharm
Biomed Anal. 113:108–120. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
González-Domínguez R, García-Barrera T and
Gómez-Ariza JL: Combination of metabolomic and
phospholipid-profiling approaches for the study of Alzheimer's
disease. J Proteomics. 104:37–47. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Psychogios N, Hau DD, Peng J, Guo AC,
Mandal R, Bouatra S, Sinelnikov I, Krishnamurthy R, Eisner R,
Gautam B, et al: The human serum metabolome. PLoS One.
6:e169572011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bouatra S, Aziat F, Mandal R, Guo AC,
Wilson MR, Knox C, Bjorndahl TC, Krishnamurthy R, Saleem F, Liu P,
et al: The human urine metabolome. PLoS One. 8:e730762013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang M, Fu G and Lei T: Two Urinary
Peptides Associated Closely With Type 2 Diabetes Mellitus. PLoS
One. 10:e01229502015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li XS, Li S and Kellermann G:
Pre-analytical and analytical validations and clinical applications
of a miniaturized, simple and cost-effective solid phase extraction
combined with LC-MS/MS for the simultaneous determination of
catecholamines and metanephrines in spot urine samples. Talanta.
159:238–247. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang Y, Sun W, Zheng J, Xu C, Wang X, Li
T, Tang Y and Li Z: Urinary metabonomic study of patients with
acute coronary syndrome using UPLC-QTOF/MS. J Chromatogr B Analyt
Technol Biomed Life Sci. 1100:122–130. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li J, Guo J, Shang E, Zhu Z, Zhu KY, Li S,
Zhao B, Jia L, Zhao J, et al: A metabolomics strategy to explore
urinary biomarkers and metabolic pathways for assessment of
interaction between Danhong injection and low-dose aspirin during
their synergistic treatment. J Chromatogr B Analyt Technol Biomed
Life Sci. 1026:168–175. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kang SM, Park JC, Shin MJ, Lee H, Oh J,
Ryu DH, Hwang GS and Chung JH: ¹H nuclear magnetic resonance based
metabolic urinary profiling of patients with ischemic heart
failure. Clin Biochem. 44:293–299. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chinese Medical Association Cardiovascular
Branch, Editorial Board of the Chinese Journal of Cardiovascular
Diseases, . Guidelines for the diagnosis and treatment of unstable
angina and non-ST-segment elevation myocardial infarction. Chin J
Cardiol. 35:25–304. 2007.
|
|
25
|
Guo N, Yang D, Wang X, Dai J, Wang M and
Lei Y: Metabonomic study of chronic heart failure and effects of
Chinese herbal decoction in rats. J Chromatogr A. 1362:89–101.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bai J, Wang MX, Chowbay B, Ching CB and
Chen WN: Metabolic profiling of HepG2 cells incubated with S(−) and
R(+) enantiomers of anti-coagulating drug warfarin. Metabolomics.
7:353–362. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Feng Q, Liu Z, Zhong S, Li R, Xia H, Jie
Z, Wen B, Chen X, Yan W, Fan Y, et al: Integrated metabolomics and
metagenomics analysis of plasma and urine identified microbial
metabolites associated with coronary heart disease. Sci Rep.
6:225252016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wyss M and Kaddurah-Daouk R: Creatine and
creatinine metabolism. Physiol Rev. 80:1107–1213. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zheng P, Gao HC, Li Q, Shao WH, Zhang ML,
Cheng K, Yang DY, Fan SH, Chen L and Xie P: Plasma metabonomics as
a novel diagnostic approach for major depressive disorder. J
Proteome Res. 11:1741–1748. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen F, Zhu K, Chen L, Ouyang L, Chen C,
Gu L, Jiang Y, Wang Z, Lin Z, Zhang Q, et al: Protein target
identification of ginsenosides in skeletal muscle tissues:
Discovery of natural small-molecule activators of muscle-type
creatine kinase. J Ginseng Res. 44:461–474. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kallioniemi E, Kärkkäinen O, Määttä S,
Könönen M, Kivimäki P, Kaarre O, Velagapudi V, Kekkonen V, Lehto
SM, Laukkanen E and Tolmunen T: Repeated transcranial magnetic
stimulation-induced motor evoked potentials correlate with the
subject-specific serum metabolic profile of creatine. J Clin
Neurophysiol. 36:229–235. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dickinson H, Ellery S, Ireland Z, LaRosa
D, Snow R and Walker DW: Creatine supplementation during pregnancy:
Summary of experimental studies suggesting a treatment to improve
fetal and neonatal morbidity and reduce mortality in high-risk
human pregnancy. BMC Pregnancy Childbirth. 14:1502014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li Z, Liu X, Wang J, Gao J, Guo S, Gao K,
Man H, Wang Y, Chen J and Wang W: Analysis of urinary metabolomic
profiling for unstable angina pectoris disease based on nuclear
magnetic resonance spectroscopy. Mol Biosyst. 11:3387–3396. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sibilitz KL, Benn M and Nordestgaard BG:
Creatinine, eGFR and association with myocardial infarction,
ischemic heart disease and early death in the general population.
Atherosclerosis. 237:67–75. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mourino-Alvarez L, Baldan-Martin M,
Gonzalez-Calero L, Martinez-Laborde C, Sastre-Oliva T, Moreno-Luna
R, Lopez-Almodovar LF, Sanchez PL, Fernandez-Aviles F, Vivanco F,
et al: Patients with calcific aortic stenosis exhibit systemic
molecular evidence of ischemia, enhanced coagulation, oxidative
stress and impaired cholesterol transport. Int J Cardiol.
225:99–106. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sharma A, Yuen D, Huet O, Pickering R,
Stefanovic N, Bernatchez P and de Haan JB: Lack of glutathione
peroxidase-1 facilitates a pro-inflammatory and activated vascular
endothelium. Vascul Pharmacol. 79:32–42. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Rosenblat M, Volkova N, Coleman R and
Aviram M: Anti-oxidant and anti-atherogenic properties of liposomal
glutathione: Studies in vitro, and in the atherosclerotic
apolipoprotein E-deficient mice. Atherosclerosis. 195:e61–e68.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Qiao M, Kisgati M, Cholewa JM, Zhu W,
Smart EJ, Sulistio MS and Asmis R: Increased expression of
glutathione reductase in macrophages decreases atherosclerotic
lesion formation in low-density lipoprotein receptor-deficient
mice. Arterioscler Thromb Vasc Biol. 27:1375–1382. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chouchani ET, Pell VR, Gaude E,
Aksentijević D, Sundier SY, Robb EL, Logan A, Nadtochiy SM, Ord
ENJ, Smith AC, et al: Ischaemic accumulation of succinate controls
reperfusion injury through mitochondrial ROS. Nature. 515:431–435.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hamel D, Sanchez M, Duhamel F, Roy O,
Honoré JC, Noueihed B, Zhou T, Nadeau-Vallée M, Hou X, Lavoie JC,
et al: G-protein-coupled receptor 91 and succinate are key
contributors in neonatal postcerebral hypoxia-ischemia recovery.
Arterioscler Thromb Vasc Biol. 34:285–293. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang L, Wei TT, Li Y, Li J, Fan Y, Huang
FQ, Cai YY, Ma GX, Liu JF, et al: Functional metabolomics
characterizes a key role for n-acetylneuraminic acid in coronary
artery diseases. Circulation. 137:1374–1390. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Miedema MD, Maziarz M, Biggs ML, Zieman
SJ, Kizer JR, Ix JH, Mozaffarian D, Tracy RP, Psaty BM, Siscovick
DS, et al: Plasma-free fatty acids, fatty acid-binding protein 4,
and mortality in older adults (from the Cardiovascular Health
Study). Am J Cardiol. 114:843–848. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Mingrone G, Castagneto-Gissey L and Macé
K: Use of dicarboxylic acids in type 2 diabetes. Br J Clin
Pharmacol. 75:671–676. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Jia P, Wang S, Xiao C, Yang L, Chen Y,
Jiang W and Zheng X, Zhao G, Zang W and Zheng X: The
anti-atherosclerotic effect of tanshinol borneol ester using fecal
metabolomics based on liquid chromatography-mass spectrometry.
Analyst. 141:1112–1120. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Nazzaro-Porro M: Azelaic acid. J Am Acad
Dermatol. 17:1033–1041. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wang SY, Wang Y, Jin XW, Zhang Y, Chen JS,
Ma WW, Wu YH and Wang DC: A urinary metabolomics study of rats
after the exposure to acrylamide by ultra performance liquid
chromatography coupled with quadrupole time-of-flight tandem mass
spectrometry. Mol Biosyst. 11:1146–1155. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Nishiumi S, Fujigaki S, Kobayashi T,
Kojima T, Ito Y, Daiko H, Kato K, Shoji H, Kodama Y, Honda K and
Yoshida M: Metabolomics-based discovery of serum biomarkers to
predict the side-effects of neoadjuvant chemoradiotherapy for
esophageal squamous cell carcinoma. Anticancer Res. 39:519–526.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Patrono C and Rocca B: Measurement of
thromboxane biosynthesis in health and disease. Front Pharmacol.
10:12442019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Hirz T, Khalaf A, El-Hachem N, Mrad MF,
Abdallah H, Créminon C, Grée R, Merhi RA, Habib A, Hachem A and
Hamade E: New analogues of 13-hydroxyocatdecadienoic acid and
12-hydroxyeicosatetraenoic acid block human blood platelet
aggregation and cyclooxygenase-1 activity. Chem Cent J. 6:1522012.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Nielsen MS, Schmidt EB, Stegger J,
Gorst-Rasmussen A, Tjonneland A and Overvad K: Adipose tissue
arachidonic acid content is associated with the risk of myocardial
infarction: A Danish case-cohort study. Atherosclerosis.
227:386–390. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Smyth EM: Thromboxane and the thromboxane
receptor in cardiovascular disease. Clin Lipidol. 5:209–219. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Martin W: The combined role of atheroma,
cholesterol, platelets, the endothelium and fibrin in heart attacks
and strokes. Med Hypotheses. 15:305–322. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Jarrar YB, Cho SA, Oh KS, Kim DH, Shin JG
and Lee SJ: Identification of cytochrome P450s involved in the
metabolism of arachidonic acid in human platelets. Prostaglandins
Leukot Essent Fatty Acids. 89:227–234. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Ameta K, Gupta A, Ameta D, Sethi R, Kumar
D, Ahmad I and Mahdi AA: 1H NMR-derived metabolomics of filtered
serum of myocardial ischemia in unstable angina patients. Clin Chim
Acta. 456:56–62. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Boyle SH, Matson WR, Velazquez EJ, Samad
Z, Williams RB Jr, Sharma S, Thomas B, Wilson JL, O'Connor C and
Jiang W: Metabolomics analysis reveals insights into biochemical
mechanisms of mental stress-induced left ventricular dysfunction.
Metabolomics. 11:571–582. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Krishnan E, Kwoh CK, Schumacher HR and
Kuller L: Hyperuricemia and incidence of hypertension among men
without metabolic syndrome. Hypertension. 49:298–303. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Baker JF, Krishnan E, Chen L and
Schumacher HR: Serum uric acid and cardiovascular disease: Recent
developments, and where do they leave us? Am J Med. 118:816–826.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Lippi G, Montagnana M, Franchini M,
Favaloro EJ and Targher G: The paradoxical relationship between
serum uric acid and cardiovascular disease. Clin Chim Acta.
392:1–7. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Ajayi AA, Mathur R and Halushka PV:
Testosterone increases human platelet thromboxane A2 receptor
density and aggregation responses. Circulation. 91:2742–2747. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Karolczak K, Konieczna L, Kostka T, Witas
PJ, Soltysik B, Baczek T and Watala C: Testosterone and
dihydrotestosterone reduce platelet activation and reactivity in
older men and women. Aging (Albany NY). 10:902–929. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Shiraki N, Nakashima A, Doi S, Carrero JJ,
Sugiya N, Ueno T, Stenvinkel P, Kohno N and Masaki T: Low serum
testosterone is associated with atherosclerosis in postmenopausal
women undergoing hemodialysis. Clin Exp Nephrol. 18:499–506. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Shores MM and Matsumoto AM: Testosterone,
aging and survival: Biomarker or deficiency. Curr Opin Endocrinol
Diabetes Obes. 21:209–216. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Chasland LC, Knuiman MW, Divitini ML,
Murray K, Handelsman DJ, Flicker L, Hankey GJ, Almeida OP, Golledge
J, Ridgers ND, et al: Higher circulating androgens and higher
physical activity levels are associated with less central adiposity
and lower risk of cardiovascular death in older men. Clin
Endocrinol (Oxf). 90:375–383. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Rosenberg MA, Shores MM, Matsumoto AM,
Bůžková P, Lange LA, Kronmal RA, Heckbert SR and Mukamal KJ: Serum
androgens and risk of atrial fibrillation in older men: The
cardiovascular health study. Clin Cardiol. 41:830–836. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Lattanzi S and Silvestrini M: Letter re:
Long-term cortisol measures predict Alzheimer disease risk.
Neurology. 89:1062017. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Maramattom BV, Zaldivar RA, Glynn SM,
Eggers SD and Wijdicks EF: Acute intermittent porphyria presenting
as a diffuse encephalopathy. Ann Neurol. 57:581–584. 2005.
View Article : Google Scholar : PubMed/NCBI
|