Introduction
Chondrosarcoma is the second most common primary
cartilage malignancy, accounting for 3.5–9% of primary bone tumors
and almost 30% of primary bone malignancies (1). These tumors affect one in every
million individuals, and typically present in the 4 and 7th decades
of life (2). Chondrosarcoma
commonly presents as gradually progressive pain specific to the
associated anatomical location (3). Axial tumors, the most common, are
accompanied by a poorer prognosis and an increased rate of
recurrence compared with acral tumors, and depending on the grade,
are associated with a 10-year survival rate of 30–80% (4,5).
Similarly, the presence of metastasis worsens prognosis, and
metastases frequently arise in the lungs as a result of
hematogenous dissemination (6,7).
Local tumor recurrence is common, and adjuvant radio- and
chemotherapy are largely ineffective; thus despite significant
morbidity, local excision has become an increasingly popular
treatment method, even for low grade lesions (4,8,9). A
notable limitation to existing management strategies is that
chondrosarcomas are impervious to chemotherapy and radiation, a
characteristic largely attributed to the presence of drug-resistant
cancer stem cells (CSCs) in the neoplastic ecosystem (2,10,11).
It has recently been established that transformed
mesenchymal stem cells, or their immediate precursors, are the most
likely cellular origin of chondrosarcoma (12). The CSC theory is based on
experimental evidence that undifferentiated CSCs exist and function
to maintain the bulk population of cells within a tumor, sustaining
the cancer and allowing for recurrence and metastasis (11). They share several key
characteristics with non-neoplastic stem cells, including
self-renewal and asymmetric division, the hallmark properties of
cancer cells (13,14).
Identifying and isolating CSCs in vitro has
been historically challenging, but was largely overcome by the use
of tumor-derived spheroids (15,16).
Spheroids act as surrogate systems to evaluate and manipulate the
CSC-associated properties of solid tumors, including tumor
resistance to chemotherapy and radiation, sustaining the cancer,
recurrence, and metastasis (17).
Experimentally, spheroids are particularly important in sarcoma
research, as their growth rates, cellular morphology, cell-cell
junctional behavior and kinase activation properties (to name a
few) closely mimic those of primary tumors (10). Additionally, spheroids are a useful
model of micrometastatic disease. Tumor-derived spheroids are
generally comprised of three structural layers: i) A central core
of hypoxic, starved necrotic cells; ii) an inner layer of
nonproliferating quiescent CSCs; and iii) an outer nutrient-rich
layer of proliferating tumor stromal cells, which interact with the
surrounding extracellular matrix (10,18).
Given that the chemoresistance and tumorigenicity of
chondrosarcomas are attributed to the CSC population, targeting
CSCs is of great significance in the realm of biologic or
chemotherapeutic management (19).
The aforementioned resistance to electromagnetic and chemical
insult is partly conferred by the infrequent replication of CSCs
and their heightened activation of DNA repair mechanisms (and
therefore, a lower apoptotic rate), an active drug efflux system,
and increased defenses against reactive oxygen species (13,14,20).
As such, CSCs are considered to be responsible for recurrence
following radiation and resection therapy, and are chiefly
accountable for tumor metastasis (21). CSCs create a resilient and
self-propagating tumor microenvironment, which in combination with
a matrix, functions to impair drug diffusion, further contributing
to chemoresistance (10,20). Though treatments that inhibit CSC
proliferation have been described in other types of sarcoma, there
are currently no methods for impeding CSCs in chondrosarcoma
(22). Furthermore, the behavior
of spheroids in chondrosarcoma has not been fully elucidated.
Therefore, the identification of novel agents which successfully
target CSCs is essential for improving the clinical management and
prognosis of chondrosarcoma.
Proline rich polypeptide-1 (PRP-1), an
antitumorigenic cytokine, is a fragment of the
neurophysin-vasopressin- associated glycoprotein that is produced
by hypothalamic neurosecretory cells. The primary structure of
PRP-1 (isolated from neurosecretory granules of bovine
neurohypophysis) comprises 15 amino acids
(Ala-Gly-Ala-Pro-Glu-Pro-Ala-Glu-Pro-Ala-Gln-Pro-Gly-Val-Tyr) with
an apparent molecular mass of 1.475 Daltons. PRP-1 has also been
described as a potent immunomodulator which inhibits mTOR and cMyc,
and suppresses cell cycle progression in high grade chondrosarcoma
(23–27). A unique feature of this potential
biologic agent is its physiological presence in the human body
(28). PRP-1 is released into
systemic circulation and has multiple organotrophic functions,
including mediating the hypothalamus-neurohypophysis-bone marrow
axis, as well as other immunomodulatory functions (25,29).
Given the lack of a dependable treatment and an
overwhelmingly poor prognosis, identification of novel therapies
for chondrosarcoma is a critical clinical priority. The efficiency
by which PRP-1 eliminates CSCs has been previously described in a
monolayer, and was flow cytometrically determined to result from
the aldehyde dehydrogenase (ALDH)high population of the
chondrosarcoma monolayer, in association with the antineoplastic
regulation of aberrant Wnt/β-catenin signaling (30). Likewise, PRP-1 was also found to
exert cytostatic properties in a chondrosarcoma monolayer by
promoting cell cycle arrest in the S phase (26). This prompted further investigation
into the effects of PRP-1; specifically, the ability to eliminate
anchorage independent colony formation (a symbol of malignancy) and
3D chondrosarcoma stem cell spheroid formation (a hallmark of the
metastatic abilities of CSC metastasis, recurrence, cancer
sustainability, and chemo- and radio-resistance). The present study
utilizes a novel in vitro 3D tumor model, (chondrosarcoma
spheroids) to determine the effects of PRP-1 on the human
chondrosarcoma CSC population.
Materials and methods
Establishing 2D and 3D cultures of
chondrosarcoma JJ012 cells
2D cell cultures were established in T-175 flasks
(353112, Falcon) and grown in 37°C, 5% CO2 incubator for
two days to achieve 80% confluency. Mycoplasma testing was
completed for the cell lines used in all experiments, the cell
lines used were authenticated, and cell culture lines were
maintained according to international guidelines on good cell
culture practice. Upon trypsinization, centrifuged cells were
pushed through 40 µm cell strainer (431750, Corning) to ensure a
healthy single cell suspension. 3D cultures were established by
seeding cells at an optimized density of 500,000 cells/well in
6-well low attachment plates (3471, Corning) in Advanced DMEM/F-12
media with reduced Fetal Bovine Serum (12634028, Thermo Fisher
Scientific, Inc.), supplemented with 10 ng/ml basic fibroblast
growth factor (AF-100-18C, Peprotech), 10 ng/ml epidermal growth
factor (AF-100-15, Peprotech), and 10 ul/ml N2 supplement
(17502048, Thermo Fisher Scientific, Inc.). Additional human
epidermal growth factor (10 ng/ml) and human basic fibroblast
growth factor (10 ng/ml) were added to each well every other day.
Sarcosphere growth was observed every day for 5–6 days and colonies
were imaged on day 6 on Leica DMI3000 B Inverted Microscope.
Aldefluor® assay and
fluorescence-activated cell sorting (FACS)
For the following experiment, we followed the
methods of our previous study, Hoyt et al (30). To measure cells with ALDH activity,
the Aldefluor® assay was carried out as described
according to manufacturer's protocol (Aldefluor kit, cat. no.
01700; StemCell Technologies). Briefly, cells were harvested and
resuspended in Aldefluor assay buffer at a concentration of
1×106/ml. To activate the Aldefluor reagent, first 25 µl
of DMSO was added and incubated for 15 min with 25 µl of 2N HCl,
then 360 µl of assay buffer was added to the vial. The cells were
then incubated with the activated Aldefluor reagent for 45 min at
37°C. Diethylaminobenzaldehyde (DEAB), a specific ALDH inhibitor,
was added as a negative control. Following incubation, all tubes
were centrifuged for 5 min at 250 × g, and the supernatant was
removed, and resuspended in Aldefluor assay buffer. The cells were
then transferred and strained onto Falcon 5 ml polystyrene round
bottom tube with a cell strainer cap (cat. no. 352235). After
labeling, the samples were sorted on a BD Biosciences Special Order
Research Product (SORP) FACSAria II, using BD FACSDiva software
(version 6.1.3) into ALDHlow and ALDHhigh
cells with and without PRP-1 treatment. Data analysis was performed
using FlowJo software (FlowJo LLC) (version 10).
Anchorage-independent cell colony growth
assay (31)
Production of 3% agarose solution
Under the hood and in a sterile 50 ml falcon tube,
0.9 g agar powder was dissolved in 30 ml distilled water in
duplicate to reach a total volume of 60 ml 3% agar. Sterile 3% agar
solution was placed in a 45°C water bath to keep solution in liquid
phase.
Production of the bottom layer (0.6%
Agarose Gel)
Pipette tips (10 ml) were pre-warmed in incubator to
prevent agarose from solidifying with handling and 9 ml of 3%
agarose solution was transferred into a sterile 50 ml conical tube,
then 36 ml warm JJ012 media was added with the tube mixed by
inversion. 2 ml of this mixture was gently added into 14 wells of
three 6-well culture plates three times for a total of 42 wells and
nine plates while avoiding bubble formation. Plates were incubated
horizontally on a flat surface at 4°C for 30 1 h to allow the
mixture to solidify, then plates were placed in a 37°C incubator
for 30 min.
Preparation of cell suspension
layer
JJ012 cells and ALDH-tagged fractions were harvested
and diluted in media to a cell concentration of 5 × 105
cells/ml to be seeded in each well of three 6 well plates per cell
group. 0.6% Agarose was prepared by mixing 6 ml of 3% agarose with
24 ml JJ012 media by inversion while avoiding bubble formation.
Each well was treated according to its assignment in a dose
response manner. In a 1:1 dilution, 500 µl cell suspension
containing 5×105 cells/ml were mixed with 0.6% agarose
solution. 1 ml of the cell-agarose solution was subsequently added
to the bottom layer of the 6-well culture plates to achieve a
seeding concentration of 2.5×105 cells/ml. Plates were
incubated at 4°C for 15 min to allow the top cell layer to solidify
and then moved to a 37°C incubator for 1 week.
Production of the feeder layer: 0.3%
agarose gel
Pre-made 3% agarose solution was heated and mixed
with warm JJ012 media by inversion to create one mixture for
control cells and one for each PRP-1 treatment group. The treatment
mixture was treated with the respective concentration of PRP-1 and
1 ml of the mixture was added to the designated wells of each
6-well culture plate containing the bottom and cell layers. Plates
were placed at 4°C for 15 min to allow the mixture to solidify and
then moved to a 37°C incubator. The feeding procedure was repeated
weekly for 3 weeks. Cell colonies were counted using a light
microscope and imaged with an inverted microscope at 400 and 100
µm.
Dose response sarcosphere formation
assay (32,33)
Human chondrosarcoma JJ012 cells were cultured in a
monolayer, then harvested with trypsin and neutralized with
serum-containing media. Cells were subsequently centrifuged and
resuspended with Thermo-Fischer Advanced (Serum-free) DMEM/F12
(Cat#12634028). Cells were centrifuged again and resuspended in 5
ml round bottom polystyrene test tubes through a strainer cap to
ensure a healthy single cell suspension.
Cells were then counted and plated in Corning Costar
Ultra-Low Attachment 6-Well Plates (Sigma-Aldrich; Merck KGaA cat#
CLS3471) at a density of 500,000 cells/well with serum-free
DMEM/F12 medium with 10 ng/ml basic fibroblast growth factor, 10
ng/ml epidermal growth factor, and 10 µl/ml N2 supplement. Cells
were treated with PRP-1 daily, in duplicate, and in a
dose-dependent manner of control, 0.05 µg/ml PRP-1, 0.5 µg/ml
PRP-1, 1 µg/ml PRP-1, 5 µg/ml PRP-1, 10 µg/ml PRP-1, 20 µg/ml
PRP-1. Additional human epidermal growth factor (10 ng/ml) and
human basic fibroblast growth factor (10 ng/ml) were added to the
media in each well every other day. Colonies were imaged after 7
days using inverted phase contrast microscopy at ×40 magnification.
Images were captured in 5 fields of view for each well. Spheroids
were quantified by two separate investigators (CG, AM) at two
separate occurrences counting spheroid number over 5 randomly
selected high-powered fields and calculating a mean value.
Discrepancies regarding the mean were re-counted by both
investigators until equal values were calculated by both
investigators to ensure reporting of true values only.
Gel electrophoresis and western
blot
JJ012 chondrosarcoma cells were cultured and
incubated to confluency. Cells were collected using trypsin and
then seeded into Petri dishes at a concentration of
1×106 cells/ml. The cells were incubated for 24 h at
37°C in a 5% CO2 incubator. The next day, an ice-cold
phosphate-buffered saline wash was performed, and protease
inhibitor was added to the cell lysis buffer (C2978; Sigma-Aldrich;
Merck KGaA) in a 1:100 ratio. After harvesting cells with a rubber
scraper and lysis of cell membranes with an 18-gauge needle, the
cells were centrifuged at 15,000 × g at 4°C. The supernatant was
then collected, and protein content was measured using
NanoDrop® spectrophotometer (Thermo Fisher Scientific,
Inc.). The supernatant was frozen at −80°C until loading onto the
gels (20 µg/lane). Polyacrylamide gel electrophoresis and western
blotting reagents were supplied by Lonza, Inc. and related
procedures were followed in accordance with the company's protocol.
The catalog numbers for the reagents and suppliers are listed
below. Pager Gold Precast Gels (59502; 10% Tris-glycine; Lonza,
Inc.); ECL reagent (RPN2109; GE Healthcare); Western Blocker
solution (W0138; Sigma-Aldrich; Merck KGaA); ProSieve Quad Color
Protein marker (4.6–300 kDa, 00193837; Lonza, Inc.); 20X reducing
agent for ProSieve ProTrack Dual Color Loading buffer (00193861;
Lonza, Inc.); ProTrack loading buffer (00193861; Lonza, Inc.);
ProSieve ProTrack Dual Color Loading buffer EX running buffer
(00200307; Lonza, Inc.); ProSieve EX Western Blot Transfer buffer
(00200309; Lonza, Inc.); Immobilon®-P polyvinylidene
difluoride membranes (P4188; Sigma-Aldrich; Merck KGaA).
Antibodies for western blotting
Mouse monoclonal antibody to CD44 was applied as a
primary antibody (Sigma-Aldrich; Merck KGaA, SAB1402714) at a
dilution of 1:1,000 and goat anti-mouse IgG peroxidase conjugate as
a secondary antibody (A4416; Sigma-Aldrich; Merck KGaA) at a
dilution of 1:5,000. Mouse monoclonal antibody to STRO-1 was
applied as a primary antibody (Life Technologies, 398401) at a
dilution of 1:250 and secondary antibody (A4416; Sigma-Aldrich;
Merck KGaA) at a dilution of 1:5,000. Mouse monoclonal antibody to
STAT3 was added as a primary antibody (Santa Cruz Biotechnology,
sc-293151) at a dilution of 1:1,000 and secondary antibody (A4416;
Sigma-Aldrich; Merck KGaA) at a dilution of 1:5,000. As
housekeeping proteins, mouse anti-tubulin antibody was used (T5168;
Sigma-Aldrich; Merck KGaA) at a dilution of 1:2,000 and anti-mouse
IgG (A4416; Sigma-Aldrich; Merck KGaA) at a dilution of 1:5,000 was
applied as a secondary antibody. Incubations for all primary
antibodies were carried out in a cold room while rocking for a
period of 24 h, while secondary antibodies were incubated for 2 h
under the same conditions.
Self-renewal assay (21)
Cultured 3D sarcospheres were dissociated into
single-cell suspensions and inoculated into medium without serum in
T-175 flasks (353112, Falcon) to ensure only cells from spheroids
would propagate and allowed to grow in 2D monolayers for
approximately 2 days. At near 80% confluency, cells were harvested
and reseeded as single cells and plated at a density of 500,000
cells/well in 6-well ultra-low attachment plates (3471, Corning)
and in 150×15 mm Petri Dishes (351058, Falcon) in Advanced
DMEM/F-12 media with reduced Fetal Bovine Serum (12634028, Thermo
Fisher Scientific, Inc.), supplemented with 10 ng/ml basic
fibroblast growth factor, 10 ng/ml epidermal growth factor, and 10
µl/ml N2 supplement. Additional human epidermal growth factor (10
ng/ml) and human basic fibroblast growth factor (10 ng/ml) were
added to each well of the 6-well plates every other day, but not
added to petri dishes. Sarcosphere growth was observed every day
for 5–6 days and colonies were imaged on day 6 on Confocal Leica
TCS SP5 microscope using ×10 and ×20 objective lenses.
Modified Annexin V/PI apoptosis assay
(invitrogen eBioscience Annexin V apoptosis detection Kit APC; Cat
#88-8007-72) (34)
Cell preparation
Human chondrosarcoma JJ012 cells were grown in a
monolayer, harvested, and centrifuged at 335 × g for 10 min. Cells
were resuspended in 2 ml 1× phosphate buffered saline (PBS) and
samples were centrifuged at 335 × g for 10 min. Cells were then
resuspended in 1 ml 1X Annexin V binding buffer. The resuspended
pellets were centrifuged at 335 × g for 10 min. Finally, pellets
were resuspended in 100 µl 1X Annexin V binding buffer.
Application of Annexin V/Pi stain
Annexin V was added according to the manufacturer's
recommendations. Tubes were incubated in the dark for 15 min at
room temperature. 1X Annexin V binding buffer (100 µl) was added to
each reaction tube to allow for a volume of 200 µl in each tube. PI
(4 µl) (Sigma-Aldrich; Merck KGaA, cat # P-4864-10ML), diluted 1:10
in 1X Annexin V binding buffer, was then added to yield a final PI
concentration of 2 µg/ml in each sample. Tubes were incubated in
the dark for 15 min at room temperature. 1X Annexin V binding
buffer (500 µl) was added to wash the cells. Samples were
centrifuged at 335 × g for 10 min. Pellets were resuspended in 500
µl 1X Annexin V binding buffer and 500 µl 2% formaldehyde to create
a 1% formaldehyde (fixative) solution. Tubes were mixed by gentle
flicking and samples then fixed on ice for 10 min. 1X PBS (1 ml)
was added to each sample and mixed gently by flicking. The
suspension was then centrifuged at 425 × g for 8 min.
Centrifugation and resuspension steps were repeated twice, and
pellet was finally resuspended by flicking the tube. Following
resuspension, 16 µl of 1:100 diluted RNase A (Sigma-Aldrich; Merck
KGaA, R4642) was added to provide a final concentration of 50
µg/ml. Samples were incubated for 15 min at 37°C. 1X PBS (1 ml) and
mixed gently by flicking. Tubes were centrifuged at 425 × g for
eight minutes. Samples were then taken for flow cytometric
analysis. Data analysis was performed using FlowJo software (FlowJo
LLC) (version 10).
Statistics
Statistical analyses were performed using two-way
analysis of variance (ANOVA) with a post hoc Tukey's multiple
comparisons test to determine cell colony growth differences at
each PRP-1 dose between cell groups and within each cell group
between PRP-1 doses. One-way analysis of variance (ANOVA) with a
post hoc Tukey's multiple comparisons test was used to determine
differences in spheroid growth at each PRP-1 dose. Post hoc
analysis expressed as a 95% confidence interval of the mean
difference. Spheroid growth experiments were repeated 2 times.
Densitometric analysis was obtained using density analysis on
ImageJ (NIH) to calculate relative optical density (OD) of
antibodies in comparison to the housekeeping protein, α-tubulin,
and bulk untreated-JJ012 was used as control. Statistical analyses
of relative optical densities were performed using individual
unpaired t-tests of all samples expressed with a 95% confidence
interval of the mean difference of relative OD. All statistical
analyses were completed using GraphPad Prism 8.3.0 (GraphPad
Software, Inc.). A P-value <0.05 was considered significant.
Error bars represent standard error of the mean (SEM) in all graphs
with *P<0.05, **P<0.01, ***P<0.001 (as indicated in the
figures and figure legends).
Results
PRP-1 significantly decreases colony
formation and anchorage independent growth in human
chondrosarcoma
The antitumorigenic effects of PRP-1 on bulk, a term
which designates tumor stromal chondrosarcoma cell monolayers, has
previously been established, including the antineoplastic
regulation of the Wnt/β-catenin pathway (30); this prompted further investigation
into the role of PRP-1 on anchorage independent growth. To ensure
the specific investigation of anchorage independent growth, cell
colonies were cultivated using an agarose colony formation assay
known to involve anchorage independence and necessitate CSC
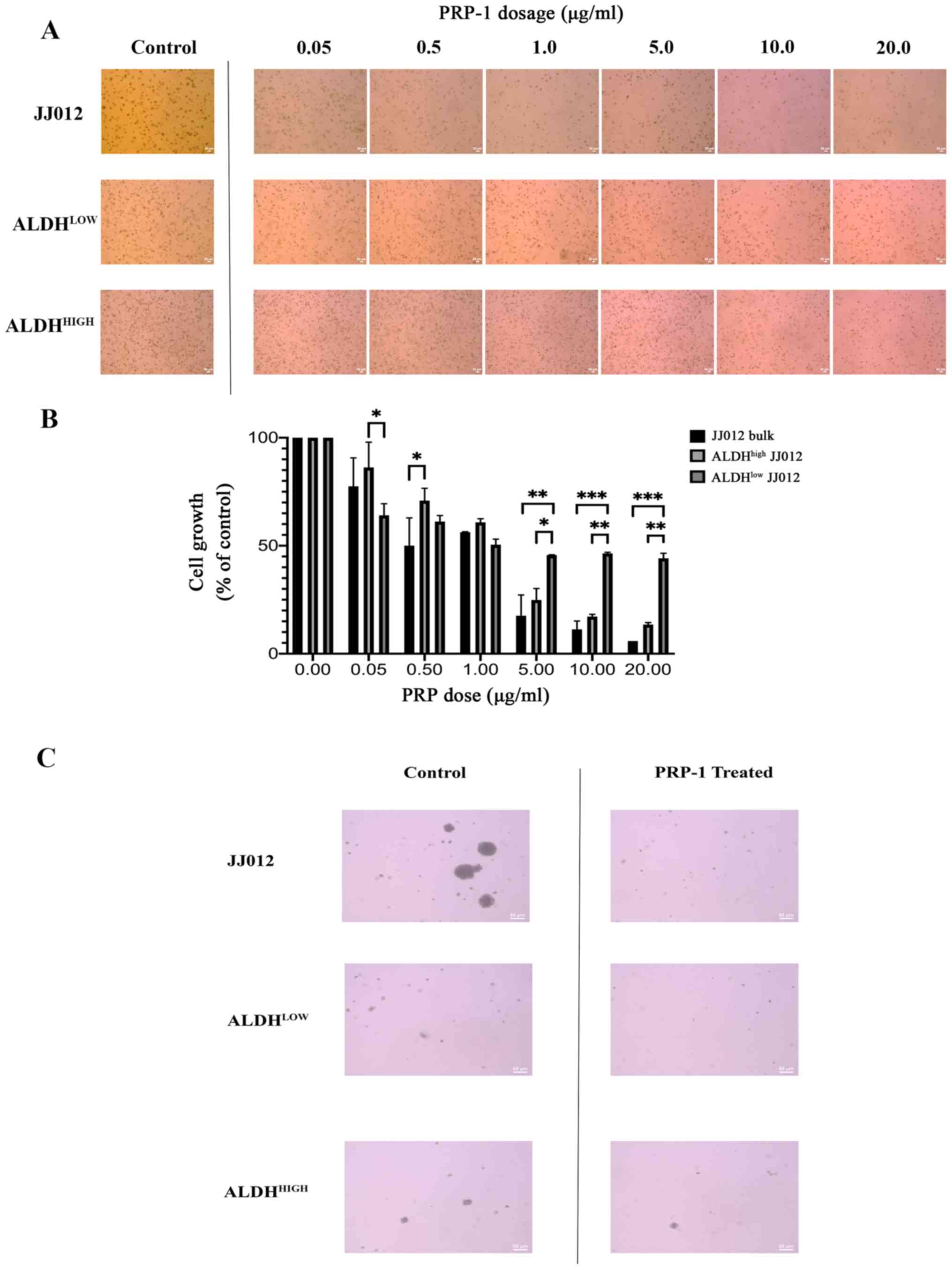
involvement as a prerequisite (31). Qualitative analysis revealed a
decrease in colony viability following treatment with PRP-1
(Fig. 1A and C). Two-way ANOVA was
conducted to determine the effects of cellular population and PRP-1
dose on spheroid growth (Fig. 1B).
Post hoc analysis of the dose response within each cell fraction
group is summarized in Table I,
which is visually reflected in Fig.
1. Of note, the number of cells in the control groups were
seeded equally, however because the exhibited images display cell
colonies which were allowed to grow for three weeks, the observed
phenomenon of the ALDHhigh group (the CSC group)
appearing to have more cells is explained by the fact that these
cells divide faster than tumor stromal/bulk cells and thus this
group grew more over the three week period than the other two
groups. There was a statistically significant interaction between
the effects of PRP-1 dose and cell population on anchorage
independent colony growth (F(12,21)=4.608; P=0.0011). Simple main
effects analysis was carried out, and significant differences in
colony growth were observed between each colony cell type at a
specified PRP-1 dose. At 0.05 µg/ml PRP-1, the mean growth of
ALDHlow JJ012 cells was significantly lower than that of
ALDHhigh cells (22.16±20.59; P=0.0334). At a dose of
0.50 µg/ml PRP-1, the mean growth of JJ012 bulk cells was
significantly lower compared with that of ALDHhigh cells
(20.82±20.32; P=0.0471). At a PRP-1 dose of 1 µg/ml, no significant
differences in mean growth were observed between any of the cell
colony groups. At 5 µg/ml PRP-1, the mean growth of JJ012 bulk
cells (28.03±20.59; P=0.0068) and ALDHhigh cells
(20.77±20.58; P=0.0477) was significantly lower than that of
ALDHlow cells. At a dose of 10 µg/ml, the mean growth of
JJ012 bulk cells (35.21±20.59; P=0.0009) and ALDHhigh
cells (29.28±20.59; P=0.0048) was significantly lower than that of
ALDHlow cells. Finally, at 20 µg/ml PRP-1, the mean
growth of JJ012 bulk cells (38.27±20.59; P=0.0004) and
ALDHhigh cells (30.66±20.59; P=0.0032) was significantly
lower than that of ALDHlow cells.
 | Table I.Post Hoc Tukey's analysis of dose
response cell colony growth. |
Table I.
Post Hoc Tukey's analysis of dose
response cell colony growth.
| Comparison | Mean
difference | 95% confidence
interval of difference | P-value |
|---|
| JJ012 Bulk |
| 0.0 vs.
0.05 | 22.50 | −4.057 to
49.05 | 0.1331 |
| 0.0 vs.
0.5 | 49.97 | 23.41 to 76.52 | <0.0001 |
| 0.0 vs.
1.0 | 43.75 | 17.19 to 70.30 | 0.0004 |
| 0.0 vs.
5.0 | 82.40 | 55.85 to 109.0 | <0.0001 |
| 0.0 vs.
10 | 88.73 | 62.18 to 115.3 | <0.0001 |
| 0.0 vs.
20 | 94.11 | 67.55 to 120.7 | <0.0001 |
| 0.05
vs. 0.5 | 27.47 | 0.9183 to
54.02 | 0.0395 |
| 0.05
vs. 1.0 | 21.25 | −5.302 to
47.80 | 0.1754 |
| 0.05
vs. 5.0 | 59.91 | 33.35 to 86.46 | <0.0001 |
| 0.05
vs. 10 | 66.24 | 39.68 to 92.79 | <0.0001 |
| 0.05
vs. 20 | 71.61 | 45.06 to 98.16 | <0.0001 |
| 0.5 vs.
1.0 | −6.220 | −32.77 to
20.33 | 0.9863 |
| 0.5 vs.
5.0 | 32.44 | 5.883 to 58.99 | 0.0105 |
| 0.5 vs.
10 | 38.77 | 12.21 to 65.32 | 0.0018 |
| 0.5 vs.
20 | 44.14 | 17.59 to 70.69 | 0.0004 |
| 1.0 vs.
5.0 | 38.66 | 12.10 to 65.21 | 0.0019 |
| 1.0 vs.
10 | 44.99 | 18.43 to 71.54 | 0.0003 |
| 1.0 vs.
20 | 50.36 | 23.81 to 76.91 | <0.0001 |
| 5.0 vs.
10 | 6.330 | −20.22 to
32.88 | 0.9850 |
| 5.0 vs.
20 | 11.71 | −14.85 to
38.26 | 0.7785 |
| 10 vs.
20 | 5.375 | −21.18 to
31.93 | 0.9936 |
| aldhhigh
jj012 |
| 0.0 vs.
0.05 | 13.75 | −12.80 to
40.30 | 0.6334 |
| 0.0 vs.
0.5 | 29.14 | 2.591 to 55.69 | 0.0255 |
| 0.0 vs.
1.0 | 39.16 | 12.60 to 65.71 | 0.0016 |
| 0.0 vs.
5.0 | 75.15 | 48.60 to 101.7 | <0.0001 |
| 0.0 vs.
10 | 82.81 | 56.25 to 109.4 | <0.0001 |
| 0.0 vs.
20 | 86.49 | 59.94 to 113.0 | <0.0001 |
| 0.05
vs. 0.5 | 15.39 | −11.16 to
41.94 | 0.5109 |
| 0.05
vs. 1.0 | 25.40 | −1.147 to
51.96 | 0.0667 |
| 0.05
vs. 5.0 | 61.40 | 34.85 to 87.95 | <0.0001 |
| 0.05
vs. 10 | 69.06 | 42.50 to 95.61 | <0.0001 |
| 0.05
vs. 20 | 72.74 | 46.19 to 99.29 | <0.0001 |
| 0.5 vs.
1.0 | 10.01 | −16.54 to
36.56 | 0.8763 |
| 0.5 vs.
5.0 | 46.01 | 19.45 to 72.56 | 0.0002 |
| 0.5 vs.
10 | 53.66 | 27.11 to 80.21 | <0.0001 |
| 0.5 vs.
20 | 57.35 | 30.80 to 83.90 | <0.0001 |
| 1.0 vs.
5.0 | 35.99 | 9.443 to 62.55 | 0.0039 |
| 1.0 vs.
10 | 43.65 | 17.10 to 70.20 | 0.0005 |
| 1.0 vs.
20 | 47.34 | 20.79 to 73.89 | 0.0002 |
| 5.0 vs.
10 | 7.656 | −18.90 to
34.21 | 0.9618 |
| 5.0 vs.
20 | 11.34 | −15.21 to
37.90 | 0.8015 |
| 10 vs.
20 | 3.688 | −22.86 to
30.24 | 0.9992 |
| aldhlow
jj012 |
| 0.0 vs.
0.05 | 35.91 | 9.358 to 62.46 | 0.0040 |
| 0.0 vs.
0.5 | 38.79 | 12.24 to 65.34 | 0.0018 |
| 0.0 vs.
1.0 | 49.53 | 22.98 to 76.08 | <0.0001 |
| 0.0 vs.
5.0 | 54.38 | 27.82 to 80.93 | <0.0001 |
| 0.0 vs.
10 | 53.53 | 26.97 to 80.08 | <0.0001 |
| 0.0 vs.
20 | 55.84 | 29.28 to 82.39 | <0.0001 |
| 0.05
vs. 0.5 | 2.880 | −23.67 to
29.43 | 0.9998 |
| 0.05
vs. 1.0 | 13.62 | −12.93 to
40.17 | 0.6431 |
| 0.05
vs. 5.0 | 18.47 | −8.087 to
45.02 | 0.3073 |
| 0.05
vs. 10 | 17.62 | −8.937 to
44.17 | 0.3583 |
| 0.05
vs. 20 | 19.93 | −6.627 to
46.48 | 0.2314 |
| 0.5 vs.
1.0 | 10.74 | −15.81 to
37.29 | 0.8376 |
| 0.5 vs.
5.0 | 15.59 | −10.97 to
42.14 | 0.4969 |
| 0.5 vs.
10 | 14.74 | −11.82 to
41.29 | 0.5597 |
| 0.5 vs.
20 | 17.05 | −9.507 to
43.60 | 0.3950 |
| 1.0 vs.
5.0 | 4.845 | −21.71 to
31.40 | 0.9963 |
| 1.0 vs.
10 | 3.995 | −22.56 to
30.55 | 0.9987 |
| 1.0 vs.
20 | 6.305 | −20.25 to
32.86 | 0.9853 |
| 5.0 vs.
10 | −0.8500 | −27.40 to
25.70 | >0.9999 |
| 5.0 vs.
20 | 1.460 | −25.09 to
28.01 | >0.9999 |
| 10 vs.
20 | 2.310 | −24.24 to
28.86 | >0.9999 |
Considering the aforementioned effect of PRP-1 in
inhibiting anchorage independent growth in chondrosarcoma cell
colonies, and its previously demonstrated cytostatic effects on
chondrosarcoma S phase arrest, the effects of PRP-1 on spheroid
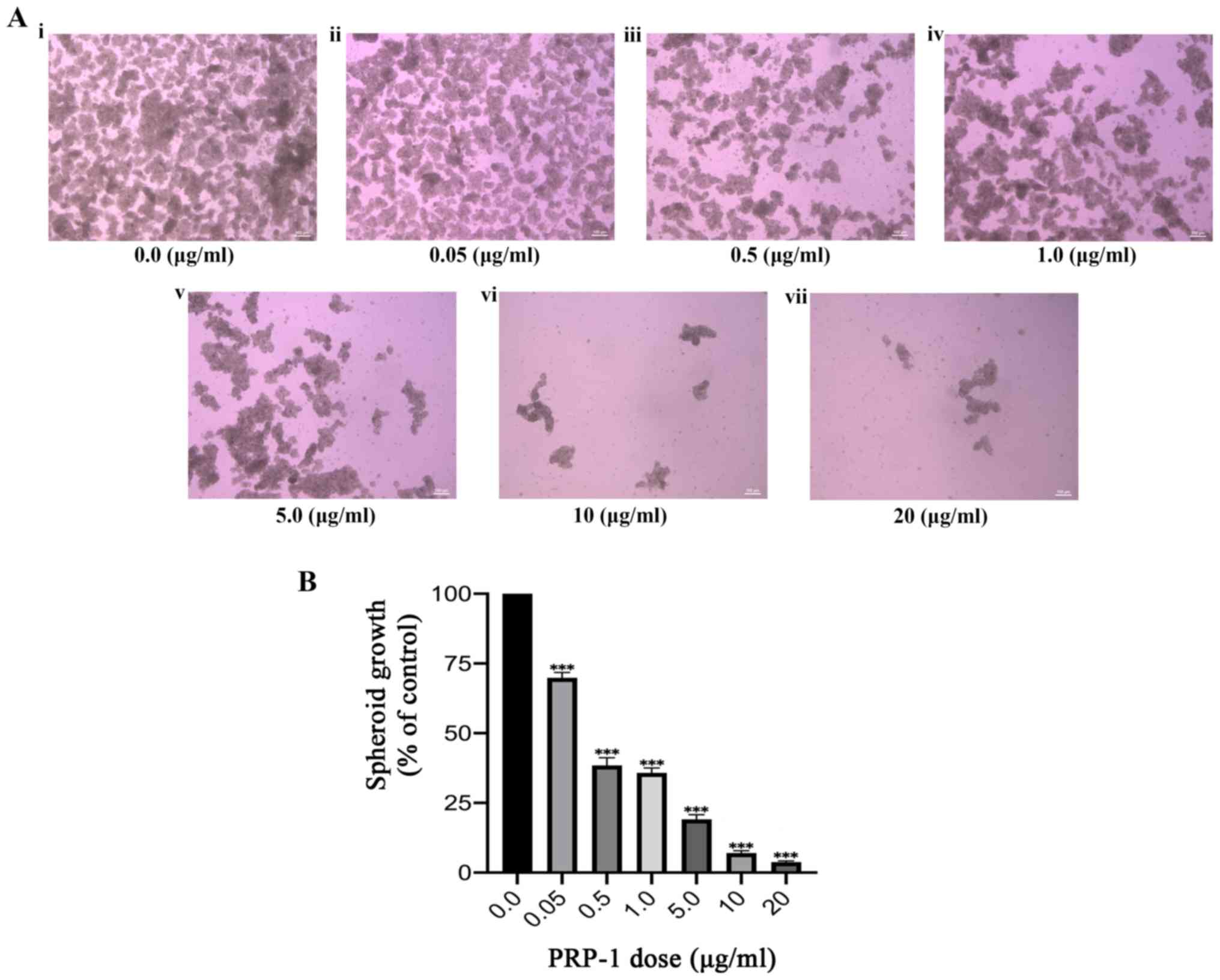
growth were subsequently investigated. Spheroids were successfully
cultured following a well-established spheroid formation assay
(described in Materials and methods), indicating the presence and
proliferation of CSCs in a hostile anchorage independent,
serum-free environment. Spheroids were formed in four separate
experiments; the last experiment utilized a dose-response
technique, which included identical doses of PRP-1 to those used in
the aforementioned colony formation experiment. Spheroid growth was
qualitatively and quantitatively evaluated, and a significant
decrease in growth was observed with increasing doses of PRP-1
(Fig. 2A). One-way ANOVA was
conducted to determine the effect of PRP-1 dosage on spheroid
growth in the bulk JJ012 cell population (Fig. 2B; Table II). A statistically significant
decrease in spheroid growth was observed with increasing doses of
PRP-1 (F(6,28)=476.7; P<0.0001). Furthermore, Tukey's post hoc
multiple comparisons test highlighted significant differences
between all doses (Table II,
visually reflected in Fig. 2),
except between 0.5 and 1 mg/ml (2.598±7.184; P=0.9075), and between
10 and 20 mg/ml PRP-1 (3.252±7.188; P=0.7781).
 | Table II.Post-Hoc Tukey's analysis of spheroid
growth. |
Table II.
Post-Hoc Tukey's analysis of spheroid
growth.
| Comparison | Mean
difference | 95% confidence
interval of difference | P-value |
|---|
| 0.0 vs. 0.05 | 30.15 | 22.97 to 37.34 | <0.0001 |
| 0.0 vs. 0.5 | 61.57 | 54.39 to 68.76 | <0.0001 |
| 0.0 vs. 1.0 | 64.17 | 56.99 to 71.35 | <0.0001 |
| 0.0 vs. 5.0 | 80.85 | 73.67 to 88.03 | <0.0001 |
| 0.0 vs. 10 | 92.93 | 85.74 to 100.1 | <0.0001 |
| 0.0 vs. 20 | 96.18 | 88.99 to 103.4 | <0.0001 |
| 0.05 vs. 0.5 | 31.42 | 24.24 to 38.60 | <0.0001 |
| 0.05 vs. 1.0 | 34.02 | 26.83 to 41.20 | <0.0001 |
| 0.05 vs. 5.0 | 50.70 | 43.51 to 57.88 | <0.0001 |
| 0.05 vs. 10 | 62.77 | 55.59 to 69.96 | <0.0001 |
| 0.05 vs. 20 | 66.03 | 58.84 to 73.21 | <0.0001 |
| 0.5 vs. 1.0 | 2.598 | −4.586 to
9.782 | 0.9075 |
| 0.5 vs. 5.0 | 19.28 | 12.09 to 26.46 | <0.0001 |
| 0.5 vs. 10 | 31.35 | 24.17 to 38.54 | <0.0001 |
| 0.5 vs. 20 | 34.61 | 27.42 to 41.79 | <0.0001 |
| 1.0 vs. 5.0 | 16.68 | 9.496 to 23.86 | <0.0001 |
| 1.0 vs. 10 | 28.76 | 21.57 to 35.94 | <0.0001 |
| 1.0 vs. 20 | 32.01 | 24.82 to 39.19 | <0.0001 |
| 5.0 vs. 10 | 12.08 | 4.892 to 19.26 | 0.0002 |
| 5.0 vs. 20 | 15.33 | 8.144 to 22.51 | <0.0001 |
| 10 vs. 20 | 3.252 | −3.932 to
10.44 | 0.7781 |
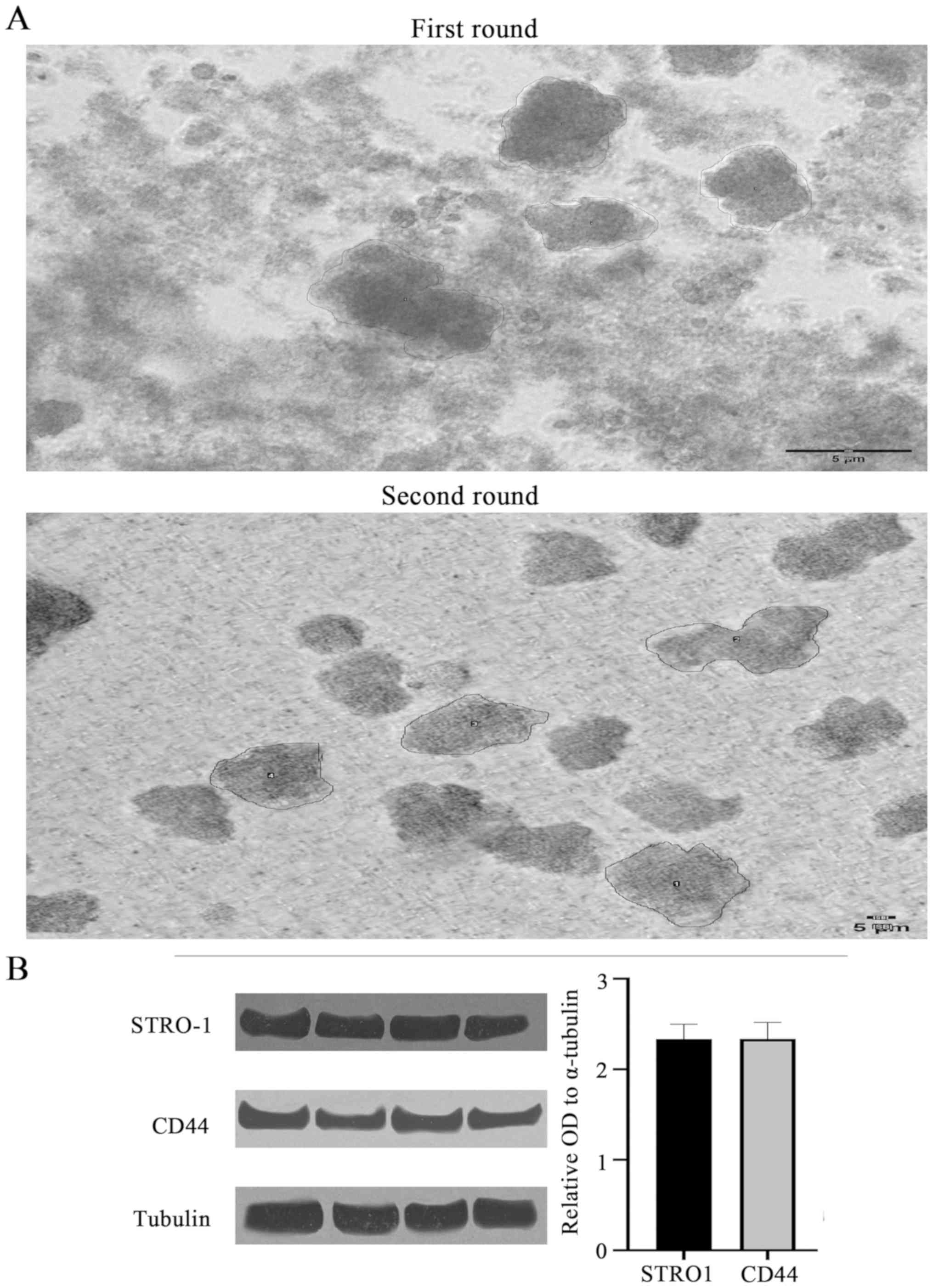
Sarcoma spheroids were grown, filtered into single
cell suspensions, transferred to a 2D flask for monolayer culture,
and their self-renewal properties were established through the
successful re-growth of spheroids from cells of the monolayer
(Fig. 3A). By breaking the first
round spheroid into single cell suspensions and then forming the
second round spheroids from these single cells, this methodology
ensures that the second round spheroids are forming from the
original first round spheroids and allows us to isolate the
property of self-renewal. Utilization of an established spheroid
self-renewal assay confirmed the presence and self-renewal capacity
of CSCs. Size measurements of n=4 spheroids revealed that
self-renewed spheroids were consistently larger than those from the
original culture (Table III;
visually represented in Fig. 3).
In terms of area measurement, the self-renewed spheroids were
consistently more than double the mean size of the original
spheroids.
 | Table III.Sarcosphere self-renewal assay. |
Table III.
Sarcosphere self-renewal assay.
| Round | Area
(mm3) | Mean diameter
(mm) | Min diameter
(mm) | Max diameter
(mm) |
|---|
| 1 | 32.64 | 81.98 | 30 | 212 |
| 1 | 22.26 | 89.02 | 35 | 244 |
| 1 | 16.66 | 116.88 | 46 | 255 |
| 1 | 12.76 | 113.80 | 49 | 255 |
| 2 | 471.36 | 190.76 | 158 | 235 |
| 2 | 469.08 | 192.83 | 158 | 232 |
| 2 | 341.43 | 190.58 | 157 | 235 |
| 2 | 345.64 | 184.40 | 154 | 231 |
Mesenchymal stem cell markers CD44,
STRO-1 and STAT3 in chondrosarcoma spheroids
Western blotting was performed to confirm the
presence of mesenchymal stem cells within the chondrosarcoma cell
and spheroid populations. The mesenchymal and CSC biomarkers CD44
and STRO-1 were identified in chondrosarcoma CSCs (Fig. 3B). The mean relative OD values for
STRO1, CD44 and STAT3 (n=4 each) were 2.33 (P<0.54), 2.34
(P<0.34) and 2.34 (P<0.26), respectively (STAT3 data not
shown). Thus, no significant difference in the expression of CSC
biomarkers was revealed between the PRP-1-treated and untreated
groups.
PRP-1 does not significantly alter the
apoptotic or late apoptotic/necrotic stages of JJ012 bulk
cells
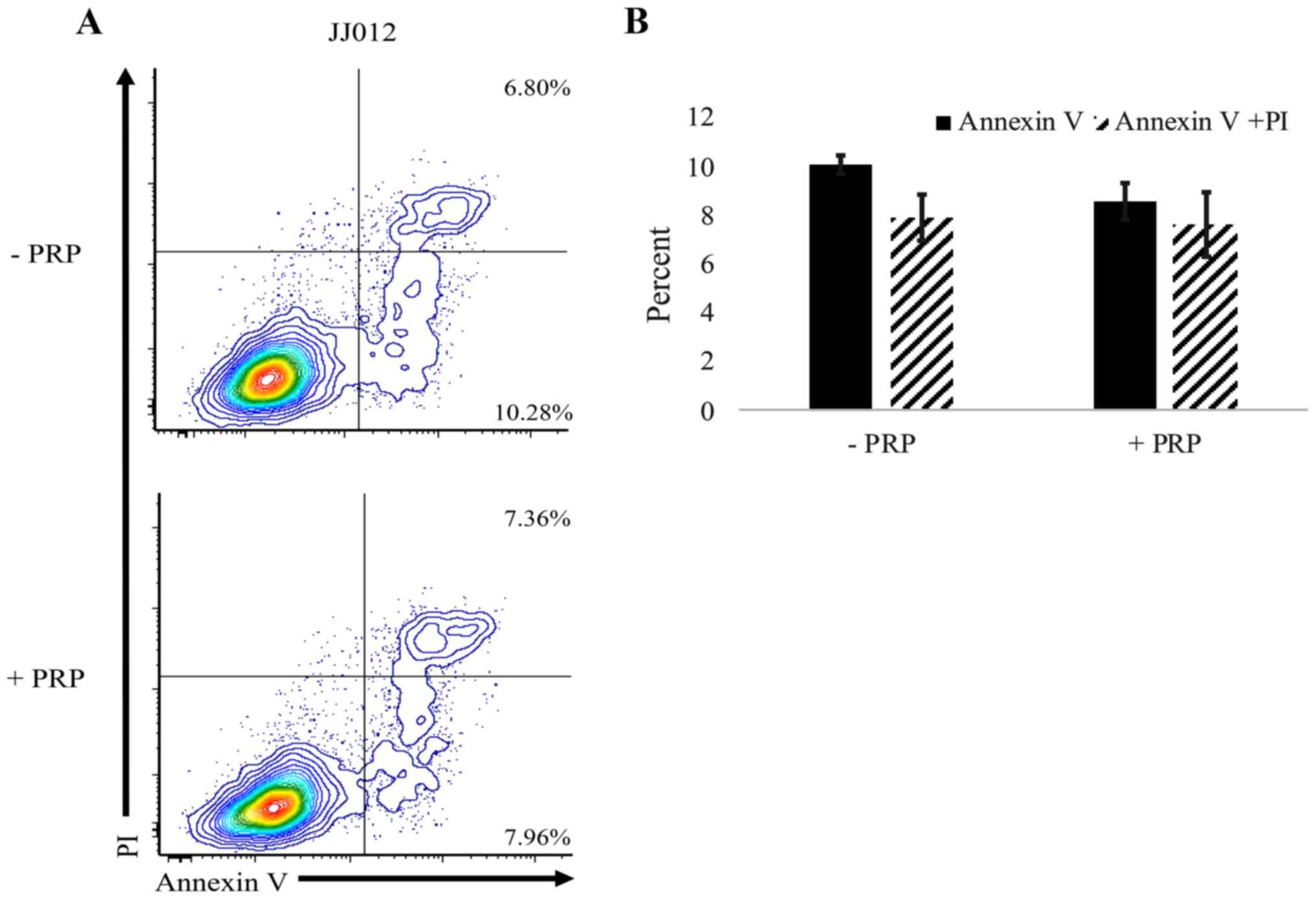
Following treatment with PRP-1, an Annexin V/PI
assay was performed to flow cytometrically investigate the
mechanism by which CSC and stromal cell death occurs in
chondrosarcoma cells. Exposure to PRP-1 did result in cell death
but did not alter the percentage of cells in the apoptotic (Annexin
V+) or late apoptotic/necrotic (Annexin V+
PI+) stages (Fig. 4);
n=3 separate cultures per group.
Discussion
The observed reduction in chondrosarcoma cell colony
formation following PRP-1 administration confirms its ability to
interfere with anchorage independent growth, a component of
metastatic potential that is inherent to the CSC population. In the
present study, the greatest decrease in colony formation was
observed in the bulk JJ012 cell group, followed closely by the
ALDHhigh CSC population. This confirms that PRP-1
selectively targets CSCs, but also effectively inhibits stromal
cell tumor growth (Fig. 1;
Table I). A reduction in tumor
stromal cells is further supported by the decreased expansion of
the ALDHlow non-CSC population, albeit to a lesser
degree than that observed in the bulk and CSC populations. In
summary, the ALDHhigh and JJ012 bulk cell populations
have a higher proliferative capacity than the ALDHlow
population. Low doses of PRP-1 did not significantly suppress
ALDHhigh or JJ012 bulk population growth, therefore
ALDHlow displayed relatively less growth at these doses.
However, greater suppression of ALDHhigh and JJ012 bulk
cells was observed with increasing doses of PRP-1, which indicates
selective inhibition of CSCs. Compared with other chemotherapeutic
approaches, this result is favorable and unique, as PRP-1 not only
decreased the CSC population, but to an extent, also reduced the
proportion of CSCs within the stromal population. In the clinic,
selective targeting of CSCs in chemo- and radio-resistant
chondrosarcomas is a great barrier to effective patient management,
though the findings of the present study indicate that the use of
PRP-1 shows promise to overcome this obstacle. Once CSC death is
induced (and their source of propagation has been diminished),
tumor stromal cells are susceptible to chemo- and/or
radiotherapeutic eradication (35).
Of note, 20 µg/ml was found to be the optimal dose
of PRP-1 for reducing cell colony formation, which is supported by
post hoc analysis (Fig. 1C).
Collectively, the present study reports that low micromolar
concentrations of PRP-1 inhibit the proliferation of chondrosarcoma
CSCs.
To confirm the presence of CSCs, western blot
analysis was used to identify the mesenchymal and CSC markers
STRO-1, CD44 and STAT3 (36,37).
The results indicate the presence of these markers with comparable
band densities and almost identical average ODs between the three
markers of interest (Fig. 4). The
consistent expression of these markers confirms the presence of
CSCs within spheroids, and subsequently, that CSCs are the primary
contributors to spheroid structure and survival (20).
Furthermore, the observed reduction in spheroid
growth after PRP-1 treatment indicates its ability to target CSCs,
and also to penetrate the outer proliferative layer and
microenvironment of the tumor-derived 3D culture in order to reach
the CSC-containing middle layer (38). Spheroids are an accepted in
vitro archetype considered to be analogous to animal models,
and require the active presence of CSCs for formation and growth
(39). Spheroids allow for the
formation of a tumor microenvironment comparable to that of in
vivo tumors. The present study revealed a reduction in spheroid
growth following PRP-1 administration, with a maximum reductive
dose of 20 µg/ml resulting in spheroid growth at only 4.82% of
control growth. Compared with a significant reduction in cell
colony formation, a higher dose of PRP-1 was required for maximum
spheroid growth reduction. This likely reflects the need for PRP-1
infiltration into the 3D spheroid microenvironment, while cell
colonies do not exhibit this same structural resilience. This may
be secondary to the central location of CSCs within the spheroid
(and in the in vivo tumor), whereas colonies and cells
cultured in a 2D monolayer do not exhibit such a spherically dense
and not easily penetrated growth pattern (40).
Notably, spheroid self-renewal occurred under
growth-restrictive conditions, suggesting that spheroids contain a
small subpopulation of self-renewing primitive/progenitor cells.
This self-renewal capacity is archetypally unique to CSCs,
non-neoplastic stem cells, and other cells demonstrating stemness
(41). With respect to average
surface area measurements, self-renewed spheroids were >2 times
the size of naively cultured spheroids. This may be due to the
resilience of CSCs in the self-renewed population, along with
readily available mRNA coding transcription and growth factors,
which are synthesized during culture of the original spheroids. In
this context, future directions may include confirming the presence
of progenitor markers in spheroid lysates derived from self-renewed
3D cultures and determining whether PRP-1 directly inhibits this
property of self-renewal.
Annexin V/PI analysis revealed an overall decrease
in spheroid viability following PRP-1 treatment and highlighted
that cell death was not occurring by apoptosis or late
apoptosis/necrosis. This finding is consistent with a previous
study, where no significant changes in apoptosis was observed in
JJ012 cells treated with PRP-1; however, the cells were found to
accumulate in the S phase of the cell cycle, indicating the
cytostatic effects of PRP-1 (26).
Further investigations may identify the exact mechanism by which
PRP-1 promotes cell death. Spheroids have reportedly been used to
investigate anoikis in anchorage independent tumor growth, as was
also conducted in the present study. Future directions should aim
to identify whether anoikis is partially or fully responsible for
PRP-1-associated cell death in chondrosarcoma (10).
The present study is not without limitation. Flow
cytometric analysis of spheroids is not always feasible, as 3D
cultures are often too large to enter the flow cytometer, limiting
Annexin V/PI analysis to isolated CSCs only. Because spheroid
growth in chondrosarcoma is not fully understood, imaging and
establishment of spheroid characteristics was considered to be of
greater importance.
Impending goals include elucidating the molecular
mechanism by which PRP-1 induces CSC death. Additionally, as
PRP-1-associated cell signaling modifications are more clearly
understood, the use of animal models may become more clinically
significant. Though 3D spheroid models are considered to be
analogous to in vivo conditions, the use of animal models
will not only elucidate a consistent effect in a living organism
but will also allow for a basic understanding of the coexistent
effects of PRP-1 in non-neoplastic tissues.
The results of the current study highlight the
inhibitory effects of PRP-1 on anchorage independent colony growth,
and in turn, the malignant potential of chondrosarcoma CSCs. These
findings indicate that PRP-1 effectively reduces CSC sustainability
in a reliable spheroid chondrosarcoma tumor model, which is
significant for the development of novel clinical approaches and
biologic agents used to manage chondrosarcoma.
The use of PRP-1 to treat metastatic chondrosarcoma
exhibits potential, notably decreasing or perhaps entirely
eliminating tumors when combined with current management
strategies, which warrants future investigation in a murine model.
If equally effective in a clinical setting, PRP-1 may
hypothetically decrease the morbidity and mortality associated with
chondrosarcoma. Further studies are required to improve our
understanding of the effects of PRP-1 on both neoplastic and
non-neoplastic tissues, the latter of which will be accomplished
using xenograft orthotopic transplantation of chondrosarcoma
spheroids into immunocompromised mice. Therefore, it behooves us to
evaluate whether PRP-1 decreases the size of the primary tumor and
prevents spontaneous metastasis to the lungs. Additional ongoing
experiments include those which will improve our understanding of
the cellular and molecular mechanisms by which PRP-1 induces CSC
death.
Acknowledgements
The authors would like to thank Dr Mark Brown
(University of Miami Department of Orthopaedic Surgery).
Funding
The present study was supported in part by a gift
from the Ratcliffe Foundation to the Miami Center of Orthopedic
Research and Education (CORE).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
CJG and KG designed the study. CJG, AKH, AM, BB, AS,
SS, JB, SAC and KG conducted the experiments and/or analyzed the
data. CJG and KG prepared the initial manuscript. CJG, AKH, BB and
AS prepared the figures and tables. CJG, AKH, AM, SAC and KG
critically reviewed and revised the manuscript for important
intellectual content. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Karpik M and Reszec J: Low grade
chondrosarcoma-epidemiology, diagnosis, treatment. Ortop Traumatol
Rehabil. 20:65–70. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Boehme KA, Schleicher SB, Traub F and
Rolauffs B: Chondrosarcoma: A rare misfortune in aging human
cartilage? The role of stem and progenitor cells in proliferation,
malignant degeneration and therapeutic resistance. Int J Mol Sci.
19:3112018. View Article : Google Scholar
|
|
3
|
Chow WA: Chondrosarcoma: Biology,
genetics, and epigenetics. F1000Res. 7:F1000 Faculty Rev-1826.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Leddy LR and Holmes RE: Chondrosarcoma of
bone. Cancer Treat Res. 162:117–130. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mery B, Espenel S, Guy JB, Rancoule C,
Vallard A, Aloy MT, Rodriguez-Lafrasse C and Magné N: Biological
aspects of chondrosarcoma: Leaps and hurdles. Crit Rev Oncol
Hematol. 126:32–36. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Andreou D, Ruppin S, Fehlberg S, Pink D,
Werner M and Tunn PU: Survival and prognostic factors in
chondrosarcoma: Results in 115 patients with long-term follow-up.
Acta Orthop. 82:749–755. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wan L, Tu C, Li S and Li Z: Regional lymph
node involvement is associated with poorer survivorship in patients
with chondrosarcoma: A SEER analysis. Clin Orthop Relat Res.
477:2508–2518. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nie Z, Lu Q and Peng H: Prognostic factors
for patients with chondrosarcoma: A survival analysis based on the
Surveillance, Epidemiology, and End Results (SEER) database
(1973–2012). J Bone Oncol. 13:55–61. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Song K, Song J, Chen F, Lin K, Ma X and
Jiang J: Does resection of the primary tumor improve survival in
patients with metastatic chondrosarcoma? Clin Orthop Relat Res.
477:573–583. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Colella G, Fazioli F, Gallo M, De Chiara
A, Apice G, Ruosi C, Cimmino A and de Nigris F: Sarcoma spheroids
and organoids-promising tools in the Era of personalized medicine.
Int J Mol Sci. 19:6152018. View Article : Google Scholar
|
|
11
|
Brown HK, Tellez-Gabriel M and Heymann D:
Cancer stem cells in osteosarcoma. Cancer Lett. 386:189–195. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rodriguez R, Rubio R and Menendez P:
Modeling sarcomagenesis using multipotent mesenchymal stem cells.
Cell Res. 22:62–77. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hanahan D and Weinberg RA: The hallmarks
of cancer. Cell. 100:57–70. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fouad YA and Aanei C: Revisiting the
hallmarks of cancer. Am J Cancer Res. 7:1016–1036. 2017.PubMed/NCBI
|
|
15
|
Ishiguro T, Ohata H, Sato A, Yamawaki K,
Enomoto T and Okamoto K: Tumor-derived spheroids: Relevance to
cancer stem cells and clinical applications. Cancer Sci.
108:283–289. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Guo X, Chen Y, Ji W, Chen X, Li C and Ge
R: Enrichment of cancer stem cells by agarose multi-well dishes and
3D spheroid culture. Cell Tissue Res. 375:397–408. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mehta P, Novak C, Raghavan S, Ward M and
Mehta G: Self-renewal and CSCs in vitro enrichment: Growth as
floating spheres. Methods Mol Biol. 1692:61–75. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ma R, Mandell J, Lu F, Heim T, Schoedel K,
Duensing A, Watters RJ and Weiss KR: Do patient-derived spheroid
culture models have relevance in chondrosarcoma research? Clin
Orthop Relat Res. May 19–2020.doi: 10.1097/CORR.0000000000001317.
(Epub ahead of print). View Article : Google Scholar
|
|
19
|
Fujii H, Honoki K, Tsujiuchi T, Kido A,
Yoshitani K and Takakura Y: Sphere-forming stem-like cell
populations with drug resistance in human sarcoma cell lines. Int J
Oncol. 34:1381–1386. 2009.PubMed/NCBI
|
|
20
|
Gao S, Shen J, Hornicek F and Duan Z:
Three-dimensional (3D) culture in sarcoma research and the clinical
significance. Biofabrication. 9:0320032017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gibbs CP, Kukekov VG, Reith JD,
Tchigrinova O, Suslov ON, Scott EW, Ghivizzani SC, Ignatova TN and
Steindler DA: Stem-like cells in bone sarcomas: Implications for
tumorigenesis. Neoplasia. 7:967–976. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tang QL, Zhao ZQ, Li JC, Liang Y, Yin JQ,
Zou CY, Xie XB, Zeng YX, Shen JN, Kang T and Wang J: Salinomycin
inhibits osteosarcoma by targeting its tumor stem cells. Cancer
Lett. 311:113–121. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Galoyan AA, Shakhlamov VA, Aghajanov MI
and Vahradyan HG: Hypothalamic proline-rich polypeptide protects
brain neurons in aluminum neurotoxicosis. Neurochem Res.
29:1349–1357. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Galoian K, Abrahamyan S, Chailyan G,
Qureshi A, Patel P, Metser G, Moran A, Sahakyan I, Tumasyan N, Lee
A, et al: Toll like receptors TLR1/2, TLR6 and MUC5B as binding
interaction partners with cytostatic proline rich polypeptide 1 in
human chondrosarcoma. Int J Oncol. 52:139–154. 2018.PubMed/NCBI
|
|
25
|
Galoian K, Luo S, Qureshi A, Patel P,
Price R, Morse AS, Chailyan G, Abrahamyan S and Temple HT: Effect
of cytostatic proline rich polypeptide-1 on tumor suppressors of
inflammation pathway signaling in chondrosarcoma. Mol Clin Oncol.
5:618–624. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Galoian KA, Temple TH and Galoyan A:
Cytostatic effect of novel mTOR inhibitor, PRP-1 (galarmin) in MDA
231 (ER-) breast carcinoma cell line. PRP-1 inhibits mesenchymal
tumors. Tumour Biol. 32:745–751. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Galoian K, Scully S and Galoyan A:
Myc-oncogene inactivating effect by proline rich polypeptide
(PRP-1) in chondrosarcoma JJ012 cells. Neurochem Res. 34:379–385.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Galoian K, Scully S, McNamara G, Flynn P
and Galoyan A: Antitumorigenic effect of brain proline rich
polypeptide-1 in human chondrosarcoma. Neurochem Res. 34:2117–2121.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Galoian K, Qureshi A, Wideroff G and
Temple HT: Restoration of desmosomal junction protein expression
and inhibition of H3K9-specific histone demethylase activity by
cytostatic proline-rich polypeptide-1 leads to suppression of
tumorigenic potential in human chondrosarcoma cells. Mol Clin
Oncol. 3:171–178. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hoyt AK, Moran A, Granger C, Sedani A,
Saigh S, Brown J and Galoian KA: PRP1 significantly decreases the
ALDHhigh cancer stem cell population and regulates the aberrant
Wnt/β-catenin pathway in human chondrosarcoma JJ012 cells. Oncol
Rep. 42:103–114. 2019.PubMed/NCBI
|
|
31
|
Horibata S, Vo TV, Subramanian V, Thompson
PR and Coonrod SA: Utilization of the soft agar colony formation
assay to identify inhibitors of tumorigenicity in breast cancer
cells. J Vis Exp. e527272015.PubMed/NCBI
|
|
32
|
Cesarz Z and Tamama K: Spheroid culture of
mesenchymal stem cells. Stem Cells Int. 2016:91763572016.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dong G, Wang S, Ge Y, Deng Q, Cao Q, Wang
Q, Shang Z, OuYang W, Li J, Liu C, et al: Serum-free culture system
for spontaneous human mesenchymal stem cell spheroid formation.
Stem Cells Int. 2019:60418162019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rieger AM, Nelson KL, Konowalchuk JD and
Barreda DR: Modified annexin V/propidium iodide apoptosis assay for
accurate assessment of cell death. J Vis Exp. 25972011.PubMed/NCBI
|
|
35
|
Whelan JS and Davis LE: Osteosarcoma,
chondrosarcoma, and chordoma. J Clin Oncol. 36:188–193. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mas A, Prusinski L, Yang Q, Diaz-Gimeno P,
Stone L, Diamond MP, Simón C and Al-Hendy A: Role of
Stro1+/CD44+ stem cells in myometrial
physiology and uterine remodeling during pregnancy. Biol Reprod.
96:70–80. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Galoczova M, Coates P and Vojtesek B:
STAT3, stem cells, cancer stem cells and p63. Cell Mol Biol Lett.
23:122018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Fang Y and Eglen RM: Three-dimensional
cell cultures in drug discovery and development. SLAS Discov.
22:456–472. 2017.PubMed/NCBI
|
|
39
|
Song L, Yuan X, Jones Z, Zhou Y, Ma T and
Li Y: Assembly of human stem cell-derived cortical spheroids and
vascular spheroids to model 3-D brain-like tissues. Sci Rep.
9:59772019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Leek R, Grimes DR, Harris AL and McIntyre
A: Methods: Using three-dimensional culture (Spheroids) as an in
vitro model of tumour hypoxia. Adv Exp Med Biol. 899:167–196. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Huang G, Ye S, Zhou X, Liu D and Ying QL:
Molecular basis of embryonic stem cell self-renewal: From signaling
pathways to pluripotency network. Cell Mol Life Sci. 72:1741–1757.
2015. View Article : Google Scholar : PubMed/NCBI
|