Introduction
Type 1 diabetes mellitus (T1DM) is a chronic
metabolic disorder that is characterized by the destruction of
islet β-cells and lack of insulin production (1). The progressive dysfunction and
decline in β-cell mass results in patients relying on exogenous
insulin to maintain glucose homeostasis. However, achieving strict
daily glycemic control is difficult for many patients (1,2).
Due to its safe and non-invasive nature, hyperbaric
oxygen (HBO) treatment has emerged since the 1990s as a potential
alternative treatment strategy for autoimmune diseases (3). HBO has been demonstrated to exhibit
anti-inflammatory properties (4),
such as mobilizing bone marrow stem cells and activating the
antioxidant enzyme system to improve tissue defense (5–7).
Clinical studies have previously revealed the improvement of
hyperglycaemia in patients with diabetes undergoing HBO treatment
(8,9). HBO has also been reported to prevent
hyperglycaemia and improve muscle oxidative capacity in rodents
with T2DM (10,11). Faleo et al (12) found that 2-week HBO treatment
preserved islet β-cell mass by stimulating proliferation,
inhibiting apoptosis and suppressing insulitis, thus decreasing the
incidence of autoimmune diabetes in non-obese diabetic mice.
Nevertheless, the mechanism of action underlying the effects of HBO
has not yet been fully elucidated. In addition to the pancreas, the
liver is also an important organ for maintaining blood glucose
balance by storing glucose as glycogen and releasing glucose by
glycogen degradation and gluconeogenesis (13). However, to date it remains unclear
whether HBO exhibits a therapeutic effect on liver glycogen storage
and glucose production in T1DM.
The ghrelin system consists of four components:
Ghrelin, ghrelin-O-acyl transferase (GOAT), growth hormone
secretagogue receptor-1a (GHSR) and liver expressed antimicrobial
peptide 2 (LEAP2) (14,15). Ghrelin is a 28-amino acid peptide
that is secreted primarily by the stomach as the endogenous ligand
for GHSR (16,17), whilst LEAP2 is the endogenous
antagonist of GHSR (15). Ghrelin
possesses a unique post-translational modification where it is
O-acylated at its serine 3 residue by GOAT, which is necessary for
binding to GHSR (18). The ghrelin
system serves a key role in regulating energy metabolism. Zhao
et al (19) previously
demonstrated that it is essential for mouse survival during calory
restriction. Ghrelin has also been found to suppress inflammation,
exert immunoregulatory (20–22)
and anti-edematous effects in the context of brain hypoxia
(23,24). Ghrelin, GOAT and GHSR are all
expressed in pancreatic islet cells, suggesting that ghrelin
mediates physiological effects on the pancreas via paracrine or
autocrine pathways (25,26). A number of studies have
demonstrated that ghrelin stimulates proliferation whilst
inhibiting apoptosis to protect cells, such as retinal ganglion
cells and osteoblastic MC3T3-E1 cells (27–30).
In particular, it has also been demonstrated that overexpression of
intra-islet ghrelin increases the proliferation of islet β-cells in
a streptozotocin (STZ)-induced β-cell injury model (31). Exogenous injection of ghrelin has
been demonstrated to delay the development of autoimmune diabetes
by mitigating insulitis and β-cell mass loss in
BioBreeding/Worcester rats (32).
Furthermore, certain studies have found that ghrelin regulates
GLUT2 expression (33,34) and stimulate hepatic gluconeogenesis
(35).
Since both ghrelin and HBO therapy have been
documented to stimulate proliferation and exhibits
anti-inflammation and anti-edematous effects, it was investigated
whether HBO influenced circulating ghrelin levels, in addition to
GOAT and GHSR protein expression in the pancreas and liver of T1DM
mice.
The present study aimed to investigate the mechanism
underlying the amelioration of hyperglycemia induced by T1DM
following 2-week HBO treatment. Subsequently, it was further
investigated if the ghrelin system lie downstream of HBO
treatment.
Materials and methods
Animals
A total of 20 C57BL/6J male mice (age, 8 weeks;
weight, 27–31 g) were obtained from Qingdao Institute of Drug
Control (Qingdao, China), and were acclimatized at the animal
facility (21–25°C; 55±20% humidity; 12-h light/dark cycle) in
specific pathogen-free conditions with ad libitum access to
food and water for 1 week prior to experimentation.
To establish a T1DM mouse model, 15 mice were
intraperitoneally (i.p.) injected with STZ (150 mg/kg) 12 h after
fasting (36), whilst the other
five mice received an equivalent volume (7.5 ml/kg) of citrate
buffer. After 7 days, blood glucose levels were measured from tail
vein blood (~50 µl) using a glucometer (B. Braun Melsungen AG). All
mice exhibiting random blood glucose ≥16.7 mmol/l accompanied by
excessive drinking, eating, urination and weight loss were
considered to be type 1 diabetic (37). These mice were then randomly
divided into three groups: i) Control (CON, n=5); ii) T1DM (n=8);
and iii) T1DM + HBO (n=7).
Animals were sacrificed following the final HBO
treatment by exsanguination following anaesthesia via an i.p.
injection of sodium pentobarbital (50 mg/kg). The tail of the
pancreas and liver were then fixed in 4% paraformaldehyde at 4°C
for 24 h and embedded in paraffin and sectioned at 5 µm for
histology assessment. Other samples of pancreas, liver and stomach
tissue were stored at −80°C for western blotting. Heparin sodium
salt (cat. no. H8060-1g; Beijing Solarbio Science & Technology
Co., Ltd.) was added to the blood samples as an anticoagulant,
following which the blood was centrifuged using a refrigerated
centrifuge (Eppendorf) to obtain the plasma (4°C, 20,000 × g, 15
min). Plasma samples were stored at −20°C for further analysis.
HBO treatment
HBO treatment was performed once per day for 2
weeks. Briefly, mice were transferred into the animal hyperbaric
oxygen chamber, which was ~40×100 cm in size (Yantai Moon Oxygen
Chamber Co., Ltd.) at 7:00 p.m. Mice in the CON and T1DM groups
were placed into a hyperbaric oxygen chamber without HBO treatment.
The hyperbaric session began with pressure rising gradually over a
5-min period, followed by continuous exposure to 100% oxygen at 2.0
atmospheres absolute (ATA) for 60 min. The chamber pressure was
then gradually decreased over another 5-min period before opening
(12).
Cumulative food intake and body
weight
On day 12 of HBO treatment, cumulative food intake
was measured. Food deprivation began at 2:00 p.m on day 12 and mice
were fasted for 5 h. Following 1-h HBO treatment, mice were fed and
cumulative food intake was measured using an electronic scale (cat.
no. TE412-L; Sartorius AG) 1, 2, 3, 4, 5, 6 and 12 h after HBO
treatment as previously described (38). During the HBO treatment, body
weight was measured every day for 11 days, except for the day of
the feeding and blood glucose testing to avoid the influence of
fasting.
Blood glucose and ghrelin levels
At the end of 2-week treatment cycle, blood glucose
levels were measured from tail vein blood using a glucometer (B.
Braun Melsungen AG) following fasting for 10 h. The mice were then
re-fed for 2 h before blood samples (~1 ml) were immediately
obtained from the inner canthus of mice for total plasma ghrelin
testing and subsequent sacrifice. Plasma total ghrelin levels were
measured using a mouse ultrasensitive ELISA kit (cat. no. CEA991Mu;
Wuhan USCN Business Co., Ltd.) according to the manufacturer's
protocols.
Histological processing
Pancreas and liver tissues were fixed in 4%
formaldehyde at 4°C for 48 h. Following dehydration with an
ascending gradient of ethanol and clearing with xylene, tissues
were embedded in paraffin and sectioned at 5 µm using a rotary
microtome (RM2016; Leica Microsystems GmbH).
Periodic acid-Schiff (PAS)
staining
Hepatic sections were stained using a PAS Staining
kit (cat. no. G1008; Wuhan Servicebio Technology Co., Ltd.) to
observe glycogen distribution according to the manufacturer's
protocols. Randomly selected hepatic sections were disposed with
α-amylase at 37°C for 30 min before PAS staining, and used as the
negative control group (cat. no. G8290; Beijing Solarbio Science
& Technology Co., Ltd.) prior to PAS staining for 30 min at
37°C.
Immunohistochemistry
Following antigen retrieval by heating at 95°C in
citrate buffer for 1 h, the pancreas and liver sections were
quenched with 0.3% hydrogen peroxide at room temperature for 30
min, blocked with 1% BSA (cat. no. 1213G057; Beijing Solarbio
Science & Technology Co., Ltd.) at room temperature for 1 h and
probed with primary antibodies for GLUT2 (rabbit polyclonal
antibody; 1:200; cat. no. ab54460; Abcam) at 37°C for 1 h. Sections
were then incubated with anti-rabbit horseradish peroxidase
(HRP)-conjugated secondary antibody (1:1; cat. no. PV-6001; OriGene
Technologies, Inc.) for 20 min at 37°C and stained using a DAB kit
(cat. no. ZLI-9018; OriGene Technologies, Inc.) according to the
manufacturer's protocols. Morphology was assessed using a light
microscope (magnification, ×400) over a spherical field of view 0.5
mm diameter (cat. no. CX31; Olympus Corporation). Negative controls
were included in each sample by substituting primary antibody with
PBS. Image Pro Plus (version 6.0; Media Cybernetics, Inc.) was used
to quantify the staining. For quantification of staining, using the
‘segmentation’ function, the measurement threshold in
Hue-Saturation-Intensity pattern was selected manually (hue, 0–30
nm; saturation, 0–255 nm; intensity, 1–170 nm), where the
integrated optical density was measured.
Immunofluorescence
The pancreas sections were firstly blocked (1% BSA +
0.1% Triton X-100 in PBS) at room temperature for 2 h. Primary
antibodies against insulin (rabbit polyclonal antibody; 1:200; cat.
no. 4590; Cell Signaling Technology, Inc.) and glucagon (mouse
monoclonal antibody; 1:2,000; cat. no. ab10988; Abcam) were added
and incubated at 4°C overnight. The slides were then incubated with
the mixture of fluorophore-conjugated secondary antibodies
(fluorescein isothiocyanate labelled goat anti-rabbit, green; and
rhodamine labelled goat anti-mouse, red; both 1:2,000; cat. nos.
ZF-0311 and ZF-0313, respectively; both OriGene Technologies, Inc.)
at room temperature for 2 h, followed by incubation with DAPI (cat.
no. D-9106; Beijing Biosynthesis Biotechnology Co., Ltd.), for 5
min. A fluorescent microscope (magnification, ×400) over a
spherical field of view 0.5 mm diameter (Axio Observer A1; Zeiss
AG) was used to measure fluorescence and perform histology
assessments using the Image J software (version 1.34.3.67; National
Institutes of Health). For quantification, fluorescent images were
converted to 8-bit gray images before the measurement threshold was
selected manually and the selected area of interest was
measured.
Western blotting
Pancreas, liver and stomach tissues was homogenized
in RIPA buffer (cat. no. P0013B; Beyotime Institute of
Biotechnology) with proteinase inhibitors. Total protein
concentrations was assessed using a bicinchoninic acid Protein
Assay kit (cat. no. P0012; Beyotime Institute of Biotechnology) and
a microplate reader (M5, MD-SpectraMax; Molecular Devices LLC).
Protein extracts were then mixed with loading buffer (cat. no.
P0015L; Beyotime Institute of Biotechnology), and 35 mg protein per
lane was loaded and separated using SDS-PAGE (10%) and transferred
onto PVDF membranes that were activated using methanol. After
blocking with 5% skimmed milk powder for 2 h at room temperature,
rabbit polyclonal anti-GOAT (cat. no. ab170690), anti-GLUT2 (cat.
no. ab54460) and anti-GHSR (cat. no. ab95250; all 1:1,000; all
Abcam) and anti-GAPDH primary antibodies (rabbit monoclonal
antibody; 1:1,000; cat. no. 5174; Cell Signaling Technology, Inc.)
were added and incubated at 4°C overnight. Goat HRP-conjugated
anti-rabbit IgG H&L secondary antibody (1:10,000; cat. no.
ZB2301; OriGene Technologies, Inc.) was then added and incubated
for 1 h at room temperature. The protein bands were developed using
the Immobilon Western Chemiluminescent Substrate (cat. no.
WBKLS0100; EMD Millipore). The intensity of bands was analyzed
using Quantity One software (version 4.5.0; Bio-Rad Laboratories,
Inc.).
Statistical analysis
Data are expressed as the mean ± SEM from three
independent repeats. Differences between two groups were compared
using one-way ANOVA. The LSD (least significance difference) test
was used as the post-hoc test. Cumulative food intake and body
weight data measured repeatedly were assessed using a mixed two-way
ANOVA followed by Sidak post hoc test. All statistical analysis was
performed using SPSS software (version 22.0; IBM Corp.). P<0.05
was considered to indicate a statistically significant
difference.
Results
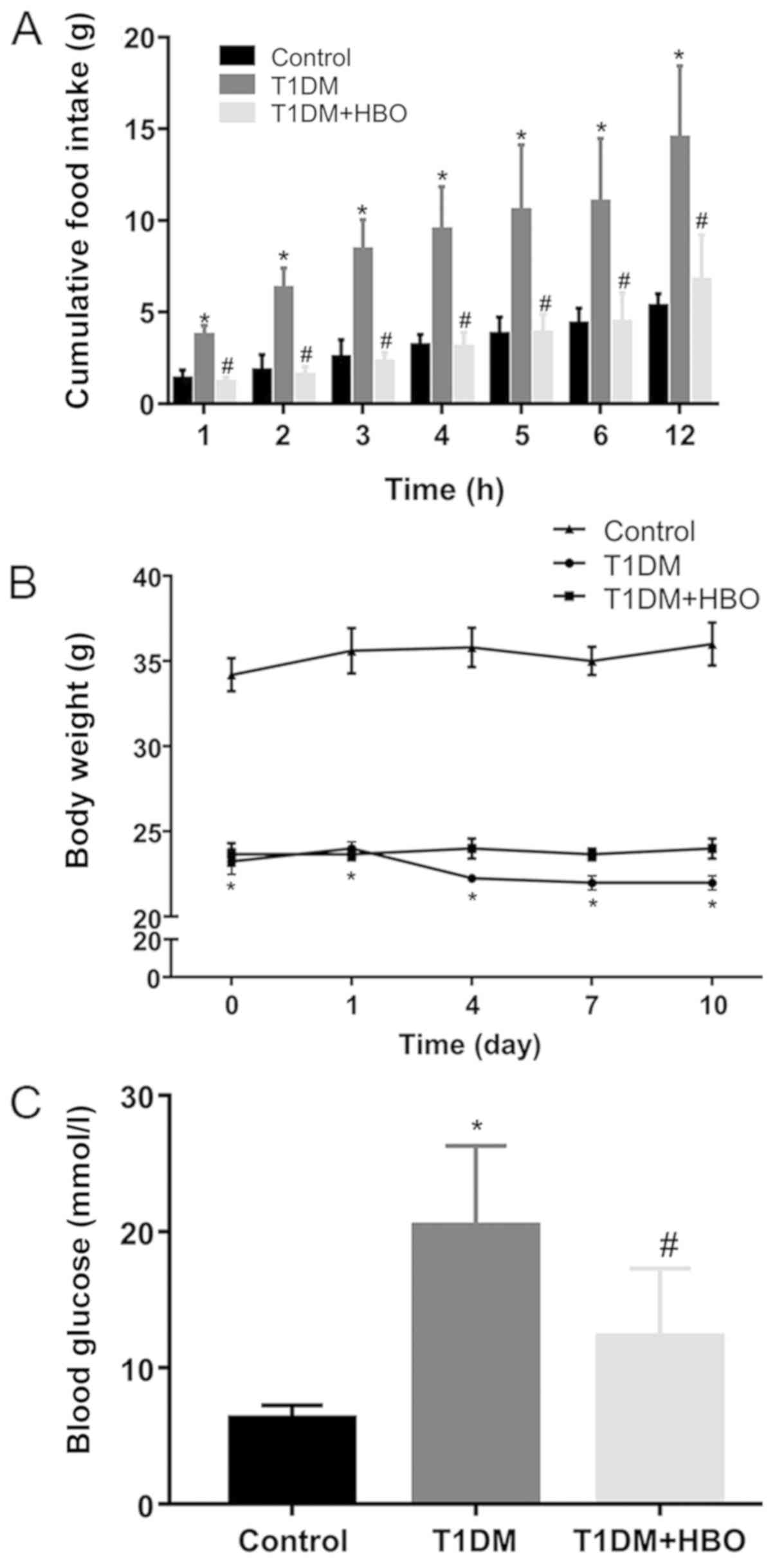
HBO suppresses the elevation of
cumulative food intake and fasting blood glucose levels in the T1DM
mouse model
Establishment of the T1DM model in the mice was
evaluated by observing their feeding behaviour, blood glucose and
body weights. Mice exhibiting random blood glucose ≥16.7 mmol/l
were considered to be T1DM.
After HBO treatment, the 12-h nocturnal cumulative
food intake (Day 12; T1DM vs. CON, 14.47±1.99 vs. 5.30±0.31 g;
P<0.05; Fig. 1A), 10-h fasting
blood glucose levels (Day 14; T1DM vs. CON, 20.40±2.23 vs.
6.28±0.44 mmol/l; P<0.05; Fig.
1C) and body weight loss (Day 10; T1DM vs. CON, 22.00±0.41 vs.
36.00±1.27 g; P<0.05; Fig. 1B)
were significantly greater in the T1DM group compared with those in
the CON group. By contrast, HBO treatment was found to
significantly reduce hyperphagia, beginning at 1 h (Day 12;
T1DM+HBO vs. T1DM, 1.14±0.11 vs. 3.70±0.24 g; P<0.05), which was
sustained for 12 h (Day 12; T1DM+HBO vs. T1DM, 6.73±0.93 g vs.
14.47±1.99 g; P<0.05; Fig. 1A),
in addition to significantly ameliorating hyperglycaemia (Day 14;
T1DM+HBO vs. T1DM, 12.30±1.89 vs. 20.40±2.23 mmol/l; P<0.05) in
T1DM mice (Fig. 1A and C).
Although HBO did not improve body weight loss significantly in mice
in the T1DM group (Day 10; T1DM+HBO vs. T1DM, 24.00±0.58 vs.
22.00±0.41 g; Fig. 1B), there was
an increasing trend in the T1DM + HBO group.
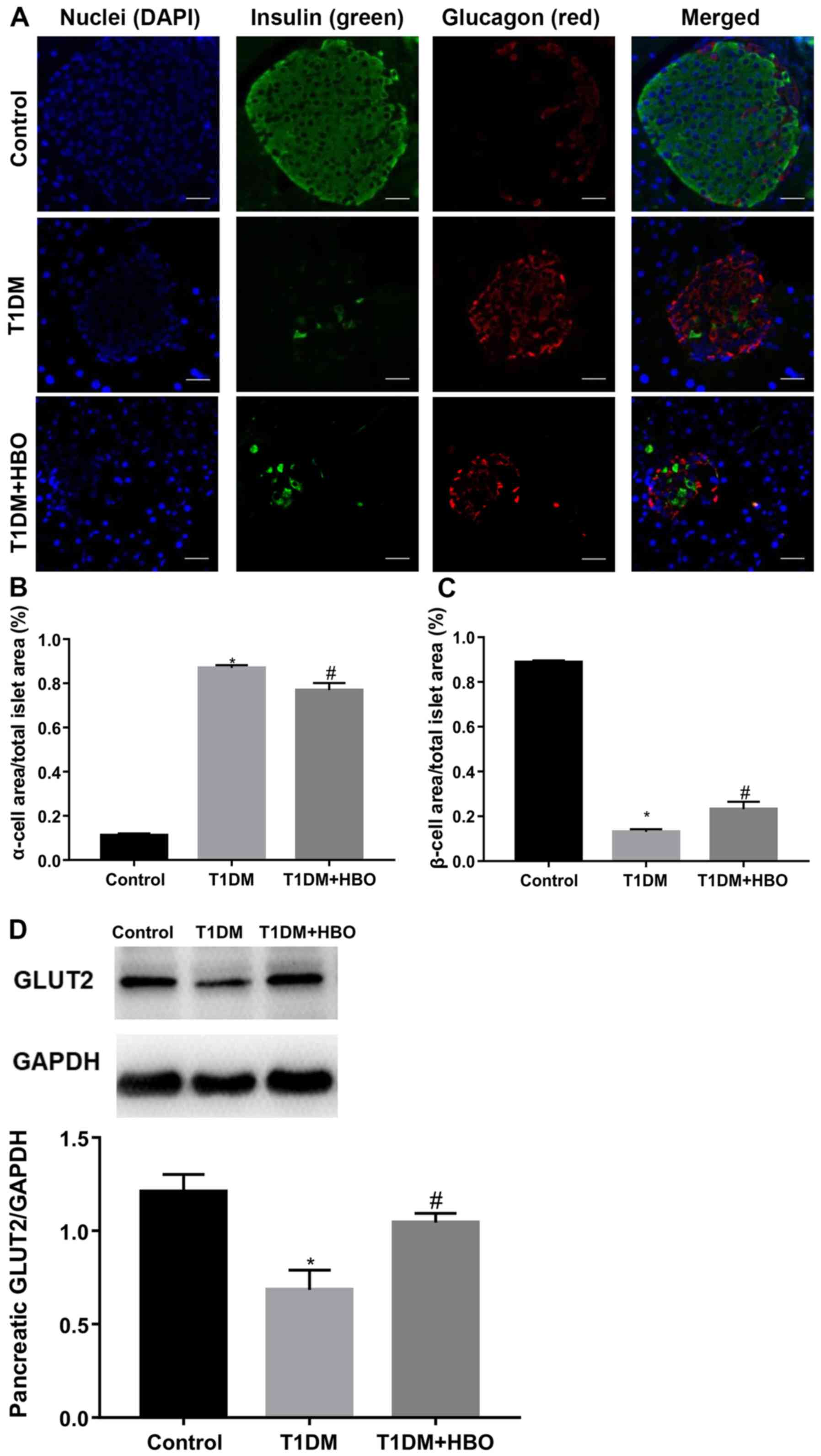
HBO treatment protects islet β-cells
and promotes pancreatic GLUT2 protein expression levels in T1DM
mice
Islet β-cell destruction is a primary feature of
T1DM. To investigate the therapeutic effects of HBO further,
insulin- and glucagon-positive areas in the pancreas were measured.
Compared with that in CON mice, pancreatic islet β-cell area in
T1DM mice was significantly decreased (T1DM vs. CON, 0.13±0.01 vs.
0.89±0.01; P<0.05; Fig. 2A and
C) whereas the islet α-cell area was significantly increased
(T1DM vs. CON, 0.87±0.01 vs. 0.11±0.01; P<0.05; Fig. 2A and B). Following 2-week HBO
treatment, β-cell area in tissues from T1DM mice was partially but
significantly increased (T1DM + HBO vs. T1DM, 0.23±0.03 vs.
0.13±0.01; P<0.05) and α-cell area was likewise partially but
significantly reduced (T1DM + HBO vs. T1DM, 0.77±0.03 vs.
0.87±0.01; P<0.05; Fig. 2A-C).
Reductions in pancreatic GLUT2 expression levels in T1DM mice were
also significantly ameliorated by HBO treatment (T1DM + HBO vs.
T1DM, 1.04±0.05 vs. 0.69±0.10; P<0.05; Fig. 2D), consistent with increased islet
β-cell area.
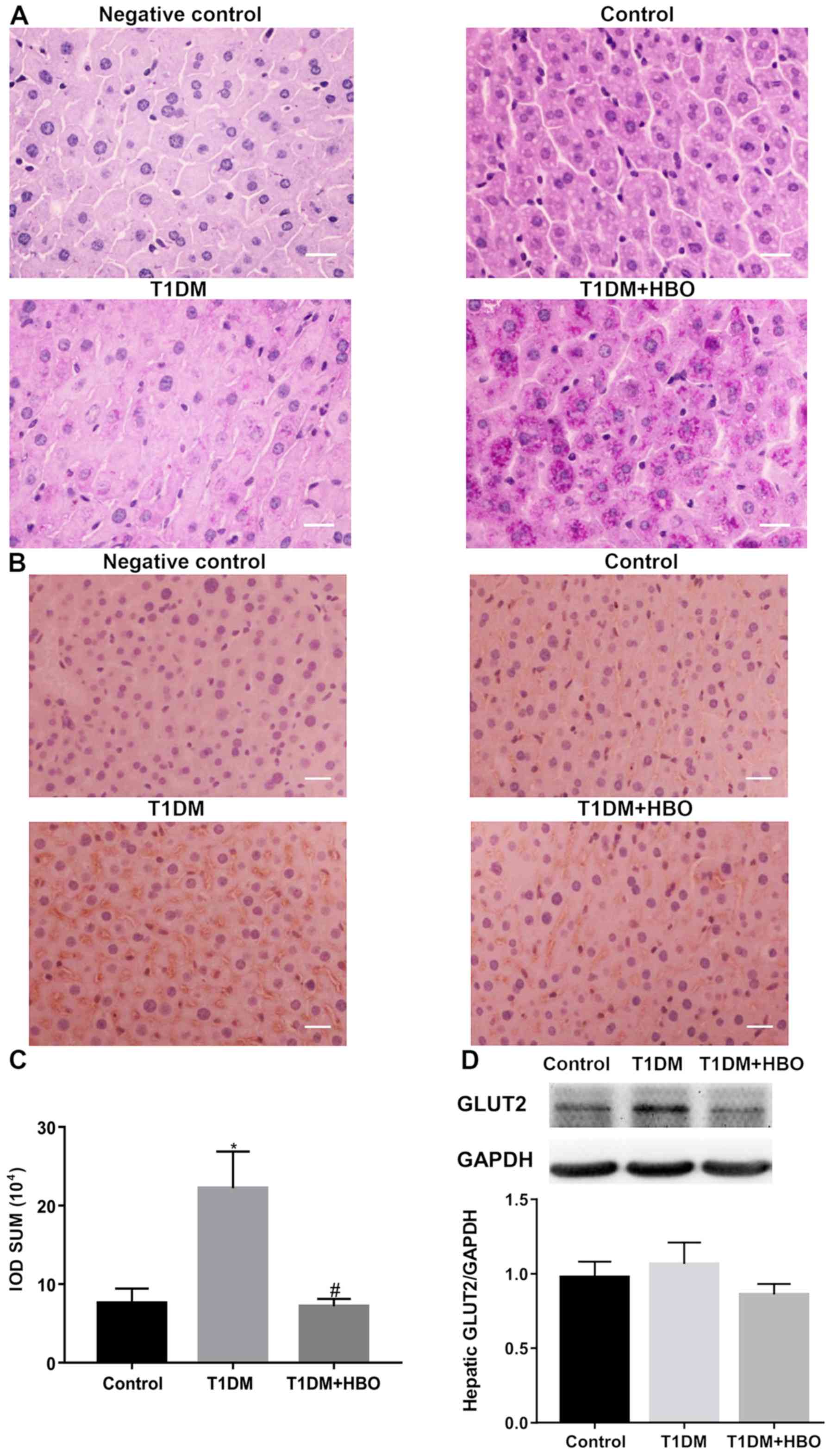
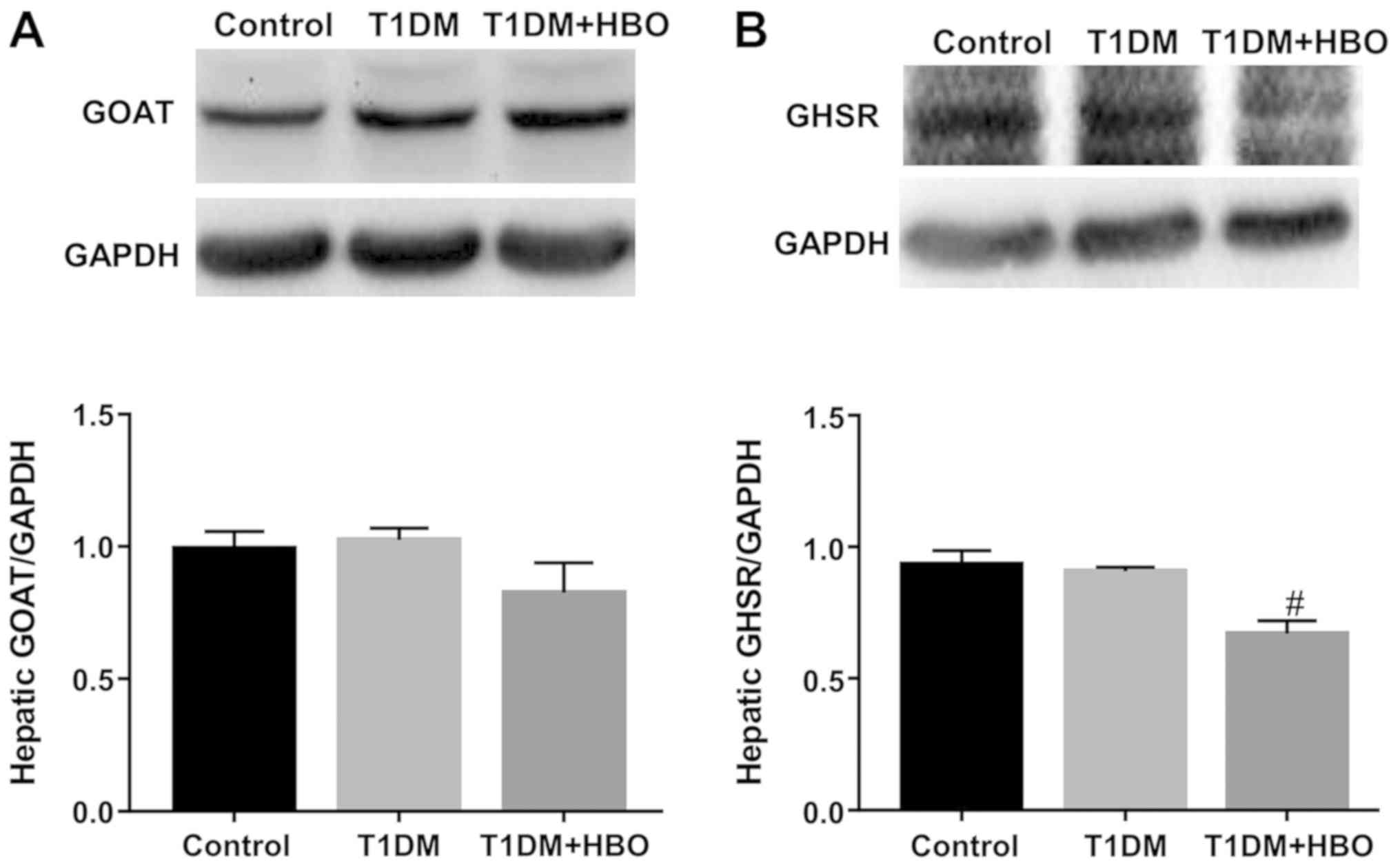
HBO enhances hepatic glycogen storage
and inhibits hepatic GLUT2 membrane trafficking in T1DM mice
To determine if the liver serves a key role in T1DM,
glycogen levels were measured as a proxy for the capacity for
glycogen storage in the liver. The present study demonstrated
significantly lower extent of glycogen storage in T1DM mice
compared with that in the CON group, whilst HBO treatment restored
hepatic glycogen storage (Fig.
3A). To investigate glucose metabolism further, hepatic GLUT2
expression and membrane trafficking levels were measured. Total
hepatic GLUT2 expression levels were not found to be significantly
altered between T1DM group and CON group, and HBO did not change
T1DM hepatic total GLUT2 expression (Fig. 3D), whereas immunohistochemistry
demonstrated that transfer of GLUT2 to the membrane was greater in
the T1DM group compared with that in the CON group (T1DM vs. CON,
222,231±46,434 vs. 75,760±18,503; P<0.05). HBO treatment
reversed this increase (T1DM + HBO vs. T1DM, 71,831±16,244 vs.
222,231±46,434; P<0.05; Fig. 3B and
C).
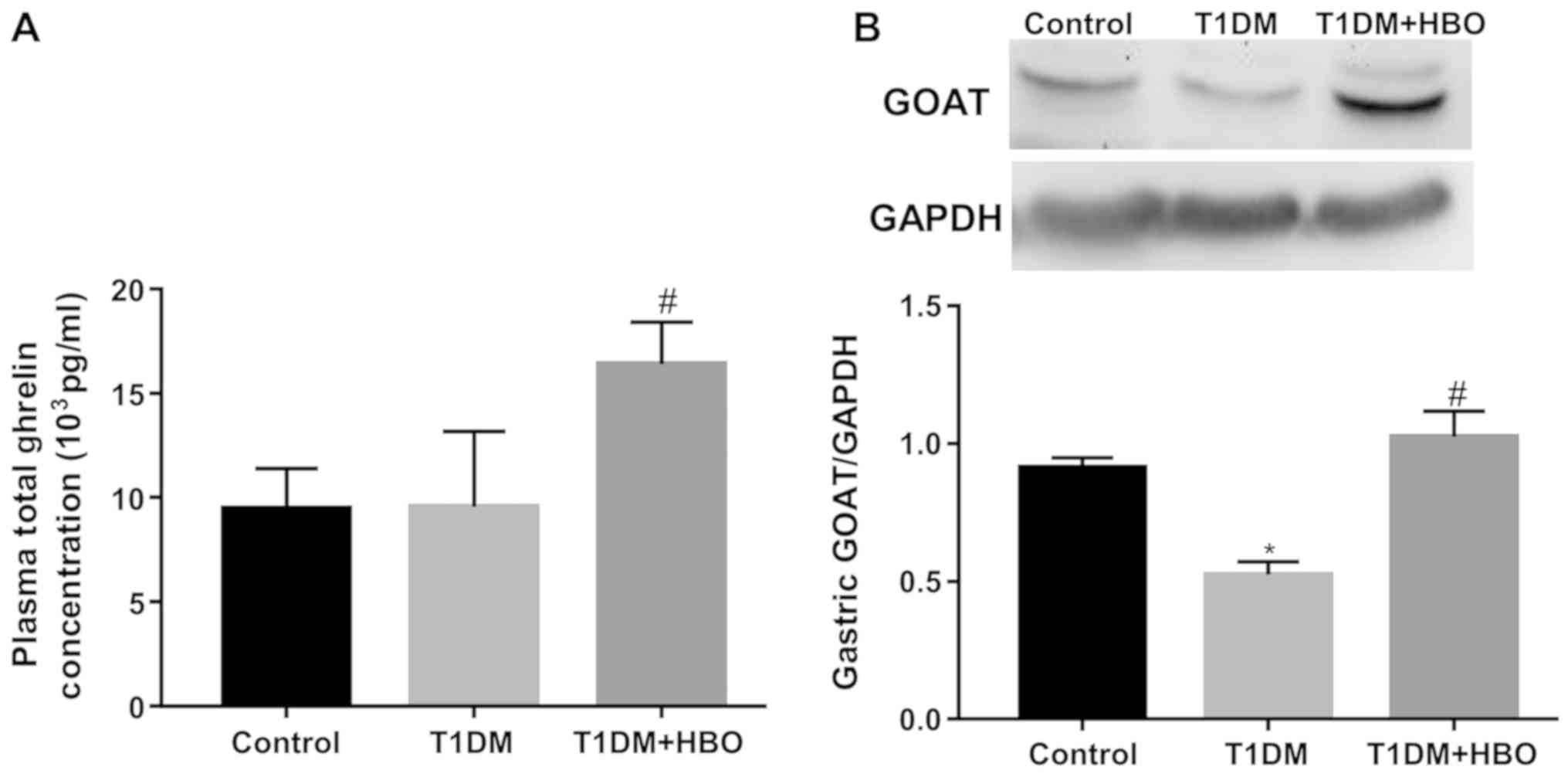
HBO elevates plasma ghrelin and
gastric GOAT protein expression levels in T1DM mice
Total ghrelin levels in the HBO group were found to
be significantly greater compared with those in mice in the T1DM
and CON groups (T1DM + HBO vs. T1DM and CON, 16,389.17±906.31 vs.
9,554.98±1,200.69 and 9,469.76±857.22 pg/ml, respectively;
P<0.05; Fig. 4A). The gastric
GOAT expression levels in the T1DM group were significantly lower
compare with those in the CON group (T1DM vs. CON, 0.52±0.05 vs.
0.91±0.03; P<0.05), which was restored by HBO treatment (T1DM +
HBO vs. T1DM, 1.02±0.09 vs. 0.52±0.05; P<0.05) to a normal level
(T1DM + HBO vs. CON 1.02±0.09 vs. 0.91±0.03; P>0.05; Fig. 4B).
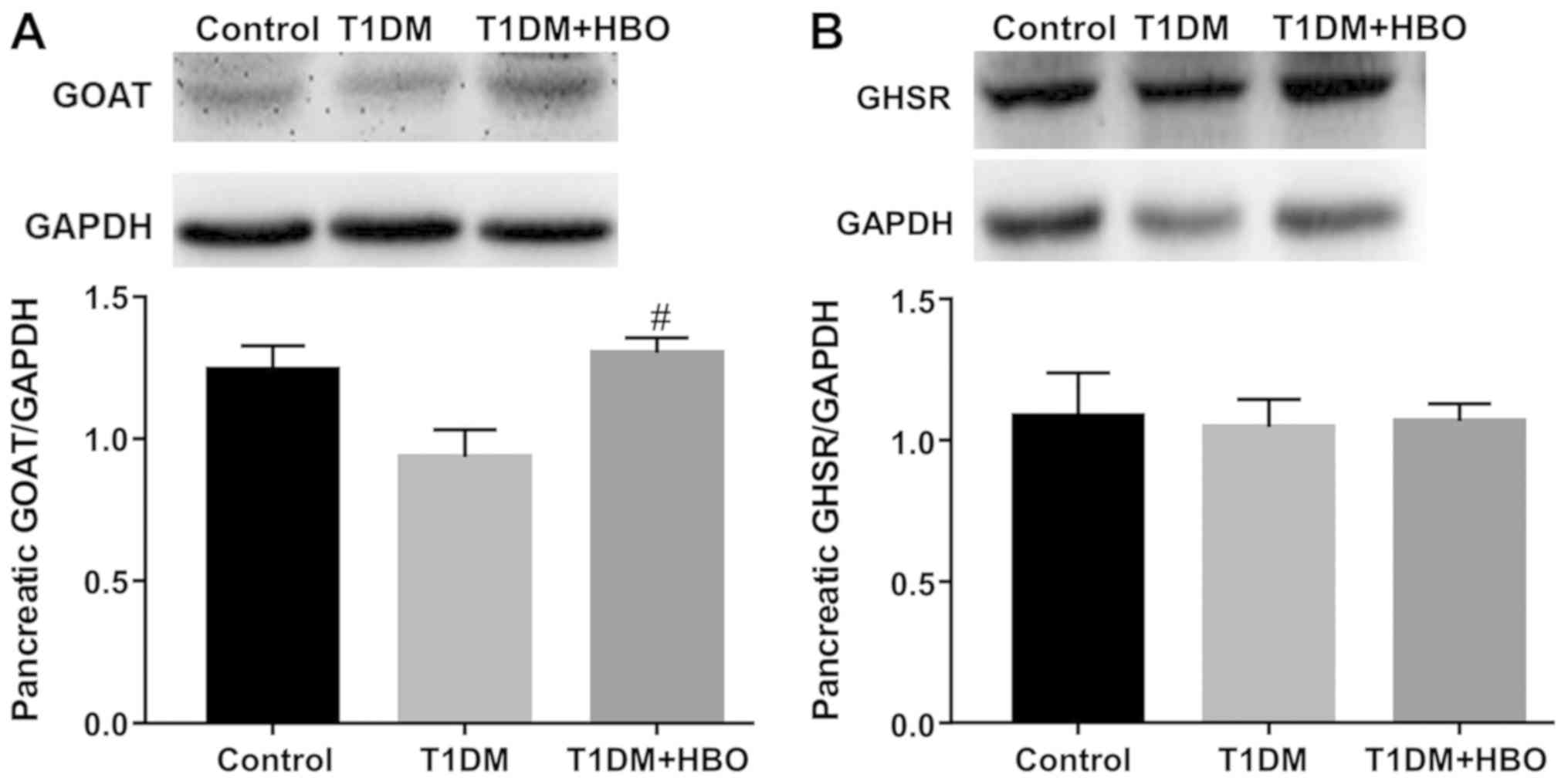
HBO promotes pancreatic GOAT
expression and downregulates hepatic GHSR expression levels in T1DM
mice
In order to investigate the role of the ghrelin
system in the changes observed thus far, GOAT and GHSR expression
levels were subsequently measured in the pancreas and liver.
Pancreatic GOAT expression in the T1DM group was not significantly
changed compared with that in the CON group (T1DM vs. CON,
0.94±0.09 vs. 1.24±0.08; P>0.05; Fig. 5A) but showed a decreasing trend.
Pancreatic GOAT expression in the T1DM group was significantly
reversed by HBO treatment (T1DM+HBO vs. T1DM; 1.30±0.05 vs.
0.94±0.09; P<0.05; Fig. 5A). No
significant difference in pancreatic GHSR expression levels was
observed among these three groups (T1DM + HBO vs. T1DM vs. CON,
1.07±0.06 vs. 1.05±0.10 vs. 1.09±0.15; Fig. 5B). In addition, no significant
differences could be observed between T1DM group and CON group in
terms of hepatic GOAT expression. HBO did not change T1DM hepatic
GOAT expression, though the GOAT expression levels in HBO + T1DM
group exhibited a decreasing trend (Fig. 6A). HBO treatment resulted in
significantly lower hepatic GHSR protein expression levels compared
with those in both the CON and T1DM groups (T1DM + HBO vs. CON and
T1DM, 0.67±0.05 vs. 0.93±0.05 and 0.91±0.02, respectively; both
P<0.05; Fig. 6B).
Discussion
HBO is commonly used to treat diabetic foot ulcers
and neuropathy in patients with diabetes mellitus (39,40).
Treatment involves intermittent administration of 100% oxygen,
usually in daily sessions of 60–90 min, at pressures of 1.5–3.0 ATA
(41,42). In the present study, a 2-week
course of HBO-100% (60-min at 2.0 ATA daily) treatment improved
diabetic symptoms in STZ-induced T1DM mice, as demonstrated by the
lower blood glucose levels and 12-h accumulative food intake. A
previous study showed that HBO influences the immune system, by
increasing resting T cells and reducing activation of dendritic
cells, to prevent autoimmune diabetes (12). Therefore, the present study
primarily focused on the regulation of glucose metabolism by HBO.
HBO significantly increased the ratio of islet β-cell area to total
islet area, whilst lowering the ratio of α-cell area to total islet
area. Furthermore, HBO upregulated GLUT2 expression levels,
consistent with the increased β-cell area, since GLUT2 is not
expressed in islet α-cells (43).
The increased β-cell area and GLUT2 expression levels in the
pancreas isolated from the T1DM + HBO group suggested that
insulin-release and glucose-sensing was improved, consistent with
the observed blood glucose level alterations following HBO
treatment. Previous studies have demonstrated that GLUT2 expression
levels are lower in islet β-cells, but not in the liver tissue,
during diabetes (43,44). In the present study, although
hepatic GLUT2 expression levels did not change among the three
groups, transfer of GLUT2 to the membrane was greater in the T1DM
group, which was reversed by HBO treatment. HBO improved
hyperglycemia in the T1DM + HBO group by increasing hepatic
glycogen storage and reducing the transfer of GLUT2 to the
membrane.
The present study also observed elevated plasma
total ghrelin levels with increased gastric expression levels of
GOAT in the T1DM + HBO group, suggesting that serum ghrelin levels
increased following 2 weeks of HBO treatment. However, there was no
difference in plasma total ghrelin levels between T1DM and control
groups. It has previously been demonstrated that plasma ghrelin
concentrations are increased in non-re-feeding STZ-treated rats
(45) whereas ghrelin levels
assessed following fasting were lower in patients with T1DM
(46). These findings suggest that
the changes in ghrelin levels in response to diet during diabetes
condition is varied. Ghrelin in the circulatory system primarily
originates from the stomach and is unstable in the circulation
(47). It has previously been
reported that gastric GOAT mRNA levels are correlated with
circulating ghrelin levels in fasted and diet-induced obese mice
(48). Therefore, both total
plasma ghrelin levels and gastric GOAT levels were correlate with
plasma ghrelin levels, which indicated that plasma ghrelin levels
increased after HBO treatment. Ghrelin is deacylated by esterases
into des-acyl ghrelin (DAG) in the circulation (47) and re-acylated locally by plasma
membrane-resident GOAT (49).
Re-acylation of ghrelin in the pancreas and liver was investigated
in the current study, and it was found that HBO upregulated
pancreatic GOAT expression without affecting hepatic GOAT
expression. Ghrelin demonstrated a protective role in islet β-cells
(31), such that HBO increased
pancreatic GOAT expression levels in T1DM mice in the present
study. Despite the fact that pancreatic GHSR expression levels did
not change, the increased GOAT expression levels suggested that
ghrelin activity was greater following HBO treatment in T1DM mice.
The gene encoding GHSR has been demonstrated to be highly expressed
and enriched in islet δ-cells rather than α- and β-cells (50), which may explain why pancreatic
GHSR levels did not change among all groups.
Ghrelin increases blood glucose levels not only via
stimulatory effects on food intake and GH secretion, but also by
decreasing insulin secretion and increasing circulating levels of
glucocorticoids, glucagon release and hepatic gluconeogenesis
(19,51–54).
Increased serum ghrelin level, lower blood glucose levels and food
intake were observed in mice in the T1DM + HBO group. A potential
explanation is that the enhanced ghrelin activity could've exerted
anti-inflammatory effects on the pancreas (32), but not to a level sufficient to
trigger feeding behavior. McFarlane et al (55) previously demonstrated that
stimulating food intake in mice via exogenous injection of ghrelin
notably increased plasma ghrelin levels. Due to the protection of
islet β-cells by HBO, increased insulin levels could be one reason
food intake was decreased in the T1DM + HBO group, while insulin
could influence food intake (56).
Ghrelin-induced protection of islet β-cells may exceed the effect
of ghrelin on decreased insulin release, increased glucagon
secretion and elevation of blood glucose levels via the central
nervous system. In addition, reduced hepatic GHSR expression levels
following HBO treatment is consistent with the altered blood
glucose levels and the effect of ghrelin on hepatic gluconeogenesis
(54). Following HBO treatment,
plasma ghrelin levels are increased and hepatic GHSR expression
levels may compensate by decreasing. In the present study, it was
found that HBO upregulated total plasma ghrelin (ghrelin and DAG)
levels. DAG has been previously demonstrated to inhibit
ghrelin-induced glucose output in hepatocytes and prevent the
hyperglycaemic effects of ghrelin (57), which may be one of the causes of
HBO-induced decrease in blood glucose. Bando et al (31) demonstrated that elevating mouse
pancreatic ghrelin levels only stimulated β-cell proliferation,
with the serum insulin levels increasing, following β-cell injury.
Chronic hyperglycaemia involves decreased numbers of
insulin-positive cells and increased numbers of glucagon-positive
cells in islets (58). HBO
decreases islet β-cell apoptosis and increases proliferation
(12), thereby decreasing blood
glucose levels by improving pancreatic islet structure and
function.
The present study investigated the therapeutic
effects of HBO on T1DM mice. The results revealed the protective
effects of HBO treatment against STZ-induced glucose metabolism
dysfunction, in addition to the involvement of the ghrelin system.
Further experiments are required to assess the effects of HBO
treatment on CON mice. However, interventions such as blocking the
action of ghrelin (e.g. using a GHSR antagonist or ghrelin/GHSR
knockout mice) and measuring the DAG and LEAP2 expression levels
were not performed due to the limited capacity of animal housing
and handling. These interventions require further
investigation.
Acknowledgements
Not applicable.
Funding
The present work was supported by the National
Natural Science Foundation of China (grant no. 31872791) and
Natural Science Foundation of Shandong Province of China (grant no.
ZR2019MC046).
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JD, LS and JY conceptualized and designed the study.
LS, JY, YuL, DZ, CZ, QL, ML, KS, RM, YaL and GG performed the
experiments. LS and JY analysed the data and wrote the manuscript.
JD, RM, LS and JY edited the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
All procedures were approved by the Ethics Committee
of the Affiliated Hospital of Qingdao University (approval no.
QYFYWZLL25600) and performed in accordance with the National
Institutes of Health guidelines.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bluestone JA, Herold K and Eisenbarth G:
Genetics, pathogenesis and clinical interventions in type 1
diabetes. Nature. 464:1293–1300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Skyler JS and Ricordi C: Stopping type 1
diabetes: Attempts to prevent or cure type 1 diabetes in man.
Diabetes. 60:1–8. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xu X, Yi H, Kato M, Suzuki H, Kobayashi S,
Takahashi H and Nakashima I: Differential sensitivities to
hyperbaric oxygen of lymphocyte subpopulations of normal and
autoimmune mice. Immunol Lett. 59:79–84. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kang N, Hai Y, Yang J, Liang F and Gao CJ:
Hyperbaric oxygen intervention reduces secondary spinal cord injury
in rats via regulation of HMGB1/TLR4/NF-κB signaling pathway. Int J
Clin Exp Pathol. 8:1141–1153. 2015.PubMed/NCBI
|
|
5
|
Tepić S, Petković A, Srejović I, Jeremić
N, Zivković V, Loncarević S, Bradić J, Jakovljević V and Zivkovć M:
Impact of hyperbaric oxygenation on oxidative stress in diabetic
patients. Undersea Hyperb Med. 45:9–17. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee YS, Chio CC, Chang CP, Wang LC, Chiang
PM, Niu KC and Tsai KJ: Long course hyperbaric oxygen stimulates
neurogenesis and attenuates inflammation after ischemic stroke.
Mediators Inflamm. 2013:5129782013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Thom SR, Bhopale VM, Velazquez OC,
Goldstein LJ, Thom LH and Buerk DG: Stem cell mobilization by
hyperbaric oxygen. Am J Physiol Heart Circ Physiol.
290:H1378–H1386. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Al-Waili NS, Butler GJ, Beale J, Abdullah
MS, Finkelstein M, Merrow M, Rivera R, Petrillo R, Carrey Z, Lee B
and Allen M: Influences of hyperbaric oxygen on blood pressure,
heart rate and blood glucose levels in patients with diabetes
mellitus and hypertension. Arch Med Res. 37:991–997. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Stevens SL, Sorita A, Narr AJ, Claus PL,
Tescher A, Millman MP, Shields RC, Buchta WG, Haddon R and Murad
MH: Applying quality improvement methods in a hyperbaric oxygen
program: Reducing unnecessary glucose testing. Undersea Hyperb Med.
43:427–435. 2016.PubMed/NCBI
|
|
10
|
Nagatomo F, Takemura A, Roy RR, Fujino H,
Kondo H and Ishihara A: Mild hyperbaric oxygen inhibits the
growth-related decline in skeletal muscle oxidative capacity and
prevents hyperglycemia in rats with type 2 diabetes mellitus. J
Diabetes. 10:753–763. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gu N, Nagatomo F, Fujino H, Takeda I,
Tsuda K and Ishihara A: Hyperbaric oxygen exposure improves blood
glucose level and muscle oxidative capacity in rats with type 2
diabetes. Diabetes Technol Ther. 12:125–133. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Faleo G, Fotino C, Bocca N, Molano RD,
Zahr-Akrawi E, Molina J, Villate S, Umland O, Skyler JS, Bayer AL,
et al: Prevention of autoimmune diabetes and induction of beta-cell
proliferation in NOD mice by hyperbaric oxygen therapy. Diabetes.
61:1769–1778. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Roden M and Bernroider E: Hepatic glucose
metabolism in humans-its role in health and disease. Best Pract Res
Clin Endocrinol Metab. 17:365–383. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mani BK and Zigman JM: Ghrelin as a
survival hormone. Trends Endocrinol Metab. 28:843–854. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ge X, Yang H, Bednarek MA, Galon-Tilleman
H, Chen P, Chen M, Lichtman JS, Wang Y, Dalmas O, Yin Y, et al:
LEAP2 Is an endogenous antagonist of the ghrelin receptor. Cell
Metab. 27:461–469.e6. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Date Y, Kojima M, Hosoda H, Sawaguchi A,
Mondal MS, Suganuma T, Matsukura S, Kangawa K and Nakazato M:
Ghrelin, a novel growth hormone-releasing acylated peptide, is
synthesized in a distinct endocrine cell type in the
gastrointestinal tracts of rats and humans. Endocrinology.
141:4255–4261. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kojima M, Hosoda H, Date Y, Nakazato M,
Matsuo H and Kangawa K: Ghrelin is a growth-hormone-releasing
acylated peptide from stomach. Nature. 402:656–660. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang J, Brown MS, Liang G, Grishin NV and
Goldstein JL: Identification of the acyltransferase that
octanoylates ghrelin, an appetite-stimulating peptide hormone.
Cell. 132:387–396. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhao TJ, Liang G, Li RL, Xie X, Sleeman
MW, Murphy AJ, Valenzuela DM, Yancopoulos GD, Goldstein JL and
Brown MS: Ghrelin O-acyltransferase (GOAT) is essential for growth
hormone-mediated survival of calorie-restricted mice. Proc Natl
Acad Sci USA. 107:7467–7472. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pereira JADS, da Silva FC and de
Moraes-Vieira PMM: The Impact of Ghrelin in Metabolic Diseases: An
Immune Perspective. J Diabetes Res. 2017:45279802017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mao Y, Tokudome T, Kishimoto I, Otani K,
Nishimura H, Yamaguchi O, Otsu K, Miyazato M and Kangawa K:
Endogenous ghrelin attenuates pressure overload-induced cardiac
hypertrophy via a cholinergic anti-inflammatory pathway.
Hypertension. 65:1238–1244. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li G, Liu J, Xia WF, Zhou CL and Lv LQ:
Protective effects of ghrelin in ventilator-induced lung injury in
rats. Int Immunopharmacol. 52:85–91. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hossienzadeh F, Babri S, Alipour MR,
Ebrahimi H and Mohaddes G: Effect of ghrelin on brain edema induced
by acute and chronic systemic hypoxia. Neuroscience Lett.
534:47–51. 2013. View Article : Google Scholar
|
|
24
|
Mohaddes G, Abdolalizadeh J, Babri S,
Abedini N and Hossienzadeh F: The anti-edematous effect of ghrelin
in brain hypoxia is associated with decreasing expression of
vascular endothelial growth factor. J Mol Neurosci. 56:273–277.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Heppner KM and Tong J: Mechanisms in
endocrinology: Regulation of glucose metabolism by the ghrelin
system: Multiple players and multiple actions. Eur J Endocrinol.
171:R21–R32. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
An W, Li Y, Xu G, Zhao J, Xiang X, Ding L,
Li J, Guan Y, Wang X, Tang C, et al: Modulation of ghrelin
O-acyltransferase expression in pancreatic islets. Cell Physiol
Biochem. 26:707–716. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim SW, Her SJ, Park SJ, Kim D, Park KS,
Lee HK, Han BH, Kim MS, Shin CS and Kim SY: Ghrelin stimulates
proliferation and differentiation and inhibits apoptosis in
osteoblastic MC3T3-E1 cells. Bone. 37:359–369. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liang QH, Liu Y, Wu SS, Cui RR, Yuan LQ
and Liao EY: Ghrelin inhibits the apoptosis of MC3T3-E1 cells
through ERK and AKT signaling pathway. Toxicol Appl Pharmacol.
272:591–597. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhu K, Zhang ML, Liu ST, Li XY, Zhong SM,
Li F, Xu GZ, Wang Z and Miao Y: Ghrelin attenuates retinal neuronal
autophagy and apoptosis in an experimental rat glaucoma model.
Invest Ophthalmol Vis Sci. 58:6113–6122. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hao Y, Liu C, Huang J, Gu Y, Li H, Yang Z,
Liu J, Wang W and Li R: Ghrelin protects against depleted
uranium-induced apoptosis of MC3T3-E1 cells through oxidative
stress-mediated p38-mitogen-activated protein kinase pathway.
Toxicol Appl Pharmacol. 290:116–125. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bando M, Iwakura H, Ariyasu H, Koyama H,
Hosoda K, Adachi S, Nakao K, Kangawa K and Akamizu T:
Overexpression of intraislet ghrelin enhances β-cell proliferation
after streptozotocin-induced β-cell injury in mice. Am J Physiol
Endocrinol Metab. 305:E140–E148. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Baena-Nieto G, Lomas-Romero IM, Mateos RM,
Leal-Cosme N, Perez-Arana G, Aguilar-Diosdado M, Segundo C and
Lechuga-Sancho AM: Ghrelin mitigates β-cell mass loss during
insulitis in an animal model of autoimmune diabetes mellitus, the
BioBreeding/Worcester rat. Diabetes Metab Res Rev. 332017.doi:
10.1002/dmrr.2813.
|
|
33
|
Blanco AM, Bertucci JI, Ramesh N, Delgado
MJ, Valenciano AI and Unniappan S: Ghrelin facilitates GLUT2-,
SGLT1- and SGLT2-mediated intestinal glucose transport in goldfish
(Carassius auratus). Sci Rep. 7:450242017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fuente-Martín E, García-Cáceres C,
Argente-Arizón P, Díaz F, Granado M, Freire-Regatillo A,
Castro-González D, Ceballos ML, Frago LM, Dickson SL, et al:
Ghrelin regulates glucose and glutamate transporters in
hypothalamic astrocytes. Sci Rep. 6:236732016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li RL, Sherbet DP, Elsbernd BL, Goldstein
JL, Brown MS and Zhao TJ: Profound hypoglycemia in starved,
ghrelin-deficient mice is caused by decreased gluconeogenesis and
reversed by lactate or fatty acids. J Biol Chem. 287:17942–17950.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhou XY, Zhang F, Hu XT, Chen J, Tang RX,
Zheng KY and Song YJ: Depression can be prevented by astaxanthin
through inhibition of hippocampal inflammation in diabetic mice.
Brain Res. 1657:262–268. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu YN, Yang Z, Kong D, Zhang Y, Yu W and
Zha W: Metformin ameliorates testicular damage in male mice with
streptozotocin-induced type 1 diabetes through the PK2/PKR Pathway.
Oxid Med Cell Longev. 2019:56817012019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang R, Yuan J, Zhang C, Wang L, Liu Y,
Song L, Zhong W, Chen X and Dong J: Neuropeptide Y-Positive neurons
in the dorsomedial hypothalamus are involved in the anorexic effect
of angptl8. Front Mol Neurosci. 11:4512018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ma L, Li P, Shi Z, Hou T, Chen X and Du J:
A prospective, randomized, controlled study of hyperbaric oxygen
therapy: Effects on healing and oxidative stress of ulcer tissue in
patients with a diabetic foot ulcer. Ostomy Wound Manage. 59:18–24.
2013.PubMed/NCBI
|
|
40
|
Veyseller B, Dogan R, Yenigun A, Aksoy F,
Tugrul S, Dogan EE and Ozturan O: Hyperbaric oxygen therapy of
olfactory dysfunction in diabetic neuropathy with type 2 diabetes
mellitus and a new definition diabetic olfactopathy. Rhinology.
54:273–277. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Stoekenbroek RM, Santema TB, Legemate DA,
Ubbink DT, van den Brink A and Koelemay MJ: Hyperbaric oxygen for
the treatment of diabetic foot ulcers: A systematic review. Eur J
Vasc Endovasc Surg. 47:647–655. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Resanovic I and Gluvic Z: Early Effects of
hyperbaric oxygen on inducible nitric oxide synthase
activity/expression in lymphocytes of type 1 diabetes patients: A
prospective pilot study. Int J Endocrinol. 2019:23285052019.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Thorens B: GLUT2, glucose sensing and
glucose homeostasis. Diabetologia. 58:221–232. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Párrizas M, Maestro MA, Boj SF, Paniagua
A, Casamitjana R, Gomis R, Rivera F and Ferrer J: Hepatic nuclear
factor 1-alpha directs nucleosomal hyperacetylation to its
tissue-specific transcriptional targets. Mol Cell Biol.
21:3234–3243. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ishii S, Kamegai J, Tamura H, Shimizu T,
Sugihara H and Oikawa S: Role of ghrelin in streptozotocin-induced
diabetic hyperphagia. Endocrinology. 143:4934–4937. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Nowak N, Hohendorff J, Solecka I, Szopa M,
Skupien J, Kiec-Wilk B, Mlynarski W and Malecki MT: Circulating
ghrelin level is higher in HNF1A-MODY and GCK-MODY than in
polygenic forms of diabetes mellitus. Endocrine. 50:643–649. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Gutierrez JA, Willency JA, Knierman MD,
Coskun T, Solenberg PJ, Perkins DR, Higgs RE and Hale JE: From
ghrelin to ghrelin's O-acyl transferase. Methods Enzymol.
514:129–146. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Gahete MD, Córdoba-Chacón J, Salvatori R,
Castaño JP, Kineman RD and Luque RM: Metabolic regulation of
ghrelin O-acyl transferase (GOAT) expression in the mouse
hypothalamus, pituitary, and stomach. Mol Cell Endocrinol.
317:154–160. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Hougland JL: Ghrelin octanoylation by
ghrelin O-acyltransferase: Unique protein biochemistry underlying
metabolic signaling. Biochem Soc Trans. 47:169–178. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Adriaenssens AE, Svendsen B, Lam BY, Yeo
GS, Holst JJ, Reimann F and Gribble FM: Transcriptomic profiling of
pancreatic alpha, beta and delta cell populations identifies delta
cells as a principal target for ghrelin in mouse islets.
Diabetologia. 59:2156–2165. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Chuang JC, Sakata I, Kohno D, Perello M,
Osborne-Lawrence S, Repa JJ and Zigman JM: Ghrelin directly
stimulates glucagon secretion from pancreatic alpha-cells. Mol
Endocrinol. 25:1600–1611. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Tong J, Prigeon RL, Davis HW, Bidlingmaier
M, Kahn SE, Cummings DE, Tschöp MH and D'Alessio D: Ghrelin
suppresses glucose-stimulated insulin secretion and deteriorates
glucose tolerance in healthy humans. Diabetes. 59:2145–2151. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Dezaki K, Hosoda H, Kakei M, Hashiguchi S,
Watanabe M, Kangawa K and Yada T: Endogenous ghrelin in pancreatic
islets restricts insulin release by attenuating Ca2+
signaling in beta-cells: Implication in the glycemic control in
rodents. Diabetes. 53:3142–3151. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Brial F, Lussier CR, Belleville K, Sarret
P and Boudreau F: Ghrelin inhibition restores glucose homeostasis
in hepatocyte nuclear factor-1α (MODY3)-Deficient Mice. Diabetes.
64:3314–3320. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
McFarlane MR, Brown MS, Goldstein JL and
Zhao TJ: Induced ablation of ghrelin cells in adult mice does not
decrease food intake, body weight, or response to high-fat diet.
Cell Metab. 20:54–60. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Kleinridders A, Ferris HA, Cai W and Kahn
CR: Insulin action in brain regulates systemic metabolism and brain
function. Diabetes. 63:2232–2243. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Özcan B, Neggers SJ, Miller AR, Yang HC,
Lucaites V, Abribat T, Allas S, Huisman M, Visser JA, Themmen AP,
et al: Does des-acyl ghrelin improve glycemic control in obese
diabetic subjects by decreasing acylated ghrelin levels? Eur J
Endocrinol. 170:799–807. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Brereton MF, Iberl M, Shimomura K, Zhang
Q, Adriaenssens AE, Proks P, Spiliotis II, Dace W, Mattis KK,
Ramracheya R, et al: Reversible changes in pancreatic islet
structure and function produced by elevated blood glucose. Nat
Commun. 5:46392014. View Article : Google Scholar : PubMed/NCBI
|