Introduction
It is currently estimated that there are >70
million people with various degrees of chronic kidney disease (CKD)
worldwide, affects 8–16% of the population worldwide (1–5).
Renal fibrosis is the final outcome in the majority of CKD cases,
despite the different etiologies (6). Renal tubular epithelial-mesenchymal
transition (EMT) and the continuous production of extracellular
matrix (ECM) proteins have been discovered to be important
characteristics of renal fibrosis (7,8). In
fact, it has been reported that 5% of myofibroblasts were derived
from tubular EMT in the fibrotic kidney (9). Moreover, renal tubular EMT was
identified to promote interstitial fibrosis by secreting
inflammatory factor, such as transforming growth factor-β1
(TGF-β1), connective tissue growth factor (CTGF), and ECM proteins
(10). In addition, ECM
aggregation was demonstrated to lead to widespread tissue fibrosis,
promoting the destruction of the renal parenchyma and eventually
leading to renal failure (7).
Nevertheless, despite the advancements made in recent decades to
understand the process of fibrogenesis, the underlying mechanisms
remain unknown.
Sox9 is a member of the highly conserved
transcription factor family defined by the similarity of the high
mobility group DNA-binding domain of SRY (11). Mutations in and around the Sox9
gene have been identified in numerous different types of disease,
including severe skeletal malformation syndrome, campomelic
dysplasia and XY sex reversal in males (12,13).
Previous studies have also demonstrated that Sox9 regulated EMT
during embryonic development and the metastasis of epithelial
tumors (14–20). During embryonic development,
multipotent neural crest cells become migratory, while in EMT,
which is induced by Sox9, these cells were discovered to
differentiate into different types of tissue, including the adrenal
medulla, cartilage, skin pigment cells and cardiac tissue (14). Moreover, Sox9 has been identified
to serve a similar role in the diseased state, particularly in
numerous types of tumor; for example, in colon (15) and breast cancer cells (16), upregulated expression levels of
Sox9 promoted EMT and cancer cell migration. In addition, previous
studies have suggested that Sox9 may serve a critical role in ECM
aggregation (17–20). These observations supported a
fundamental role for Sox9 in the processes of EMT and ECM
aggregation.
Transcriptome analysis using RNA-sequencing
(RNA-seq) technology has revealed that the expression levels of
Sox9 mRNA were markedly upregulated in the obstructed kidneys of an
unilateral ureteral obstruction (UUO) mouse model (21). In addition, the microarray
detection of human sclerotic glomeruli captured using laser
microscopy discovered that the expression levels of Sox9 were
markedly upregulated, as well as those of collagen α-1(I) chain and
collagen α-2(I) chain (22).
Furthermore, in mesangial cells, Sox9, as a downstream target of
transforming growth factor (TGF)-β1, activated collagen α-2(IV)
chain transcription (23). Based
on these previous studies, the present study hypothesized that Sox9
may increase the susceptibility of renal tubular EMT and ECM
aggregation.
In order to determine the role and potential
mechanism of Sox9 in renal fibrosis, the present study used a UUO
rat model and revealed that Sox9 expression levels were
significantly upregulated in renal tubular epithelial cells
following obstructive injury. In addition, the overexpression of
Sox9 in NRK-52E cells promoted tubular EMT and ECM aggregation, and
significantly increased the phosphorylation levels of AKT.
Conversely, blocking the PI3K/AKT signaling pathway alleviated the
phenotype of fibrosis in NRK-52E cells induced by the
overexpression of Sox9. Thus, the present study identified Sox9 as
an important regulator of renal fibrosis.
Materials and methods
Animal model
All experiments were approved by the Ethics
Committee for the Use of Experimental Animals at Jinling Hospital
(Nanjing, China). Male Wister rats (age, 5–6 weeks; weight, 180–200
g) were obtained from the Experimental Animal Center of Jinling
Hospital. Rats were housed in the animal facility, which was
maintained at 20–25°C with 55% relative humidity and an automatic
12-h light/dark cycle. All rats received a standard laboratory diet
and tap water ad libitum. A total of 24 rats was used. To
investigate Sox9 expression levels following obstructive injury,
rats were randomly divided into four groups (6/group): i) Sham
control; ii) 3 days after UUO; iii) 7 days after UUO; and iv) 14
days after UUO. Rats were anesthetized with an intraperitoneal
injection of 5% chloral hydrate (400 mg/kg), and vital signs (heart
rate, respiratory rate and depth, and body temperature) and
reflexes (toe and tail pinch) were monitored to ensure the rats
were fully anesthetized. For rats in the UUO groups, the left
ureter was exposed using a midline incision and completely ligated
15-mm below the renal pelvis using 4.0 silk. The same procedure was
performed in the sham control group, but without ureteral ligation.
Rats were sacrificed by cervical dislocation following anesthesia
with an intraperitoneal injection of 5% chloral hydrate (500 mg/kg)
at 3, 7 or 14 days following UUO and 14 days following sham
operation, and the kidney tissues were harvested and stored at
−80°C for subsequent experiments.
Cell culture and treatments
The rat NRK-52E proximal tubular epithelial cell
line was purchased from The Cell Bank of Type Culture Collection of
the Chinese Academy of Sciences. Cells were cultured in high
glucose DMEM (Gibco; Thermo Fisher Scientific, Inc.), supplemented
with 8% FBS (Gibco; Thermo Fisher Scientific, Inc.) and
penicillin/streptomycin (penicillin 100 U/ml, streptomycin 0.1
mg/ml; Gibco; Thermo Fisher Scientific, Inc.), and maintained at
37°C in 5% CO2 and 95% air.
Cells were cultured at 37°C in six-well culture
plates until 60–70% confluence, then incubated with serum-free DMEM
at 37°C for 12 h. Following the incubation, NRK-52E cells were
treated at 37°C with 10 ng/ml recombinant TGF-β1 (PeproTech, Inc.)
or PI3K inhibitors, LY29002 (10 µM; Selleck Chemicals) or
wortmannin (1 µM; Selleck Chemicals), for 24 or 48 h and
subsequently harvested for further analysis.
Lentivirus transduction
The Sox9 overexpression (LV-Sox9) and negative
control (NC; empty vector) lentiviruses were constructed by
Shanghai GeneChem Co., Ltd., and lentiviral infections were
performed according to the manufacturer's instructions. Briefly,
growing cells were seeded into 6-well plates and incubated at 37°C
for 48 h. Then, 20 µl polybrene (5 mg/ml) and 50 µl virus (storage
concentration, 6.0×108 TU/ml for Sox9 overexpression,
6.2×108 TU/ml for negative control) was added to 20 ml
high glucose DMEM with 8% FBS and mixed gently. Cells were cultured
with the mixture medium (1 ml/well for 6-well plates) at 37°C for
24 h. At 24 h after infection, the transfection mixture was
replaced with high glucose DMEM with 8% FBS and cultured for a
further 48 h at 37°C. The transfection efficiency was verified
using reverse transcription-quantitative PCR (RT-qPCR) and western
blotting in virus-infected cells that were GFP-positive 72 h
following transduction. The infected cells expressing green
fluorescent protein (GFP) were observed under a fluorescence
microscope (magnification, ×400 magnification). Puromycin (1 µg/ml,
Shanghai GeneChem Co., Ltd.) was used to select for stably
transfected cell lines.
RT-qPCR
Total RNA was extracted from NRK-52E cells and
kidney tissue using TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.), according to the manufacturer's protocol.
Total RNA was reverse transcribed into cDNA using a PrimeScript™ RT
Master Mix (cat. no. RR036A; Takara Bio, Inc.), using the following
conditions: 37°C for 15 min, followed by 85°C for 5 sec. qPCR was
subsequently performed using SYBR® Premix Ex Taq™ (cat.
no. RR420A; Takara Bio, Inc.) according to the manufacturer's
protocol, using the following conditions: 94°C for 5 min, and
followed by 30 cycles of 94°C for 30 sec and 58–61°C for 3 sec. The
primer sequences used for the qPCR are presented in Table I. The relative expression levels of
the mRNAs were quantified using the 2−ΔΔCq method
(24) and normalized to GAPDH.
 | Table I.Primers used for reverse
transcription-quantitative PCR. |
Table I.
Primers used for reverse
transcription-quantitative PCR.
| Gene | Primer sequence
(5′→3′) |
|---|
| GAPDH | F:
TCTCTTGTGACAAAGTGGACAT |
|
| R:
CCCATTCTCAGCCTTGACTGT |
| Sox9 | F:
AGCACAAGAAAGACCACCCC |
|
| R:
CGCCTTGAAGATGGCGTTAG |
| E-cadherin | F:
CACCGTGGTTTCTTGCGTTT |
|
| R:
TCAGGTTCACTGGCATGCTT |
| Vimentin | F:
CAGTCACTCACCTGCGAAGT |
|
| R:
AGTTAGCAGCTTCAAGGGCA |
| Plasminogen 1 | F:
CGTCTTCCTCCACAGCCATT |
| activator
inhibitor | R:
GTTGGATTGTGCCGAACCAC |
| Collagen 1 | F:
ACATGCCGTGACCTCAAGAT |
|
| R:
ATGTCCATTCCGAATTCCTG |
| Fibronectin | F:
TGGAGAGACAGGAGGAAATAGC |
|
| R:
CAGTGACAGCATACAGGGTGAT |
| Connective
tissue | F:
TGGCTTGCTCAGGGTAACTG |
| growth factor | R:
AACTGCCTCCCAAACCAGTC |
| β-catenin | F:
ATCATTCTGGCCAGTGGTGG |
|
| R:
GACAGCACCTTCAGCACTCT |
| Frizzled-5 | F:
ACTCGCTACGAGGCTTTGTC |
|
| R:
CTTAGTGCCACCCTGCTTGA |
| c-Myc | F:
GCTACGTCCTTCTCCCCAAG |
|
| R:
GGTCTCATCGTCAGGATCGC |
| Transcription
4 | F:
ATCACAGCAGTGACCCTTGG |
| factor | R:
CCGAGGAGTGCGATGGATAG |
Western blotting
Western blotting was performed to analyze the
protein expression levels. Briefly, total protein was extracted
from kidney tissue or harvested cells using 400 µl tissue and cell
lysis buffer (RIPA lysis buffer; Beyotime Institute of
Biotechnology), supplemented with phosphatase inhibitors and a
protease inhibitor (Beyotime Institute of Biotechnology) on ice.
Tissue and cell lysates were centrifuged at 4°C for 5 min at 12,000
× g, total protein was quantified using a bicinchoninic acid
protein assay kit (Beyotime Institute of Biotechnology) and 40 µg
protein/lane was separated via 8% SDS-PAGE. The separated proteins
were subsequently transferred onto PVDF membranes and blocked with
5% skimmed milk at room temperature for 1 h. The membranes were
then incubated overnight at 4°C with the following primary
antibodies: Anti-Sox9 (1:5,000; cat. no. ab185966; Abcam),
anti-E-cadherin (1:1,000; cat. no. ab76055; Abcam), anti-Vimentin
(1:5,000; cat. no. ab92547; Abcam), anti-plasminogen activator
inhibitor 1 (PAI-1; 1:2,000; cat. no. ab66705; Abcam),
anti-collagen 1 (COL1; 1:1,000; cat. no. AF7001; Affinity
Biosciences), anti-fibronectin (1:5,000; cat. no. ab45688; Abcam),
anti-β-catenin (1:5,000; cat. no. ab32572; Abcam), anti-AKT
(1:2,000; cat. no. AF6261; Affinity Biosciences),
anti-phosphorylated (p)-AKT (1:5,000; cat. no. ab81283; Abcam) and
anti-β-actin (1:10,000; cat. no. bsm-33036M; BIOSS). Following the
primary antibody incubation, the membranes were incubated with
either an anti-mouse IgM horseradish peroxidase (HRP)-conjugated
secondary antibody (1:5,000; cat. no. bs-0368R-HRP; BIOSS) or an
anti-rabbit IgG HRP-conjugated secondary antibody (1:5,000; cat.
no. bs-0295G-HRP; BIOSS) at room temperature for 2 h. Protein bands
were visualized using an enhanced chemiluminescence reagent
(Beyotime Institute of Biotechnology) and the FluorChem M system
(ProteinSimple). ImageJ software (version 1.5.1; National
Institutes of Health) was used to perform densitometric analysis.
The expression levels were analyzed and normalized to β-actin, the
internal loading control.
Histology and
immunohistochemistry
The fresh kidney tissue was fixed in 4%
paraformaldehyde at 4°C overnight, subsequently embedded in
paraffin and sliced into sections (thickness, 5 µm). For
histological analysis, the kidney sections were deparaffinized and
rehydrated in a descending ethanol series (100 and 75% ethanol at
room temperature for 5 min). The sections were subsequently stained
with Masson's trichrome stain kit (cat. no. BSBA-4079A; OriGene
Technologies, Inc.). Briefly, the sections were stained with
hematoxylin iron solution for 3 min at room temperature, followed
by 0.5% acid alcohol, and washed in water once more. Samples were
stained with Ponceau S solution for 5 min at room temperature, and
subsequently rinsed in water. Finally, samples were stained using
phosphomolybdic acid for 1 min at room temperature, followed by
aniline blue solution for 3 min at room temperature, and washed
with 1% acetic acid solution at room temperature. Masson staining
evaluated kidney histological changes, such as the fibrotic area,
indicated by blue staining, and the degree of tubular atrophy. The
samples were examined by light microscopy (magnification,
×200).
Immunohistochemistry was performed using the
paraffin-embedded kidney samples. The paraffin sections were
subsequently de-waxed in water, and incubated with 3% hydrogen
peroxide at room temperature for 10 min. Subsequently, the sections
were blocked with 5% goat serum (cat. no. 16210064; Thermo Fisher
Scientific, Inc.) in PBS at room temperature for 1 h and then
incubated overnight at 4°C with the anti-Sox9 primary antibody
(1:2,000). Following the primary antibody incubation, the sections
were incubated with an HRP-conjugated goat anti-rabbit secondary
antibody (1:5,000; cat. no. bs-40295G-HRP; BIOSS) at room
temperature for 2 h. The slides were visualized using
3,3′-diaminobenzidine (Beyotime Institute of Biotechnology) and
counterstained with hematoxylin at room temperature for 2 min. The
samples were examined by light microscopy (magnification,
×400).
Immunofluorescence staining
Cells at 80% confluence were cultured on coverslips
were fixed with 4% paraformaldehyde at room temperature for 15 min
and blocked with 5% goat serum (Gibco; Thermo Fisher Scientific,
Inc.) in PBS at room temperature for 30 min. The coverslips were
then incubated overnight at 4°C with the following primary
antibodies: Anti-COL1 (1:1,500) and anti-fibronectin (1:500).
Following the primary antibody incubation, the coverslips were
washed 3 times with PBS and incubated with a goat anti-rabbit IgG
antibody labeled with Alexa Fluor® 546 (4 µg/ml; cat.
no. A-11035; Invitrogen; Thermo Fisher Scientific, Inc.) for 1 h at
room temperature in the dark. The nuclei were counterstained with
DAPI (Thermo Fisher Scientific, Inc.) at room temperature for 5
min. Stained slides were visualized using a fluorescence microscope
(magnification, ×400) (Imager A2; Zeiss GmbH).
RNA-seq and bioinformatics
analysis
Total RNA was extracted using TRIzol®
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) from SV-Sox9-
or NC-transfected NRK-52E cells and sequenced by CapitalBio
Technology Inc.. The mRNA profiling was performed using the
Illumina HiSeq 2500 platform (Illumina, Inc.). The lists of
differentially expressed genes were analyzed via Cuffdiff software
(version 2; cufflinks.cbcb.umd.edu) (25). The differentially expressed genes
(|fold-change|>2 and P<0.05) were subjected to functional
term and signaling pathway enrichment analysis using Gene Ontology
(GO; geneontology.org) (26) and Kyoto Encyclopedia of Genes and
Genomes (KEGG) (27) databases,
respectively.
Statistical analysis
Statistical analysis was performed using SPSS 20
software (IBM Corp.) and data are presented as the mean ± SD of ≥3
independent experiments. A Student's t-test or a one-way ANOVA,
followed by a Tukey's post-hoc test was used to determine the
statistical significances between 2 and >2 groups, respectively.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Expression levels of Sox9 are
upregulated in the tubules of UUO model rats
The obstructive nephropathy induced after UUO is a
classic model of renal interstitial fibrosis, characterized by
tubular EMT and high levels of ECM aggregation (28). The expression levels of E-cadherin
were downregulated and those of Vimentin, COL1 and fibronectin were
upregulated in a time-dependent manner in UUO model rats, which
indicated that the model of renal fibrosis had been successfully
established (Fig. 1A and B).
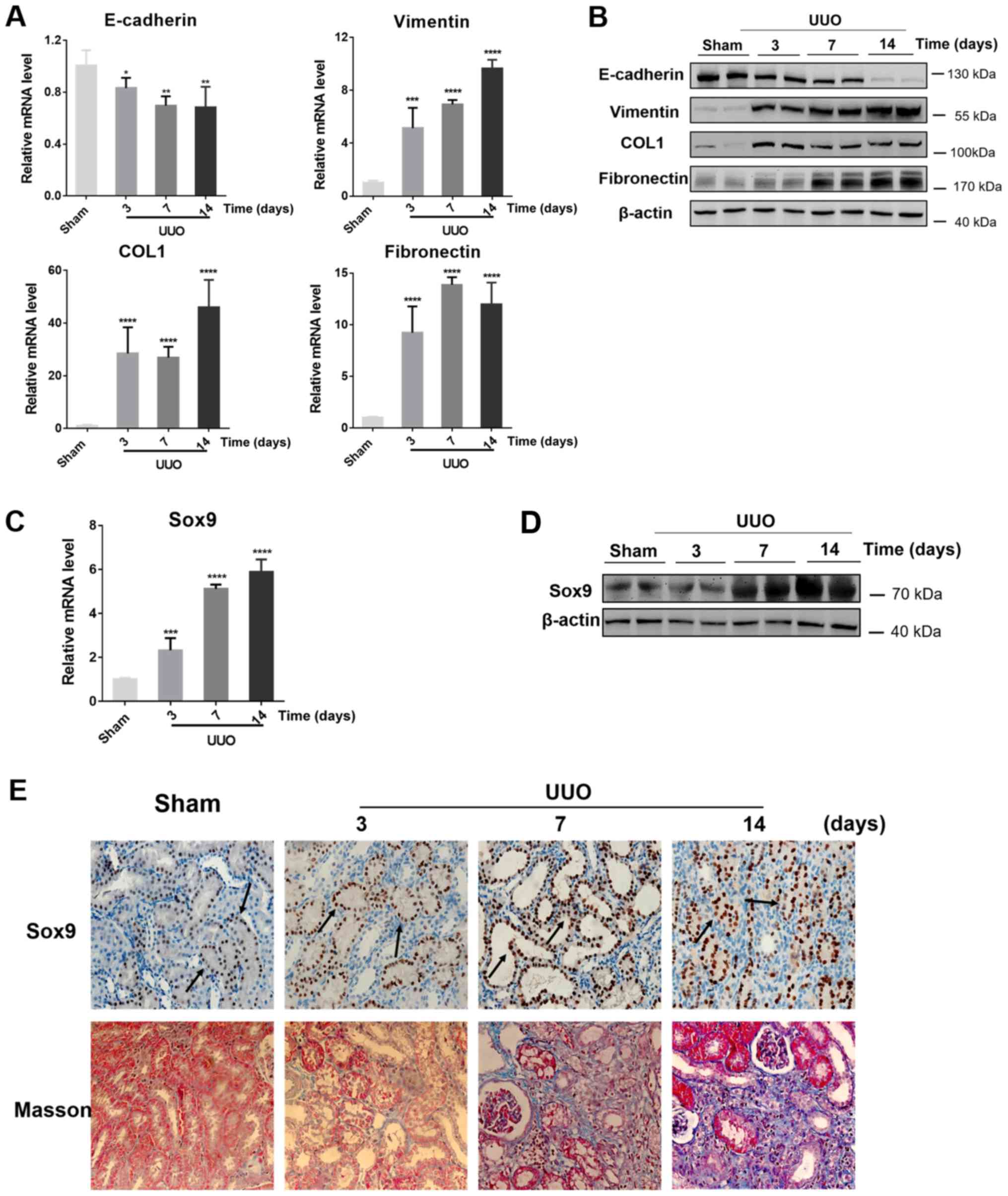 | Figure 1.Expression levels of EMT and fibrotic
markers and Sox9 in UUO-induced kidney fibrosis. (A) mRNA and (B)
protein expression levels of E-cadherin, Vimentin, fibronectin and
COL1 in the sham control and the obstructed kidneys following UUO
for 3, 7 or 14 days. (C) mRNA and (D) protein expression levels of
Sox9 in the obstructed kidneys in the sham control and the
obstructed kidneys following UUO for 3, 7 or 14 days. n=6
rats/group, western blotting experiments were repeated once. (E)
Immunohistochemical staining revealed the expression levels and
localization of Sox9 protein in the sham control and the obstructed
kidneys following UUO for 3, 7 or 14 days. Masson's trichrome
staining was used to identify areas of fibrosis (blue staining) in
the sham control and the obstructed kidneys following UUO for 3, 7
or 14 days. Arrows indicate positive staining of Sox9.
Magnification, ×400 for immunohistochemical staining, ×200 for
Masson's trichrome staining. Data are presented as the mean ± SD.
*P<0.05, **P<0.01, ***P<0.001, ****P<0.0001 vs. sham.
UUO, unilateral ureteral obstruction; COL1, collagen 1. |
To determine whether the expression levels of Sox9
in renal fibrosis were altered, the expression levels of Sox9 in
the UUO model rats were analyzed. The experimental data revealed
that the mRNA expression levels of Sox9 were upregulated by >2-,
4- and 5-fold at 3, 7 and 14 days following UUO, respectively,
compared with the sham group (Fig.
1C). In addition, western blotting demonstrated that the
protein expression levels of Sox9 were upregulated in the
obstructed kidneys at 7 and 14 days following UUO compared with the
sham group (Fig. 1D).
To further determine the type of cell in which Sox9
protein expression levels were upregulated, Sox9 protein expression
levels were investigated using immunohistochemical staining in the
fibrotic kidneys of the UUO model rats. Sox9 protein expression
levels were markedly upregulated in the obstructed kidneys by 7 and
14 days following UUO compared with the sham group (Fig. 1E), which were positively associated
with the severity of kidney fibrosis stained with Masson's staining
Notably, Sox9 protein expression levels were identified to be
markedly upregulated in renal tubular epithelial cells (black
arrows; Fig. 1E).
Overexpression of Sox9 in tubular
epithelial cells induces renal tubular EMT and ECM aggregation
As Sox9 protein was identified to be abundantly
expressed in renal tubular epithelial cells following obstructive
injury, NRK-52E cells were transfected with LV-Sox9 to investigate
the role of Sox9; the transfection efficiency was revealed to be
90% in NRK-52E cells infected with the lentivirus for 72 h
following the observation with a fluorescence microscope (Fig. 2A). The transfection efficiency was
also analyzed using RT-qPCR and western blotting; the mRNA and
protein expression levels of Sox9 were significantly upregulated in
LV-Sox9-transfected cells compared with the NC-transfected cells
(Fig. 2B and C).
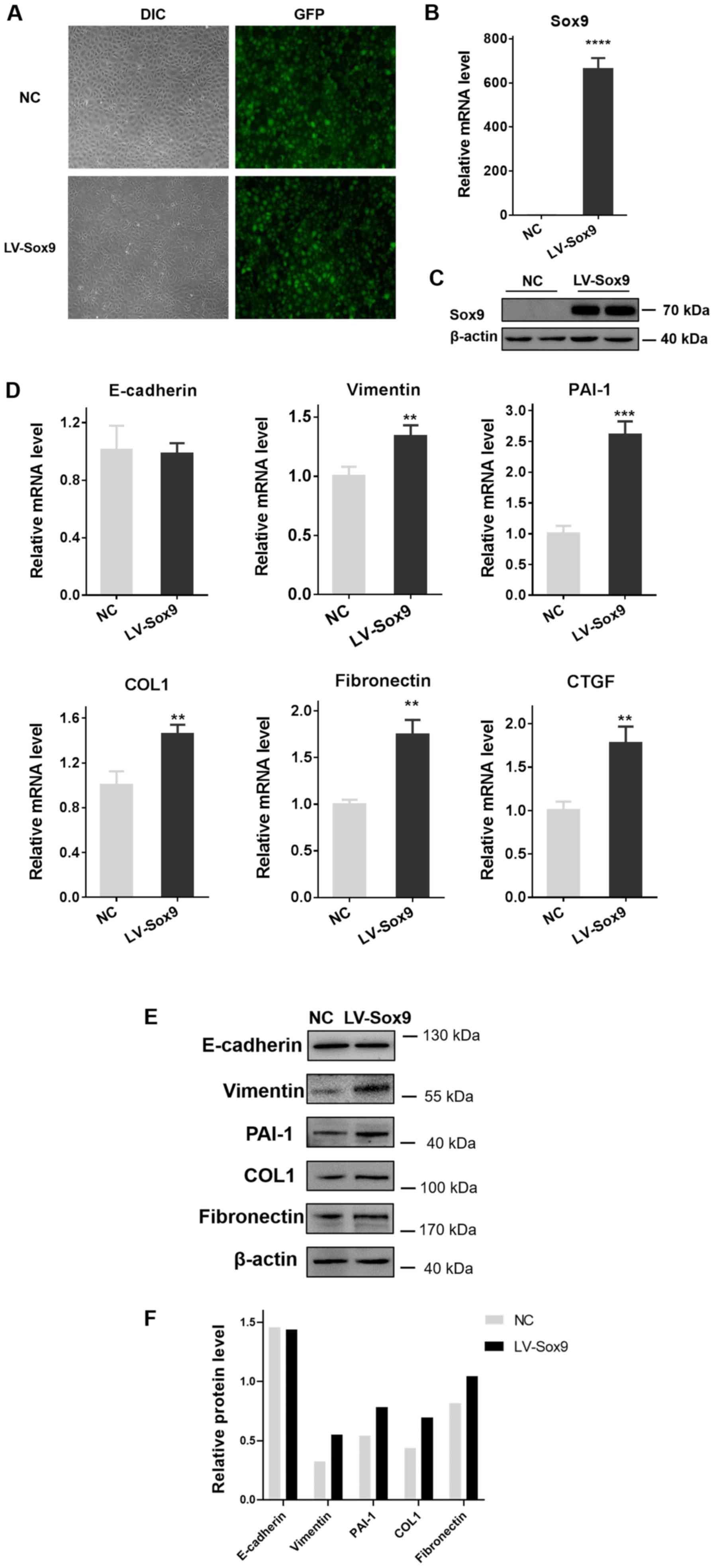 | Figure 2.Overexpression of Sox9 in tubular
epithelial cells induces renal tubular epithelial-mesenchymal
transition and extracellular matrix aggregation. NRK-52E cells were
transfected with LV-Sox9 or NC. (A) Transfection efficiency was
observed under a fluorescence microscope. Magnification, ×400. The
transfection efficiency of LV-Sox9 was also analyzed using (B)
RT-qPCR and (C) western blotting. (D) RT-qPCR was used to analyze
the expression levels of E-cadherin, Vimentin, PAI-1, COL1,
fibronectin and CTGF in tubular epithelial cells following the
overexpression of Sox9. n=3/group. Overexpression of Sox9 in
tubular epithelial cells induces renal tubular
epithelial-mesenchymal transition and extracellular matrix
aggregation. NRK-52E cells were transfected with LV-Sox9 or NC. (E)
Western blotting was used to analyze the expression levels of
E-cadherin, Vimentin, PAI-1, COL1 and fibronectin in tubular
epithelial cells following the overexpression of Sox9. n=3/group,
western blotting experiments were repeated once. (F)
Semi-quantification of the expression levels presented in part (E).
Data are presented as the mean ± SD. **P<0.01, ***P<0.001,
****P<0.0001 vs. NC. LV-Sox9, stabilized Sox9 expressing
lentivirus; NC, negative control; DIC, differential interference
contrast microscope; PAI-1, plasminogen activator inhibitor 1;
COL1, collagen 1; CTGF, connective tissue growth factor; RT-qPCR,
reverse transcription-quantitative PCR. |
The overexpression of Sox9 significantly upregulated
the mRNA expression levels of Vimentin, PAI-1, COL1, fibronectin
and connective tissue growth factor (CTGF) compared with the
NC-transfected cells, which is consistent with EMT and ECM
production (7–9) (Fig.
2D). In addition, compared with the NC-transfected cells, the
LV-Sox9-transfected cells demonstrated upregulated protein
expression levels of Vimentin, PAI-1, COL1 and fibronectin in the
tubular epithelial cells (Fig. 2E and
F). However, there was no effect on the expression levels of
E-cadherin compared with NC-transfected cells.
Sox9 aggravates tubular EMT and ECM
aggregation induced by TGF-β1 in tubular epithelial cells
To determine whether the overexpression of Sox9
aggravated the renal tubular EMT and ECM aggregation induced by
TGF-β1, LV-Sox9-transfected NRK-52E cells were treated with TGF-β1.
RT-qPCR analysis revealed that the overexpression of Sox9
potentiated the TGF-β1-induced downregulation of E-cadherin
expression levels, and upregulation of Vimentin, PAI-1, COL1,
fibronectin and CTGF expression levels (Fig. 3A). Similarly, western blotting
revealed that the overexpression of Sox9 enhanced the
TGF-β1-induced downregulation of E-cadherin protein expression
levels and upregulation of Vimentin, PAI-1, COL1 and fibronectin
protein expression levels (Fig. 3B and
C). Compared with the NRK-52E cells without TGF-β1 treatment,
the cells treated with TGF-β1 had upregulated expression levels of
Vimentin, PAI-1, COL1 and fibronectin and downregulated E-cadherin
expression levels. Immunofluorescence staining to determine COL1
and fibronectin expression levels following the treatment with
TGF-β1 revealed similar results to the RT-qPCR and western blotting
data (Fig. 3C). Therefore, these
findings suggested that the overexpression of Sox9 may potentiate
renal tubular EMT and ECM aggregation induced by TGF-β1 in tubular
epithelial cells.
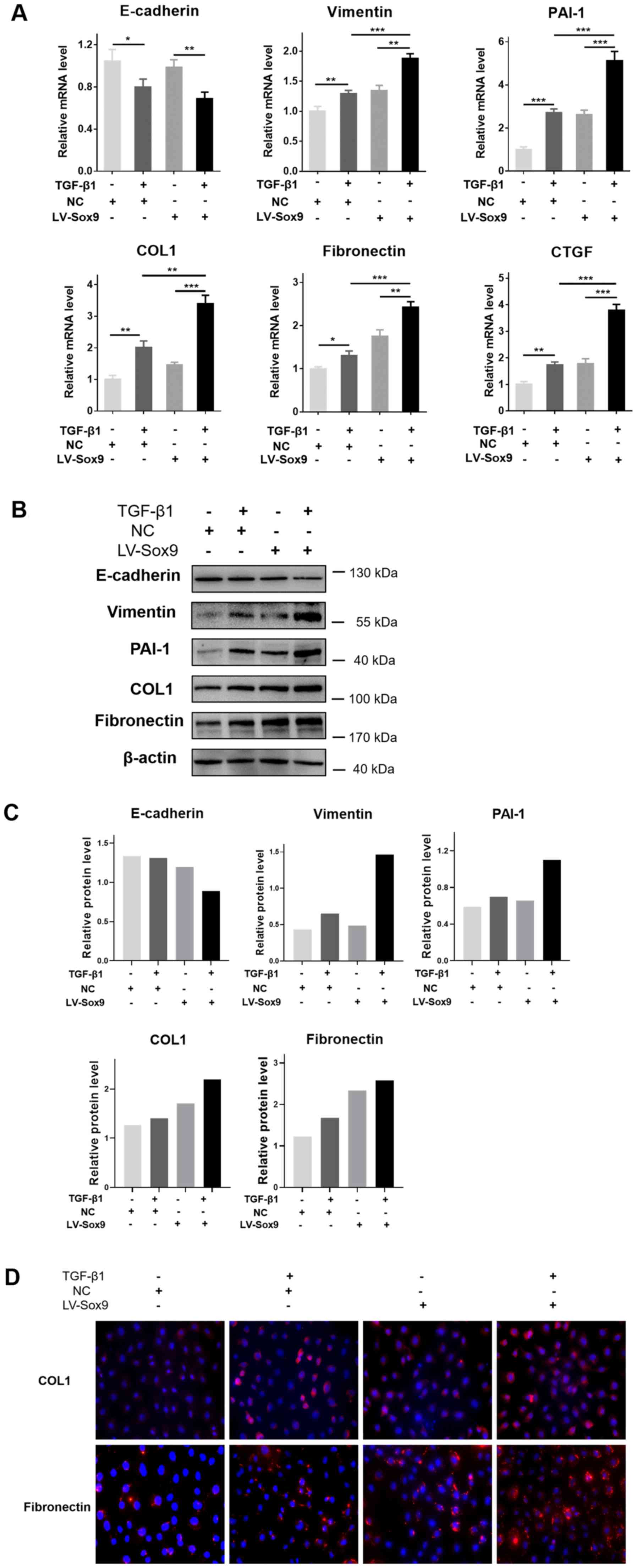 | Figure 3.Sox9 potentiates the tubular
epithelial-mesenchymal transition and extracellular matrix
aggregation induced by TGF-β1 in tubular epithelial cells. NRK-52E
cells were transfected with LV-Sox9 or NC and then treated with
TGF-β1 (10 ng/ml) for various periods of time as indicated. (A)
Reverse transcription-quantitative PCR was used to analyze the
expression levels of E-cadherin, Vimentin, PAI-1, COL1, fibronectin
and CTGF in LV-Sox9- or NC-transfected NRK-52E cells treated with
TGF-β1 for 24 h. (B) Western blotting was used to analyze the
expression levels of E-cadherin, Vimentin, PAI-1, COL1 and
fibronectin in LV-Sox9- or NC-transfected NRK-52E cells treated
with TGF-β1 for 48 h. n=3/group, western blotting experiments were
repeated once. Sox9 potentiates the tubular epithelial-mesenchymal
transition and extracellular matrix aggregation induced by TGF-β1
in tubular epithelial cells. NRK-52E cells were transfected with
LV-Sox9 or NC and then treated with TGF-β1 (10 ng/ml) for various
periods of time as indicated. (C) Semi-quantification of the
expression levels presented in part (B). (D) Immunofluorescence
staining was used to analyze the protein expression levels of COL1
and fibronectin in the LV-Sox9- or NC-transfected NRK-52E cells
treated with TGF-β1 for 48 h. Magnification, ×400. Data are
presented as the mean ± SD. *P<0.05, **P<0.01, ***P<0.001.
LV-Sox9, stabilized Sox9 expressing lentivirus; NC, negative
control; PAI-1, plasminogen activator inhibitor 1; COL1, collagen
1; CTGF, connective tissue growth factor; TGF-β1, transforming
growth factor β1. |
PI3K/AKT signaling pathway is
activated following Sox9 overexpression in tubular epithelial
cells
An increasing number of studies have revealed that
Sox9 acts primarily through the Wnt/β-catenin signaling pathway
(29–32). Therefore, to identify molecular
responders of Sox9, it was investigated whether Sox9 could activate
Wnt signaling molecules in NRK-52E cells. The results revealed that
no statistical differences were observed in the mRNA and protein
expression levels of β-catenin, a crucial mediator of canonical Wnt
signaling, between NC- and LV-Sox9-transfected cells (Fig. S1A and S1B). In addition, the overexpression of
Sox9 did not affect the mRNA expression levels of Wnt receptors,
such as Frizzled-5 (FZD5), or their target genes, including c-Myc
and transcription factor 4 (TCF4; Fig. S1C).
To further determine how Sox9 may regulate renal
tubular EMT and ECM aggregation, mRNA transcriptome analysis using
RNA-seq technology was performed in NRK-52E cells overexpressing
Sox9. A total of 317 differentially expressed genes were identified
(|fold-change|>2 and P<0.05), including 174 upregulated and
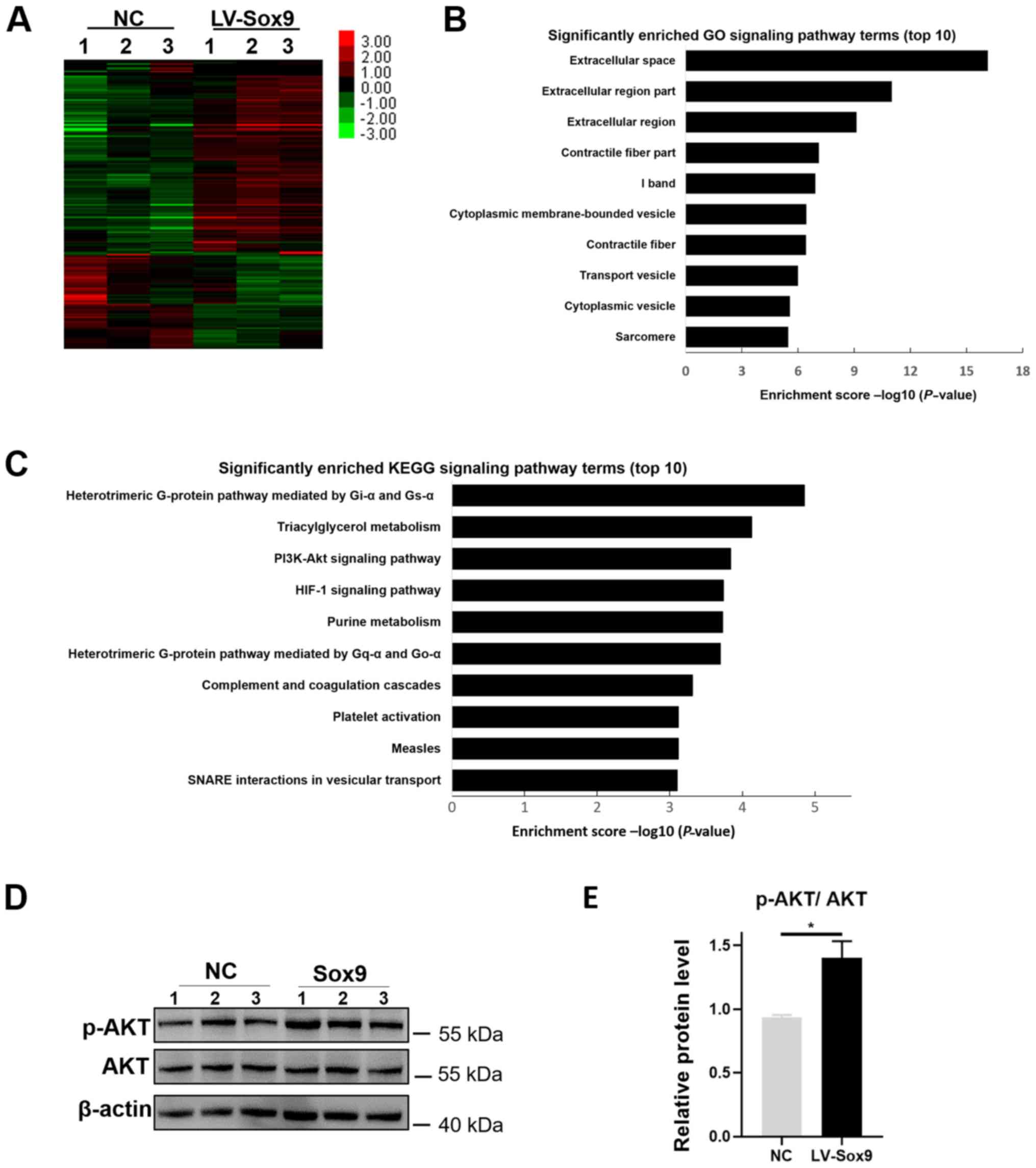
143 downregulated genes (Fig. 4A).
Subsequently, GO functional term and KEGG signaling pathway
enrichment analysis were performed to identify the potential
physiological functions of the differentially expressed mRNAs. GO
analysis discovered that the differentially expressed genes were
highly associated with ‘Extracellular space’, ‘Extracellular region
part’, ‘Extracellular region’ and ‘Contractile fiber part’
(Fig. 4B). KEGG analysis also
identified that the differentially expressed genes was involved in
the ‘PI3K-Akt signaling pathway’ and the ‘HIF-1 signaling pathway’
(Fig. 4C). Previous studies have
reported that in chondrocytes and human lung fibroblast cell line,
the knockdown of Sox9 downregulated the phosphorylation levels of
AKT (33,34). Therefore, it was investigated
whether Sox9 regulated AKT activity in NRK-52E cells. The
overexpression of Sox9 did not upregulate the protein expression
levels of total AKT in NRK-52E cells; however, the protein
expression levels of p-AKT were upregulated in LV-Sox9-transfected
cells compared with NC-transfected cells (Fig. 4D).
Blockade of PI3K/AKT signaling pathway
with PI3K inhibitors alleviates the tubular EMT and ECM aggregation
caused by the overexpression of Sox9 in tubular epithelial
cells
The inhibitors of PI3K, LY29002 and wortmannin, were
used to investigate whether the Sox9-induced renal tubular EMT and
ECM aggregation was caused by activation of the PI3K/AKT signaling
pathway. The protein and/or mRNA expression levels of Vimentin,
PAI-1, COL1 and fibronectin were significantly downregulated in
cells overexpressing Sox9 following the treatment with LY294002 or
wortmannin compared with the LV-Sox9-transfected cells alone
(Fig. 5A-C). In addition, the
treatment of LV-Sox9-transfected cells with wortmannin or LY29002
downregulated the protein expression levels of p-AKT compared with
LV-Sox9-transfected cells (Fig. 5B and
C). These results suggested that the overexpression of Sox9 may
induce tubular epithelial EMT and ECM aggregation, at least in
part, through activation of the PI3K/AKT signaling pathway.
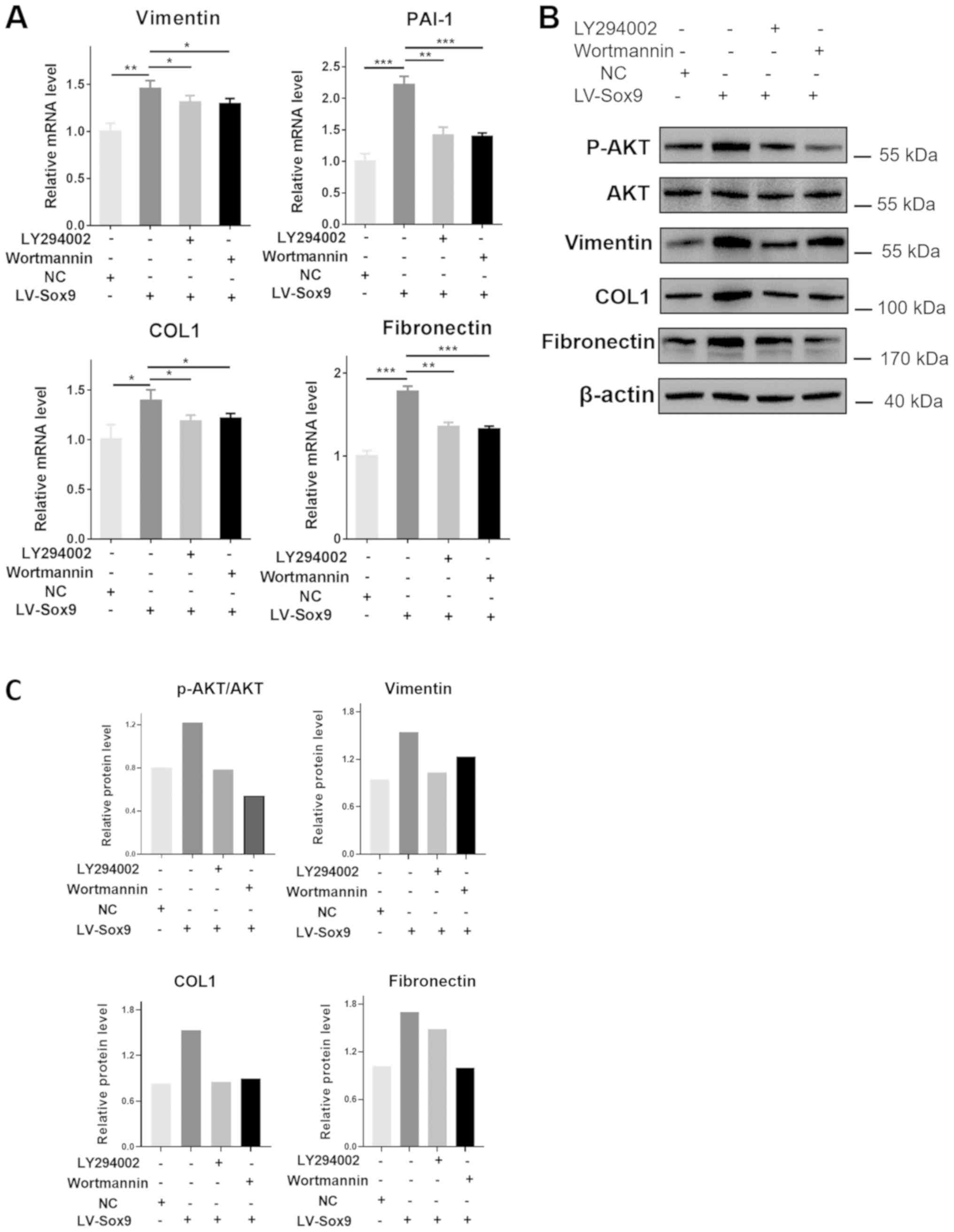 | Figure 5.Blockade of PI3K/AKT signaling
pathway by PI3K inhibitors alleviates tubular
epithelial-mesenchymal transition and extracellular matrix
aggregation induced by the overexpression of Sox9 in tubular
epithelial cells. NRK-52E cells transfected with LV-Sox9 or NC were
treated with 10 µM LY29002 or 1 µM wortmannin for 48 h. (A) Reverse
transcription-quantitative PCR was used to analyze the expression
levels of Vimentin, PAI-1, COL1 and fibronectin in LY294002- or
wortmannin-treated NRK-52E cells. (B) Western blotting was used to
analyze the expression levels of p-AKT, AKT, Vimentin, COL1 and
fibronectin in LY294002- or wortmannin-treated NRK-52E cells. (C)
Semi-quantification of the expression levels presented in part (B).
n=3/group, western blotting experiments were repeated once.
*P<0.05, **P<0.01, ***P<0.001. Data are presented as the
mean ± SD. LV-Sox9, stabilized Sox9 expressing lentivirus; NC,
negative control; PAI-1, plasminogen activator inhibitor 1; COL1,
collagen 1; p-, phosphorylated. |
Discussion
Numerous studies have demonstrated that Sox9 is as
an important transcription factor involved in organ development,
including the development of the kidney (35,36).
Sox9 activation has also been associated with chronic fibrotic
disorders in the liver (17,20)
and the heart (18). However, to
the best of our knowledge, the role of Sox9 in renal interstitial
fibrosis remains poorly understood. The present study revealed that
the expression levels of Sox9 were upregulated in renal tubular
epithelial cells following obstructive injury, and that the
overexpression of Sox9 in NRK-52E cells promoted tubular EMT and
ECM aggregation. Furthermore, the overexpression of Sox9
upregulated the phosphorylation levels of AKT, while the PI3K
inhibitors, LY29002 and wortmannin, attenuated the phenotype of
fibrosis in NRK-52E cells induced by the overexpression of Sox9.
Thus, these findings indicated that Sox9 may be an important
regulator of renal tubular EMT and ECM aggregation, suggesting its
potential as a novel target for therapeutic interference in
CKD.
Firstly, a UUO model of renal fibrosis was
established and confirmed via detecting EMT and fibrotic markers
and Masson's staining. The expression levels of E-cadherin protein
were notably downregulated at 14 days following UUO, whereas the
mRNA expression levels remained stable. This was consistent with a
previous study by Geng et al (37), which reported that numerous
post-translational modifications may regulate the protein
expression levels of E-cadherin in human breast cancer cells.
Secondly, the role of Sox9 in the development of renal fibrosis
during obstructive injury was investigated. The mRNA and protein
expression levels of Sox9 were significantly upregulated in a
time-dependent manner in the kidney tissue and in tubular
epithelial cells following renal fibrosis. These data suggested a
possible association between Sox9 and renal fibrosis, and are
consistent with a previous study by Kumar et al (38), which reported that the expression
levels of Sox9 were upregulated in renal tubular epithelial cells
in an acute kidney injury (AKI) model. In addition, the activation
of Sox9 was involved in the repair process of renal tubular
epithelial cells following AKI (38). These findings demonstrated the
potential role of Sox9 in the repair process of renal tubular
epithelial cells following AKI; however, the role and underlying
mechanism of action of Sox9 in renal fibrogenesis remains unknown.
An additional study comparing RNA-seq analysis at various time
points during the process from AKI to chronic kidney injury
revealed that the expression levels of Sox9 were maintained, even
weeks after AKI, indicating that Sox9 may not only be involved in
the repair process, but also in the process of chronic fibrosis
(39).
Renal tubular epithelial cells are the primary
target of a variety of metabolic, immunological, ischemic and toxic
insults (40,41). In the tubulointerstitium, renal
tubular epithelial cells were also discovered to serve a
significant role in promoting the release of inflammatory factors
and ECM aggregation through epithelial-mesenchymal communication
(42). In the present study, Sox9
expression levels were upregulated in the renal tubular epithelium
in obstructive nephropathy; thus, indicating a potential important
role of Sox9 in promoting renal tubular EMT and ECM aggregation
following the overexpression of Sox9 in NRK-52E cells. Furthermore,
the overexpression of Sox9 in tubular epithelial cells upregulated
the mRNA and protein expression levels of Vimentin, PAI-1, COL1,
fibronectin and the mRNA expression levels of CTGF, which is
consistent with the characteristic features of renal tubular EMT
and ECM aggregation under renal fibrosis (7–9).
Notably, previous studies have also reported a role for Sox9 in the
process of EMT and ECM aggregation; for example, Sox9 was found
expressed in the cardiac cushions, where it promoted EMT processes
to form valve structures (43). In
addition, Sox9 was identified as a crucial regulator of cardiac
fibrosis during ischemic injury (18,36).
Furthermore, the genetic disruption of Sox9 attenuated liver
fibrosis by downregulating the expression levels of osteopontin,
which is an important component of the ECM and can also promote
fibrosis (20). Notably, in the
present study, the overexpression of Sox9 was discovered to
potentiate the tubular EMT and ECM aggregation induced by TGF-β1.
This finding further indicated that Sox9 may serve a critical role
in the processes of EMT and ECM aggregation in tubular epithelial
cells, following potential upregulation of phosphorylated Sox9
expression levels induced by TGF-β1. These hypotheses were
consistent with a previous study, which reported that TGF-β
promoted the phosphorylation of Sox9 protein expression levels in
chondrocytes (44).
Numerous previous studies have revealed that Sox9
primarily acts through the Wnt/β-catenin signaling pathway in
tumorigenesis and metastasis (45,46).
In addition, the sustained activation of this signaling pathway was
linked to CKD and renal fibrosis in patients and experimental
animal models (47). However, the
present study revealed that the changes in the mRNA and protein
expression levels of β-catenin were not statistically different to
the NC-transfected cells when Sox9 was overexpressed in renal
tubular epithelial cells. Interestingly, it has been suggested that
the detection of β-catenin expression level may not be a sensitive
indicator of the Wnt/β-catenin signaling pathway activation
(48,49). Therefore, additional important
molecules involved in the Wnt/β-catenin signaling pathway were
detected. Unfortunately, no significant differences were observed
in the mRNA expression levels of the Wnt receptor, FZD5, and its
target genes, c-Myc and TCF4, following the overexpression of Sox9
in renal tubular epithelial cells, which indicated that Sox9 may
not activate the Wnt/β-catenin signaling pathway to promote renal
tubular EMT and ECM aggregation in renal tubular epithelial
cells.
The present study identified that Sox9 was involved
in the PI3K/AKT signaling pathway from mRNA transcriptome analysis
using RNA-seq technology. In addition, Sox9 upregulated the
phosphorylation levels of AKT without affecting the total protein
expression levels of AKT. These results are consistent with a
previous study, in which the phosphorylation levels of AKT and the
PI3K subunit, PI3K catalytic subunit α isoform, were downregulated
in chondrocytes following the knockdown of Sox9 (34). Thus, to confirm whether the
phosphorylation of AKT mediated renal tubular EMT and ECM
aggregation in NRK-52E cells overexpressing Sox9, LY29002 and
wortmannin PI3K inhibitors were used; the results revealed that the
inhibition of the PI3K/AKT signaling pathway by the inhibitors
downregulated expression levels of Vimentin, PAI-1, COL1 and
fibronectin in NRK-52E cells overexpressing Sox9. Notably, PI3K
inhibitors have been demonstrated to alleviate tubular EMT and
renal fibrosis (50,51). These observations indicated that
Sox9 may promote tubular EMT and ECM aggregation in NRK-52E cells
through the PI3K/AKT signaling pathway.
Nonetheless, there are several limitations to the
present study. First, the study only investigated the effects of
Sox9 in one cell line; therefore, further studies should be
conducted using other cell lines, such as the NRK-49F cell line,
which are rat renal interstitial fibroblasts, to provide more
definitive evidence on the role of Sox9 in renal fibrosis.
Secondly, the phosphorylation of AKT was also observed in
NC-transfected cells (Fig. 4D).
The PI3K inhibitors, LY29002 and wortmannin, may constantly
suppress the phosphorylation of AKT, therefore further experiments,
such as RNA interference or genetic knockout studies, are required
to determine the association between Sox9 and the PI3K/AKT
signaling pathway.
In conclusion, the results of the present study
revealed that the expression levels of Sox9 were upregulated in the
renal tubular epithelium following obstructive injury and that the
overexpression of Sox9 may promote tubular EMT and ECM aggregation
in vitro through regulating the PI3K/AKT signaling pathway.
These findings have implications for the development of novel
therapeutic approaches to suppress tubular EMT and ECM aggregation
in the kidney.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the Pediatric
Medical Innovation Team of Jiangsu Province (grant. no.
CXTDA2017022), the Primary Research & Development Plan of
Jiangsu Province (grant no. BE2017719) and Longgang District
Economic and Technology Development Special Fund for Medical and
Health Technology Plan Projects of Shenzhen (grant no.
LGKCYLWS2018000128).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
ZX and CG designed the study and analyzed the data;
ZZ and WW performed the majority of the experiments, analyzed the
data and wrote the manuscript; XF and ML helped to perform the
animal experiments; XF and HW helped to perform the histological
and laboratory analysis; and CG provided constructive suggestions.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All experiments were approved by the Ethics
Committee for the Use of Experimental Animals at Jinling Hospital
(Nanjing, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Braun L, Sood V, Hogue S, Lieberman B and
Copley-Merriman C: High burden and unmet patient needs in chronic
kidney disease. Int J Nephrol Renovasc Dis. 5:151–163.
2012.PubMed/NCBI
|
|
2
|
Coresh J, Selvin E, Stevens LA, Manzi J,
Kusek JW, Eggers P, Van Lente F and Levey AS: Prevalence of chronic
kidney disease in the United States. JAMA. 298:2038–2047. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hsu CY, Vittinghoff E, Lin F and Shlipak
MG: The incidence of end-stage renal disease is increasing faster
than the prevalence of chronic renal insufficiency. Ann Intern Med.
141:95–101. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Plantinga LC, Boulware LE, Coresh J,
Stevens LA, Miller ER III, Saran R, Messer KL, Levey AS and Powe
NR: Patient awareness of chronic kidney disease: Trends and
predictors. Arch Intern Med. 168:2268–2275. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jha V, Garcia-Garcia G, Iseki K, Li Z,
Naicker S, Plattner B, Saran R, Wang AY and Yang CW: Chronic kidney
disease: Global dimension and perspectives. Lancet. 382:260–272.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Eddy AA: Overview of the cellular and
molecular basis of kidney fibrosis. Kidney Int Suppl (2011). 4:2–8.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Duffield JS: Cellular and molecular
mechanisms in kidney fibrosis. J Clin Invest. 124:2299–2306. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lovisa S, Zeisberg M and Kalluri R:
Partial epithelial-to-mesenchymal transition and other new
mechanisms of kidney fibrosis. Trends Endocrinol Metab. 27:681–695.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
LeBleu VS, Taduri G, O'Connell J, Teng Y,
Cooke VG, Woda C, Sugimoto H and Kalluri R: Origin and function of
myofibroblasts in kidney fibrosis. Nat Med. 19:1047–1053. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nieto MA, Huang RY, Jackson RA and Thiery
JP: EMT: 2016. Cell. 166:21–45. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gordon CT, Tan TY, Benko S, Fitzpatrick D,
Lyonnet S and Farlie PG: Long-range regulation at the SOX9 locus in
development and disease. J Med Genet. 46:649–656. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Foster JW, Dominguez-Steglich MA, Guioli
S, Kwok C, Weller PA, Stevanović M, Weissenbach J, Mansour S, Young
ID, Goodfellow PN, et al: Campomelic dysplasia and autosomal sex
reversal caused by mutations in an SRY-related gene. Nature.
372:525–530. 1994. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wagner T, Wirth J, Meyer J, Zabel B, Held
M, Zimmer J, Pasantes J, Bricarelli FD, Keutel J, Hustert E, et al:
Autosomal sex reversal and campomelic dysplasia are caused by
mutations in and around the SRY-related gene SOX9. Cell.
79:1111–1120. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Haldin CE and LaBonne C: SoxE factors as
multifunctional neural crest regulatory factors. Int J Biochem Cell
Biol. 42:441–444. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bowen KA, Doan HQ, Zhou BP, Wang Q, Zhou
Y, Rychahou PG and Evers BM: PTEN loss induces
epithelial-mesenchymal transition in human colon cancer cells.
Anticancer Res. 29:4439–4449. 2009.PubMed/NCBI
|
|
16
|
Endo Y, Deonauth K, Prahalad P, Hoxter B,
Zhu Y and Byers SW: Role of Sox-9, ER81 and VE-cadherin in retinoic
acid-mediated trans-differentiation of breast cancer cells. PLoS
One. 3:e27142008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hanley KP, Oakley F, Sugden S, Wilson DI,
Mann DA and Hanley NA: Ectopic SOX9 mediates extracellular matrix
deposition characteristic of organ fibrosis. J Biol Chem.
283:14063–14071. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lacraz GPA, Junker JP, Gladka MM, Molenaar
B, Scholman KT, Vigil-Garcia M, Versteeg D, de Ruiter H, Vermunt
MW, Creyghton MP, et al: Tomo-seq identifies SOX9 as a key
regulator of cardiac fibrosis during ischemic injury. Circulation.
136:1396–1409. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Oh CD, Lu Y, Liang S, Mori-Akiyama Y, Chen
D, de Crombrugghe B and Yasuda H: SOX9 regulates multiple genes in
chondrocytes, including genes encoding ECM proteins, ECM
modification enzymes, receptors, and transporters. PLoS One.
9:e1075772014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pritchett J, Harvey E, Athwal V, Berry A,
Rowe C, Oakley F, Moles A, Mann DA, Bobola N, Sharrocks AD, et al:
Osteopontin is a novel downstream target of SOX9 with diagnostic
implications for progression of liver fibrosis in humans.
Hepatology. 56:1108–1116. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Arvaniti E, Moulos P, Vakrakou A,
Chatziantoniou C, Chadjichristos C, Kavvadas P, Charonis A and
Politis PK: Whole-transcriptome analysis of UUO mouse model of
renal fibrosis reveals new molecular players in kidney diseases.
Sci Rep. 6:262352016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bennett MR, Czech KA, Arend LJ, Witte DP,
Devarajan P and Potter SS: Laser capture microdissection-microarray
analysis of focal segmental glomerulosclerosis glomeruli. Nephron
Exp Nephrol. 107:e30–e40. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sumi E, Iehara N, Akiyama H, Matsubara T,
Mima A, Kanamori H, Fukatsu A, Salant DJ, Kita T, Arai H and Doi T:
SRY-related HMG box 9 regulates the expression of Col4a2 through
transactivating its enhancer element in mesangial cells. Am J
Pathol. 170:1854–1864. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Trapnell C, Hendrickson DG, Sauvageau M,
Goff L, Rinn JL and Pachter L: Differential analysis of gene
regulation at transcript resolution with RNA-seq. Nat Biotechnol.
31:46–53. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ashburner M, Ball CA, Blake JA, Botstein
D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT,
et al: Gene ontology: Tool for the unification of biology. The gene
ontology consortium. Nat Genet. 25:25–29. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kanehisa M, Goto S, Sato Y, Kawashima M,
Furumichi M and Tanabe M: Data, information, knowledge and
principle: Back to metabolism in KEGG. Nucleic Acids Res.
42((Database Issue)): D199–D205. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chevalier RL, Forbes MS and Thornhill BA:
Ureteral obstruction as a model of renal interstitial fibrosis and
obstructive nephropathy. Kidney Int. 75:1145–1152. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Santos JC, Carrasco-Garcia E, Garcia-Puga
M, Aldaz P, Montes M, Fernandez-Reyes M, de Oliveira CC, Lawrie CH,
Araúzo-Bravo MJ, Ribeiro ML and Matheu A: SOX9 elevation acts with
canonical WNT signaling to drive gastric cancer progression. Cancer
Res. 76:6735–6746. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kawai T, Yasuchika K, Ishii T, Miyauchi Y,
Kojima H, Yamaoka R, Katayama H, Yoshitoshi EY, Ogiso S, Kita S, et
al: SOX9 is a novel cancer stem cell marker surrogated by
osteopontin in human hepatocellular carcinoma. Sci Rep.
6:304892016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu JA, Wu MH, Yan CH, Chau BK, So H, Ng
A, Chan A, Cheah KS, Briscoe J and Cheung M: Phosphorylation of
Sox9 is required for neural crest delamination and is regulated
downstream of BMP and canonical Wnt signaling. Proc Natl Acad Sci
USA. 110:2882–2887. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Blache P, van de Wetering M, Duluc I,
Domon C, Berta P, Freund JN, Clevers H and Jay P: SOX9 is an
intestine crypt transcription factor, is regulated by the Wnt
pathway, and represses the CDX2 and MUC2 genes. J Cell Biol.
166:37–47. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ikegami D, Akiyama H, Suzuki A, Nakamura
T, Nakano T, Yoshikawa H and Tsumaki N: Sox9 sustains chondrocyte
survival and hypertrophy in part through Pik3ca-Akt pathways.
Development. 138:1507–1519. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhu Z, Dai J, Liao Y and Wang T: Sox9
protects against human lung fibroblast cell apoptosis induced by
LPS through activation of the AKT/GSK3β pathway. Biochemistry
(Mosc). 82:606–612. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chaboissier MC, Kobayashi A, Vidal VI,
Lützkendorf S, van de Kant HJ, Wegner M, de Rooij DG, Behringer RR
and Schedl A: Functional analysis of Sox8 and Sox9 during sex
determination in the mouse. Development. 131:1891–1901. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Stolt CC, Lommes P, Sock E, Chaboissier
MC, Schedl A and Wegner M: The Sox9 transcription factor determines
glial fate choice in the developing spinal cord. Genes Dev.
17:1677–1689. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Geng F, Zhu W, Anderson RA, Leber B and
Andrews DW: Multiple post-translational modifications regulate
E-cadherin transport during apoptosis. J Cell Sci. 125:2615–2625.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kumar S, Liu J, Pang P, Krautzberger AM,
Reginensi A, Akiyama H, Schedl A, Humphreys BD and McMahon AP: Sox9
activation highlights a cellular pathway of renal repair in the
acutely injured mammalian kidney. Cell Rep. 12:1325–1338. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liu J, Kumar S, Dolzhenko E, Alvarado GF,
Guo J, Lu C, Chen Y, Li M, Dessing MC, Parvez RK, et al: Molecular
characterization of the transition from acute to chronic kidney
injury following ischemia/reperfusion. JCI Insight. 2:e947162017.
View Article : Google Scholar
|
|
40
|
Liu BC, Tang TT, Lv LL and Lan HY: Renal
tubule injury: A driving force toward chronic kidney disease.
Kidney Int. 93:568–579. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Schiessl IM: The role of
tubule-interstitial crosstalk in renal injury and recovery. Semin
Nephrol. 40:216–231. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Tan RJ, Zhou D and Liu Y: Signaling
crosstalk between tubular epithelial cells and interstitial
fibroblasts after kidney injury. Kidney Dis (Basel). 2:136–144.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Garside VC, Cullum R, Alder O, Lu DY,
Vander Werff R, Bilenky M, Zhao Y, Jones SJ, Marra MA, Underhill TM
and Hoodless PA: SOX9 modulates the expression of key transcription
factors required for heart valve development. Development.
142:4340–4350. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Coricor G and Serra R: TGF-β regulates
phosphorylation and stabilization of Sox9 protein in chondrocytes
through p38 and Smad dependent mechanisms. Sci Rep. 6:386162016.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wang H, He L, Ma F, Regan MM, Balk SP,
Richardson AL and Yuan X: SOX9 regulates low density lipoprotein
receptor-related protein 6 (LRP6) and T-cell factor 4 (TCF4)
expression and Wnt/β-catenin activation in breast cancer. J Biol
Chem. 288:6478–6487. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ma F, Ye H, He HH, Gerrin SJ, Chen S,
Tanenbaum BA, Cai C, Sowalsky AG, He L, Wang H, et al: SOX9 drives
WNT pathway activation in prostate cancer. J Clin Invest.
126:1745–1758. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wang Y, Zhou CJ and Liu Y: Wnt signaling
in kidney development and disease. Prog Mol Biol Transl Sci.
153:181–207. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zong Y, Huang J, Sankarasharma D, Morikawa
T, Fukayama M, Epstein JI, Chada KK and Witte ON: Stromal
epigenetic dysregulation is sufficient to initiate mouse prostate
cancer via paracrine Wnt signaling. Proc Natl Acad Sci USA.
109:E3395–E3404. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Placencio VR, Sharif-Afshar AR, Li X,
Huang H, Uwamariya C, Neilson EG, Shen MM, Matusik RJ, Hayward SW
and Bhowmick NA: Stromal transforming growth factor-beta signaling
mediates prostatic response to androgen ablation by paracrine Wnt
activity. Cancer Res. 68:4709–4718. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Du R, Xia L, Ning X, Liu L, Sun W, Huang
C, Wang H and Sun S: Hypoxia-induced Bmi1 promotes renal tubular
epithelial cell-mesenchymal transition and renal fibrosis via
PI3K/Akt signal. Mol Biol Cell. 25:2650–2659. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zeng R, Yao Y, Han M, Zhao X, Liu XC, Wei
J, Luo Y, Zhang J, Zhou J, Wang S, et al: Biliverdin reductase
mediates hypoxia-induced EMT via PI3-kinase and Akt. J Am Soc
Nephrol. 19:380–387. 2008. View Article : Google Scholar : PubMed/NCBI
|