Introduction
The introduction of synthetic small interfering RNAs
(siRNAs) into cells can elicit RNA interference (RNAi), thereby
inhibiting the expression of a targeted mRNA in the cells (1). Therefore, siRNA therapeutics has
great potential to become the next generation of medicines. The
effectiveness of siRNA therapeutics relies on an siRNA delivery
system that can protect siRNAs from serum nucleases and deliver
siRNAs efficiently into cells in a target tissue (2). In particular, cationic liposomes have
been widely investigated for in vitro and in vivo
siRNA delivery (3,4). For systemic siRNA delivery with
cationic liposomes, siRNA/cationic liposome complexes (siRNA
lipoplexes) must be stabilized in the blood by avoiding their
interaction with blood components such as erythrocytes (5). Therefore, polyethylene glycol
(PEG)-modified cationic liposomes are often used for in vivo
siRNA transfection, and a PEG-lipid derivative is incorporated into
the liposomal membrane via a lipid-anchor during the preparation of
liposomes. PEG modification (PEGylation) on the surface of siRNA
lipoplexes can protect siRNA lipoplexes from interaction with blood
components and macrophage capture, and consequently prolong
retention in the blood circulation (6). Despite the advantages of PEGylation,
the presence of PEG on the surface of siRNA lipoplexes severely
decreases the gene-silencing efficiency of siRNA lipoplexes by
preventing cellular uptake and endosomal escape processes, which is
known as the PEG dilemma (6,7).
Generally, PEGylation of siRNA lipoplexes can reduce
siRNA accumulation in the lungs by avoiding agglutination with
blood components. However, PEGylation with 1–2 mol%
PEG-1,2-distearoyl-sn-glycero-3-phosphoethanolamine
(PEG-DSPE) strongly inhibited the gene-silencing effects by siRNA
lipoplexes (8), indicating that
PEGylation with PEG-DSPE abolished gene-silencing by siRNA
lipoplexes with increasing content of PEG-DSPE in liposomal
formulations. Therefore, for the development of siRNA lipoplexes
without the loss of gene-silencing activity by PEGylation, an
optimal amount of PEG-lipid must be included in the PEGylated
liposomal formulation. Regarding PEG-lipid derivatives,
1,2-distearoyl-rac-glycero-3-methylpolyoxyethylene
(PEG-DSG),
1,2-dimyristoyl-rac-glycero-3-methylpolyoxyethylene
(PEG-DMG), and poly(ethylene glycol) cholesteryl ether (PEG-Chol)
have also been used for the PEGylation of cationic liposomes.
Inclusion of PEG-lipid with longer saturated diacyl chains into
siRNA lipoplexes increased the plasma concentration of lipoplexes
after intravenous injection (9),
indicated that the length and saturation of the acyl chain in
PEG-lipid derivatives strongly affected the stability of PEGylated
siRNA lipoplexes in the blood circulation after intravenous
injection. PEGylated siRNA lipoplexes with PEG-DSPE showed
excellent blood circulation compared with those incorporating
PEG-DMG (10). In addition,
PEG-DMG dissociated rapidly from PEGylated lipid
nanoparticle-encapsulated siRNA, but PEG-DSG dissociated slowly
(11). Furthermore, incorporation
of PEG-Chol into cationic liposomes was stabilized in human urine
and enhanced cellular uptake, whereas the incorporation of PEG-DSPE
or PEG-DSG into cationic liposomes effectively prevented the
formation of agglomeration but decreased cellular uptake (12). These findings suggested that
anchors of PEG derivatives in PEGylated siRNA lipoplexes strongly
affected cellular association and stability in the blood
circulation. However, to the best of our knowledge, there are still
few reports about the effects of PEG anchors in PEGylated siRNA
lipoplexes on gene-silencing activity and siRNA
biodistribution.
In this study, to examine the effects of PEG anchors
in PEGylated siRNA lipoplexes on in vitro gene-silencing and
siRNA biodistribution after intravenous injection, we used various
kinds of PEG derivatives, and prepared PEGylated siRNA lipoplexes.
Here, we found that PEGylation of siRNA lipoplexes with PEG-DSG,
PEG-Chol, and PEG-chondroitin sulfate conjugate (PEG-CS) might
improve the systemic stability without loss of transfection
activity by PEGylation.
Materials and methods
Materials
1,2-Dioleoyl-3-trimethylammonium-propane methyl
sulfate salt (DOTAP) was obtained from Avanti Polar Lipids Inc.
(Alabaster, AL, USA). Dimethyldioctadecylammonium bromide (DDAB,
product name: DC-1-18) and
11-((1,3-bis(dodecanoyloxy)-2-((dodecanoyloxy)
methyl)propan-2-yl)amino)-N,N,N-trimethyl-11-oxoundecan-1-aminium
bromide (product name: TC-1-12) were obtained from Sogo
Pharmaceutical Co., Ltd. (Tokyo, Japan).
N-(Methylpolyoxyethylene
oxycarbonyl)-1,2-distearoyl-sn-glycero-3-phosphoethanolamine,
sodium salt (PEG mean molecular weight (MW): 2000, PEG-DSPE,
SUNBRIGHT® DSPE-020CN), N-(Methylpolyoxyethylene
oxycarbonyl)-1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine,
sodium salt (PEG mean MW: 2000, PEG-DPPE, SUNBRIGHT®
PP-020CN), N-(Methylpolyoxyethylene
oxycarbonyl)-1,2-dimyristoyl-sn-glycero-3-phosphoethanolamine,
sodium salt (PEG mean MW: 2000, PEG-DMPE, SUNBRIGHT®
PM-020CN),
1,2-distearoyl-rac-glycero-3-methylpolyoxyethylene (PEG mean
MW: 2000, PEG-DSG, SUNBRIGHT® GS-020),
1,2-dipalmitoyl-rac-glycero-3-methylpolyoxyethylene (PEG
mean MW: 2000, PEG-DPG, SUNBRIGHT® GP-020),
1,2-dimyristoyl-rac-glycero-3-methylpolyoxyethylene (PEG
mean MW: 2000, PEG-DMG, SUNBRIGHT® GM-020),
poly(ethylene glycol) cholesteryl ether (PEG mean MW: 2000,
PEG-Chol, SUNBRIGHT® CS-020), and
1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE,
COATSOME ME-8181) were obtained from NOF Co., Ltd. All other
chemicals were of the highest grade available.
Small interfering RNAs
siRNAs targeting nucleotides of firefly
luciferase (Luc siRNA), non-silencing siRNA (control [Cont] siRNA)
as a negative control for Luc siRNA, and cyanine 5.5
(Cy5.5)-labeled pGL3 luciferase siRNA (Cy5.5-siRNA) were
synthesized by Sigma Genosys. The siRNA sequences of the Luc siRNA
were: Sense strand: 5′-CCGUGGUGUUCGUGUCUAAGA-3′, and antisense
strand: 5′-UUAGACACGAACACCACGGUA-3 (13). The siRNA sequences of the Cont
siRNA were: Sense strand: 5′-GUACCGCACGUCAUUCGUAUC-3′, and
antisense strand: 5′-UACGAAUGACGUGCGGUACGU-3′ (14). The siRNA sequence of Cy5.5-siRNA
was as reported previously (15).
Synthesis of PEG-CS
Methoxypolyethylene glycol-chondroitin sulfate
conjugate (PEG-CS) was produced by periodate oxidation of
chondroitin sulfate C sodium salt (CS, MW 35,000; Wako Pure
Chemical Industries, Ltd.) (16)
and subsequent reductive amination with methoxypolyethylene glycol
amine (mPEG-NH2; MW 2,000, SUNBRIGHT®
MEPA-20H; NOF Co., Ltd.) (17) as
described as follows. After CS (1 g) was dissolved in water (20
ml), NaIO4 (0.3 g) was added and stirred in the dark at
room temperature for 4 h. The mixture was chromatographed with a
Sephadex G50 (GE Healthcare Bio-Science AB) column (3 cm in inner
diameter ×20 cm in length) using 0.1 M NaCl aqueous solution. The
high molecular weight fractions were put into a cellulose tube (MW
cut-off 12,000), and dialyzed against water at 4°C to obtain
aqueous solution of oxidized CS (oxCS). A certain volume of the
remaining solution was freeze-dried, and the amount of the
resultant powder was weighed to determine the yield of oxCS.
The volume of the aqueous solution containing 200 mg
of oxCS was set at 20 ml by evaporation of and/or addition of
water. Then, mPEG-NH2 (100 mg) was added, and
NaBH3CN was added gradually, when the pH of the mixture
was adjusted at 6.4–6.6 with 0.1 M HCl and 0.1 M NaOH. After the
mixture was stirred overnight, NaBH3CN (50 mg) was
further added, and pH of the mixture was adjusted to 6.4–6.6. After
6 h, the mixture was chromatographed using a Sephadex G50 column in
a similar manner. The high molecular weight fractions were gathered
and dialyzed against water in a similar manner. Methoxypolyethylene
glycol amine-introduced CS (PEG-CS) was obtained as powder by
lyophilization of the aqueous solution remaining in the dialysis
tube. The chemical structure of PEG-CS (Fig. 1) was investigated by
1H-NMR measurement, in which the substitution degree was
1:6.7 (mol/mol) of mPEG:(Glc-GalNAc) unit.
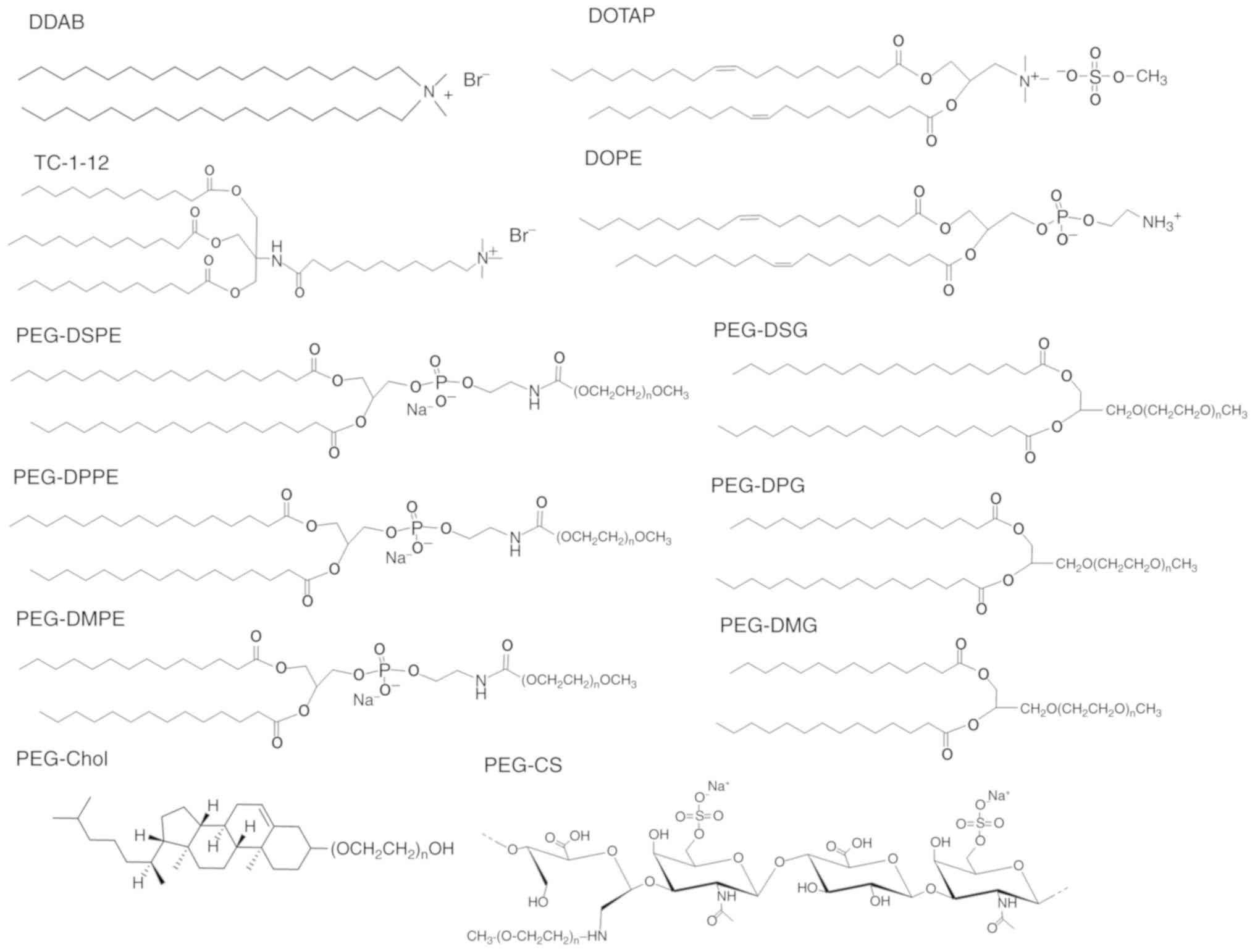 | Figure 1.Structure of cationic lipids, neutral
lipids and PEG-derivatives (n=45 for PEG-DSPE, PEG-DPPE, PEG-DMPE,
PEG-DSG, PEG-DPG, PEG-DMG, PEG-Chol and PEG-CS). DOTAP,
1,2-dioleoyl-3-trimethylammonium-propane methyl sulfate salt; DDAB,
dimethyldioctadecylammonium bromide; TC-1-12,
11-((1,3-bis(dodecanoyloxy)-2-((dodecanoyloxy)methyl)propan-2-yl)amino)-N,N,N-trimethyl-11-oxoundecan-1-aminium
bromide; DOPE,
1,2-dioleoyl-sn-glycero-3-phosphoethanolamine; PEG-DSPE,
N-(methylpolyoxyethylene
oxycarbonyl)-1,2-distearoyl-sn-glycero-3-phosphoethanolamine;
PEG-DPPE, N-(methylpolyoxyethylene
oxycarbonyl)-1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine
sodium salt; PEG-DMPE,
N-(methylpolyoxyethyleneoxycarbonyl)-1,2-dimyristoyl-sn-glycero-3-phosphoethanolamine
sodium salt; PEG-DSG,
1,2-distearoyl-rac-glycero-3-methylpolyoxyethylene; PEG-DPG,
1,2-dipalmitoyl-rac-glycero-3-methylpolyoxyethylene;
PEG-DMG,
1,2-dimyristoyl-rac-glycero-3-methylpolyoxyethylene;
PEG-Chol, poly(ethylene glycol) cholesteryl ether; PEG-CS,
polyoxyethylene-chondroitin sulfate conjugate. |
Preparation of PEGylated cationic
liposomes and siRNA lipoplexes
Cationic liposomes were prepared from DOTAP/DOPE
(composition designated as LP-DOTAP), DDAB/DOPE (composition
designated as LP-DDAB), and TC-1-12/DOPE (composition designated as
LP-TC-1-12), at molar ratios of 1:1. PEGylated cationic liposomes
were incorporated with 1, 2, or 3 mol% PEG-DSPE, PEG-DPPE,
PEG-DMPE, PEG-DSG, PEG-DPG, PEG-DMG, or PEG-Chol into each
liposomal formulation.
For the preparation of cationic liposomes and
PEGylated cationic liposomes using a thin-film hydration method,
cationic lipid, DOPE, and PEG-lipid were dissolved in chloroform,
and chloroform was evaporated under vacuum in a rotary evaporator
at 60°C to obtain a thin film. The thin film was hydrated with
water at 60°C by vortex mixing. The liposomes were sonicated in a
bath-type sonicator (Bransonic® 2510J-MTH, 100 W;
Branson UL Trasonics Co.) for 5–10 min at room temperature.
To prepare cationic liposome/siRNA complexes (siRNA
lipoplexes), each cationic liposome was added to siRNA at a charge
ratio (+:-) of 4:1 with vortex-mixing for 10 sec and left at room
temperature for 15 min. The theoretical charge ratio (+:-) of
cationic liposomes to siRNA is expressed as the molar ratio of
nitrogen of cationic lipid to siRNA phosphate.
To prepare siRNA lipoplexes covered with PEG-CS, the
cationic liposome suspension was mixed with siRNA by vortex-mixing
for 10 s at a charge ratio (+:-) of 4:1, and left for 15 min at
room temperature. To prepare ternary complexes with PEG-CS, siRNA
lipoplexes were mixed with PEG-CS to be 1, 2, or 3 mol% PEGylation
(charge ratios (+:-) of 4:0.9, 4:1.8, and 4:2.7, respectively). The
theoretical charge ratio (+:-) of cationic liposomes to CS in
PEG-CS was calculated as the molar ratio of nitrogen of cationic
lipid to sulfate and carboxylic acid of CS (two negative charges
per disaccharide unit).
Size and ζ-potential of PEGylated
cationic liposomes and siRNA lipoplexes
The particle size distributions of non-PEGylated and
PEGylated cationic liposomes and siRNA lipoplexes were measured by
the cumulant method using a light-scattering photometer (ELS-Z2,
Otsuka Electronics Co., Ltd.) at 25°C after diluting the dispersion
to an appropriate volume with water. The ζ-potentials were measured
using electrophoresis light-scattering methods with the ELS-Z2 at
25°C after diluting the dispersion with an appropriate volume of
water.
Accessibility of siRNA in siRNA
lipoplexes
The association of siRNAs with cationic liposomes
was analyzed using an exclusion assay with SYBR® Green I
Nucleic Acid Gel Stain (Takara Bio Inc.) (13). siRNA lipoplexes were formed at
charge ratios (+:-) of 1:1, 2:1, 3:1, and 4:1. siRNA lipoplexes
with 1 µg of siRNA in a volume of 100 µl of Tris-HCl buffer (pH
8.0) were mixed with 100 µl of 2,500-fold diluted SYBR®
Green I Nucleic Acid Gel Stain solution in Tris-HCl buffer, and
then incubated for 30 min. Fluorescence was measured at an emission
wavelength of 535 nm with an excitation wavelength of 485 nm using
a fluorescence plate reader (ARVO X2; Perkin Elmer). As a control,
the value of fluorescence obtained upon addition of free siRNA
solution was set as 100%. The amount of siRNA available to interact
with the SYBR® Green I was expressed as a percentage of
the control.
Cell culture
Human breast cancer MCF-7 cells stably expressing
firefly luciferase (MCF-7-Luc) constructed by transfection
of plasmid pcDNA3 containing the firefly luciferase (hLuc)
gene from plasmid psiCHECK2 (Promega, Madison, USA) were donated by
Dr. Kenji Yamato (University of Tsukuba, Tsukuba, Japan). MCF-7-Luc
cells were grown in RPMI-1640 medium, supplemented with 10%
heat-inactivated fetal bovine serum (FBS) and 1.2 mg/ml G418 at
37°C in a 5% CO2 humidified atmosphere.
Gene-silencing effects by PEGylated
siRNA lipoplexes in cultured cells
MCF-7-Luc cells were seeded in a 6-well culture
plate at a density of 3×105 cells per well 24 h prior to
transfection. Non-PEGylated and PEGylated siRNA lipoplexes were
formed by the addition of non-PEGylated and PEGylated cationic
liposomes, respectively, into 50 pmol Cont siRNA or Luc siRNA at a
charge ratios (+:-) of 4:1 with vortex-mixing for 10 sec, and left
at room temperature for 15 min. In PEGylated siRNA lipoplexes with
PEG-CS, non-PEGylated lipoplexes with 50 pmol Cont siRNA or Luc
siRNA were mixed with PEG-CS at an indicated mol%, and further left
at room temperature for 15 min. For transfection, each siRNA
lipoplex was diluted in 1 ml of medium supplemented with 10% FBS
and then the mixture was added to the cells (50 pmol siRNA/well).
Forty-eight hours after transfection, the cells were lysed by the
addition of 250 µl of cell lysis buffer (Pierce™ Luciferase Cell
Lysis Buffer, ThermoFisher Scientific Inc.) after washing with PBS,
and subjected to one cycle of freezing (−80°C) and thawing at 37°C,
followed by centrifugation at 15,000 g for 10 sec. Aliquots of 10
µl of the supernatants of cell lysates were mixed with 50 µl of
PicaGene MelioraStar-LT Luminescence Reagent (Toyo Ink Mfg. Co.
Ltd., Tokyo, Japan), and the luminescence was measured as counts
per sec (cps) with a chemoluminometer (ARVO X2). Protein
concentrations of the supernatants were determined with BCA reagent
(Pierce™ BCA Protein Assay kit; Thermo Fisher Scientific, Inc.),
using bovine serum albumin as a standard, and the luciferase
activity (cps/µg protein) was calculated. Luciferase activity (%)
was calculated as relative to the luciferase activity (cps/µg
protein) of untransfected cells.
Cytotoxicity by PEGylated siRNA
lipoplexes
MCF-7-Luc cells were seeded in a 96-well plate 24 h
prior to transfection. Each siRNA lipoplex with 50 pmol Cont siRNA
was diluted in 1 ml of medium supplemented with 10% FBS, and then
the mixture (100 µl) was added to the cells at 50% confluency in
the well (final 50 nM siRNA concentration). After a 24 h incubation
period, cell numbers were determined using a Cell Counting Kit-8
(Dojindo Laboratories). Cell viability was expressed as relative to
the absorbance at 450 nm of untransfected cells.
Agglutination assay
Blood (0.3 ml) was collected from the jugular vein
of one female BALB/c mice (8 weeks of age; Sankyo Labo Service
Corp. Inc.) while under anesthesia by an intraperitoneal injection
of 50 mg/kg body weight of pentobarbital (Nembutal, Dainippon
Pharmaceutical Co., Ltd.). Erythrocytes were collected from the
whole blood at 4°C by centrifugation at 300 g for 3 min and
resuspended in PBS as a 2% (v/v) suspension of erythrocytes.
Non-PEGylated and PEGylated siRNA lipoplexes with 2 µg siRNA were
added to 100 µl of 2% (v/v) erythrocyte suspension, respectively.
After incubation for 15 min at 37°C, the sample was placed on a
glass plate and agglutination was observed by microscopy.
Biodistribution of siRNA after
intravenous injection of PEGylated siRNA lipoplexes into mice
A total of 43 female BALB/c mice (18–20 g, 8 weeks
of age; Sankyo Labo Service Corp. Inc.) were housed in a
temperature (24°C) and humidity (55%) controlled room with a 12 h
light/dark cycle (lights on at 8:00 a.m.) with ad libitum
access to food and water. For all experiments, health and behavior
of the mice were checked daily. Non-PEGylated and PEGylated siRNA
lipoplexes were formed by the addition of non-PEGylated and
PEGylated cationic liposomes, respectively, into 20 µg of
Cy5.5-siRNA with vortex mixing for 10 sec and left at room
temperature for 15 min. In PEGylated siRNA lipoplexes with PEG-CS,
non-PEGylated lipoplexes with 20 µg of Cy5.5-siRNA were mixed with
PEG-CS at an indicated mol%, and further left at room temperature
for 15 min. The non-PEGylated or PEGylated siRNA lipoplexes were
administered intravenously via the lateral tail vein into female
BALB/c mice (n=1 for each siRNA lipoplex). Based on our previous
study (18), mice were sacrificed
at 1 h after intravenous injection of siRNA lipoplexes into mice
(pre-defined endpoint). One hour after injection, mice were
sacrificed by cervical dislocation (a total of 43 mice), and
euthanasia after cervical dislocation was confirmed by careful
assessment of the mice for unambiguous signs of death such as
cardiac arrest. Cy5.5 fluorescence imaging of the tissues was
performed using a NightOWL LB981 NC100 system (Berthold
Technologies). In Cy5.5 fluorescence imaging, the excitation and
emission filters were set at 630/20 and 680/30 nm, respectively.
The exposure time for fluorescence was 5 sec. A grayscale
body-surface reference image was collected using a NightOWL LB981
CCD camera. The images were analyzed using IndiGo2 software
(version 2.0.1.0) provided with the in vivo imaging system
(Berthold Technologies). The tissues after fluorescence imaging
were frozen on dry ice and sliced into 16-µm sections. The
localization of Cy5.5-siRNA was examined using an Eclipse TS100-F
microscope (Nikon).
Statistical analysis
Data are presented as the mean + standard deviation
(SD) of triple determinations. The statistical significance of
differences between mean values was determined by Student's t-test
using GraphPad Prism 4.0 (GraphPad Software Inc.). Multiple
measurement comparisons were performed by analysis of variance
followed by one-way analysis of variance on ranks with post hoc
Tukey test using GraphPad Prism 4.0. P<0.05 was considered to
indicate a statistically significant difference.
Results and Discussion
Characterization of cationic liposomes
and siRNA lipoplexes
For the PEGylation of liposomes, a commonly used
lipid derivative of PEG is PEG-DSPE, and PEG-DSPE is incorporated
into the liposomal membrane via the DSPE anchor during the
preparation of cationic liposomes. In this study, first, to examine
whether the anchor of PEG-lipid derivatives in PEGylated siRNA
lipoplexes affected in vitro gene silencing, we prepared
PEGylated cationic liposomes with various types of PEG-lipid
derivatives. Here, we used DDAB and DOTAP as dialkyl cationic
lipids; and TC-1-12 as a trialkyl cationic lipid (Fig. 1). For the preparation of
non-PEGylated cationic liposomes, LP-DDAB, LP-DOTAP, and LP-TC-1-12
were prepared from DDAB/DOPE, DOTAP/DOPE, and TC-1-12/DOPE,
respectively, at a molar ratio of 1:1. For the preparation of
PEGylated cationic liposomes, 1, 2, or 3 mol% PEG-DSPE, PEG-DPPE,
PEG-DMPE, PEG-DSG, PEG-DPG, PEG-DMG, and PEG-Chol were included
into the formulations of cationic liposomes. For example,
LP-DDAB-PEG-DSPE, LP-DDAB-PEG-DPPE, LP-DDAB-PEG-DMPE,
LP-DDAB-PEG-DSG, LP-DDAB-PEG-DPG, LP-DDAB-PEG-DMG, and
LP-DDAB-PEG-Chol included PEG-DSPE, PEG-DPPE, PEG-DMPE, PEG-DSG,
PEG-DPG, PEG-DMG, and PEG-Chol, respectively, at an indicated mol%
into the formulation of LP-DDAB (Table
I). The sizes of PEGylated cationic liposomes were
approximately 90–120 nm, and the ζ-potentials were approximately
+37–53 mV (Tables I–III). When the liposomes were mixed with
siRNA, the lipoplex sizes were approximately 150–310 nm and their
ζ-potentials were approximately +29–48 mV (Tables I–III).
 | Table I.Particle size and ζ-potential of
DDAB-based liposomes and lipoplexes of small interfering RNA. |
Table I.
Particle size and ζ-potential of
DDAB-based liposomes and lipoplexes of small interfering RNA.
|
| Liposomes |
Lipoplexesb |
|---|
|
|
|
|
|---|
| Liposome | Sizea (nm) | PDI |
ζ-potentiala (mV) | Sizea (nm) | PDI |
ζ-potentiala (mV) |
|---|
| LP-DDAB | 104.2±0.8 | 0.21±0.01 | 48.0±3.6 | 177.3±1.8 | 0.17±0.02 | 43.1±1.0 |
| LP-DDAB-PEG-DSPE (1
mol%) | 100.5±0.6 | 0.23±0.01 | 49.5±0.3 | 198.0±1.4 | 0.22±0.01 | 37.3±0.7 |
| LP-DDAB-PEG-DSPE (2
mol%) | 103.3±0.3 | 0.24±0.01 | 52.5±3.1 | 180.1±0.9 | 0.19±0.01 | 33.4±0.4 |
| LP-DDAB-PEG-DSPE (3
mol%) | 105.9±1.2 | 0.23±0.01 | 43.5±4.1 | 224.1±2.5 | 0.26±0.01 | 28.7±0.6 |
| LP-DDAB-PEG-DPPE (1
mol%) | 107.2±0.9 | 0.26±0.01 | 45.3±0.7 | 151.6±1.4 | 0.19±0.01 | 42.2±1.9 |
| LP-DDAB-PEG-DPPE (2
mol%) |
95.5±1.1 | 0.24±0.01 | 43.6±0.2 | 160.4±2.1 | 0.18±0.01 | 38.9±0.9 |
| LP-DDAB-PEG-DPPE (3
mol%) |
94.0±0.8 | 0.24±0.01 | 43.1±0.9 | 170.5±3.1 | 0.19±0.02 | 38.0±0.2 |
| LP-DDAB-PEG-DMPE (1
mol%) | 104.3±1.0 | 0.26±0.00 | 50.4±0.6 | 189.0±2.6 | 0.21±0.01 | 42.2±0.3 |
| LP-DDAB-PEG-DMPE (2
mol%) | 124.5±3.5 | 0.27±0.00 | 49.3±0.8 | 187.2±4.0 | 0.23±0.01 | 39.4±0.5 |
| LP-DDAB-PEG-DMPE (3
mol%) | 114.5±0.8 | 0.29±0.01 | 48.6±0.8 | 171.0±3.2 | 0.22±0.01 | 37.9±0.5 |
| LP-DDAB-PEG-DSG (1
mol%) |
96.6±2.2 | 0.23±0.01 | 38.0±0.8 | 178.1±0.8 | 0.21±0.01 | 39.3±1.6 |
| LP-DDAB-PEG-DSG (2
mol%) |
94.2±0.6 | 0.22±0.00 | 41.9±3.1 | 188.8±3.4 | 0.23±0.02 | 35.0±0.9 |
| LP-DDAB-PEG-DSG (3
mol%) |
95.1±2.5 | 0.24±0.01 | 45.7±1.5 | 179.9±3.5 | 0.22±0.01 | 35.1±0.9 |
| LP-DDAB-PEG-DPG (1
mol%) | 104.0±1.3 | 0.25±0.01 | 48.5±0.9 | 211.8±1.4 | 0.22±0.01 | 42.1±0.6 |
| LP-DDAB-PEG-DPG (2
mol%) | 113.9±0.9 | 0.25±0.01 | 48.3±0.4 | 209.8±4.3 | 0.25±0.01 | 36.7±2.3 |
| LP-DDAB-PEG-DPG (3
mol%) | 109.0±1.3 | 0.25±0.01 | 50.1±3.7 | 184.6±3.1 | 0.26±0.01 | 34.3±0.7 |
| LP-DDAB-PEG-DMG (1
mol%) | 116.5±0.7 | 0.25±0.01 | 52.3±0.9 | 192.9±1.9 | 0.20±0.01 | 39.8±0.4 |
| LP-DDAB-PEG-DMG (2
mol%) |
93.7±0.7 | 0.23±0.00 | 52.8±0.4 | 234.6±3.3 | 0.24±0.01 | 37.8±0.4 |
| LP-DDAB-PEG-DMG (3
mol%) | 116.7±0.7 | 0.25±0.00 | 50.2±0.7 | 180.5±0.9 | 0.23±0.01 | 34.6±0.3 |
| LP-DDAB-PEG-Chol (1
mol%) | 102.1±1.1 | 0.21±0.01 | 47.6±3.1 | 196.2±4.2 | 0.23±0.01 | 48.3±1.0 |
| LP-DDAB-PEG-Chol (2
mol%) | 108.1±2.6 | 0.15±0.02 | 42.7±1.6 | 177.8±3.2 | 0.19±0.01 | 39.7±1.0 |
| LP-DDAB-PEG-Chol (3
mol%) |
95.4±1.2 | 0.22±0.02 | 43.4±2.2 | 191.6±5.3 | 0.21±0.01 | 35.3±0.9 |
 | Table III.Particle size and ζ-potential of
TC-1-12-based liposomes and lipoplexes of siRNA. |
Table III.
Particle size and ζ-potential of
TC-1-12-based liposomes and lipoplexes of siRNA.
|
| Liposomes |
Lipoplexesb |
|---|
|
|
|
|
|---|
| Liposome | Sizea (nm) | PDI |
ζ-potentiala (mV) | Sizea (nm) | PDI |
ζ-potentiala (mV) |
|---|
| LP-TC-1-12 | 114.9±0.9 | 0.24±0.01 | 46.6±0.6 | 168.6±2.8 | 0.18±0.01 | 41.0±0.9 |
| LP-TC-1-12-PEG-DSPE
(1 mol%) | 98.6±1.1 | 0.24±0.01 | 44.6±1.0 | 314.2±16.3 | 0.31±0.01 | 37.3±0.4 |
| LP-TC-1-12-PEG-DSPE
(2 mol%) | 96.2±1.8 | 0.23±0.00 | 38.5±3.8 | 184.3±2.9 | 0.27±0.01 | 39.1±0.4 |
| LP-TC-1-12-PEG-DSPE
(3 mol%) | 90.9±2.2 | 0.26±0.02 | 48.4±0.9 | 164.0±1.5 | 0.21±0.01 | 34.8±0.2 |
| LP-TC-1-12-PEG-DSG
(1 mol%) | 88.0±0.4 | 0.25±0.01 | 40.1±3.4 | 138.9±1.0 | 0.13±0.01 | 40.1±0.7 |
| LP-TC-1-12-PEG-DSG
(2 mol%) | 107.9±1.8 | 0.24±0.00 | 40.4±1.8 | 157.4±0.8 | 0.15±0.02 | 35.9±0.5 |
| LP-TC-1-12-PEG-DSG
(3 mol%) | 91.1±0.6 | 0.24±0.00 | 50.6±1.4 | 151.7±2.2 | 0.16±0.00 | 42.2±1.3 |
| LP-TC-1-12-PEG-Chol
(1 mol%) | 110.7±2.0 | 0.22±0.02 | 37.9±2.2 | 180.0±4.2 | 0.18±0.02 | 37.1±0.5 |
| LP-TC-1-12-PEG-Chol
(2 mol%) | 118.0±1.0 | 0.25±0.00 | 40.8±1.6 | 182.9±2.5 | 0.16±0.01 | 34.6±0.8 |
| LP-TC-1-12-PEG-Chol
(3 mol%) | 100.7±1.0 | 0.23±0.01 | 47.2±0.7 | 169.6±2.2 | 0.16±0.02 | 34.0±1.0 |
Previously, we developed anionic polymer-coated
siRNA lipoplexes and found that chondroitin sulfate (CS) coatings
for siRNA lipoplexes produced safe systemic vectors (19,20).
Therefore, for the development of PEG-coating via electrostatic
interaction between positively charged siRNA lipoplexes and
negatively charged CS, we synthesized PEG-chondroitin sulfate
conjugate (PEG-CS). CS consists of linear repeating disaccharide
units of glucuronic acid and sulfated galactosamine, and PEG-CS has
linear PEG2000 molecules introduced onto glucuronic acid
of CS in a PEG2000 molecule per approximately 6–7
disaccharide units of CS (Fig. 1).
For the preparation of siRNA lipoplexes coated with PEG-CS,
LP-DOTAP, LP-DDAB, and LP-TC-1-12 were mixed with siRNA a charge
ratio (+:-) of 4:1, and then PEG-CS solution was added to these
siRNA lipoplexes at 1, 2, or 3 mol% PEG in PEG-CS in the
formulation of cationic liposomes. The ζ-potentials of the ternary
complexes after the addition of PEG-CS were almost consistently
negative around 2 mol% PEG in PEG-CS (Table IV), indicating that nitrogen of
the siRNA lipoplex was completely covered with a sulfate group or a
carboxyl group of CS. The sizes of PEGylated siRNA lipoplexes with
PEG-CS were approximately 160–250 nm regardless of the cationic
lipid type in the cationic liposomes.
 | Table IV.Particle size and ζ-potential of
PEG-CS coated siRNA lipoplexes. |
Table IV.
Particle size and ζ-potential of
PEG-CS coated siRNA lipoplexes.
| Lipoplex | Charge
ratioa (+:-:-) | Sizeb (nm) | PDI |
ζ-potentialb (mV) |
|---|
| LP-DOTAP-PEG-CS (1
mol%) | 4:1:0.9 | 250.0±1.7 | 0.20±0.01 | 39.7±0.1 |
| LP-DOTAP-PEG-CS (2
mol%) | 4:1:1.8 | 192.5±2.3 | 0.15±0.03 | −26.0±0.5 |
| LP-DOTAP-PEG-CS (3
mol%) | 4:1:2.7 | 183.6±2.1 | 0.12±0.00 | −32.4±1.1 |
| LP-DDAB-PEG-CS (1
mol%) | 4:1:0.9 | 211.0±0.9 | 0.11±0.01 |
41.6±0.5 |
| LP-DDAB-PEG-CS (2
mol%) | 4:1:1.8 | 165.8±0.6 | 0.12±0.01 | −28.9±1.2 |
| LP-DDAB-PEG-CS (3
mol%) | 4:1:2.7 | 166.8±1.7 | 0.12±0.01 | −39.2±1.0 |
| LP-TC-1-12-PEG-CS
(1 mol%) | 4:1:0.9 | 168.6±1.5 | 0.13±0.02 |
27.0±1.8 |
| LP-TC-1-12-PEG-CS
(2 mol%) | 4:1:1.8 | 212.1±41.1 | 0.10±0.02 | −28.3±1.9 |
| LP-TC-1-12-PEG-CS
(3 mol%) | 4:1:2.7 | 161.6±1.5 | 0.10±0.02 | −26.9±1.1 |
Next, we examined the association of siRNA with each
PEGylated cationic liposome using an exclusion assay with
SYBR® Green I. SYBR® Green I is a
DNA/RNA-intercalating agent whose fluorescence is dramatically
enhanced upon binding to unbound siRNA in cationic liposome
suspension. As a result, in DDAB- and DOTAP-based PEGylated
cationic liposomes, the fluorescence of SYBR® Green I
was markedly decreased by the addition of PEGylated cationic
liposomes into the siRNA solution above charge ratios (+:-) of 2:1,
compared with that in siRNA solution (Fig. 2). This result suggested that siRNAs
were completely bound to each cationic liposome regardless of the
type of PEG-lipid derivatives in DDAB- and DOTAP-based PEGylated
cationic liposomes. In contrast, in TC-1-12-based PEGylated
cationic liposomes, the decreases in fluorescence after the
addition of PEGylated cationic liposomes into the siRNA solution
were saturated at around 20–50% of free siRNA, indicating that
PEGylation in LP-TC-1-12 may partially inhibit the interaction
between siRNA and cationic liposomes. Furthermore, in PEGylated
siRNA lipoplexes with PEG-CS, an increase in unbound siRNA (~30%)
was observed with increasing amounts of PEG-CS added to the siRNA
lipoplexes, suggesting that some siRNA molecules may be released
from the siRNA lipoplexes by the addition of negatively charged
PEG-CS.
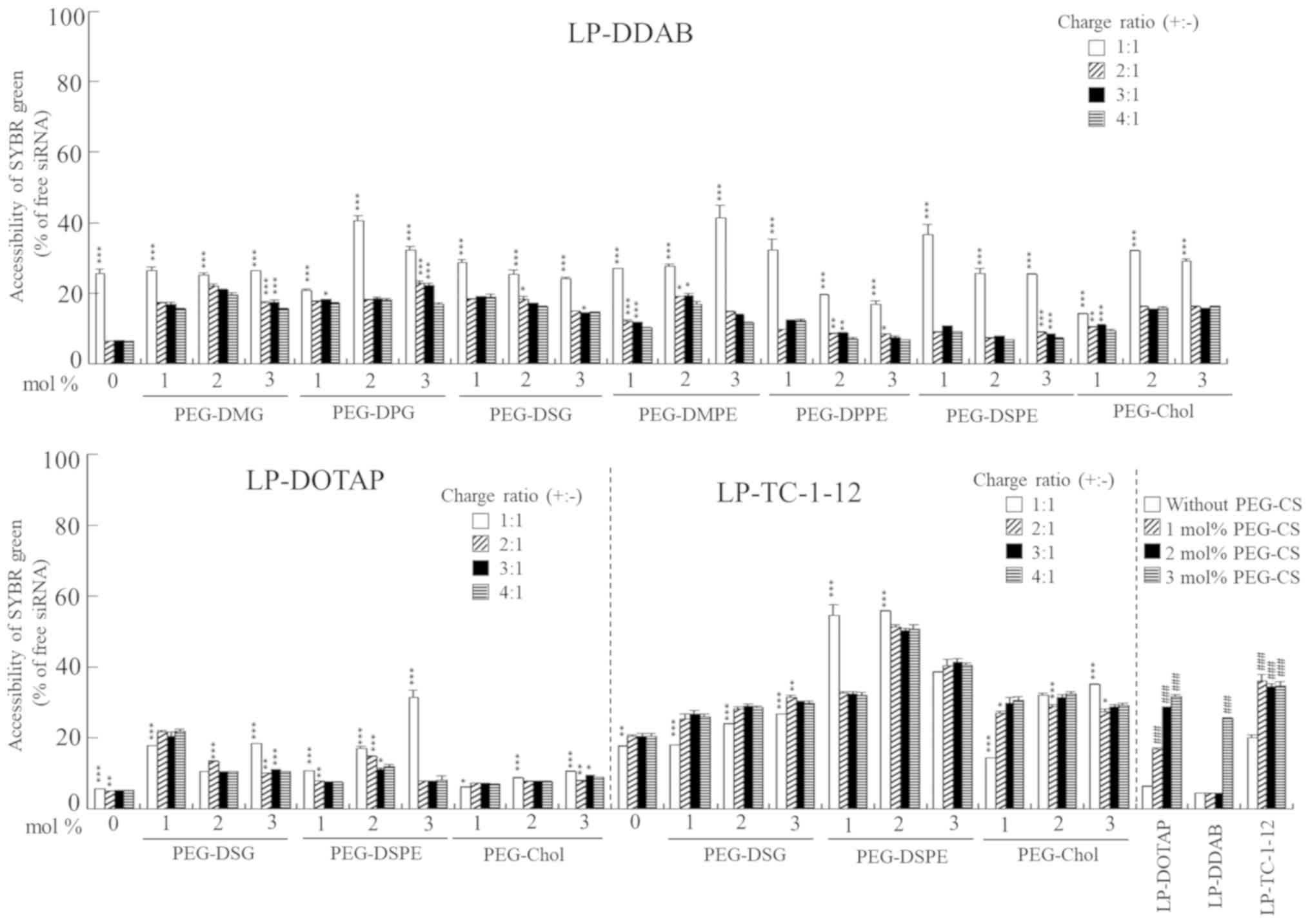 | Figure 2.Association of siRNA with PEGylated
cationic liposomes in an exclusion assay using SYBR®
Green I Nucleic Acid Gel Stain. PEGylated siRNA lipoplexes were
prepared by mixing siRNA with 1, 2 or 3 mol% PEGylated cationic
liposomes at charge ratios (+:-) of 1:1, 2:1, 3:1 and 4:1,
respectively. In PEGylated siRNA lipoplexes with PEG-CS, siRNA
lipoplexes were prepared by mixing siRNA with cationic liposomes at
a charge ratio (+:-) of 4:1. PEG-CS was subsequently added at 1, 2
or 3 mol% PEGylation. As a control, the value of fluorescence
obtained after the addition of free siRNA solution was set as 100%.
The amount of siRNA available to interact with SYBR®
Green I is expressed as a percentage of the control. Each column
represents the mean + SD (n=3). *P<0.05, **P<0.01 and
***P<0.001 vs. a charge ratio (+:-) of 4:1 in PEGylated siRNA
liposomes with PEG-DMG, PEG-DPG, PEG-DSG, PEG-DMPE, PEG-DPPE,
PEG-DSPE and PEG-Chol. ###P<0.001 vs. non-PEGylated
siRNA lipoplexes in PEGylated siRNA lipoplexes with PEG-CS. siRNA,
small interfering RNA; PEG, polyethylene glycol; LP-DDAB, DDAB
liposome; LP-DOTAP, DOTAP liposome; LP-TC-1-12, TC-1-12
liposome. |
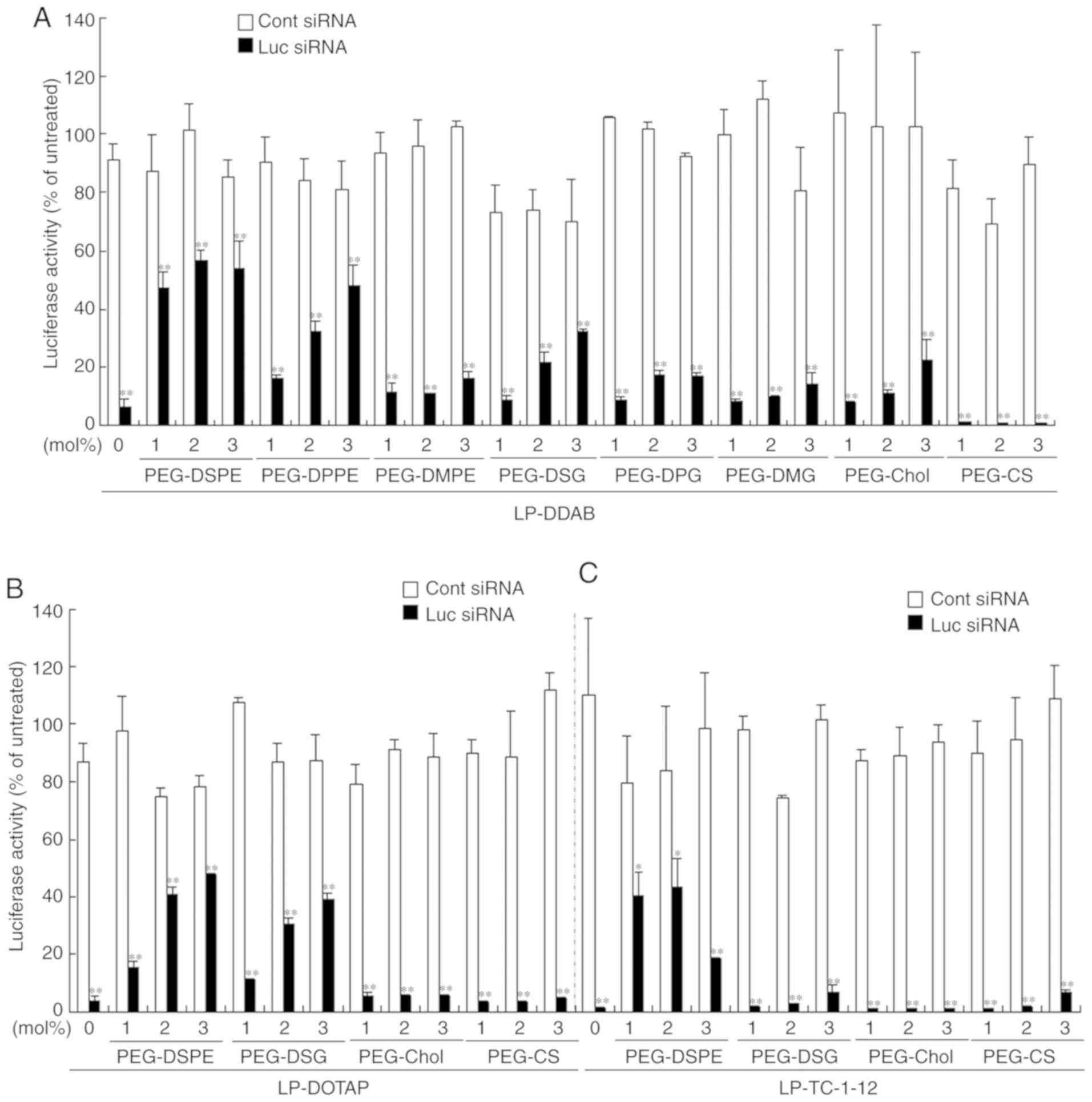
Effects of anchor type of the PEG
derivative in PEGylated siRNA lipoplexes on in vitro gene knockdown
efficacy
To examine the effects of the PEG anchor in
PEGylated siRNA lipoplexes on gene knockdown, MCF-7-Luc cells were
incubated with 1, 2, or 3 mol% PEGylated siRNA lipoplexes at a
final concentration of 50 nM siRNA, and the gene-silencing effects
were assessed by assaying luciferase activity. Non-PEGylated
LP-DOTAP, LP-DDAB, and LP-TC-1-12 lipoplexes with Luc siRNA
strongly suppressed luciferase activity (Fig. 3). In DDAB-based PEGylated siRNA
lipoplexes, LP-DDAB-PEG-DSPE and LP-DDAB-PEG-DPPE lipoplexes
strongly decreased the gene-silencing activity with increasing
amounts of PEG-DSPE and PEG-DPPE, respectively, and LP-DDAB-PEG-DSG
moderately with increasing amounts of PEG-DSG. However,
LP-DDAB-PEG-DMPE, LP-DDAB-PEG-DPG, LP-DDAB-PEG-DMG,
LP-DDAB-PEG-Chol, and LP-DDAB-PEG-CS lipoplexes did not greatly
affect the gene-silencing effects by PEGylation, compared with
non-PEGylated LP-DDAB lipoplexes (Fig.
3A). PEGylation with PEG-lipids with long dialkyl chains
(C16-C18) and a phosphate group trended to inhibit the
gene-silencing effects in the cells by siRNA lipoplexes, indicating
that PEG-lipids with short dialkyl chains and/or without phosphate
groups may be easily detached from PEGylated siRNA lipoplexes in
culture medium. In DOTAP-based PEGylated siRNA lipoplexes,
LP-DOTAP-PEG-DSPE and LP-DOTAP-PEG-DSG lipoplexes decreased the
gene-silencing activity with increasing amounts of PEG-DSPE and
PEG-DSG, respectively; however, LP-DOTAP-PEG-Chol and
LP-DOTAP-PEG-CS lipoplexes did not show an affect by PEGylation,
compared with non-PEGylated LP-DOTAP lipoplexes (Fig. 3B). Furthermore, in TC-1-12-based
PEGylated siRNA lipoplexes, LP-TC-1-12-PEG-DSPE lipoplexes
decreased the gene-silencing activity with increasing amounts of
PEG-DSPE; however, LP-TC-1-12-DSG, LP-TC-1-12-PEG-Chol, and
LP-TC-1-12-PEG-CS lipoplexes did not show an affect by PEGylation,
compared with non-PEGylated LP-TC-1-12 lipoplexes (Fig. 3C). As a result, PEGylation of siRNA
lipoplexes with PEG-DSG, PEG-Chol, and PEG-CS tended not to
markedly inhibit the gene-silencing effects compared with
PEGylation with PEG-DSPE (inhibitory effects in gene-silencing by
PEGylation: PEG-DSPE > PEG-DSG > PEG-Chol > PEG-CS),
indicating that PEG-DSG, PEG-Chol, and PEG-CS may be more easily
released from PEGylated siRNA lipoplexes, compared with PEG-DSPE.
In particular, PEG-CS coating of siRNA lipoplexes did not inhibit
gene-silencing effects by siRNA lipoplexes regardless of the
cationic lipid type, suggesting that PEG coating via electrostatic
interaction may be easily detached from siRNA lipoplexes compared
with those by incorporation of PEG-lipid derivatives into the
liposomal membrane via a lipid anchor. Regarding the PEG-DSPE, the
electrostatic interaction between the phosphate group of PEG-DSPE
and amine group of cationic lipids can remain stably on the surface
of siRNA lipoplexes (10). From
these results, the inhibition of the gene-silencing effects by
PEGylation was largely affected by the anchor of PEG derivatives in
PEGylated siRNA lipoplexes.
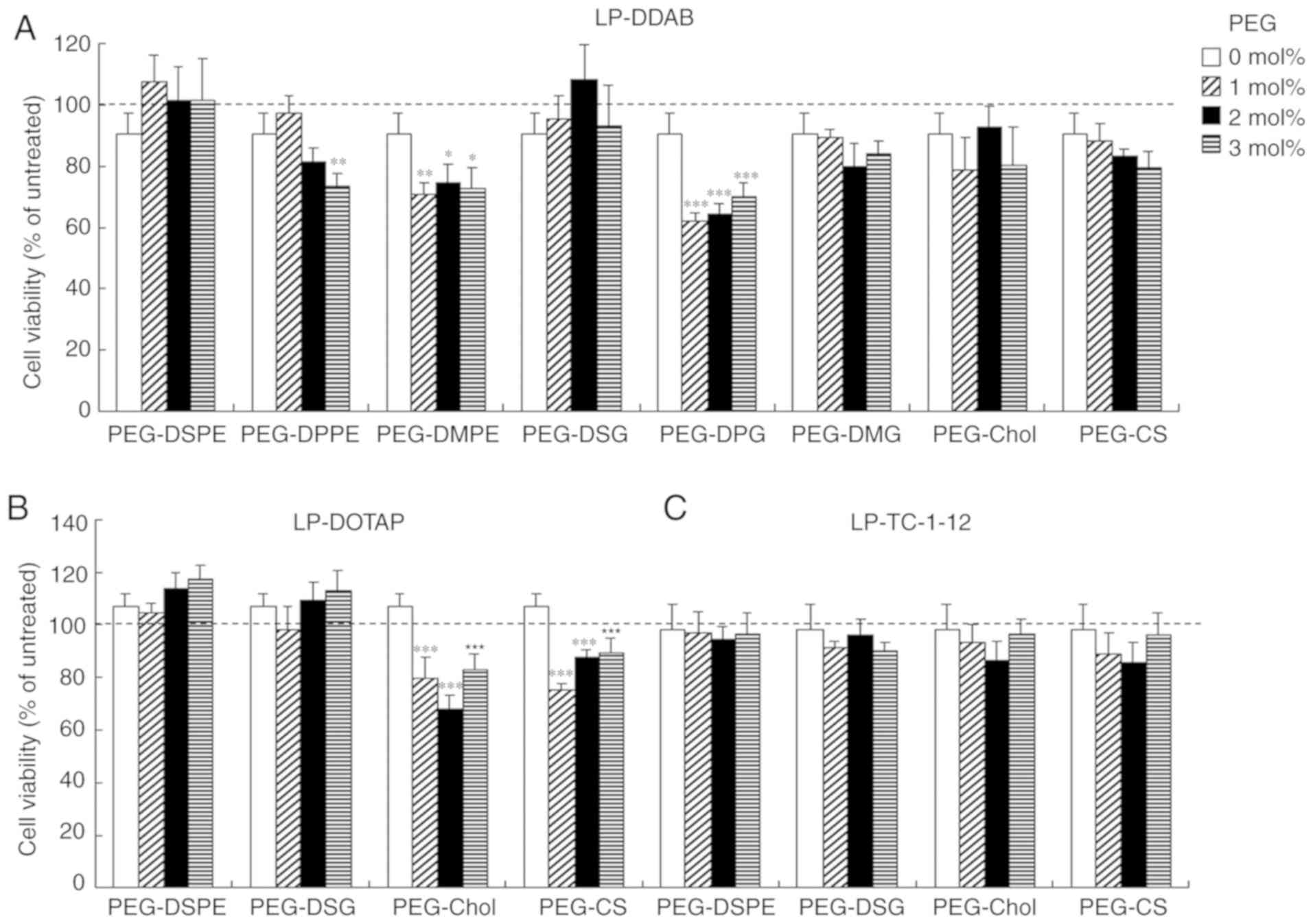
Cytotoxicity by PEGylated siRNA
lipoplexes
To examine the effects of the anchor type of the PEG
derivatives on cytotoxicity by PEGylated siRNA lipoplexes, we
investigated cell viabilities at 24 h after transfection into MCF-7
cells with PEGylated siRNA lipoplexes. Non-PEGylated siRNA
lipoplexes did not induce cytotoxicity regardless of the cationic
lipid types in cationic liposomes (>90% in cell viability)
(Fig. 4). However,
LP-DDAB-PEG-DMPE, LP-DDAB-PEG-DPPE, LP-DDAB-PEG-DPG,
LP-DOTAP-PEG-Chol, and LP-DOTAP-PEG-CS lipoplexes increased
cytotoxicity by PEGylation (60–80% cell viability). In contrast,
the other PEGylated lipoplexes did not show significant
cytotoxicity.
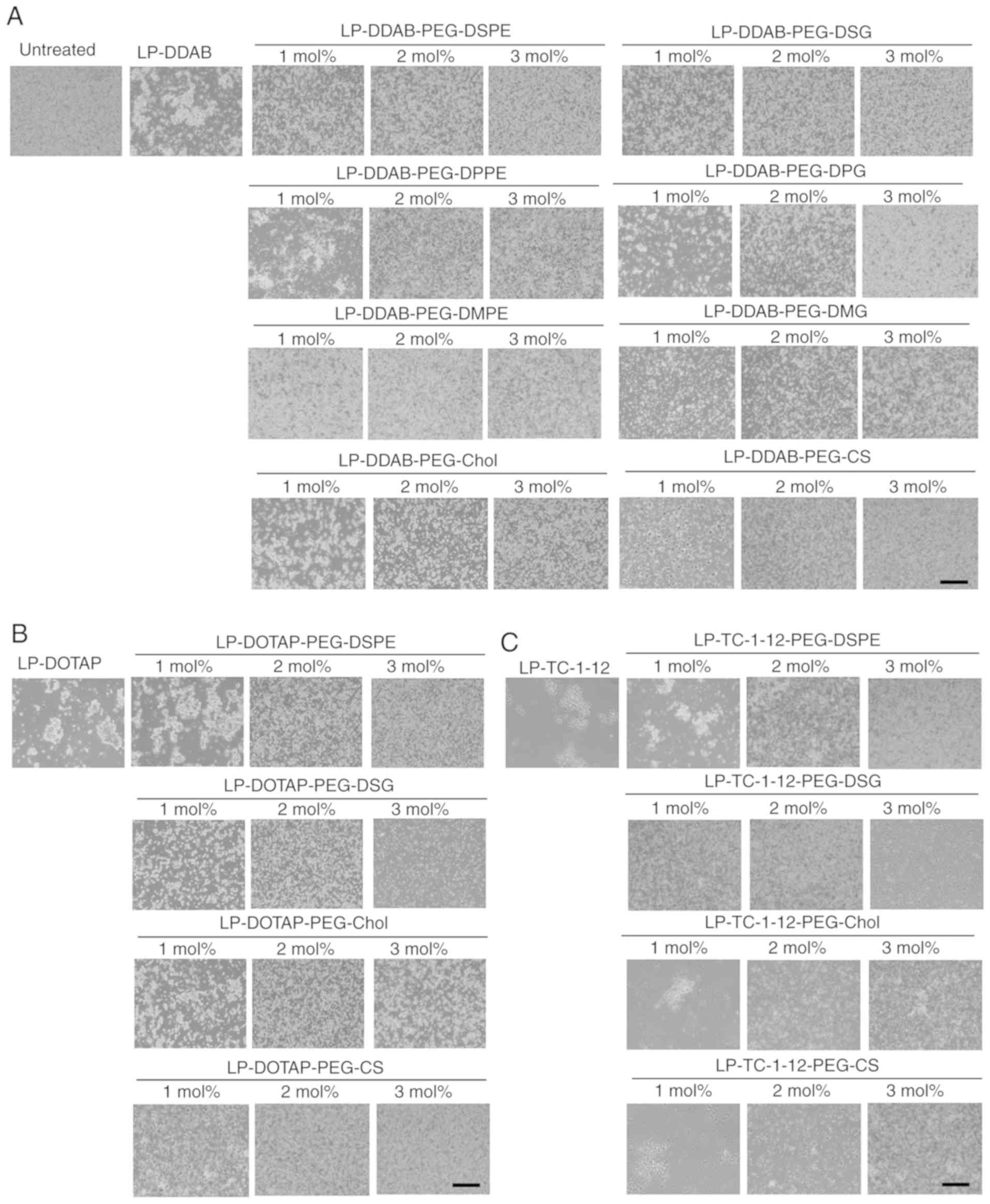
Interaction with erythrocytes and
PEGylated siRNA lipoplexes
To prevent aggregation of siRNA lipoplexes with
blood components such as erythrocytes after systemic injection, the
surface of siRNA lipoplexes has often been modified with PEG. To
examine this effect, non-PEGylated and PEGylated siRNA lipoplexes
were added into erythrocyte suspensions. Of the non-PEGylated
cationic liposomes, all the siRNA lipoplexes induced agglutination
after mixing with the erythrocyte suspension regardless of the
cationic lipid type in the liposomal formulation (Fig. 5). However, in PEGylated siRNA
lipoplexes, PEG on the surface of siRNA lipoplexes may prevent
agglutination with erythrocytes with increasing amounts of
PEG-lipid derivatives or PEG-CS in the liposomal formulation
(Fig. 5). From this result, all
the PEGylated siRNA lipoplexes were able to prevent aggregation
with erythrocytes regardless of the anchor type of the PEG
derivatives.
Biodistribution of siRNA after
intravenous injection of PEGylated siRNA lipoplexes
To investigate the effects of the anchor of PEG
derivatives in PEGylated siRNA lipoplexes on the biodistribution of
siRNA after intravenous injection, we intravenously injected
non-PEGylated and PEGylated lipoplexes with Cy5.5-siRNA into mice
and observed the biodistribution of siRNA at 1 h after injection.
In non-PEGylated siRNA lipoplexes, LP-DOTAP, LP-DDAB, and
LP-TC-1-12 lipoplexes largely accumulated in the lungs (Figs. 6 and 7), indicating that positively charged
siRNA lipoplexes bound to blood components such as erythrocytes in
the blood circulation, and the agglutinates were entrapped in the
highly extended lung capillaries. In contrast, in DDAB-based
PEGylated siRNA lipoplexes, PEGylation of LP-DDAB lipoplexes with
PEG-DSPE, PEG-DSG, PEG-Chol, or PEG-CS largely accumulated in the
liver with increasing amounts of the derivatives (Figs. 6A and 7A), suggested that PEGylation can prevent
agglutination in the blood circulation. However, PEGylation of
LP-DDAB lipoplexes with PEG-DPG, or PEG-DMG did not largely affect
the biodistribution of siRNA even at 3 mol% PEGylation, compared
with non-PEGylated LP-DDAB lipoplexes (Figs. 6A and 7A). These results indicated that PEG-DPG
and PEG-DMG were rapidly released from PEGylated siRNA lipoplexes
in the blood circulation, and did not prevent the interaction with
blood components. In DOTAP-based PEGylated siRNA lipoplexes,
PEGylation of LP-DOTAP lipoplexes with PEG-DSPE, PEG-DSG, PEG-Chol,
or PEG-CS largely accumulated in the liver with increasing amounts
of the PEG derivatives (Figs. 6B
and 7B). Furthermore, in
TC-1-12-based PEGylated siRNA lipoplexes, PEGylation of LP-TC-1-12
lipoplexes with PEG-DSPE largely accumulated in the liver even at 1
mol% PEGylation, and PEGylation of LP-TC-1-12 lipoplexes with
PEG-DSG or PEG-Chol reduced the accumulation of siRNA in the lungs
with increasing amounts of the PEG derivatives (2–3 mol%
PEGylation) (Figs. 6C and 7C). These results suggested that
PEGylation with PEG-DSG, PEG-Chol, and PEG-CS can prevent
aggregation with blood components as well as that with PEG-DSPE
regardless of the cationic lipid types in PEGylated siRNA
lipoplexes.
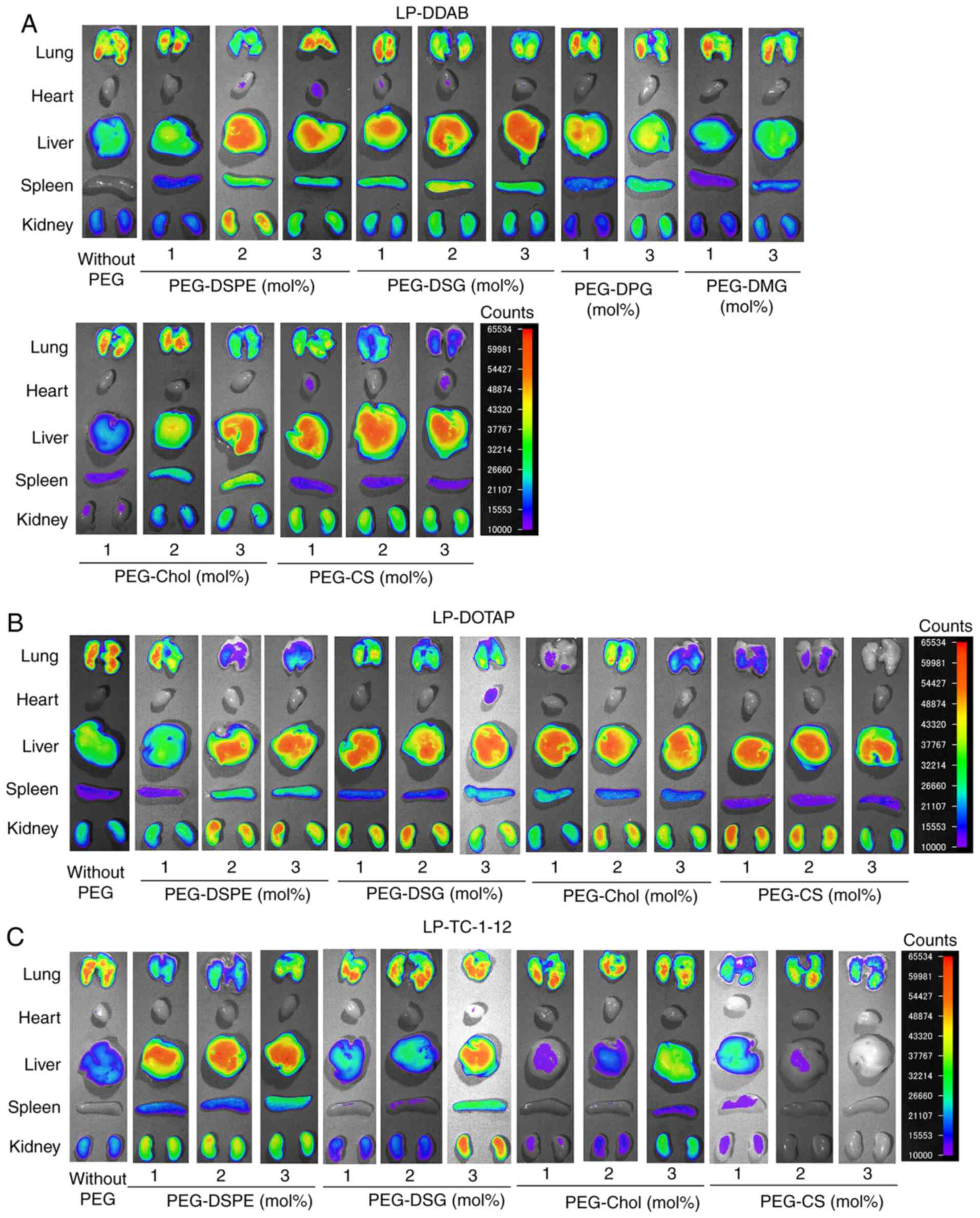 | Figure 6.Effects of PEG-derivatives in
cationic liposomes on the biodistribution of siRNA in mice at 1 h
after intravenous injection with PEGylated siRNA lipoplexes.
Non-PEGylated and PEGylated siRNA lipoplexes were prepared by
mixing non-PEGylated and PEGylated cationic liposomes,
respectively, with 20 µg Cy5.5-siRNA, and were administered
intravenously to mice. Ex vivo images of dissected tissues
were obtained 1 h after intravenous injection. The exposure time
for the detection of Cy5.5 fluorescence was 5 sec. Fluorescence
intensity is illustrated using a color-coded scale (red is maximum,
purple is minimum). (A) LP-DDAB lipoplexes, (B) LP-DOTAP lipoplexes
and (C) LP-TC-1-12 lipoplexes were used. PEG, polyethylene glycol;
siRNA, small interfering RNA; LP-DDAB, DDAB liposome; LP-DOTAP,
DOTAP liposome; LP-TC-1-12, TC-1-12 liposome. |
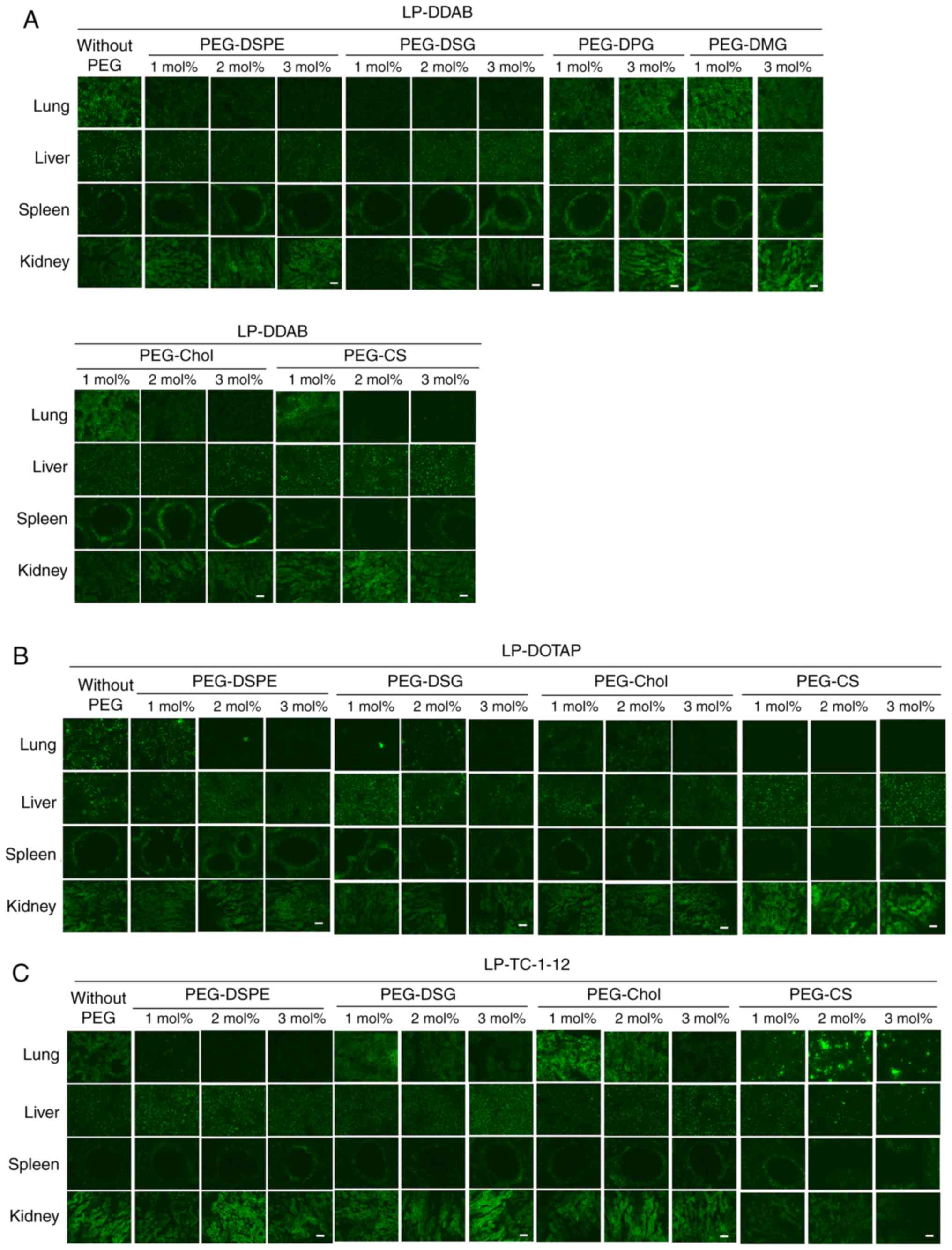 | Figure 7.Effects of PEG-derivatives in
cationic liposomes on the biodistribution of siRNA in mice at 1 h
after intravenous injection of PEGylated siRNA lipoplexes.
Non-PEGylated and PEGylated siRNA lipoplexes were prepared by
mixing non-PEGylated and PEGylated cationic liposomes,
respectively, with 20 µg Cy5.5-siRNA, and were administered
intravenously to mice. (A) LP-DDAB lipoplexes, (B) LP-DOTAP
lipoplexes and (C) LP-TC-1-12 lipoplexes were used. The
localization of Cy5.5-siRNA in tissues was examined 1 h after
intravenous injection. Green signals indicated localization of
Cy5.5-siRNA (scale bar, 100 µm). PEG, polyethylene glycol; siRNA,
small interfering RNA; LP-DDAB, DDAB liposome; LP-DOTAP, DOTAP
liposome; LP-TC-1-12, TC-1-12 liposome. |
In DDAB-based siRNA lipoplexes, injection of
PEGylated siRNA lipoplexes with PEG lipid derivatives with dialkyl
(C14-C16) chains induced siRNA accumulation in the lungs, whereas
dialkyl (C18) chains induced siRNA accumulation in the liver
(Figs. 6A and 7A). LP-DDAB-PEG-DSPE and LP-DDAB-PEG-DSG
lipoplexes prevented agglutination with erythrocytes in the blood
circulation and led to an increase in the accumulation of siRNA in
the liver. In contrast, LP-DDAB-PEG-DPG and LP-DDAB-PEG-DMG
lipoplexes accumulated in the lungs after intravenous injection,
indicated that PEG-DPG and PEG-DMG may be quickly released from
siRNA lipoplexes in blood circulation, resulting in agglutination
with erythrocytes. These results indicated that siRNA
biodistribution after intravenous injection of siRNA lipoplexes was
strongly affected by the length of the PEG anchor in PEGylated
siRNA lipoplexes. Moreover, regardless of the cationic lipid types
in cationic liposomes, PEGylation with PEG-DSG, PEG-Chol, and
PEG-CS can prevent aggregation with erythrocytes (Fig. 5) and decrease the accumulation in
the lungs (Figs. 6 and 7) as well as that with PEG-DSPE although
they did not markedly inhibit in vitro gene-silencing
effects (Fig. 3). These results
suggested that PEG-DSG, PEG-Chol, and PEG-CS in PEGylated siRNA
lipoplexes may be gradually released from the lipoplexes;
therefore, PEGylation with PEG-DSG, PEG-Chol, and PEG-CS may
temporally stabilize siRNA lipoplexes in the blood circulation,
resulting in a decrease in siRNA accumulation in the lungs. As a
result, the PEGylated siRNA lipoplexes with PEG-DSG, PEG-Chol, and
PEG-CS can accumulate in the liver without a loss of transfection
activity by PEGylation; therefore, they may have potential as
vectors for the delivery of siRNA therapeutics to the liver or
liver metastasis. However, further studies should be performed to
examine the effects of PEG anchors in PEGylated siRNA lipoplexes on
hepatic toxicity and in vivo gene-silencing activity after
intravenous injection.
Generally, stable PEGylation of siRNA lipoplexes
strongly inhibits cellular uptake and endosomal escape, which
result in a significant loss of RNAi activity. For successful siRNA
delivery in vivo, a crucial problem associated with the use
of PEG must be overcome. One way to solve this problem is to use
cleavable PEG-lipid derivatives that release the PEG moiety by
cleavage of a linker between PEG and the anchor lipid when exposed
to the appropriate stimulus at the target site (7,21).
Most cleavable PEG derivatives have been designed to be cleaved in
response to the extracellular or intracellular microenvironment
such as temperature, pH, specific enzymes, etc. However, the
cleavage of a linker between PEG and the anchor lipid in response
to a stimulus may be inefficient in some cases because PEG on the
surface of siRNA lipoplexes will reduce association with stimuluses
such as specific enzymes. Another strategy is to use releasable PEG
derivatives that are released from siRNA lipoplexes as time
advances although these can prevent non-specific association in
blood circulation. The advantage of using releasable PEG
derivatives is the ease and relatively low cost of preparation of
PEGylated liposomes. Therefore, PEGylation of siRNA lipoplexes with
releasable PEG-DSG, PEG-Chol, and PEG-CS may have the potential to
improve systemic stability without a loss of transfection activity
by PEGylation.
PEGylation of siRNA lipoplexes generally lowers the
efficiency of siRNA-mediated gene-silencing in vitro and
in vivo. In this study, we examined the effects of PEG
anchors in PEGylated siRNA lipoplexes on in vitro
gene-silencing effects and biodistribution after intravenous
injection. PEGylation of siRNA lipoplexes with PEG-DSG, PEG-Chol,
and PEG-CS tended not to greatly inhibit the gene-silencing effects
compared with PEGylation with PEG-DSPE. However, PEG-DSG, PEG-Chol,
and PEG-CS in PEGylated siRNA lipoplexes may prevent agglutination
in the blood circulation and decrease the accumulation of siRNA in
the lungs after systemic injection. From these findings, releasable
PEGylation of siRNA lipoplexes with PEG-DSG, PEG-Chol, and PEG-CS
may improve the systemic stability after intravenous injection
without loss of transfection activity by PEGylation.
Acknowledgements
The authors would like to thank Ms. Naho Egashira,
Ms. Asuka Sasaki, Ms. Yui Sugawara, Ms. Huka Nakajima, Ms. Azumi
Otake, Mr. Ryota Shimizu, Ms. Yukiha, Mori and Ms. Haruka Takahashi
(Department of Molecular Pharmaceutics, Hoshi University) for
assistance with experimental work (in vitro gene-silencing
effect).
Funding
The current project was supported in part by a
Grant-in-Aid for Scientific Research (C) from the Japan Society for
the Promotion of Science (grant no. 20K07142).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YH conceived and designed the study. Experiments
were performed by KT, SS, KO and HO. YH wrote the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
All animal experiments were conducted in accordance
with the ‘Guide for the Care and Use of Laboratory Animals’ adopted
by the Institutional Animal Care and Use Committee of Hoshi
University (Tokyo, Japan; accredited by the Ministry of Education,
Culture, Sports, Science and Technology). Ethical approval for the
current study was obtained from the Institutional Animal Care and
Use Committee of Hoshi University (approval no. 30-072).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wilson RC and Doudna JA: Molecular
mechanisms of RNA interference. Annu Rev Biophys. 42:217–239. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen X, Mangala LS, Rodriguez-Aguayo C,
Kong X, Lopez-Berestein G and Sood AK: RNA interference-based
therapy and its delivery systems. Cancer Metastasis Rev.
37:107–124. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang S, Zhi D and Huang L: Lipid-based
vectors for siRNA delivery. J Drug Target. 20:724–735. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zatsepin TS, Kotelevtsev YV and
Koteliansky V: Lipid nanoparticles for targeted siRNA
delivery-going from bench to bedside. Int J Nanomedicine.
11:3077–3086. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang Y, Satterlee A and Huang L: In vivo
gene delivery by nonviral vectors: Overcoming hurdles? Mol Ther.
20:1298–1304. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xia Y, Tian J and Chen X: Effect of
surface properties on liposomal siRNA delivery. Biomaterials.
79:56–68. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hatakeyama H, Akita H and Harashima H: The
polyethyleneglycol dilemma: Advantage and disadvantage of
PEGylation of liposomes for systemic genes and nucleic acids
delivery to tumors. Biol Pharm Bull. 36:892–899. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hattori Y, Shimizu S, Ozaki KI and Onishi
H: Effect of cationic lipid type in Folate-PEG-modified cationic
liposomes on folate receptor-mediated siRNA transfection in tumor
cells. Pharmaceutics. 11:1812019. View Article : Google Scholar
|
|
9
|
Sonoke S, Ueda T, Fujiwara K, Sato Y,
Takagaki K, Hirabayashi K, Ohgi T and Yano J: Tumor regression in
mice by delivery of Bcl-2 small interfering RNA with pegylated
cationic liposomes. Cancer Res. 68:8843–8851. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Song F, Sakurai N, Okamoto A, Koide H, Oku
N, Dewa T and Asai T: Design of a novel PEGylated liposomal vector
for systemic delivery of siRNA to solid tumors. Biol Pharm Bull.
42:996–1003. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen S, Tam YY, Lin PJ, Leung AK, Tam YK
and Cullis PR: Development of lipid nanoparticle formulations of
siRNA for hepatocyte gene silencing following subcutaneous
administration. J Control Release. 196:106–112. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nakamura T, Noma Y, Sakurai Y and
Harashima H: Modifying cationic liposomes with cholesteryl-PEG
prevents their aggregation in human urine and enhances cellular
uptake by bladder cancer cells. Biol Pharm Bull. 40:234–237. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hattori Y, Nakamura T, Ohno H, Fujii N and
Maitani Y: siRNA delivery into tumor cells by lipid-based
nanoparticles composed of hydroxyethylated cholesteryl triamine.
Int J Pharm. 443:221–229. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hattori Y, Hara E, Shingu Y, Minamiguchi
D, Nakamura A, Arai S, Ohno H, Kawano K, Fujii N and Yonemochi E:
siRNA delivery into tumor cells by cationic cholesterol
derivative-based nanoparticles and liposomes. Biol Pharm Bull.
38:30–38. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hattori Y, Arai S, Kikuchi T, Ozaki K,
Kawano K and Yonemochi E: Therapeutic effect for liver-metastasized
tumor by sequential intravenous injection of anionic polymer and
cationic lipoplex of siRNA. J Drug Target. 24:309–317. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dawlee S, Sugandhi A, Balakrishnan B,
Labarre D and Jayakrishnan A: Oxidized chondroitin
sulfate-cross-linked gelatin matrixes: A new class of hydrogels.
Biomacromolecules. 6:2040–2048. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sasatsu M, Onishi H and Machida Y:
Preparation of a PLA-PEG block copolymer using a PLA derivative
with a formyl terminal group and its application to nanoparticulate
formulation. Int J Pharm. 294:233–245. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hattori Y, Nakamura A, Hanaya S, Miyanabe
Y, Yoshiike Y, Kikuchi T, Ozaki KI and Onishi H: Effect of
chondroitin sulfate on siRNA biodistribution and gene silencing
effect in mice after injection of siRNA lipoplexes. J Drug Deliv
Sci Tech. 41:401–409. 2017. View Article : Google Scholar
|
|
19
|
Hattori Y, Nakamura A, Arai S, Nishigaki
M, Ohkura H, Kawano K, Maitani Y and Yonemochi E: In vivo siRNA
delivery system for targeting to the liver by poly-l-glutamic
acid-coated lipoplex. Results Pharma Sci. 4:1–7. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hattori Y, Yamasaku H and Maitani Y:
Anionic polymer-coated lipoplex for safe gene delivery into tumor
by systemic injection. J Drug Target. 21:639–647. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fang Y, Xue J, Gao S, Lu A, Yang D, Jiang
H, He Y and Shi K: Cleavable PEGylation: A strategy for overcoming
the ‘PEG dilemma’ in efficient drug delivery. Drug Deliv. 24
(sup1):22–32. 2017. View Article : Google Scholar : PubMed/NCBI
|